Abstract
Background and Aim
Biliary tract infection (BTI) is an inflammatory disease and commonly associated with bacteremia. Delays in diagnosis or treatment of BTI cause high morbidity and mortality. However, an early diagnosis depends on appropriate clinical investigations. Appropriate biomarkers are urgently needed to improve the BTI diagnostic rate. We hypothesized that intestinal fatty acid‐binding protein (I‐FABP) might be a potential biomarker for BTI diagnosis.
Methods
We examined data from subjects aged ≥18 years diagnosed with BTI, including cholangitis and cholecystitis, whose blood samples were adequate for I‐FABP and zonulin assessment. We also collected blood samples from healthy volunteers as the control group. We excluded subjects in both groups who received steroids, antibiotics, or probiotics within 1 month before hospital admission (BTI cohort) or participation in this research (controls). The main study endpoint was to compare the diagnostic ability of I‐FABP to detect BTI in comparison with high‐sensitivity C‐reactive protein (hs‐CRP) and zonulin.
Results
The study collected the data of 51 patients with BTI and 35 healthy subjects. The receiver operating characteristic (ROC) area under the curve (AUC) for I‐FABP was 0.884 (95% confidence interval [CI]: 0.814–0.954), numerically higher than that for hs‐CRP (0.880; 0.785–0.976) and zonulin (0.570; 0.444–0.697). We estimated that the optimal cutoff value of I‐FABP was 2.1 ng/mL (sensitivity: 0.804; specificity: 0.829) for the diagnosis of BTI.
Conclusions
In summary, this study suggests that I‐FABP may be a potential alternative biomarker to hs‐CRP for diagnosing BTI. Further research should verify the use of I‐FABP as a marker for BTI diagnosis, but also for other inflammatory diseases.
Keywords: biliary tract infection, biomarker, diagnosis, intestinal fatty acid‐binding protein
Our study revealed the diagnostic performance of intestinal fatty acid‐binding protein (I‐FABP) was comparable to that of C‐reactive protein (CRP) and superior to zonulin for detecting biliary tract infection (BTI).
Introduction
Biliary tract infection (BTI), including cholangitis and cholecystitis, is an inflammatory disease mainly caused by bacterial infections. High morbidity and mortality in BTI are attributed to delays in diagnosis or treatment, especially in patients with BTI‐related septic shock.1 A large‐scale Asian study noted an increase in the 30‐day all‐cause mortality rate with increasing severity of acute cholangitis (low severity: 2.4%; high severity: 8.4%).2 Diagnosis of acute cholangitis used to be based solely on Charcot's cholangitis triad (jaundice, fever, and pain).3 The Tokyo Guidelines 2013 (TG13) and international practice guidelines for diagnostic criteria for acute cholangitis have been reviewed and revised in the Tokyo Guidelines 2018 (TG18).4, 5 These more sensitive diagnostic criteria include more clinical findings such as systemic inflammation markers (e.g., abnormal white blood cell [WBC] counts, increasing serum C‐reactive protein [CRP] levels), cholestasis, and imaging findings (e.g., abdominal ultrasound, computed tomography, and magnetic resonance imaging).5 However, underdiagnosis of mild or early‐stage BTI remains a challenge, especially in patients with normal WBC or CRP levels. Moreover, elevated WBC and CRP levels lack specificity for BTI, as they are also upregulated in other infectious and noninfectious diseases.6 Thus, new biomarkers are critical for the diagnosis of BTI.
Zonulin, an analog of zonula occludens toxin secreted by Vibrio cholerae, increases intestinal permeability of small bowel epitheliums by altering intestinal barrier functioning.7 Measurement of serum zonulin levels has therefore been recommended as a biomarker of intestinal permeability.8, 9 Increased intestinal permeability has been recorded in patients with sepsis, systemic inflammatory response syndrome (SIRS), or those who are critically ill,10, 11 and higher zonulin levels have been found in patients with sepsis compared with healthy subjects.12
Intestinal fatty acid‐binding protein (I‐FABP), an intracellular binding protein, plays a role in transporting long‐chain fatty acids. I‐FABP is elevated after abdominal trauma and in patients with intestinal necrosis after aortic surgery,13 and is considered to be a reliable biomarker for the detection of intestinal injury,14, 15 acute intestinal ischemia,16, 17 and gut barrier failure.18 BTI elicits acute inflammation; however, no investigation to date has evaluated the association between I‐FABP and BTI. We, therefore, sought to determine the reliability of I‐FABP in the detection of BTI and its potential as a biomarker for the diagnosis of BTI, compared with levels of high‐sensitivity C‐reactive protein (hs‐CRP) and zonulin expression.
Methods
Study design
Demographic and clinical data were obtained from 51 patients with BTI and 35 healthy volunteers (controls) attending Xiamen Chang Gung Hospital between 3 March 2020 and 27 July 2020. The study aimed to determine the diagnostic ability of I‐FABP for BTI, by comparing the sensitivity and specificity of I‐FABP with those of hs‐CRP and zonulin. CRP is listed as one of the diagnostic criteria for acute cholangitis in the TG18,5 while zonulin is a well‐known biomarker for gut permeability, which is usually elevated in older people and is associated with chronic inflammation,19, 20 as well as sepsis and SIRS.12
Ethical considerations
This study was approved by the Institutional Review Board (IRB) of Xiamen Chang Gung Hospital (IRB number: XMCGIRB2020027) and conducted according to local regulations and the ethical principles outlined in the Declaration of Helsinki. Blood samples were obtained for research purposes, and each study participant provided informed written consent.
Subjects
Eligible BTI patients were enrolled who met the following criteria: (i) aged at least 18 years at the time of blood sample collection; (ii) were diagnosed with cholangitis and cholecystitis, as according to the TG18 criteria21, 22; and (iii) residual blood samples were sufficient for quantifying I‐FABP and zonulin. Healthy subjects satisfying the following criteria were recruited: (i) aged at least 18 years and (ii) provided signed informed consent before data collection and blood samples. Study subjects were excluded if they had received steroids, antibiotics, or probiotics within 1 month before blood sample collection (BTI cohort) or participation in this research (controls).
The diagnostic criteria for acute cholangitis include: (A) systemic inflammation, such as (A‐1) fever and/or shaking chills and (A‐2) evidence of inflammatory response judged by laboratory data; (B) cholestasis, such as (B‐1) jaundice and (B‐2) abnormal liver function tests judged by laboratory data; (C) imaging, such as (C‐1) biliary dilatation and (C‐2) evidence of the etiology on imaging (stricture, stone, stent, etc.). Patients who had one item in each of (A), (B), and (C) were diagnosed with acute cholangitis.21
The diagnostic criteria of cholecystitis are as follows: (A) local signs of inflammation, including (A‐1) Murphy's sign and (A‐2) right upper quadrant mass/pain/tenderness; (B) systemic signs of inflammation, including (B‐1) fever, (B‐2) elevated CRP, and (3) elevated WBC count; (C) imaging findings, including evidence characteristic of acute cholecystitis. Patients who had one item in (A) and one item in (B + C) were diagnosed with acute cholecystitis.22
Variables
Data collected from medical charts included demographics, diagnosis, disease characteristics, laboratory results (i.e., WBC, I‐FABP, hs‐CRP, zonulin), SIRS scores, use of antibiotics, and hospitalization records. BTI severity was determined based on TG18 severity assessment criteria.21, 22
Measurement of I‐FABP and zonulin
Stored blood samples from BTI patients and freshly obtained samples from healthy subjects in ethylenediaminetetraacetic acid tubes were centrifuged at 2000 rpm for 10 min, before they were frozen and stored at −80°C. Frozen samples were analyzed in the Xiamen Chang Gung Hospital laboratory with the Human I‐FABP ELISA Kit (Elabscience, Wuhan, China) and Human Zonulin ELISA Kit (Elabscience, Wuhan, China), and I‐FABP and zonulin levels were determined using the Absorbance Microplate Reader (Molecular Devices, Shanghai, China), according to the manufacturer's instructions.
Each sample was measured once. The limit of detection for I‐FABP was 0.10 ng/mL, in the range of 0.16–10 ng/mL; the limit of detection for zonulin was 0.47 ng/mL, in the range of 0.78–50 ng/mL.
Statistical methods
We compared continuous variables using two‐sample t‐tests or Wilcoxon signed‐rank tests and compared categorical variables using the Chi‐square test. The receiver operating characteristic (ROC) curves were plotted to present the true positive rate (i.e., sensitivity) versus the false positive rate (1‐specificity) for detecting BTI with I‐FABP, hs‐CRP, and zonulin concentrations. The results of the ROC curves were demonstrated using the area under the curve (AUC) with 95% confidence intervals (CIs), as a measure of how well a parameter can distinguish between two diagnostic groups (i.e., BTI and healthy cohorts). The ROC AUC varies between 0 and 1, where a higher AUC represents a better performance.
ROC curves were also used to compare patients with varying severity of BTI (mild vs moderate/severe) with healthy subjects to evaluate the predictive ability of I‐FABP, hs‐CRP, and zonulin in detecting BTI. The odds ratio (OR) for the association between potential diagnostic biomarkers and BTI was acquired using univariate logistic regression models. A significant difference was defined as a P value <0.05. Data analyses were performed using Statistical Analysis Software (SAS®) version 9.4 (SAS Institute, Cary, NC, USA).
Results
Table 1 presents the demographics and laboratory data of the overall population (n = 86), patients with BTI (n = 51; BTI group) and healthy subjects (n = 35; control group). For the overall population, the mean age (±SD) was 48.5 ± 15.08 years, and half of the subjects were male. Serum measurements (mean ± SD) of inflammatory biomarkers included I‐FABP, hs‐CRP, zonulin, and WBC, with levels of 2.8 ± 1.91 ng/mL, 17.2 ± 45.81 mg/dL, 9.9 ± 12.96 ng/mL, and 8.2 ± 4.00 × 103/μL, respectively. In the BTI group, the levels of I‐FABP (3.8 ng/mL), hs‐CRP (22.0 mg/dL), and total bilirubin (36.2 μmol/L) were all significantly higher than corresponding values in the control group (1.4 ng/mL, P < 0.001; 1.0 mg/dL, P < 0.001; and 12.7 μmol/L, P = 0.032). No significant between‐group differences were observed for demographic characteristics, zonulin, hemoglobin, or platelet levels. Based on the physician's judgment, 42 patients had mild BTI and 9 had moderate/severe BTI. Twenty‐four patients (47.1%) had been treated with antibiotics for >7 days and 28 (54.9%) had been hospitalized for >7 days. Twenty‐four patients (47.1%) had an average SIRS score of ≥1 (data not shown).
Table 1.
Characteristics and laboratory results of patients with BTI and healthy subjects
| Overall n = 86 | BTI n = 51 | Control n = 35 | P value† | |
|---|---|---|---|---|
| Age, mean ± SD (years) | 48.5 ± 15.08 | 50.9 ± 16.63 | 44.9 ± 11.83 | 0.117 |
| Gender, male/female | 43/43 | 27/24 | 16/19 | 0.510 |
| Laboratory tests | ||||
| I‐FABP (ng/mL) | <0.001* | |||
| Mean ± SD | 2.8 ± 1.91 | 3.8 ± 1.77 | 1.4 ± 0.99 | |
| Median (IQR) | 2.4 (37.0–60.0) | 3.8 (2.4–5.0) | 1.1 (0.7–1.9) | |
| WBCs (×103/μL) | <0.001* | |||
| Mean ± SD | 8.2 ± 4.00 | 9.6 ± 4.14 | 5.4 ± 1.24 | |
| Median (IQR) | 7.0 (5.4–10.1) | 8.7 (6.8–10.8) | 5.1 (4.3–6.3) | |
| hs‐CRP (mg/dL) | <0.001* | |||
| Mean ± SD | 17.2 ± 45.81 | 22.0 ± 51.24 | 1.0 ± 1.44 | |
| Median (IQR) | 2.4 (0.8–10.2) | 3.5 (1.2–16.8) | 0.4 (0.2–0.9) | |
| Total bilirubin (μmol/L) | 0.032* | |||
| Mean ± SD | 31.1 ± 45.82 | 36.2 ± 50.62 | 12.7 ± 3.84 | |
| Median (IQR) | 15.4 (10.9–25.5) | 17.6 (10.9–30.4) | 12.0 (10.2–14.5) | |
| Zonulin (ng/mL) | 0.274 | |||
| Mean ± SD | 9.9 ± 12.96 | 9.5 ± 14.22 | 10.5 ± 11.08 | |
| Median (IQR) | 5.9 (1.5–17.9) | 3.6 (0.3–22.1) | 11.0 (3.3–14.8) | |
| Hemoglobin (g/L) | 0.854 | |||
| Mean ± SD | 134.5 ± 23.31 | 134.5 ± 24.06 | 134.1 ± 21.15 | |
| Median (IQR) | 139.0 (125.0–147.0) | 139.0 (125.0–146.0) | 133.5 (125.0–149.0) | |
| Platelets (×103/μL) | 0.570 | |||
| Mean ± SD | 236.9 ± 58.86 | 234.7 ± 61.89 | 244.9 ± 47.31 | |
| Median (IQR) | 239.0 (201.0–269.0) | 240.0 (192.0–272.0) | 238.0 (222.0–261.0) | |
Significant difference.
To compare differences between the BTI and control groups, two‐sample t‐test or Wilcoxon signed‐rank test was applied for continual variables; Chi‐square test was applied for categorical variables.
BTI, biliary tract infection; hs‐CRP, high‐sensitivity C‐reactive protein; I‐FABP, intestinal fatty acid‐binding protein; IQR, interquartile range; WBC, white blood cells.
Prognostic ability of I‐FABP, hs‐CRP, and zonulin for BTI detection
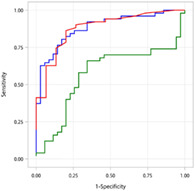
ROC AUC curves for detecting BTI with I‐FABP, hs‐CRP, and zonulin are illustrated in Figure 1. The predictive ability of I‐FABP seemed to be comparable to that of hs‐CRP and superior to zonulin. Among all the biomarkers, the highest ROC AUC was observed with I‐FABP (0.884 vs 0.880 for hs‐CRP and 0.570 for zonulin). The optimal cutoff value of I‐FABP was estimated to be 2.1 ng/mL in the diagnosis of BTI, with a sensitivity of 0.804 and a specificity of 0.829 (Table 2).
Figure 1.
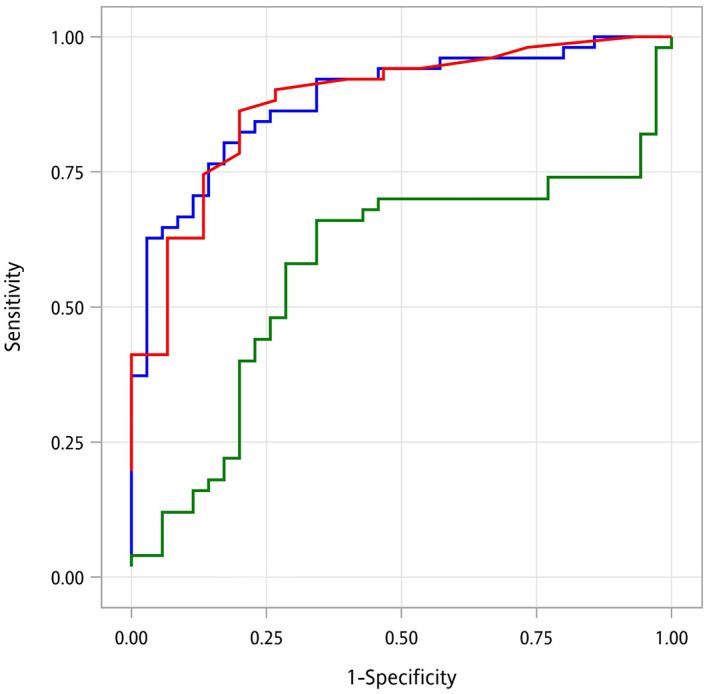
ROC AUC curves for I‐FABP, hs‐CRP, and zonulin for the prediction of BTI. AUC, area under the curve; BTI, biliary tract infection; hs‐CRP, high‐sensitivity C‐reactive protein; I‐FABP, intestinal fatty acid‐binding protein; ROC, receiver operating characteristic.  , AUC of iFABP: 0.884;
, AUC of iFABP: 0.884;  , AUC of Zonulin: 0.57;
, AUC of Zonulin: 0.57;  , AUC of Hs‐CRP: 0.88.
, AUC of Hs‐CRP: 0.88.
Table 2.
Sensitivity and specificity of I‐FABP, hs‐CRP, and zonulin for diagnosis of BTI
| Index | AUCROC | Cutoff | Sensitivity | Specificity | 95% CI | PPV | NPV |
|---|---|---|---|---|---|---|---|
| I‐FABP | 0.884 | 2.1 | 0.804 | 0.829 | 0.814–0.954 | 0.872 | 0.744 |
| hs‐CRP | 0.880 | 1.0 | 0.863 | 0.800 | 0.785–0.976 | 0.963 | 0.632 |
| Zonulin | 0.570 | 6.9 | 0.660 | 0.657 | 0.444–0.697 | 0.733 | 0.575 |
AUCROC, area under ROC curves; BTI, biliary tract infection; CI, confidence interval; hs‐CRP, high‐sensitivity C‐reactive protein; I‐FABP, intestinal fatty acid‐binding protein; NPV, negative predictive value; PPV, positive predictive value; ROC, receiver operating characteristic.
We also sought to determine whether the diagnostic abilities of I‐FABP, hs‐CRP, and zonulin differed among patients with different severity of BTI (Fig. 2). We found that the ROC AUC for I‐FABP in the detection of BTI was 0.861 for mild BTI and 0.994 for moderate/severe BTI, both of which were comparable to the ROC AUC values for hs‐CRP (mild BTI: 0.859; moderate/severe BTI: 0.981) and higher than zonulin (mild BTI: 0.559; moderate/severe BTI: 0.622). The results suggested that I‐FABP performed similarly to hs‐CRP in identifying BTI, regardless of disease severity, which may shed light on its application on early diagnosis (i.e., mild BTI).
Figure 2.
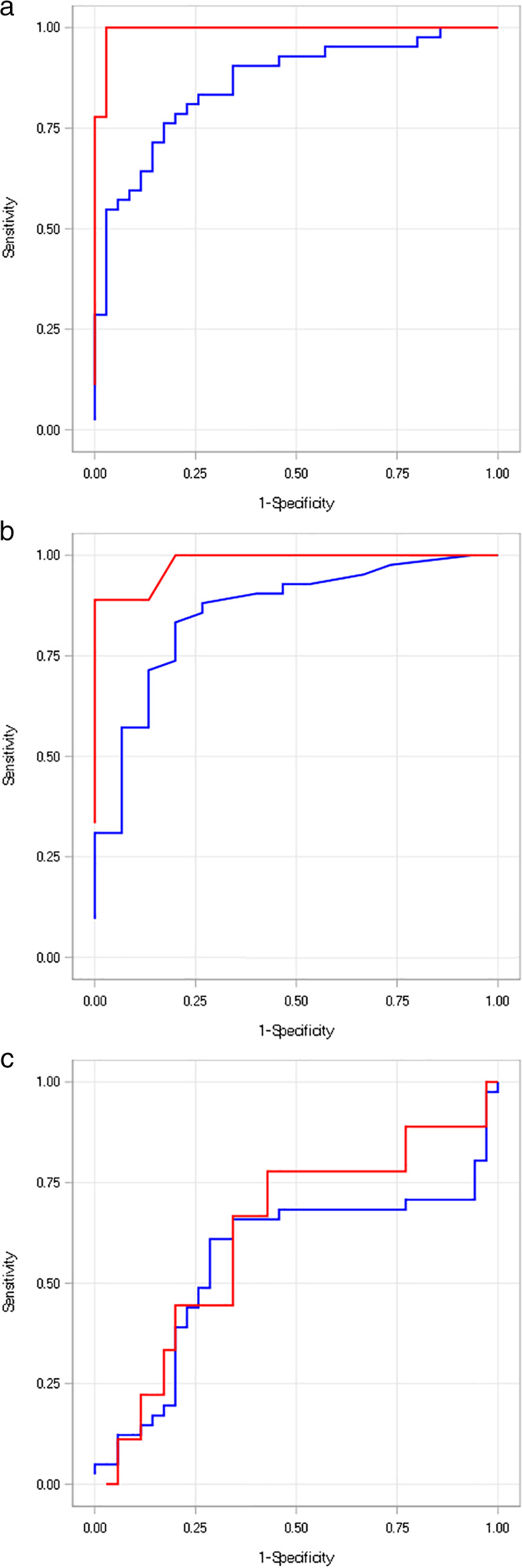
ROC AUC curves for I‐FABP, hs‐CRP, and zonulin for evaluating the predictive ability to diagnose patients with different severities of BTI compared with healthy subjects. (a) I‐FABP.  , AUC of mild: 0.861;
, AUC of mild: 0.861;  , AUC of moderate/severe 0.994. (b) Hs‐CRP.
, AUC of moderate/severe 0.994. (b) Hs‐CRP.  , AUC of mild: 0.859;
, AUC of mild: 0.859;  , AUC of moderate/severe, 0.981. (c) Zonulin. Forty‐two patients had mild BTI; 9 had moderate/severe BTI.
, AUC of moderate/severe, 0.981. (c) Zonulin. Forty‐two patients had mild BTI; 9 had moderate/severe BTI.  , AUC of mild: 0.559;
, AUC of mild: 0.559;  , AUC of moderate/severe, 0.622. AUC, area under the curve; BTI, biliary tract infection; hs‐CRP, high‐sensitivity C‐reactive protein; I‐FABP, intestinal fatty acid‐binding protein; ROC, receiver operating characteristic.
, AUC of moderate/severe, 0.622. AUC, area under the curve; BTI, biliary tract infection; hs‐CRP, high‐sensitivity C‐reactive protein; I‐FABP, intestinal fatty acid‐binding protein; ROC, receiver operating characteristic.
Associations between BTI and I‐FABP, hs‐CRP, and zonulin
Table 3 shows the outcomes of logistic regression analyses predicting the associations between potential biomarkers and BTI. I‐FABP (OR: 3.36; 95 CI: 2.02–5.59; P < 0.001) and hs‐CRP (2.04; 1.06–3.93; P = 0.032) were significantly associated with the diagnosis of BTI, whereas zonulin was not (0.99; 0.96–1.03; P = 0.725).
Table 3.
Logistic regression model for diagnosis of BTI
| Odds ratio | 95% CI | P value† | |
|---|---|---|---|
| I‐FABP | 3.36 | 2.02–5.59 | <0.001* |
| hs‐CRP | 2.04 | 1.06–3.93 | 0.032* |
| Zonulin | 0.99 | 0.96–1.03 | 0.725 |
Significant difference.
Logistic regression models.
BTI, biliary tract infection; CI, confidence interval; hs‐CRP, high‐sensitivity C‐reactive protein; I‐FABP, intestinal fatty acid‐binding protein.
Discussion
This research investigated the potential of I‐FABP serving as a diagnostic biomarker for BTI. According to the study's ROC AUC evidence, I‐FABP discriminates in favor of BTI, with an optimal cutoff value of 2.1 ng/mL. The diagnostic ability of I‐FABP was comparable to that of hs‐CRP and numerically greater than zonulin, regardless of disease severity. Univariate logistic regression analysis demonstrated a strong association between I‐FABP and BTI. These study data indicate that I‐FABP may be a useful additional biochemical marker to hs‐CRP for diagnosis of inflammatory diseases. Moreover, an assessment of I‐FABP expression might assist with a physician diagnosis of BTI in individuals without clear signs of systemic inflammation, especially in those with early‐stage disease.
Research has established that serum I‐FABP concentrations of 2 ng/mL or less can be used as normal reference values for healthy Japanese and can be used to differentiate between them and patients with intestinal diseases.23 This reference value is consistent with the estimated cutoff value of 2.1 ng/mL for serum I‐FABP concentration in the diagnosis of BTI in our study. However, the application of serum I‐FABP concentration values may vary from one race of people to another. For instance, lower serum I‐FABP concentrations have been observed in Western countries compared with our data (patients with BTI: 3.8 ng/mL; healthy subjects: 1.4 ng/mL), with research from Poland recording mean serum I‐FABP concentrations of 166.9 pg/mL in patients with ulcerative colitis and 61.3 pg/mL in healthy individuals,24 while German research has documented mean serum I‐FABP concentrations of 2101.0 pg/mL in patients with severe intestinal injury and 351.4 pg/mL in healthy individuals.25 Moreover, Uzun et al.13 and Güzel et al.26 have recorded cutoff values of I‐FABP for acute mesenteric ischemia of >90–144.9 pg/mL in Turkey, mostly based on patients with mild BTI.
To our knowledge, this study is the first to investigate the diagnostic ability of I‐FABP for BTI and may provide useful clinical reference data for physicians. However, some limitations exist. Firstly, as I‐FABP examinations were not regularly performed as a part of routine practice, we could only enroll BTI patients who had sufficient residual blood samples, which limited our sample size. Our findings should accordingly be interpreted with caution, and they require validation in a large‐scale study. Secondly, this study was conducted at a single center, restricting its generalizability. Lastly, we did not investigate the difference in I‐FABP concentrations between BTI and other abdominal inflammatory diseases. The specificity of I‐FABP for diagnosing BTI should be investigated further.
Conclusion
In summary, the evidence from this study suggests that I‐FABP may serve as a potential additional diagnostic biomarker to hs‐CRP for diagnosis of inflammatory diseases. Although these findings should be verified by further studies, I‐FABP assessment may be an appropriate marker for BTI diagnosis and may provide more diagnostic information in patients with normal CRP levels.
ACKNOWLEDGMENTS
The authors acknowledge assistance from the CRO Division of Formosa Biomedical Technology Corporation, Taipei, Taiwan, for statistical analysis and editorial support. Guiding project of Xiamen science and Technology Bureau, grant number: 3502Z20209098.
Declaration of conflict of interest: The authors declare that they have no competing interests.
Author contribution: Conceptualization, Chih‐Jung Chang and Yang‐Bor Lu. Data curation, Yu‐Chieh Weng, Jung‐Chieh Lee, and Yung‐Ning Huang. Formal analysis, Hui‐Shan Hsieh. Funding acquisition, Yang‐Bor Lu. Methodology, Yang‐Bor Lu and Chih‐Jung Chang. Project administration, Wei‐Ting Chen and Yang‐Bor Lu. Visualization, Hui‐Shan Hsieh. Writing—original draft, Yu‐Chieh Weng and Yang‐Bor Lu. Writing—review and editing: Yang‐Bor Lu. Approval of final manuscript: all authors.
Contributor Information
Chih‐Jung Chang, Email: chan.chih.jung@gmail.com.
Yang‐Bor Lu, Email: bentleylu@gmail.com.
REFERENCES
- 1.Melzer M, Toner R, Lacey S, Bettany E, Rait G. Biliary tract infection and bacteraemia: presentation, structural abnormalities, causative organisms and clinical outcomes. Postgrad. Med. J. 2007; 83: 773–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gomi H, Takada T, Hwang TLet al. Updated comprehensive epidemiology, microbiology, and outcomes among patients with acute cholangitis. J. Hepatobiliary Pancreat. Sci. 2017; 24: 310–18. [DOI] [PubMed] [Google Scholar]
- 3.Rumsey S, Winders J, MacCormick AD. Diagnostic accuracy of Charcot's triad: a systematic review. ANZ J. Surg. 2017; 87: 232–8. [DOI] [PubMed] [Google Scholar]
- 4.Kiriyama S, Takada T, Strasberg SMet al. TG13 guidelines for diagnosis and severity grading of acute cholangitis (with videos). J. Hepatobiliary Pancreat. Sci. 2013; 20: 24–34. [DOI] [PubMed] [Google Scholar]
- 5.Kiriyama S, Kozaka K, Takada Tet al. Tokyo Guidelines 2018: diagnostic criteria and severity grading of acute cholangitis (with videos). J. Hepatobiliary Pancreat. Sci. 2018; 25: 17–30. [DOI] [PubMed] [Google Scholar]
- 6.Ali FT, Ali MA, Elnakeeb MM, Bendary HN. Presepsin is an early monitoring biomarker for predicting clinical outcome in patients with sepsis. Clin. Chim. Acta Int. J. Clin. Chem. 2016; 460: 93–101. [DOI] [PubMed] [Google Scholar]
- 7.Wang W, Uzzau S, Goldblum SE, Fasano A. Human zonulin, a potential modulator of intestinal tight junctions. J. Cell Sci. 2000; 113(24): 4435–40. [DOI] [PubMed] [Google Scholar]
- 8.Sapone A, de Magistris L, Pietzak Met al. Zonulin upregulation is associated with increased gut permeability in subjects with type 1 diabetes and their relatives. Diabetes. 2006; 55: 1443–9. [DOI] [PubMed] [Google Scholar]
- 9.Smecuol E, Sugai E, Niveloni Set al. Permeability, zonulin production, and enteropathy in dermatitis herpetiformis. Clin. Gastroenterol. Hepatol. 2005; 3: 335–41. [DOI] [PubMed] [Google Scholar]
- 10.Doig CJ, Sutherland LR, Sandham JD, Fick GH, Verhoef M, Meddings JB. Increased intestinal permeability is associated with the development of multiple organ dysfunction syndrome in critically ill ICU patients. Am. J. Respir. Crit. Care Med. 1998; 158: 444–51. [DOI] [PubMed] [Google Scholar]
- 11.Herbert MK, Holzer P. Standardized concept for the treatment of gastrointestinal dysmotility in critically ill patients—current status and future options. Clin. Nutr. 2008; 27: 25–41. [DOI] [PubMed] [Google Scholar]
- 12.Klaus DA, Motal MC, Burger‐Klepp Uet al. Increased plasma zonulin in patients with sepsis. Biochem. Med. 2013; 23: 107–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Uzun O, Turkmen S, Eryigit Uet al. Can intestinal fatty acid binding protein (I‐FABP) be a marker in the diagnosis of abdominal pathology? Turk. J. Emerg. Med. 2014; 14: 99–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pelsers MM, Namiot Z, Kisielewski Wet al. Intestinal‐type and liver‐type fatty acid‐binding protein in the intestine. Tissue distribution and clinical utility. Clin. Biochem. 2003; 36: 529–35. [DOI] [PubMed] [Google Scholar]
- 15.Funaoka H, Kanda T, Kajiura S, Ohkaru Y, Fujii H. Development of a high‐specificity sandwich ELISA system for the quantification of human intestinal fatty acid‐binding protein (I‐FABP) concentrations. Immunol. Invest. 2011; 40: 223–42. [DOI] [PubMed] [Google Scholar]
- 16.Kanda T, Fujii H, Fujita M, Sakai Y, Ono T, Hatakeyama K. Intestinal fatty acid binding protein is available for diagnosis of intestinal ischaemia: immunochemical analysis of two patients with ischaemic intestinal diseases. Gut. 1995; 36: 788–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Girish TU, Hegde A. Intestinal fatty acid binding protein (I‐FABP) as a marker for acute intestinal ischemia. Int. Surg. J. 2019; 6(2): 374. [Google Scholar]
- 18.Tornai T, Palyu E, Vitalis Zet al. Gut barrier failure biomarkers are associated with poor disease outcome in patients with primary sclerosing cholangitis. World J. Gastroenterol. 2017; 23: 5412–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sturgeon C, Fasano A. Zonulin, a regulator of epithelial and endothelial barrier functions, and its involvement in chronic inflammatory diseases. Tissue Barriers. 2016; 4: e1251384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qi Y, Goel R, Kim Set al. Intestinal permeability biomarker zonulin is elevated in healthy aging. J. Am. Med. Dir. Assoc. 2017; 18: 810.e1–4. 810.e811‐810.e814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gomi H, Solomkin JS, Schlossberg Det al. Tokyo Guidelines 2018: antimicrobial therapy for acute cholangitis and cholecystitis. J. Hepatobiliary Pancreat. Sci. 2018; 25: 3–16. [DOI] [PubMed] [Google Scholar]
- 22.Yokoe M, Hata J, Takada Tet al. Tokyo Guidelines 2018: diagnostic criteria and severity grading of acute cholecystitis (with videos). J. Hepatobiliary Pancreat. Sci. 2018; 25: 41–54. [DOI] [PubMed] [Google Scholar]
- 23.Funaoka H, Kanda T, Fujii H. Intestinal fatty acid‐binding protein (I‐FABP) as a new biomarker for intestinal diseases. Rinsho byori . Jpn. J. Clin. Pathol. 2010; 58: 162–8. [PubMed] [Google Scholar]
- 24.Wiercinska‐Drapalo A, Jaroszewicz J, Siwak E, Pogorzelska J, Prokopowicz D. Intestinal fatty acid binding protein (I‐FABP) as a possible biomarker of ileitis in patients with ulcerative colitis. Regul. Pept. 2008; 147: 25–8. [DOI] [PubMed] [Google Scholar]
- 25.Voth M, Duchene M, Auner B, Lustenberger T, Relja B, Marzi I. I‐FABP is a novel marker for the detection of intestinal injury in severely injured trauma patients. World J. Surg. 2017; 41: 3120–7. [DOI] [PubMed] [Google Scholar]
- 26.Güzel M, Sözüer EM, Salt Ö, İkizceli İ, Akdur O, Yazıcı C. The value of the serum I‐FABP level for diagnosing acute mesenteric ischemia. Surg. Today. 2014; 44: 2072–6. [DOI] [PubMed] [Google Scholar]


