Abstract
Structurally complex benzo- and spiro-fused N-polyheterocycles can be accessed via intramolecular Pd(0)-catalyzed alkene 1,2-aminoarylation reactions. The method uses N-(pentafluorobenzoyloxy)carbamates as the initiating motif, and this allows aza-Heck-type alkene amino-palladation in advance of C–H palladation of the aromatic component. The chemistry is showcased in the first total synthesis of the complex alkaloid (+)-pileamartine A, which has resulted in the reassignment of its absolute stereochemistry.
Pd-catalyzed cascade reactions are widely used for the rapid assembly of structurally complex ring systems, especially within the context of total synthesis.1 A valuable framework for accessing complex N-polyheterocycles resides in intramolecular Pd-catalyzed alkene aminocarbonations, where C–H palladation is used to install the new C–C bond (Scheme 1A). Building upon Hegedus’ seminal report,2a Yang and co-workers have developed several oxidative 1,2-aminocarbonation processes,2b including variants that involve aryl C–H palladation (Scheme 1B).3a,3b Enantioselective 1,2-aminoarylations of this type have been reported by Liu and co-workers.3c Mechanistically distinct processes that exploit external (hetero)aryl C–H units have been developed by the groups of Michael4a,4b and Sigman.4c External 1,3-dienes undergo 1,2-aminoarylation via the intermediacy of Pd(II)-π-allyls, as reported by Lloyd-Jones, Booker-Milburn, and co-workers.5,6
Scheme 1. Introduction.
A key feature of the processes in Scheme 1B is that they usually require (a) relatively acidic NH units and (b) a high degree of conformational bias.3 The former presumably aids NH palladation,7 whereas the latter enhances the efficiency of one or both of the cyclization steps. Consequently, these oxidative processes offer very specific scope, such that selective examples require systems where the aromatic unit is attached directly to the amide NH unit (i.e., anilide-based systems). Nevertheless, the value of these cascade reactions is clear, and so the development of complementary or broader scope alternatives is a pressing and worthwhile objective. To this end, we considered whether redox neutral processes might be developed that exploit a N–O bond as an internal oxidant (Scheme 1C). In this design, N–H palladation is replaced by N–O oxidative addition, which alleviates, at least in part, the requirement for an acidifying functionality. Further, by using an internal oxidant, substrate binding and catalyst oxidation are united (cf. Scheme 1B). Accordingly, catalysis should be more robust, and less conformationally biased cyclizations might be achievable. Indeed, we have recently shown that activated N-hydroxy-sulfonamides8a and -carbamates8b,8c are viable substrates for aza-Heck cyclizations and that these methods offer enhanced scope versus oxidative alternatives.9 Watson and co-workers have outlined similar benefits using other types of N–O-based functionality as the initiating unit.8d−8f In this report, we describe the first examples of processes where aza-Heck cyclization of activated N-hydroxycarbamates is used to trigger intramolecular aryl C–H functionalization cascades (Scheme 1C).10 The method provides a new and powerful framework for the 1,2-aminoarylation of alkenes and, in so doing, provides direct access to alkaloid-like scaffolds. This is demonstrated through the first total synthesis of the complex alkaloid pileamartine A, which has led to the stereochemical reassignment of this natural product.11
The envisaged cascade amino-arylation processes are mechanistically complex, and key considerations are outlined in Scheme 2A. Following N–O oxidative addition to I, efficient cyclization requires dissociation of pentafluorobenzoate to access cationic aza-Pd species I′, as supported by earlier studies.8a,8b For substrates of type 1, aza-palladation is expected to be selective for trans-II; this diastereoselectivity has been observed for processes involving external aryl boronic ester nucleophiles (Scheme 2A, gray box). However, aryl C–H palladation from trans-II is expected to be demanding due to geometric constraints. Consequently, the establishment of a Curtin–Hammett scenario is required wherein reversible aza-palladation allows access to cis-II, which, although thermodynamically disfavored, is geometrically set up for aryl C–H palladation. Although not exploited as a design tactic, reversible alkene aza-palladation has been established in other contexts12 and requires a free coordination site, which can, in principle, be provided by maintaining a cationic Pd center.13 At the stage of cis-II, efficient C–H palladation likely requires association of a carboxylate ligand to facilitate concerted metalation deprotonation (CMD).14 One option would be for the Pd center to reengage the pentafluorobenzoate leaving group; however, this species is expected to be suboptimal because it dissociates readily and its carbonyl unit is not especially basic. To address this, we considered evaluating external benzoate additives (ArCO2M) to improve CMD efficiency. A beneficial aspect of this strategy is that it releases ArCO2H, which can then trigger Et3N-mediated protodecarboxylation of the pentafluorobenzoate leaving group.8a,15 This (a) drives equilibrium access to the requisite cationic aza-Pd-intermediate I′ and (b) allows the benzoate additive to be used catalytically. The latter is important because stoichiometric quantities of strongly coordinating benzoate additives are expected to inhibit alkene aza-palladation by preventing access to cationic intermediate I′.
Scheme 2. Mechanistic Analysis and Optimization of the Cascade Process.
Isolated yield.
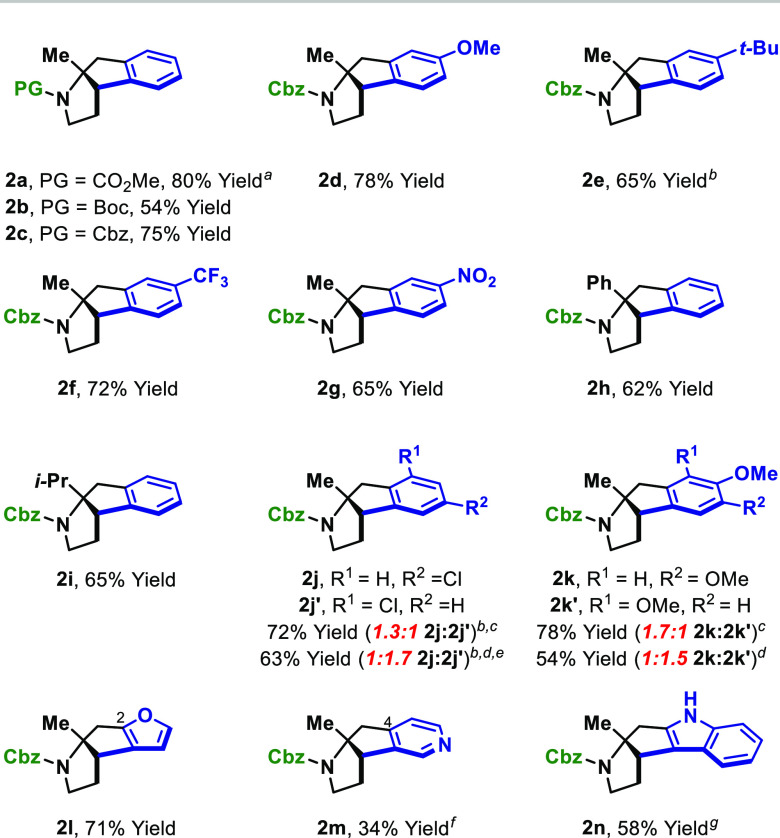
In early efforts toward the envisaged cascades, we established that exposure of 1a (LG = OFBz) to a CgPPh-ligated Pd catalyst (10 mol %) at 130 °C generates tricyclic system 2a in 62% yield and as a single diastereomer (Scheme 2B, entry 1). Under these conditions, n-Bu2O was the most effective solvent. To optimize the process, we evaluated a variety of carboxylate additives leading to the observation that addition of 25 mol % NaOBz improves the yield of 2a to 80% (entry 3). The use of (in situ generated) triethylammonium benzoate was substantially less effective (entry 4), and a control experiment established that Et3N is required for optimal yields (entry 5).15 With optimized components in hand, we reassessed catalyst and additive loadings to provide the conditions in entry 6, which deliver 2a in 80% yield using 5 mol % of the Pd catalyst and 10 mol % of the NaOBz and Et3N cocatalysts. Other leaving groups (entries 7 and 8), P-ligands, and carboxylate additives were less efficient, and the N-Ts analogue of 1a (see the SI) did not undergo cyclization. The failure of system 1a″ (entry 8) supports the notion that a cationic manifold is required; previous studies indicate that O-tosyl activated systems cyclize in “neutral” mode.8c Note that the C–N bond of 1a is easily installed in 68% yield via Mitsunobu reaction of the corresponding alcohol with MeO2CNHOFBz (see the SI).
Having established optimized conditions with 1a, other carbamate protecting groups were evaluated (Table 1). Cyclization of N-Boc and N-Cbz systems 1b and 1c delivered 2b and 2c in 54% and 75% yield, respectively; notably, these processes were slower than with methyl carbamate 1a (48 vs 24 h). Nevertheless, further scope studies were pursued using an N-Cbz group because this offered the best balance between yield and synthetic utility. A variety of electronically distinct para-substituted arenes (1d–g) engaged with minimal variation in efficiency. The process offers a good degree of flexibility for the alkene R-group, as evidenced by efficient cyclizations of systems possessing more bulky (2i) or conjugated substituents (2h). Meta-substituted arenes 1j and 1k have two different positions available for C–C bond formation, and the use of NaOBz as the additive offered no selectivity (1:1 r.r.). To address this, further carboxylate additives were screened, and these studies revealed that, in both cases, 2-MeOC6H4CO2Na favors C–C bond formation at the para-position with respect to the substituent (1.3:1 2j:2j′ and 1.7:1 2k:2k′). Conversely, use of 2-NO2C6H4CO2Na switched this selectivity to provide 2j′ and 2k′ preferentially. These results are consistent with the carboxylate additive playing a key role in C–H palladation, although there is insufficient data to offer a precise rationalization for the observed regioselectivities. C–C bond formation was highly selective for heteroaromatics 2l and 2m using NaOBz as the carboxylate additive, presumably because these systems offer a substantial electronic bias for metalation. For 2l, complete selectivity for the furan C2-position was observed, whereas C4 selectivity was observed for C3-pyridyl system 2m. An unprotected indole participated efficiently to provide 2n in 58% yield.
Table 1. Cascades to Access Benzofused Polyheterocycles.
The reaction time was 24 h.
The reaction time was 72 h.
10 mol % 2-MeOC6H4CO2Na was used in place of NaOBz.
10 mol % 2-NO2C6H4CO2Na was used in place of NaOBz.
5 mol % Pd2dba3 and 30 mol % CgPPh were used.
5 mol % Pd2dba3, 50 mol % CgPPh, and 150 °C were used.
5 mol % Pd2dba3, 30 mol % CgPPh, and 200 mol % Et3N were used.
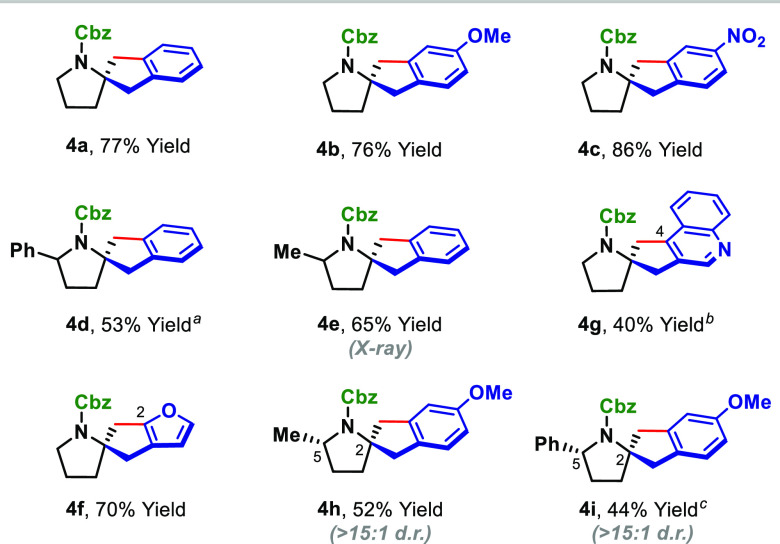
We next evaluated distinct processes where the aromatic unit is appended to the internal position of the alkene (Table 2). For phenyl-substituted precursor 3a, cyclization proceeded efficiently under optimized conditions to deliver spirocycle 4a in 77% yield. As seen earlier, these processes are relatively insensitive to the electronics of the aromatic unit, such that methoxy and nitro variants 3b and 3c participated with similar levels of efficiency. Systems 4d and 4e, which possess α-substituents, were tolerated; the structure of product 4e was confirmed by single-crystal X-ray diffraction. Cyclizations involving 3-quinolinyl (4g) and 3-furyl (4f) acceptors were also feasible, and C–C bond formation was completely selective for C4 and C2, respectively. To probe the possibility of diastereoselective processes, cyclizations of α-substituted systems 3h and 3i, which possess a para-methoxy substituent on the arene, were evaluated. In these cases, 4h and 4i were generated as single diastereomers, whose structures were verified by NOE analysis (see the SI). The outcome of these processes reflects the very high C2–C5 cis-diastereoselectivity associated with the alkene aza-palladation step. This enables control of the diastereoselectivity of the product even though the two desymmetrizing elements (the α-substituents and the methoxy groups) are distant from one another.
Table 2. Cascades to Access Spirofused Polyheterocycles.
150 °C, 3.75 mol % Pd2dba3, 30 mol % CgPPh.
150 °C, 5 mol % Pd2dba3, 50 mol % CgPPh.
160 °C, 5 mol % Pd2dba3, 40 mol % CgPPh.
Pileamartines A and B were recently isolated from the leaves of Pilea aff. martinii by Thanh, Pham, and co-workers and possess a stereochemically rich and compact framework.11 To demonstrate the utility of the aza-Heck cascades described here, we targeted a synthesis of the proposed natural enantiomer (17) (Scheme 3). This required an enantio- and diastereoselective synthesis of alcohol 11. After extensive experimentation, we developed an efficient four-pot procedure for the installation of the challenging 1,3-stereorelationship. Exposure of epoxide 5 (>99% e.e.), which can be prepared in two steps,16 to the sodium enolate of 6 provided lactone 7 in 83% yield and 3:1 d.r.; the desired cis-diastereomer (cis-7) could be isolated in 61% yield. Optimization studies revealed three key observations: (1) a dilute DMF solution (0.04 M) is optimal for diastereoselectivity, (2) the diastereoselectivity is likely under kinetic control,17 and (3) the isopropyl ester of 6 is more efficient than the corresponding methyl ester. The latter is consistent with a bulkier ester suppressing a competing Claisen reaction. Lactone 7 was converted to p-bromobenzyl ester 8, whose absolute stereochemistry was confirmed by single-crystal X-ray diffraction.
Scheme 3. Total Synthesis of (+)-Pileamartine A.

Conversion of 7 to alcohol 11 was nontrivial. In the event, we discovered that monoselective addition of Grignard 9 to lactone 7 can be achieved under Knochel conditions (LaCl3·LiCl),18 which presumably forms a mixture of 10 and 10′ in situ. Indeed, the protonated form of 10 was unstable and readily underwent dehydration to the corresponding dihydrofuran. To circumvent this, the subsequent olefination step was telescoped by direct exposure of 10/10′ to the Tebbe reagent at 50 °C, which provided alkene 11 (>20:1 d.r.) in 60% yield. The alcohol of 11 is relatively hindered, such that Mitsunobu reaction with 12 occurred in only 21% yield under standard conditions (PPh3, DIAD), with the mass balance consisting predominantly of elimination products. To address this, we sought to improve the leaving group ability of the oxyphosphonium intermediate by replacing PPh3 with a more electron poor phosphine [P(3,4,5-(F)3C6H2)3], and this modification provided 13 in 67% yield and >20:1 d.r.
The pivotal aza-Heck cascade to form 15 performed poorly under the conditions outlined in Table 1 (19% yield, 1:1 r.r.). Ultimately, by switching to a more electron poor phosphine [(CgP(4-NO2C6H4)] and using CsOPiv as the carboxylate additive, the core ring system 15a/b could be accessed in 40% yield, albeit as a 1:1 mixture of regioisomers, resulting from nonselective C–H palladation at the stage of 14. Efforts to improve selectivity by exploring alternative carboxylate additives were partially successful, but resulted in lower yields (see the SI). Nevertheless, the efficiency of the process is notable given it simultaneously installs the tetrasubstituted stereocenter and key C–C and C–N bonds. The process also serves to validate α-substituted substrates in cascades of this type (cf. Table 1). The desired regioisomer 15a was advanced to the proposed structure of the natural product (17) via hydrogenative removal of the O-benzyl and N-Cbz units (to 16) and subsequent cyclization under Appel conditions.
The 1H and 13C NMR data of 17 were in agreement with reported data; however, the specific rotation value [17: [α]25D = +144.1 (c 0.32, CHCl3); natural pileamartine A [α]25D = −141.2 (c 0.33, CHCl3)] and ECD spectrum (see the SI) were opposite of those determined for natural material. These data indicate that the original absolute stereochemical assignment of the natural product, which was made by comparison of experimental and calculated ECD spectra,11 is incorrect. In view of this, we recalculated the ECD spectrum of the 6S,8R,9S enantiomer at the B3PW91/cc-pVTZ level of theory and obtained a convincing match to the data for 17 (Figure S1).19 Measured and calculated VCD spectra were also in complete agreement (Figure S2). Taken together with X-ray data for 8, there can be little doubt about the absolute configuration of 17, and so we conclude that the configuration of natural pileamartine A should be reassigned as 6R,8S,9R (boxed structure).20
In summary, we show that aza-Heck cyclization of activated N-hydroxycarbamates can be used to trigger intramolecular aryl C–H functionalization cascades. These processes offer a counterpoint to oxidative Pd-catalyzed alkene 1,2-aminoarylations, and their utility has been validated in an eight-step synthesis of pileamartine A. Efforts to develop related methodologies are ongoing and will be reported in due course.
Acknowledgments
We thank the European Research Council for financial support via the EU’s Horizon 2020 Programme (ERC Grant 639594 CatHet), AstraZeneca, the Leverhulme Trust (Philip Leverhulme Prize to J.F.B.), EPSRC (EP/M506473/1, EP/N509619/1) for studentships (to I.R.H. and B.T.J.), and the Alfonso Martın Escudero Foundation for a Postdoctoral Fellowship (to J.G.-C.). We thank the University of Bristol, School of Chemistry X-ray crystallography service, for analysis.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacs.1c08615.
Experimental details, characterization data, and crystallographic data (PDF)
Accession Codes
CCDC 2103391–2103392 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif, or by emailing data_request@ccdc.cam.ac.uk, or by contacting The Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: +44 1223 336033.
The authors declare no competing financial interest.
Supplementary Material
References
- a Ohno H.; Inuki S. Recent Progress in Palladium-Catalyzed Cascade Cyclizations for Natural Product Synthesis. Synthesis 2018, 50, 700. 10.1055/s-0036-1589165. [DOI] [Google Scholar]; b Biemolt J.; Ruijter E. Advances in Palladium-Catalyzed Cascade Cyclizations. Adv. Synth. Catal. 2018, 360, 3821. 10.1002/adsc.201800526. [DOI] [Google Scholar]
- a Hegedus L. S.; Allen G. F.; Olsen D. J. Palladium-Assisted Cyclization-lnsertion Reactions. Synthesis of Functionalized Heterocycles. J. Am. Chem. Soc. 1980, 102, 3583. 10.1021/ja00530a044. [DOI] [Google Scholar]; b Leading reference:Yip K.-T.; Yang M.; Law K.-L.; Zhu N.-Y.; Yang D. Pd(II)-Catalyzed Enantioselective Oxidative Tandem Cyclization Reactions. Synthesis of Indolines through C-N and C-C Bond Formation. J. Am. Chem. Soc. 2006, 128, 3130. 10.1021/ja060291x. [DOI] [PubMed] [Google Scholar]
- a Yip K.-T.; Yang D. Pd(II)-Catalyzed Intramolecular Amidoarylation of Alkenes with Molecular Oxygen as Sole Oxidant. Org. Lett. 2011, 13, 2134. 10.1021/ol2006083. [DOI] [PubMed] [Google Scholar]; b Du W.; Gu Q.; Li Z.; Yang D. Palladium(II)-Catalyzed Intramolecular Tandem Aminoalkylation via Divergent C(sp3)–H Functionalization. J. Am. Chem. Soc. 2015, 137, 1130. 10.1021/ja5102739. [DOI] [PubMed] [Google Scholar]; c Zhang W.; Chen P.; Liu G. Enantioselective Palladium(II)-Catalyzed Intramolecular Aminoarylation of Alkenes by Dual N-H and Aryl C-H Bond Cleavage. Angew. Chem., Int. Ed. 2017, 56, 5336. 10.1002/anie.201700889. [DOI] [PubMed] [Google Scholar]
- a Rosewall C. F.; Sibbald P. A.; Liskin D. V.; Michael F. E. Palladium-Catalyzed Carboamination of Alkenes Promoted by N-Fluorobenzenesulfonimide via C-H Activation of Arenes. J. Am. Chem. Soc. 2009, 131, 9488. 10.1021/ja9031659. [DOI] [PubMed] [Google Scholar]; b Sibbald P. A.; Rosewall C. F.; Swartz R. D.; Michael F. E. Mechanism of N-Fluorobenzenesulfonimide Promoted Diamination and Carboamination Reactions: Divergent Reactivity of a Pd(IV) Species. J. Am. Chem. Soc. 2009, 131, 15945. 10.1021/ja906915w. [DOI] [PubMed] [Google Scholar]; c Jana R.; Pathak T. P.; Jensen K. H.; Sigman M. S. Palladium(II)-Catalyzed Enantio- and Diastereoselective Synthesis of Pyrrolidine Derivatives. Org. Lett. 2012, 14, 4074. 10.1021/ol3016989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houlden C. E.; Bailey C. D.; Ford J. G.; Gagné M. R.; Lloyd-Jones G. C.; Booker-Milburn K. I. Distinct Reactivity of Pd(OTs)2: The Intermolecular Pd(II)-Catalyzed 1,2-Carboamination of Dienes. J. Am. Chem. Soc. 2008, 130, 10066. 10.1021/ja803397y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxidative alkene 1,2-aminoarylations can also be achieved under radical-based Cu-catalysis:; Zeng W.; Chemler S. R. Copper(II)-Catalyzed Enantioselective Intramolecular Carboamination of Alkenes. J. Am. Chem. Soc. 2007, 129, 12948. 10.1021/ja0762240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G.; Stahl S. S. Two-Faced Reactivity of Alkenes: cis- versus trans-Aminopalladation in Aerobic Pd-Catalyzed Intramolecular Aza-Wacker Reactions. J. Am. Chem. Soc. 2007, 129, 6328. 10.1021/ja070424u. [DOI] [PubMed] [Google Scholar]
- a Hazelden I. R.; Carmona R. C.; Langer T.; Pringle P. G.; Bower J. F. Pyrrolidines and Piperidines by Ligand Enabled Aza Heck Cyclizations and Cascades of N-(Pentafluorobenzoyloxy)carbamates. Angew. Chem., Int. Ed. 2018, 57, 5124. 10.1002/anie.201801109. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Hazelden I. R.; Ma X.; Langer T.; Bower J. F. Diverse N-Heterocyclic Ring Systems via Aza Heck Cyclizations of N-(Pentafluorobenzoyloxy)sulfonamides. Angew. Chem., Int. Ed. 2016, 55, 11198. 10.1002/anie.201605152. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Ma X.; Hazelden I. R.; Langer T.; Munday R. H.; Bower J. F. Enantioselective Aza-Heck Cyclizations of N-(Tosyloxy)carbamates: Synthesis of Pyrrolidines and Piperidines. J. Am. Chem. Soc. 2019, 141, 3356. 10.1021/jacs.8b12689. [DOI] [PubMed] [Google Scholar]; d Shuler S. A.; Yin G.; Krause S. B.; Vesper C. M.; Watson D. A. Synthesis of Secondary Unsaturated Lactams via an Aza-Heck Reaction. J. Am. Chem. Soc. 2016, 138, 13830. 10.1021/jacs.6b08932. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Xu F.; Shuler S. A.; Watson D. A. Synthesis of N-H Bearing Imidazolidinones and Dihydroimidazolones Using Aza-Heck Cyclizations. Angew. Chem., Int. Ed. 2018, 57, 12081. 10.1002/anie.201806295. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Xu F.; Korch K. M.; Watson D. A. Synthesis of Indolines and Derivatives by Aza-Heck Cyclization. Angew. Chem., Int. Ed. 2019, 58, 13448. 10.1002/anie.201907758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reviews encompassing aza-Wacker cyclizations:; a Minatti A.; Muñiz K. Intramolecular Aminopalladation of Alkenes as a Key Step to Pyrrolidines and Related Heterocycles. Chem. Soc. Rev. 2007, 36, 1142. 10.1039/B607474J. [DOI] [PubMed] [Google Scholar]; b McDonald R. I.; Liu G.; Stahl S. S. Palladium (II)-Catalyzed Alkene Functionalization via Nucleopalladation: Stereochemical Pathways and Enantioselective Catalytic Applications. Chem. Rev. 2011, 111, 2981. 10.1021/cr100371y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- During the preparation of this manuscript, conceptually related but synthetically distinct processes were reported that use oxime esters as the initiating motif and generate four-membered rings:; a Wei W.-X.; Li Y.; Wen Y.-T.; Li M.; Li X.-S.; Wang C.-T.; Liu H.-C.; Xia Y.; Zhang B.-S.; Jiao R.-Q.; Liang Y.-M. Experimental and Computational Studies of Palladium-Catalyzed Spirocyclization via a Narasaka–Heck/C(sp3 or sp2)–H Activation Cascade Reaction. J. Am. Chem. Soc. 2021, 143, 7868. 10.1021/jacs.1c04114. [DOI] [PubMed] [Google Scholar]; For related processes involving C–H metalation of an external heteroarene, see:; b Bao X.; Wang Q.; Zhu J. Palladium-Catalyzed Enantioselective Narasaka-Heck Reaction/Direct C-H Alkylation of Arenes: Iminoarylation of Alkenes. Angew. Chem., Int. Ed. 2017, 56, 9577. 10.1002/anie.201705641. [DOI] [PubMed] [Google Scholar]
- Thuy A. D. T.; Thanh V. T. T.; Mai H. D. T.; Le H. T.; Litaudon M.; Chau V. M.; Pham V. C. Pileamartines A and B: Alkaloids from Pilea aff. martinii with a New Carbon Skeleton. Tetrahedron Lett. 2018, 59, 1909. 10.1016/j.tetlet.2018.03.070. [DOI] [Google Scholar]
- White P. B.; Stahl S. S. Reversible Alkene Insertion into the Pd–N Bond of Pd(II)-Sulfonamidates and Implications for Catalytic Amidation Reactions. J. Am. Chem. Soc. 2011, 133, 18594. 10.1021/ja208560h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dissociation of an ancillary phosphine ligand is another option.
- Gorelsky S. I.; Lapointe D.; Fagnou K. Analysis of the Concerted Metalation-Deprotonation Mechanism in Palladium-Catalyzed Direct Arylation Across a Broad Range of Aromatic Substrates. J. Am. Chem. Soc. 2008, 130, 10848. 10.1021/ja802533u. [DOI] [PubMed] [Google Scholar]
- Et3N mediates protodecarboxylation:; Gierczyk B.; Wojciechowski G.; Brzenzinski B.; Greah E.; Schroeder G. Study of the decarboxylation mechanism of fluorobenzoic acids by strong N-bases. J. Phys. Org. Chem. 2001, 14, 691. 10.1002/poc.419. [DOI] [Google Scholar]
- Titanium Salalen Catalysts Based on cis-1,2-Diaminocyclohexane: Enantioselective Epoxidation of Terminal Non-Conjugated Olefins with H2O2. Angew. Chem. Int. Ed.2013, 52, 8467. In practice, we used Jacobsen resolution to provide 5 in higher e.e. (see the SI).
- For example, epimerization of 7 using NaH (50 mol%) in DMF at 130 °C lowered the d.r. from 2.5:1 to 2:1.
- Krasovskiy A.; Kopp F.; Knochel P. Soluble Lanthanide Salts (LnCl3·2 LiCl) for the Improved Addition of Organomagnesium Reagents to Carbonyl Compounds. Angew. Chem., Int. Ed. 2006, 45, 497. 10.1002/anie.200502485. [DOI] [PubMed] [Google Scholar]; To the best of our knowledge, this procedure has not been applied previously to lactones. In the absence of LaCl3·2LiCl, 11 was formed in 1:1 d.r. and 10% yield.
- For clarity, the carbon numbering system used in ref (11) is employed here.
- The SI details a synthesis of the natural enantiomer [[α]25D = −127.1 (c 0.20, CHCl3)].
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.