Abstract
Background:
Despite advances in surgical techniques, the use of maximal repair to treat large or massive rotator cuff tears results in a high retear rate postoperatively. Currently, no randomized controlled trials have compared the outcomes of maximal repair with interposition dermal allograft bridging reconstruction.
Hypothesis:
We hypothesized that large or massive rotator cuff tendon tears reconstructed using bridging dermal allograft would have better clinical outcomes 2 years postoperatively, as measured using the Western Ontario Rotator Cuff (WORC) index, than would those receiving the current gold standard treatment of debridement and maximal repair alone. We also expected that patients treated via bridging reconstruction using dermal allograft would have fewer postoperative failures as assessed using postoperative magnetic resonance imaging scans.
Study Design:
Randomized controlled trial; Level of evidence 1.
Methods:
A sample size of 30 patients (determined using a priori sample size calculation) with massive, retracted rotator cuff tears were randomly allocated to 1 of 2 groups: maximal repair or bridging reconstruction using dermal allograft. All patients completed questionnaires (WORC and Disabilities of the Arm, Shoulder and Hand [DASH]) preoperatively and postoperatively at 3 months, 6 months, 1 year, and 2 years. The primary outcome of this study was the WORC index at 2 years. Secondary outcomes included healing rate, progression of rotator cuff arthropathy, and postoperative acromiohumeral distance in both groups.
Results:
Patients treated via bridging reconstruction using dermal allograft had better postoperative WORC and DASH scores (23.93 ± 24.55 and 15.77 ± 19.27, respectively) compared with patients who received maximal repair alone (53.36 ± 31.93 and 34.32 ± 23.31, respectively). We also noted increased progression to rotator cuff arthropathy in the maximal repair group with an increased retear rate when compared with the reconstruction group (87% and 21%, respectively; P < .001). The acromiohumeral distance was maintained in the reconstruction group but significantly decreased in the maximal repair group.
Conclusion:
Rotator cuff bridging reconstruction using a dermal allograft demonstrated improved patient-reported outcomes as measured using the WORC index 2 years postoperatively. This technique also showed favorable structural healing rates and decreased progression to arthropathy compared with maximal repair.
Trial Registration:
ClinicalTrials.gov (NCT01987973)
Keywords: massive rotator cuff tear, maximal repair, bridging reconstruction, interposition graft, dermal allograft, patient-reported outcome
Injuries to the rotator cuff are some of the most common shoulder injuries. Degenerative or chronic rotator cuff tears often result from an age-related progression, whereas others can be classified as acute rotator cuff tears resulting from a specific incident. Along with their specific onset, rotator cuff tears are also often classified by their anterior to posterior size and by the number of involved tendons. Tear sizes of <1, 1-3, 3-5, and >5 cm are considered small, medium, large, and massive, respectively.31
Maximal repair of large and massive rotator cuff tears has been the gold standard treatment.3,6,16,35 This treatment results in pain relief and improved function in the short term, but the retear rates remain high, ranging from 50% to 90%.1,6,16 The retear rates are correlated with the size of the rotator cuff tear as well as other factors including muscle atrophy, tendon quality, and postoperative rehabilitation protocol.32 The general concept behind using maximal repair, also known as partial repair, is to re-create the rotator cable to regain the function of the rotator cuff in a construct that resembles a suspension bridge. This concept was first described by Burkhart et al10 in 1993. This technique puts increased tension on the areas being repaired, and most failures of rotator cuff repairs are at the tendon-anchor interface.39,43
In an effort to improve healing rates, a number of techniques have been introduced to manage rotator cuff tears.2 One recently developed and highly used technique is superior capsular reconstruction. Originally, this technique used fascia lata autograft, and the original publication by Mihata et al38 cited improved patient-reported outcome scores and range of motion. North American surgeons have preferred the use of allografts as popularized by Burkhart et al9,11 and have not reproduced the same positive outcomes as the results of Mihata et al. Despite low-level evidence, many surgeons continue to use superior capsular reconstruction as a treatment option for patients with massive cuff tears.9,48 Included in these options is the use of acellular dermal allografts that can be used for interposition or bridging reconstruction. It is thought that using dermal allograft to perform bridging reconstruction can contribute to increasing load to failure and that this technique provides a superior suture pull-out strength compared with that of the native remnant cuff.1,4 In addition, acellular dermal allograft can act as a scaffold to allow healing to occur from the cuff remnant to the graft and from the graft to the bone.21,24 Few surgeons have performed bridging dermal allograft reconstructions, and only a few low-level studies have been published on patient outcomes.‡
As mentioned, orthopaedic surgeons have used dermal allograft implants to perform bridging reconstruction to improve the outcomes for rotator cuff tears.§ Early studies using this technique demonstrated favorable patient outcomes in small patient cohorts.8,41 In a level 4 study, Wong et al49 assessed 45 patients with massive rotator cuff tears that were managed using arthroscopic bridging dermal allograft reconstruction. At the 2-year follow-up, it was found that patients treated using bridging reconstruction had improved Western Ontario Rotator Cuff (WORC) scores, American Shoulder and Elbow Surgeon (ASES) questionnaire scores, and University of California–Los Angeles (UCLA) scores postoperatively. This study demonstrated that bridging reconstruction is a safe procedure with high patient satisfaction. A systematic review demonstrated that using a graft for an anatomic bridging rotator cuff reconstruction resulted in improved function on objective testing and was functionally better than was nonanatomic or partial repair of large or massive rotator cuff tears.32 A prospective study on open bridging reconstruction in patients with irreparable cuff tears reported better outcomes for patients treated using this technique compared with maximal repair alone.42 Three other studies have reported on the healing of various interposition bridging reconstruction techniques (open, arthroscopic, and mini-open) with variable healing results.18,40,45 We are unaware of any randomized controlled trials comparing bridging reconstruction using dermal allograft versus maximal repair alone in the context of treating large or massive rotator cuff tears.
We hypothesized that large or massive rotator cuff tears reconstructed using bridging dermal allograft would have better patient-reported outcome scores 2 years postoperatively than would those treated using the current gold standard treatment of debridement and maximal repair alone. We also hypothesized that patients who received bridging reconstruction would have superior healing rates because we believed that the graft would distribute the load more evenly than would the anchor points of the maximal repair. This study presents the clinical and radiographic outcomes of our randomized controlled trial comparing bridging reconstruction versus maximal repair in patients with large or massive rotator cuff tears.
Methods
This was a single-center, blinded-observer, randomized controlled trial in which 30 patients were prospectively enrolled at the Halifax Infirmary, Queen Elizabeth II Health Sciences Centre in Halifax, Nova Scotia, Canada. All of the patients were enrolled prospectively between 2014 and 2017 (REB Approval No. 1015440). Eligible participants were recruited between January 2015 and March 2018 and attended clinical follow-ups at baseline preoperatively and at 6 weeks, 3 months, 6 months, 12 months, and 24 months postoperatively, as per current clinical practice. The study was registered with ClinicalTrials.gov (NCT01987973). This study was funded by Dalhousie University Department of Surgery. The sample size was based on a priori sample size calculations regarding differences in WORC score at the 2-year follow-up. In accordance with data published by Kirkley et al,29 we used a clinical difference of 15% (or 15 points on a 100-point scale) to calculate the sample size with a power of 80% and an alpha of .05. Our calculations showed that we required 15 patients per group to show a 15-point difference in means between WORC scores in each treatment group at the 2-year follow-up.
Inclusion Criteria
The inclusion criteria entailed magnetic resonance imaging (MRI)–proven diagnosis of a large or massive (>3 cm) 2-tendon (supraspinatus and infraspinatus) tear of the rotator cuff in a competent adult (>18 years of age).
Exclusion Criteria
Patients with any of the following conditions were excluded:
Uncontrolled diabetes (hemoglobin A1C >7%)
Pregnancy
Presence of local or systemic infection
Inability to cooperate with and/or comprehend postoperative instructions
Nonvascular surgical sites (MRI proven)
Poor nutritional status (albumin <30 g/L)
Cancer
Paralysis of the shoulder
Contracture of the shoulder
Cuff tear arthropathy or osteoarthritis of the shoulder
Inability to provide informed consent for the study
Patients with large or massive rotator cuff tears were approached by the primary investigator (I.W.) regarding the study during a clinical office visit. After a detailed discussion of the risks, benefits, and alternatives of the study, we provided the patient with a copy of the informed consent to review if he or she demonstrated interest in the study. If the patient then wanted to be enrolled in the study, they provided informed consent to a member of the research team as per Division of Orthopaedic Surgery and Nova Scotia Health Authority standard operating procedures.
Preoperatively, routine radiographs including anteroposterior (AP) and Y views of the shoulder and an MRI scan were obtained for all patients enrolled in the study. Patients were not exposed to any additional radiation. All patients completed a structured clinical examination conducted by a sports medicine fellowship–trained orthopaedic surgeon (C.M.C.). The examination consisted of range of motion testing (including forward flexion, lateral elevation, and internal and external rotation) via goniometer and strength testing via handheld dynamometer (Hoggan Scientific, Salt Lake City, UT). Each patient also completed the WORC and Disabilities of the Arm, Shoulder and Hand (DASH) questionnaires.22,23,27,28,33,44
Randomization and Surgical Technique
All patients underwent a 15-point arthroscopic shoulder examination and arthroscopic acromioplasty as per the technique outlined by Snyder et al.47 The bursal side of the tear was evaluated, and the tear dimensions were measured in both AP and mediolateral planes (see the online Video Supplement for this technique). At this stage, the randomization was done using a computerized random number generator. The patients enrolled in the maximal repair group underwent single-row rotator cuff repair performed under adequate tension as described by Dini and Snyder14 with re-creation of the rotator cable as described by Burkhart et al10 (Healicoil tripled-loaded suture anchors; Smith & Nephew) (Figure 1). The biceps tendon was left intact if there was no damage, but a biceps tenotomy was performed if there was >50% tear. The patients randomized to the reconstruction group underwent maximal repair performed along with a rotator cuff reconstruction using a dermal allograft tissue (GraftJacket; Wright Medical) as described by the senior author (I.W.) previously49 (Figure 2).
Figure 1.

Intraoperative images showing final construction after maximal repair of a left shoulder. (A) View from the lateral portal showing repair to the anterior anchor. The defect is shown between the anterior and posterior anchors, with margin convergence sutures in between. (B) View from the same portal; microfracture site of the greater tuberosity is visible and was used to create a crimson duvet. The defect is also visible from this view.
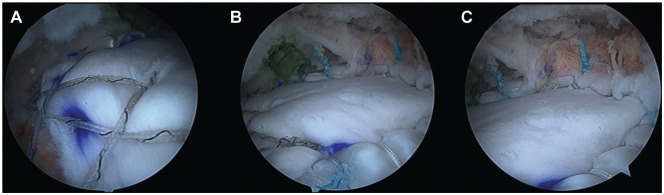
Figure 2.
Intraoperative images of final bridging reconstruction using dermal allograft. (A) View of lateral attachment of graft. (B) View of middle graft. (C) View of graft attachment to remaining cuff. All views are from the posterolateral portal.
Postoperative Protocol
The shoulder was immobilized in a sling for 6 to 8 weeks postoperatively, and only passive forward flexion and external rotation were allowed for the next 4 weeks. The patients then progressed to active assisted and active shoulder motion for another 4 weeks. Strengthening began at 16 weeks postoperatively.
Patients followed up with the attending surgeon (I.W.) at 2 weeks postoperatively for a wound check. Further follow-up appointments occurred 6 weeks, 3 months, 6 months, 12 months, and 24 months postoperatively, as per current clinical practice. At the 3-, 6-, 12-, and 24-month visits, the patients underwent a structured clinical examination conducted by a sports medicine fellowship–trained orthopaedic surgeon who was blinded to the patients’ treatment group (C.M.C.). The patients also completed the WORC and DASH questionnaires at these follow-up appointments. Routine postoperative AP radiographs were obtained 2 weeks, 12 months, and 24 months postoperatively. A musculoskeletal radiologist (J.P.K.) reviewed these radiographs and measured the acromiohumeral distance (AHD) and progression of rotator cuff arthropathy (RCA) for each patient. RCA was graded as per the classification method suggested by Hamada et al.20 A postoperative 1.5-T MRI scans was obtained for each patient at the 12-month postoperative time point, as this was previously established as a sufficient timepoint to assess tendon healing.24 Postoperative MRI scans were reviewed by a musculoskeletal radiologist who was aware that each patient had undergone treatment for a massive rotator cuff tear but did not know which treatment each patient received (J.P.K.). Retears were defined as tears that were larger than those at baseline on preoperative MRI scans with no cuff attached at the level of the anchors.
Analysis of Data
The paired-samples t test was used to test for differences within each group in terms of WORC scores, DASH scores, and healing rates. Changes in WORC and DASH scores were assessed using the independent 2-sample t test. The chi-square test was performed to determine the difference between the 2 groups regarding the incidence of failure and to assess the number of patients in each group who met the minimal clinically important difference (MCID) for both WORC (MCID = 15)29 and DASH (MCID = 10.83).15 Repeated-measures analysis of variance was used to perform a within-subjects analysis to assess differences in WORC scores within each group between time points. An analysis of covariance was conducted to determine whether there was a statistically significant covariate effect of sex on the postoperative outcome (WORC, DASH, and AHD). All of the statistical tests were performed using α = .05. Due to the small sample size of each group, the Hedges g was used as an effect size measure for continuous data, and the Cramer V was used as an effect size measure for categorical data. For the Hedges g, an effect size ≤0.2 was considered small, between 0.2 and 0.8 was considered medium, and ≥0.8 was considered large. For the Cramer V, a value <0.3 was considered a weak association, between 0.3 and 0.5 was considered a moderate association, and >0.5 was considered a strong association.
Results
A total of 30 patients were recruited and randomized to either the maximal repair group (n = 15) or the bridging reconstruction group (n = 15). In the reconstruction group, one patient did not complete follow-up, bringing the final sample size at analysis to 29. Descriptive characteristics were similar in both groups with the exception of the distribution of males and females in each group (Table 1). All patients in the maximal repair group received partial repair because their cuffs were not repairable.
Table 1.
Descriptive Information
| Maximal Repaira (n = 15) | Bridging Reconstructionb (n = 14) | P Value (α = .05) | |
|---|---|---|---|
| Time after surgery, y, mean ± SD | 3.72 ± 0.72 | 3.34 ± 0.90 | .219 |
| Age, y, mean ± SD | 60.86 ± 6.67 | 59.10 ± 8.81 | .550 |
| Sex, n | |||
| Male | 13 | 6 | .013 |
| Female | 2 | 8 | |
| Side, n | |||
| Left | 5 | 3 | .474 |
| Right | 10 | 11 | |
All repairs resulted in partial repair.
One patient was lost to follow-up.
At the 2-year follow-up, there was a significant difference from preoperatively in DASH scores in the repair group (P = .007; Hedges g = 0.569) but no difference in the WORC scores in the repair group (P = .087; Hedges g = 0.484). Patients in the reconstruction group had improved WORC and DASH scores postoperatively (P < .001 for both). These results are summarized in Table 2.
Table 2.
Comparison of Pre- and Postoperative WORC and DASH Scores in Each Groupa
| Group | Score | Mean ± SD | P Value (α = .05) | Effect Size (Hedges g) |
|---|---|---|---|---|
| Maximal repair | Preoperative WORC | 66.10 ± 19.15 | .087 | 0.484 |
| Postoperative WORC | 53.36 ± 31.93 | |||
| Preoperative DASH | 46.67 ± 19.99 | .007 | 0.569 | |
| Postoperative DASH | 34.32 ± 23.31 | |||
| Bridging reconstruction | Preoperative WORC | 65.36 ± 18.73 | <.001 | 1.897 |
| Postoperative WORC | 23.93 ± 24.55 | |||
| Preoperative DASH | 48.96 ± 19.77 | <.001 | 1.700 | |
| Postoperative DASH | 15.77 ± 19.27 |
DASH, Disabilities of the Arm, Shoulder and Hand; WORC, Western Ontario Rotator Cuff.
When assessing changes in WORC and DASH scores, we observed a significant difference between the repair and reconstruction groups (Table 3).
Table 3.
Comparison of ΔWORC and ΔDASH Scores in Each Groupa
| Group | ΔWORC | ΔDASH |
|---|---|---|
| Maximal repair, mean ± SD | −12.74 ± 26.77 | −12.35 ± 13.05 |
| Bridging reconstruction, mean ± SD | −41.42 ± 27.02 | −33.19 ± 22.40 |
| P value (α = .05) | .008 | .012 |
| Effect size (Hedges g) | 1.067 | 1.147 |
DASH, Disabilities of the Arm, Shoulder and Hand; WORC, Western Ontario Rotator Cuff.
Pre- and postoperative WORC and DASH scores were compared between each group, and although the preoperative values were similar for each score in each group (P = .917 and .780, respectively; Hedges g = 0.039 and 0.115, respectively), there was a significant difference between postoperative WORC and DASH scores between the groups (P = .010 and .004, respectively; Hedges g = 1.028 and 0.864, respectively), with significantly better scores observed in the reconstruction group.
We assessed the number of patients in each treatment group who met the MCID. Significantly more patients met the MCID for WORC in the reconstruction group (79%) compared with the repair group (40%) (P = .035). No significant difference was found in the number of patients meeting the MCID for DASH between the reconstruction and repair groups (83% and 58%, respectively; P = .178).
Passive and active ranges of motion along with strength measurements were similar between the groups at baseline (P > .05) (Table 4). Postoperatively, the patients in each group improved with respect to those measures (P < .05); however, there was no difference between the 2 groups (P > .05).
Table 4.
Comparison of Pre- and Postoperative Active Range of Motion (ROM) Between the Repair and Reconstruction Groups
| Group | ROM, deg, Mean ± SD | P Value | |
|---|---|---|---|
| Preoperative forward flexion | Repair | 84.8 ± 72.55 | .520 |
| Reconstruction | 66 ± 82.83 | ||
| Postoperative forward flexion | Repair | 128.57 ± 65.55 | .672 |
| Reconstruction | 138.86 ± 61.64 | ||
| Preoperative lateral elevation | Repair | 71.93 ± 59.90 | .735 |
| Reconstruction | 63.07 ± 78.95 | ||
| Postoperative lateral elevation | Repair | 121.07 ± 64.65 | .318 |
| Reconstruction | 143.36 ± 50.42 |
Postoperative MRI evaluations were performed on average 1.22 ± 0.52 years postoperatively in the repair group and 1.21 ± 0.68 years postoperatively in the reconstruction group. The reconstruction group had 3 of 14 (21%) patients with complete retears, whereas the repair group had 13 of 15 (87%) patients with complete retears. The difference between the 2 groups was statistically significant (P < .001). Pre- and postoperative imaging results for the maximal repair group and reconstruction group are shown in Figures 3 and 4, respectively.
Figure 3.
Preoperative computed tomography arthrogram demonstrating a 2-tendon tear of the supraspinatus and infraspinatus tendons with retraction to the level of the glenoid: (A) coronal view; (B) sagittal view; and (C) sagittal view, showing 50% atrophy of the supraspinatus tendon. Postoperative magnetic resonance imaging scan taken 2 years after maximal repair: (D) coronal view, (E) sagittal view, and (F) sagittal view. There is a complete retear of the supraspinatus and infraspinatus tendons from the anchor attachments on the greater tuberosity. Coronal view (D) shows increased arthritis in the glenohumeral joint. Sagittal Y view (F) shows increased atrophy with marked fatty infiltration of supraspinatus and infraspinatus muscles.
Figure 4.

Preoperative magnetic resonance imaging (MRI) scan demonstrating a tear of the supraspinatus and infraspinatus tendons with retraction to the level of the glenoid rim: (A) coronal view and (B) sagittal view. Postoperative MRI scan after bridging reconstruction using dermal allograft showing that the graft is intact, connecting the remnant cuff to the greater tuberosity: (C) coronal view and (D) sagittal view.
The reconstruction group required 2 revision surgeries, only 1 of which was a result of a graft tear. Conversely, the repair group had 7 patients who underwent revision surgeries. A total of 3 patients were not eligible for revision bridging reconstruction because of progression of glenohumeral osteoarthritis, whereas the other 4 patients had revision bridging reconstruction.
The average pre- and postoperative AHDs are reported in Table 5. The AHD was significantly lower postoperatively in the repair group (P = .003) (Figure 5) than in the reconstruction group, in which the AHD was maintained (P = .797) (Figure 6). We noted a statistically significant difference in the change in AHD between groups (repair group, –3.15 ± 3.39 mm; reconstruction group, 0.15 ± 2.00 mm; P = .005; Hedges g = 1.115).
Table 5.
Summary of the Pre- and Postoperative Acromiohumeral Distance (AHD) as Measured on Radiographsa
| Surgery Type | Preoperative AHD, mm | 1-y Postoperative AHD, mm | P Value Between Pre- and Postoperative (α = .05) | Effect Size (Hedges g) |
|---|---|---|---|---|
| Maximal repair | 8.15 ± 1.87 | 5.00 ± 2.92 | .003 | 1.305 |
| Bridging reconstruction | 6.89 ± 1.80 | 7.04 ± 2.44 | .797 | 0.070 |
| P value (α = .05) | .083 | .037 |
Data are presented as mean ± SD.
Figure 5.
Preoperative (A) anteroposterior, (B) axillary, and (C) lateral radiographs of the left shoulder, showing minimal glenohumeral arthritis and no bony pathologies. Postoperative (D) anteroposterior and (E) lateral radiographs of left shoulder 2 years after surgery, showing superior migration of the humeral head.
Figure 6.
Preoperative (A) anteroposterior, (B) axillary, (C) and lateral radiographs of the right shoulder, showing minimal glenohumeral arthritis and no bony pathologies. Postoperative (D) anteroposterior and (E) lateral radiographs of right shoulder 2 years after bridging reconstruction surgery, showing maintenance of acromiohumeral distance with no osteoarthritic changes.
The results of the 2-way analysis of covariance showed no significant effect of sex on postoperative outcomes for WORC, DASH, and AHD (P = .071, P = .619, and P = .251, respectively).
The incidence of RCA in each group was assessed using the Hamada grading (Table 6).20 The majority of patients in both groups were classified as having Hamada grade 1 preoperatively. Significantly more patients in the repair group progressed with respect to RCA compared to the reconstruction group (P = .017). Upon further radiographic assessment for each patient, it was noted that 3 of 15 (20%) patients in the repair group and 0 of 14 (0%) patients in the reconstruction group proceeded to end-stage glenohumeral osteoarthritis (P < .001) (Figure 7).
Table 6.
Incidence of RCA as per Hamada Classification on Anteroposterior Radiographs of the Shouldera
| Surgery Type | Hamada Grade 1 | Hamada Grade 2 | Hamada Grade 3 | Hamada Grade 4 | Patients With RCA Progression, n/N (%) | P Value (α = .05) | Effect Size (Cramer V) | |
|---|---|---|---|---|---|---|---|---|
| Maximal repair | Pre | 13 | 2 | 0 | 0 | 6/15 (40) | .039 | 0.384 |
| Post | 8 | 4 | 0 | 3 (4b) | ||||
| P value | .016 | |||||||
| Bridging reconstruction | Pre | 13 | 1 | 0 | 0 | 1/14 (7.1) | ||
| Post | 12 | 2 | 0 | 0 | ||||
| P value | .317 |
Data are presented as n unless otherwise indicated. Pre, preoperative; Post, postoperative; RCA, rotator cuff arthropathy.
Figure 7.

Postoperative (A) anteroposterior and (B) lateral radiographs of the left shoulder, showing extensive glenohumeral arthritis with obliteration of the glenohumeral space.
Discussion
The results of this prospective, randomized controlled trial show that bridging reconstruction improved patient-reported outcomes postoperatively but also resulted in superior outcomes compared with maximal repair—the current gold standard. This is similar to findings from the largest case series on arthroscopic bridging reconstruction that was previously published, which demonstrated improved patient outcomes as measured using the visual analog scale, Simple Shoulder Test, and modified UCLA score.25 A mini-open bridging reconstruction technique published in 2014 found that all patients experienced significant pain relief, improved range of motion, and improved ASES score.30 One study compared the outcomes of 13 patients treated using mini-open maximal repair with those of 13 patients who received mini-open bridging reconstruction and found improved Constant scores (P < .01) and Oxford Shoulder Scores (P < .02) at the 2-year follow-up.42 An observational study in 2012 found improved pain, function, strength, and ASES score in their cohort of 24 patients who received bridging reconstruction.19 Although all of these studies used different patient-reported outcome scores, they indicated that bridging reconstruction improves pain and function for patients with large or massive cuff tears.
In the current study, the maximal repair group had a retear rate of 87% compared with 21% in the reconstruction group. This high retear rate may be due to the increased forces at the anchor points in the maximal repair construct. This technique attempts to regain cuff function by re-creating the rotator cable like a suspension bridge, as described by Burkhart et al.10 The anchors used to suspend the rotator cable will have increased forces from the sutures holding the anterior and posterior cuff edges to the tuberosity. Margin convergence sutures between the anchors may also contribute to increased tension. It is possible that this increased force on the anchors increases the chance for retear, which not only decreases patient outcomes but may affect the quality of the tissue for a future revision surgery.13 Conversely, the use of a dermal allograft in the bridging reconstruction technique results in a redistribution of forces over the entire tuberosity and graft edges, which decreases the tension on the native cuff. We used 9 sutures to fix our graft to the medial, anterior, and posterior aspects of the rotator cuff, which may explain the low retear rate. The number of sutures and the distribution of forces may allow for increased healing and revascularization, which will help to decrease the retear rate.
Our retear rate of 21% is comparable to that reported by Jones and Synder25 in their case series of 109 shoulders that were treated using this technique. At a 3-month follow-up, those investigators reported their graft integrity (as measured using MRI) to be 85%, and at 1 year this value was reported to be 74% (ie, a 26% retear rate). Gupta et al19 reported that 76% of their patients had fully intact grafts after this procedure; the remaining 24% of their patients had partially intact grafts, and no patients had complete graft tears, as confirmed using ultrasonography. Modi et al40 reported an 85.7% healing rate in their cohort of 14 patients with pre- and postoperative MRI scans who underwent mini-open bridging reconstruction; 14.3% of their patients had a partial tear. Conversely, studies by Rhee and Oh45 and Gouk et al18 demonstrated higher retear rates (75% and 84%, respectively). Those 2 studies reported on smaller patient cohorts, which may explain the discrepancy between their results and the ones presented herein. The Gouk et al study introduced a strengthening protocol at 6 weeks postoperatively, whereas our patients did not begin strengthening until 16 weeks after surgery. It is possible that early strengthening compromised the results of the surgical reconstruction. Our technique resulted in 3 complete cuff retears that were detected on MRI scans. Despite this radiographic finding, only 1 revision surgery was required as a result of a retear. This is in keeping with other studies that have found that patient function and satisfaction are maintained despite radiographic evidence of cuff tears.37
Our retear rate for the repair group (87%) is similar to that reported by others.3,6,16,26,35-37 The incidence of postoperative rotator cuff tears was reported to be 94% using ultrasonography in a 2004 study of patients with large or massive rotator cuff tears.16 Despite the high retear rate, patient satisfaction remained high, and excellent pain relief was maintained in the short term. Kim et al26 reported a 91% retear rate in their 2013 study of patients who received a complete repair of their large or massive contracted rotator cuff tears.
Previous studies using dermal allograft augmentation have found improved patient outcomes and improved healing after an allograft augmentation procedure compared with maximal repair alone.3,17 One study found improved ASES and Constant scores in the group treated using a graft augment but found no differences in UCLA score between the 2 groups.3 Similarly, Gilot et al17 reported improved WORC, 12-Item Short Form, and ASES scores in both groups but found that their results favored the augmented group. Barber et al3 reported a 15% retear rate for their patients treated using allograft augmentation, whereas their nonaugmented group had a 60% retear rate. Gilot et al17 reported a 10% retear rate for their augmented group. Although augmentation and bridging reconstruction using acellular dermal allograft are 2 different techniques, their similar results with respect to healing may suggest that the allograft promotes infiltration of native tendon matrix and revascularization.
Progression to severe glenohumeral arthritis occurred in 20% of our patients undergoing repair, and these patients were not candidates for revision graft bridging reconstruction. Few studies have reported this finding. Traditionally, it has been believed that maximal repair is ideal in the context of treating large or massive cuff tears. No natural history has been reported, aside from a study that found that failed partial rotator cuff repairs can progress to osteoarthritis more quickly.34 We believe this demonstrates that maximal repair may not be as benign as once thought but rather can eliminate future treatment options. Instead of being able to undergo another arthroscopic procedure, these patients can be treated only using reverse total shoulder arthroplasty (rTSA), which has a complication rate that is estimated to be as high as 30%.5,12 A study in 2009 showed that patients who received rTSA after a failed rotator cuff surgery had inferior outcomes compared with those receiving primary rTSA.7
We believe that the difference in outcomes in our study is a result of differences in joint biomechanics and healing. We maintain that in bridging reconstruction, the dermal allograft reattaches the native cuff to the tuberosity using less tension, allowing for better healing to occur and thereby functioning as a musculotendinous unit that improves patients’ function and subsequently their outcome. The graft allows better distribution of forces in the rotator cuff, which may be the reason that the reconstruction group had fewer complete retears. It is possible that the graft acts as a spacer within the shoulder, a speculation backed up by our data showing maintenance of AHD in the reconstruction group. We believe that this is also the reason for decreased progression of RCA, which will inevitably improve patient function. Improved patient function and decreased pain will be reflected in postoperative DASH and WORC scores.
The strengths of this study include that it is a level 1, prospective, randomized controlled trial. We used blinded observers for postoperative clinical and radiological assessment, and the patients were blinded to their treatment group, thereby decreasing the perceived bias in their questionnaire responses. We obtained postoperative MRI scans to confirm healing for all patients and radiographs to assess RCA. All of these images were assessed by a musculoskeletal radiologist. By having a single surgeon, we ensured consistent operative techniques. The main limitation is that our study was a single-center study with all surgical interventions performed by the principal investigator (I.W.).
Conclusion
This is the first randomized controlled trial comparing maximal repair versus bridging reconstruction using dermal allograft in the treatment of large or massive rotator cuff tears. The results of the investigation show that this technique resulted in superior patient-reported outcome scores (WORC and DASH). We also found that this technique resulted in a lower retear rate, decreased progression to RCA, and better maintenance of AHD compared with maximal repair. Given that 20% of our patients with maximal repair progressed to severe glenohumeral arthritis and were not candidates for subsequent arthroscopic procedures, we believe that maximal repair is not a benign procedure and may limit future treatment options. Further studies are required to assess the longevity of these positive outcomes. Future studies should aim to investigate MRI results from this patient population. Moreover, it would be beneficial to compare bridging reconstruction and superior capsular reconstruction, which is a prevalent treatment option for patients with massive cuff tears.
Supplementary Material
Acknowledgments
The authors thank Ryland Murphy and Anjaneyulu Purnachandra Tejaswi Ravipati for their assistance in data collection and Jie Ma for assistance in data analysis and manuscript submission.
Submitted July 9, 2020; accepted April 9, 2021.
The authors declared that they have no conflicts of interest in the authorship and publication of this contribution. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
A Video Supplement for this article is available online.
References
- 1.Adams JE, Zobitz ME, Reach JS, Jr, An K-N, Steinmann SP. Rotator cuff repair using an acellular dermal matrix graft: an in vivo study in a canine model. Arthroscopy. 2006;22(7):700-709. doi: 10.1016/j.arthro.2006.03.016 [DOI] [PubMed] [Google Scholar]
- 2.American Academy of Orthopaedic Surgeons. Management of Rotator Cuff Injuries Clinical Practice Guideline. American Academy of Orthopaedic Surgeons; 2019. [Google Scholar]
- 3.Barber FA, Burns JP, Deutsch A, Labbé MR, Litchfield RB. A prospective, randomized evaluation of acellular human dermal matrix augmentation for arthroscopic rotator cuff repair. Arthroscopy. 2012;28(1):8-15. doi: 10.1016/j.arthro.2011.06.038 [DOI] [PubMed] [Google Scholar]
- 4.Barber FA, Herbert MA, Boothby MH. Ultimate tensile failure loads of a human dermal allograft rotator cuff augmentation. Arthroscopy. 2008;24(1):20-24. doi: 10.1016/j.arthro.2007.07.013 [DOI] [PubMed] [Google Scholar]
- 5.Barco R, Savvidou OD, Sperling JW, Sanchez-Sotelo J, Cofield RH. Complications in reverse shoulder arthroplasty. EFORT Open Reviews. 2016;1(3):72-80. doi: 10.1302/2058-5241.1.160003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boileau P, Brassart N, Watkinson DJ, Carles M, Hatzidakis AM, Krishnan SG. Arthroscopic repair of full-thickness tears of the supraspinatus: does the tendon really heal? J Bone Joint Surg Am. 2005;87(6):1229-1240. doi: 10.2106/JBJS.D.02035 [DOI] [PubMed] [Google Scholar]
- 7.Boileau P, Gonzalez J-F, Chuinard C, Bicknell R, Walch G. Reverse total shoulder arthroplasty after failed rotator cuff surgery. J Shoulder Elbow Surg. 2009;18(4):600-606. doi: 10.1016/j.jse.2009.03.011 [DOI] [PubMed] [Google Scholar]
- 8.Bond JL, Dopirak RM, Higgins J, Burns J, Snyder SJ. Arthroscopic replacement of massive, irreparable rotator cuff tears using a GraftJacket allograft: technique and preliminary results. Arthroscopy. 2008;24(4):403.e1-403.e8. doi: 10.1016/j.arthro.2007.07.033 [DOI] [PubMed] [Google Scholar]
- 9.Burkhart SS, Denard PJ, Adams CR, Brady PC, Hartzler RU. Arthroscopic superior capsular reconstruction for massive irreparable rotator cuff repair. Arthrosc Tech. 2016;5(6):e1407-e1418. doi: 10.1016/j.eats.2016.08.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burkhart SS, Esch JC, Jolson RS. The rotator crescent and rotator cable: an anatomic description of the shoulder’s “suspension bridge.” Arthroscopy. 1993;9(6):611-616. doi: 10.1016/s0749-8063(05)80496-7 [DOI] [PubMed] [Google Scholar]
- 11.Burkhart SS, Pranckun JJ, Hartzler RU. Superior capsular reconstruction for the operatively irreparable rotator cuff tear: clinical outcomes are maintained 2 years after surgery. Arthroscopy. 2020;36(2):373-380. doi: 10.1016/j.arthro.2019.08.035 [DOI] [PubMed] [Google Scholar]
- 12.Craig RS, Lane JCE, Carr AJ, Furniss D, Collins GS, Rees JL. Serious adverse events and lifetime risk of reoperation after elective shoulder replacement: population based cohort study using hospital episode statistics for England. BMJ. 2019;364:l298. doi: 10.1136/bmj.l298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Desmoineaux P. Failed rotator cuff repair. Orthop Traumatol Surg Res. 2019;105(1S):S63-S73. doi: 10.1016/j.otsr.2018.06.012 [DOI] [PubMed] [Google Scholar]
- 14.Dini AA, Snyder SJ. Rotator cuff repair—the SCOI row method. Medicina Fluminensis. 2015;51(1):114-126. [Google Scholar]
- 15.Franchignoni F, Vercelli S, Giordano A, Sartorio F, Bravini E, Ferriero G. Minimal clinically important difference of the Disabilities of the Arm, Shoulder and Hand Outcome Measure (DASH) and its shortened version (QuickDASH). J Orthop Sports Phys Ther. 2014;44(1):30-39. doi: 10.2519/jospt.2014.4893 [DOI] [PubMed] [Google Scholar]
- 16.Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86(2):219-224. [DOI] [PubMed] [Google Scholar]
- 17.Gilot GJ, Alvarez-Pinzon AM, Barcksdale L, Westerdahl D, Krill M, Peck E. Outcome of large to massive rotator cuff tears repaired with and without extracellular matrix augmentation: a prospective comparative study. Arthroscopy. 2015;31(8):1459-1465. doi: 10.1016/j.arthro.2015.02.032 [DOI] [PubMed] [Google Scholar]
- 18.Gouk CJC, Shulman RM, Buchan C, Thomas MJE, Taylor FJ. Failure of dermal allograft repair of massive rotator cuff tears in magnetic resonance imaging and clinical assessment. Clin Orthop Surg. 2019;11(2):200-208. doi: 10.4055/cios.2019.11.2.200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gupta AK, Hug K, Berkoff DJ, et al. Dermal tissue allograft for the repair of massive irreparable rotator cuff tears. Am J Sports Med. 2012;40(1):141-147. doi: 10.1177/0363546511422795 [DOI] [PubMed] [Google Scholar]
- 20.Hamada K, Yamanaka K, Uchiyama Y, Mikasa T, Mikasa M. A radiographic classification of massive rotator cuff tear arthritis. Clin Orthop Relat Res. 2011;469(9):2452-2460. doi: 10.1007/s11999-011-1896-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hirahara AM, Andersen WJ, Panero AJ. Ultrasound assessment of the superior capsular reconstruction with dermal allograft: an evaluation of graft thickness and vascularity. Arthroscopy. 2019;35(12):3194-3202. doi: 10.1016/j.arthro.2019.06.042 [DOI] [PubMed] [Google Scholar]
- 22.Holtby R, Razmjou H. Measurement properties of the Western Ontario rotator cuff outcome measure: a preliminary report. J Shoulder Elbow Surg. 2005;14(5):506-510. doi: 10.1016/j.jse.2005.02.017 [DOI] [PubMed] [Google Scholar]
- 23.Huang H, Grant JA, Miller BS, Mirza FM, Gagnier JJ. A systematic review of the psychometric properties of patient-reported outcome instruments for use in patients with rotator cuff disease. Am J Sports Med. 2015;43(10):2572-2582. doi: 10.1177/0363546514565096 [DOI] [PubMed] [Google Scholar]
- 24.Iannotti JP, Deutsch A, Green A, et al. Time to failure after rotator cuff repair. J Bone Joint Surg Am. 2013;95(11):965-971. doi: 10.2106/JBJS.L.00708 [DOI] [PubMed] [Google Scholar]
- 25.Jones CR, Snyder SJ. Massive irreparable rotator cuff tears: a solution that bridges the gap. Sports Med Arthrosc Rev. 2015;23(3):130-138. doi: 10.1097/JSA.0000000000000064 [DOI] [PubMed] [Google Scholar]
- 26.Kim S-J, Kim S-H, Lee S-K, Seo J-W, Chun Y-M. Arthroscopic repair of massive contracted rotator cuff tears. J Bone Joint Surg Am. 2013;95(16):1482-1488. doi: 10.2106/JBJS.L.01193 [DOI] [PubMed] [Google Scholar]
- 27.Kirkley A, Griffin S, Dainty K. Scoring systems for the functional assessment of the shoulder. Arthroscopy. 2003;19(10):1109-1120. doi: 10.1016/j.arthro.2003.10.030 [DOI] [PubMed] [Google Scholar]
- 28.Kirkley A, Griffin S, McLintock H, Ng L. The development and evaluation of a disease-specific quality of life measurement tool for shoulder instability: the Western Ontario Shoulder Instability Index (WOSI). Am J Sports Med. 1998;26(6):764-772. doi: 10.1177/03635465980260060501 [DOI] [PubMed] [Google Scholar]
- 29.Kirkley A, Griffin S, Richards C, Miniaci A, Mohtadi N. Prospective randomized clinical trial comparing the effectiveness of immediate arthroscopic stabilization versus immobilization and rehabilitation in first traumatic anterior dislocations of the shoulder. Arthroscopy. 1999;15(5):507-514. doi: 10.1053/ar.1999.v15.015050 [DOI] [PubMed] [Google Scholar]
- 30.Kokkalis ZT, Mavrogenis AF, Scarlat M, et al. Human dermal allograft for massive rotator cuff tears. Orthopedics. 2014;37(12):e1108-e1116. doi: 10.3928/01477447-20141124-59 [DOI] [PubMed] [Google Scholar]
- 31.Lädermann A, Burkhart SS, Hoffmeyer P, et al. Classification of full-thickness rotator cuff lesions: a review. EFORT Open Reviews. 2016;1(12):420-430. doi: 10.1302/2058-5241.1.160005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lewington MR, Ferguson DP, Smith TD, Burks R, Coady C, Wong IH-B. Graft utilization in the bridging reconstruction of irreparable rotator cuff tears: a systematic review. Am J Sports Med. 2017;45(13):3149-3157. doi: 10.1177/0363546517694355 [DOI] [PubMed] [Google Scholar]
- 33.Makhni EC, Hamamoto JT, Higgins JD, et al. How comprehensive and efficient are patient-reported outcomes for rotator cuff tears? Orthop J Sports Med. 2017;5(3):2325967117693223. doi: 10.1177/2325967117693223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matsuba T, Hata Y, Ishigaki N, Nakamura K, Kato H. Osteoarthritis progression of the shoulder: a long-term follow-up after mini-open rotator cuff repair. J Orthop Surg (Hong Kong). 2018;26(2):2309499018768106. doi: 10.1177/2309499018768106 [DOI] [PubMed] [Google Scholar]
- 35.Mellado JM, Calmet J, Olona M, et al. MR assessment of the repaired rotator cuff: prevalence, size, location, and clinical relevance of tendon rerupture. Eur Radiol. 2006;16(10):2186-2196. doi: 10.1007/s00330-006-0147-z [DOI] [PubMed] [Google Scholar]
- 36.Mellado JM, Calmet J, Olona M, et al. Surgically repaired massive rotator cuff tears: MRI of tendon integrity, muscle fatty degeneration, and muscle atrophy correlated with intraoperative and clinical findings. AJR Am J Roentgenol. 2005;184(5):1456-1463. doi: 10.2214/ajr.184.5.01841456 [DOI] [PubMed] [Google Scholar]
- 37.Meyer M, Klouche S, Rousselin B, Boru B, Bauer T, Hardy P. Does arthroscopic rotator cuff repair actually heal? Anatomic evaluation with magnetic resonance arthrography at minimum 2 years follow-up. J Shoulder Elbow Surg. 2012;21(4):531-536. doi: 10.1016/j.jse.2011.02.009 [DOI] [PubMed] [Google Scholar]
- 38.Mihata T, Lee TQ, Watanabe C, et al. Clinical results of arthroscopic superior capsule reconstruction for irreparable rotator cuff tears. Arthroscopy. 2013;29(3):459-470. doi: 10.1016/j.arthro.2012.10.022 [DOI] [PubMed] [Google Scholar]
- 39.Miller BS, Downie BK, Kohen RB, et al. When do rotator cuff repairs fail? Serial ultrasound examination after arthroscopic repair of large and massive rotator cuff tears. Am J Sports Med. 2011;39(10):2064-2070. doi: 10.1177/0363546511413372 [DOI] [PubMed] [Google Scholar]
- 40.Modi A, Singh HP, Pandey R, Armstrong A. Management of irreparable rotator cuff tears with the Graftjacket allograft as an interpositional graft. Shoulder Elbow. 2013;5(3):188-194. doi: 10.1111/sae.12021 [DOI] [Google Scholar]
- 41.Moore DR, Cain EL, Schwartz ML, Clancy WG. Allograft reconstruction for massive, irreparable rotator cuff tears. Am J Sports Med. 2006;34(3):392-396. doi: 10.1177/0363546505281237 [DOI] [PubMed] [Google Scholar]
- 42.Pandey R, Tafazal S, Shyamsundar S, Modi A, Singh HP. Outcome of partial repair of massive rotator cuff tears with and without human tissue allograft bridging repair. Shoulder Elbow. 2016;9(1):23-30. doi: 10.1177/1758573216665114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ponce BA, Hosemann CD, Raghava P, Tate JP, Sheppard ED, Eberhardt AW. A biomechanical analysis of controllable intraoperative variables affecting the strength of rotator cuff repairs at the suture-tendon interface. Am J Sports Med. 2013;41(10):2256-2261. doi: 10.1177/0363546513499228 [DOI] [PubMed] [Google Scholar]
- 44.Raman J, Macdermid JC. Western Ontario Rotator Cuff Index. J Physiother. 2012;58(3):201. doi: 10.1016/S1836-9553(12)70115-7 [DOI] [PubMed] [Google Scholar]
- 45.Rhee SM, Oh JH. Bridging graft in irreparable massive rotator cuff tears: autogenic biceps graft versus allogenic dermal patch graft. Clin Orthop Surg. 2017;9(4):497-499. doi: 10.4055/cios.2017.9.4.497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sharma N, Refaiy El A, Sibly TF. Short-term results of rotator cuff repair using GraftJacket as an interpositional tissue-matched thickness graft. J Orthop. 2018;15(2):732-735. doi: 10.1016/j.jor.2018.05.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Snyder SJ, Karzel RP, Getelman MH, et al. Shoulder Arthroscopy. 3rd ed.Wolters Kluwer Health; 2015. [Google Scholar]
- 48.Tokish JM, Makovicka JL. The superior capsular reconstruction: lessons learned and future directions. J Am Acad Orthop Surg. 2020;28(13):528-537. doi: 10.5435/JAAOS-D-19-00057 [DOI] [PubMed] [Google Scholar]
- 49.Wong I, Burns J, Snyder S. Arthroscopic GraftJacket repair of rotator cuff tears. J Shoulder Elbow Surg. 2010;19(2 suppl):104-109. doi: 10.1016/j.jse.2009.12.017 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.