Abstract
Preclinical studies suggest mesenchymal stromal cell extracellular vesicles (MSC‐EVs) reduce inflammation and improve organ function in lung diseases; however, an objective analysis of all available data is needed prior to translation. Using rigorous meta‐research methods, we determined the effectiveness of MSC‐EVs for preclinical respiratory diseases and identified experimental conditions that may further refine this therapy. A systematic search of MEDLINE/Embase identified 1167 records. After screening, 52 articles were included for data extraction and evaluated for risk of bias and quality of reporting in study design. A random effects meta‐analysis was conducted for acute lung injury (ALI; N = 23), bronchopulmonary dysplasia (BPD; N = 8) and pulmonary arterial hypertension (PAH; N = 7). Subgroup analyses identified EV methods/characteristics that may be associated with improved efficacy. Data is presented as standardized mean differences (SMD) or risk ratios (RR) with 95% confidence intervals (CI). For ALI, MSC‐EVs markedly reduced lung injury (SMD ‐4.33, CI ‐5.73 to ‐2.92), vascular permeability (SMD ‐2.43, CI ‐3.05 to ‐1.82), and mortality (RR 0.39, CI 0.22 to 0.68). Small EVs were more consistently effective than large EVs whereas no differences were observed between tissue sources, immunocompatibility or isolation techniques. For BPD, alveolarization was improved by MSC‐EVs (SMD ‐1.45, CI ‐2.08 to ‐0.82) with small EVs more consistently beneficial then small/large EVs. In PAH, right ventricular systolic pressure (SMD ‐4.16, CI ‐5.68 to ‐2.64) and hypertrophy (SMD ‐2.80, CI ‐3.68 to ‐1.91) were significantly attenuated by EVs. In BPD and PAH, EVs isolated by ultracentrifugation demonstrated therapeutic benefit whereas tangential flow filtration (N = 2) displayed minimal efficacy. Lastly, risk of bias and quality of reporting for experimental design were consistently unclear across all studies. Our findings demonstrate clear potential of MSC‐EVs to be developed as therapy for acute and chronic lung diseases. However, greater transparency in research design and direct comparisons of isolation technique and EV subtypes are needed to generate robust evidence to guide clinical translation.
Protocol Registration: PROSPERO CRD42020145334
Keywords: exosomes, lung, mesenchymal stem cells, mesenchymal stromal cells extracellular vesicles, meta‐analysis, respiratory, systematic review
1. INTRODUCTION
Mesenchymal stromal cells (MSCs) are a heterogeneous population of cells with multipotent differentiation potential and diverse secretory, immunomodulatory, and therapeutic functions (Le Blanc & Mougiakakos, 2012; Viswanathan et al., 2019). They can be derived from readily available tissues, such as bone marrow, umbilical cord, and adipose tissue (Jung et al., 2012; Romanov, 2003). Many in vitro and in vivo studies show that MSCs can modulate inflammatory pathways, improve organ function and prolong survival (Harrell et al., 2019; Jung et al., 2012), and there are currently over 1000 clinical trials registered on ClinicalTrials.gov investigating MSCs as an intervention across a variety of diseases. Despite this widespread interest, MSCs have received approval for only two conditions, graft versus host disease and perianal fistulas in Crohn's disease (Galipeau & Sensébé, 2018). The lack of successful clinical translation is multifactorial but may include technical challenges associated with generating a consistent, viable and effective cell therapy (Caplan et al., 2019; Lukomska et al., 2019; Rizk et al., 2016). For example, post‐hoc analysis from a clinical trial of MSCs for moderate to severe acute respiratory distress syndrome demonstrated an extensive range in cell viability from 36 to 85% (Matthay et al., 2019). Interestingly, it has now been suggested that MSCs exert their therapeutic effects at least in part by secretion of extracellular vesicles (EVs), which may represent a more robust therapeutic product than delivery of intact cells (Akyurekli et al., 2015; Allan et al., 2019; Börger et al., 2017; Colombo et al., 2014; Gnecchi et al., 2005).
Respiratory disorders, whether acute or chronic, remain a leading cause of death worldwide (WHO, 2020). Greater than 500 million people are affected by chronic lung diseases with minimal curative options (Soriano et al., 2020). Moreover, COVID‐19 related acute respiratory distress syndrome has shed light on the lack of available specific therapies to combat severe respiratory infections/injury. Given the anti‐inflammatory and regenerative properties of MSC‐derived EVs (MSC‐EVs), pulmonary diseases have become a prominent focus for preclinical development of EV therapeutics (Guo et al., 2020). Administration of MSC‐EVs improved outcomes in both acute (e.g. acute lung injury, the preclinical correlate of acute respiratory distress syndrome) and chronic (e.g. pulmonary arterial hypertension) conditions, as well as neonatal diseases (e.g. bronchopulmonary dysplasia) (Harrell et al., 2019; Lee et al., 2019; Monsel et al., 2016; Worthington & Hagood, 2020). MSC‐EVs carry a variety of key mRNAs, microRNAs and proteins known to mediate cell processes including inflammation, angiogenesis, apoptosis and fibrosis (Tieu et al., 2020), all of which are critical to lung recovery. Hence, EVs can potentially harness the benefits of whole cells while overcoming many of the common issues that accompany live cell therapies. These advantages include EVs potentially having lower immunogenicity, enhanced ability to cross important biological barriers (e.g. lung endothelium and epithelium), and favourable manufacturing/storage procedures (Chen et al., 2011).
As EV therapies have garnered interest over the past decade, there remain challenges and unanswered questions associated with developing this cell‐free therapy. In our previous systematic review, we analysed the methodology and outcomes of more than 200 in vivo studies investigating MSC‐EVs as a therapy (Tieu et al., 2020). There was high heterogeneity in EV nomenclature, experimental approach, interventional traits, dosage regimen and study design. The Minimal Information for Studies of Extracellular Vesicles (MISEV 2018) attempts to address many of these issues (Théry et al., 2018). However, given the diversity of EV methodology available, there is no consensus as to which isolation technique, interventional traits (e.g. MSC source or EV subtype) or administration protocols (e.g. delivery route, timing of treatment) will result in greatest therapeutic benefit. A systematic and comprehensive understanding of these experimental details and the overall impact of MSC‐EVs on lung repair are needed prior to considering clinical translation.
In the clinical world, scientifically rigorous systematic reviews and meta‐analyses are the gold standard approach for evaluating the efficacy of interventions objectively and comprehensively (Crowther et al., 2010; Gurevitch et al., 2018; Murad et al., 2016; Sena et al., 2014). They objectively assess the quality of research being conducted, elucidate specific therapy or patient population characteristics that affect treatment outcomes, and identify knowledge gaps. By applying meta‐research methods in preclinical EV research, our study aims to determine the efficacy of MSC‐EVs as treatment for various acute and chronic respiratory conditions. A meta‐analysis of acute lung injury, bronchopulmonary dysplasia, and pulmonary arterial hypertension was completed. We also conducted subgroup analyses to explore the specific EV characteristics and methodology that may be associated with greater benefits to help refine MSC‐EV therapy. Lastly, we assessed the quality of reporting experimental parameters and risk of bias in study design that currently exists in MSC‐EV research.
2. MATERIALS AND METHODS
2.1. Protocol and registration
Our protocol was registered in the International Prospective Registry of Systematic Reviews (PROSPERO CRD42020145334). Reporting of this systematic review follows the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) 2020 guidelines (Supplemental File 1).
2.2. Eligibility criteria
Eligibility criteria for study inclusion was created a priori and defined according to five parameters: population, intervention, comparator, outcomes and study design (PICOS). Predefining PICOS parameters is standard practice in meta‐research methodology that enables unbiased search, inclusion, and assessment of pertinent articles.
2.2.1. Population
We included any in vivo animal models of respiratory disease or injury. Invertebrate animals, in vitro or ex vivo preclinical studies were excluded.
2.2.2. Intervention
Studies must have administered MSC‐EVs for inclusion. All animal or tissue source of MSCs were included. MSCs or EVs may have been pre‐treated, co‐treated or modified (e.g. gene transfection). All routes, doses, timing and frequency of administration were included. Studies may have administered EVs as xenogeneic, allogeneic or autologous products. Studies in which EVs were not derived from MSCs, or were not administered to animals directly, were excluded.
2.2.3. Comparator
All comparators including (but not limited to) placebo, vehicle control, MSCs and/or fibroblasts were considered.
2.2.4. Outcome
Primary, secondary and tertiary outcomes were selected for acute lung injury (ALI), bronchopulmonary dysplasia (BPD), and pulmonary arterial hypertension (PAH), the three most studied lung conditions for MSC‐EV therapy. For lung conditions outside of ALI, BPD, and PAH, the primary and secondary outcomes reported in the articles were extracted for narrative synthesis. Studies in which outcomes did not assess EV efficacy (e.g. only the biodistribution of EVs was assessed) were excluded.
2.2.5. Study Design
We included controlled interventional studies (randomized, pseudo‐randomized, or non‐randomized), while unpublished grey literature, abstracts, review articles, editorials, commentaries, and letters were excluded. Studies were not excluded based on language or publication date.
2.3. Search strategy
A systematic search of Ovid MEDLINE, Ovid MEDLINE In‐Process & Other Non‐Indexed Citations, and Embase Classic + Embase was conducted until March 6, 2019 using a broad search strategy for any in vivo study investigating MSC‐EVs. To update our study, an additional search was conducted on 4 August, 2020 with lung disease filters. Both search strategies (Supplemental File 2) were designed with the help of an information specialist and assessed by a second information specialist through the Peer Review of Electronic Search Strategies (PRESS) (McGowan et al., 2016). The search strategies were modified according to database and used controlled vocabulary, MeSH terms (e.g. mesenchymal stem cells, mesenchymal stromal cells, extracellular vesicles, exosomes, microvesicles), abbreviations (e.g. MSCs, EVs, MVs), and filters for preclinical animal models. No restrictions were applied for publication date.
2.4. Study selection and data extraction
Titles and abstracts were independently screened in duplicate (AT, MS, CG and/or KH) using the Distiller Systematic Review Software (DistillerSR, Evidence Partners, Ottawa, Canada). A calibration test using 10 studies was performed before beginning the formal screening process to ensure high inter‐rater correlation (kappa 0.8). Titles and abstracts that met criteria or that could not be excluded with certainty moved on to full‐text screening. Following a calibration test involving 10 studies, two reviewers (AT, MS, CG and/or KH) independently reviewed the full‐text articles. Reasons for exclusion were recorded at this stage. Disagreements between reviewers were resolved through discussion. The selection process is presented in a PRISMA flow diagram (Figure 1).
FIGURE 1.

Preferred Reporting Items for Systematic Reviews and Meta‐Analysis (PRISMA) flow diagram detailing study screening and selection
Standardized data extraction forms were created in DistillerSR. Data pertaining to study characteristics, risk of bias, and quality assessment were extracted independently and in duplicate (AT, CG and/or KH). A calibration exercise for data extraction was conducted on the first three studies. Data was collected on study characteristics, including: general study characteristics, animal model characteristics (e.g. species, sex, model of disease or injury), MSC and EV isolation and characterization techniques, MSC or EV modifications, and dosage. Details for all data collected can be found in our registered PROSPERO protocol. We used the generally accepted size‐based definitions of EV subtypes, i.e. exosomes or small EVs (30‐150 nm in diameter) and microvesicles or large EVs (150‐1000 nm in diameter) (Théry et al., 2018; Tieu et al., 2020), to determine whether authors use of these terms was consistent with MISEV recommendations.
Since MSCs are a heterogeneous population of cells widely accessible from a variety of tissue sources, the International Society for Cell Therapy (ISCT) published guidelines to standardize MSC characterization. Studies were assessed for their adherence to the ISCT criteria including: (1) adherence to plastic in standard culture conditions, (2) positive and negative expression of specific surface antigens, and (3) multipotent differentiation potential. Furthermore, international guidelines for investigating EVs were published in 2018 entitled “Minimal Information for Studies of EVs” (MISEV 2018) (Théry et al., 2018). Researchers are encouraged to characterize EVs by amount, two measures of single vesicle analysis, and at least three positive and one negative protein marker. These criteria were used to assess study adherence to EV characterization.
2.5. Primary and secondary outcomes
Outcomes were predetermined by consulting experts within the field of regenerative medicine and lung disease. Primary outcomes included histological lung injury scores for ALI, mean linear intercept (a measure of alveolarization) for BPD, and right ventricular systolic pressure for PAH. Secondary outcomes for ALI and BPD included protein concentration and neutrophil count in bronchoalveolar lavage fluid (measures of alveolar‐capillary membrane permeability and inflammation, respectively). For BPD and PAH, Fulton's index (a measure of right ventricular hypertrophy and indicator of pulmonary hypertension) was extracted as a secondary outcome. Tertiary outcomes, if reported, included measures of survival and adverse events of EV therapy. The corresponding authors of the individual studies included in our review were contacted to obtain relevant missing data.
2.6. Risk of bias and quality of reporting
Risk of bias was evaluated by two independent reviewers (AT, CG) using the SYRCLE (Systematic Review Centre for Laboratory Animal Experimentation) risk of bias tool (Hooijmans et al., 2014). The SYRCLE tool features 10 different parameters including randomization, blinding and outcome reporting, each of which was scored as having a low, high, or unclear risk of bias for each study. Quality of reporting was evaluated by two independent reviewers (AT, CG) using the NIH standards for transparency in reporting preclinical research. Each element in the NIH standards for transparency was scored as having been reported or not reported. Disagreements between reviewers were resolved through discussion (Fergusson et al., 2019a; NIH 2014).
2.7. Data analysis
Extracted data on study characteristics, methodology and intervention characteristics are presented as frequencies, proportions and/or percentages. Studies may have used multiple techniques and interventions, either as separate experiments or in combination. Hence, the cumulative percentage of certain categories may be greater than 100%.
Study outcomes were pooled using Comprehensive Meta‐Analyst (version 3; BioStat Inc., USA) and represented by forest plots. Continuous outcome measures were expressed as standardized mean differences (SMDs) with 95% confidence intervals (CIs), which were calculated using a random effects inverse variance meta‐analysis. Standardized mean difference was chosen due to the expected heterogeneity in the measurement techniques of outcomes. Dichotomous outcome measures were expressed as risk ratios (RRs) with 95% confidence intervals (CIs), which were calculated using a random effects meta‐analysis based on the DerSimonian Laird model. We corrected for the multiple use of a single control group using the method described by Vesterinen et al (Vesterinen et al., 2014). Statistical heterogeneity was assessed using the Cochrane I2 test. Planned subgroup analyses included subgroups based on animal characteristics (e.g. sex, age, species), intervention characteristics (e.g. tissue source of MSCs, subtype of EVs, method of EV isolation), timing of treatment and respiratory disease modelled (e.g. ALI, BPD, PAH).
3. RESULTS
3.1. Study characteristics
Our systematic searches yielded a total of 1167 records (Figure 1). Following level 1 screening of titles and abstracts, 434 articles underwent level 2 full‐text screening which resulted in a total of 52 studies included for our systematic review (Ahn et al., 2018; Aliotta et al., 2017, 2016; Bandeira et al., 2018; Braun et al., 2018; Chaubey et al., 2018; Chen et al., 2014, 2019, 2020; Choi et al., 2014; Cruz et al., 2015; de Castro et al., 2017; Deng et al., 2020; Dinh et al., 2020; Fang et al., 2020a, 2020b; Gao et al., 2020; Hao et al., 2019; Harrell et al., 2020; Hogan et al., 2019; Huang et al., 2019; Khatri et al., 2018; Kim et al., 2020; Klinger et al., 2020; Lee et al., 2012; Lei et al., 2020; Li et al., 2015, 2019a, 2019b, 2020; Liu et al., 2018, 2019a, 2019b; Mansouri et al., 2019; Maremanda et al., 2019; Monroe et al., 2020; Monsel et al., 2015; Porzionato et al., 2019; Potter et al., 2018; Silva et al., 2019; Stone et al., 2017; Tang et al., 2017; Varkouhi et al., 2019; Wang et al., 2020; Willis et al., 2018, 2020; Xu et al., 2019, 2020; Yi et al., 2019; Zhang et al., 2020; Zhaorigetu et al., 2020; Zhu et al., 2014). Our meta‐analysis focussed on the three most frequently investigated diseases (ALI, BPD and PAH).
Baseline characteristics of the included studies are reported in Table 1. A majority of studies were conducted in the United States (N = 22; 42%) or China (N = 21; 42%) (Figure S3.1). The first paper examining MSC‐EVs in a model of lung disease was published in 2012. Since then, there has been a marked growth in the number of preclinical animal studies investigating MSC‐EVs with over 60% of studies (N = 32) published in 2019 and 2020 (Figure S3.1). A variety of lung conditions have been investigated including ALI (N = 23; 44%), PAH (N = 8; 15%), BPD (N = 7; 13%), asthma / allergic inflammation (N = 5; 10%), pulmonary fibrosis (N = 5; 10%), congenital diaphragmatic hernia (N = 2; 4%) and chronic obstructive pulmonary disease (N = 2; 4%). Most studies utilized either a mouse (N = 31; 60%) and/or rat (N = 21; 40%) model, with only one study applying a pig model (2%). Nearly half of the studies used only male animals (N = 24; 46%), with 8 studies using females only (15%), 2 studies using both sexes (4%), and 18 studies not reporting of the sex of their animals (35%).
TABLE 1.
Summary of study characteristics of all included articles
| First author | Year | Country | Animal model | Human disease | MSC source | EV isolation and size / morphology analysis | Terminology consistency | Positive surface markers | Negative surface markers | Dosage regimen (dose, no. of doses, route and immunocompatibility) |
|---|---|---|---|---|---|---|---|---|---|---|
| Acute Respiratory Distress Syndrome / Acute Lung Injury | ||||||||||
| Zhu, YG | 2014 | United States | Mouse | Acute Respiratory Distress Syndrome | Human bone marrow | Ultracentrifugation; SEM; 150‐ 1000 nm (large EVs) | Yes | Not described | Not described | 3e6 MSC equivalent over 48h; 1 dose; Intravenous, Intratracheal; Xenogeneic |
| Li, L | 2015 | China | Mouse | Acute Respiratory Distress Syndrome | Human bone marrow | Isolation kit / PEG; Not described; Not described | Unclear | CD63 | Not described | 1.5ug/g; 1 dose; Intravenous; Xenogeneic |
| Tang, XD | 2017 | China | Mouse | Acute Respiratory Distress Syndrome | Human bone marrow | Ultracentrifugation; SEM, TEM; 150–1000 nm (large EVs) | Yes | Not described | Not described | 30uL (equivalent to 3e6 MSCs over 48h); 1 dose; Intratracheal; Xenogeneic |
| Khatri, M | 2018 | United States | Pig | Acute Respiratory Distress Syndrome | Pig bone marrow | Ultracentrifugation; TEM; 30‐ 150 nm (small EVs) | No | CD63, CD9, CD81, CD29 (Integrin B1/B2) | Not described | 80ug/kg; 1 dose; Intratracheal; Allogeneic |
| Potter, DR | 2018 | United States | Mouse | Acute Respiratory Distress Syndrome | Human bone marrow | Ultracentrifugation; NTA; 30‐ 1000 nm (both small/large EVs) | Yes | CD63, CD9, CD81, MSC markers | Not described | 30ug; 1 dose; Not Described; Xenogeneic |
| Yi, X | 2019 | China | Mouse | Acute Respiratory Distress Syndrome | Mouse bone marrow | High speed centrifugation; NTA, TEM; 30–150 nm (small EVs) | Yes | CD63 | Not described | 100ug; 1 dose; Intravenous; Allogeneic |
| Xu, N | 2019 | China | Rat | Acute Respiratory Distress Syndrome | Rat bone marrow | Ultracentrifugation; NTA, TEM; 30–150 nm (small EVs) | Yes | CD63, CD9, CD81 | Not described | 5e6 MSC equivalent; 1 dose; Intratracheal; Allogeneic |
| Varkouhi, AK | 2019 | Canada | Rat | Acute Respiratory Distress Syndrome | Human umbilical cord | Anion‐exchange chromatography; TEM; 30–150 nm (small EVs) | No | Not described | Not described | 1e8 particles/kg; 1 dose; Intravenous; Xenogeneic |
| Silva, JD | 2019 | Brazil | Mouse | Acute Respiratory Distress Syndrome | Mouse bone marrow | Ultracentrifugation; NTA, DLS; 30–1000 nm (both small/large EVs) | Yes | Not described | Not described | 1e5 MSC equivalent; 1 dose; Intravenous; Allogeneic |
| Li, JS | 2019 | China | Rat | Acute Respiratory Distress Syndrome | Human umbilical cord | Isolation kit / PEG; DLS, TEM; 30–150 nm (small EVs) | Yes | CD63, CD9 | Not described | 800 ug of RNA; 1 dose; Intravenous; Xenogeneic |
| Liu, J | 2019 | China | Rat | Acute Respiratory Distress Syndrome | Rat bone marrow | Ultracentrifugation; TEM; 30‐ 150 nm (small EVs) | Yes | CD63, CD81, TSG101, MSC markers | Not described | 5‐10ug; 1 dose; Intravenous; Allogeneic |
| Li, QC | 2019 | China | Rat | Acute Respiratory Distress Syndrome | Rat bone marrow | High speed centrifugation; NTA, TEM; 30–150 nm (small EVs) | Yes | CD63, CD9, CD81 | Not described | 25ug; 4 doses; Intravenous; Allogeneic |
| Huang, R | 2019 | China | Mouse | Acute Respiratory Distress Syndrome | Human adipose tissue | Ultracentrifugation; NTA, TEM; 30–1000 nm (both small/large EVs) | Yes | CD63, CD81, MSC markers | Calnexin, GM130 | 100ug; 1 dose; Intravenous; Xenogeneic |
| Chen, W | 2019 | China | Rat | Acute Respiratory Distress Syndrome | Human umbilical cord | Ultracentrifugation; TEM; Not described | Unclear | MSC markers | Not described | Not described; 1 dose; Intratracheal; Xenogeneic |
| Wang, J | 2020 | China | Mouse | Acute Respiratory Distress Syndrome | Human adipose tissue | Ultracentrifugation; DLS, TEM; 30–150 nm (small EVs) | Yes | CD63, CD81, CD44, CD105, CD40 | Calnexin, GM130 | 50ug; 1 dose; Intravenous, Intratracheal; Xenogeneic |
| Deng, H | 2020 | China | Mouse | Acute Respiratory Distress Syndrome | Mouse bone marrow | Ultracentrifugation, Ultrafiltration; NTA, TEM; 30‐150 nm (small EVs) | Yes | CD63, CD81 | Not described | 50ug or 100ug; 1 dose; Intratracheal; Allogeneic |
| Chen, WX | 2020 | China | Rat | Acute Respiratory Distress Syndrome | Human umbilical cord | Ultracentrifugation; TEM; Not described | Unclear | Not described | Not described | 1e6 particles; 1 dose; Intratracheal; Xenogeneic |
| Asthma / Allergic Airway Inflammation | ||||||||||
| Cruz, FF | 2015 | United States | Mouse | Asthma / Allergic Airway Inflammation | Human and mouse bone marrow | Ultracentrifugation; NTA, TEM; 30–1000 nm (both small/large EVs) | Yes | Not described | Not described | 3e6 MSC equivalent; 1 dose; Intravenous; Xenogeneic, Allogeneic |
| de Castro, LL | 2017 | Brazil | Mouse | Asthma / Allergic Airway Inflammation | Human adipose tissue | Ultracentrifugation; DLS; 30‐ 1000 nm (both small/large EVs) | Yes | Not described | Not described | 37ug; 1 dose; Intravenous; Xenogeneic |
| Kim, SD | 2020 | South Korea | Mouse | Asthma / Allergic Airway Inflammation | Mouse adipose tissue | Ultracentrifugation, Ultrafiltration; TEM; Not described | Unclear | CD81, CD40 | Not described | 10ug; 4 doses; Intranasal; Allogeneic |
| Fang, SB | 2020 | China | Mouse | Asthma / Allergic Airway Inflammation | Human iPSCs | Anion‐exchange chromatography; NTA, TEM; 30–150 nm (small EVs) | Yes | CD63, CD9, CD81, TSG101, Alix | Calnexin | 2e10 particles; 1 dose; Intravenous; Xenogeneic |
| Fang, SB | 2020 | China | Mouse | Asthma / Allergic Airway Inflammation | Human iPSCs | Anion‐exchange chromatography; NTA, TEM; 30–150 nm (small EVs) | Yes | CD63, CD9, CD81, TSG101, Alix | Calnexin | 1.5e10 particles; 3 doses; Intravenous; Xenogeneic |
| Bronchopulmonary Dysplasia | ||||||||||
| Ahn, SY | 2018 | South Korea | Rat | Bronchopulmonary Dysplasia | Human umbilical cord | Ultracentrifugation; NTA, TEM; 30–150 nm (small EVs) | No | CD63, CD9 | Cytochrome C, GM130, Fibrillarin | 20ug; 1 dose; Intratracheal; Xenogeneic |
| Willis, GR | 2018 | United States | Mouse | Bronchopulmonary Dysplasia | Human bone marrow, human umbilical cord | Tangential flow filtration, IDX density gradient ultracentrifugation; NTA, TEM; 30–150 nm (small EVs) | Yes | CD63, CD9, Flotillin‐1 | Not described | 0.5e6 MSC equivalent over 36 h; 1 dose; Intravenous; Xenogeneic |
| Chaubey, S | 2018 | United States | Mouse | Bronchopulmonary Dysplasia | Human umbilical cord | Ultracentrifugation; NTA, TEM; 30–150 nm (small EVs) | Yes | CD63, CD81, Alix | TGN48 | 0.7e6 MSC equivalent over 24h (2.4‐ 2.8ug); 2 doses; intraperitoneal; Xenogeneic |
| Brain, RK | 2018 | United States | Rat | Bronchopulmonary Dysplasia | Rat bone marrow | Ultracentrifugation; NTA, TEM; 30–1000 nm (both small/large EVs) | No | CD63, CD9, CD81 | Not described | 15ug or 3.4e9 particles; 14 or 12 doses; intraperitoneal; Allogeneic |
| Porizonato, A | 2019 | Italy | Rat | Bronchopulmonary Dysplasia | Human umbilical cord | Tangential flow filtration; NTA; 30–1000 nm (both small/large EVs) | Yes | CD63, CD9, CD81, Annexin V | HLAs | 8e8 particles/g for P3, 4.5e8 for P7, and 3e8 for P10; 3 doses; Intratracheal; Xenogeneic |
| Willis, GR | 2020 | United States | Mouse | Bronchopulmonary Dysplasia | Human umbilical cord | Tangential flow filtration, IDX density gradient ultracentrifugation; NTA, TEM; 30–150 nm (small EVs) | Yes | CD63, CD9, CD81, TSG101, Alix, Flotillin‐1 | GM130 | 0.5e6 MSC equivalent, or 1e6 MSC equivalent; 1 or 4 doses; Intravenous; Xenogeneic |
| Li, Z | 2020 | China | Rat | Bronchopulmonary Dysplasia | Human placenta | Ultracentrifugation; TEM; 30‐ 150 nm (small EVs) | Yes | CD63, CD9, CD81, HSP70/90 | Not described | 0.3ug; 1 dose; Intratracheal; Xenogeneic |
| Chronic Obstructive Pulmonary Disease | ||||||||||
| Maremanda, K | 2019 | United States | Mouse | Chronic Obstructive Pulmonary Disease | Mouse bone marrow | Isolation kit / PEG; TRPS, TEM; 30–150 nm (small EVs) | Yes | CD63, Alix, Flotillin‐1 | Calnexin | 15ug; 10 doses; intraperitoneal; Allogeneic |
| Harrell, CR | 2020 | Serbia | Mouse | Chronic Obstructive Pulmonary Disease | Human placenta | Ultracentrifugation, Isolation kit / PEG; Not described; Not described | Unclear | Not described | Not described | 0.1 ml; 15 doses; intraperitoneal; Xenogeneic |
| Congenital Diaphragmatic Hernia | ||||||||||
| Zhaorigetu, S | 2020 | United States | Rat | Congenital Diaphragmatic Hernia | Human bone marrow | Tangential flow filtration; TRPS, TEM; 30–150 nm (small EVs) | Yes | Not described | Not described | 1e10 particles/ml; 1 dose; Intravascular; Xenogeneic |
| Monroe, MN | 2020 | United States | Rat | Congenital Diaphragmatic Hernia | Human bone marrow | Tangential flow filtration; NTA, TEM; Not described | Unclear | Not described | Not described | 1e10 particles/ml; 1 dose; Direct tissue injection; Xenogeneic |
| Ischemia‐Reperfusion Injury | ||||||||||
| Stone, ML | 2017 | United States | Mouse | Ischemia‐Reperfusion Injury | Human umbilical cord | Ultracentrifugation; NTA; 150‐1000 nm (large EVs) | No | MSC markers | Not described | 1e6 particles; 1 dose; Intratracheal; Xenogeneic |
| Li, JW | 2019 | China | Mouse | Ischemia‐Reperfusion Injury | Mouse bone marrow | Ultracentrifugation, Isolation kit / PEG; NTA; Not described | Unclear | CD63, CD9, CD81 | Not described | 30uL (2e6 MSC equivalent); 1 dose; Intratracheal; Allogeneic |
| Pneumonia | ||||||||||
| Monsel, A | 2015 | United States | Mouse | Pneumonia | Human bone marrow | Ultracentrifugation; SEM; 150‐ 1000 nm (large EVs) | Yes | CD44 | Not described | EVs made by 9e6 MSCs over 48h; 1 dose; Intravenous, Intratracheal; Xenogeneic |
| Hao, Q | 2019 | United States | Mouse | Pneumonia | Human bone marrow | Ultracentrifugation; NTA, SEM; 30–1000 nm (both small/large EVs) | Yes | CD44 | Not described | 1e10 particles; 1 dose; Intravenous; Xenogeneic |
| Pulmonary Arterial Hypertension | ||||||||||
| Lee, C | 2012 | United States | Mouse | Pulmonary Arterial Hypertension | Mouse bone marrow | Ultracentrifugation, Size exclusion chromatography; TEM; 30–150 nm (small EVs) | Yes | CD63, CD9, CD81, TSG101, Alix, HSP70/90, Flotillin‐1 | Not described | 0.1ug (low dose), 10ug (high dose); 1 or 2 doses; Intravenous; Allogeneic |
| Chen, JY | 2014 | United States | Rat | Pulmonary Arterial Hypertension | Rat bone marrow | Ultracentrifugation; NTA, TEM; 30–1000 nm (both small/large EVs) | No | CD29 (Integrin B1/B2), Annexin V | Not described | 30ug; 8 doses; Intravenous; Allogeneic |
| Aliotta, JM | 2016 | United States | Mouse | Pulmonary Arterial Hypertension | Human and mouse bone marrow | Ultracentrifugation; NTA, TEM; 30–150 nm (small EVs), 30 ‐1000 nm (both EVs) | No | CD63, CD9, CD81 | Not described | 25ug; 3 or 4 doses; Intravenous; Xenogeneic, Allogeneic |
| Aliotta, JM | 2017 | United States | Mouse | Pulmonary Arterial Hypertension | Mouse bone marrow | Ultracentrifugation; NTA, TEM; 30–1000 nm (both small/large EVs) | Yes | CD63 | Not described | 25ug; 3 doses; Intravenous; Allogeneic |
| Liu, Z | 2018 | China | Rat | Pulmonary Arterial Hypertension | Rat bone marrow | Ultracentrifugation; TEM; Not described | Unclear | Not described | Not described | 30ug; 8 doses; Intravenous; Allogeneic |
| Hogan, SE | 2019 | United States | Mouse | Pulmonary Arterial Hypertension | Human bone marrow | Tangential flow filtration, Size exclusion chromatography; NTA, TEM; 30–150 nm (small EVs) | Yes | CD63, CD81, TSG101, Alix, Flotillin‐1, Annexin V, EpCAM, ICAM | GM130 | 2e7 particles in 200uL; 1 or 9 doses; Intravenous; Xenogeneic |
| Zhang, S | 2020 | China | Rat | Pulmonary Arterial Hypertension | Human umbilical cord | Ultracentrifugation; TEM; 30‐150 nm (small EVs) | Yes | CD63, CD81, TSG101, Alix | Not described | 25ug; 3 doses; Intravenous; Xenogeneic |
| Klinger, JR | 2020 | United States | Rat | Pulmonary Arterial Hypertension | Human bone marrow | Ultracentrifugation; NTA, TEM; 30–1000 nm (both small/large EVs) | Yes | CD63, CD9, CD81, TSG101 | Albumin | 100ug/kg; 3 doses; Intravenous; Xenogeneic |
| Pulmonary Fibrosis | ||||||||||
| Choi, M | 2014 | South Korea | Mouse | Pulmonary Fibrosis | Human bone marrow | Isolation kit / PEG; Not described; Not described | Unclear | Not described | Not described | 10ug; 2 doses; Intravenous; Xenogeneic |
| Gao, Y | 2016 | China | Rat | Pulmonary Fibrosis | Rat adipose tissue | Ultracentrifugation; NTA, TEM; 30–1000 nm (both small/large EVs) | Yes | CD63, TSG101, Alix | GM130 | 2.5‐2.8e10 particles; 1 dose; Intratracheal; Allogeneic |
| Bandeira, E | 2018 | Brazil | Mouse | Pulmonary Fibrosis | Mouse adipose tissue | Ultracentrifugation; NTA, SEM, TEM; 30–1000 nm (both small/large EVs) | Yes | CD63, CD9, CD81, Lamp1 | Not described | Two doses: 1) 1e5 MSC equivalent over 24h; 2) 1e6 MSC equivalent over 24h; 1 dose; Intratracheal; Allogeneic |
| Mansouri, N | 2019 | United States | Mouse | Pulmonary Fibrosis | Human bone marrow | IDX density gradient ultracentrifugation; NTA, TEM; 30–150 nm (small EVs) | Yes | CD63, CD9, Alix, Flotillin‐1 | GM130 | 5e6 MSC equivalent; 1 dose; Intravenous; Xenogeneic |
| Dinh, PC | 2020 | United States | Rat | Pulmonary Fibrosis | Human bone marrow | Ultrafiltration; NTA, TEM; 30‐150 nm (small EVs) | Yes | CD63, CD9, CD81, TSG101 | Not described | 10e9 particles/kg; 7 doses; Inhalation; Xenogeneic |
| Other Models of Lung Injury | ||||||||||
| Lei, X | 2020 | China | Mouse | Radiation‐Induced Lung Injury | Human placenta | Not described; NTA, TEM; 30‐150 nm (small EVs) | No | CD63, CD9, TSG101, Alix | GM130 | 100ug/dose; 4 doses; Intravenous; Xenogeneic |
| Xu, B | 2020 | China | Rat | Smoke Inhalation Lung Injury | Rat bone marrow | Isolation kit / PEG; NTA, TEM; 30–150 nm (small EVs) | Yes | CD63, CD9, CD81 | Not described | 250ug; 1 dose; Intravenous; Allogeneic |
3.2. EV methodology and interventional characteristics
The animal source of MSCs used included human (N = 34; 65%), mouse (N = 11; 21%), rat (N = 8; 15%) and pig (N = 1; 2%) (Figure S3.2). MSCs were derived from a variety of tissue sources including bone marrow (N = 31; 60%), umbilical cord (N = 11; 21%), adipose tissue (N = 6; 12%), placenta (N = 3; 6%) and induced pluripotent stem cells (iPSCs) (N = 2; 4%) (Figure S3.2). Studies administered either xenogeneic EVs (N = 34; 65%) or allogeneic EVs (N = 20; 38%). Based on the ISCT guidelines, 30 studies (58%) met all three criteria for MSC characterization. In vitro trilineage differentiation and the positive/negative markers used in each study are described in Figure S3.2.
The most common techniques used for EV isolation were ultracentrifugation (N = 34; 65%), isolation kits (N = 7; 13%) and tangential flow filtration (N = 6; 12%) (Figure S3.3). EVs were characterized by quantification (N = 47; 90%), size distribution (N = 45; 87%), morphological analysis (N = 45; 87%) and/or surface marker expression (N = 41; 79%) in most studies (Figure S3.3). Based on the MISEV 2018 guidelines, 10 studies (19%) adequately characterized their EV therapies. Importantly, 52% (N = 27) of studies did not report assessment of negative markers to demonstrate specific isolation of EVs. Regarding EV nomenclature, we assessed whether the terminology used was consistent with reported size distribution of EVs (e.g. small, large or both small/large EVs combined). Thirty‐five (67%) studies utilized consistent terminology, whereas 8 studies (15%) used discordant terms and 9 studies (17%) did not provide size analysis. More details regarding EV isolation and characterization, including specific techniques and markers used, can be found in Figure S3.3.
The two most common routes of administration for MSC‐EV delivery included intravenous (N = 31; 60%) and intratracheal (N = 16; 31%) delivery (Figure S3.4). The units applied for dosing EVs varied considerably and included absolute protein amount (N = 23; 44%), particle number (N = 11; 21%) or amount of EVs released by a certain number of MSCs (N = 8; 15%), as well as EVs released over time or dosed by weight of animal (Figure S3.4). Most studies delivered a single dose of therapy (N = 35; 67%); studies with multiple administrations used a median value of 4 doses (ranging from 2–15 doses). EV modifications were conducted in 33 studies (63%) which included modifications before EV isolation (N = 17; 33%), modifications to EVs directly (N = 8; 15%) and co‐treatments (N = 8; 15%). Lastly, dose‐response and biodistribution analyses were examined in 8 (15%) and 7 (13%) studies, respectively (Figure S3.4).
3.3. Meta‐analyses of acute lung injury studies
Method of ALI induction included intratracheal endotoxin (e.g. lipopolysaccharide; N = 8), intratracheal E. Coli (N = 3), ischemia‐reperfusion injury (N = 2), bleomycin injection (N = 2), smoke inhalation (N = 1), radiation (N = 1), viral induction (N = 1), shock (N = 1), phosgene (N = 1), burn (N = 1), trauma (N = 1) or intestinal ischemia‐reperfusion (N = 1). Histological lung injury was reported in 7 studies investigating preclinical ALI. All 7 studies demonstrated a significant reduction in lung injury from MSC‐EV therapy (pooled analysis, SMD ‐4.33, 95%CI ‐5.73 to ‐2.92, I2 = 74%) (Figure 2). Subgroup analyses demonstrated that small EVs consistently result in a significant attenuation of lung injury whereas the efficacy of large EVs is highly variable and similar to control treatments from pooled analysis (Figure 3). Studies that isolated EVs by ultracentrifugation did not demonstrate a difference in effect size as compared to the single study that utilized an isolation kit (Figure 3). Lastly, MSC‐EVs may demonstrate greater benefits (P = 0.004) to attenuate lung injury in female rodents as compared to males, however only one study used female animals (Figure 3). No difference in effect size was observed between MSC tissue source (bone marrow vs. umbilical cord), species source of MSCs, immunocompatibility (allogeneic vs. xenogeneic), timing of treatment, route of administration or animal model (Figure 3).
FIGURE 2.
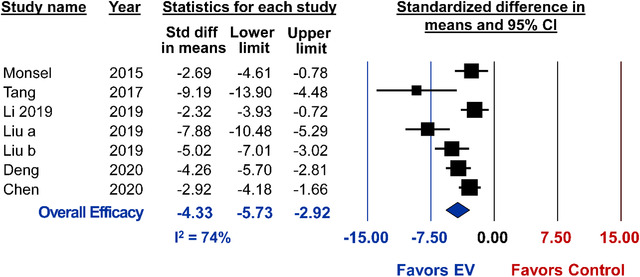
Meta‐analysis for all included studies of acute lung injury that reported the primary outcome of lung injury score. Data is presented as a forest plot with standardized mean difference and 95% confidence intervals. Effect sizes < 0 favours EV treatment and > 0 favours control. Subscript denotes a separate study or article that was published within the same year. The ‘Overall Efficacy’ represents a pooled estimate of MSC‐EV effect on lung injury score from all studies combined. I2 value represents the statistical heterogeneity
FIGURE 3.

Subgroup analysis for all included studies of acute lung injury that reported the primary outcome of lung injury score. Each row represents pooled estimate data from studies within that subgroup. Data is presented as a forest plot with standardized mean difference and 95% confidence intervals. I2 value represents the statistical heterogeneity within each subgroup. Effect sizes < 0 favours EV treatment and > 0 favours control. The ‘Overall Efficacy’ is a pooled estimate effect of MSC‐EVs on lung injury score from all studies combined
Changes to alveolar‐capillary barrier permeability measured by bronchoalveolar lavage fluid (BALF) protein concentration were reported in 28 studies. Overall, MSC‐EV administration was associated with a significant attenuation of lung permeability (SMD ‐2.43, 95%CI ‐3.05 to ‐1.82, I2 = 83%) (Figure 4). From subgroup analysis, lung permeability was reduced to a greater extent (P < 0.001) by delivery of small EVs or both small/large as compared to large EVs alone (Figure 5). Allogeneic administration of MSC‐EVs also showed a greater reduction in lung permeability compared to xenogeneic delivery (P = 0.001). Sex (P = 0.009) and species (P = 0.03) may be associated with greater EV efficacy as female animals and rat models demonstrated better outcomes (Figure 5). Lastly, the single study that used an isolation kit displayed a greater improvement (P < 0.001) to lung permeability as compared to studies that utilized ultracentrifugation. Similar to lung injury score, no difference in effect size was observed when considering MSC tissue source, route of administration or timing of treatment.
FIGURE 4.
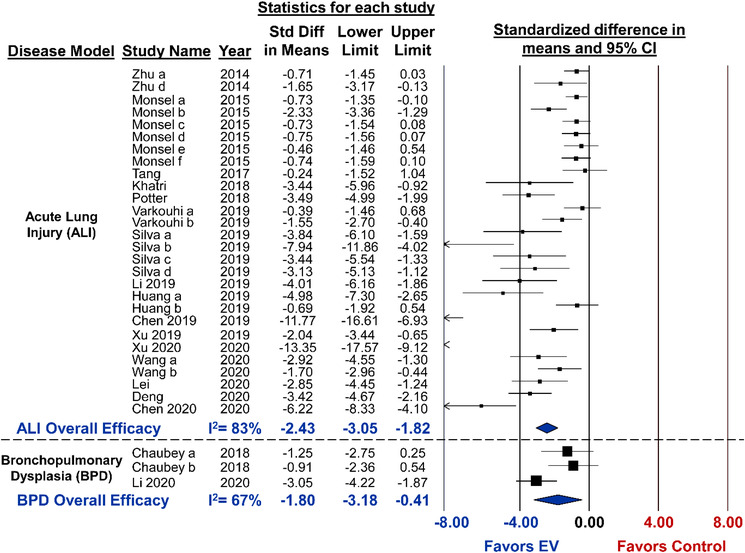
Meta‐analysis for all included studies of acute lung injury and bronchopulmonary dysplasia that reported the secondary outcome of alveolar capillary permeability. Data is presented as a forest plot with standardized mean difference and 95% confidence intervals. Effect sizes < 0 favours EV treatment and > 0 favours control. Subscript denotes a separate study or article that was published within the same year. The ‘Overall Efficacy’ represents a pooled estimate of MSC‐EV effect on lung vascular permeability from all studies combined. I2 value represents the statistical heterogeneity
FIGURE 5.
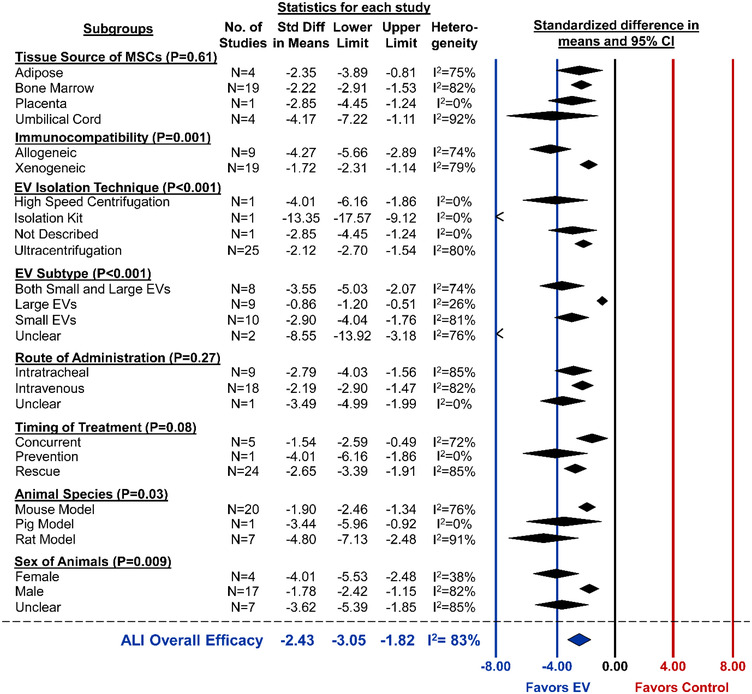
Subgroup analysis for all included studies of acute lung injury that reported the secondary outcome of alveolar capillary permeability. Each row represents pooled estimate data from studies within that subgroup. Data is presented as a forest plot with standardized mean difference and 95% confidence intervals. I2 value represents the statistical heterogeneity within each subgroup. Effect sizes < 0 favours EV treatment and > 0 favours control. The ‘Overall Efficacy’ is a pooled estimate effect of MSC‐EVs on lung vascular permeability from all studies of acute lung injury combined
Other secondary outcomes considered for ALI studies included mortality (RR 0.39, 95%CI 0.22 to 0.68, I2 = 14%) (Figure S4.1) and inflammatory response as measured by neutrophil infiltration in BALF (SMD ‐1.42, 95%CI ‐1.83 to ‐1.02, I2 = 66%) (Figure S4.2), both of which were significantly improved by MSC‐EV therapy. Lastly, a dose‐response analysis was conducted for studies administering EVs by protein amount and reporting lung vascular permeability as an outcome. Despite many studies delivering similar doses, the effect size varied considerably and was not correlated to dose (P = 0.311) (Figure S4.3).
3.4. Meta‐analyses of bronchopulmonary dysplasia studies
All animal models of BPD were induced by housing new‐born mice or rats under hyperoxia conditions, ranging from 60–95% oxygen. Alveolarization was reported in 11 studies from 7 different articles and pooled analysis demonstrated marked improvements from MSC‐EV administration (SMD ‐1.45, 95%CI ‐2.08 to ‐0.82, I2 = 63%) (Figure 6). Similar to ALI, subgroup analysis indicated that small EVs consistently improve alveolarization, whereas the combination of small/large EVs were highly variable and not statistically different as compared to control (Figure 7). One study that isolated EVs by TFF did not exhibit any benefits from EVs. However, when TFF was combined with density gradient ultracentrifugation or ultracentrifugation was used alone, MSC‐EVs significantly improved lung alveolarization (P = 0.008) (Figure 7). Immunocompatibility (allogeneic, P = 0.045), route of administration (intraperitoneal, P = 0.040) and timing of treatment (concurrent, P = 0.044) may result in differential effects, whereas no differences in efficacy was observed when comparing tissue source of MSCs or animal species.
FIGURE 6.
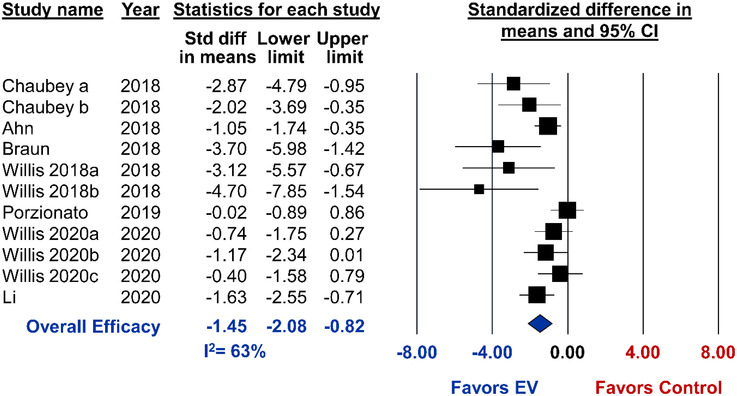
Meta‐analysis for all included studies of bronchopulmonary dysplasia that reported the primary outcome of lung alveolarization. Data is presented as a forest plot with standardized mean difference and 95% confidence intervals. Effect sizes < 0 favours EV treatment and > 0 favours control. Subscript denotes a separate study or article that was published within the same year. The ‘Overall Efficacy’ represents a pooled estimate of MSC‐EV effect on alveolarization from all studies combined. I2 value represents the statistical heterogeneity
FIGURE 7.
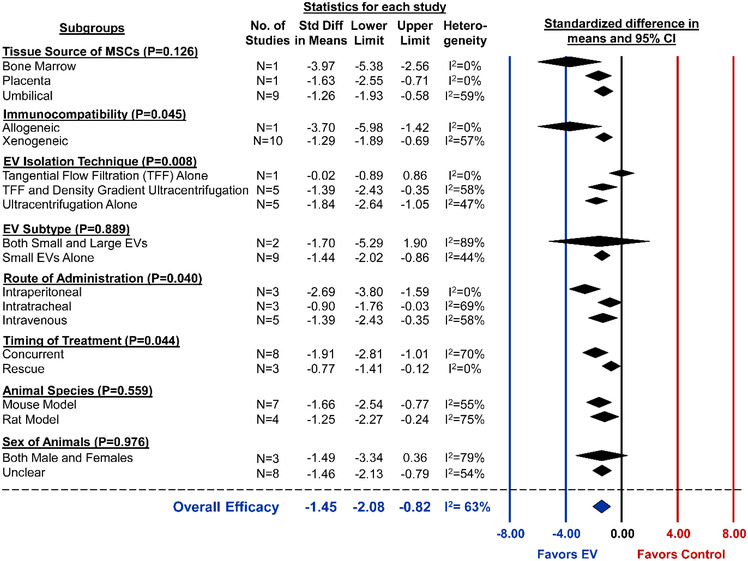
Subgroup analysis for all included studies of bronchopulmonary dysplasia that reported the primary outcome of lung alveolarization. Each row represents pooled estimate data from studies within that subgroup. Data is presented as a forest plot with standardized mean difference and 95% confidence intervals. I2 value represents the statistical heterogeneity within each subgroup. Effect sizes < 0 favours EV treatment and > 0 favours control. The ‘Overall Efficacy’ is a pooled estimate effect of MSC‐EVs on alveolarization from all studies combined
One study evaluated the survival benefits of MSC‐EVs in experimental BPD; however, mortality was shown to be unaltered as compared to control (RR 0.86, 95%CI 0.41 to 1.80) (Figure S4.1). Other secondary outcomes of experimental BPD that were assessed include alveolar permeability (SMD ‐1.80, 95%CI ‐3.18 to ‐0.41, I2 = 67%) (Figure 4) and lung inflammation as measured by BALF neutrophil (SMD ‐2.48, 95%CI ‐3.41 to ‐1.75, I2 = 66%) (Figure S4.2), both of which demonstrated significant improvements from MSC‐EV delivery. Furthermore, MSC‐EV delivery resulted in marked reductions to both Fulton's Index (measure of right ventricular hypertrophy; SMD ‐0.99, 95%CI ‐1.49 to ‐0.49, I2 = 0%) (Figure S4.4) and RVSP (SMD ‐1.20, 95%CI ‐2.07 to ‐0.34, I2 = 0%) (Figure S4.5).
3.5. Meta‐analyses of pulmonary arterial hypertension studies
Method for PAH induction included monocrotaline injection (N = 5), chronic hypoxia(N = 2), or the combination of semaxanib (SU5416) and chronic hypoxia in rats (N = 2). Ten distinct studies from 5 articles reported RVSP, each of which demonstrated a significant reduction after MSC‐EV administration (pooled analysis, SMD ‐4.16, 95%CI ‐5.68 to ‐2.64, I2 = 84%) (Figure 8). Exploratory subgroup analysis showed allogeneic MSC‐EVs improved RVSP to a significantly greater extent than xenogeneic administration (P < 0.001) (Figure 9). Interestingly, in the single report of EVs isolated by combining TFF and size exclusion chromatography, EVs were less efficacious (P < 0.001) as compared to ultracentrifugation or ultracentrifugation/chromatography combined (Figure 9). Studies that did not report sex showed a significantly greater reduction in RVSP as compared to male‐only studies (P < 0.001). Despite the well‐known sex dependency of PAH, no preclinical study explored MSC‐EV therapy in female animals. Unlike ALI or BPD, EV subtype did not demonstrate variable effects to RVSP. No differences in EV efficacy were observed when comparing MSC tissue source or timing of treatment (Figure 9). Lastly, 17 studies reported the secondary outcome of Fulton's Index. In these studies, MSC‐EV administration led to a significant reduction in right ventricular hypertrophy (SMD ‐2.80, 95%CI ‐3.68 to ‐1.91, I2 = 78%) (Figure S4.4).
FIGURE 8.
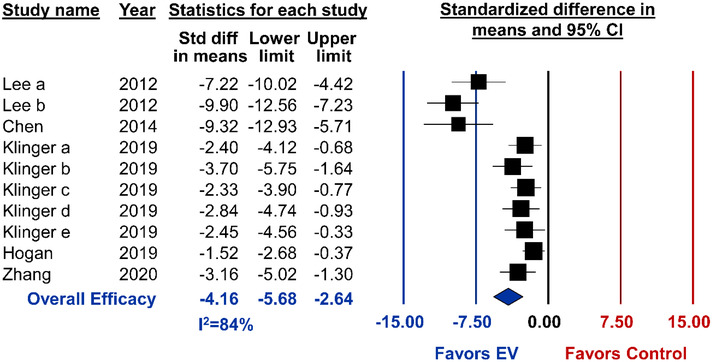
Meta‐analysis for all included studies of pulmonary arterial hypertension that reported the primary outcome of right ventricular systolic pressure. Data is presented as a forest plot with standardized mean difference and 95% confidence intervals. Effect sizes < 0 favours EV treatment and > 0 favours control. Subscript denotes a separate study or article that was published within the same year. The ‘Overall Efficacy’ represents a pooled estimate of MSC‐EV effect on right ventricular systolic pressure from all studies combined. I2 value represents the statistical heterogeneity
FIGURE 9.
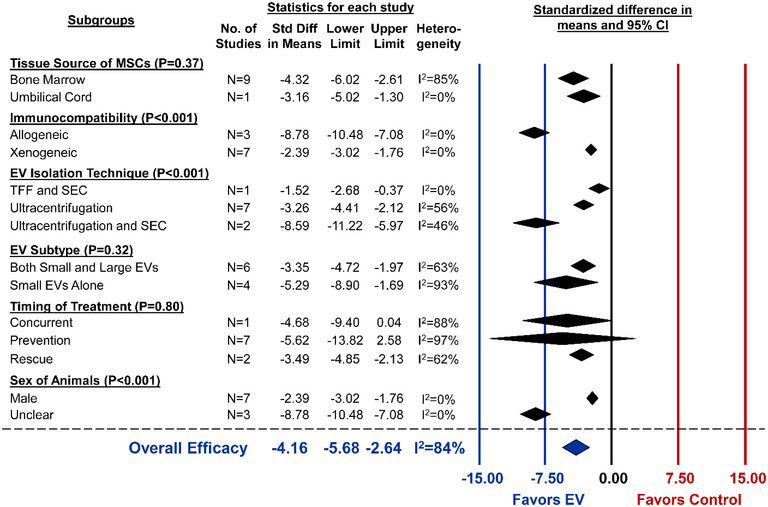
Subgroup analysis for all included studies of pulmonary arterial hypertension that reported the primary outcome of right ventricular systolic pressure. Each row represents pooled estimate data from studies within that subgroup. Data is presented as a forest plot with standardized mean difference and 95% confidence intervals. I2 value represents the statistical heterogeneity within each subgroup. Effect sizes < 0 favours EV treatment and > 0 favours control. The ‘Overall Efficacy’ is a pooled estimate effect of MSC‐EVs on right ventricular systolic pressure from all studies combined
3.6. Studies of other respiratory conditions
3.6.1. Asthma / allergic airway inflammation (N = 5 studies)
Disease was induced by intraperitoneal delivery of ovalbumin (N = 3) (De Castro et al., 2017; Fang et al., 2020a; Kim et al., 2020), Aspergillus fumigatus hyphal extract (N = 1) (Cruz et al., 2015), or IL‐33 (N = 1) (Fang et al., 2020b). MSC‐EVs were administered by intravenous injection in 4 studies, with one study using intranasal delivery for more localized lung distribution. Four studies assessed in vivo outcomes, all of which reported a significant reduction to BALF neutrophils, eosinophils and pro‐inflammatory cytokines from MSC‐EV delivery. Three studies reported a marked attenuation in lung inflammation score. One study showed improvements to lung mechanics, and another demonstrated a reduction in lung hyperresponsiveness to allergens (e.g. Aspergillus hyphal). Lastly, one study assessed differential gene expression in lung tissues post‐EV administration and found the most upregulated genes were those related to antioxidation (Pon1), mitochondrial function (Bex2) and lung epithelium protection (Scgb1c1). Of note, the inhibition of miR‐146a abolished the therapeutic efficacy of MSC‐EVs, thereby illustrating its critical role within EVs for immunomodulation.
3.6.2. Pulmonary fibrosis (N = 5 studies)
Fibrosis was induced by intratracheal silica (N = 2) (Bandeira et al., 2018; Choi et al., 2014), bleomycin (N = 2) (Dinh et al., 2020; Mansouri et al., 2019), or fine particulate matter (N = 1) (Gao et al., 2020). All studies delivered MSC‐EVs as a rescue treatment which led to reduced lung collagen content in three studies, one of which was still significantly elevated as compared to control. In the other two studies, MSC‐EV treated animals did not attenuate collagen deposition, whereas MSCs themselves or lung spheroid cell exosomes led to marked improvements. The effect of MSC‐EVs on BALF macrophage expression was variable. Two studies reported no changes in number of lung macrophages, whereas one study observed a rescued expression of anti‐inflammatory macrophages. Most studies demonstrated reduced expression of pro‐inflammatory cytokines including IL‐1β, TGFβ1 and TNFα. Lastly, one study found that transplantation of bone marrow derived monocytes preconditioned with MSC‐EVs attenuated both pulmonary fibrosis and lung inflammation.
3.6.3. Chronic obstructive pulmonary disease (COPD)
Two articles (4%) studied the effects of MSC‐EVs in a cigarette smoke induced mouse model of COPD (Harrell et al., 2020; Maremanda et al., 2019). In one study, the combination of MSCs and exosomes were more effective than either treatment alone to reduce BALF cell count and cytokine levels (Maremanda et al., 2019). The mechanism of action was found to be recovery of mitochondrial transfer and mitochondrial fusion gene expression. The second study of COPD found intraperitoneal delivery of MSC‐EVs resulted in marked reductions to plasma pro‐inflammatory cytokines, histological lung injury and lung influx of inflammatory immune cells (Harrell et al., 2020).
3.7. Quality of reporting in MSC‐EV research
All 52 lung articles were assessed for their completeness of reporting study design domains based on the NIH Principles and Guidelines for Reporting in Preclinical Research (NIH 2014). These guidelines identify parameters that should be included in preclinical publications to enhance scientific rigor, reproducibility and transparency. No articles used established reporting guidelines (e.g. Animal Research: Reporting In Vivo Experiments, ARRIVE) (Figure S6). Findings were substantiated under a range of conditions (e.g. different dosages, routes of administration, modifications) in 31 studies (60%). Forty studies (77%) stated N‐values for at least one in vivo outcome and 31 studies (60%) for all outcomes. A priori sample size determination was described in seven studies (13%); however, all seven studies had inadequate information necessary to reproduce calculations. The total number of animals used in all experiments was reported in 17 studies (33%) (Figure S6).
A primary outcome was not explicitly stated in any of the 52 studies (Figure S6). The number of measurements per subject was described for at least one outcome in 30 studies (58%), two of which reported the number of replicates in all findings. Randomization of experimental groups was mentioned in less than half of the articles (N = 24); however, the method of randomization was not described in any article. No studies reported blinding of the personnel conducting experiments. Blinding of data analysis was described for all outcomes in two studies (4%) and some outcomes in 16 studies (31%), with no blinding of outcome assessment mentioned for 33 studies (66%). Lastly, the NIH recommends reporting the criteria for data exclusion to minimize selection bias. Seven studies (13%) provided reasoning for data omission, which included excluding animals with heart rates < 300 beats per minute for hemodynamic measurements (Willis et al., 2018), low expression of genes in RNA sequencing (Hogan et al., 2019), or statistical outliers (Varkouhi et al., 2019). Forty‐five studies (87%) did not provide any criteria for data exclusion (Figure S6).
3.8. Risk of bias assessment
A majority of studies were deemed as having an ‘unclear’ risk of bias across most domains (Figure 10). Although 24 studies (46%) reported that animals were randomized to experimental groups, no studies provided details regarding method of randomization (i.e. to reduce selection bias) which is essential to assess adequate random sequence generation. Thirty‐two studies (62%) displayed a low risk for reporting baseline characteristics (i.e. to reduce selection bias). However, the risk of bias was unclear across all studies for the domains of allocation concealment (i.e. selection bias), random housing (i.e. selection bias), blinding of personnel (i.e. performance bias) and selective outcome reporting (i.e. reporting bias). Seventeen studies reported blinded assessments (i.e. detection bias) for some outcomes (e.g. histological/pathological injury) and 2 studies were blinded for all outcomes. Incomplete outcome data (i.e. attrition bias) was assessed as whether the N‐value was consistent between the methods and results sections. Fourteen studies (27%) exhibited low risk, 25 studies (48%) had unclear risk and 13 studies (25%) were deemed high risk. Lastly, other potential sources of bias considered included source of funding, sample size calculation and conflict of interests. Eleven studies (21%) had a high risk in at least one of these additional categories. (Figure 10).
FIGURE 10.
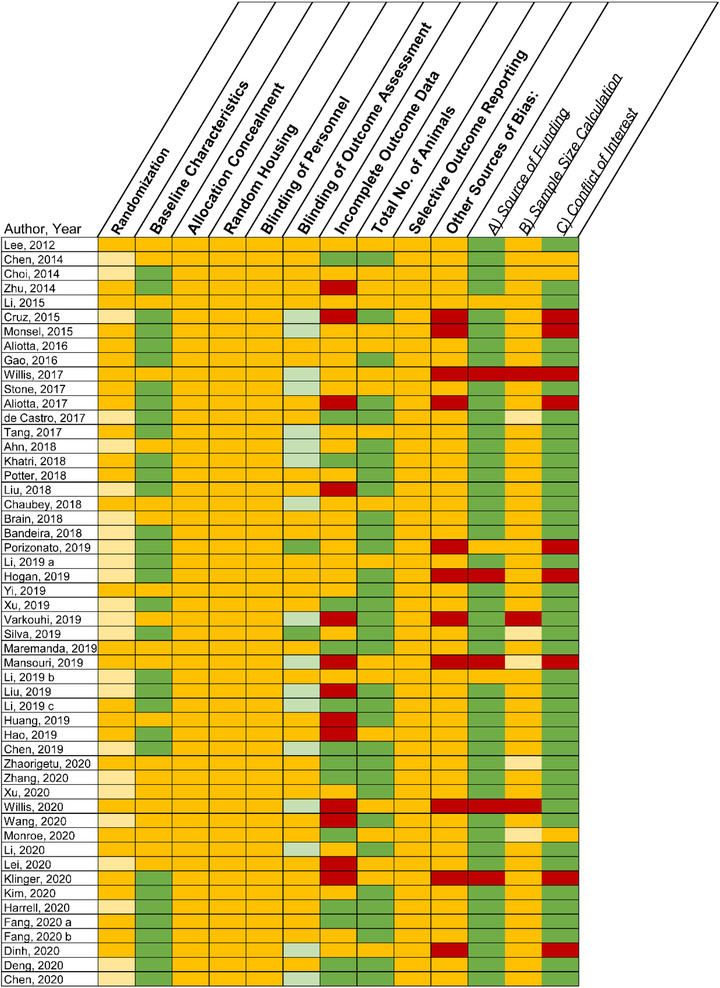
Risk of bias assessment in accordance with the SYRCLE tool. Yellow represents unclear, green represents low risk of bias and red represents high risk of bias. Light yellow represents a study that reported randomization or sample size calculations, but did not provide detailed methodology on how these parameters were achieved. Light green under ‘blinding of outcome’ represents studies that blinded some outcomes, whereas dark green represents blinding of all outcomes
4. DISCUSSION
Our systematic review provides a comprehensive synthesis of the preclinical efficacy, methodology, study design and preclinical reporting of MSC‐EV studies of acute and chronic respiratory diseases. Pooled estimates from meta‐analyses indicate that MSC‐EV administration significantly improves primary outcomes in ALI (i.e. lung injury score), BPD (i.e. alveolarization) and PAH (i.e. right ventricular systolic pressure). With the exception of mortality for BPD, MSC‐EVs were also markedly effective at improving secondary outcomes including survival (ALI), lung vascular permeability (ALI and BPD), BALF neutrophil count (ALI and BPD), RVSP (BPD) and Fulton's Index (BPD and PAH). These findings demonstrate the therapeutic efficacy of MSC‐EVs to treat both acute and chronic lung diseases.
Our previous systematic analysis of all animal studies investigating MSC‐EVs found high heterogeneity in how EVs are being isolated and characterized (Tieu et al., 2020). Moreover, there were significant inconsistencies in nomenclature being used to describe EV therapy and variability in interventional characteristics including tissue source of MSCs and treatment regimen. After updating our search and limiting our review to publications related to lung disorders, we still observed similar heterogeneity. When assessing whether groups were characterizing their MSCs and EVs according to international guidelines (e.g. ISCT criteria and MISEV 2018) (Dominici et al., 2006; Théry et al., 2018), we found that 58% and 19% of studies met the recommendations, respectively. This is a significant increase from our previous reports of only 2% adherence to MISEV 2018 (Tieu et al., 2020) which highlights the impact of these guidelines for improving EV characterization. EV nomenclature remains inconsistent despite the suggestions from MISEV 2018 to describe EV therapies by physical parameters (e.g. small EVs 30–150 nm, large EVs 150–1000 nm). Based on size alone, we found that over 30% of studies are using discordant terminology. This finding may be problematic for two reasons: (1) inconsistent terms will hinder between study comparisons of different EV populations, and (2) our finding likely is an underestimate as we only considered size and did not include analysis of EV cellular origin (e.g. exosomes originate as intraluminal vesicles within late endosomes). Greater standardization in reporting of size and applying accurate terminology in the EV field can help identify specific EV populations that serve as a more effective therapeutic product.
Given the many variables involved in developing an MSC‐EV therapy, we conducted exploratory subgroup analyses to determine if specific EV parameters may be associated with improved efficacy. Across studies of ALI, BPD and PAH, the different tissue sources of MSCs (e.g. bone marrow, umbilical cord, placenta) and routes of administration (e.g. intravenous, intraperitoneal, intratracheal) were equally efficacious, despite beliefs that intratracheal delivery may allow for greater lung distribution. Moreover, umbilical cord tissue is known to be more efficient in producing EVs with potentially less variability from donor demographics (e.g. age, sex, comorbidities) and serves as a non‐invasive, abundant source for MSCs given its designation as biohazardous waste (Curley et al., 2017; Fong et al., 2012; Hua et al., 2014). Hence, the similar efficacy and ease in collection of umbilical cord tissue suggests some potential advantages when considering clinical translation. EVs isolated by TFF appeared to be less effective than those enriched by ultracentrifugation for preclinical BPD and PAH (no studies used TFF in ALI). However, these findings were from only two studies that utilized TFF and more direct comparisons of TFF and ultracentrifugation are warranted to establish the relative in vivo efficacy of EVs, especially given TFF's greater efficiency in EV yield and potential for clinical scaleup (Haraszti et al., 2018). Lastly, we conducted subgroup analysis by EV subtype/size to investigate whether distinct EV populations may exhibit variable outcomes. Small EVs or the combination of small/large EVs were found to be more consistently beneficial as compared to large EVs in ALI and BPD (no studies used large EVs in PAH). Moreover, small EVs led to a significant improvement in lung vascular permeability as compared to large EVs. These results may indicate that there are differential effects between small and large EVs, which may not be dependent on acuity/chronicity of the disease state.
Our study had limitations which warrant discussion. Firstly, the findings from subgroup analyses should be considered exploratory. We show that specific EV characteristics are associated with greater efficacy in these investigations. However, some subgroup categories were limited by the number of available studies and/or the sample size. Hence, updated analyses when more studies are published will be required to generate definitive conclusions. Our findings identify the need for robust data from head‐to‐head comparisons of EV methodology and/or subtypes to identify the most effective approaches for clinical translation. Next, data generated from studies with a low risk of bias may serve as a more accurate indicator for the efficacy of MSC‐EVs. Unfortunately, most studies included in our analysis demonstrated an “unclear” risk across a majority of design parameters, which is commonly observed in many fields of preclinical in vivo research (Avey et al., 2016; Bailey et al., 2021a, 2021b; Begley & Ioannidis, 2015; Fergusson et al., 2019a, 2019b). Future analyses will provide greater insights once researchers and journals adopt more complete reporting of preclinical study design (Collins & Tabak, 2014; Henderson et al., 2013; Landis et al., 2012). Lastly, although no adverse events were reported, none of the included studies conducted formal experiments to investigate MSC‐EV safety.
Although MSC‐EV therapy demonstrates great promise, several questions still need to be answered to advance the field. We found dose‐response and biodistribution studies to be infrequently conducted in respiratory diseases, which is also the case for the MSC‐EV field as a whole (Tieu et al., 2020). These experiments are urgently needed to better understand the optimal approach for dosing EVs before attempting clinical translation. Moreover, there are no standardized guidelines on how EV dose should be measured and reported (e.g. protein, particle or RNA amount). We now know that MSC‐EVs contain many bioactive factors including mRNas, microRNAs and proteins that modulate critical cell processes ranging from inflammation to apoptosis to angiogenesis (Tieu et al., 2020). Although many studies show the importance of specific molecules by inhibiting their expression, few have attempted to compare whether overexpressing one bioactive factor is more important than others. Lastly, quality of reporting and potential risk of bias in study design is consistently unclear in preclinical EV research. Greater rigor and transparent detailing of experimental parameters including randomization, blinding, and sample size estimates will generate more robust evidence for EV efficacy to guide clinical translation.
CONFLICT OF INTEREST
Duncan J. Stewart is an advisor to Northern Therapeutics (Montreal, QC, Canada). The remaining authors have no competing interests to declare.
Supporting information
Supplemental File 1. PRISMA 2020 Checklist.
Supplemental File 2. Systematic Review Search Strategies.
Supplemental File 3. Supporting Figures for Study Characteristics, EV Methodology and Interventional Traits
Supplemental File 4. Meta‐Analysis of Secondary Outcomes
Supplemental File 5. Subgroup Analyses of Outcomes.
Supplemental File 6. Quality of Reporting in MSC‐EV Lung Studies.
ACKNOWLEDGEMENTS
AT is an MD/PhD candidate supported as a Vanier Scholar with the Canadian Institutes of Health Research (CIHR), a Canadian Vascular Network Scholar and an Audrey J Boyce MD/PhD Fellow. MML is supported by the Ottawa Hospital Anesthesia Alternate Funds Association and holds a University of Ottawa Junior Research Chair in Innovative Translational Research. The authors thank Risa Shorr, librarian and information specialist within the Ottawa Hospital Research Institute who designed the search strategy for this systematic review.
Tieu, A., Hu, K., Gnyra, C., Montroy, J., Fergusson, D. A., Allan, D. S., Stewart, D. J., Thebaud, B., & Lalu, M. M. (2021). Mesenchymal stromal cell extracellular vesicles as therapy for acute and chronic respiratory diseases: A meta‐analysis. Journal of Extracellular Vesicles, 10, e12141. 10.1002/jev2.12141
Funding information
This work was not supported by any external or internal sources of funding.
REFERENCES
- Akyurekli, C., Le, Y., Richardson, R. B., Fergusson, D., Tay, J., & Allan, D. S. (2015). A systematic review of preclinical studies on the therapeutic potential of mesenchymal stromal cell‐derived microvesicles. Stem Cell Reviews and Reports, 11, 150–160 [DOI] [PubMed] [Google Scholar]
- Allan, D., Tieu, A., Lalu, M., & Burger, D. (2019). Concise review: Mesenchymal stromal cell‐derived extracellular vesicles for regenerative therapy and immune modulation: progress and challenges toward clinical application. Stem Cells Translational Medicine, 9, 39–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Börger, V., Bremer, M., Ferrer‐Tur, R., Gockeln, L., Stambouli, O., Becic, A., & Giebel, B. (2017). Mesenchymal stem/stromal cell‐derived extracellular vesicles and their potential as novel immunomodulatory therapeutic agents. International Journal of Molecular Sciences, 18, 1450. 10.3390/ijms18071450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn, S. Y., Park, W. S., Kim, Y. E., Sung, D. K., Sung, S. I., Ahn, J. Y., Chang, Y. S. (2018). Vascular endothelial growth factor mediates the therapeutic efficacy of mesenchymal stem cell‐derived extracellular vesicles against neonatal hyperoxic lung injury. Experimental & Molecular Medicine, 50(4), 1–12. 10.1038/s12276-018-0055-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aliotta, J. M., Pereira, M., Wen, S., Dooner, M. S., Del Tatto, M., Papa, E., Cheng, Y., Goldberg, L., Ventetuolo, C. E., Liang, O., Klinger, J. R., & Quesenberry, P. J. (2017). Bone marrow endothelial progenitor cells are the cellular mediators of pulmonary hypertension in the murine monocrotaline injury model. Stem Cells Translational Medicine, 6, 1595–1606. 10.1002/sctm.16-0386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aliotta, J. M., Pereira, M., Wen, S., Dooner, M. S., Del Tatto, M., Papa, E., Goldberg, L. R., Baird, G. L., Ventetuolo, C. E., Quesenberry, P. J., & Klinger, J. R. (2016). Exosomes induce and reverse monocrotaline‐induced pulmonary hypertension in mice. Cardiovascular Research, 110, 319–330. 10.1093/cvr/cvw054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avey, M. T., Moher, D., Sullivan, K. J., Fergusson, D., Griffin, G., Grimshaw, J. M., Hutton, B., Lalu, M. M., Macleod, M., Marshall, J., Mei, S. H. J., Rudnicki, M., Stewart, D. J., Turgeon, A. F., & Mcintyre, L. (2016). The devil is in the details: Incomplete reporting in preclinical animal research. Plos One, 11, e0166733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey, A. J. M., Li, H., Kirkham, A. M., Tieu, A., Maganti, H. B., Shorr, R., Fergusson, D. A., Lalu, M. M., Elomazzen, H., Allan, D. S. (2021a). MSC‐Derived Extracellular Vesicles to Heal Diabetic Wounds: a Systematic Review and Meta‐Analysis of Preclinical Animal Studies. Stem Cell Reviews and Reports, 1–12. 10.1007/s12015-021-10164-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey, A. J. M., Tieu, A., Gupta, M., Slobodian, M., Shorr, R., Ramsay, T., Rodriguez, R. A., Fergusson, D. A., Lalu, M. M., Allan, D. S. (2021b). Mesenchymal Stromal Cell‐derived Extracellular Vesicles in Preclinical Animal Models of Tumor Growth: Systematic Review and Meta‐analysis. Stem Cell Reviews and Reports, 1–14. 10.1007/s12015-021-10163-5 [DOI] [PubMed] [Google Scholar]
- Bandeira, E., Oliveira, H., Silva, J. D., Menna‐Barreto, R. F. S., Takyia, C. M., Suk, J. S., Witwer, K. W., Paulaitis, M. E., Hanes, J., Rocco, P. R. M., Morales, M. M. (2018). Therapeutic effects of adipose‐tissue‐derived mesenchymal stromal cells and their extracellular vesicles in experimental silicosis. Respiratory Research, 19(1), 1–10. 10.1186/s12931-018-0802-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begley, C. G., & Ioannidis, J. P. A. (2015). Reproducibility in science: Improving the standard for basic and preclinical research. Circulation Research, 116, 116–126 [DOI] [PubMed] [Google Scholar]
- Braun, R. K., Chetty, C., Balasubramaniam, V., Centanni, R., Haraldsdottir, K., Hematti, P., & Eldridge, M. W. (2018). Intraperitoneal injection of MSC‐derived exosomes prevent experimental bronchopulmonary dysplasia. Biochemical and Biophysical Research Communications, 503, 2653–2658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan, H., Olson, S. D., Kumar, A., George, M., Prabhakara, K. S., Wenzel, P., Bedi, S., Toledano‐Furman, N. E., Triolo, F., Kamhieh‐Milz, J., Moll, G., Cox, C. S. (2019). Mesenchymal Stromal Cell Therapeutic Delivery: Translational Challenges to Clinical Application. Frontiers in Immunology, 10. 10.3389/fimmu.2019.01645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaubey, S., Thueson, S., Ponnalagu, D., Alam, M. A., Gheorghe, C. P., Aghai, Z., Singh, H., Bhandari, V. (2018). Early gestational mesenchymal stem cell secretome attenuates experimental bronchopulmonary dysplasia in part via exosome‐associated factor TSG‐6. Stem Cell Research & Therapy, 9(1), 1–26. 10.1186/s13287-018-0903-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, J. Y., An, R., Liu, Z. J., Wang, J. J., Chen, S. Z., Hong, M. M., Liu, J. H., Xiao, M. Y., & Chen, Y. F. (2014). Therapeutic effects of mesenchymal stem cell‐derived microvesicles on pulmonary arterial hypertension in rats. Acta Pharmacologica Sinica, 35, 1121–1128. 10.1038/aps.2014.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, T. S., Arslan, F., Yin, Y., Tan, S. S., Lai, R. C., Choo, A. B. H., Padmanabhan, J., Lee, C. N., De, K. D. P., & Lim, S. K. (2011). Enabling a robust scalable manufacturing process for therapeutic exosomes through oncogenic immortalization of human ESC‐derived MSCs. Journal of Translational Medicine, 9, 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, W., Wang, S., Xiang, H., Liu, J., Zhang, Y., Zhou, S., Du, T., & Shan, L. (2019). Microvesicles derived from human Wharton's Jelly mesenchymal stem cells ameliorate acute lung injury partly mediated by hepatocyte growth factor. International Journal of Biochemistry & Cell Biology, 112, 114–122 [DOI] [PubMed] [Google Scholar]
- Chen, W.‐X., Zhou, J., Zhou, S.‐S., Zhang, Y.‐D., Ji, T.‐Y., Zhang, X.‐L., Wang, S.‐M., Du, T., & Ding, D.‐G. (2020). Microvesicles derived from human Wharton's jelly mesenchymal stem cells enhance autophagy and ameliorate acute lung injury via delivery of miR‐100. Stem Cell Research & Therapy, 11, 113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi, M., Ban, T., & Rhim, T. (2014). Therapeutic use of stem cell transplantation for cell replacement or cytoprotective effect of microvesicle released from mesenchymal stem cell. Molecules and Cells, 37, 133–139. 10.14348/molcells.2014.2317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins, F. S., & Tabak, L. A. (2014). Policy: NIH plans to enhance reproducibility. Nature News, 505, 612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo, M., Raposo, G., & Théry, C. (2014). Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annual Review of Cell and Developmental Biology, 30, 255–289 [DOI] [PubMed] [Google Scholar]
- Crowther, M., Lim, W., & Crowther, M. A. (2010). Systematic review and meta‐analysis methodology. Blood, 116, 3140–3146 [DOI] [PubMed] [Google Scholar]
- Cruz, F. F., Borg, Z. D., Goodwin, M., Sokocevic, D., Wagner, D. E., Coffey, A., Antunes, M., Robinson, K. L., Mitsialis, S. A., Kourembanas, S., Thane, K., Hoffman, A. M., McKenna, D. H., Rocco, P. R. M., & Weiss, D. J. (2015). Systemic administration of human bone marrow‐derived mesenchymal stromal cell extracellular vesicles ameliorates aspergillus hyphal extract‐induced allergic airway inflammation in immunocompetent mice. Stem Cells Translational Medicine, 4, 1302–1316. 10.5966/sctm.2014-0280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curley, G. F., Jerkic, M., Dixon, S., Hogan, G., Masterson, C., O'toole, D., Devaney, J., & Laffey, J. G. (2017).Cryopreserved, xeno‐free human umbilical cord mesenchymal stromal cells reduce lung injury severity and bacterial burden in rodent Escherichia coli–induced acute respiratory distress syndrome. Critical Care Medicine, 45, e202–e212 [DOI] [PubMed] [Google Scholar]
- de Castro, L. L., Xisto, D. G., Kitoko, J. Z., Cruz, F. F., Olsen, P. C., Redondo, P. A. G., Ferreira, T. P. T., Weiss, D. J., Martins, M. A., Morales, M. M., Rocco, P. R. M. (2017). Human adipose tissue mesenchymal stromal cells and their extracellular vesicles act differentially on lung mechanics and inflammation in experimental allergic asthma. Stem Cell Research & Therapy, 8(1), 1–12. 10.1186/s13287-017-0600-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng, H., Wu, L., Liu, M., Zhu, L., Chen, Y., Zhou, H., Shi, X., Wei, J., Zheng, L., Hu, X., Wang, M., He, Z., Lv, X., & Yang, H. (2020). Bone marrow mesenchymal stem cell‐derived exosomes attenuate LPS‐induced ARDS by modulating macrophage polarization through inhibiting glycolysis in macrophages. Shock (Augusta, Ga.), 18, 18. [DOI] [PubMed] [Google Scholar]
- Dinh, P.‐U. C., Paudel, D., Brochu, H., Popowski, K. D., Gracieux, M. C., Cores, J., Huang, K., Hensley, M. T., Harrell, E., Vandergriff, A.C., George, A. K., Barrio, R. T., Hu, S., Allen, T. A., Blackburn, K., Caranasos, T. G., Peng, X., Schnabel, L. V., Adler, K. B., … Cheng, K. (2020). Inhalation of lung spheroid cell secretome and exosomes promotes lung repair in pulmonary fibrosis. Nature Communications, 11, 1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominici, M., Le Blanc, K., Mueller, I., Slaper‐Cortenbach, I., Marini, F. C., Krause, D. S., Deans, R. J., Keating, A., Prockop, D. J., & Horwitz, E. M. (2006). Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8, 315–317 [DOI] [PubMed] [Google Scholar]
- Fang, S.‐B., Zhang, H.‐Y., Meng, X.‐C., Wang, C., He, B.‐X., Peng, Y.‐Q., Xu, Z.‐B., Fan, X.‐L., Wu, Z.‐J., Wu, Z.‐C., Zheng, S.‐G., & Fu, Q.‐L. (2020a). Small extracellular vesicles derived from human MSCs prevent allergic airway inflammation via immunomodulation on pulmonary macrophages. Cell Death & Disease, 11, 1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang, S.‐B., Zhang, H.‐Y., Wang, C., He, B.‐X., Liu, X.‐Q., Meng, X.‐C., Peng, Y.‐Q., Xu, Z.‐B., Fan, X.‐L., Wu, Z.‐J., Chen, D., Zheng, L., Zheng, S. G., & Fu, Q.‐L. (2020b). Small extracellular vesicles derived from human mesenchymal stromal cells prevent group 2 innate lymphoid cell‐dominant allergic airway inflammation through delivery of miR‐146a‐5p. Journal of Extracellular Vesicles, 9, 1723260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fergusson, D. A., Avey, M. T., Barron, C.y C., Bocock, M., Biefer, K. E., Boet, S., Bourque, S. L., Conic, I., Chen, K., Dong, Y. Y., Fox, G. M., George, R. B., Goldenberg, N. M., Gragasin, F. S., Harsha, P., Hong, P. J., James, T. E., Larrigan, S. M., Macneil, J. L., … Lalu, M. M. (2019a). Reporting preclinical anesthesia study (REPEAT): Evaluating the quality of reporting in the preclinical anesthesiology literature. PLOS ONE, 14, e0215221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fergusson, D. A., Wesch, N. L., Leung, G. J., Macneil, J. L., Conic, I., Presseau, J., Cobey, K. D., Diallo, J.‐S., Auer, R., Kimmelman, J., Kekre, N., El‐Sayes, N., Krishnan, R., Keller, B. A., Ilkow, C., & Lalu, M. M. (2019b). Assessing the completeness of reporting in preclinical oncolytic virus therapy studies. Molecular Therapy Oncolytics, 14, 179–187. 10.1016/j.omto.2019.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong, C. Y., Gauthaman, K., Cheyyatraivendran, S., Lin, H. D., Biswas, A., & Bongso, A. (2012). Human umbilical cord Wharton's jelly stem cells and its conditioned medium support hematopoietic stem cell expansion ex vivo. Journal of Cellular Biochemistry, 113, 658–668 [DOI] [PubMed] [Google Scholar]
- Galipeau, J., & Sensébé, L. (2018). Mesenchymal stromal cells: Clinical challenges and therapeutic opportunities. Cell Stem Cell, 22, 824–833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao, Y., Sun, J., Dong, C., Zhao, M., Hu, Y., & Jin, F. (2020). Extracellular vesicles derived from adipose mesenchymal stem cells alleviate PM2. 5‐Induced lung injury and pulmonary fibrosis. Medical Science Monitor: International Medical Journal of Experimental and Clinical Research, 26, e922782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnecchi, M., He, H., Liang, O. D., Melo, L. G., Morello, F., Mu, H., Noiseux, N., Zhang, L., Pratt, R. E., Ingwall, J. S., & Dzau, V. J. (2005). Paracrine action accounts for marked protection of ischemic heart by Akt‐modified mesenchymal stem cells. Nature Medicine, 11, 367 [DOI] [PubMed] [Google Scholar]
- Guo, H., Su, Y., & Deng, F. (2020). Effects of mesenchymal stromal cell‐derived extracellular vesicles in lung diseases: Current status and future perspectives. Stem cell reviews Repors, 17, 440–458. 10.1007/s12015-020-10085-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurevitch, J., Koricheva, J., Nakagawa, S., & Stewart, G. (2018). Meta‐analysis and the science of research synthesis. Nature, 555, 175–182 [DOI] [PubMed] [Google Scholar]
- Hao, Q., Gudapati, V., Monsel, A., Park, J. H., Hu, S., Kato, H., Lee, J. H., Zhou, L., He, H., & Lee, J. W. (2019). Mesenchymal stem cell‐derived extracellular vesicles decrease lung injury in mice. Journal of Immunology, 203, 1961–1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haraszti, R. A., Miller, R., Stoppato, M., Sere, Y. Y., Coles, A., Didiot, M.‐C., Wollacott, R., Sapp, E., Dubuke, M. L., Li, X., Shaffer, S. A., Difiglia, M., Wang, Y., Aronin, N., & Khvorova, A. (2018). Exosomes produced from 3D cultures of MSCs by tangential flow filtration show higher yield and improved activity. Molecular Therapy, 26, 2838–2847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrell, C. R., Miloradovic, D., Sadikot, R., Fellabaum, C., Markovic, B. S., Miloradovic, D., Acovic, A., Djonov, V., Arsenijevic, N., & Volarevic, V. (2020).Molecular and cellular mechanisms responsible for beneficial effects of mesenchymal stem cell‐derived product “Exo‐d‐MAPPS” in attenuation of chronic airway inflammation. Analytical Cellular Pathology, 2020, 13153891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrell, C. R., Jovicic, N., Djonov, V., Arsenijevic, N., & Volarevic, V. (2019). Mesenchymal stem cell‐derived exosomes and other extracellular vesicles as new remedies in the therapy of inflammatory diseases. Cells, 8, 1605. 10.3390/cells8121605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson, V. C., Kimmelman, J., Fergusson, D., Grimshaw, J. M., & Hackam, D. G. (2013). Threats to validity in the design and conduct of preclinical efficacy studies: A systematic review of guidelines for in vivo animal experiments. Plos Medicine, 10, e1001489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan, S. E., Salazar, M. P. R., Cheadle, J., Glenn, R., Medrano, C., Petersen, T. H., & Ilagan, R. M. (2019). Mesenchymal stromal cell‐derived exosomes improve mitochondrial health in pulmonary arterial hypertension. American Journal of Physiology Lung Cellular & Molecular Physiology, 17, 17. [DOI] [PubMed] [Google Scholar]
- Hogan, S. E., Rodriguez Salazar, M. P., Cheadle, J., Glenn, R., Medrano, C., Petersen, T. H., & Ilagan, R. M. (2019). Mesenchymal stromal cell‐derived exosomes improve mitochondrial health in pulmonary arterial hypertension. American Journal of Physiology‐Lung Cellular and Molecular Physiology, 316, L723–L737 [DOI] [PubMed] [Google Scholar]
- Hooijmans, C. R., Rovers, M. M., De Vries, R. B., Leenaars, M., Ritskes‐Hoitinga, M., & Langendam, M. W. (2014). SYRCLE's risk of bias tool for animal studies. Bmc Medical Research Methodology [Electronic Resource], 14, 43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua, J., Gong, J., Meng, H., Xu, B., Yao, L., Qian, M., He, Z., Zou, S., Zhou, B., & Song, Z. (2014). Comparison of different methods for the isolation of mesenchymal stem cells from umbilical cord matrix: Proliferation and multilineage differentiation as compared to mesenchymal stem cells from umbilical cord blood and bone marrow. Cell Biology International, 38, 198–210 [DOI] [PubMed] [Google Scholar]
- Huang, R., Qin, C., Wang, J., Hu, Y., Zheng, G., Qiu, G., Ge, M., Tao, H., Shu, Q., & Xu, J. (2019). Differential effects of extracellular vesicles from aging and young mesenchymal stem cells in acute lung injury. Aging: Clinical and Experimental Research, 11, 7996–8014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung, Y., Bauer, G., & Nolta, J. A. (2012). Concise review: Induced pluripotent stem cell‐derived mesenchymal stem cells: Progress toward safe clinical products. Stem Cells, 30, 42–47. 10.1002/stem.727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatri, M., Richardson, L. A., & Meulia, T. (2018).Mesenchymal stem cell‐derived extracellular vesicles attenuate influenza virus‐induced acute lung injury in a pig model. Stem Cell Research & Therapy, 9, 1–13. 10.1186/s13287-018-0774-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, S. D., Kang, S. A., Kim, Y. W., Yu, H. S., Cho, K. S., & Roh, H. J. (2020).Screening and functional pathway analysis of pulmonary genes associated with suppression of allergic airway inflammation by adipose stem cell‐derived extracellular vesicles. Stem Cells International, 2020, 15684250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinger, J. R., Pereira, M., Del Tatto, M., Brodsky, A. S., Wu, K. Q., Dooner, M. S., Borgovan, T., Wen, S., Goldberg, L. R., Aliotta, J. M., Ventetuolo, C. E., Quesenberry, P. J., & Liang, O. D. (2020). Mesenchymal stem cell extracellular vesicles reverse sugen/hypoxia pulmonary hypertension in rats. American Journal of Respiratory Cell and Molecular Biology, 62, 577–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landis, S. C., Amara, S. G., Asadullah, K., Austin, C. P., Blumenstein, R., Bradley, E. W., Crystal, R. G., Darnell, R. B., Ferrante, R. J., Fillit, H., Finkelstein, R., Fisher, M., Gendelman, H. E., Golub, R. M., Goudreau, J. L., Gross, R. A., Gubitz, A. K., Hesterlee, S. E., Howells, D. W., … Silberberg, S. D. (2012). A call for transparent reporting to optimize the predictive value of preclinical research. Nature 490, 187–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Blanc, K., & Mougiakakos, D. (2012). Multipotent mesenchymal stromal cells and the innate immune system. Nature Reviews Immunology, 12, 383–396 [DOI] [PubMed] [Google Scholar]
- Lee, C., Mitsialis, S. A., Aslam, M., Vitali, S. H., Vergadi, E., Konstantinou, G., Sdrimas, K., Fernandez‐Gonzalez, A., & Kourembanas, S. (2012). Exosomes mediate the cytoprotective action of mesenchymal stromal cells on hypoxia‐induced pulmonary hypertension. Circulation, 126, 2601–2611. 10.1161/CIRCULATIONAHA.112.114173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, J. H., Park, J., & Lee, J.‐W. (2019). Therapeutic use of mesenchymal stem cell–derived extracellular vesicles in acute lung injury. Transfusion, 59, 876–883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei, X., He, N., Zhu, L., Zhou, M., Zhang, K., Wang, C., Huang, H., Chen, S., Li, Y., Liu, Q., Han, Z., Guo, Z., Han, Z., & Li, Z. (2020). Mesenchymal Stem Cells Derived Extracellular Vesicles Attenuate Radiation‐Induced Lung Injury via miRNA‐214‐3p. Antioxidants & Redox Signaling, 14, 14. [DOI] [PubMed] [Google Scholar]
- Li, J. W., Wei, L., Han, Z., & Chen, Z. (2019a).Mesenchymal stromal cells‐derived exosomes alleviate ischemia/reperfusion injury in mouse lung by transporting anti‐apoptotic miR‐21‐5p. European Journal of Pharmacology, 852, 68–76. [DOI] [PubMed] [Google Scholar]
- Li, L., Jin, S., & Zhang, Y. (2015). Ischemic preconditioning potentiates the protective effect of mesenchymal stem cells on endotoxin‐induced acute lung injury in mice through secretion of exosome. International Journal of Clinical and Experimental Medicine, 8, 3825–3832 [PMC free article] [PubMed] [Google Scholar]
- Li, Q. C., Liang, Y., & Su, Z. B. (2019b). Prophylactic treatment with MSC‐derived exosomes attenuates traumatic acute lung injury in rats. American Journal of Physiology‐Lung Cellular & Molecular Physiology, 316, L1107–L1117. [DOI] [PubMed] [Google Scholar]
- Li, Z., Gong, X., Li, D., Yang, X., Shi, Q., & Ju, X. (2020). Intratracheal transplantation of amnion‐derived mesenchymal stem cells ameliorates hyperoxia‐induced neonatal hyperoxic lung injury via aminoacyl‐peptide hydrolase. International Journal of Stem Cells, 13, 221–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, J., Chen, T., Lei, P., Tang, X., & Huang, P. (2019a). Exosomes released by bone marrow mesenchymal stem cells attenuate lung injury induced by intestinal ischemia reperfusion via the TLR4/NF‐kappaB pathway. International Journal of Medical Sciences, 16, 1238–1244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, J.‐S., Du, J., Cheng, X., Zhang, X.‐Z., Li, Y., & Chen, X.‐L. (2019b). Exosomal miR‐451 from human umbilical cord mesenchymal stem cells attenuates burn‐induced acute lung injury. Journal of the Chinese Medical Association: JCMA, 82, 895–901 [DOI] [PubMed] [Google Scholar]
- Liu, Z., Liu, J., Xiao, M., Wang, J., Yao, F., Zeng, W., Yu, L., Guan, Y., Wei, W., Peng, Z., Zhu, K., Wang, J., Yang, Z., Zhong, J., & Chen, J. (2018). Mesenchymal stem cell‐derived microvesicles alleviate pulmonary arterial hypertension by regulating renin‐angiotensin system. Journal of the American Society of Hypertension : JASH, 12, 470–478 [DOI] [PubMed] [Google Scholar]
- Lukomska, B., Stanaszek, L., Zuba‐Surma, E., Legosz, P., Sarzynska, S., & Drela, K. (2019). Challenges and controversies in human mesenchymal stem cell therapy. Stem Cells International, 2019, 19628536. 10.1155/2019/9628536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansouri, N., Willis, G. R., Fernandez‐Gonzalez, A., Reis, M., Nassiri, S., Mitsialis, S. A., & Kourembanas, S. (2019). Mesenchymal stromal cell exosomes prevent and revert experimental pulmonary fibrosis through modulation of monocyte phenotypes. JCI Insight, 4, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maremanda, K. P., Sundar, I. K., & Rahman, I. (2019). Protective role of mesenchymal stem cells and mesenchymal stem cell‐derived exosomes in cigarette smoke‐induced mitochondrial dysfunction in mice. Toxicology and Applied Pharmacology, 385, 114788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthay, M. A., Calfee, C. S., Zhuo, H., Thompson, B. T., Wilson, J. G., Levitt, J. E., Rogers, A. J., Gotts, J. E., Wiener‐Kronish, J. P., Bajwa, E. K., Donahoe, M. P., Mcverry, B. J., Ortiz, L. A., Exline, M., Christman, J. W., Abbott, J., Delucchi, K. L., Caballero, L., Mcmillan, M., … Liu, K. D. (2019). Treatment with allogeneic mesenchymal stromal cells for moderate to severe acute respiratory distress syndrome (START study): A randomised phase 2a safety trial. The Lancet. Respiratory Medicine, 7, 154–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan, J., Sampson, M., Salzwedel, D. M., Cogo, E., Foerster, V., & Lefebvre, C. (2016). PRESS Peer Review of Electronic Search Strategies: 2015 Guideline Statement. Journal of Clinical Epidemiology, 75, 40–46. 10.1016/j.jclinepi.2016.01.021 [DOI] [PubMed] [Google Scholar]
- Monroe, M. N., Zhaorigetu, S., Gupta, V. S., Jin, D., Givan, K. D., Curylo, A. L., Olson, S. D., CoxJr, C. S., Segura, A., Buja, L. M., Grande‐Allen, K. J., & Harting, M. T. (2020). Extracellular vesicles influence the pulmonary arterial extracellular matrix in congenital diaphragmatic hernia. Pediatric Pulmonology, 22, 22. [DOI] [PubMed] [Google Scholar]
- Monsel, A., Zhu, Y. G., Gennai, S., Hao, Q., Hu, S., Rouby, J. J., Rosenzwajg, M., Matthay, M. A., & Lee, J. W. (2015). Therapeutic effects of human mesenchymal stem cell‐derived microvesicles in severe pneumonia in mice. American Journal of Respiratory and Critical Care Medicine, 192, 324–336. 10.1164/rccm.201410-1765OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsel, A., Zhu, Y.‐G., Gudapati, V., Lim, H., & Lee, J. W. (2016). Mesenchymal stem cell derived secretome and extracellular vesicles for acute lung injury and other inflammatory lung diseases. Expert Opinion on Biological Therapy, 16, 859–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murad, M. H., Asi, N., Alsawas, M., & Alahdab, F. (2016). New evidence pyramid. BMJ Evidence‐Based Medicine, 21, 125–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIH . Principles and guidelines for reporting preclinical research. https://www.nih.gov/research‐training/rigor‐reproducibility/principles‐guidelines‐reporting‐preclinical‐research. (2014).
- Porzionato, A., Zaramella, P., Dedja, A., Guidolin, D., Van Wemmel, K., Macchi, V., Jurga, M., Perilongo, G., De Caro, R., Baraldi, E., & Muraca, M. (2019). Intratracheal administration of clinical‐grade mesenchymal stem cell‐derived extracellular vesicles reduces lung injury in a rat model of bronchopulmonary dysplasia. American Journal of Physiology‐Lung Cellular and Molecular Physiology, 316, L6–L19 [DOI] [PubMed] [Google Scholar]
- Potter, D. R., Miyazawa, B. Y., Gibb, S. L., Deng, X., Togaratti, P. P., Croze, R. H., Srivastava, A. K., Trivedi, A., Matthay, M., Holcomb, J. B., Schreiber, M. A., & Pati, S. (2018). Mesenchymal stem cell‐derived extracellular vesicles attenuate pulmonary vascular permeability and lung injury induced by hemorrhagic shock and trauma. The Journal of Trauma and Acute Care Surgery, 84, 245–256. 10.1097/TA.0000000000001744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizk, M., Monaghan, M., Shorr, R., Kekre, N., Bredeson, C. N., & Allan, D. S. (2016). Heterogeneity in studies of mesenchymal stromal cells to treat or prevent graft‐versus‐host disease: A scoping review of the evidence. Biology of Blood and Marrow Transplantation, 22, 1416–1423. 10.1016/j.bbmt.2016.04.010 [DOI] [PubMed] [Google Scholar]
- Romanov, Y. A. (2003). Searching for alternative sources of postnatal human mesenchymal stem cells: Candidate MSC‐like cells from umbilical cord. Stem Cells, 21, 105–110. 10.1634/stemcells.21-1-105 [DOI] [PubMed] [Google Scholar]
- Sena, E. S., Currie, G. L., Mccann, S. K., Macleod, M. R., & Howells, D. W. (2014). Systematic reviews and meta‐analysis of preclinical studies: Why perform them and how to appraise them critically. Journal of Cerebral Blood Flow and Metabolism, 34, 737–742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva, J. D., de Castro, L. L., Braga, C. L., Oliveira, G. P., Trivelin, S. A., Barbosa‐Junior, C. M., Morales, M. M., Dos Santos, C. C., Weiss, D. J., Lopes‐Pacheco, M., Cruz, F. F., & Rocco, P. R. M. (2019).Mesenchymal stromal cells are more effective than their extracellular vesicles at reducing lung injury regardless of acute respiratory distress syndrome etiology. Stem Cells International, 2019, 18262849.1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano, J. B., Kendrick, P. J., Paulson, K. R., Gupta, V., Abrams, E. M., Adedoyin, R. A., Adhikari, T. B., Advani, S. M., Agrawal, A., Ahmadian, E., Alahdab, F., Aljunid, S. M., Altirkawi, K. A., Alvis‐Guzman, N., Anber, N. H., Andrei, C. L., Anjomshoa, M., Ansari, F., Antó, J. M., … Vos, T. (2020). Prevalence and attributable health burden of chronic respiratory diseases, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. The Lancet. Respiratory Medicine, 8, 585–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone, M. L., Zhao, Y., Robert Smith, J., Weiss, M. L., Kron, I. L., Laubach, V. E., & Sharma, A. K. (2017).Mesenchymal stromal cell‐derived extracellular vesicles attenuate lung ischemia‐reperfusion injury and enhance reconditioning of donor lungs after circulatory death. Respiratory Research, 18, 1–12. 10.1186/s12931-017-0704-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, X. D., Shi, L., Monsel, A., Li, X. Y., Zhu, H. L., Zhu, Y. G., & Qu, J. M. (2017). Mesenchymal stem cell microvesicles attenuate acute lung injury in mice partly mediated by Ang‐1 mRNA. Stem Cells, 35, 1849–1859. 10.1002/stem.2619 [DOI] [PubMed] [Google Scholar]
- Théry, C., Witwer, K. W., Aikawa, E., Alcaraz, M. J., Anderson, J. D., Andriantsitohaina, R., Antoniou, A., Arab, T., Archer, F., Atkin‐Smith, G. K., Ayre, D. C., Bach, J.‐M., Bachurski, D., Baharvand, H., Balaj, L., Baldacchino, S., Bauer, N. N., Baxter, A. A., Bebawy, M., … Zuba‐Surma, E. K. (2018). Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. Journal of Extracellular Vesicles, 7, 1535750. 10.1080/20013078.2018.1535750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tieu, A., Lalu, M. M., Slobodian, M.l, Gnyra, C., Fergusson, D. A., Montroy, J., Burger, D., Stewart, D. J., & Allan, D. S. (2020). An analysis of mesenchymal stem cell‐derived extracellular vesicles for preclinical use. ACS Nano, 14, 9728–9743. 10.1021/acsnano.0c01363 [DOI] [PubMed] [Google Scholar]
- Varkouhi, A. K., Jerkic, M., Ormesher, L., Gagnon, S., Goyal, S., Rabani, R., Masterson, C., Spring, C.s, Chen, P. Z., Gu, F. X., Dos Santos, C. C., Curley, G. F., & Laffey, J. G. (2019). Extracellular vesicles from interferon‐γ–primed human umbilical cord mesenchymal stromal cells reduce Escherichia coli–induced acute lung injury in rats. Anesthesiology, 130, 778–790 [DOI] [PubMed] [Google Scholar]
- Vesterinen, H. M., Sena, E. S., Egan, K. J., Hirst, T. C., Churolov, L., Currie, G. L., Antonic, A., Howells, D. W., & Macleod, M. R. (2014). Meta‐analysis of data from animal studies: A practical guide. Journal of Neuroscience Methods, 221, 92–102. 10.1016/j.jneumeth.2013.09.010 [DOI] [PubMed] [Google Scholar]
- Viswanathan, S., Shi, Y., Galipeau, J., Krampera, M., Leblanc, K., Martin, I., Nolta, J., Phinney, D. G., & Sensebe, L. (2019). Mesenchymal stem versus stromal cells: International Society for Cell & Gene Therapy (ISCT®) Mesenchymal Stromal Cell committee position statement on nomenclature. Cytotherapy, 21, 1019–1024 [DOI] [PubMed] [Google Scholar]
- Wang, J., Huang, R., Xu, Q., Zheng, G., Qiu, G., Ge, M., Shu, Q., & Xu, J. (2020). Mesenchymal stem cell‐derived extracellular vesicles alleviate acute lung injury via transfer of miR‐27a‐3p. Critical Care Medicine, 48, e599–e610 [DOI] [PubMed] [Google Scholar]
- Willis, G. R., Fernandez‐Gonzalez, A., Anastas, J., Vitali, S. H., Liu, X., Ericsson, M., Kwong, A., Mitsialis, S. A., & Kourembanas, S. (2018). Mesenchymal stromal cell exosomes ameliorate experimental bronchopulmonary dysplasia and restore lung function through macrophage immunomodulation. American Journal of Respiratory and Critical Care Medicine, 197, 104–116. 10.1164/rccm.201705-0925OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis, G. R., Fernandez‐Gonzalez, A., Reis, M., Yeung, V., Liu, X., Ericsson, M., Andrews, N. A., Mitsialis, S. A., Kourembanas, S. (2020). Mesenchymal stromal cell‐derived small extracellular vesicles restore lung architecture and improve exercise capacity in a model of neonatal hyperoxia‐induced lung injury. Journal of Extracellular Vesicles, 9(1), 1–14. 10.1080/20013078.2020.1790874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Heath Organization (WHO) . The top 10 causes of death. (2020). https://www.who.int/news‐room/fact‐sheets/detail/the‐top‐10‐causes‐of‐death
- Worthington, E. N., & Hagood, J. S. (2020). Therapeutic use of extracellular vesicles for acute and chronic lung disease. International Journal of Molecular Sciences, 21, 2318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, Bin, Gan, Chun‐xia, Chen, Si‐si, Li, Jia‐qi, Liu, Ming‐zhuo, Guo, Guang‐hua (2020). BMSC‐derived exosomes alleviate smoke inhalation lung injury through blockade of the HMGB1/NF‐κB pathway. Life Sciences, 257, 1–10. 10.1016/j.lfs.2020.118042 [DOI] [PubMed] [Google Scholar]
- Xu, N., Shao, Y., Ye, K., Qu, Y., Memet, O., He, D., & Shen, J. (2019). Mesenchymal stem cell‐derived exosomes attenuate phosgene‐induced acute lung injury in rats. Inhalation Toxicology, 31, 52–60 [DOI] [PubMed] [Google Scholar]
- Yi, X., Wei, X., Lv, H., An, Y., Li, L., Lu, P., Yang, Y., Zhang, Q., Yi, H., & Chen, G. (2019). Exosomes derived from microRNA‐30b‐3p‐overexpressing mesenchymal stem cells protect against lipopolysaccharide‐induced acute lung injury by inhibiting SAA3. Experimental Cell Research, 383, 111454 [DOI] [PubMed] [Google Scholar]
- Zhang, S., Liu, X., Ge, L. L., Li, K., Sun, Y., Wang, F., Han, Y., Sun, C., Wang, J., Jiang, W., Xin, Q., Xu, C., Chen, Y., Chen, O., Zhang, Z., & Luan, Y. (2020). Mesenchymal stromal cell‐derived exosomes improve pulmonary hypertension through inhibition of pulmonary vascular remodeling. Respiratory Research, 21, 71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhaorigetu, S., Bair, H., Jin, D., Gupta, V. S., Pandit, L. M., Bryan, R. M., Lally, K. P., Olson, S. D., Cox, C. S., & Harting, M. T. (2020). Extracellular vesicles attenuate nitrofen‐mediated human pulmonary artery endothelial dysfunction: Implications for congenital diaphragmatic hernia. Stem Cells and Development, 27, 27. [DOI] [PubMed] [Google Scholar]
- Zhu, Y. G., Feng, X. M., Abbott, J., Fang, X. H., Hao, Q., Monsel, A., Qu, J. M., Matthay, M. A., & Lee, J. W. (2014). Human mesenchymal stem cell microvesicles for treatment of Escherichia coli endotoxin‐induced acute lung injury in mice. Stem Cells, 32, 116–125. 10.1002/stem.1504 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental File 1. PRISMA 2020 Checklist.
Supplemental File 2. Systematic Review Search Strategies.
Supplemental File 3. Supporting Figures for Study Characteristics, EV Methodology and Interventional Traits
Supplemental File 4. Meta‐Analysis of Secondary Outcomes
Supplemental File 5. Subgroup Analyses of Outcomes.
Supplemental File 6. Quality of Reporting in MSC‐EV Lung Studies.


