Abstract
Background
The antitumor efficacy of human epidermal growth factor receptor 2 (HER2)-targeted therapies, such as humanized monoclonal antibody trastuzumab (Herceptin®, Roche), in patients with breast-to-brain cancer metastasis is hindered by the low permeability of the blood-brain barrier (BBB). NEO100 is a high-purity version of the natural monoterpene perillyl alcohol, produced under current good manufacturing practice (cGMP) regulations, that was shown previously to reversibly open the BBB in rodent models. Here we investigated whether NEO100 could enable brain entry of trastuzumab to achieve greater therapeutic activity.
Methods
An in vitro BBB, consisting of human astrocytes and brain endothelial cells, was used to determine trastuzumab penetration in the presence or absence of NEO100. For in vivo studies, we administered intravenous (IV) trastuzumab or the trastuzumab-drug conjugate ado-trastuzumab emtansine (T-DM1; Kadcyla®, Roche), to mouse models harboring intracranial HER2+ breast cancer, with or without BBB opening via IA NEO100. Brain and tumor tissues were examined for the presence of trastuzumab and infiltration of immune cells. Therapeutic impact was evaluated based on overall survival.
Results
NEO100 greatly increased trastuzumab penetration across an in vitro BBB. In vivo, IA NEO100-mediated BBB opening resulted in brain tumor-selective accumulation of trastuzumab, without detectable presence in normal brain tissue, along with increased presence of immune cell populations. IV delivery of trastuzumab or T-DM1 achieved significantly greater overall survival of tumor-bearing mice when combined with IA NEO100.
Conclusion
IA NEO100 facilitates brain tumor entry of trastuzumab and T-DM1 and significantly enhances their therapeutic efficacy, along with increased antibody-dependent immune cell recruitment.
Keywords: blood-brain barrier, brain metastasis, HER2+ breast cancer, Herceptin, Kadcyla, perillyl alcohol
Key Points.
Intra-arterially administered NEO100 opens the BBB in a preclinical HER2+ brain cancer model.
BBB opening by NEO100 enables IV trastuzumab and T-DM1 to accumulate in brain tumors.
Trastuzumab and T-DM1 achieve therapeutic impact against HER2+ brain tumors after BBB opening.
Importance of the Study.
The blood-brain barrier (BBB) prevents many potential therapeutic agents from exerting their activity against central nervous system (CNS) malignancies. Therefore, there is an urgent medical need for drug delivery methods that support increased brain entry of therapeutics. Intra-arterial (IA) NEO100, a highly pure version of the natural monoterpene perillyl alcohol, has shown preliminary evidence of its ability to transiently open the BBB, enabling brain entry of therapeutic agents of different molecular sizes. In the present study, we demonstrate that this approach can achieve effective therapeutic activity in preclinical models of intracranial HER2+ breast cancer, where mice received intravenous trastuzumab or T-DM1, two commonly used targeted therapies for HER2+ breast cancer. These observations bode well for future clinical application in HER2+ breast cancer patients with brain metastases and hold the promise to expand the therapeutic reach of these targeted therapies from the periphery into the CNS.
The majority of morbidity and mortality from breast cancer is associated with metastatic disease.1–3 Once tumor cells have entered the brain, prognosis becomes particularly poor. Treatment options for brain-metastatic lesions often are restricted to cranial radiotherapy and surgical resection, although surgery is not always possible and radiotherapy comes at the expense of potential neurological toxicity via radiation necrosis and other side effects. The impact of chemotherapy is severely limited by the blood-brain barrier (BBB), which prevents brain entry of most drugs. In micrometastases (<1 mm), the BBB retains its normal function, whereas in larger brain lesions, its barrier capacity may be compromised along the so-called blood-tumor barrier (BTB).3,4 Yet even in this latter scenario, increased “leakiness” of the BTB is insufficient to support effective therapeutic access to brain lesions.5,6 Furthermore, some malignant foci appear to grow around preexisting blood vessels with intact BBB—a process called co-option—which also shields them from the reach of circulating drugs.7
Despite the introduction of better treatments for breast cancer in general, the incidence of brain metastasis from breast cancer has increased in recent years.1,8 Local and extracranial malignancies can be treated more effectively, which results in longer survival of patients. However, cancerous seeds in the brain, for the most part, are protected from systemic therapeutic intervention and eventually trigger cancer recurrence. This is particularly evident in the case of HER2-positive (HER2+) breast cancer, a subtype that displays overexpressed epidermal growth factor receptor 2 (EGFR2/HER2/Neu) and has a predilection to spread to the brain.3,9 Median survival times for patients with HER2+ brain-metastatic breast cancer are highly variable with a range of 6-34 months,10–14 depending on a number of factors, as considered by breast cancer-specific prognostic models15,16 and the treatment regimen that is applied.2,3,17 The poor prognosis for these patients is compounded by the high risk of significant morbidity due to potentially progressive neurologic deficits that lead to diminished quality of life.
Several HER2-targeted therapies are approved for the treatment of metastatic HER2+ breast cancer, including small-molecule inhibitors such as lapatinib, neratinib, and tucatinib, antibodies such as trastuzumab, pertuzumab, and margetuximab, and antibody-drug conjugates (ADC) such as ado-trastuzumab emtansine/T-DM1 and fam-trastuzumab deruxtecan. While these targeted treatments have been successfully integrated into the combination treatment of patients with HER2+ breast cancer and have incrementally improved control of systemic disease, their therapeutic impact on brain metastases has been muted due to insufficient penetration of the BBB.3,18,19 As a result, the incidence of breast cancer patients suffering from metastatic spread to the brain has increased, and novel treatment approaches are required to address this substantial medical need.
To overcome the BBB obstacle and achieve better brain entry of systemically circulating therapeutics, different approaches can be considered, but they generally involve significant risks and limitations. The best-known clinical method to open the BBB consists of the intracarotid injection of mannitol. However, this procedure has led to variable and unpredictable responses in BBB breakdown, seizures, risk of brain embolism, and catastrophic bleeds.20 As a result, the use of intracarotid mannitol is restricted to specialized centers and has not found widespread acceptance. Transcranial focused ultrasound coupled with intravenously delivered microbubbles is currently explored as another BBB disruption technique,21 and clinical trials of this approach have been initiated.4 At this time, it is not yet clear whether this procedure will become clinically useful. As well, the effects of radiation on BBB permeability have long been recognized,22 but many details, including time course and magnitude of increased opening, still remain to be established.4
In our studies, we are developing intra-arterial (IA) injection of NEO100 as a novel means to reversibly and safely open the BBB. NEO100 is a high-purity version of the natural monoterpene perillyl alcohol (POH), a naturally occurring monoterpene and metabolite of limonene found in the peel of citrus fruits and many other botanical sources.23 A large number of Phase I and II clinical studies using oral POH explored its activity against numerous solid cancers, but the results were generally disappointing.23 Clinical trials in Brazil are investigating an intranasally delivered formulation of POH in malignant glioma patients,24 and NEO100, produced under current good manufacturing practices (cGMP), is undergoing clinical testing as an intranasal formulation for recurrent glioblastoma in the United States [ClinicalTrials.gov Identifier: NCT02704858].
Delivering POH/NEO100 via the intranasal route showed that it was very well tolerated, even after several years of four-times daily application.24 In addition, this molecule’s amphipathic nature, high partition coefficient, and its presumed interaction along the acyl-lipid tails of the cell membrane bilayer25,26 indicated to us that it might be able to safely and reversibly open the BBB. We therefore investigated this hypothesis in mouse models where we performed a sequence of two injections: the first injection administered NEO100 by IA delivery, with the objective to open the BBB; the second injection administered a non-BBB permeable tracer or drug via intravenous (IV) delivery, with the expectation that now these agents would be able to enter the brain from the systemic circulation. Indeed, our prior study established that IA injection of NEO100 is feasible and able to safely and reversibly open the BBB, enabling brain entry of different-sized molecules present in the systemic circulation.27 In the following, we now present data addressing the critical question as to whether this novel method can achieve superior therapeutic outcomes. We used preclinical models of intracranial HER2+ breast cancer, where we opened the BBB with IA NEO100, followed by IV delivery of therapeutic antibodies, trastuzumab or T-DM1, and evaluation of their distribution and activity against the malignant brain lesions.
Materials and Methods
Cell Isolation and Culture
Human tissues were obtained following written informed consent from patients in accordance with the Declaration of Helsinki guidelines and the Institutional Review Board at the Keck School of Medicine of University of Southern California (USC). Brain endothelial cells (BECs) were isolated as previously described28 and used between passages 3-8. Human astrocytes were purchased from ScienCell Research Laboratories (Carlsbad, CA, USA). Details of the two mouse mammary tumor lines, D2F2 and 4T1, and verification of their HER2+ status, as well as culture conditions for all cells are included in the Supplementary Materials.
In Vitro BBB Model
Primary cultures of astrocytes (1.5 × 105/insert) and BECs (1.5 × 105/insert) were co-seeded on top of 0.4-µm pore-size Transwell inserts (Millipore, Temecula, CA) pre-coated with Matrigel (Corning, New York, NY) and grown to complete confluence to mimic BBB conditions. Inserts were placed in 24-well plates with glass coverslips at the bottom. These coverslips had been coated with 1% gelatin (Sigma-Aldrich, St. Louis, MA, USA) and seeded with 4T1-HER2+ or D2F2-HER2+ cells at a density of 5 × 104 cells/well (see sketch in Figure 1A). Trastuzumab was added to the top chamber in the presence or absence of NEO100. An equivalent volume of dimethyl sulfoxide (DMSO, Sigma-Aldrich) was used as vehicle control for NEO100. After 10 min of incubation, top inserts (BBB cells) were removed and bottom glass coverslips (HER2+ tumor cells) were processed for immunostaining.
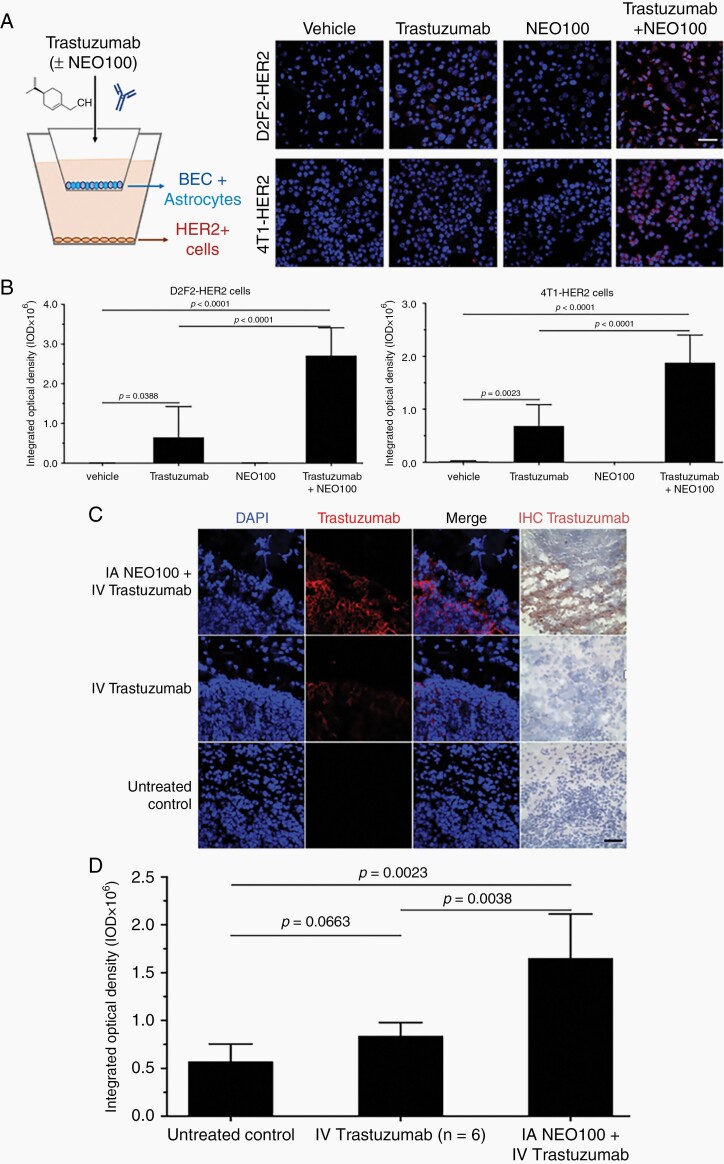
Fig. 1.
NEO100 facilitates blood-brain barrier (BBB) penetration of trastuzumab. (A) Left: Illustration of in vitro Transwell BBB set up. Brain endothelial cells (BEC) + astrocytes formed the BBB, whereas HER2+ cells at the bottom represented the target for trastuzumab binding. Right panels: Representative confocal pictures of 4T1-HER2+ and D2F2-HER2+ cells after immunostaining with red fluorophore-conjugated anti-human IgG antibody (detecting trastuzumab). As indicated, the top chambers received vehicle, trastuzumab, NEO100, or a combination of trastuzumab + NEO100. Concentration of NEO100 was 1 mM, which was confirmed to be non-cytotoxic (not shown). After 10 min, HER2+ cells were removed and stained. Red fluorescent staining denotes trastuzumab bound to cells; blue staining (DAPI) identifies nuclei. Scale bar, 50 µm. (B) Red fluorescent staining was quantitated and presented in bar graphs, representing the average of three independent experiments performed in triplicate. Left: D2F2-HER2+ cells; right: 4T1-HER2+ cells. (C) Balb/c mice bearing intracranial 4T1-HER2+ cells were treated with intra-arterial (IA) NEO100 (or vehicle), immediately followed by intravenous (IV) trastuzumab, or remained untreated (control). Twenty-four hours later, perfused brains were collected and sections were subjected to immunostaining with a biotinylated antibody recognizing trastuzumab (right panel; brown stain), or a fluorescently labeled antibody recognizing human IgG (second panel from left; red stain). DAPI was used as the counterstain. Representative confocal images are shown. (Brain sections from mice treated with NEO100 alone are not shown, as they yielded results identical to untreated control.). Scale bar, 50 µm. (D) Red fluorescent staining (indicating cell-bound trastuzumab) from C was quantitated and presented in bar graphs, representing the averages from multiple independent images.
NEO100, Trastuzumab, and T-DM1
NEO100 is 99% pure POH manufactured under compliance with cGMP regulations, as set by the US Food and Drug Administration (FDA). It was produced at Norac Pharma (Azusa, CA, USA) as described in the Supplementary Materials. A working solution of 0.3% was made by mixing pure NEO100 and 0.9% sodium chloride (obtained as Sodium Chloride Injection, USP, sterile and nonpyrogenic, from Fresenius Kabi, Lake Zurich, IL, USA) and vortexing the solution immediately before use. (Additional details are provided in the Supplementary Materials). Trastuzumab and T-DM1 were obtained as Herceptin and Kadcyla, respectively, from the USC/Norris Comprehensive Cancer Center Pharmacy in powder form and reconstituted in aqueous solution as described in the Supplementary Materials.
Experimental Animals and Brain Tissue Collection
All animal protocols were approved by the Institutional Animal Care and Use Committee (IACUC) of USC. About 6- to 8-week-old male and female BLAB/c mice (The Jackson Laboratory, Bar Harbor, ME, USA) were used. A syngeneic brain metastasis model was established by intracranial implantation of 1 × 104 4T1 or 4T1-HER2+ murine breast cancer cells. Some cell lines harbored firefly luciferase to enable noninvasive monitoring of tumor development by optical imaging. Animal survival times were determined starting from the date at which tumor cells were implanted. For survival experiments, body weight and clinical scores were monitored on a regular basis to avoid death as an endpoint criterion.29 Mice were euthanized in compliance with applicable standard operating procedures for rodent euthanasia and as approved by IACUC. At the time of euthanasia, mice were perfused with saline to eliminate circulatory components. Brains were collected, immediately frozen in Clear Frozen Section Compound (VWR, Radnor, PA, USA), and stored at −80°C until further analysis.
IA and IV Injections
IA injection of 40 µL NEO100 (0.3%, v/v in 0.9% sodium chloride) was accomplished by ultrasound-guided intracardiac injection into the left ventricle, as previously described.27 Additional details are provided in the Supplementary Materials. IV injections of 40 µL trastuzumab (8 mg/kg) or 40 µL T-DM1 (3.6 mg/kg) were performed via tail vein catheter, which was put in place before the mice were subjected to the IA procedure. IV injection was performed right after completion of IA injection of NEO100.
Immunostaining
Detailed protocols for immunohistochemistry and immunocytochemistry were described elsewhere.30,31 The primary antibodies and their catalog numbers are provided in the Supplementary Materials, along with details of picture acquisition. Three independent experiments were performed, and images were analyzed and quantitated using ImageJ software of the National Institutes of Health.
Statistical Analyses
Statistical analyses were performed using GraphPad Prism 7. A P value <.05 was considered significant. Two-tailed t-test or 1-way ANOVA followed by Dunnett’s or Tukey’s multiple comparison tests were used, accordingly. For in vitro experiments with primary cell cultures, each patient-derived sample was considered the unit of analysis and accounted for an independent value, assayed in triplicate. At least three different patient-derived samples were used per experiment. Data were presented as mean ± standard deviation (SD).
Results
NEO100 Enhances BBB Penetration of Trastuzumab
For our in vitro studies, we used a BBB model that consisted of co-cultures of patient-derived BECs and astrocytes that were seeded into the upper Transwell inserts of a 2-chamber system.32 The bottom of the lower chamber contained 4T1 or D2F2 murine breast cancer cells overexpressing human HER2 (Figure 1A). To determine barrier permeability, trastuzumab was added to the upper chamber in the presence or absence of NEO100, and antibody penetration through the BBB was analyzed by detecting trastuzumab that had bound to the HER2+ cells in the bottom chamber. Trastuzumab was visualized by immunostaining with a fluorescently labeled goat antibody recognizing human immunoglobulin G (IgG). As shown in Figure 1A, B, after the addition of trastuzumab to the upper chamber, its binding to HER2+ cells in the lower chamber could be detected. However, the extent of bound trastuzumab was significantly (P < .0001) increased in the presence of NEO100, indicating greater permeability of the BBB as a result of NEO100 addition.
To investigate whether a similar effect could be achieved in vivo, we performed a single intracardiac injection of NEO100 into the left ventricle of a mouse as a means to target NEO100 to the cerebral arteries, ie, to the proximity of the BBB. This IA injection was immediately followed by IV (tail vein) injection of trastuzumab. We hypothesized that any BBB-opening effect of NEO100 would allow circulating trastuzumab to enter the brain. To simulate a HER2+ brain-metastatic breast cancer model, we implanted 4T1-HER2+ cells into the brains of mice. Two weeks after implantation, the mice received IA NEO100 in combination with IV trastuzumab. Twenty-four hours later, mice were euthanized. Perfused brains were collected, and sections were analyzed by immunochemistry with anti-human IgG and anti-trastuzumab antibodies. As shown in Figure 1C, D, brain tumor sections from mice that were co-treated with NEO100 and trastuzumab showed strong positive staining for trastuzumab, whereas sections from mice that had received trastuzumab in the absence of NEO100 showed very little trastuzumab positivity. Combined, the results shown in Figure 1 indicate that NEO100 enabled trastuzumab to cross the BBB and bind to its HER2+ target sites.
Trastuzumab Preferentially Accumulates Within Tumor Tissue After BBB Opening
We next investigated the general distribution of trastuzumab after NEO100-mediated BBB opening in mouse brains harboring HER2+ breast cancer lesions. As before, trastuzumab was delivered IV immediately after IA delivery of NEO100 (or vehicle control). The next day, brains were collected and stained for the presence of trastuzumab, as well as for the proliferation marker Ki-67 to distinguish tumor tissue from normal brain tissue. As before, brain tissues from mice receiving IV trastuzumab in combination with IA NEO100 revealed the prominent presence of the humanized antibody. Trastuzumab staining principally overlapped with staining for Ki-67, but was noticeably absent from Ki-67-negative brain regions (Figure 2A), demonstrating that trastuzumab highly selectively accumulated in tumor tissue over normal brain tissue.
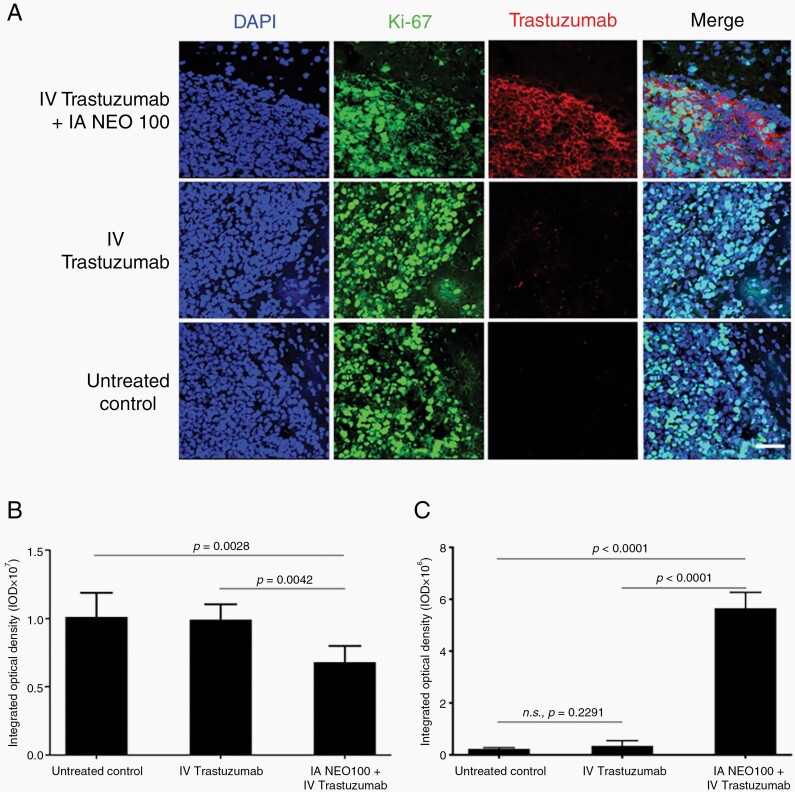
Fig. 2.
Trastuzumab accumulates within tumor tissue, rather than normal brain tissue. Balb/c mice bearing intracranial 4T1-HER2+ cells were treated with intra-arterial (IA) NEO100 (or vehicle), immediately followed by intravenous (IV) trastuzumab, or remained untreated (control). Twenty-four hours later, brains were collected and sections were subjected to immunostaining with fluorescently labeled antibodies recognizing Ki-67 (green) or human IgG (red). DAPI was used as the counterstain (blue). (A) Representative confocal images are shown (scale bar, 50 µm). (B) Quantification of green anti-Ki-67 fluorescence (averages from multiple independent images). (C) Quantification of red anti-human IgG fluorescence (averages from multiple independent images).
The strongly elevated presence of trastuzumab within brain tumor tissue in response to co-treatment with IA NEO100 + IV trastuzumab correlated with significantly (P = .0042) decreased Ki-67 staining, as compared to IV trastuzumab treatment alone (Figure 2B, C). This finding suggested that the increased presence of the humanized antibody at the tumor target site might exert therapeutic activity.
BBB Opening by NEO100 Enables Trastuzumab to Unfold Its Therapeutic Activity in the Brain
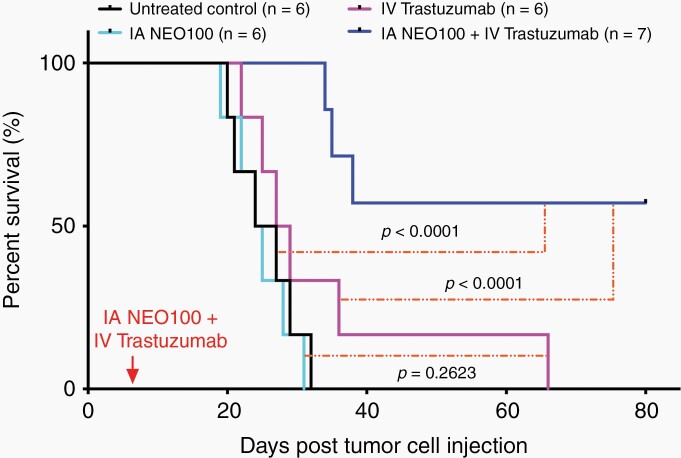
In order to test the veracity of our hypothesis that BBB opening by NEO100 can enable brain-targeted therapeutic impact of systemically delivered trastuzumab, we determined survival times of mice with HER2+ breast cancer cells implanted in their brains. After confirmation of tumor take via noninvasive bioluminescent imaging, the animals were randomly separated into four treatment groups: (i) IA NEO100 only, (ii) IV trastuzumab only, (iii) IA NEO100 followed by IV trastuzumab, and (iv) no treatment (control). Following a one-time treatment, survival of animals was monitored (Figure 3A). Animals in groups (i), (ii), and (iv) rapidly succumbed to disease, with median overall survival (mOS) of 25, 29, and 25 days, respectively. In particular, there was no difference in survival between untreated animals and those that had received trastuzumab without NEO100 (P = .26). In contrast, animals in the combination group (iii) survived much longer, with mOS of >80 days (P = .0001).
Fig. 3.
NEO100-facilitated brain entry of trastuzumab prolongs survival of mice with intracranial HER2+ breast cancer. Mice were implanted intracranially with 4T1-HER2+ cells. Seven days later, the animals received a single round of treatment, consisting of intra-arterial (IA) NEO100 alone, intravenous (IV) trastuzumab alone, or a combination of the two. Some mice remained untreated (control). Shown is Kaplan-Meier survival plot with overall survival of animals in the different treatment groups and P values added.
As an additional control, we performed a similar in vivo experiment with intracranially implanted parental 4T1 cells, ie, cells lacking transfected HER2. In this case, however, combination treatment of IA NEO100 with IV trastuzumab did not result in detectable survival benefit, ie, these mice died as rapidly as mice in the untreated control group (results not shown). Thus, unlike mice harboring HER2+ tumors, mice harboring tumors without transfected human HER2 did not reap the benefit of NEO100-mediated antitumor trastuzumab activity in the brain. This outcome further confirmed the specificity of this treatment, ie, that therapeutic activity was dependent on the interaction of the humanized antibody with its HER2 target.
NEO100-Mediated Brain Entry of Trastuzumab Triggers Enhanced Immune Response
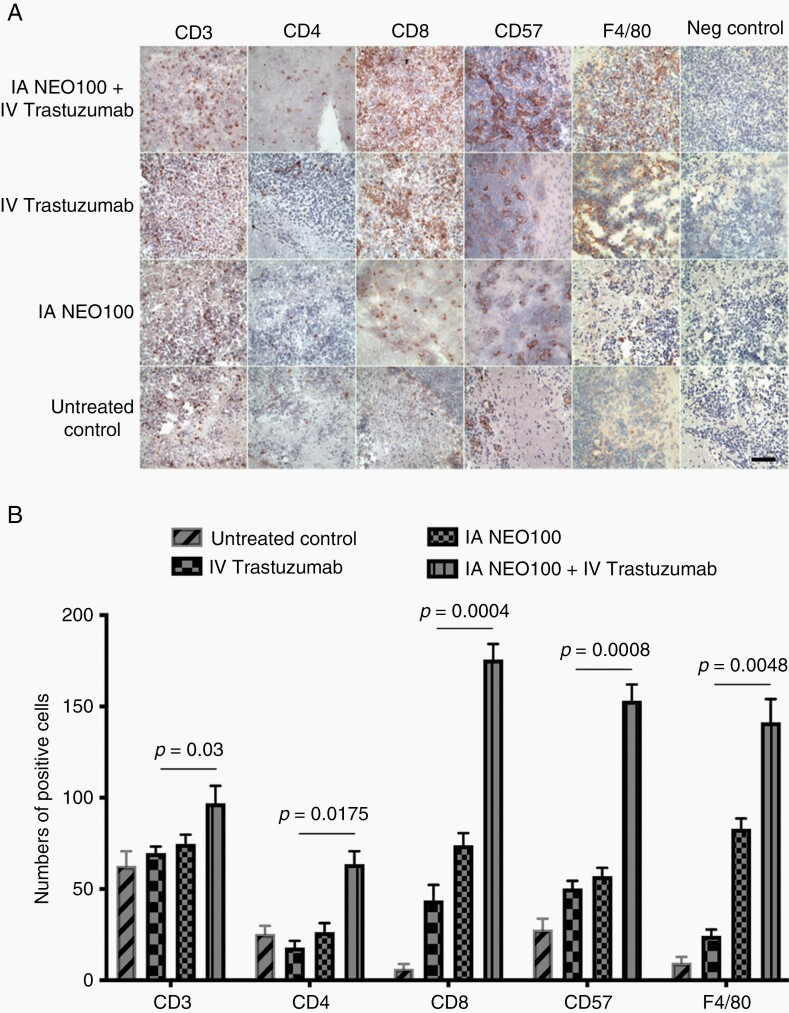
Innate and adaptive immune mechanisms are emerging as contributing players in the modulation of the effects of HER2-targeted therapeutic antibodies.33–35 To gain some preliminary insight into how BBB opening by NEO100 might affect these mechanisms, we investigated the presence of different immune effector cells in HER2+ brain tumor lesions. Tumor-bearing animals received a single dose of IV trastuzumab, with or without prior BBB opening by IA NEO100, or IA NEO100 alone, or remained untreated. Twenty-four hours later, brains were collected and analyzed for the presence of natural killer (NK) cells (CD57+), macrophages (F4/80+), and T cells (CD3+, CD4+, CD8+) (Figure 4). As compared to untreated animals, there was an increase in several markers for these immune effector cells in mice treated with trastuzumab alone or NEO100 alone. In comparison, however, the combination treatment resulted in the highest accumulation of all five markers, which was greater (P < .05) than what resulted from individual treatments. These results indicate that BBB opening by NEO100, combined with the administration of trastuzumab, triggers an enhanced immune response, ie, substantially increased recruitment of immune effector cells to the brain-localized tumor.
Fig. 4.
NEO100-facilitated brain entry of trastuzumab enhances the recruitment of immune cells. (A) Mice harboring intracranial 4T1-HER2+ tumors received a single dose of intra-arterial (IA) NEO100 alone, intravenous (IV) trastuzumab alone, a combination of the two, or remained untreated (control). Twenty-four hours later, perfused brains were collected and sections were subjected to immunohistochemical analysis with antibodies against CD3, CD4, and CD8 to detect T lymphocytes, against CD57 to detect mature natural killer (NK) cells, and against F4/80 to detect macrophages. Sections were also processed in the absence of primary antibodies (shown as Negative control). Scale bar, 50 µm. (B) The number of positive cells per field was determined in multiple sections, and averages (±SD) are shown in the bar graph. Statistical comparisons were done between tissues from mice treated with IV trastuzumab alone and those from mice treated with IV trastuzumab in combination with IA NEO100. In all cases, the combination treatment resulted in the greater presence of immune cells (P < .05).
NEO100 Enhances T-DM1 Accumulation and Apoptosis in Brain Tumor Tissue
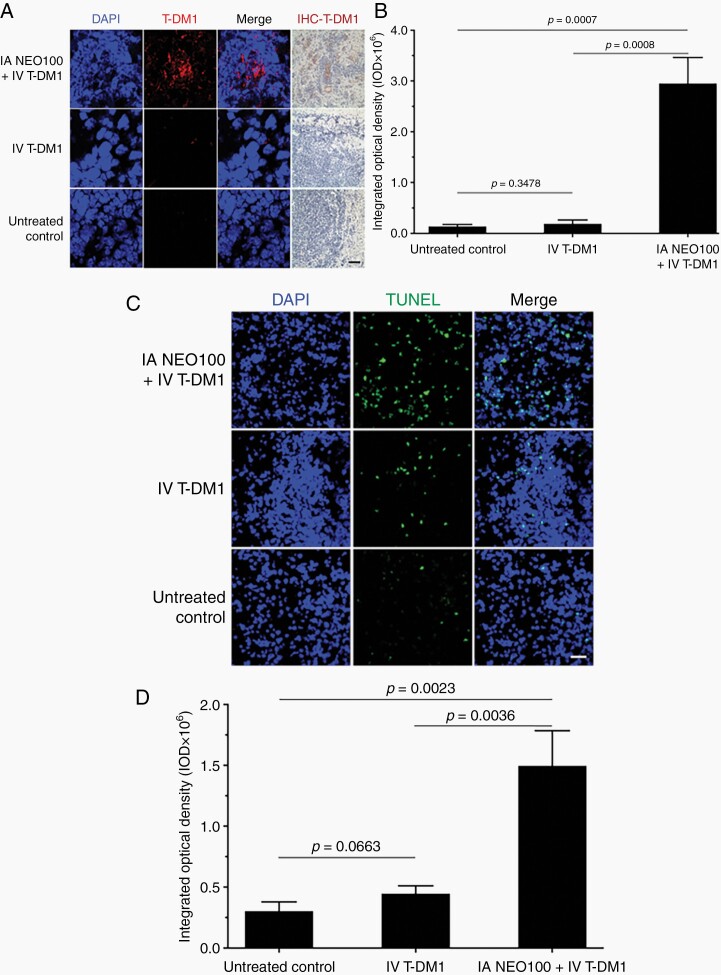
The ADC T-DM1 represents a new generation of cytotoxic drugs. However, similar to trastuzumab, it has revealed severely limited ability to reach brain tumor lesions from the systemic circulation. We therefore investigated whether IA NEO100 would facilitate brain entry and intracranial therapeutic activity of this ADC, similar to what we documented with trastuzumab above. Mice with HER2+ brain lesions were treated with a single dose of IV T-DM1, with or without prior BBB opening by IA NEO100. A control group of mice received no treatment. Twenty-four hours later, perfused brains were collected, and sections were analyzed by immunochemistry with anti-human IgG and anti-trastuzumab antibodies. As shown in Figure 5A, B, brain tumor sections from mice that were co-treated with NEO100 and T-DM1 showed strong positive staining for trastuzumab, whereas sections from mice that had received T-DM1 in the absence of NEO100 showed barely detectable trastuzumab positivity (P = .0008).
Fig. 5.
Intra-arterial (IA) NEO100 enhances brain entry of T-DM1 and tumor cell apoptosis. Mice harboring intracranial 4T1-HER2+ tumors received a single dose of IA NEO100 alone, intravenous (IV) T-DM1 alone, a combination of the two, or remained untreated (control). Twenty-four hours later, perfused brains were collected and sections were analyzed. (A) The presence of T-DM1 was detected by IHC staining with a biotinylated antibody recognizing trastuzumab (right panel; brown stain), or a fluorescently labeled antibody recognizing human IgG (second panel from left; red stain). DAPI was used as the counterstain. Representative confocal images are shown. (Brain sections from mice treated with NEO100 alone are not shown, as they yielded results identical to untreated control.). Scale bar, 50 µm. (B) Quantification of red fluorescent staining of conjugated trastuzumab (averages from multiple independent images). (C) Brain sections were subjected to TUNEL staining to detect apoptotic cells (green), with DAPI (blue) as the counterstain. Shown are representative images from the brain area harboring tumor tissue. Scale bar, 50 µm. (D) Quantification of green fluorescent TUNEL staining (averages from multiple independent images).
Brain tissue sections were further subjected to TUNEL staining as an indicator of apoptotic cell death. Results in Figure 5C, D show that combination treatment achieved significantly (P = .0036) higher TUNEL positivity than T-DM1 treatment in the absence of NEO100. Combined, the results in Figure 5 indicate that the presence of NEO100 enabled T-DM1 to cross the BBB, bind to its HER2+ target sites, and trigger tumor cell death.
BBB Opening by NEO100 Enables T-DM1 to Unfold Its Therapeutic Activity in the Brain
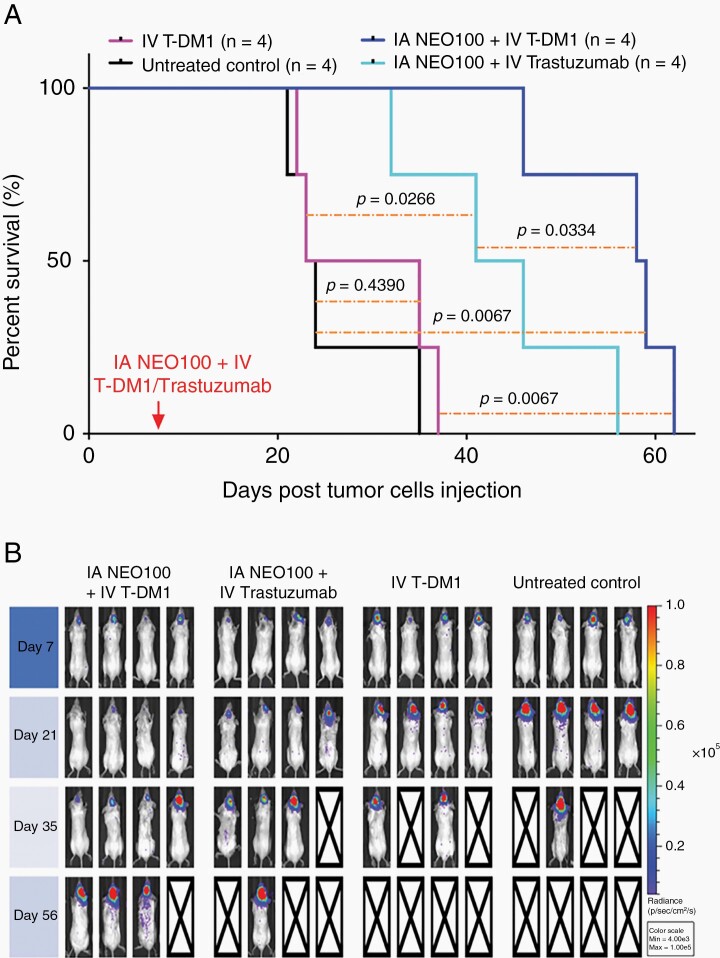
Lastly, we determined therapeutic activity of T-DM1 with or without prior BBB opening by NEO100. Mice harboring HER2+ breast cancer cells in their brains were randomly separated into four treatment groups: (i) IV T-DM1 only, (ii) IV T-DM1 preceded by IA NEO100, (iii) trastuzumab preceded by IA NEO100, and (iv) no treatment (control). After a one-time treatment, survival of animals was monitored (Figure 6). IV T-DM1 alone was unable to generate a survival benefit, ie, these animals succumbed to disease as quickly as untreated animals. In stark contrast, when mice received T-DM1 in combination with NEO100, their survival was prolonged to a median of 58 days, as compared to 29 days of T-DM1-only treated mice (P = .0067). Group (iii), treated with NEO100 in combination with trastuzumab, also presented with significantly (P = .0266) increased survival as compared to the untreated control group (mOS = 24 days), although their mOS of 44 days was less than the 58 days in response to T-DM1 with NEO100.
Fig. 6.
NEO100-facilitated brain entry of T-DM1 prolongs survival of mice with intracranial HER2+ breast cancer. Mice were implanted intracranially with 4T1-HER2+ cells. Eight days later, the animals received a single round of treatment, consisting of intravenous (IV) T-DM1 (alone or combined with intra-arterial (IA) NEO100), a combination of IV trastuzumab with IA NEO100, or remained untreated (control). (A) Shown is Kaplan-Meier survival plot with overall survival of animals in the different treatment groups, along with statistical analysis of significance (P values). (B) Tumor cells harbored firefly luciferase and therefore noninvasive optical imaging of tumors could be performed on a regular basis. Shown are imaging results from the group of mice described in A.
Because in this experiment, we used 4T1-HER2+ cells that harbored luciferase, it was possible to monitor tumor take and development over time. The imaging results (Figure 6B) were consistent with the outcome shown in the Kaplan-Meier plot and further confirmed that brain-targeted activity of both therapeutic antibodies was significantly improved by IV NEO100.
Discussion
The incidence of brain metastases in HER2+ breast cancer has increased over the past two decades, primarily because new targeted treatments more effectively control peripheral disease, but do not effectively cross the BBB to also attack brain lesions. Many small-molecule inhibitors, such as lapatinib, are substrates for different efflux pumps that are highly active in the BBB, whereas water-soluble antibodies, such as trastuzumab, are hindered due to their large surface area.17,21 While the challenge of effective brain delivery of therapeutic agents is not restricted to central nervous system (CNS) lesions of HER2+ breast cancer, we have used this cancer type as a preclinical model to determine whether BBB opening by our novel method of IA NEO100 delivery would enable brain-targeted therapeutic activity of large therapeutic molecules present in the systemic circulation. In the present study, we were able to provide proof of principle that BBB opening by IA NEO100 enabled access of IV trastuzumab and IV T-DM1 to intracranial HER2+ tumor tissue, resulting in striking anticancer activity. In the absence of NEO100, both antibody-based therapeutic regimens were inactive, consistent with their known inability to cross the BBB.
In our mouse models, we used intracardiac injection as a means for IA delivery of NEO100. While this method represents the most appropriate and suitable IA approach for small rodents, it would not generally be feasible in patients. To translate this method to human patients, we envision cannulation of the femoral artery with selective threading to the tumor-feeding intracranial arteries (performed by interventional neuroradiology), representing an appropriate human equivalent of intracardiac injections in mice. This approach constitutes a routine procedure that is commonly performed by endovascular neurosurgeons in the clinic in the context of cerebral angiograms, aneurysm coiling, tumor embolization, and thrombectomies.36 In comparison to intracarotid injection of mannitol, it represents a technique that is much more common, associated with significantly lower risk, and not restricted to highly specialized medical centers. As such, IA NEO100 has the potential to become a safer, easier, and more widely available method to open the BBB of patients.
It is noteworthy that we used a one-time application of this method to deliver a single dose of trastuzumab or T-DM1 to the brain. The resulting significant increase in survival of HER2+ tumor-bearing animals with IA NEO100 is even more striking in view of the many repeat applications that are performed in the clinic with IV trastuzumab alone. For metastatic breast cancer, trastuzumab is given weekly, whereas T-DM1 is given in 3-week cycles. The pronounced benefit of our single dose is highly encouraging because it points to the possibility that intracranial effectiveness of these therapeutic antibodies in patients might be achievable with only very few applications. Although transfemoral IA cannulation is a standard procedure, it still is more invasive than simple IV infusion, and as such, it would be desirable to keep its application infrequent. It will require further detailed studies of trastuzumab neuro-pharmacokinetics to shed light on its surprisingly strong benefit after a single application. One might speculate that perhaps antibody turnover in the brain is slower than in the periphery.
Furthermore, innate and adaptive immune mechanisms are emerging as contributing players in the modulation of the effects of HER2-targeted therapeutic antibodies.33–35 In this context, we found that BBB opening by NEO100 increased the recruitment of CD8+ T cells, mature NK cells, and macrophages to the tumor microenvironment, and this effect was compounded in the presence of trastuzumab. Here as well, the dynamics of these immune effector cells and their potential continued presence requires further study, which may contribute to understand the mechanism behind the pronounced therapeutic benefit of single trastuzumab dosing in the brain.
In summary, our study presents proof of principle that IA NEO100 can enable access of IV therapeutic antibodies to intracranial tumor lesions, resulting in pronounced therapeutic activity. These observations bode well for future clinical applications in HER2+ breast cancer patients with brain involvement and hold the promise to expand the therapeutic reach of targeted therapies from the periphery into the CNS. Toward this goal, additional supportive studies are required along the translational path. For instance, additional safety studies are required to validate that BBB opening by NEO100 is well tolerated and without significant long-term detrimental sequelae. Combination approaches with other established therapeutic agents, as currently used for peripheral disease in the clinic, should be explored for potentially further optimized anticancer activity. We are optimistic that our current study will serve as a solid platform from which such important next steps can be initiated.
Supplementary Material
Acknowledgments
We appreciate thoughtful input from Mrs. Diana Chingos and Mrs. Tricia Russo, former breast cancer patients and now cancer patient advocates and appreciated members of our team. We are grateful to Alan Epstein (University of Southern California) and Wei-Zen Wei (Wayne State University) for providing HER2-overexpressing mouse mammary tumor cell lines. Also, we appreciate Ivetta Vorobyova at MIC (Molecular Imaging Center) USC for the performance of optical imaging (NIH S10OD021785) and ultrasound-guided intracardiac injection (NIH S10OD018096) in the in-vivo study.
Funding
Funding for these studies was provided by NeOnc Technologies, Inc. (T.C.C.), the USC Wright Foundation (W.W.), the National Cancer Institute (1R41CA217551) (W.W., T.C.C.), Sounder Foundation (T.C.C.), Sharyl and Oscar Garza Research Fund (T.C.C), and METAvivor Research and Support, Inc. (A.H.S., T.C.C.).
Conflict of interest statement.
T.C.C. is the founder and stakeholder of NeOnc Technologies (Los Angeles, California). No potential conflict of interest was disclosed by the other authors.
Authorship statement.
W.W., H.H., and N.I.M.-R designed and performed experiments, contributed to data collection and analysis, and drafted the manuscript. S.Z., H.-Y.C., and S.D.S. performed experiments and contributed to data collection and analysis. J.F. contributed to data collection and analysis. P.M.B. contributed to study design and interpretation. J.N. and L.C. contributed to study design and materials. A.H.S. contributed to study design and data analysis, acquired funding, and finalized the manuscript. T.C.C. conceived of the study, acquired funding, contributed to study design, data analysis, and supervision. All authors reviewed and approved the final version of the manuscript.
References
- 1.Krishnan M, Krishnamurthy J, Shonka N. Targeting the sanctuary site: options when breast cancer metastasizes to the brain. Oncology (Williston Park). 2019;33(8):683730. [PubMed] [Google Scholar]
- 2.Mills MN, Figura NB, Arrington JA, et al. Management of brain metastases in breast cancer: a review of current practices and emerging treatments. Breast Cancer Res Treat. 2020;180(2):279–300. [DOI] [PubMed] [Google Scholar]
- 3.Soffietti R, Ahluwalia M, Lin N, Rudà R. Management of brain metastases according to molecular subtypes. Nat Rev Neurol. 2020;16(10):557–574. [DOI] [PubMed] [Google Scholar]
- 4.Sprowls SA, Arsiwala TA, Bumgarner JR, et al. Improving CNS delivery to brain metastases by blood-tumor barrier disruption. Trends Cancer. 2019;5(8):495–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Babak MV, Zalutsky MR, Balyasnikova IV. Heterogeneity and vascular permeability of breast cancer brain metastases. Cancer Lett. 2020;489:174–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lockman PR, Mittapalli RK, Taskar KS, et al. Heterogeneous blood-tumor barrier permeability determines drug efficacy in experimental brain metastases of breast cancer. Clin Cancer Res. 2010;16(23):5664–5678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Osswald M, Blaes J, Liao Y, et al. Impact of blood-brain barrier integrity on tumor growth and therapy response in brain metastases. Clin Cancer Res. 2016;22(24):6078–6087. [DOI] [PubMed] [Google Scholar]
- 8.Nieder C, Spanne O, Mehta MP, Grosu AL, Geinitz H. Presentation, patterns of care, and survival in patients with brain metastases: what has changed in the last 20 years? Cancer. 2011;117(11):2505–2512. [DOI] [PubMed] [Google Scholar]
- 9.Bendell JC, Domchek SM, Burstein HJ, et al. Central nervous system metastases in women who receive trastuzumab-based therapy for metastatic breast carcinoma. Cancer. 2003;97(12):2972–2977. [DOI] [PubMed] [Google Scholar]
- 10.Dawood S, Ueno NT, Valero V, et al. Incidence of and survival following brain metastases among women with inflammatory breast cancer. Ann Oncol. 2010;21(12):2348–2355. [DOI] [PubMed] [Google Scholar]
- 11.Laakmann E, Witzel I, Neunhoffer T, et al. Characteristics and clinical outcome of breast cancer patients with asymptomatic brain metastases. Cancers (Basel). 2020;12(10):2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Niikura N, Hayashi N, Masuda N, et al. Treatment outcomes and prognostic factors for patients with brain metastases from breast cancer of each subtype: a multicenter retrospective analysis. Breast Cancer Res Treat. 2014;147(1):103–112. [DOI] [PubMed] [Google Scholar]
- 13.Xu Z, Marko NF, Chao ST, et al. Relationship between HER2 status and prognosis in women with brain metastases from breast cancer. Int J Radiat Oncol Biol Phys. 2012;82(5):e739–e747. [DOI] [PubMed] [Google Scholar]
- 14.Gori S, Puglisi F, Moroso S, et al. The HERBA study: a retrospective multi-institutional Italian study on patients with brain metastases from HER2-positive breast cancer. Clin Breast Cancer. 2019;19(4):e501–e510. [DOI] [PubMed] [Google Scholar]
- 15.Sperduto PW, Kased N, Roberge D, et al. Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol. 2012;30(4):419–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Subbiah IM, Lei X, Weinberg JS, et al. Validation and development of a modified breast graded prognostic assessment as a tool for survival in patients with breast cancer and brain metastases. J Clin Oncol. 2015;33(20):2239–2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khan M, Zhao Z, Arooj S, Zheng T, Liao G. Lapatinib plus local radiation therapy for brain metastases from HER-2 positive breast cancer patients and role of trastuzumab: a systematic review and meta-analysis. Front Oncol. 2020;10:576926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mortimer JE, Kruper L, Cianfrocca M, et al. Use of HER2-directed therapy in metastatic breast cancer and how community physicians collaborate to improve care. J Clin Med. 2020;9(6):1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paracha N, Reyes A, Diéras V, Krop I, Pivot X, Urruticoechea A. Evaluating the clinical effectiveness and safety of various HER2-targeted regimens after prior taxane/trastuzumab in patients with previously treated, unresectable, or metastatic HER2-positive breast cancer: a systematic review and network meta-analysis. Breast Cancer Res Treat. 2020;180(3):597–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joshi S, Meyers PM, Ornstein E. Intracarotid delivery of drugs: the potential and the pitfalls. Anesthesiology. 2008;109(3):543–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arvanitis CD, Askoxylakis V, Guo Y, et al. Mechanisms of enhanced drug delivery in brain metastases with focused ultrasound-induced blood-tumor barrier disruption. Proc Natl Acad Sci U S A. 2018;115(37):E8717–E8726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lundqvist H, Rosander K, Lomanov M, et al. Permeability of the blood-brain barrier in the rat after local proton irradiation. Acta Radiol Oncol. 1982;21(4):267–271. [DOI] [PubMed] [Google Scholar]
- 23.Chen TC, Fonseca CO, Schönthal AH. Preclinical development and clinical use of perillyl alcohol for chemoprevention and cancer therapy. Am J Cancer Res. 2015;5(5):1580–1593. [PMC free article] [PubMed] [Google Scholar]
- 24.da Fonseca CO, Teixeira RM, Silva JC, et al. Long-term outcome in patients with recurrent malignant glioma treated with perillyl alcohol inhalation. Anticancer Res. 2013;33(12):5625–5631. [PubMed] [Google Scholar]
- 25.Chen TC, da Fonseca CO, Schönthal AH. Perillyl alcohol and its drug-conjugated derivatives as potential novel methods of treating brain metastases. Int J Mol Sci. 2016;17(9):1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.da Fonseca CO, Khandelia H, Salazar MD, Schönthal AH, Meireles OC, Quirico-Santos T. Perillyl alcohol: dynamic interactions with the lipid bilayer and implications for long-term inhalational chemotherapy for gliomas. Surg Neurol Int. 2016;7:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang W, Marín-Ramos NI, He H, et al. NEO100 enables brain delivery of blood-brain barrier impermeable therapeutics. Neuro Oncol. 2021;23(1):63–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marín-Ramos NI, Thein TZ, Ghaghada KB, Chen TC, Giannotta SL, Hofman FM. miR-18a inhibits BMP4 and HIF-1α normalizing brain arteriovenous malformations. Circ Res. 2020;127(9):e210–e231. [DOI] [PubMed] [Google Scholar]
- 29.Helgers SOA, Talbot SR, Riedesel AK, et al. Body weight algorithm predicts humane endpoint in an intracranial rat glioma model. Sci Rep. 2020;10(1):9020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Virrey JJ, Golden EB, Sivakumar W, et al. Glioma-associated endothelial cells are chemoresistant to temozolomide. J Neurooncol. 2009;95(1):13–22. [DOI] [PubMed] [Google Scholar]
- 31.Marín-Ramos NI, Balabasquer M, Ortega-Nogales FJ, et al. A potent isoprenylcysteine carboxylmethyltransferase (ICMT) inhibitor improves survival in Ras-driven acute myeloid leukemia. J Med Chem. 2019;62(13):6035–6046. [DOI] [PubMed] [Google Scholar]
- 32.Kaisar MA, Sajja RK, Prasad S, Abhyankar VV, Liles T, Cucullo L. New experimental models of the blood-brain barrier for CNS drug discovery. Expert Opin Drug Discov. 2017;12(1):89–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bellati F, Napoletano C, Ruscito I, Liberati M, Panici PB, Nuti M. Cellular adaptive immune system plays a crucial role in trastuzumab clinical efficacy. J Clin Oncol. 2010;28(21):e369–e370; author reply e371. [DOI] [PubMed] [Google Scholar]
- 34.Bianchini G, Gianni L. The immune system and response to HER2-targeted treatment in breast cancer. Lancet Oncol. 2014;15(2):e58–e68. [DOI] [PubMed] [Google Scholar]
- 35.Triulzi T, Regondi V, De Cecco L, et al. Early immune modulation by single-agent trastuzumab as a marker of trastuzumab benefit. Br J Cancer. 2018;119(12):1487–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sattenberg RJ, Meckler J, Saver JL, Gobin YP, Liebeskind DS. Cerebral angiography. In: Grotta JC, ed. Stroke: Pathophysiology, Diagnosis, and Management. Amsterdam: Elsevier; 2016:790–805. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.