Abstract
To date, only one case of pediatric type II negative pressure pulmonary edema (NPPE) caused by removal of an endobronchial foreign body has been documented. We report another case of type II NPPE that developed after extraction of inhaled peanuts. A 21-month-old boy who presented with wheezing and intermittent cough for 1 month after eating peanuts was admitted to our department. A chest computed tomographic scan showed foreign bodies lodged in the right main bronchus. Fiberoptic bronchoscopy was performed, and three pieces of peanuts were removed. Fifteen minutes after this procedure, the child grew restless and started coughing with frothy pink sputum. Tachypnea and rales were observed. A chest radiograph showed patchy opacification in both lungs, especially in the right lower zone, leading to the diagnosis of type II NPPE. Intravenous furosemide and dexamethasone were immediately administered, followed by non-invasive continuous positive airway pressure ventilation. Twelve hours later, the patient recovered uneventfully and was discharged home the following day. In conclusion, pediatric type II NPPE rapidly occurs following the relief of upper airway obstruction. Clinicians need to be aware of the acuteness and manifestations of type II NPPE to make an early diagnosis and initiate prompt treatment.
Keywords: Type II negative pressure pulmonary edema, pediatric patient, foreign body aspiration, fiberoptic bronchoscopy, lung, upper airway obstruction
Introduction
Negative pressure pulmonary edema (NPPE), which is also known as post-obstructive pulmonary edema, occurs most frequently at the onset or relief of upper airway obstruction.1,2 There are two types of NPPE called types I and II. Type I NPPE occurs following an episode of acute airway obstruction, while type II develops after the relief of upper airway obstruction.2 NPPE is not commonly found in children.3 While pediatric type I NPPE has been documented in many case/case series reports in the last two decades,4–14 significantly fewer type II NPPE cases in children have been described.15–19 To date, only one case of type II NPPE caused by the removal of an endobronchial foreign body in a child has been reported.18 We present another case of type II NPPE that developed after extraction of inhaled peanuts in a 21-month-old boy.
Case report
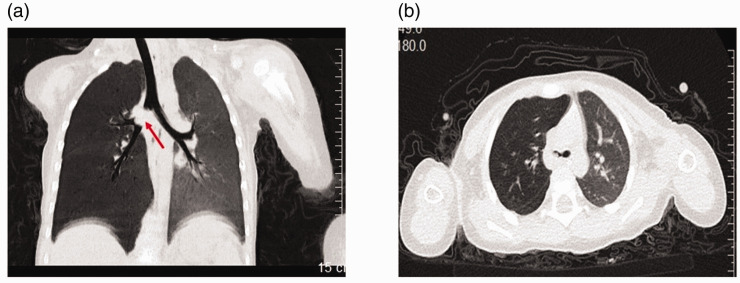
The reporting of this study conforms to the CARE guidelines.20 A 21-month-old boy who presented with wheezing and intermittent cough for 1 month after eating peanuts was admitted to our department. Upon admission, the patient had a respiratory rate of 48 breaths/minute and oxygen saturation of 93% on room air. Marked respiratory effort and the use of accessory muscles were noted. Auscultation showed wheezing in the right lung. A chest computed tomographic scan showed foreign bodies lodged in the right main bronchus that caused overinflation of the right lung secondary to partial bronchial obstruction (Figure 1a, b). The diagnosis of foreign body aspiration was made. The patient was administered 100 mL of 5% glucose intravenously. Fiberoptic bronchoscopy was then performed under anesthesia with intravenous administration of propofol (2 mg/kg), which was repeated once during the procedure. The bronchoscope was moved down the trachea until the carina was seen. The opening of the left main bronchus was found to be normal, while three pieces of peanuts that were lodged slightly down the opening of the main right bronchus were identified and extracted. Immediately after the removal of the peanuts, breathing difficulties and wheezing in the patient vanished, and the respiratory rate was recorded at 30 breaths/minute.
Figure 1.
Computed tomographic scan findings before the fibrotic bronchoscopic procedure. Computed tomographic images show a foreign body (or foreign bodies) (red arrow) lodged at the upper one third of the right main bronchus (a) causing overinflation in the right lung (b).
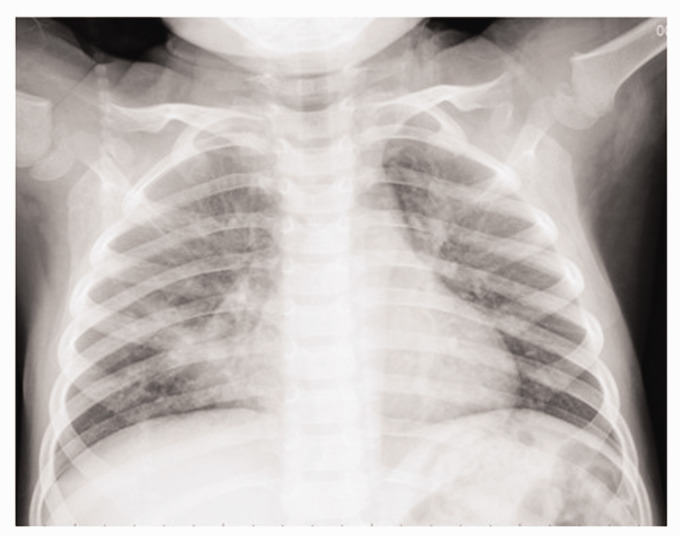
Approximately 15 minutes after the fiberoptic bronchoscopic procedure, the boy grew restless and started coughing with frothy pink sputum. His respiratory rate increased to 46 breaths/minute, oxygen saturation was 90% in room air, and rales were heard in both lungs, especially in the right side. Bronchospasm and physical bronchial obstruction were excluded. A chest X-ray revealed patchy opacification in both lungs, especially in the right lower zone (Figure 2), which led to the diagnosis of type II NPPE. Intravenous furosemide (10 mg) and dexamethasone (2.5 mg) were administered immediately, followed by non-invasive continuous positive airway pressure (CPAP) ventilation. The coughing ceased approximately 30 minutes after the medications and CPAP. Three hours after CPAP, the boy’s respiratory rate was reduced to 38 breaths/minute, and CPAP was maintained for 12 hours until his respiratory rate was stabilized at approximately 28 breaths/minutes and oxygen saturation was maintained at > 98%. The patient was discharged home the following day.
Figure 2.
A chest radiogram shows patchy opacification in both lungs, especially in the right lower zone, 15 minutes after the fibrotic bronchoscopic procedure.
Discussion
Type II NPPE develops after relief of upper airway obstruction. Chronic upper airway obstruction is believed to result in an increased effort for breathing, which can create a positive end-expiratory alveolar pressure, leading to a reduced capillary wall pressure gradient.3 Once the airway obstruction is relieved, the positive end-expiratory alveolar pressure is lost, resulting in an increase in the capillary wall pressure gradient and capillary leakage, thereby producing pulmonary edema (i.e., type II NPPE).3
Type II NPPE in children is rare.3 Only five reports of cases of pediatric type II NPPE that described its diagnosis and treatment have been documented in the last two decades.15–19 Most of these cases occurred after tonsillectomy/adenoidectomy.15,16,19 Only one case was reported to be caused by the removal of a foreign body in the bronchus.18 With regard to the onset of type II NPPE, Moser et al.15 observed the symptoms of NPPE 45 minutes after tonsillectomy/adenoidectomy in a previously healthy 14-year-old girl. Masuda et al.18 treated a 5-year-old boy with foreign body aspiration and observed NPPE 15 minutes after removal of an inhaled peanut. Van Kooy and Gargiulo reported a girl who started to experience NPPE upon arrival to the post-anesthesia care unit after tonsillectomy/adenoidectomy.19 These data are in line with our finding that type II NPPE developed within 1 hour following re-patency of the airway.
The diagnosis of type II NPPE relies on the medical history, clinical characteristics, and radiological findings. The presence of agitation, tachypnea, frothy pink sputum, rales, and oxygen desaturation upon the relief of upper airway obstruction strongly suggests the diagnosis of type II NPPE, especially in a vigorous child.19 In the current case, the patient had all clinical manifestations consistent with type II NPPE after extraction of inhaled peanuts. The final diagnosis of type II NPPE was made in our patient with the support of chest radiographic findings as reported in all previous cases in the literature.15–19 Notably, a chest X-ray also showed patchy opacification in the left upper zone in the present case, but the mechanism behind this phenomenon remains unknown.
The first treatment priority for patients with type II NPPE is the correction of hypoxemia. Non-invasive ventilation was applied to treat our patient, while all children with type II NPPE described in previous reports underwent ventilation involving intubation.15–19 Approximately half of the patients, including our patient, received diuretics15,16,19 and corticosteroids.16,18 Because of the limited amount of studies, the effect of diuretics and corticosteroids in managing type II NPPE in children remains unknown. Determination of this issue will be challenging because of the rarity of type II NPPE.
Masuda et al.18 observed that most of the opacification in the lungs disappeared in less than 1 hour after treatment as shown by chest radiograms. Moser et al.15 reported that complete resolution of pulmonary edema was achieved 37 hours after treatment with ventilation and diuretics in a 14-year-old girl. Improvement of type II NPPE 24 hours after ventilation in a 12-year-old boy was reported by Austin et al.17 We observed disappearance of type II NPPE symptoms 12 hours after treatment. However, we did not perform a chest radiograph to examine the resolution of pulmonary edema because the child recovered uneventfully. Taken together, these findings suggest that prompt management is crucial for rapid improvement and resolution of type II NPPE in children.
Conclusion
Type II NPPE can rapidly occur following the relief of upper airway obstruction in children. Therefore, clinicians need to be aware of the acuteness and manifestations of type II NPPE to make an early diagnosis and initiate prompt treatment.
Supplemental Material
Supplemental material, sj-pdf-1-imr-10.1177_03000605211047779 for A rare case of type II negative pressure pulmonary edema following extraction of inhaled peanuts in a 21-month-old boy by Qin Li and Liang Zhou in Journal of International Medical Research
Supplemental material, sj-pdf-2-imr-10.1177_03000605211047779 for A rare case of type II negative pressure pulmonary edema following extraction of inhaled peanuts in a 21-month-old boy by Qin Li and Liang Zhou in Journal of International Medical Research
Acknowledgements
We would like to thank Drs Weiran Dong and Fan Wang from the Department of Respirology, Children’s Hospital of Hebei Province for their participation in the care of the patient.
Ethics statement: According to our hospital regulations, ethics approval was not required for this case report study because a case report involving only a single individual does not meet the definition of research. Informed written consent was obtained from the parents of the patient for publication.
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD: Liang Zhou https://orcid.org/0000-0003-2415-6977
References
- 1.Bhattacharya M, Kallet RH, Ware LB, et al. Negative-pressure pulmonary edema. Chest 2016; 150: 927–933. [DOI] [PubMed] [Google Scholar]
- 2.Guffin TN, Har-el G, Sanders A, et al. Acute postobstructive pulmonary edema. Otolaryngol Head Neck Surg 1995; 112: 235–237. [DOI] [PubMed] [Google Scholar]
- 3.Thiagarajan RR, Laussen PC.Negative pressure pulmonary edema in children-pathogenesis and clinical management. Paediatr Anaesth 2007; 17: 307–310. [DOI] [PubMed] [Google Scholar]
- 4.Toukan Y, Gur M, Keshet D, et al. Negative pressure pulmonary edema in a child following laryngospasm triggered by a laryngeal mask. Isr Med Assoc J 2019; 21: 56–57. [PubMed] [Google Scholar]
- 5.Xiong J, Sun Y.Negative pressure pulmonary edema: a case report. BMC Anesthesiol 2019; 19: 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee JH, Lee JH, Lee MH, et al. Postoperative negative pressure pulmonary edema following repetitive laryngospasm even after reversal of neuromuscular blockade by sugammadex: a case report. Korean J Anesthesiol 2017; 70: 95–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bashir A, Ahmad SQ, Silverman J, et al. Post-obstructive pulmonary edema from aspirated nuts. SAGE Open Med Case Rep 2017; 5: 2050313X17717391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Toukan Y, Gur M, Bentur L.Negative pressure pulmonary edema following choking on a cookie. Pediatr Pulmonol 2016; 51: E25–E27. [DOI] [PubMed] [Google Scholar]
- 9.Dubey PK.Post extubation negative pressure pulmonary edema due to posterior mediastinal cyst in an infant. Ann Card Anaesth 2014; 17: 161–163. [DOI] [PubMed] [Google Scholar]
- 10.Berdai AM, Labib S, Harandou M.Postobstructive pulmonary edema following accidental near-hanging. Am J Case Rep 2013; 14: 350–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mehta VM, Har-El G, Goldstein NA.Postobstructive pulmonary edema after laryngospasm in the otolaryngology patient. Laryngoscope 2006; 116: 1693–1696. [DOI] [PubMed] [Google Scholar]
- 12.Silva PS, Monteiro Neto H, Andrade MM, et al. Negative-pressure pulmonary edema: a rare complication of upper airway obstruction in children. Pediatr Emerg Care 2005; 21: 751–754. [DOI] [PubMed] [Google Scholar]
- 13.Ringold S, Klein EJ, Del Beccaro MA.Postobstructive pulmonary edema in children. Pediatr Emerg Care 2004; 20: 391–395. [DOI] [PubMed] [Google Scholar]
- 14.Devys JM, Cadi P, Nivoche Y.Protein concentration in pulmonary oedema fluid for negative pressure pulmonary oedema in children. Paediatr Anaesth 2000; 10: 557–558. [DOI] [PubMed] [Google Scholar]
- 15.Moser JJ, O'Connell M, McAllister DL.Case report: Post obstructive pulmonary edema (POPE) Type II following elective adenotonsillectomy requiring novel use of high frequency oscillatory ventilation (HFOV). F1000Res 2018; 7: 1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Powell T, Sharma N, McKie KT.Postobstructive pulmonary edema following tonsillectomy/adenoidectomy in a 2-year-old with Poland-Moebius syndrome. Case Rep Otolaryngol 2016; 2016: 5431809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Austin AL, Kon A, Matteucci MJ.Respiratory failure in a child due to type 2 postobstructive pulmonary edema. Pediatr Emerg Care 2016; 32: 23–24. [DOI] [PubMed] [Google Scholar]
- 18.Masuda A, Asano F, Tsuzuku A, et al. Postobstructive pulmonary edema that developed immediately after the removal of an endobronchial foreign body. Intern Med 2015; 54: 497–502. [DOI] [PubMed] [Google Scholar]
- 19.Van Kooy MA, Gargiulo RF.Postobstructive pulmonary edema. Am Fam Physician 2000; 62: 401–404. [PubMed] [Google Scholar]
- 20.Gagnier JJ, Kienle G, Altman DG, et al. The CARE guidelines: consensus-based clinical case reporting guideline development. Headache 2013; 53: 1541–1547. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-imr-10.1177_03000605211047779 for A rare case of type II negative pressure pulmonary edema following extraction of inhaled peanuts in a 21-month-old boy by Qin Li and Liang Zhou in Journal of International Medical Research
Supplemental material, sj-pdf-2-imr-10.1177_03000605211047779 for A rare case of type II negative pressure pulmonary edema following extraction of inhaled peanuts in a 21-month-old boy by Qin Li and Liang Zhou in Journal of International Medical Research