Abstract
For numerous enabling features and strategic virtues, contemporary alkyne metathesis is increasingly recognized as a formidable synthetic tool. Central to this development was the remarkable evolution of the catalysts during the past decades. Molybdenum alkylidynes carrying (tripodal) silanolate ligands currently set the standards; their functional group compatibility is exceptional, even though they comprise an early transition metal in its highest oxidation state. Their performance is manifested in case studies in the realm of dynamic covalent chemistry, advanced applications to solid-phase synthesis, a revival of transannular reactions, and the assembly of complex target molecules at sites, which one may not intuitively trace back to an acetylenic ancestor. In parallel with these innovations in material science and organic synthesis, new insights into the mode of action of the most advanced catalysts were gained by computational means and the use of unconventional analytical tools such as 95Mo and 183W NMR spectroscopy. The remaining shortcomings, gaps, and desiderata in the field are also critically assessed.
Introduction
Alkyne metathesis, that is, the redistribution of the alkylidyne units of a pair of acetylene derivatives with the aid of a transition-metal catalyst, has been known since the late 1960s.1 Although barely younger than olefin metathesis, it took a long time for this transformation to step out of the shadow of its exceptionally impactful sibling in order to gain a sharp profile in its own right.
As the early phase of alkyne metathesis has been thoroughly reviewed, it may suffice here to recapitalize only the major lines of development.2−9 The rather long initial “latency” period certainly has to do with the fact that the lead findings were somewhat fuzzy and incongruent.
The reaction was discovered with a heterogeneous catalyst (WO3/silica) that required very high temperatures (200–450 °C) to be operative.1 As such conditions hardly apply to any elaborate substrate, this system had little impact and truly heterogeneous catalysts in general remained largely unexplored.2−9
The first alkyne metathesis reaction in homogeneous phase used Mo(CO)6 and a phenol additive.10 This recipe was well received for its preparative convenience and continues to find occasional applications.11 Once again, however, fairly high temperatures are mandatory (160 °C in the original report), at which excess phenol is not necessarily an innocent bystander. It is hence hardly surprising that this method applies to robust substrates only and its functional group compatibility is inherently limited. Moreover the active species generated in situ defied direct inspection; therefore, this catalyst system did not lend itself to rational (rather than empirical) optimization.
This stunning lack of information on a reasonably popular catalyst system stood in stark contrast to the very detailed understanding of the organometallic principles. The advent of high-valent transition-metal alkylidyne complexes (“Schrock alkylidynes”) laid a solid ground for thorough mechanistic investigations.2,12,13 These studies provided compelling evidence for a sequence of [2 + 2] cycloaddition/cycloreversion steps being accountable for the reaction, as had been hypothesized before (Scheme 1).14 More precisely, the square-pyramidal metallacyclobutadiene tautomer A primarily formed converts into tautomer C, from which the product is released by [2 + 2] cycloreversion; this reorganization passes through a trigonal-bipyramidal intermediate (or transition state) B.15 Moreover, it became very soon very clear that the proper choice of ancillary ligands about the active alkylidyne unit is a critical determinant of catalytic activity.2,12,13
Scheme 1. General Reaction Mechanism (Degenerate Setting) and Primordial Catalysts.
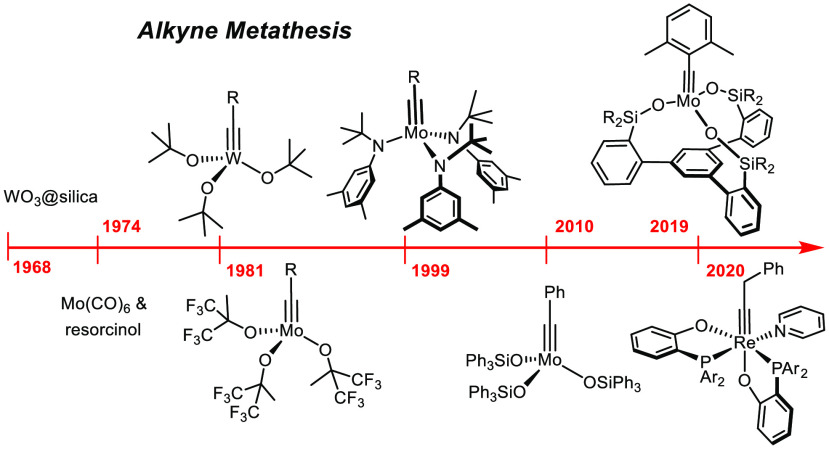
The tungsten alkylidyne complex 1a (R = tBu) (and its close relatives) developed by Schrock and co-workers was the historically first well-defined alkyne metathesis catalyst.16−19 The same group also showed that certain molybdenum alkylidynes are competent, provided they carry poorer π-donor ligands such as (per)fluorinated tert-alkoxides;18,20,21 as the original synthesis of complexes of type 2 was much less efficient than of their tungsten counterparts, this class of catalysts largely fell into oblivion and was rediscovered only much later (see below). Finally, rhenium alkylidynes such as 3 were found to be (moderately) active.22−24
The catalysts have undergone massive evolution since then; yet, all relevant alkyne metathesis catalysts used to date continue to be tungsten, molybdenum or rhenium alkylidynes; they are hence variations on the themes originally invented by Schrock in the (early) 1980s.
Why Bother?
For the tremendous success of contemporary olefin metathesis,25 one might argue that there is little need for alkyne metathesis in general, not least because most (internal) alkynes are chemically more “expensive” than analogous olefins. Considering the certainly unequal substrate basis only, however, may lead to a distorted picture.
Notwithstanding some skepticism in the early literature whether or not olefin metathesis applies to the formation of macrocyclic rings,26 our group was able to demonstrate that even conformationally unbiased dienes such as 4 cyclize well when treated with the then brand-new (and at the time not yet commercial) first-generation Grubbs catalyst;27 subsequent hydrogenation of product 5 furnished the musk odorant exaltolide in excellent overall yield (Scheme 2).28,29 While this discovery went a long way since then in our laboratory and elsewhere,25,30−34 this lead discovery actually revealed an important limitation too: cycloalkene 5 was obtained as an almost 1:1 mixture of the two alkene isomers, likely because the reversibility of RCM entails thermodynamic control. Though inconsequential in this particular case, it was immediately clear that applications of RCM-based macrocyclizations to more elaborate substrates will eventually face a serious handicap: substantial or even complete loss of precious material is possible as long as no control over the configuration of the newly formed double bond can be exerted.
Scheme 2. Lead Findings in Macrocycle Synthesis via RCM (A) or RCAM (B). Model Substrates Incompatible with the Tungsten Alkylidyne 1 (C).
The development of kinetically Z- or E-selective catalysts is arguably the best way forward. While much progress has been made in this direction,35−37 there is still considerable room for improvement; most notably, kinetic E-selectivity continues to lack a reasonably general solution.38,39 For this reason, we started to contemplate whether alkyne metathesis followed by stereoselective semireduction could be an alternative answer or not.
Proof-of-concept for the formation of macrocyclic rings by ring closing alkyne metathesis (RCAM) was attained with diyne 6 (Scheme 2).40,41 This particular substrate was transformed with the aid of 1a into cycloalkyne 7, which could then be converted into Z-alkene 9 or the corresponding E-alkene 8(42,43) in high yield and excellent selectivity.
The ability to set the desired alkene geometry with high fidelity even at a late stage of a synthesis endeavor is arguably an asset of potentially strategic relevance.44 What is more, a triple bond offers innumerous possibilities for postmetathetic transformations other than semireduction. It was therefore reasonable for us to believe at the outset of our project that alkyne metathesis could serve as a springboard that allows a plethora of structural motifs to be attained. The reaction should certainly be considered as a serious alternative to stereoselective olefin metathesis; at the same time, however, it might become relevant far beyond this frontier as a valuable synthetic tool in its own right.
For this vista to become true, however, a number of massive challenges had to be met. As the formation of 7 had shown, the activity of complex 1 was rather modest; warming was necessary to ensure reasonable rates and the yield of product was not overly high either.40 A much more serious limitation was the narrow functional group tolerance of 1 and analogues.41,45 Although a few applications to target-oriented synthesis proved successful,6,7,9,46−50 substrates as simple as 10–13 were inadequate likely because the donor sites quench the activity of the Lewis-acidic tungsten catalyst and/or get activated and destroyed upon coordination to 1 (Scheme 2); electron-rich or -deficient substrates were not compliant either.41 An attempt to make the anticancer agent epothilone C by RCAM of diyne 18 followed by Lindlar reduction was therefore inconceivable with the toolbox available before the turn of the millennium (see below).
A Knight’s Move
A much improved compatibility of the catalysts with functional groups of all sorts hence constituted the single most important goal to be attained at the outset of our venture. To this end, we decided early on to defer work on tungsten alkylidynes and redirect our efforts toward molybdenum-based systems for the lower intrinsic Lewis acidity of this transition metal.
A first step was taken when we found that treatment of complex 14(51) with CH2Cl2 generates an active species, which indeed shows a much improved tolerance, notably toward common heteroatom donor sites (Scheme 3).52−54 This aspect was illustrated by the successful application of 14/CH2Cl2 to the formation of epothilone C which the tungsten alkylidyne 1a was unable to reach (Scheme 4);53,55 additional case studies followed shortly thereafter.6 Even today, 14/CH2Cl2 remains an indispensable tool, especially in cases in which encumbered alkynes need to be activated.56−58
Scheme 3. Molybdenum Alkylidynes Derived from a Mo(+3) Precursor: Basic and Advanced Format.
Scheme 4. Total Synthesis of Epothilone as an Early Illustration of the Superior Tolerance of [Mo]-Based Catalysts.
On the basis of mechanistic studies which had shown that the C atom of CH2Cl2 gets transformed into the methylidyne unit of 15 on reaction with 14,53 Moore and co-workers developed an important modification.59,60 By using higher gem-dihalides as the activating agents, they managed to obtain and isolate the corresponding alkylidyne complexes such as 17; in combination with a reductive recycling strategy, this approach is also “economical” with regard to the molybdenum source. If one desires so, protonolysis of the fairly basic amide ligands in 17 with a phenol of choice (or, later, with an appropriate silanol) allows the ligand sphere to be adjusted and the catalytic properties to be fine-tuned. This system found numerous applications in polymer chemistry and material science.5,61
The chemical virtues notwithstanding, all catalysts derived from 14 come at a high “price”: this complex is extremely sensitive and must be prepared and handled with rigorous Schlenk techniques; moreover, it mandates an Ar atmosphere because it is even capable of activating N2 under mild conditions.51 The procedures for its preparation have to be strictly followed since the (redox) chemistry of low-valent molybdenum is intricate and by no means fully understood.62 Likewise, the solvents must be scrupulously dried; the presence of protic sites in the substrate to be metathesized is inconceivable. The step forward taken with 14/CH2Cl2 or complexes such as 17 in terms of functional group compatibility hence came along with a step sideward (backward) with regard to handling.
Silanolate Molybdenum Alkylidynes
Although the practicality of 14/CH2Cl2 is poor, this system provided compelling evidence that alkyne metathesis reactions can be performed in complex settings without interference of common polar and apolar substituents. However, it was also clear that better access to the relevant catalysts had to be found without need to resort to 14 as the molybdenum source.
Apprehensive of the fact that the very first alkyne metathesis had used a catalyst adsorbed on silica,1 we conjectured that silanolates might be suitable ancillary ligands.63−66 They are poorer π-donors than (tertiary) alkoxides;67 moreover, the Si–O–Mo linkage is floppy and hence easy to stretch and bend (Figure 1): in so doing, the oxygen atom formally shuttles between the extremes of sp and sp3 hybridization, which gently tunes its donor ability.68
Figure 1.
Angle-dependent metal–ligand bonding.
The adaptive electronic character of a silanolate ligand is arguably ideal in the context of alkyne metathesis.63,64,69 Note that the catalytic cycle shown in Scheme 1 consists of a sequence of elementary steps with alternating electronic optima: substrate binding and metallacycle formation are favored by a more Lewis-acidic central atom, whereas the retro-[2 + 2] step and product release are easier at a more electron-rich site. To ensure efficient turnover, these opposing needs must be properly balanced; the adaptiveness of silanolates provides an intrinsic handle to do so.63,64 At the same time, the Lewis acidity of the molybdenum alkylidyne unit itself will likely be tempered such that an attractive overall application profile might ensue. The fact that various (alkyne) metathesis catalysts had been successfully immobilized on silica surfaces or attached to polyoligomeric silsesquioxanes was also deemed encouraging:70,71 it is the silyloxy group of the first coordination sphere that basically determines the electronic structure of the metal center.72 In any case, the reasoning that silanolates might synergize with the operative molybdenum alkylidyne fragment largely proved correct: in terms of functional group compatibility, silanolate-bearing catalysts brought alkyne metathesis to a previously unknown level and continue to set the standards in the field.6,63−65
In parallel, more proficient entries into molybdenum alkyidyne complexes were established. The currently best way adopts a route originally developed by Mayr and co-workers in which readily accessible Fischer-carbyne complexes are oxidized with Br2 to furnish Schrock tribromoalkylidynes such as 20 (Scheme 5).73,74 Although this step needs careful temperature control, it can be performed on a multigram scale. Compound 20 itself is catalytically inactive, as is the derived alkoxide complex 21. Treatment of 20 with triphenylsilanolate affords the ate complex 22 (unless the addition is carefully controlled); because the uptake of the fourth ligand is reversible, 22 serves as a reservoir for the neutral alkylidyne 23 as the actual catalyst.63,64 Ate-complex formation is therefore no impediment; in certain cases, the slow release of the active species in solution is even beneficial (see below). If one so desires, however, this issue can be avoided by resorting to 21 as the substrate: treatment with the silanol of choice results in quantitative ligand exchange; no salt is formed and the liberated tert-butanol can simply be removed in vacuo. This procedure is presently our preferred route to the parent complexes 23 as well as to the more modern “canopy catalysts” discussed below.75 In this context, it is also important to mention that the renaissance of molybdenum alkylidynes endowed with partly fluorinated or perfluorinated alkoxide ligands, which the Schrock group had already pioneered but which had then found little echo, was also brought about by the improved access route passing through tribromides such as 20.8,76
Scheme 5. Improved Synthesis of Molybdenum Alkylidynes Exemplified by the Parent Silanolate Catalysts and the Bench-Stable Phenanthroline Adduct.

Figure 2 illustrates the high activity of these new catalysts by comparison with the Schrock alkylidyne 1a as the classical reference point.63,64 The reasons for this improved performance are fairly well understood: it has recently been possible, for example, to isolate the metallacyclobutadiene 24 derived from 23 on reaction with excess 3-hexyne.77
Figure 2.
Benchmarking of the activity of complexes 23a and 1a (1 mol % each): formation PhC≡CPh from PhC≡CMe in the presence of MS 5 Å as 2-butyne scavenger in toluene at ambient temperature.
The structure of this very sensitive complex in the solid state is highly instructive (Figure 3): it adopts a geometry in between square-pyramidal and trigonal-bipyramidal; although the bond lengths are uneven, the differences are small. In solution, the interconversion of this tautomer with the second tautomer necessary for productive cycloreversion (corresponding to A → C in Scheme 1) could not be frozen out even at −90 °C.77 It obviously takes very little for 24 to pass through the trigonal-bipyramidal rendition (B) at which the intermediates responsible for productive turn over converge. These spectroscopic and crystallographic data hence nicely explain the excellent reactivity of complex 23 and congeners.
Figure 3.

Core region of metallacyclobutadiene 24 in the solid state; Mo = yellow, O = red, Si = green.
For a reaction that is inherently reversible, it is necessary to perturb the equilibrium in order to reach quantitative conversion. We showed that addition of molecular sieves is a convenient way to do so, as they are capable of trapping 2-butyne (released when working with methyl-capped alkynes as the most common substrates) and hence allow the reaction to proceed to completion even at ambient temperature.63,64 This practice has been widely embraced.6−9,78
Figure 4 gives a noncomprehensive overview over functional groups compatible with 23 and relatives.63−65 A few comments may help to calibrate these results: while the tungsten complex 1 had failed with substrates carrying even moderately basic N atoms, S-donor sites, or common heterocyclic rings (Scheme 2), 23 is largely undisturbed by their presence. Likewise, 1 is incapable of metathesizing electron-rich (e.g., alkynylsilanes, -phosphines) or electron-deficient substrates (e.g., alkynoates),41,79 whereas 23 does so; this latter aspect proved enabling as witnessed by several total synthesis projects.65,80−82 At the meta level, this catalyst can even be compared to the famous ruthenium carbene complexes for olefin metathesis, the exquisite tolerance of which is highly appreciated;25 however, Grubbs-type catalysts often fail if the substrates contain amines, sulfides, phosphines. or nitriles. Moreover, phosphine-bearing ruthenium carbenes endanger azides, alkyl halides, epoxides, and the like.25 When seen against this backdrop, the compatibility of 23 comprising a non-noble metal atom in its highest oxidation state with all of these functionalities is deemed remarkable. On the other hand, Grubbs-type catalysts truly excel in the presence of protic groups and remain operative even in aqueous media.25 Although 23 does tolerate certain protic sites, especially when sterically hindered (see below), the sensitivity toward (moderately) acidic functionality in general remains the most eminent Achilles heel.
Figure 4.
Survey of compatible functional groups
Complex 23 and its relatives have an additional bonus in that they form bench-stable adducts with 1,10-phenanthroline or 2,2′-bipyridine (Scheme 5).63,64 Because the trans-effect of the alkylidyne weakens the opposing N···Mo bond in 23·phen and the orbital overlap between Mo1 and N2 is not perfect on geometric grounds either (Figure 6), the stabilizing chelate ligand can be pulled off with the aid of ZnCl2 or MnCl2, and the active catalyst be released in solution.63,64 To be able to fill a highly active and superbly selective catalyst into a bottle (Scheme 5)—though in masked form—seemed elusive at the outset of our project. For the first time, adducts such as 23·phen enable those practitioners who are less experienced with and/or equipped for the handling of highly sensitive complexes to leverage the power of contemporary alkyne metathesis catalysts. Recent total syntheses of haliclonin A,83,84 nakadomarin A,85 crysophaentin F,86 the tubulin inhibitor WF-1360F87 and a study toward crassin acetate88 illustrate the utility of these stabilized catalysts (Figure 5).
Figure 6.

Core region of complex 23·phen (Ar = 2,6-Me2C6H3): the uneven Mo–N bonds, reflecting the suboptimal orbital overlap between Mo1 and N2 and the trans-effect of the alkylidyne, render binding of the chelate ligand reversible.
Figure 5.
Selected applications of the bench-stable phenanthroline adduct 23·phen in target-oriented synthesis.
A Functional Group Paradox
The comparison shown in Scheme 6 sheds light on a certain paradox: thus, the cyclization of the densely decorated diyne 25 with the aid of ate complex 22 (slowly releasing complex 23 in solution) to give 26 was fast (<30 min) and high yielding.89 Deprotection of the two −OPMB groups at C13 and C21 followed by spirocyclization upon activation of the triple bond with a gold catalyst paved the way to the intricate protein phosphatase inhibitor spirastrellolide F.89
Scheme 6. Functional Group “Paradox”.
In striking contrast, a substrate as simple as 27 carrying nothing but an unhindered primary −OH group proved unmanageable: if the alcohol replaces the silanolate ligands in 23, the catalyst gradually or completely loses activity.90,91
The “Canopy” Series
We conjectured that recourse to the chelate effect might help remedy this issue, at least in part, since simultaneous cleavage of three silanolate linkages is statistically less likely and partial solvolysis potentially reversible. A first foray, which used the readily available trisilanoles 28 and 29, was partly unsuccessful yet encouraging.92 Partial cross-linking occurs on reaction of these conformationally flexible ligands with complex 17: the resulting mixture of dimeric/oligomeric species, however, exhibits excellent catalytic properties. As expected, an improved—though certainly not perfect—compatibility with free −OH groups and other protic sites was noticed.92
Hence, the ligand design was revisited with the hope of forming catalysts that retain these virtues yet are structurally well-defined. The platform shown in Scheme 7 proved adequate: trisilanols 30 are sufficiently preorganized to ligate a single metal center but flexible enough to support the different intermediates passed through during a catalytic turnover.75,77,93 The preparation of ligands of this type is straightforward, scalable, and modular; the same design was independently pursued by the group of Lee.94,95
Scheme 7. Evolution of the Tripodal Silanolate Ligand Framework: The “Canopy” Series”.
The derived “canopy” complexes of type 31 or 32 are indeed privileged catalysts. They combine the advantages of the parent silanolate-supported molybdenum alkylidynes 23 with a higher robustness against protic substituents including unprotected alcohols (for specific examples, see Schemes 16 and 17).75 Even a certain stability toward moisture was noticed; although there remains much room for improvement, the ability to perform alkyne metathesis reactions in technical grade solvents is deemed an important step toward a truly practical methodology.75 Their compatibility with numerous Lewis-basic groups is equally astounding if one considers that the operative metal alkylidyne comprises an early transition metal in its highest oxidation state: the finish of a total synthesis of nominal njaoamine I proves that complex 31 (R = Me) remains fully operative even in the presence of two different tertiary amines and a quinoline (Scheme 8).96 To properly assess this result, it may suffice to say that a single tertiary amine sufficed to quench the activity of first and second generation Grubbs-type catalysts in a very closely related RCM-setting en route to the sibling alkaloid ingenamine.96
Scheme 16. Key Steps of a Total Synthesis of the Nor-Cembranoid Sinulariadiolide.
Scheme 17. Final Act En Route to Amphidinolide F.
Scheme 8.
For this promising application profile, the canopy catalysts have already been subject to intense experimental and computational scrutiny.69,75,77,95 Since a comprehensive discussion is beyond the scope of this Perspective, a portrait in “al fresco” style must suffice. Thus, the geometric constraints of the podand ligand framework lead to a slightly more Lewis-acidic Mo center, which favors substrate binding. This step is also strongly affected by the size of the substituents on the Si atoms forming a fence about the alkylidyne unit; small unbranched alkyl groups therefore provide a kinetic advantage. Complex 31 (R = Me) carrying lateral methyl substituents is currently the most active member of this series,75 although the homologues with higher unbranched alkyl groups retain appreciable reactivity.97 The missing steric protection, however, renders complex 31 susceptible to degradation by bimolecular coupling,97 which may explain why fairly high loadings had to be used in some demanding applications.
An Unorthodox Mechanism
The arguably most striking aspect, however, concerns the reaction mechanism itself. The corset of the ligand framework prevents the isomerization of the metallacyclobutadiene D primarily formed into a second tautomer from occurring; therefore, the canonical course of alkyne metathesis as shown in Scheme 1 is blocked.
Pseudorotation about the adjacent Mo–O bond allows this handicap to be circumvented (Scheme 9):77,95 it results in exchange of the R1and R3substituents on the metallacyclic ring while maintaining the original tautomeric form; in so doing, an unprecedented gateway to product formation is opened. The actual pseudorotation passes through a “bent” metallacyclic intermediate E that can also connect to a metallatetrahedrane G.69,77 In line with early reasoning, however, G was shown to be an off-cycle intermediate, which could even be isolated in certain settings.94
Scheme 9. Pseudorotation is Mandatory for Productive Turnover of Canopy Catalysts.
A remarkable analogy to observations previously made in the context of alkene metathesis deserves mentioning. In case of Schrock-type molybdenum and tungsten alkylidene complexes, the catalytic cycle passes through a trigonal-bipyramidal metallacyclobutane, which can convert by turnstile rotation into a square-pyramidal isomer with a bent metallacyclic ring;98 again, the latter is off the catalytic cycle and presents a potential doorway for catalyst deactivation. The mechanistic similarity to the way the canopy catalysis operates (Scheme 9) is further illustrated by the fact that the catalytically competent metallacycles in ether case are planar and allow the Cα-atoms to retain noticeable alkylidene (alkylidyne in D/F) character.99−101
Underappreciated Tools: 95Mo and 183W NMR
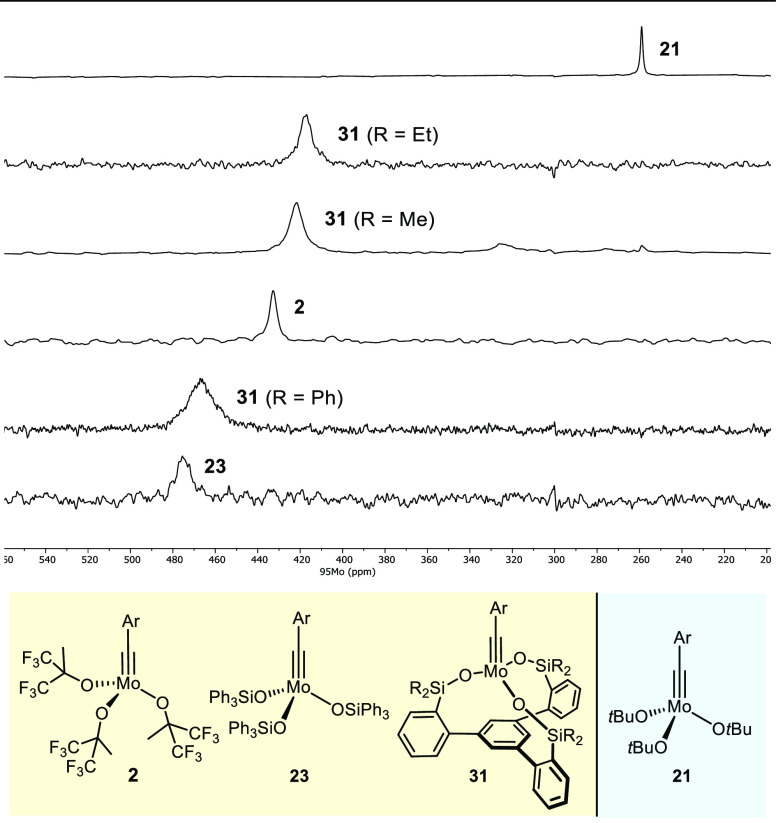
The 13C NMR data of alkylidynes are informative, especially when the recorded isotropic shift is deconvoluted into the individual components of the shift tensor by computational means.100 Such an analysis allows the energy differences between filled and empty orbitals (including the HOMO/LUMO gap) to be assessed. Complementary information can be gained by inspecting the other end of the alkylidyne, that is the molybdenum center. Despite a poor gyromagnetic ratio and low abundance, the spin 5/2 nucleus 95Mo lends itself for this very purpose;102,103 good spectra were obtained in short acquisition times at slightly elevated temperatures to slow down quadrupolar relaxation (Figure 7).
Figure 7.
95Mo NMR spectra ([D8]-toluene, 60 °C) of different molybdenum alkylidyne complexes; Ar = 2,6-Me2C6H3.
The resonances of the catalytically active complexes 2, 23, and 31 are distinct from that of their inactive cousin 21.75,93 If one takes the 95Mo chemical shift as a proxy for the Lewis acidity, the comparison shown in Figure 7 is intuitive: qualitatively, it suggests that molybdenum alkylidynes with fluorinated alkoxide or silanolate ligands are notably more Lewis acidic than those comprising ordinary alkoxides, which tallies well with the conclusions drawn from UV/vis and DFT data.69 Importantly, 95Mo NMR is able to pick up remote and hence subtle effects such as changes in the periphery: thus, δMo of complexes 31 with R = Me and R = Ph are no less than 45 ppm apart, whereas their 13C NMR signals show little difference (ΔδC = 7.5 ppm). More quantitative interpretations of the 95Mo shifts, however, mandate computational assistance. This is particularly true since the electrophilic character of the metal center is strongly geometry-dependent, most notably on the Mo–O–Si bond angles which modulate the donor strengths of the silanolate ligands (see above). For the floppiness of silanolates, the 95Mo NMR shifts in solution necessarily average over many conformations. It will therefore be of interest to record complementary solid state 95Mo NMR data where the dynamic is largely frozen out and a clearer picture of the inherent nature of the different catalysts might emerge; the experimental challenge, however, is considerable.
Unequal Twins
Whereas (tripodal) silanolates synergize remarkably well with molybdenum alkylidynes, they proved largely inadequate in the tungsten series. In essence, these poor π-donors render the W(+6) center in complexes such as 32 too Lewis acidic (Figure 8). As a consequence, the derived metallacycles are overstabilized and turnover comes to a halt (the same is true for highly fluorinated alkoxide ligands).69,77,104 Moreover, competing polymerization of the alkyne substrate is a serious issue.104 Once again, it proved highly informative to interrogate the operative transition metal entity directly, in this case via 183W NMR, which proved sensitive even to small changes in the chemical environment; the strongly deshielded signals of 32 and congeners are indicative of an overly electrophilic alkylidyne.104 This conclusion was confirmed by a computational analysis of the 13C shift tensors.
Figure 8.
Tungsten alkylidynes with tripodal silanolate or alkoxide ligand frameworks exhibit strikingly different activities.
Therefore, the ligand design was revisited and the silicon linkers were replaced altogether.104 Although one might think of complex 33 as a tethered variant of the classical Schrock catalyst 1, the constrained ligand geometry pays valuable dividends. Thus, 33 shows a notably better functional group tolerance than 1, although it does not rival the best molybdenum canopy catalysts available to date.97,104
Alternative Catalyst Designs
An alternative design pursued by the Zhang group is also heading toward more tolerant catalysts with chelate ligand frameworks. It relies on phenolate units held together by different types of tethers;105−107 the most advanced manifestation features a simple CH group as the central linker (Scheme 10).108 The actual catalyst is generated in situ upon reaction of 34 with complex 17. Although a firm proof of formation of the presumed monomeric species 35 is missing, the reactivity of this two-component system is high enough to allow certain applications to be performed under quasi “open air” conditions; however, CCl4 is required as solvent or cosolvent for optimal results.108 A number of challenging functional groups were found to be compatible, including pyridine, thiophene, aromatic aldehydes, nitriles, nitro groups, and an arylpinacolboronate. That a phenol-based ligand set is adequate for phenol-bearing substrates is perhaps unsurprising yet worth mentioning.
Scheme 10. Lexicon of Alternative Catalysts.
A recent disclosure by Jia and co-workers deserves special emphasis for two reasons:109 first, the d2 Re(+5) complex 36 is an extremely rare case of a non-d0 alkylidyne showing catalytic activity.110 Moreover, 36 is air-stable and tolerates various groups that had been problematic in the past: specifically, it is the only catalyst known to date that works even in the presence of an unprotected carboxylic acid. Other protic groups including primary alcohols, phenols, or aniline are equally compatible, as are certain donor sites.109 Although the reactions catalyzed by 36 are rather slow and require high temperatures (≥100 °C in toluene), further scrutiny of this lead compound is warranted.
A myriad of other molybdenum and tungsten alkylidyne complexes was published during the last decades (Scheme 10). The revival of catalysts such as 3 or 37 carrying partly fluorinated or perfluorinated alkoxide ligands has already been mentioned. A “volcano-type” correlation between the degree of fluorination and catalytic activity was established, which has to do with the fact that, from a certain degree of peripheral fluorination onward, the central atoms are too Lewis acidic and the pertinent intermediates on the catalytic cycle overstabilized.8,111−113 Yet other catalyst families are distinguished by heteroleptic ligand spheres, comprising, for example, imidazolin-2-iminato or N-heterocyclic carbenes. Some members of this series such as 38 show impressive turnover numbers and rates;114−119 their relevance for advanced synthesis, however, is currently difficult to assess as the set of test substrates is (too) narrow and does not contain any of the truly challenging functional groups.
Complex 40 denotes the other extreme (Scheme 11):120 its reactivity is tempered to the extent that only a highly strained cycloalkyne such as 39 gets activated, whereas the alkyne units in the resulting polymer 41 remain untouched; for this striking selectivity, chain transfer is precluded. The promise of (living) ring-opening alkyne metathesis polymerization (ROAMP) in general for the formation of precision polymers was recognized only recently;120−125 it is expected to provide many opportunities for material science in the future.5,126
Scheme 11. Example of a “Living” ROAMP Reaction.
Strategy Level Applications
This Perspective does not intend to provide a comprehensive coverage of the applications of alkyne metathesis in organic synthesis and material science. Rather, the following examples are solely meant to illustrate aspects of strategic relevance and encourage further studies in this field.
Shape-Persistent Objects
Alkyne metathesis is an inherently reversible process: provided the chosen catalyst is sufficiently active and long-lived, the reaction is under thermodynamic control. For this reason, it qualifies for applications to dynamic covalent chemistry (DCC) which mandates that a system is able to correct initial “mistakes” by scrambling of the mixture until the most stable product (distribution) has been reached. In so doing, DCC provides access to molecular objects beyond reach of more conventional approaches.127
Molybdenum alkylidynes, most notably those supported by silanolate ligands, meet the stringent criteria of activity and stability. The breathtakingly simple and efficient synthesis of 43, a shape-persistent molecular object of Möbius topology, provides a captivating illustration (Scheme 12).128 All it took was to react diyne 42 at 60 °C with a catalyst generated in situ from 17 upon exchange of the original amide ligands for triphenylsilanolates. Together with other similarly intricate applications in the literature,129−132 this example suggests that alkyne metathesis has a bright future in the context of material science in general and DCC in particular.5,126,127
Scheme 12. One-Step Formation of a Molecular Möbius Strip.
π-Bond Selectivity
It is well-known that the standard catalysts for olefin metathesis react with double bonds and triple bonds with similar ease; this indiscriminative behavior is at the very heart of enyne metathesis.25,133 In striking contrast, alkyne metathesis catalysts are rigorously selective in that they leave double bonds of all sorts untouched.134,135
Two examples must suffice to illustrate how this orthogonality can be leveraged in different chemical context. Neurymenolide A is a structurally unique antibacterial agent and mitotic spindle poison comprising four skipped and hence highly isomerization-prone alkenes. Any attempt to forge the macrocyclic scaffold with the aid of a (Z-selective) alkene metathesis catalyst copes with indiscriminate activation of all ((Z)-configured) double bonds. RCAM in combination with semireduction allowed the problem to be avoided, and this exceptionally sensitive product to be reached with excellent yield and selectivity (Scheme 13).136 Suffice it to say that the power and mildness of gold catalysis for the formation of the 2-pyrone ring was equally critical for success.137
Scheme 13. Total Synthesis of Neurymenolide.
The formation of GTPase targeting stapled peptides by solid-phase synthesis is similarly instructive (Figure 9).138,139 Thus, compound 47 comprising an “edge-on” macrobicyclic backbone was obtained in a one-pot operation on reaction of the corresponding diene/diyne substrate with a mixture of first-generation Grubbs catalyst and complex 23.138 This example corroborates the notion that the functional group tolerance of the molybdenum alkylidyne and a classical ruthenium carbene are comparable.
Figure 9.
Stapled peptide formed by concomitant yet orthogonal metathetic catenation.
Trisubstituted Alkenes and Late-Stage Diversity
As described in the Introduction, the formation of stereodefined olefins by alkyne metathesis/semireduction had initially motivated us to engage in this field. In fact, this strategy allowed us to conquer many structurally complex 1,2-disubstituted Z- and E-alkenes;6,7,44 the neurymenolide case alluded to above is representative.
It is important to recognize, however, that alkyne metathesis reaches far beyond this initial goal in that it also provides an excellent gateway to trisubstituted alkenes. Much of this progress relates to the fact that propargyl alcohols are compliant and lend themselves to hydroxy-directed trans-hydrometalation reactions catalyzed by [Cp*RuCl]4 (G → H, Scheme 14).140−142 These stereochemically unorthodox transformations afford products of type I, which, in turn, can be elaborated into numerous structural motifs by taking advantage of the rich chemistry of the Csp2-ER3 (E = Si, Ge, Sn) bond.
Scheme 14. Hydroxy-Directed trans-Hydrometalation.
Ring closure of diyne 48 proceeded well with the molybdenum alkylidyne 22 (Scheme 15).143 The resulting propargylic cycloalkyne 49 was transformed into stannane 50, which was then cross coupled with methyl iodide.144 This approach to the antibiotic 5,6-dihydrocineromycin B compares favorably to a previous synthesis in which the macrocycle had been closed at the trisubstituted olefinic site by RCM: 25 mol % of the second-generation Grubbs catalyst was necessary to obtain the target in 40% yield.145 As the 2-methyl-but-2-en-1-ol motif is commonplace in natural products, this new and stereoselective approach to trisubstituted alkenes is of more general relevance and has already served other total synthesis endeavors in the polyketide, diterpene, and depsipeptide series (Figure 10).57,146−148
Scheme 15. (Diverted) Total Synthesis of a Macrolide Antibiotic.
Figure 10.
Further applications of RCAM/trans-hydrometalation to the synthesis of trisubstituted alkenes.
At the same time, a metalated intermediate such as 50 provides ample opportunity for late-stage diversification. The small “library” of non-natural analogues 51–54 of the dihydrocineromycin estate illustrates this aspect (Scheme 15).143,149,150
A Transannular Rendition
The success of transannular reactions critically hinges on the availability of stereochemically defined macrocyclic precursors. While this “macrocyclic challenge” had been a serious impediment in the past, RCAM in combination with appropriate downstream chemistry is able to revitalize the field.151
A recent total synthesis of the marine nor-cembranoid sinulariadiolide may illustrate this point (Scheme 16).152 Specifically, the intricate tricyclic skeleton comprising an 11-membered nexus was forged by a strain-driven transannular Michael addition reaction. The required precursor 58 comprising a trisubstituted enoate subunit was made in configurationally defined format via RCAM followed by trans-hydrostannation/methoxycarbonylation. Ring closure was initially performed with the two-component catalyst system 17/28 but later found to be equally efficient with the canopy catalyst 32 developed in parallel in our laboratory.75 The compatibility with an unprotected secondary −OH group, a hydroxylamine, an elimination-prone tert-aldol substructure, and two different olefinic sites attests to the mildness and chemofidelity of the method. The subsequent ruthenium-catalyzed trans-hydrostannation furnished product 57,141,142,153 which was elaborated into the stereodefined Michael acceptor by palladium catalyzed methoxycarbonylation.154 The derived cyclic carbonate 58, on treatment with Cs2CO3 in MeOH, succumbed to a cascade commencing with cleavage of the enol acetate; this step, in turn, triggered the crucial transannular Michael addition, followed by decarboxylative cleavage of the carbonate, in situ formation of a strained butenolide, and final oxa-Michael addition of external MeOH; as the back-side of the acceptor 59 is shielded by the macrocyclic skeleton, even this intermolecular step proceeded with impeccable selectivity.152
Details apart, this example shows that RCAM can help leverage the still underutilized power of transannular reactivity in that it provides a reliable entry into highly decorated and configurationally well-defined macrocyclic substrates. At the same time, it illustrates that alkyne metathesis can make structural patterns available, the descent of which from a triple bond may not be immediately obvious.
Carbonyl Equivalents
This notion is also exemplified by other similarly intricate case studies. The C atoms of an alkyne have the same formal oxidation state as a carbonyl group, and π-acid catalysis is uniquely capable of harnessing this synthetic equivalence;155 once again, transannular settings can help secure the appropriate regioselectivity.156
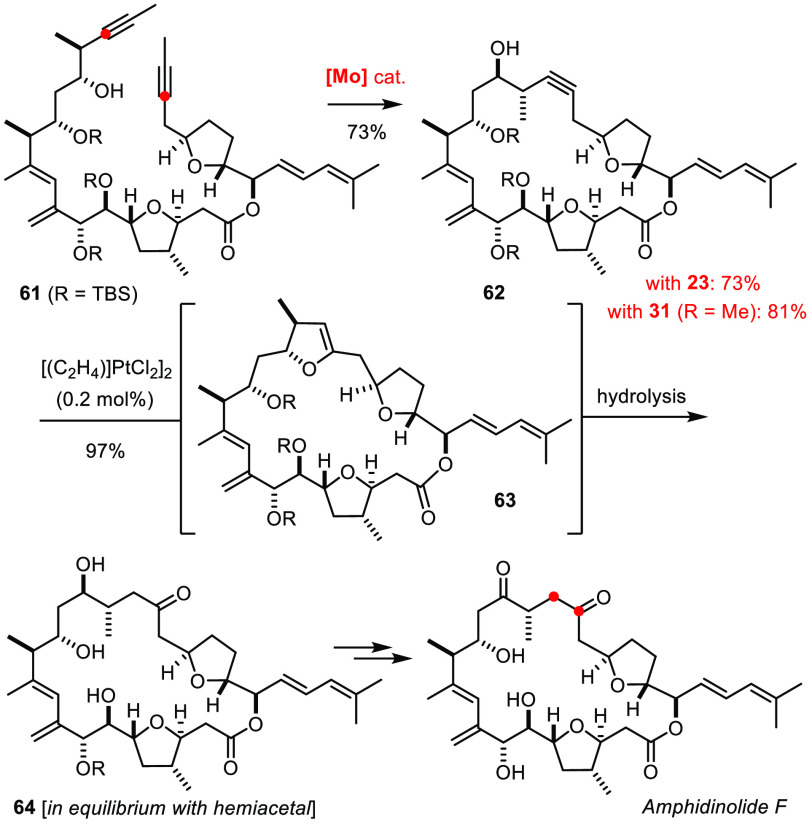
The synthesis of spirastrellolide F by RCAM followed by transannular spiroketalization referred to above illustrates this aspect (Scheme 6).89 No less challenging is the case shown in Scheme 17:157,158 RCAM of the polysubstituted diyne 61 gave product 62; the reaction was initially performed with 23 as the catalyst and later repeated with the canopy variant 31.75 Activation of 62 with catalytic Pt(+2) entailed a transannular hydroalkoxylation with formation of the labile enol ether 63, which was hydrolyzed upon workup to reveal the peculiar “umpoled” 1,4-oxygenation pattern of amphidinolide F.157,158
An RCAM-based approach to enigmazole plays with the equivalence of a propargyl acetate and an enone (Scheme 18).159 Slow release of the active catalyst 23 from the ate-complex 22 gave the best yield of cycloalkyne 66, probably because the propargylic acetate in this particular cyclization precursor is exceptionally elimination-prone in the presence of a Lewis acid. Activation of the derived compound 67 with a chiral gold catalyst caused a 3,3-sigmatropic rearrangement with formation of allenyl acetate 68, which, once formed, gets activated by the very same catalyst and succumbs to transannular hydroalkoxylation. Hydrolysis of the resulting enol ester 69 unveils the Michael addition product 70 as immediate precursor of the targeted natural product.159
Scheme 18. Total Synthesis of Enigmazole A by RCAM in Concert with a Gold-Catalyzed Reaction Cascade.
Brief reference is also made in this context to lythranidine (Scheme 19).160 Once again, it was the tolerance of the molybdenum alkylidyne 23 toward unprotected alcohol and phenol groups that proved enabling. Redox isomerization161 of product 72 furnished enone 73 in readiness for a transannular aza-Michael addition. The ability to encode a 1,3-aminoalcohol motif in form of an alkyne may not be immediately apparent. These examples showcase that the cornucopia of alkyne metathesis is filled with a multitude of structural patterns of preparative relevance.
Scheme 19. An RCAM/Redox-Isomerization Sequence.
Heterocyclic Targets
The formation of heterocyclic motifs from alkyne precursors has to be seen in a similar vein. An unconventional approach to the bacterial metabolite kendomycin represents an elaborate example (Scheme 20).162,163 RCAM of diyne 75 with formation of 76 set the stage for a subsequent π-acid catalyzed annelation of the benzofuran nucleus (77),164 which was later oxidized to unveil the quinone-methide/lactol chromophore of this prominent target.
Scheme 20. Assembly of Kendomycin at an Unconventional Site.
Terminal Alkyne Metathesis
All examples discussed so far relied on the use of internal alkynes; methyl caps are by far most common. An expansion of the substrate pool in general is highly desirable; terminal alkynes in particular are potentially lucrative starting points.
The Schrock group had already uncovered why their use is challenging:165 the critical step follows the first [2 + 2] cycloaddition in that the resulting complex is prone to transannular C–H insertion with formation of a deprotio-metallacyclobutadiene; this process destroys catalyst and substrate alike. It was much later that Tamm and co-workers noticed that Schrock-type molybdenum alkylidynes 3 endowed with poorly basic hexafluoro-tert-butoxide ligands allow this destructive step to be avoided and certain terminal aliphatic alkynes to be metathesized;166,167 the chosen test set, however, was small.
Shortly thereafter, complex 23 was found to be equally suited.65,168 Upon more comprehensive screening, however, we noticed that this transformation is highly substrate dependent for reasons that are not entirely clear; a late-stage implementation into target-oriented synthesis is therefore still deemed (overly) risky.
Gratifyingly, we could show that substrates comprising one terminal alkyne and one internal alkyne are much better behaved. In this case, RCAM reactions with the aid of 23 and analogues proved robust and high yielding. Our campaign leading to the structure revision of mandelalide A highlights this aspect (Scheme 21).169,170 Moreover, this example reiterates the fact that alkene and alkyne metathesis are orthogonal: the 1,3-enyne primarily formed was transformed into the nonthermodynamic Z,E-configured 1,3-diene subunit of this particular product. Suffice it to say that enyne/yne metathesis followed by appropriate semireduction allows all possible 1,3-diene configurations to be reached in a stereoselective manner, without any scrambing or ring contraction interfering (Figure 11); sensitive skipped 1,4-dienes are equally accessible.44,136,171−179 Likewise, 1,3-dienes comprised of vic-methylene branches are within reach when RCAM is combined with cross-enyne metathesis with ethylene, as exemplified by the conquest of amphidinolide V.171,172
Scheme 21. Manifestation of the Reliable Terminal/Internal Alkyne Setting.
Figure 11.
RCAM provides selective entry into all diene motifs.
New Formats
Even 1,3-diynes and 1,3,5-triynes can be made by alkyne metathesis, although one might expect that conjugated triple bonds get scambled.180−182 This transformation has empowered the total syntheses of the immunomodulatory macrolides ivorenolide A and B (Scheme 22), which further confirm the compliance of substrates comprising one terminal and one internal alkyne.92,183
Scheme 22. Advanced 1,3-Diyne Metathesis Reaction.
Equally useful is controlled head-to-tail cyclodimerization. Recent conquests of disorazole C1184 and the antimalarial agent samroiyotmycin A (using the newest canopy catalyst 31, Scheme 23)185 illustrate this reaction format.
Scheme 23. Selective Head-to-Tail Cyclodimerization.
Heteroatom-Containing Triple Bonds
While the power and relevance of catalytic alkyne metathesis is by now largely undisputed, there remains much room for improvement when it comes to reactions of heteroatom-containing triple bonds. Proof-of-concept for nitrile/alkyne metathesis reactions is available;186 the major challenge to be met en route to truly efficient settings is the high thermodynamic stability of the resulting metal nitride complexes. The fact, however, that certain such complexes do react with internal alkynes, though fairly slowly and under rather forcing conditions, provides an encouraging outlook.66,187
Another unorthodox case is the metathetic activation of N≡N bonds. To the best of our knowledge, all attempts at direct cleavage of molecular nitrogen itself have so far met with failure.188 When seen against this backdrop, the exceptional ease with which the triple bond of aryl diazonium salts is activated by ate-complexes 22 (M = Mo, W) is all the more surprising (Scheme 24); the reaction proceeds within minutes even below 0 °C.189 For the time being, this reaction is a stoichiometric process, since in situ recycling of the resulting imido complex 84 into an alkylidyne is currently not possible. Although much of the chemistry of aryl diazonium salts is rooted in the exceptional ease with which they lose dinitrogen, 22 exclusively activates the thermodynamically much more stable triple bond. One might hope that this transformation anticipates future cleavage of N2, e.g., when bound end-on to an appropriate metal center; if so, it would open a conceptually different foray toward N2 activation devoid of any redox steps.
Scheme 24. Selective Cleavage of the N≡N Triple Bond of the “World’s Best Leaving Group”.
Conclusions and Outlook
Before the turn of the millennium, alkyne metathesis faced the paradox of being well understood but hardly relevant. This situation has changed since then; the reaction is increasingly recognized as truly enabling and relevant from the strategy point of view; it is clearly more than a subordinate relative of olefin metathesis. At the same time, this development may help correct the common misconception that high-valent early transition metals, other than their “noble” cousins, provide too limited opportunities when polysubstituted, densely functionalized, and/or fragile compounds need to be addressed.
Most applications in the realm of target-oriented synthesis cited above implemented RCAM at a very late stage of a multistep endeavor. The fact that we are willing to subject very precious materials to this methodology illustrates our confidence in the reliability and performance of the catalysts.
The impressive advances of the past decade notwithstanding, a number of issues remain to be tackled. The perhaps most obvious ones concern the current inability to perform reactions in water or other protic media and the still largely missing compatibility with strongly acidic groups. A better availability and even greater ease of handling of the catalysts is also desirable, as are higher turnover numbers when working with (poly)functionalized substrates. Finally, the development of truly catalytic ways of activating heteroatom-containing triple bonds deserves more attention, as such methods would increase the substrate pool to a considerable extent. That the future role of alkyne metathesis will also hinge on the development of ever more effective and selective ways of making and manipulating triple bonds is obvious: π-acid catalysis provides an excellent example for how such concurrent method development can enlarge the scope;155 the emergence of catalytic alkyne trans- and gem-hydrogenation as well as the related trans-hydrometalation also needs to be quoted in this context.140 Further such advances are desirable, necessary, and arguably feasible.
Acknowledgments
I sincerely thank all students, postdocs, staff members, and collaboration partners involved in this long-term project for their invaluable contributions; their names appear in the references. Generous financial support by the Max-Planck-Society, the German Science Foundation, and the Alexander-von-Humboldt Foundation is gratefully acknowledged.
Open access funded by Max Planck Society.
The author declares the following competing financial interest(s): Patent WO 2011/120508 A1 covering catalysts with silanolate ligands
References
- Pennella F.; Banks R. L.; Bailey G. C. Disproportionation of Alkynes. Chem. Commun. 1968, 1548–1549. 10.1039/c19680001548. [DOI] [Google Scholar]
- Schrock R. R. Multiple Metal–Carbon Bonds for Catalytic Metathesis Reactions. Angew. Chem., Int. Ed. 2006, 45, 3748–3759. 10.1002/anie.200600085. [DOI] [PubMed] [Google Scholar]
- Fürstner A.; Davies P. W. Alkyne Metathesis. Chem. Commun. 2005, 2307–2320. 10.1039/b419143a. [DOI] [PubMed] [Google Scholar]
- Schrock R. R.; Czekelius C. Recent Advances in the Syntheses and Applications of Molybdenum and Tungsten Alkylidene and Alkylidyne Catalysts for the Metathesis of Alkenes and Alkynes. Adv. Synth. Catal. 2007, 349 (1–2), 55–77. 10.1002/adsc.200600459. [DOI] [Google Scholar]
- Zhang W.; Moore J. S. Alkyne Metathesis: Catalysts and Synthetic Applications. Adv. Synth. Catal. 2007, 349, 93–120. 10.1002/adsc.200600476. [DOI] [Google Scholar]
- Fürstner A. Alkyne Metathesis on the Rise. Angew. Chem., Int. Ed. 2013, 52 (10), 2794–2819. 10.1002/anie.201204513. [DOI] [PubMed] [Google Scholar]
- Fürstner A., Alkyne Metathesis in Organic Synthesis. In Modern Alkyne Chemistry: Catalytic and Atom-Economic Transformations; Trost B. M., Li C. J., Eds.; Wiley: Weinheim, 2015; pp 69–111. [Google Scholar]
- Ehrhorn H.; Tamm M. Well-Defined Alkyne Metathesis Catalysts: Developments and Recent Applications. Chem. - Eur. J. 2019, 25, 3190–3208. 10.1002/chem.201804511. [DOI] [PubMed] [Google Scholar]
- Lee D.; Volchkov I.; Yun S. Y. Alkyne Metathesis. Org. React. 2020, 102, 613–931. 10.1002/0471264180.or102.02. [DOI] [Google Scholar]
- Mortreux A.; Blanchard M. Metathesis of alkynes by a molybdenum hexacarbonyl–resorcinol catalyst. J. Chem. Soc., Chem. Commun. 1974, 19, 786–787. 10.1039/C39740000786. [DOI] [Google Scholar]
- Mortreux A.; Coutelier O. Alkyne Metathesis Catalysts: Scope and Future. J. Mol. Catal. A: Chem. 2006, 254, 96–104. 10.1016/j.molcata.2006.03.054. [DOI] [Google Scholar]
- Schrock R. R. High Oxidation State Multiple Metal-Carbon Bonds. Chem. Rev. 2002, 102, 145–179. 10.1021/cr0103726. [DOI] [PubMed] [Google Scholar]
- Schrock R. R. High-Oxidation-State Molybdenum and Tungsten Alkylidyne Complexes. Acc. Chem. Res. 1986, 19, 342–348. 10.1021/ar00131a003. [DOI] [Google Scholar]
- Katz T. J.; McGinnis J. Mechanism of the Olefin Metathesis Reaction. J. Am. Chem. Soc. 1975, 97, 1592–1594. 10.1021/ja00839a063. [DOI] [Google Scholar]
- Cui M.; Lin R.; Jia G. Chemistry of Metallacyclobutadienes. Chem. - Asian J. 2018, 13, 895–912. 10.1002/asia.201701814. [DOI] [PubMed] [Google Scholar]
- Wengrovius J. H.; Sancho J.; Schrock R. R. Metathesis of Acetylenes by Tungsten(VI)-Alkylidyne Complexes. J. Am. Chem. Soc. 1981, 103, 3932–3934. 10.1021/ja00403a058. [DOI] [Google Scholar]
- Pedersen S. F.; Schrock R. R.; Churchill M. R.; Wasserman H. J. Reaction of Tungsten(VI) Alkylidyne Complexes with Acetylenes to Give Tungstenacyclobutadiene and Cyclopentadienyl Complexes. J. Am. Chem. Soc. 1982, 104, 6808–6809. 10.1021/ja00388a068. [DOI] [Google Scholar]
- Schrock R. R.; Murdzek J. S.; Freudenberger J. H.; Churchill M. R.; Ziller J. W. Preparation of Molybdenum and Tungsten Neopentylidyne Complexes of the Type M(CCMe3)(O2CR)3, Their Reactions with Acetylenes, and the X-ray Structure of the η3-Cyclopropenyl Complex W[C3(CMe3)Et2](O2CCH3)3. Organometallics 1986, 5, 25–33. 10.1021/om00132a005. [DOI] [Google Scholar]
- Listemann M. L.; Schrock R. R. Multiple metal carbon bonds. 35. A general route to tri-tert-butoxytungsten alkylidyne complexes. Scission of acetylenes by ditungsten hexa-tert-butoxide. Organometallics 1985, 4 (1), 74–83. 10.1021/om00120a014. [DOI] [Google Scholar]
- McCullough L. G.; Schrock R. R. Metathesis of Acetylenes by Molybdenum(VI) Alkylidyne Complexes. J. Am. Chem. Soc. 1984, 106, 4067–4068. 10.1021/ja00326a051. [DOI] [Google Scholar]
- McCullough L. G.; Schrock R. R.; Dewan J. C.; Murdzek J. C. Preparation of Trialkoxymolybdenum(VI) Alkylidyne Complexes, Their Reactions with Acetylenes, and the X-ray Structure of Mo[C3(CMe3)2][OCH(CF3)2]2(C5H5N)2. J. Am. Chem. Soc. 1985, 107, 5987–5998. 10.1021/ja00307a025. [DOI] [Google Scholar]
- Schrock R. R.; Weinstock I. A.; Horton A. D.; Liu A. H.; Schofield M. H. Preparation of Rhenium(VII) Monoimido Alkylidyne Complexes and Metathesis of Acetylenes via Rhenacyclobutadiene Intermediates. J. Am. Chem. Soc. 1988, 110, 2686–2687. 10.1021/ja00216a071. [DOI] [Google Scholar]
- Weinstock I. A.; Schrock R. R.; Davis W. M. Rhenium(VII) Monoimido Alkylidyne Complexes. The Importance of Face Selectivity in the Metathesis of Acetylenes via Rhenacyclobutadiene Intermediates. J. Am. Chem. Soc. 1991, 113, 135–144. 10.1021/ja00001a021. [DOI] [Google Scholar]
- Chabanas M.; Baudouin A.; Copéret C.; Basset J.-M. A Highly Active Well-Defined Rhenium Heterogeneous Catalyst for Olefin Metathesis Prepared via Surface Organometallic Chemistry. J. Am. Chem. Soc. 2001, 123 (9), 2062–2063. 10.1021/ja000900f. [DOI] [PubMed] [Google Scholar]
- Handbook of Metathesis, 2nd ed.; Grubbs R. H., O’Leary D. J., Eds.; Wiley-VCH: Weinheim, 2015. [Google Scholar]
- Grubbs R. H.; Miller S. J.; Fu G. C. Ring-Closing Metathesis and Related Processes in Organic Synthesis. Acc. Chem. Res. 1995, 28 (11), 446–452. 10.1021/ar00059a002. [DOI] [Google Scholar]
- Nguyen S. T.; Grubbs R. H.; Ziller J. W. Syntheses and activities of new single-component, ruthenium-based olefin metathesis catalysts. J. Am. Chem. Soc. 1993, 115 (21), 9858–9859. 10.1021/ja00074a086. [DOI] [Google Scholar]
- Fürstner A.; Langemann K. Conformationally Unbiased Macrocyclization Reactions by Ring Closing Metathesis. J. Org. Chem. 1996, 61 (12), 3942–3943. 10.1021/jo960733v. [DOI] [PubMed] [Google Scholar]
- Fürstner A.; Langemann K. Macrocycles by Ring-Closing Metathesis. Synthesis 1997, 1997, 792–803. 10.1055/s-1997-4472. [DOI] [Google Scholar]
- Fürstner A. Olefin Metathesis and Beyond. Angew. Chem., Int. Ed. 2000, 39 (17), 3012–3043. . [DOI] [PubMed] [Google Scholar]
- Hoveyda A. H.; Zhugralin A. R. The remarkable metal-catalysed olefin metathesis reaction. Nature 2007, 450 (7167), 243–251. 10.1038/nature06351. [DOI] [PubMed] [Google Scholar]
- Nicolaou K. C.; Bulger P. G.; Sarlah D. Metathesis Reactions in Total Synthesis. Angew. Chem., Int. Ed. 2005, 44 (29), 4490–4527. 10.1002/anie.200500369. [DOI] [PubMed] [Google Scholar]
- Fürstner A. Metathesis in Total Synthesis. Chem. Commun. 2011, 47 (23), 6505–6511. 10.1039/c1cc10464k. [DOI] [PubMed] [Google Scholar]
- Fürstner A. Catalysis for Total Synthesis: A Personal Account. Angew. Chem., Int. Ed. 2014, 53, 8587–8598. 10.1002/anie.201402719. [DOI] [PubMed] [Google Scholar]
- Hoveyda A. H. Evolution of Catalytic Stereoselective Olefin Metathesis: From Ancillary Transformation to Purveyor of Stereochemical Identity. J. Org. Chem. 2014, 79 (11), 4763–4792. 10.1021/jo500467z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogba O. M.; Warner N. C.; O’Leary D. J.; Grubbs R. H. Recent advances in ruthenium-based olefin metathesis. Chem. Soc. Rev. 2018, 47 (12), 4510–4544. 10.1039/C8CS00027A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery T. P.; Ahmed T. S.; Grubbs R. H. Stereoretentive Olefin Metathesis: An Avenue to Kinetic Selectivity. Angew. Chem., Int. Ed. 2017, 56 (37), 11024–11036. 10.1002/anie.201704686. [DOI] [PubMed] [Google Scholar]
- Shen X.; Nguyen T. T.; Koh M. J.; Xu D.; Speed A. W. H.; Schrock R. R.; Hoveyda A. H. Kinetically E-selective macrocyclic ring-closing metathesis. Nature 2017, 541, 380–386. 10.1038/nature20800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen T. T.; Koh M. J.; Shen X.; Romiti F.; Schrock R. R.; Hoveyda A. H. Kinetically controlled E-selective catalytic olefin metathesis. Science 2016, 352 (6285), 569–575. 10.1126/science.aaf4622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fürstner A.; Seidel G. Ring-closing Metathesis of Functinalized Acetylene Derivatives: A New Entry into Cycloalkynes. Angew. Chem., Int. Ed. 1998, 37, 1734–1736. . [DOI] [PubMed] [Google Scholar]
- Fürstner A.; Guth O.; Rumbo A.; Seidel G. Ring Closing Alkyne Metathesis. Comparative Investigation of Two Different Catalyst Systems and Application to the Stereoselective Synthesis of Olfactory Lactones, Azamacrolides, and the Macrocyclic Perimeter of the Marine Alkaloid Nakadomarin A. J. Am. Chem. Soc. 1999, 121, 11108–11113. 10.1021/ja992074k. [DOI] [Google Scholar]
- Radkowski K.; Sundararaju B.; Fürstner A. A Functional-Group-Tolerant Catalytic trans-Hydrogenation of Alkynes. Angew. Chem., Int. Ed. 2013, 52, 355–360. 10.1002/anie.201205946. [DOI] [PubMed] [Google Scholar]
- Fürstner A.; Radkowski K. A chemo- and stereoselective reduction of cycloalkynes to (E)-cycloalkenes. Chem. Commun. 2002, 18, 2182–2183. 10.1039/B207169J. [DOI] [PubMed] [Google Scholar]
- Fürstner A. Teaching Metathesis “Simple” Stereochemistry. Science 2013, 341 (6152), 1229713. 10.1126/science.1229713. [DOI] [PubMed] [Google Scholar]
- Schrock et al. had already shown that certain tungsten alkylidynes react in a Wittig-like manner with benzaldeyhde, acetone, ethyl acetate, and even DMF; see:Freudenberger J. H.; Schrock R. R. Multiple metal-carbon bonds. 41. Wittig-like reactions of tungsten alkylidyne complexes. Organometallics 1986, 5 (2), 398–400. 10.1021/om00133a041. [DOI] [Google Scholar]
- Fürstner A.; Dierkes T. Concise Synthesis of (S,S)-(+)-Dehydrohomoancepsenolide. Org. Lett. 2000, 2 (16), 2463–2465. 10.1021/ol006122d. [DOI] [PubMed] [Google Scholar]
- Fürstner A.; Grela K.; Mathes C.; Lehmann C. W. Novel and Flexible Entries into Prostaglandins and Analogues Based on Ring Closing Alkyne Metathesis or Alkyne Cross Metathesis. J. Am. Chem. Soc. 2000, 122 (48), 11799–11805. 10.1021/ja003119g. [DOI] [Google Scholar]
- Fürstner A.; Stelzer F.; Rumbo A.; Krause H. Total Synthesis of the Turrianes and Evaluation of Their DNA-Cleaving Properties. Chem. - Eur. J. 2002, 8 (8), 1856–1871. . [DOI] [PubMed] [Google Scholar]
- Fürstner A.; Castanet A.-S.; Radkowski K.; Lehmann C. W. Total Synthesis of (S)-(+)-Citreofuran by Ring Closing Alkyne Metathesis. J. Org. Chem. 2003, 68 (4), 1521–1528. 10.1021/jo026686q. [DOI] [PubMed] [Google Scholar]
- Ghalit N.; Poot A. J.; Fürstner A.; Rijkers D. T. S.; Liskamp R. M. J. Ring-Closing Alkyne Metathesis Approach toward the Synthesis of Alkyne Mimics of Thioether A-, B-, C-, and DE-Ring Systems of the Lantibiotic Nisin Z. Org. Lett. 2005, 7 (14), 2961–2964. 10.1021/ol0508781. [DOI] [PubMed] [Google Scholar]
- Cummins C. C. Reductive cleavage and related reactions leading to molybdenum-element multiple bonds: new pathways offered by three-coordinate molybdenum(III). Chem. Commun. 1998, 1777–1786. 10.1039/a802402b. [DOI] [Google Scholar]
- Fürstner A.; Mathes C.; Lehmann C. W. Mo[N(tBu)(Ar)]3 Complexes as Catalyst Precursors: In Situ Activation and Application to Metathesis Reactions of Alkynes and Diynes. J. Am. Chem. Soc. 1999, 121, 9453–9454. 10.1021/ja991340r. [DOI] [Google Scholar]
- Fürstner A.; Mathes C.; Lehmann C. W. Alkyne Metathesis: Development of a Novel Molybdenum-Based Catalyst System and its Application to the Total Synthesis of Epothilone A and C. Chem. - Eur. J. 2001, 7, 5299–5315. . [DOI] [PubMed] [Google Scholar]
- Fürstner A.; Mathes C. Alkyne Cross Metathesis Reactions of Extended Scope. Org. Lett. 2001, 3 (2), 221–223. 10.1021/ol0068795. [DOI] [PubMed] [Google Scholar]
- Fürstner A.; Mathes C.; Grela K. Concise Total Syntheses of Epothilone A and C Based on Alkyne Metathesis. Chem. Commun. 2001, 1057–1059. 10.1039/b101669p. [DOI] [Google Scholar]
- For some instructive cases, see refs (57 and 58) and the following:Mailhol D.; Willwacher J.; Kausch-Busies N.; Rubitski E. E.; Sobol Z.; Schuler M.; Lam M. H.; Musto S.; Loganzo F.; Maderna A.; Fürstner A. Synthesis, Molecular Editing, and Biological Assessment of the Potent Cytotoxin Leiodermatolide. J. Am. Chem. Soc. 2014, 136, 15719–15729. 10.1021/ja508846g. [DOI] [PubMed] [Google Scholar]
- Karier P.; Ungeheuer F.; Ahlers A.; Anderl F.; Wille C.; Fürstner A. Metathesis at an Implausible Site: A Formal Total Synthesis of Rhizoxin D. Angew. Chem., Int. Ed. 2019, 58 (1), 248–253. 10.1002/anie.201812096. [DOI] [PubMed] [Google Scholar]
- Schulthoff S.; Hamilton J. Y.; Heinrich M.; Kwon Y.; Wirtz C.; Fürstner A. The Formosalides: Structure Determination by Total Synthesis. Angew. Chem., Int. Ed. 2021, 60 (1), 446–454. 10.1002/anie.202011472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W.; Kraft S.; Moore J. S. A reductive recycle strategy for the facile synthesis of molybdenum(VI) alkylidyne catalysts for alkyne metathesis. Chem. Commun. 2003, 7, 832–833. 10.1039/b212405j. [DOI] [PubMed] [Google Scholar]
- Zhang W.; Kraft S.; Moore J. S. Highly Active Trialkoxymolybdenum(VI) Alkylidyne Catalysts Synthesized by a Reductive Recycle Strategy. J. Am. Chem. Soc. 2004, 126 (1), 329–335. 10.1021/ja0379868. [DOI] [PubMed] [Google Scholar]
- Pattillo C. C.; Cencer M. M.; Moore J. S. Discussion Addendum for: Preparation of a Carbazole-Based Macrocycle Via Precipitation-Driven Alkyne Metathesis. Org. Synth. 2018, 95, 231–239. 10.15227/orgsyn.095.0231. [DOI] [Google Scholar]
- Hillenbrand J.; van Gastel M.; Bill E.; Neese F.; Fürstner A. Isolation of a Homoleptic Non-oxo Mo(V) Alkoxide Complex: Synthesis, Structure, and Electronic Properties of Penta-tert-Butoxymolybdenum. J. Am. Chem. Soc. 2020, 142 (38), 16392–16402. 10.1021/jacs.0c07073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heppekausen J.; Stade R.; Goddard R.; Fürstner A. Practical New Silyloxy-Based Alkyne Metathesis Catalysts with Optimized Activity and Selectivity Profiles. J. Am. Chem. Soc. 2010, 132, 11045–11057. 10.1021/ja104800w. [DOI] [PubMed] [Google Scholar]
- Heppekausen J.; Stade R.; Kondoh A.; Seidel G.; Goddard R.; Fürstner A. Optimized Synthesis, Structural Investigations, Ligand Tuning and Synthetic Evaluation of Silyloxy-Based Alkyne Metathesis Catalysts. Chem. - Eur. J. 2012, 18, 10281–10299. 10.1002/chem.201200621. [DOI] [PubMed] [Google Scholar]
- Persich P.; Llaveria J.; Lhermet R.; de Haro T.; Stade R.; Kondoh A.; Fürstner A. Increasing the Structural Span of Alkyne Metathesis. Chem. - Eur. J. 2013, 19 (39), 13047–13058. 10.1002/chem.201302320. [DOI] [PubMed] [Google Scholar]
- The original discovery was made with the aid of silanolate-based molybdenum nitrides, which convert into the catalytically active alkylidynes on reaction with an alkyne substrate; see:Bindl M.; Stade R.; Heilmann E. K.; Picot A.; Goddard R.; Fürstner A. Molybdenum Nitride Complexes with Ph3SiO- Ligands are Exceedingly Practical and Tolerant Precatalysts for Alkyne Metathesis and Efficient Nitrogen Transfer Agents. J. Am. Chem. Soc. 2009, 131, 9468–9470. 10.1021/ja903259g. [DOI] [PubMed] [Google Scholar]
- Krempner C. Role of Siloxides in Transition Metal Chemistry and Homogeneous Catalysis. Eur. J. Inorg. Chem. 2011, 2011, 1689–1698. 10.1002/ejic.201100044. [DOI] [Google Scholar]
- Because sp-hybridization enhances the O–Mo π-donation, it was originally believed that stretched silanolates are stronger donors, see refs (63 and 64). Recent computations, however, corrected this view in that they showed that enhanced π-donation is actually overcompensated by weakening of the Mo–O σ-bond. Silanolates, in the sp-hybridized extreme, are hence weaker rather than stronger net donor ligands; see ref (69).
- Haack A.; Hillenbrand J.; van Gastel M.; Fürstner A.; Neese F. Spectroscopic and Theoretical Study on Siloxy-Based Molybdenum and Tungsten Alkylidyne Catalysts for Alkyne Metathesis. ACS Catal. 2021, 11, 9086–9101. 10.1021/acscatal.1c01587. [DOI] [Google Scholar]
- Weissman H.; Plunkett K. N.; Moore J. S. A Highly Active, Heterogeneous Catalyst for Alkyne Metathesis. Angew. Chem., Int. Ed. 2006, 45 (4), 585–588. 10.1002/anie.200502840. [DOI] [PubMed] [Google Scholar]
- Cho H. M.; Weissman H.; Wilson S. R.; Moore J. S. A Mo(VI) Alkylidyne Complex with Polyhedral Oligomeric Silsesquioxane Ligands: Homogeneous Analogue of a Silica-Supported Alkyne Metathesis Catalyst. J. Am. Chem. Soc. 2006, 128 (46), 14742–14743. 10.1021/ja065101x. [DOI] [PubMed] [Google Scholar]
- For a detailed discussion and compilation of relevant literature, see:Solans-Monfort X.; Filhol J.-S.; Copéret C.; Eisenstein O. Structure, spectroscopic and electronic properties of a well defined silica supported olefin metathesis catalyst, [(≡ SiO)Re(≡ CR)(=CHR)(CH2R)], through DFT periodic calculations: silica is just a large siloxy ligand. New J. Chem. 2006, 30 (6), 842–850. 10.1039/B603426H. [DOI] [Google Scholar]
- Mayr A.; McDermott G. A. Oxidative transformation of monobromotetracarbonyl(alkylidyne) complexes of molybdenum and tungsten into tribromo(alkylidyne) complexes. J. Am. Chem. Soc. 1986, 108 (3), 548–549. 10.1021/ja00263a054. [DOI] [PubMed] [Google Scholar]
- McDermott G. A.; Dorries A. M.; Mayr A. Synthesis of carbyne complexes of chromium, molybdenum, and tungsten by formal oxide abstraction from acyl ligands. Organometallics 1987, 6 (5), 925–931. 10.1021/om00148a005. [DOI] [Google Scholar]
- Hillenbrand J.; Leutzsch M.; Yiannakas E.; Gordon C. P.; Wille C.; Nöthling N.; Copéret C.; Fürstner A. Canopy Catalysts” for Alkyne Metathesis: Molybdenum Alkylidyne Complexes with a Tripodal Ligand Framework. J. Am. Chem. Soc. 2020, 142 (25), 11279–11294. 10.1021/jacs.0c04742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberlag B.; Wu X.; Brandhorst K.; Grunenberg J.; Daniliuc C. G.; Jones P. G.; Tamm M. Preparation of Imidazolin-2-iminato Molybdenum and Tungsten Benzylidyne Complexes: A New Pathway to Highly Active Alkyne Metathesis Catalysts. Chem. - Eur. J. 2010, 16, 8868–8877. 10.1002/chem.201000597. [DOI] [PubMed] [Google Scholar]
- Haack A.; Hillenbrand J.; Leutzsch M.; van Gastel M.; Neese F.; Fürstner A. Productive Alkyne Metathesis with “Canopy Catalysts” Mandates Pseudorotation. J. Am. Chem. Soc. 2021, 143 (15), 5643–5648. 10.1021/jacs.1c01404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- It was recently shown that higher internal alkynes (derived from substrates carrying alkyl rather than methyl caps) can also be trapped with the aid of molecular sieves; see:Kiel G. R.; Bergman H. M.; Tilley T. D. Site-selective [2 + 2 + n] cycloadditions for rapid, scalable access to alkynylated polycyclic aromatic hydrocarbons. Chem. Sci. 2020, 11 (11), 3028–3035. 10.1039/C9SC06102A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollner A.; Altmann K.-H.; Gertsch J.; Mulzer J. The Laulimalide Family: Total Synthesis and Biological Evaluation of Neolaulimalide, Isolaulimalide, Laulimalide and a Nonnatural Analogue. Chem. - Eur. J. 2009, 15 (24), 5979–5997. 10.1002/chem.200802605. [DOI] [PubMed] [Google Scholar]
- Wölfl B.; Mata G.; Fürstner A. Total Synthesis of Callyspongiolide, Part 2: The Ynoate Metathesis/cis-Reduction Strategy. Chem. - Eur. J. 2019, 25 (1), 255–259. 10.1002/chem.201804988. [DOI] [PubMed] [Google Scholar]
- Mata G.; Wölfl B.; Fürstner A. Synthesis and Molecular Editing of Callyspongiolide, Part 1: The Alkyne Metathesis/trans-Reduction Strategy. Chem. - Eur. J. 2019, 25 (1), 246–254. 10.1002/chem.201804987. [DOI] [PubMed] [Google Scholar]
- Schaubach S.; Michigami K.; Fürstner A. Hydroxyl-Assisted trans-Reduction of 1,3-Enynes: Application to the Formal Synthesis of (+)-Aspicilin. Synthesis 2016, 49 (01), 202–208. 10.1055/s-0035-1562381. [DOI] [Google Scholar]
- Guo L. D.; Huang X. Z.; Luo S. P.; Cao W. S.; Ruan Y. P.; Ye J. L.; Huang P. Q. Organocatalytic, Asymmetric Total Synthesis of (−)-Haliclonin A. Angew. Chem., Int. Ed. 2016, 55, 4064–4068. 10.1002/anie.201512005. [DOI] [PubMed] [Google Scholar]
- Luo S.-P.; Huang X.-Z.; Guo L.-D.; Huang P.-Q. Catalytic Asymmetric Total Synthesis of Macrocyclic Marine Natural Product (−)-Haliclonin A. Chin. J. Chem. 2020, 38 (12), 1723–1736. 10.1002/cjoc.202000291. [DOI] [Google Scholar]
- Boeckman R. K.; Wang H.; Rugg K. W.; Genung N. E.; Chen K.; Ryder T. R. A Scalable Total Synthesis of (−)-Nakadomarin A. Org. Lett. 2016, 18, 6136–6139. 10.1021/acs.orglett.6b03137. [DOI] [PubMed] [Google Scholar]
- Vendeville J. B.; Matters R. E.; Chen A.; Light M. E.; Tizzard G. J.; Chai C. L. L.; Harrowven D. C. A Synthetic Approach to Chrysophaerntin F. Chem. Commun. 2019, 55, 4837–4840. 10.1039/C9CC01666J. [DOI] [PubMed] [Google Scholar]
- Neuhaus C. M.; Liniger M.; Stieger M.; Altmann K. H. Total Synthesis of the Tubulin Inhibitor WF-1360F Based on Macrocycle Formation through Ring-Closing Alkyne Metathesis. Angew. Chem., Int. Ed. 2013, 52 (22), 5866–5870. 10.1002/anie.201300576. [DOI] [PubMed] [Google Scholar]
- Herstad G.; Molesworth P. P.; Miller C.; Benneche T.; Tius M. A. Ring-Closing Metathesis in an Enantioselective Synthesis of the Macrocyclic Core of Crassin Acetate. Tetrahedron 2016, 72, 2084–2093. 10.1016/j.tet.2016.02.050. [DOI] [Google Scholar]
- Benson S.; Collin M.-P.; Arlt A.; Gabor B.; Goddard R.; Fürstner A. Second-Generation Total Synthesis of Spirastrellolide F Methyl Ester: The Alkyne Route. Angew. Chem., Int. Ed. 2011, 50 (37), 8739–8744. 10.1002/anie.201103270. [DOI] [PubMed] [Google Scholar]
- Although silanoles are more acidic than alcohols, partial ligand exchange may occur because the substrate is present in large excess, especially at the beginning of the reaction.
- Protonation/solvolysis of the alkylidyne unit is a second pathway by which the activity can be lost; while strongly acidic groups may act in this way, this process does not seem to play a major role when working with substrates carrying free −OH groups.
- Schaubach S.; Gebauer K.; Ungeheuer F.; Hoffmeister L.; Ilg M. K.; Wirtz C.; Fürstner A. A Two-Component Alkyne Metathesis Catalyst System with an Improved Substrate Scope and Functional Group Tolerance: Development and Applications to Natural Product Synthesis. Chem. - Eur. J. 2016, 22 (25), 8494–8507. 10.1002/chem.201601163. [DOI] [PubMed] [Google Scholar]
- Hillenbrand J.; Leutzsch M.; Fürstner A. Molybdenum Alkylidyne Complexes with Tripodal Silanolate Ligands. The Next Generation of Alkyne Metathesis Catalysts. Angew. Chem., Int. Ed. 2019, 58, 15690–15696. 10.1002/anie.201908571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson R. R.; Rotella M. E.; Du P.; Zhou X.; Fronczek F. R.; Kumar R.; Gutierrez O.; Lee S. Siloxide Podand Ligand Scaffold for Molybdenum-Catalyzed Alkyne Metathesis and Isolation of a Dynamic Metallatetrahedrane Intermediate. Organometallics 2019, 38, 4054–4059. 10.1021/acs.organomet.9b00430. [DOI] [Google Scholar]
- Thompson R. R.; Rotella M. E.; Zhou X.; Fronczek F. R.; Gutierrez O.; Lee S. Impact of Ligands and Metals on the Formation of Metallacyclic Intermediates and a Nontraditional Mechanism for Group VI Alkyne Metathesis Catalysts. J. Am. Chem. Soc. 2021, 143 (24), 9026–9039. 10.1021/jacs.1c01843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Z.; Spohr S. M.; Tobegen S.; Farès C.; Fürstner A. A Unified Approach to Polycyclic Alkaloids of the Ingenamine Estate: Total Syntheses of Keramaphidin B, Ingenamine, and Nominal Njaoamine I. J. Am. Chem. Soc. 2021, 143, 14402–14414. 10.1021/jacs.1c07955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillenbrand J.; Korber J. N.; Leutzsch M.; Nothling N.; Furstner A. Canopy Catalysts for Alkyne Metathesis: Investigations into a Bimolecular Decomposition Pathway and the Stability of the Podand Cap. Chem. - Eur. J. 2021, 10.1002/chem.202102080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solans-Monfort X.; Copéret C.; Eisenstein O. Metallacyclobutanes from Schrock-Type d0 Metal Alkylidene Catalysts: Structural Preferences and Consequences in Alkene Metathesis. Organometallics 2015, 34 (9), 1668–1680. 10.1021/acs.organomet.5b00147. [DOI] [Google Scholar]
- Gordon C. P.; Yamamoto K.; Liao W.-C.; Allouche F.; Andersen R. A.; Copéret C.; Raynaud C.; Eisenstein O. Metathesis Activity Encoded in the Metallacyclobutane Carbon-13 NMR Chemical Shift Tensors. ACS Cent. Sci. 2017, 3 (7), 759–768. 10.1021/acscentsci.7b00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon C. P.; Raynaud C.; Andersen R. A.; Copéret C.; Eisenstein O. Carbon-13 NMR Chemical Shift: A Descriptor for Electronic Structure and Reactivity of Organometallic Compounds. Acc. Chem. Res. 2019, 52, 2278–2289. 10.1021/acs.accounts.9b00225. [DOI] [PubMed] [Google Scholar]
- For a recent case study from this laboratory on a reaction that involves two interconverting yet distinctly different metallacyclobutanes with/without noticable alkylidene character at the α-C-atoms, see:Peil S.; Bistoni G.; Goddard R.; Fürstner A. Hydrogenative Metathesis of Enynes via Piano-Stool Ruthenium Carbene Complexes Formed by Alkyne gem-Hydrogenation. J. Am. Chem. Soc. 2020, 142 (43), 18541–18553. 10.1021/jacs.0c07808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann B. E. The Cinderella Nuclei. Annu. Rep. NMR Spectrosc. 1991, 23, 141–207. 10.1016/S0066-4103(08)60277-X. [DOI] [Google Scholar]
- Young C. G.; Kober E. M.; Enemark J. H. 95Mo and 183W NMR Studies of Triply Bonded Dinuclear M(III) and Related M≡C (M = Mo or W) Complexes. Polyhedron 1987, 6, 255–259. 10.1016/S0277-5387(00)80794-9. [DOI] [Google Scholar]
- Hillenbrand J.; Leutzsch M.; Gordon C. P.; Copéret C.; Fürstner A. 183W NMR Spectroscopy Guides the Search for Tungsten Alkylidyne Catalysts for Alkyne Metathesis. Angew. Chem., Int. Ed. 2020, 59, 21758–21768. 10.1002/anie.202009975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jyothish K.; Wang Q.; Zhang W. Highly Active Multidentate Alkyne Metathesis Catalysts: Ligand-Activity Relationship and Their Applications in Efficient Synthesis of Porphyrin-Based Aryleneethynylene Polymers. Adv. Synth. Catal. 2012, 354, 2073–2078. 10.1002/adsc.201200243. [DOI] [Google Scholar]
- Yang H.; Liu Z.; Zhang W. Multidentate Triphenolsilane-Based Alkyne Metathesis Catalysts. Adv. Synth. Catal. 2013, 355, 885–890. 10.1002/adsc.201201105. [DOI] [Google Scholar]
- Du Y.; Yang H.; Zhu C.; Ortiz M.; Okochi K. D.; Shoemaker R.; Jin Y.; Zhang W. Highly Active Multidentate Ligand-Based Alkyne Metathesis Catalysts. Chem. - Eur. J. 2016, 22, 7959–7963. 10.1002/chem.201505174. [DOI] [PubMed] [Google Scholar]
- Ge Y.; Huang S.; Hu Y.; Zhang L.; He L.; Krajewski S.; Ortiz M.; Jin Y.; Zhang W. Highly active alkyne metathesis catalysts operating under open air condition. Nat. Commun. 2021, 12 (1), 1136. 10.1038/s41467-021-21364-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui M.; Bai W.; Sung H. H. Y.; Williams I. D.; Jia G. Robust Alkyne Metathesis Catalyzed by Air Stable d2 Re(V) Alkylidyne Complexes. J. Am. Chem. Soc. 2020, 142, 13339–13344. 10.1021/jacs.0c06581. [DOI] [PubMed] [Google Scholar]
- Bai W.; Wei W.; Sung H. H. Y.; Williams I. D.; Lin Z.; Jia G. Syntheses of Re(V) Alkylidyne Complexes and Ligand Effect on the Reactivity of Re(V) Alkylidyne Complexes toward Alkynes. Organometallics 2018, 37, 559–569. 10.1021/acs.organomet.7b00877. [DOI] [Google Scholar]
- Ehrhorn H.; Bockfeld D.; Freytag M.; Bannenberg T.; Kefalidis C. E.; Maron C.; Tamm M. Studies on Molybdena- and Tungstenacyclobutadiene Complexes Supported by Fluoroalkoxy Ligands as Intermediates of Alkyne Metathesis. Organometallics 2019, 38, 1627–1639. 10.1021/acs.organomet.9b00068. [DOI] [Google Scholar]
- Estes D. P.; Gordon C. P.; Fedorov A.; Liao W. C.; Ehrhorn H.; Bittner C.; Zier M. L.; Bockfeld D.; Chan K. W.; Eisenstein O.; Raynaud C.; Tamm M.; Copéret C. Molecular and Silica-Supported Molybdenum Alkyne Metathesis Catalysts: Influence of Electrons and Dynamics on Activity Revealed by Kinetics, Solid-State NMR, and Chemical Shift Analysis. J. Am. Chem. Soc. 2017, 139, 17597–17607. 10.1021/jacs.7b09934. [DOI] [PubMed] [Google Scholar]
- Estes P. D.; Bittner C.; Arias O.; Casey M.; Fedorov A.; Tamm M.; Copéret C. Alkyne Metathesis with Silica-Supported and Molecular Catalysts at Parts-per-Million Loadings. Angew. Chem., Int. Ed. 2016, 55, 13960–13964. 10.1002/anie.201605129. [DOI] [PubMed] [Google Scholar]
- Beer S.; Hrib C. G.; Jones P. G.; Brandhorst K.; Grunenberg J.; Tamm M. Efficient Room-Temperature Alkyne Metathesis with Well-Defined Imidazolin-2-iminato Tungsten Alkylidyne Complexes. Angew. Chem., Int. Ed. 2007, 46, 8890. 10.1002/anie.200703184. [DOI] [PubMed] [Google Scholar]
- Bittner C.; Ehrhorn H.; Bockfeld D.; Brandhorst K.; Tamm M. Tuning the Catalytic Alkyne Metathesis Activity of Molybdenum and Tungsten 2,4,6-Trimethylbenzylidyne Complexes with Fluoroalkoxide Ligands OC(CF3)nMe3–n. Organometallics 2017, 36, 3398–3406. 10.1021/acs.organomet.7b00519. [DOI] [Google Scholar]
- Groos J.; Hauser P. M.; Koy M.; Frey W.; Buchmeiser M. R. Highly Reactive Cationic Molybdenum Alkylidyne N-Heterocyclic Carbene Catalysts for Alkyne Metathesis. Organometallics 2021, 40, 1178–1184. 10.1021/acs.organomet.1c00175. [DOI] [Google Scholar]
- Hauser P. M.; Hunger M.; Buchmeiser M. R. Silica-Supported Molybdenum Alkylidyne N-Heterocyclic Carbene Catalysts: Relevance of Site Isolation to Catalytic Performance. ChemCatChem 2018, 10, 1829–1834. 10.1002/cctc.201701654. [DOI] [Google Scholar]
- Koy M.; Elser I.; Meisner J.; Frey W.; Wurst K.; Kästner J.; Buchmeiser M. R. High Oxidation State Molybdenum N-Heterocyclic Carbene Alkylidyne Complexes: Synthesis, Mechanistic Studies, and Reactivity. Chem. - Eur. J. 2017, 23, 15484–15490. 10.1002/chem.201703313. [DOI] [PubMed] [Google Scholar]
- Roland C. D.; VenkatRamani S.; Jakhar V. K.; Ghiviriga J.; Abboud K. A.; Veige A. S. Synthesis and Characterization of a Molybdenum Alkylidyne Supported by a Trianionic OCO3– Pincer Ligand. Organometallics 2018, 37, 4500–4505. 10.1021/acs.organomet.8b00677. [DOI] [Google Scholar]
- von Kugelgen S.; Piskun I.; Griffin J. H.; Eckdahl C. T.; Jarenwattananon N. N.; Fischer F. R. Templated Synthesis of End-Functionalized Graphene Nanoribbons through Living Ring-Opening Alkyne Metathesis Polymerization. J. Am. Chem. Soc. 2019, 141, 11050–11058. 10.1021/jacs.9b01805. [DOI] [PubMed] [Google Scholar]
- Fischer F. R.; Nuckolls C. Design of Living Ring-Opening Alkyne Metathesis. Angew. Chem., Int. Ed. 2010, 49 (40), 7257–7260. 10.1002/anie.201003549. [DOI] [PubMed] [Google Scholar]
- Bellone D. E.; Bours J.; Menke E. H.; Fischer F. R. Highly Selective Molybdenum ONO Pincer Complex Initiates the Living Ring-Opening Metathesis Polymerization of Strained Alkynes with Exceptionally Low Polydispersity Indices. J. Am. Chem. Soc. 2015, 137, 850–856. 10.1021/ja510919v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paley D. W.; Sedbrook D. F.; Decatur J.; Fischer F. R.; Steigerwald M. L.; Nuckolls C. Alcohol-Promoted Ring-Opening Alkyne Metathesis Polymerization. Angew. Chem., Int. Ed. 2013, 52, 4591–4594. 10.1002/anie.201300758. [DOI] [PubMed] [Google Scholar]
- Jeong H.; von Kugelgen S.; Bellone D.; Fischer F. R. Regioselective Termination Reagents for Ring-Opening Alkyne Metathesis Polymerization. J. Am. Chem. Soc. 2017, 139 (43), 15509–15514. 10.1021/jacs.7b09390. [DOI] [PubMed] [Google Scholar]
- von Kugelgen S.; Bellone D. E.; Cloke R. R.; Perkins W. S.; Fischer F. R. Initiator Control of Conjugated Polymer Topology in Ring-Opening Alkyne Metathesis Polymerization. J. Am. Chem. Soc. 2016, 138 (19), 6234–6239. 10.1021/jacs.6b02422. [DOI] [PubMed] [Google Scholar]
- Yang H.; Jin Y.; Du Y.; Zhang W. Application of Alkyne Metathesis in Polymer Synthesis. J. Mater. Chem. A 2014, 2, 5986–5993. 10.1039/c3ta14227b. [DOI] [Google Scholar]
- Huang S.; Lei Z.; Jin Y.; Zhang W. By-design molecular architectures via alkyne metathesis. Chem. Sci. 2021, 12, 9591–9606. 10.1039/D1SC01881G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X.; Laffoon J. D.; Chen D.; Pérez-Estrada S.; Danis A. S.; Rodríguez-López J.; Garcia-Garibay M. A.; Zhu J.; Moore J. S. Kinetic Control in the Synthesis of a Möbius Tris((ethynyl)[5]helicene) Macrocycle Using Alkyne Metathesis. J. Am. Chem. Soc. 2020, 142 (14), 6493–6498. 10.1021/jacs.0c01430. [DOI] [PubMed] [Google Scholar]
- Sisco S. W.; Larson B. M.; Moore J. S. Relaxing Conformational Constraints in Dynamic Macrocycle Synthesis. Macromolecules 2014, 47 (12), 3829–3836. 10.1021/ma500673x. [DOI] [Google Scholar]
- Kiel G. R.; Bay K. L.; Samkian A. E.; Schuster N. J.; Lin J. B.; Handford R. C.; Nuckolls C.; Houk K. N.; Tilley T. D. Expanded Helicenes as Synthons for Chiral Macrocyclic Nanocarbons. J. Am. Chem. Soc. 2020, 142 (25), 11084–11091. 10.1021/jacs.0c03177. [DOI] [PubMed] [Google Scholar]
- Moneypenny T. P.; Yang A.; Walter N. P.; Woods T. J.; Gray D. L.; Zhang Y.; Moore J. S. Product Distribution from Precursor Bite Angle Variation in Multitopic Alkyne Metathesis: Evidence for a Putative Kinetic Bottleneck. J. Am. Chem. Soc. 2018, 140 (17), 5825–5833. 10.1021/jacs.8b02184. [DOI] [PubMed] [Google Scholar]
- Wang Q.; Yu C.; Zhang C.; Long H.; Azarnoush S.; Jin Y.; Zhang W. Dynamic covalent synthesis of aryleneethynylene cages through alkyne metathesis: dimer, tetramer, or interlocked complex?. Chem. Sci. 2016, 7 (5), 3370–3376. 10.1039/C5SC04977F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diver S. T.; Giessert A. J. Enyne Metathesis (Enyne Bond Reorganization). Chem. Rev. 2004, 104 (3), 1317–1382. 10.1021/cr020009e. [DOI] [PubMed] [Google Scholar]
- For a rare exception, see ref (135) and the following:Chuprun S.; Acosta C. M.; Mathivathanan L.; Bukhryakov K. V. Molybdenum Benzylidyne Complexes for Olefin Metathesis Reactions. Organometallics 2020, 39 (19), 3453–3457. 10.1021/acs.organomet.0c00491. [DOI] [Google Scholar]
- Nadif S. S.; Kubo T.; Gonsales S. A.; VenkatRamani S.; Ghiviriga I.; Sumerlin B. S.; Veige A. S. Introducing “Ynene” Metathesis: Ring-Expansion Metathesis Polymerization Leads to Highly Cis and Syndiotactic Cyclic Polymers of Norbornene. J. Am. Chem. Soc. 2016, 138 (20), 6408–6411. 10.1021/jacs.6b03247. [DOI] [PubMed] [Google Scholar]
- Chaladaj W.; Corbet M.; Fürstner A. Total Synthesis of Neurymenolide A Based on a Gold-Catalyzed Synthesis of 4-Hydroxy-2-pyrones. Angew. Chem., Int. Ed. 2012, 51, 6929–6933. 10.1002/anie.201203180. [DOI] [PubMed] [Google Scholar]
- Fürstner A. Gold Catalysis for Heterocyclic Chemistry: A Representative Case Study on Pyrone Natural Products. Angew. Chem., Int. Ed. 2018, 57 (16), 4215–4233. 10.1002/anie.201707260. [DOI] [PubMed] [Google Scholar]
- Cromm P. M.; Schaubach S.; Spiegel J.; Fürstner A.; Grossmann T. N.; Waldmann H. Orthogonal Ring-Closing Alkyne and Olefin Metathesis for the Synthesis of Small GTPase-targeting Bicyclic Peptides. Nat. Commun. 2016, 7, 11300. 10.1038/ncomms11300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cromm P. M.; Wallraven K.; Glas A.; Bier D.; Fürstner A.; Ottmann C.; Grossmann T. N. Contraining an Irregular Peptide Secondary Structure through Ring-Closing Alkyne Metathesis. ChemBioChem 2016, 17, 1915–1919. 10.1002/cbic.201600362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fürstner A. trans-Hydrogenation, gem-Hydrogenation, and trans-Hydrometalation of Alkynes: An Interim Report on an Unorthodox Reactivity Paradigm. J. Am. Chem. Soc. 2019, 141 (1), 11–24. 10.1021/jacs.8b09782. [DOI] [PubMed] [Google Scholar]
- Rummelt S. M.; Radkowski K.; Roşca D.-A.; Fürstner A. Interligand Interactions Dictate the Regioselectivity of trans-Hydrometalations and Related Reactions Catalyzed by [Cp*RuCl]. Hydrogen Bonding to a Chloride Ligand as a Steering Principle in Catalysis. J. Am. Chem. Soc. 2015, 137 (16), 5506–5519. 10.1021/jacs.5b01475. [DOI] [PubMed] [Google Scholar]
- Roşca D.-A.; Radkowski K.; Wolf L. M.; Wagh M.; Goddard R.; Thiel W.; Fürstner A. Ruthenium-Catalyzed Alkyne trans-Hydrometalation: Mechanistic Insights and Preparative Implications. J. Am. Chem. Soc. 2017, 139 (6), 2443–2455. 10.1021/jacs.6b12517. [DOI] [PubMed] [Google Scholar]
- Rummelt S. M.; Preindl J.; Sommer H.; Fürstner A. Selective Formation of a Trisubstituted Alkene Motif by trans-Hydrostannation/Stille Coupling: Application to the Total Synthesis and Late-Stage Modification of 5,6-Dihydrocineromycin B. Angew. Chem., Int. Ed. 2015, 54 (21), 6241–6245. 10.1002/anie.201501608. [DOI] [PubMed] [Google Scholar]
- Huwyler N.; Radkowski K.; Rummelt S. M.; Fürstner A. Two Enabling Strategies for the Stereoselective Conversion of Internal Alkynes into Trisubstituted Alkenes. Chem. - Eur. J. 2017, 23 (50), 12412–12419. 10.1002/chem.201702470. [DOI] [PubMed] [Google Scholar]
- Reddy G. V.; Kumar R. S. C.; Siva B.; Babu K. S.; Rao J. M. Formal Total Synthesis of (−)-5,6-Dihydrocineromycine B. Synlett 2012, 23 (18), 2677–2681. 10.1055/s-0032-1317346. [DOI] [Google Scholar]
- Meng Z.; Souillart L.; Monks B.; Huwyler N.; Herrmann J.; Müller R.; Fürstner A. A “Motif-Oriented” Total Synthesis of Nannocystin Ax. Preparation and Biological Assessment of Analogues. J. Org. Chem. 2018, 83 (13), 6977–6994. 10.1021/acs.joc.7b02871. [DOI] [PubMed] [Google Scholar]
- Kwon Y.; Schulthoff S.; Dao Q. M.; Wirtz C.; Fürstner A. Total Synthesis of Disciformycin A and B: Unusually Exigent Targets of Biological Significance. Chem. - Eur. J. 2018, 24 (1), 109–114. 10.1002/chem.201705550. [DOI] [PubMed] [Google Scholar]
- Löffler L. E.; Wirtz C.; Fürstner A. Collective Total Synthesis of Casbane Diterpenes: One Strategy, Multiple Targets. Angew. Chem., Int. Ed. 2021, 60 (10), 5316–5322. 10.1002/anie.202015243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer H.; Fürstner A. Stereospecific Synthesis of Fluoroalkenes by Silver-Mediated Fluorination of Functionalized Alkenylstannanes. Chem. - Eur. J. 2017, 23 (3), 558–562. 10.1002/chem.201605444. [DOI] [PubMed] [Google Scholar]
- Sommer H.; Hamilton J. Y.; Fürstner A. A Method for the Late-Stage Formation of Ketones, Acyloins, and Aldols from Alkenylstannanes: Application to the Total Synthesis of Paecilonic Acid A. Angew. Chem., Int. Ed. 2017, 56 (22), 6161–6165. 10.1002/anie.201701391. [DOI] [PubMed] [Google Scholar]
- For a general discussion and compilation of relevant literature, see:Fürstner A. Lessons from Natural Product Total Synthesis: Macrocyclization and Postcyclization Strategies. Acc. Chem. Res. 2021, 54 (4), 861–874. 10.1021/acs.accounts.0c00759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Z.; Fürstner A. Total Synthesis of (−)-Sinulariadiolide. A Transannular Approach. J. Am. Chem. Soc. 2019, 141, 805–809. 10.1021/jacs.8b12185. [DOI] [PubMed] [Google Scholar]
- Rummelt S. M.; Fürstner A. Ruthenium-Catalyzed trans-Selective Hydrostannation of Alkynes. Angew. Chem., Int. Ed. 2014, 53, 3626–3630. 10.1002/anie.201311080. [DOI] [PubMed] [Google Scholar]
- Sommer H.; Fürstner A. Hydroxyl-Assisted Carbonylation of Alkenyltin Derivatives: Development and Application to a Formal Synthesis of Tubelactomicin A. Org. Lett. 2016, 18 (13), 3210–3213. 10.1021/acs.orglett.6b01431. [DOI] [PubMed] [Google Scholar]
- Fürstner A.; Davies P. W. Catalytic Carbophilic Activation: Catalysis by Platinum and Gold π-Acids. Angew. Chem., Int. Ed. 2007, 46, 3410–3449. 10.1002/anie.200604335. [DOI] [PubMed] [Google Scholar]
- Fürstner A. From Understanding to Prediction: Gold and Platinum-Based π-Acid Catalysis in Target Oriented Synthesis. Acc. Chem. Res. 2014, 47, 925–938. 10.1021/ar4001789. [DOI] [PubMed] [Google Scholar]
- Valot G.; Regens C. S.; O’Malley D. P.; Godineau E.; Takikawa H.; Fürstner A. Total Synthesis of Amphidinolide F. Angew. Chem., Int. Ed. 2013, 52 (36), 9534–9538. 10.1002/anie.201301700. [DOI] [PubMed] [Google Scholar]
- Valot G.; Mailhol D.; Regens C. S.; O’Malley D. P.; Godineau E.; Takikawa H.; Philipps P.; Fürstner A. Concise Total Syntheses of Amphidinolides C and F. Chem. - Eur. J. 2015, 21 (6), 2398–2408. 10.1002/chem.201405790. [DOI] [PubMed] [Google Scholar]
- Ahlers A.; de Haro T.; Gabor B.; Fürstner A. Concise Total Synthesis of Enigmazole A. Angew. Chem., Int. Ed. 2016, 55, 1406–1411. 10.1002/anie.201510026. [DOI] [PubMed] [Google Scholar]
- Gebauer K.; Fürstner A. Total Synthesis of the Biphenyl Alkaloid (−)-Lythranidine. Angew. Chem., Int. Ed. 2014, 53, 6393–6396. 10.1002/anie.201402550. [DOI] [PubMed] [Google Scholar]
- Trost B. M.; Livingston R. C. Two-metal catalyst system for redox isomerization of propargyl alcohols to enals and enones. J. Am. Chem. Soc. 1995, 117 (37), 9586–9587. 10.1021/ja00142a036. [DOI] [Google Scholar]
- Hoffmeister L.; Persich P.; Fürstner A. Formal Total Synthesis of Kendomycin by Way of Alkyne Metathesis/Gold Catalysis. Chem. - Eur. J. 2014, 20 (15), 4396–4402. 10.1002/chem.201304580. [DOI] [PubMed] [Google Scholar]
- Citreofuran was an early manifestation of this logic, then using the classical Schrock tungsten alkylidyne complex 1; see ref (49).
- Fürstner A.; Davies P. W. Heterocycles by PtCl2-Catalyzed Intramolecular Carboalkoxylation or Carboamination of Alkynes. J. Am. Chem. Soc. 2005, 127 (43), 15024–15025. 10.1021/ja055659p. [DOI] [PubMed] [Google Scholar]
- McCullough L. G.; Listemann M. L.; Schrock R. R.; Churchill M. R.; Ziller J. W. Multiple metal-carbon bonds. 34. Why terminal alkynes cannot be metathesized. Preparation and crystal structure of a deprotonated tungstacyclobutadiene complex, W(η5-C5H5)[C3(CMe3)2]Cl. J. Am. Chem. Soc. 1983, 105 (22), 6729–6730. 10.1021/ja00360a040. [DOI] [Google Scholar]
- Haberlag B.; Freytag M.; Daniliuc C. G.; Jones P. G.; Tamm M. Efficient Metathesis of Terminal Alkynes. Angew. Chem., Int. Ed. 2012, 51 (52), 13019–13022. 10.1002/anie.201207772. [DOI] [PubMed] [Google Scholar]
- For even earlier attempts with structurally undefined catalysts generated in situ, see:Coutelier O.; Mortreux A. Terminal Alkyne Metathesis: A Further Step Towards Selectivity. Adv. Synth. Catal. 2006, 348 (15), 2038–2042. 10.1002/adsc.200606116. [DOI] [Google Scholar]
- Lhermet R.; Fürstner A. Cross-Metathesis of Terminal Alkynes. Chem. - Eur. J. 2014, 20 (41), 13188–13193. 10.1002/chem.201404166. [DOI] [PubMed] [Google Scholar]
- Willwacher J.; Fürstner A. Catalysis-Based Total Synthesis of Putative Mandelalide A. Angew. Chem., Int. Ed. 2014, 53 (16), 4217–4221. 10.1002/anie.201400605. [DOI] [PubMed] [Google Scholar]
- Willwacher J.; Heggen B.; Wirtz C.; Thiel W.; Fürstner A. Total Synthesis, Stereochemical Revision, and Biological Reassessment of Mandelalide A: Chemical Mimicry of Intrafamily Relationships. Chem. - Eur. J. 2015, 21 (29), 10416–10430. 10.1002/chem.201501491. [DOI] [PubMed] [Google Scholar]
- Fürstner A.; Larionov O.; Flügge S. What is Amphidinolide V? Report on a Likely Conquest. Angew. Chem., Int. Ed. 2007, 46, 5545–5548. 10.1002/anie.200701640. [DOI] [PubMed] [Google Scholar]
- Fürstner A.; Flügge S.; Larionov O.; Takahashi Y.; Kubota T.; Kobayashi J. Total Synthesis and Biological Evaluation of Amphidinolide V and Analogues. Chem. - Eur. J. 2009, 15, 4011–4029. 10.1002/chem.200802068. [DOI] [PubMed] [Google Scholar]
- Willwacher J.; Kausch-Busies N.; Fürstner A. Divergent Total Synthesis of the Antimitotic Agent Leiodermatolide. Angew. Chem., Int. Ed. 2012, 51 (48), 12041–12046. 10.1002/anie.201206670. [DOI] [PubMed] [Google Scholar]
- Hickmann V.; Kondoh A.; Gabor B.; Alcarazo M.; Fürstner A. Catalysis-based and Protecting-Group-free Total Syntheses of the Marine Oxylipins Hybridalactone and the Ecklonialactones A, B, and C. J. Am. Chem. Soc. 2011, 133, 13471–13480. 10.1021/ja204027a. [DOI] [PubMed] [Google Scholar]
- Fürstner A.; Turet L. Concise and Practical Synthesis of Latrunculin A by Ring-Closing Enyne–Yne Metathesis. Angew. Chem., Int. Ed. 2005, 44 (22), 3462–3466. 10.1002/anie.200500390. [DOI] [PubMed] [Google Scholar]
- Fürstner A.; De Souza D.; Turet L.; Fenster M. D. B.; Parra-Rapado L.; Wirtz C.; Mynott R.; Lehmann C. W. Total Syntheses of the Actin-Binding Macrolides Latrunculin A, B, C, M, S and 16-epi-Latrunculin B. Chem. - Eur. J. 2007, 13 (1), 115–134. 10.1002/chem.200601135. [DOI] [PubMed] [Google Scholar]
- Fürstner A.; Bonnekessel M.; Blank J. T.; Radkowski K.; Seidel G.; Lacombe F.; Gabor B.; Mynott R. Total Synthesis of Myxovirescin A1. Chem. - Eur. J. 2007, 13 (31), 8762–8783. 10.1002/chem.200700926. [DOI] [PubMed] [Google Scholar]
- Micoine K.; Fürstner A. Concise Total Synthesis of the Potent Translation and Cell Migration Inhibitor Lactimidomycin. J. Am. Chem. Soc. 2010, 132 (40), 14064–14066. 10.1021/ja107141p. [DOI] [PubMed] [Google Scholar]
- Micoine K.; Persich P.; Llaveria J.; Lam M.-H.; Maderna A.; Loganzo F.; Fürstner A. Total Syntheses and Biological Reassessment of Lactimidomycin, Isomigrastatin and Congener Glutarimide Antibiotics. Chem. - Eur. J. 2013, 19 (23), 7370–7383. 10.1002/chem.201300393. [DOI] [PubMed] [Google Scholar]
- Lysenko S.; Volbeda J.; Jones P. G.; Tamm M. Catalytic Metathesis of Conjugated Diynes. Angew. Chem., Int. Ed. 2012, 51 (27), 6757–6761. 10.1002/anie.201202101. [DOI] [PubMed] [Google Scholar]
- Schnabel T. M.; Melcher D.; Brandhorst K.; Bockfeld D.; Tamm M. Unraveling the Mechanism of 1,3-Diyne Cross-Metathesis Catalyzed by Silanolate-Supported Tungsten Alkylidyne Complexes. Chem. - Eur. J. 2018, 24 (36), 9022–9032. 10.1002/chem.201801651. [DOI] [PubMed] [Google Scholar]
- Curbet I.; Colombel-Rouen S.; Manguin R.; Clermont A.; Quelhas A.; Müller D. S.; Roisnel T.; Baslé O.; Trolez Y.; Mauduit M. Expedient synthesis of conjugated triynes via alkyne metathesis. Chem. Sci. 2020, 11 (19), 4934–4938. 10.1039/D0SC01124J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungeheuer F.; Fürstner A. Concise Total Synthesis of Ivorenolide B. Chem. - Eur. J. 2015, 21, 11387–11392. 10.1002/chem.201501765. [DOI] [PubMed] [Google Scholar]
- Ralston K. J.; Ramstadius H. C.; Brewster R. C.; Niblock H. S.; Hulme A. N. Self-Assembly of Disorazole C1 through a One-Pot Alkyne Metathesis Homodimerization Strategy. Angew. Chem., Int. Ed. 2015, 54 (24), 7086–7090. 10.1002/anie.201501922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yiannakas E.; Grimes M. I.; Whitelegge J. T.; Fürstner A.; Hulme A. N. An Alkyne-Metathesis-Based Approach to the Synthesis of the Anti-Malarial Macrodiolide Samroiyotmycin A. Angew. Chem., Int. Ed. 2021, 60 (34), 18504–18508. 10.1002/anie.202105732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer A. M.; Wiedner E. S.; Gary J. B.; Gdula R. L.; Kuhlmann N. C.; Johnson M. J. A.; Dunietz B. D.; Kampf J. W. Synthetic, Mechanistic, and Computational Investigations of Nitrile-Alkyne Cross-Metathesis. J. Am. Chem. Soc. 2008, 130 (28), 8984–8999. 10.1021/ja800020w. [DOI] [PubMed] [Google Scholar]
- Gdula R. L.; Johnson M. J. A. Highly Active Molybdenum–Alkylidyne Catalysts for Alkyne Metathesis: Synthesis from the Nitrides by Metathesis with Alkynes. J. Am. Chem. Soc. 2006, 128 (30), 9614–9615. 10.1021/ja058036k. [DOI] [PubMed] [Google Scholar]
- For an indirect approach, see:Song J.; Liao Q.; Hong X.; Jin L.; Mézailles N. Conversion of Dinitrogen into Nitrile: Cross-Metathesis of N2-Derived Molybdenum Nitride with Alkynes. Angew. Chem., Int. Ed. 2021, 60 (22), 12242–12247. 10.1002/anie.202015183. [DOI] [PubMed] [Google Scholar]
- Lackner A.; Fürstner A. The Triple-Bond Metathesis of Aryldiazonium Salts: A Prospect for Dinitrogen Cleavage. Angew. Chem., Int. Ed. 2015, 54, 12814–12818. 10.1002/anie.201506546. [DOI] [PubMed] [Google Scholar]