Abstract
Objectives
In this prospective case–control study, we explored the regulatory roles of the NLRP3 inflammasome in hepatitis B virus-associated acute-on-chronic liver failure (HBV-ACLF).
Methods
Thirty patients with HBV-ACLF, 30 patients with chronic hepatitis B, and 30 healthy individuals were enrolled. Real-time reverse transcription polymerase chain reaction was used to assess mRNA levels in peripheral blood mononuclear cells and serum protein levels were assessed by enzyme-linked immunosorbent assay.
Results
Serum levels of alanine aminotransferase, asparagine aminotransferase, total bilirubin, and direct bilirubin in patients with HBV-ACLF were increased. Transcript levels of NLRP3 and ASC and protein levels of interleukin (IL)-1β, IL-18, and sCD40L were elevated in patients with HBV-ACLF. Expression of the NLRP3 inflammasome signaling pathway components procaspase-1 and pro-IL-1β was elevated in patients with HBV-ACLF.
Conclusions
This prospective case-control study demonstrated that significant activation of the NLRP3 inflammasome occurs in patients with HBV-ACLF. The activated NLRP3 inflammasome mediated liver failure by stimulating procaspase-1 and pro-IL-1 β and regulating downstream CD40-CD40L signaling.
Keywords: NLRP3, hepatitis B virus, liver failure, inflammasome, CD40-CD40L, case–control study
Introduction
Liver failure occurs when the liver is damaged and is no longer able to function because of hepatocyte necrosis.1 Liver failure develops rapidly and leads to severe illness and high mortality.2 The pathogenesis of liver failure is complex and is related to many factors including viral infections, alcohol, drugs, autoimmune diseases, hepatotoxic substance damage, and inflammatory immune damage.3,4 The major cause of liver failure in China is hepatotropic viral infection, especially by hepatitis B virus (HBV). The main clinical manifestation of hepatotropic viral infection is acute-on-chronic liver failure (ACLF), which has showed increasing incidence in recent years.5 The mechanisms underlying HBV-associated ACLF (HBV-ACLF) are complex.6 Therefore, studies investigating the mechanisms of HBV-ACLF are needed.6
Inflammatory responses are characteristic manifestations of acute and chronic liver diseases.7,8 Inflammasomes are multi-protein complexes in cells that are involved in inflammation and immune responses during infection, diabetes, atherosclerosis, and cancer.9 Inflammasomes can prevent infection and maintain homeostasis by promoting activation of cellular factors such as interleukin (IL)-18 and IL-1β, inducing programmed cell death, initiating inflammatory responses, and activating immune responses.10 There are two main receptor proteins for inflammasomes: the NOD-like receptor (NLR) protein family and the AIM2-like receptor (ALR) protein family.11 NLRs are mainly expressed in immune cells and modulate inflammatory responses and natural immune responses through recognition of intracellular pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns.12,13 There are many activation signals for NLR family proteins. The activation signals for NLRP3 are the most fully understood and include components of pathogens (e.g., bacteria, viruses, and fungi)14,15 as well as non-pathogen molecules (e.g., adenosine triphosphate, uric acid, silicon crystal, and amyloid β).16,17 Recognition of PAMPs by NLRP3 inflammasomes plays important roles in antibacterial immune responses.18
NLRP3 is an important component of inflammasomes, and can stimulate the maturation of precursors of the caspase-1-dependent cytokines IL-1β and IL-18 (pro-IL-1β and pro-IL-18).19 The NLRP3 inflammasome has been connected to various hepatopathies, and its activation is implicated in hepatic injury.20 Levels of NLRP3 in normal hepatic tissue are low. However, following injury, the numbers of neutrophils and monocytes in liver tissue are significantly increased, and NLRP3 expression in nonparenchymal cells such as Kupffer cells was increased in a CCL4-induced model of liver injury.21 Following ischemia/reperfusion injury, reactive oxygen species mediate the activation of NLRP3 and ALR inflammasomes to induce inflammatory responses. Kupffer cells also play important roles in this process.22 NLRP3 inflammasomes can potentiate inflammatory responses induced by galactosamine (GalN)/lipopolysaccharide (LPS) following the interaction between thioredoxin interacting protein and NLRP3. By contrast, heme oxygenase-1 can protect the liver by inhibiting NLRP3-associated signaling and antagonizing inflammation induced by GalN/LPS.23 Therefore, NLRP3 may be an important regulator of oxidative stress and inflammatory diseases. After stimulation of monocytes with LPS, expression of IL-1β in the hepatic tissues of patients with ACLF was elevated compared with healthy individuals, while IL-1β levels in patients with advanced ACLF are decreased.24 Therefore, understanding the role of NLRP3 in hepatic failure is crucial.
This prospective case–control study investigated the role of NLRP3 inflammasome activation in liver failure. The results may be helpful in developing novel therapies for hepatic failure.
Materials and methods
Patients
HBV-ACLF patients were selectively recruited. In addition, patients with chronic hepatitis B (CHB) with a similar age range were also enrolled. Healthy individuals were enrolled as the healthy control (HC) group. There was no special screening for sex in the three groups. We used the Model for End Stage Liver Disease (MELD) scoring system to grade liver disease. The system is mainly based on the international normalized ratio (INR), serum total bilirubin (TBIL), and serum creatinine. Patients with cirrhosis had clinical manifestations of decompensated liver function prior to inclusion. Patients with a previous history of HBV or hepatitis B surface antigen (HBsAg) positive test results for more than 6 months as well as current positive results for HBsAg and/or HBV DNA were diagnosed with CHB. The study was approved by The First Affiliated Hospital of Guangxi Medical University (ID: IACUC-20170301-03). Written informed consent was obtained from all patients and/or their families.
The inclusion criteria were: (i) age 18 to 65 years; (ii) history of HBV or positive HBsAg test for more than 6 months; and (iii) clinical manifestations of decompensated liver function (TBIL ≥ 171.1 mol/L and INR ≥ 1.5) meeting the standards of the Guideline of Prevention and Treatment for CHB (2015 update) and Guidelines for Diagnosis and Treatment of Liver Failure (2018 edition). The exclusion criteria were: (i) other hepatitis virus infections; (ii) alcoholic, drug or autoimmune liver diseases; (iii) tumors; and (iv) serious systemic diseases. Peripheral blood was collected and centrifuged at 3500 × g for 5 minutes to separate serum or 1000 × g for 10 minutes to isolate plasma. To isolate peripheral blood mononuclear cells (PBMCs), 5 mL of plasma was thoroughly mixed with 5 mL of phosphate-buffered saline, then gently dispensed on top of 4 mL of Ficoll. After centrifugation at 2500 × g for 20 minutes, the middle PBMC layer was aspirated and diluted to 10 mL with phosphate-buffered saline. After centrifuging the sample at 1500 × g for 10 minutes, the upper layer of liquid was removed. PBMCs were mixed with cryopreservation solution and stored at −80°C.
We followed the EQUATOR guidelines for reporting observational studies (https://www.equator-network.org/reporting-guidelines/reporting-participation-in-case-control-studies/).
Quantitative reverse transcription polymerase chain reaction (RT-qPCR)
TRIzol reagent (1 mL) was used to lyse 3 × 106 cells. Total RNA was obtained by phenol chloroform extraction. The concentration and quality of RNA was assessed using a Nanodrop ND2000 spectrophotometer (Thermo Scientific, Waltham, MA, USA). RNA (1 μg) was reverse transcribed into cDNA using the TIANScript II cDNA First Strand Synthesis Kit (Tiangen, Beijing, China) and stored at −20°C.
To assess mRNA abundance of NLRP3, ASC, procaspase-1, and pro-IL-1β, RT-qPCR was carried out using the SuperReal PreMix (SYBR Green) qRT-PCR kit (Tiangen). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as the internal reference. The primer sequences for NLRP3 were 5′-CTATCTGTTCTATATCCACTGTCG-3′ (forward) and 5′-GAGGAAGAGGATTCTGGAGGGT-3′ (reverse). The primer sequences for ASC were 5′-CGCCGAGGAGCTCAAGAAGT-3′ (forward) and 5′-GCTCGGCGCCGTAGGTCTC-3′ (reverse). The primer sequences for GAPDH were 5′-TGACGTGGACATCCGCAAAG-3′ (forward) and 5′-CTGGAAGGTGGACAGCGAGG-3′ (reverse). Each RT-qPCR reaction consisted of 10 μL of SYBR Premix EXTaq, 0.5 μL of forward primer, 0.5 μL of reverse primer, 2 μL of cDNA, and 7 μL of ddH2O. The reactions were incubated at 94°C for 52 minutes (initial denaturation) followed by 46 cycles of 94°C for 30 s (denaturation), 55°C for 30 s (annealing); and 72°C for 30 s (elongation). To determine relative abundance of NLRP3 mRNA compared with GAPDH mRNA, the 2−ΔΔCq method25 was employed (n = 3).
Enzyme-linked immunosorbent assay (ELISA)
To measure the amount of IL-18, procaspase-1 and pro-IL-1β in liquid samples, ELISA kits (Abcam, Cambridge, UK) were used according to the manufacturer’s instructions. First, serum samples were diluted in microplate wells and incubated at room temperature for 2.5 hours. The plate was washed, and biotin-labeled antibody was added. After incubating at room temperature for 1 hour, the plate was washed again and horseradish peroxidase-streptavidin was added. After additional incubation at room temperature for 45 minutes, the plate was washed again. After addition of chromogenic solution into each well, the plate was incubated at room temperature for 30 minutes. Following addition of stop solution to wells, a microplate reader was immediately used to read the absorbance of each microplate well at 450 nm. Standard curves were used to calculate concentrations.
Statistical analyses
All data were analyzed using SPSS version 22.0 (IBM Corp., Armonk, NY, USA). Data were expressed as means ± standard deviations. To assess differences between two groups, independent sample t-tests were carried out. Comparisons among more than two groups was performed using one-way analysis of variance followed by Student–Newman–Keuls post-hoc tests. Values of P < 0.05 were considered statistically significant. Sample size was calculated using a sample calculator (https://www.powerandsamplesize.com/) and yielded an estimate of at least 24 samples.
Results
Patient characteristics
Thirty patients with HBV-ACLF were selectively recruited (18 men and 12 women). The HBV-ACLF patients were aged from 18 to 65 years old (mean 45.6 ± 8.6 years). Thirty patients with CHB with a similar age range as well as 30 healthy individuals were also enrolled. Among the patients with HBV-ACLF, eight showed cirrhosis with decompensated liver function. The clinical characteristics of participants are shown in Table 1.
Table 1.
Clinical characteristics of study participants.
| Indicators | HC | CHB | HBV-ACLF |
|---|---|---|---|
| MELD | Not tested | 6.32 ± 1.45 | 23.43 ± 3.76 |
| INR | Not tested | 0.87 ± 0.56 | 2.34 ± 0.39 |
| WBC (109/L) | 6.45 ± 2.67 | 5.98 ± 1.45 | 8.27 ± 6.19 |
| Ascites | No | No | Yes |
| Previous decompensations | No | No | Yes |
Data represent means ± standard deviations.
HC, healthy control; CHB, chronic hepatitis B; HBV-ACLF, hepatitis B virus-associated acute-on-chronic liver failure; MELD, model for end stage liver disease; INR, international normalized ratio; WBC, white blood cells.
Biochemical indicators of liver function are elevated in patients with HBV-ACLF
Patients with HBV-ACLF had higher levels of alanine aminotransferase, asparagine aminotransferase, total bilirubin, and direct bilirubin compared with patients with CHB and HCs (P < 0.05) (Figure 1a–d). These data suggested that biochemical indices of liver function were elevated in patients with HBV-ACLF.
Figure 1.
Biochemical indicators of liver function in patients with HBV-ACLF, patients with CHB, and HCs. Serum levels of (a) ALT, (b) TB, (c) AST and (d) DB were measured. *, P < 0.05; **, P < 0.01; n.s., not significant.
HC, healthy control; CHB, chronic hepatitis B; HBV-ACLF, hepatitis B-associated acute-on-chronic liver failure; ALT, alanine aminotransferase; AST, asparagine aminotransferase; TB, total bilirubin; DB, direct bilirubin.
NLRP3 and ASC mRNA and protein levels are increased in patients with HBV-ACLF
We used RT-qPCR and ELISA to assess levels of NLRP3 and ASC mRNA and protein. PBMCs from patients with HBV-ACLF had higher levels of NLRP3 and ASC mRNA compared with those of patients with CHB or HCs (P < 0.05) (Figure 2a and b). ELISA showed that NLRP3 and ASC levels in the sera of patients with HBV-ACLF were significantly increased compared with those of patients with CHB or HCs (P < 0.05) (Figure 2c and d). These data showed that levels of NLRP3 and ASC were increased in patients with HBV-ACLF.
Figure 2.
Levels of NLRP3 and ASC in patients with HBV-ACLF, patients with CHB, and HCs. (a–b) Relative levels of (a) NLRP3 and (b) ASC mRNA in PBMCs measured by RT-qPCR. (c–d) Levels of (c) NLRP3 and (d) ASC protein in serum measured by ELISA. *, P < 0.05; **, P < 0.01; n.s., not significant.
HC, healthy control; CHB, chronic hepatitis B; HBV-ACLF, hepatitis B-associated acute-on-chronic liver failure; ELISA, enzyme-linked immunosorbent assay; PBMC, peripheral blood mononuclear cell; RT-qPCR, quantitative reverse transcription polymerase chain reaction.
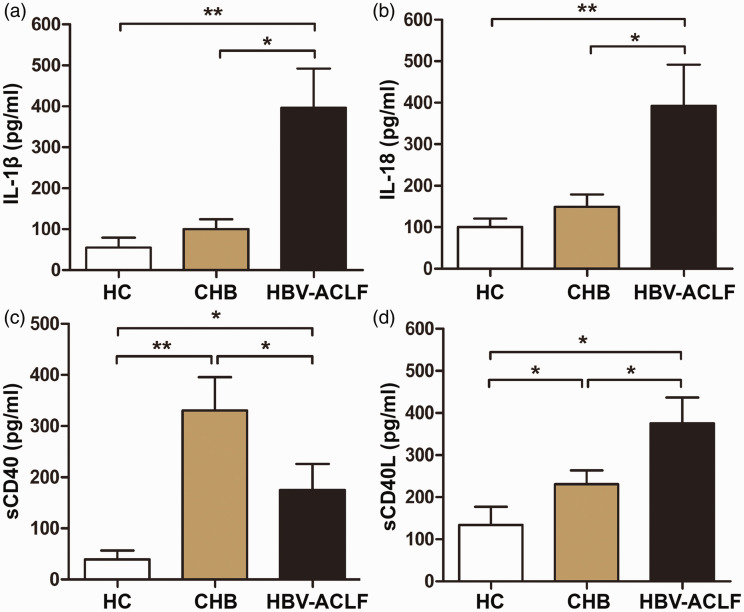
Patients with HBV-ACLF have elevated levels of IL-1β, IL-18, and sCD40L
Levels of immune-related molecules were assessed by ELISA. The sera of patients with HBV-ACLF had increased levels of IL-1β, IL-18 and sCD40L compared with those of patients with CHB and HCs (P < 0.05) (Figure 3a, b and d). Interestingly, serum levels of sCD40 in patients with HBV-ACLF were increased compared with HCs but decreased compared with patients with CHB (P < 0.05) (Figure 3c). These data show that levels of the immune-related molecules IL-1β, IL-18, and sCD40L were increased in patients with HBV-ACLF.
Figure 3.
Levels of immune-related molecules in sera from patients with HBV-ACLF, patients with CHB, and HCs. Serum levels of (a) IL-1β, (b) IL-18, (c) sCD40 and (d) sCD40L were determined by ELISA. *, P < 0.05; **, P < 0.01.
HC, healthy control; CHB, chronic hepatitis B; HBV-ACLF, hepatitis B-associated acute-on-chronic liver failure; IL, interleukin; ELISA, enzyme-linked immunosorbent assay.
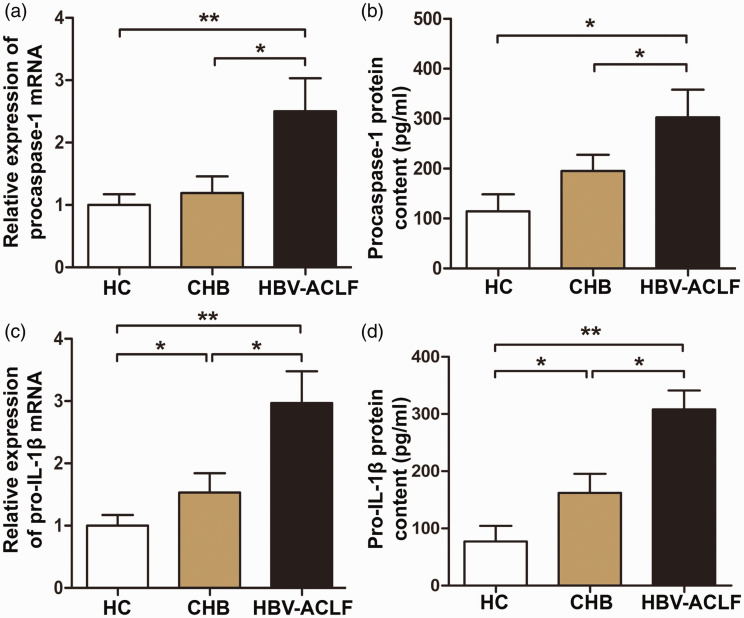
Expression of NLRP3 inflammasome signaling pathway molecules is increased in patients with HBV-ACLF
To further study the mechanisms of NLRP3 in HBV-ACLF, we examined mRNA and protein levels of the NLRP3 inflammasome signaling pathway molecules, procaspase-1 and pro-IL-1β, in PBMCs and serum. Procaspase-1 and pro-IL-1β mRNA and protein levels were significantly increased in PBMCs from patients with HBV-ACLF compared with those of patients with CHB or HCs (P < 0.05) (Figure 4a–d). These data showed that levels of NLRP3 inflammasome signaling pathway molecules were increased in patients with HBV-ACLF compared with patients with CHB or HCs.
Figure 4.
Levels of procaspase-1 and pro-IL-1β in patients with HBV-ACLF, patients with CHB, and HCs. (a and c) Relative expression of (a) procaspase-1 and (c) pro-IL-1β mRNA in PBMCs measured by RT-qPCR. (b and d) Levels of (b) procaspase-1 and (d) pro-IL-1β proteins in serum measured by ELISA. *, P < 0.05; **, P < 0.01.
HC, healthy control; CHB, chronic hepatitis B; HBV-ACLF, hepatitis B-associated acute-on-chronic liver failure; IL, interleukin; PBMC, peripheral blood mononuclear cell; RT-qPCR, quantitative reverse transcription polymerase chain reaction; ELISA, enzyme-linked immunosorbent assay.
Discussion
Liver failure is a heterogeneous and serious disease that is characterized by major harms, treatment difficulties, poor prognosis, and high mortality.26,27 Liver failure can be categorized into acute liver failure, subacute liver failure, ACLF, and chronic liver failure; ACLF is the most common.28 HBV-ACLF can lead to metabolic disorders and further impairment of multiple organ functions.29 The mechanisms underlying HBV-ACLF are complex and include immune damage and inflammatory responses.
The NLRP3 inflammasome is an important intracellular protein complex and plays important roles in liver diseases.15 Following stimulation, NLRP3 combines with ASC and procaspase-1 to form inflammasomes.18 Inactive procaspase-1 is activated to form caspase-1, which then converts inactive IL-1β and IL-18 into their active state and mediating inflammatory apoptosis.18 NLRP3 inflammasomes can be activated by many different stimuli including LPS, nucleic acids, hyaluronic acid, and heparin sulfate. However, the specific molecular mechanisms of activation remain unclear.30,31 Activation of the NLRP3 inflammasome is associated with intracellular potassium outflow, lysosomal damage, intracellular cAMP reduction, and increased calcium concentration.32 The occurrence of ACLF is associated with many confounding factors. For example, high dynamic and low dynamic circulation in patients with liver cirrhosis, as well as systemic infection, are high risk factors for fatal ACLF.33 There appears to be no correlation between severity of liver disease and cardiac insufficiency.34 Acute decompensation can lead to deposition of type III collagen and poor prognosis in patients with liver cirrhosis.35 When CD40L expressed on activated T cells interacts with CD40 on macrophages, this can inhibit the activation of NLRP3 inflammasomes in macrophages. CD40L alone can also inhibit the activation of macrophage NLRP3 inflammasomes by activators such as alum.36 Our data demonstrated that the mRNA levels of the NLRP3 inflammasome-associated molecules ASC, NLRP3, and caspase-1 were significantly increased in PBMCs from patients with HBV-ACLF compared with patients with CHB and HCs.
The regulatory network of the CD40-CD40L costimulatory pathway is also complex. Serum sCD40L and sCD40 have key functions in ACLF.37 sCD40L can bind to CD40 on monocytes, stimulating CD40-CD40L signaling and activating the cell.37 The interaction between sCD40 and CD40L inhibits immunoglobulin production and T cell activation, playing an immunosuppressive role.38 When inflammation and necrosis are aggravated in liver tissues of patients with CHB, levels of sCD40 are increased significantly in serum. However, levels of sCD40 in the sera of patients with liver failure are similar to those of CHB patients with level 1 inflammation and necrosis, but lower than those of CHB patients with level 2 or 3 inflammation and necrosis.39 Another study showed that CD40 and CD40L expression was significantly higher in liver tissue from patients with fulminant liver failure compared with patients with chronic liver disease or healthy individuals. LPS stimulates human liver macrophages to up-regulate CD40L expression.39 It has recently been shown that inflammasome activation in both PBMCs and in the liver results in IL-1 production.40,41 We did not investigate liver-derived mediators in this study. Additionally, we did not examine levels of IL-1α or gasdermin, which will be the focus of a future study. We also plan to carry out animal experiments to explore the mechanisms of NLRP3 in liver disease. The role of NLRP3 in HBV-ACLF needs to be further investigated. In future studies, we plan to investigate how NLRP3 regulates the CD40-CD40L pathway and how caspase-1, IL-1β, and NLRP3 interact with the CD40-CD40L pathway to regulate the occurrence and development of HBV-ACLF.
In conclusion, this prospective case-control study showed significant activation of the NLRP3 inflammasome in patients with HBV-ACLF. In addition, the activated NLRP3 inflammasome may participate in liver failure by activating procaspase-1 and pro-IL-1β as well as by regulating downstream CD40-CD40L signaling. Future studies will address how NLRP3 interacts with procaspase-1 and pro-IL-1β to regulate the CD40-CD40L pathway.
Acknowledgment
The authors wish to thank their department and research team for their help and dedication.
Footnotes
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Availability of data and materials: The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author contributions: ZL and JJ contributed to the design of the study. ZL performed the experiments. JJ analyzed the data. ZL and JJ interpreted results and prepared the manuscript. The final version of the manuscript was read and approved by both authors.
ORCID iD: Jianning Jiang https://orcid.org/0000-0002-4814-4148
References
- 1.Yoshida Y, Okada Y, Suzuki A, et al. Fatal acute hepatic failure in a family infected with the hepatitis A virus subgenotype IB: A case report. Medicine (Baltimore) 2017; 96: e7847. DOI: 10.1097/md.0000000000007847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee W, Hwang MH, Lee Y, et al. Protective effects of zingerone on lipopolysaccharide-induced hepatic failure through the modulation of inflammatory pathways. Chem Biol Interact 2018; 281: 106–110. DOI: 10.1016/j.cbi.2017.12.031. [DOI] [PubMed] [Google Scholar]
- 3.Wang H, Zhang H, Zhang Y, et al. Plumbagin protects liver against fulminant hepatic failure and chronic liver fibrosis via inhibiting inflammation and collagen production. Oncotarget 2016; 7: 82864–82875. DOI: 10.18632/oncotarget.12655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu M, Wang P, Zhao M, et al. Intestinal dendritic cells are altered in number, maturity and chemotactic ability in fulminant hepatic failure. PLoS One 2016; 11: e0166165. DOI: 10.1371/journal.pone.0166165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin S, Chen J, Wang M, et al. Prognostic nomogram for acute-on-chronic hepatitis B liver failure. Oncotarget 2017; 8: 109772–109782. DOI: 10.18632/oncotarget.21012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhong Y, Guo Y, Liu X, et al. Serum glycopatterns as novel potential biomarkers for diagnosis of acute-on-chronic hepatitis B liver failure. Sci Rep 2017; 7: 45957. DOI: 10.1038/srep45957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou Q, Tang H.The progress of antiviral therapy in patients with HBV-related liver failure. Hepatogastroenterology 2013; 60: 1877–1880. [PubMed] [Google Scholar]
- 8.Reinholz M, Clanner-Engelshofen BM, Heppt MV, et al. Dermal fillers do not induce upregulation of NLRP3 inflammasomes or expression of inflammatory cytokines in granulomas. J Cosmet Dermatol 2020: 19: 2838–2844. DOI: 10.1111/jocd.13341. [DOI] [PubMed] [Google Scholar]
- 9.Spel L, Martinon F.Inflammasomes contributing to inflammation in arthritis. Immunol Rev 2020; 294: 48–62. DOI: 10.1111/imr.12839. [DOI] [PubMed] [Google Scholar]
- 10.McCoy AJ, Koizumi Y, Higa N, et al. Differential regulation of caspase-1 activation via NLRP3/NLRC4 inflammasomes mediated by aerolysin and type III secretion system during Aeromonas veronii infection. J Immunol 2010; 185: 7077–7084. DOI: 10.4049/jimmunol.1002165. [DOI] [PubMed] [Google Scholar]
- 11.Skeldon A, Saleh M.The inflammasomes: molecular effectors of host resistance against bacterial, viral, parasitic, and fungal infections. Front Microbiol 2011; 2: 15. DOI: 10.3389/fmicb.2011.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Strowig T, Henao-Mejia J, Elinav E, et al. Inflammasomes in health and disease. Nature 2012; 481: 278–286. DOI: 10.1038/nature10759. [DOI] [PubMed] [Google Scholar]
- 13.Conforti-Andreoni C, Ricciardi-Castagnoli P, Mortellaro A.The inflammasomes in health and disease: from genetics to molecular mechanisms of autoinflammation and beyond. Cell Mol Immunol 2011; 8: 135–145. DOI: 10.1038/cmi.2010.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Craven RR, Gao X, Allen IC, et al. Staphylococcus aureus alpha-hemolysin activates the NLRP3-inflammasome in human and mouse monocytic cells. PLoS One 2009; 4: e7446. DOI: 10.1371/journal.pone.0007446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wong KW, Jacobs WR., Jr.Critical role for NLRP3 in necrotic death triggered by Mycobacterium tuberculosis. Cell Microbiol 2011; 13: 1371–1384. DOI: 10.1111/j.1462-5822.2011.01625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xiang Y, Wang X, Yan C, et al. Adenosine-5'-triphosphate (ATP) protects mice against bacterial infection by activation of the NLRP3 inflammasome. PLoS One 2013; 8: e63759. DOI: 10.1371/journal.pone.0063759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hornung V, Bauernfeind F, Halle A, et al. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol 2008; 9: 847–856. DOI: 10.1038/ni.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang WJ, Fang ZM, Liu WQ.NLRP3 inflammasome activation from Kupffer cells is involved in liver fibrosis of Schistosoma japonicum-infected mice via NF-κB. Parasit Vectors 2019; 12: 29. DOI: 10.1186/s13071-018-3223-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu X, Dong L, Lin X, et al. Relevance of the NLRP3 inflammasome in the pathogenesis of chronic liver disease. Front Immunol 2017; 8: 1728. DOI: 10.3389/fimmu.2017.01728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Del Campo JA, Gallego P, Grande L.Role of inflammatory response in liver diseases: Therapeutic strategies. World J Hepatol 2018; 10: 1–7. DOI: 10.4254/wjh.v10.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pan CW, Pan ZZ, Hu JJ, et al. Mangiferin alleviates lipopolysaccharide and D-galactosamine-induced acute liver injury by activating the Nrf2 pathway and inhibiting NLRP3 inflammasome activation. Eur J Pharmacol 2016; 770: 85–91. DOI: 10.1016/j.ejphar.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 22.Kim HY, Kim SJ, Lee SM.Activation of NLRP3 and AIM2 inflammasomes in Kupffer cells in hepatic ischemia/reperfusion. FEBS J 2015; 282: 259–270. DOI: 10.1111/febs.13123. [DOI] [PubMed] [Google Scholar]
- 23.Kim SJ, Lee SM.NLRP3 inflammasome activation in D-galactosamine and lipopolysaccharide-induced acute liver failure: Role of heme oxygenase-1. Free Radic Biol Med 2013; 65: 997–1004. DOI: 10.1016/j.freeradbiomed.2013.08.178. [DOI] [PubMed] [Google Scholar]
- 24.Lv H, Fan X, Wang L, et al. Daphnetin alleviates lipopolysaccharide/d-galactosamine-induced acute liver failure via the inhibition of NLRP3, MAPK and NF-κB, and the induction of autophagy. Int J Biol Macromol 2018; 119: 240–248. DOI: 10.1016/j.ijbiomac.2018.07.101. [DOI] [PubMed] [Google Scholar]
- 25.Martinon F, Petrilli V, Mayor A, et al. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 2006; 440: 237–241. DOI: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 26.Taguchi K, Yamasaki K, Seo H, et al. Potential use of biological proteins for liver failure therapy. Pharmaceutics 2015; 7: 255–274. DOI: 10.3390/pharmaceutics7030255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu Y, Xiong Y, Xing F, et al. Precise regulation of miR-210 is critical for the cellular homeostasis maintenance and transplantation efficacy enhancement of mesenchymal stem cells in acute liver failure therapy. Cell Transplant 2017; 26: 805–820. DOI: 10.3727/096368916x694274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schroder K, Tschopp J.The inflammasomes. Cell 2010; 140: 821–832. DOI: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 29.Menu P, Vince JE.The NLRP3 inflammasome in health and disease: The good, the bad and the ugly. Clin Exp Immunol 2011; 166: 1–15. DOI: 10.1111/j.1365-2249.2011.04440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qu J, Yuan Z, Wang G, et al. The selective NLRP3 inflammasome inhibitor MCC950 alleviates cholestatic liver injury and fibrosis in mice. Int Immunopharmacol 2019; 70: 147–155. DOI: 10.1016/j.intimp.2019.02.016. [DOI] [PubMed] [Google Scholar]
- 31.Abbasi-Oshaghi E, Mirzaei F, Pourjafar M.NLRP3 inflammasome, oxidative stress, and apoptosis induced in the intestine and liver of rats treated with titanium dioxide nanoparticles: in vivo and in vitro study. Int J Nanomedicine 2019; 14: 1919–1936. DOI: 10.2147/ijn.s192382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang L, Zou ZQ, Liu CX, et al. Immunotherapeutic interventions in chronic hepatitis B virus infection: A review. J Immunol Methods 2014; 407: 1–8. DOI: 10.1016/j.jim.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 33.Praktiknjo M, Monteiro S, Grandt J, et al. Cardiodynamic state is associated with systemic inflammation and fatal acute-on-chronic liver failure. Liver Int 2020; 40: 1457–1466. DOI: 10.1111/liv.14433. [DOI] [PubMed] [Google Scholar]
- 34.Merli M, Calicchia A, Ruffa A, et al. Cardiac dysfunction in cirrhosis is not associated with the severity of liver disease. Eur J Intern Med 2013; 24:172–176. DOI: 10.1016/j.ejim.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 35.Praktiknjo M, Lehmann J, Nielsen MJ, et al. Acute decompensation boosts hepatic collagen type III deposition and deteriorates experimental and human cirrhosis. Hepatol Commun 2018; 2: 211–222. DOI: 10.1002/hep4.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wolf D, Jehle F, Ortiz Rodriguez A, et al. CD40L deficiency attenuates diet-induced adipose tissue inflammation by impairing immune cell accumulation and production of pathogenic IgG-antibodies. PLoS One 2012; 7: e33026. DOI: 10.1371/journal.pone.0033026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tseng M, Ge S, Roberts R, et al. Liver transplantation in a patient with CD40 ligand deficiency and hyper-IgM syndrome: Clinical and immunological assessments. Am J Transplant 2016; 16: 1626–1632. DOI: 10.1111/ajt.13580. [DOI] [PubMed] [Google Scholar]
- 38.Marquez M, Fernandez-Gutierrez C, Montes-De-Oca M, et al. Chronic antigenic stimuli as a possible explanation for the immunodepression caused by liver cirrhosis. Clin Exp Immunol 2009; 158: 219–229. DOI: 10.1111/j.1365-2249.2009.04005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shen HH, Bai BK, Wang YQ, et al. Serum soluble CD40 is associated with liver injury in patients with chronic hepatitis B. Exp Ther Med 2015; 9: 999–1005. DOI: 10.3892/etm.2015.2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Praktiknjo M, Schierwagen R, Monteiro S, et al. Hepatic inflammasome activation as origin of interleukin-1α and interleukin-1β in liver cirrhosis. Gut 2020: gutjnl-2020-322621. doi: 10.1136/gutjnl-2020-322621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Monteiro S, Grandt J, Uschner FE, et al. Differential inflammasome activation predisposes to acute-on-chronic liver failure in human and experimental cirrhosis with and without previous decompensation. Gut 2021; 70: 379–387. DOI: 10.1136/gutjnl-2019-320170. [DOI] [PMC free article] [PubMed] [Google Scholar]