Abstract
Background
Global variations in survival for brain tumors are very wide when all histological types are considered together. Appraisal of international differences should be informed by the distribution of histology, but little is known beyond Europe and North America.
Methods
The source for the analysis was the CONCORD database, a program of global surveillance of cancer survival trends, which includes the tumor records of individual patients from more than 300 population-based cancer registries. We considered all patients aged 0-99 years who were diagnosed with a primary brain tumor during 2000-2014, whether malignant or nonmalignant. We presented the histology distribution of these tumors, for patients diagnosed during 2000-2004, 2005-2009, and 2010-2014.
Results
Records were submitted from 60 countries on 5 continents, 67 331 for children and 671 085 for adults. After exclusion of irrelevant morphology codes, the final study population comprised 60 783 children and 602 112 adults. Only 59 of 60 countries covered in CONCORD-3 were included because none of the Mexican records were eligible. We defined 12 histology groups for children, and 11 for adults. In children (0-14 years), the proportion of low-grade astrocytomas ranged between 6% and 50%. Medulloblastoma was the most common subtype in countries where low-grade astrocytoma was less commonly reported. In adults (15-99 years), the proportion of glioblastomas varied between 9% and 69%. International comparisons were made difficult by wide differences in the proportion of tumors with unspecified histology, which accounted for up to 52% of diagnoses in children and up to 65% in adults.
Conclusions
To our knowledge, this is the first account of the global histology distribution of brain tumors, in children and adults. Our findings provide insights into the practices and the quality of cancer registration worldwide.
Keywords: epidemiological study, health care disparities, histology, International Classification of Diseases, population-based cancer registries, primary brain tumor
Key Points.
A study on the histology distribution of brain tumors spanning 59 countries in 5 continents.
Wide international variation suggesting disparities in registration practices and data quality.
Robust evidence for actions aimed to improve data quality and to harmonize data collection worldwide.
Importance of the Study.
To our knowledge, this is the first study of the histology distribution of brain tumors worldwide. We analyzed individual records for nearly 700 000 patients diagnosed with a primary brain tumor in 59 countries, during 2000-2014. Many countries were included for the first time in international comparisons. Data were collected using the same protocol to ensure robustly comparable information. We considered children and adults separately, using distinct histology groupings. The global variation in the histology distribution was remarkable. We provided evidence that such variation may be mainly due to international differences in cancer registration practices but also to wide disparities in the quality of data. This study population will be used for further global comparisons of brain tumor survival by histology. Our study should prompt cancer registries to improve data quality and to cooperate internationally for the harmonization of data collection.
Central nervous system (CNS) tumors encompass more than 50 histological subtypes, with distinct genetic hallmarks, clinical behavior, and survival.1
CNS tumors represent an important cause of cancer-related death in children, adolescents, and young adults.2,3 Given that most of the patients live in low- and middle-income countries, the social burden of brain tumors is disproportionately great in countries that are generally least well equipped to deal with that burden.2,4,5
In order to make a robust international comparison of the frequency of the various histological types of brain tumor, it is first necessary to define suitable histology groupings. The standard framework for presenting data on tumors in children is the International Classification of Childhood Cancer, third edition (ICCC-36), based on the International Classification of Diseases for Oncology, third edition (ICD-O-3). A separate framework for adolescents and young adults was devised by Barr et al.7 Further schemes for grouping brain tumors by their histology include those used in Cancer Incidence in Five Continents (CI5), the Central Brain Tumor Registry of the United States (CBTRUS), and the European Information Network on Rare Cancers (RARECARENet).8–10 Such strategies are not specific to children or adults, however, and the level of granularity varies.
The distribution of brain tumors by histology has only been described as part of analyses of incidence or survival by histology in a given country, region, or territory,11 but differences in study design do not allow valid comparisons. Large international population-based studies, such as the Automated Childhood Cancer Information System (ACCIS) and the European Cancer Registry-based study on survival and care of cancer patients (EUROCARE), used standardized data collection, but they only include European countries.12–14 African, Central and South American, and Asian countries are substantially under-represented in brain tumor studies by histology.11
The CONCORD program established global surveillance of trends in cancer survival in 2015.15 The third cycle, in 2018 (CONCORD-3), included individual data for more than 37 million patients from 71 countries, diagnosed with 1 of the 18 common tumors during 2000-2014. CONCORD-3 highlighted the wide global disparities in survival from all brain tumors combined.
Knowledge of the histology distribution in cohorts of cancer patients used for population-based survival analyses is key to interpreting the worldwide disparities in survival for all brain tumor subtypes combined. Limited access to care is likely to be the main reason for the global inequalities in survival, but international differences could also arise by confounding if the distribution of histological subtypes varies worldwide and there are international differences in survival between the histological subtypes. For health care systems aiming to track cancer outcomes, clinically relevant survival data by histology are crucial. Only estimates that are based on accurate registration of brain tumors can safely be used by public health officials for cancer control planning at the national and international levels. Robust survival estimates may also be used by clinicians and epidemiologists to monitor adherence to clinical guidelines.
Using the CONCORD-3 database, we aimed to assess international differences in reporting of the histology of brain tumors and the main indicators of data quality in cancer registration, in children and adults. This study aims to help appraise the validity of future global comparisons of survival from brain tumors using CONCORD-3 data.
Patients and Methods
Records were obtained from data supplied by 286 of the 322 population-based cancer registries participating in CONCORD-3. Data were collected using the same protocol and centrally validated for protocol adherence and consistency through a rigorous 3-phase data quality control procedure (details published elsewhere).15,16 In brief, registrations based on a death certificate or autopsy, age out of range and those with invalid date sequences were excluded. Possible errors included implausible combinations of age, sex, site, and morphology. Each registry was invited to confirm or correct records with possible errors.
The study population comprised children (0-14 years) and adults (15-99 years), diagnosed during 2000-2014 with a tumor originating in the brain (ICD-O-3 topography code C71), and for whom a morphology code was available. We included both primary, malignant tumors (ICD-O-3 behavior code 3) and nonmalignant tumors, whether benign or of uncertain behavior (code 0 or 1, respectively).
We used ICD-O-3 to select the morphology codes and the World Health Organisation (WHO) Classification of Central Nervous System Tumors (fourth edition) for the definition of pathology.1,17
Morphology codes in ICD-O-3 such as 9400/3 with the attribute “not otherwise specified” (NOS) can be used in cancer registration. Rule G of ICD-O-3 allows the use of a sixth digit in the morphology code to define the histological grading or degree of differentiation.17 We used this rule to re-classify tumors coded to “astrocytoma NOS” (ICD-O-3 code 9400/3) to one of the more specific astrocytic subtypes. We did not recode “astrocytoma NOS” with an undetermined grade (grade 9); these were analyzed separately. The sixth digit of the morphology code is assigned by the pathologist or the registrar, while the WHO grade is part of the tumor subtype definition.
The London School of Hygiene and Tropical Medicine’s Ethics Committee approved the project.
Results
CONCORD-3 included 67 331 children and 671 085 adults diagnosed with a primary brain tumor in 60 countries during 2000-2014 (Supplementary Tables 1 and 2).
We defined distinct histology groupings for children and adults. For children, we mainly followed ICCC-3,6 but we made three changes: (1) we introduced a third, more granular tier for astrocytic tumors; (2) three of the four ICCC-3 subgroups of embryonal tumors (atypical teratoid/rhabdoid tumor, medulloepithelioma, and primitive neuroectodermal tumor) were grouped together, and (3) oligoastrocytoma was included in the “oligodendroglial tumor” histology group. For adults, there are no bespoke classification systems, so we based our definitions on advice from expert pathologists. The 12 histology groupings adopted for children, and the 11 groupings adopted for adults are set out in Supplementary Tables 3 and 4, respectively.
We excluded from analysis 6548 children (9.7% of eligible tumor records) and 68 973 adults (10.3%) because the morphology code was (1) not consistent with the WHO classification; (2) consistent with the WHO classification but relevant only for the meninges or the pituitary gland, or (3) missing (Supplementary Tables 5 and 6).
The final study population comprised 60 783 children (90.3% of eligible submissions) and 602 112 adults (89.7%). The study covered only 59 of the 60 countries included in CONCORD-3: Mexico was excluded because none of the records had a valid morphology code. Supplementary Tables 1 and 2 present detailed trends for 2000-2004, 2005-2009, and 2010-2014, by country and histology group.
We focus our comments mainly on the histology distribution for 2005-2009 when proportions were more robust than for 2000-2004 and 2010-2014 because more registries contributed data for the central period. The comments are broadly applicable to earlier and later periods.
Children (0-14 Years)—2005-2009
The proportion of brain tumors classified as low-grade astrocytoma (WHO grade I or II) varied from less than 10% to more than 30%. The proportion was below 10% in African countries, in Brazil, Costa Rica, Ecuador, China, Korea, Thailand, the Russian Federation, and New Zealand; in the range 10%-19% in Argentina, Colombia, Japan, Jordan, Taiwan, Poland, and Australia, and in the range 20%-29% in Chile, Canada, Israel, Singapore, Turkey, Croatia, Denmark, France, Germany, Italy, Norway, Portugal, Spain, and Sweden. These tumors accounted for more than 30% of brain tumors in Puerto Rico, the United States, Belarus, Czech Republic, Finland, Ireland, the Netherlands, Slovakia, Switzerland, and the United Kingdom (Supplementary Table 1, Figure 1).
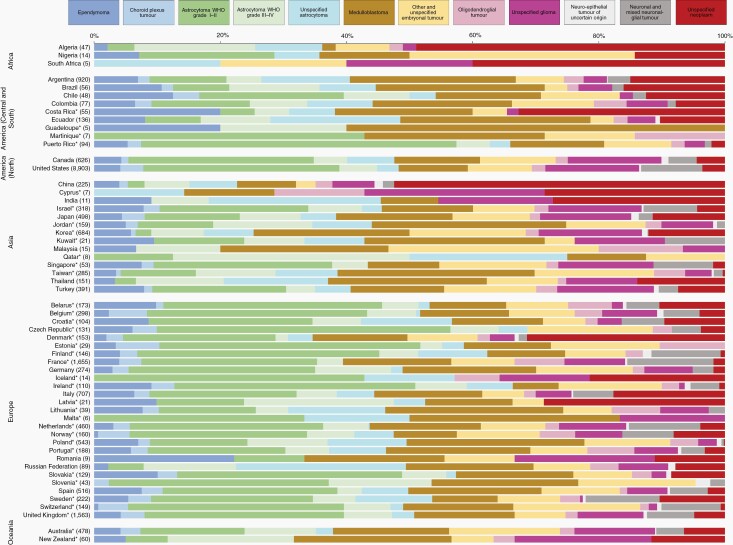
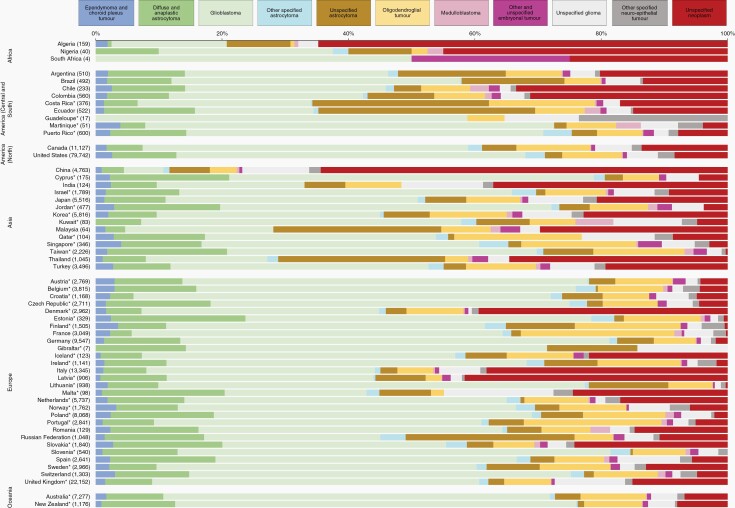
Fig. 1.
Histology distribution (%) by country, children (0-14 years), 2005-2009. Numbers in brackets are counts (all brain tumors combined). *Data with 100% coverage of the national population.
High-grade astrocytomas (WHO grade III or IV) comprised less than 10% of all brain tumors in Argentina, Colombia, Costa Rica, Puerto Rico, Canada, the United States, China, Israel, Japan, Korea, Singapore, Turkey, and in 17 of 28 participating European countries. The proportion was in the range 10%-20% in African countries, and in Brazil, Ecuador, Jordan, Taiwan, Thailand, Germany, Poland, the Russian Federation, Australia, and New Zealand (Supplementary Table 1, Figure 1).
Unspecified astrocytomas (ICD-O-3 code 9400/39) accounted for less than 10% of brain tumors in 30 of 59 countries, but the proportion was in the range 10%-19% in African countries, and in Argentina, Colombia, Thailand, Croatia, Finland, Poland, the Russian Federation, and Sweden. The highest levels were seen in Ecuador (20%) and the Russian Federation (27%). Unspecified astrocytoma was ungraded (sixth digit of the ICD-O-3 morphology code) in less than 50% of the cases in Puerto Rico, Israel, Belarus, Belgium, Germany, the Netherlands, Slovakia, and New Zealand; in 50%-99% of the cases in African countries, Ecuador, Canada, the United States, China, Japan, Jordan, Taiwan, Turkey, Czech Republic, Italy, Norway, Poland, Portugal, the Russian Federation, Spain, Switzerland, the United Kingdom, and Australia; 100% ungraded elsewhere. Most cases with known grade were assigned grade 1 or 2 in the United States, Israel, Taiwan, Turkey, France, Germany, the Netherlands, the United Kingdom, and Australia. In Jordan, however, there were slightly more tumors with grade 3-4 than 1-2 (Supplementary Tables 1 and 7, Figure 1).
Medulloblastomas represented less than 10% of brain tumors in African countries, and in China and Ireland. The proportion was in the range 10%-19% in 21 of 59 countries; and in the range 20%-29% in Argentina, Brazil, Korea, Thailand, Poland, and New Zealand; the proportion was 30% in Ecuador, 31% in Jordan in Taiwan (Supplementary Table 1, Figure 1).
Unspecified tumors represented less than 10% of brain tumors in most countries. The proportion was in the range 10%-20% in Argentina, Brazil, Ecuador, Japan, Korea, Thailand, Belarus, Italy, and New Zealand. The proportion was 41% in African countries, 33% in Costa Rica, 52% in China, and 31% in Denmark (Supplementary Table 1, Figure 1).
Adults (15-99 Years)—2005-2009
Diffuse and anaplastic astrocytomas accounted for less than 10% of brain tumors in 26 of 59 countries. The proportion was in the range 10%-19% in Argentina, Brazil, Chile, Ecuador, Puerto Rico, the United States, Cyprus, Israel, Jordan, Qatar, Singapore, Taiwan, Austria, Belgium, Czech Republic, Germany, Latvia, Malta, the Netherlands, Poland, Romania, the Russian Federation, Slovakia, Slovenia, Spain, Switzerland, and New Zealand; the proportion was 21% in Estonia (Supplementary Table 2, Figure 2).
Fig. 2.
Histology distribution (%) by country, adults (15-99 years), 2005-2009. Numbers in brackets are counts (all brain tumors combined). *Data with 100% coverage of the national population.
The proportion of brain tumors classified as glioblastoma varied from less than 10% to more than 50%. The proportion was below 10% only in China; in the range 10%-29% in Algeria, Nigeria, Costa Rica, Ecuador, India, Malaysia, Thailand, Malta, and the Russian Federation; in the range 30%-49% in Argentina, Brazil, Chile, Colombia, Japan, Korea, Qatar, Singapore, Taiwan, Turkey, Denmark, Iceland, Italy, Latvia, Romania, Slovakia, and Spain; in the range 50%-70% in Martinique, Puerto Rico, North America, Cyprus, Israel, Jordan, Kuwait, in 21 of 28 participating European countries and in Oceania (Supplementary Table 2, Figure 2).
Unspecified astrocytoma encompassed less than 10% of brain tumors in 43 of 59 countries. The proportion was in the range 10%-19% in Algeria, Argentina, Brazil, Colombia, Finland, and Lithuania, and in the range 20%-29% in Costa Rica, Malaysia, Thailand, and the Russian Federation. The highest level was seen in Ecuador (34%). Unspecified astrocytoma was ungraded (sixth digit of the ICD-O-3 morphology code) in less than 50% of the cases in Puerto Rico, the United States, Cyprus, Israel, Jordan, Qatar, Singapore, Turkey, Belgium, Czech Republic, the Netherlands, Slovakia, Slovenia, Spain, Switzerland, the United Kingdom, and New Zealand; in 50%-99% of the cases in 23 countries; and 100% ungraded in Nigeria, Brazil, Costa Rica, Martinique, Korea, Malaysia, Croatia, Denmark, Estonia, Finland, France, Iceland, Ireland, Latvia, and Sweden. Most cases with known grade were assigned grade 1 in Norway and the Russian Federation, grade 2 or 3 in 30 countries, and grade 4 in Canada (Supplementary Tables 2 and 8, Figure 2).
Brain tumors of unspecified histology accounted for less than 10% of brain tumors in 28 countries. The proportion was in the range 10%-19% in Brazil, Costa Rica, Ecuador, Canada, Turkey, the Netherlands, Romania, the Russian Federation, Sweden, and the United Kingdom; in the range 30%-50% in Nigeria, Chile, Colombia, India, Thailand, Denmark, Italy, and Latvia. The highest levels were seen in Algeria (65%) and China (65%) (Supplementary Table 2, Figure 2).
Basis of Diagnosis—2000-2014
In children, the vast majority of low-grade astrocytomas were histologically verified. The proportion was in the range 90%-94% in Canada and Australia; in the range 95%-99% in the United States, Israel, Japan, Singapore, Turkey, Belgium, Croatia, France, Germany, Italy, the Netherlands, Spain, and the United Kingdom; and 100% in the remaining 38 countries (Supplementary Table 9).
For childhood unspecified neoplasms, a diagnostic confirmation was mostly not available in Central and South America, North America, Asia, Europe, and Oceania (10%-25%), while diagnoses were largely confirmed in Africa (74%) (Supplementary Table 9).
In adults, glioblastomas were mostly histologically verified. The proportion was 79% in Malta; in the range 80%-89% in Canada, Croatia, Norway, the United Kingdom, and New Zealand; in the range 90%-94% in the United States, Israel, Korea, Kuwait, Austria, Germany, Switzerland, and Australia; in the range 95%-99% in 26 countries; glioblastomas were reported as 100% histologically verified in the remaining 13 countries (Supplementary Table 10).
The proportion of histological verification for unspecified neoplasms, in adults, varied between 4% in Oceania and 65% in Africa (Supplementary Table 10).
Time Trends
The proportion of low-grade astrocytomas in children was fairly stable in all continents during the 15 years between 2000 and 2014. The proportion of unspecified neoplasms rose from 2% to 6% in North America.
In adults, the proportion of glioblastomas during 2000-2014 rose only in Europe (from 46% to 56%) and Oceania (from 57% to 65%). Increasing trends for unspecified neoplasms were observed in Central and South America, while the proportions for both subtypes subsided in Europe and Oceania. In North America, the proportion of unspecified neoplasms rose from 6% to 12%.
Discussion
To our knowledge, this is the first global study of the distribution of brain tumor histology. It spans 60 countries in 5 continents and includes countries, regions, or territories not previously represented in international comparisons. We analyzed individual patient records from 286 population-based cancer registries. Data were collected using the same study protocol and checked using the same data quality procedures to ensure high-quality and robustly comparable information.
There is wide international variation in the distribution of brain tumor subtypes around the world. There were striking international differences in the proportion of low-grade astrocytomas in children (ranging from 6% to 50% in 2005-2009). The proportion of childhood medulloblastomas also varied widely between countries, in several of which it offset the low proportion of low-grade astrocytomas. In adults, the largest international variation was for glioblastomas (from 9% to 69%).
We found wide international disparities in some of the quality indicators, such as the proportion of tumors with an unspecified histology, up to 52% in children and 65% in adults, and the proportion of histologically verified tumors.
Nonmalignant brain tumors should be recorded by all cancer registries because the location of brain tumors is a determinant of the outcome as well as histology. We have provided compelling evidence that remarkable international differences exist in the registration of nonmalignant brain tumors. This was mostly seen for low-grade astrocytomas in children, and for childhood neuronal and mixed neuronal-glial tumors, both of which are mainly nonmalignant subtypes. For instance, Ecuador started recording nonmalignant brain tumors in 2011, while in New South Wales, Australia, only malignant brain tumor subtypes are registered, by law.
ICCC-3 is a well-established standard for conducting studies on childhood tumors, but it does not allow for stratification of astrocytic tumors by WHO grade.6 Studies on survival from childhood brain tumors published to date have generally adopted ICCC-3, but the interpretation of time trends and international differences is complicated by changes in coding over time and the inconsistent registration of nonmalignant tumors between countries or regions.11 ICD-O underwent a major change in 2000, coinciding with the release of the third edition. Pilocytic astrocytoma was attributed a behavior code of 3 (malignant) in ICD-O-2 and a behavior code of 1 (borderline) in ICD-O-3.17,18 In the United States, where registration of nonmalignant tumors has been mandatory since 2004,19 pilocytic astrocytoma (WHO grade I) alone represented 30% of all childhood gliomas (2007-2011).20 In countries where nonmalignant tumors are inconsistently recorded, pilocytic astrocytoma has become potentially ineligible for cancer registration since 2000. Failing to record pilocytic astrocytoma, the single most common childhood brain tumor, and any other nonmalignant tumors could potentially lead to underestimation of both incidence and survival for all childhood astrocytic tumors combined, regardless of behavior. If these international differences in cancer registration practices are not properly considered, global disparities in survival for all astrocytic tumors may be wrongly interpreted. Survival in countries or regions that only include malignant brain tumors will be systematically lower than in countries where nonmalignant tumors are also registered. CONCORD-3 showed wide international disparities in survival from childhood brain tumors. For instance, among children diagnosed during 2005-2009, age-standardized 5-year net survival varied from less than 40% in Brazil, 60% in Australia, and close to 80% in Sweden.15 Those disparities persisted substantially unchanged among children diagnosed during 2010-2014. In our study, during 2005-2009, the proportion of low-grade astrocytoma in Brazil, Australia, and Sweden were 9%, 17%, and 26%, respectively. The use of misleading survival estimates may have huge implications when the public is engaged in research, because it may lead to a distorted perception of cancer burden and risk.
The CONCORD-3 protocol required data to be coded according to ICD-O-3, and both malignant and nonmalignant brain tumors were eligible. In this study, however, pilocytic astrocytoma was still coded as malignant (ICD-O-3 behavior code 3) in 7194 children and 5344 adults. For instance, the proportion of miscoded childhood pilocytic astrocytoma was 70% in Czech Republic and 100% in Canada, the United States, Israel, and Taiwan (data not shown).
Glioblastomas comprised 80% of astrocytic tumors in the 40-99 years age group in North America, Europe, and Oceania during 2000-2014, but only 60% or less in Central and South America (data not shown). Glioblastoma incidence was considerably higher in non-Hispanic Whites than in other ethnicities in the United States during 2000-2014, suggesting that risk alleles are more common in populations of predominantly European ancestry.9,21 Alternatively, a higher proportion of cases in a given population could reflect an older population, because the incidence of glioblastoma increases with age. In countries where glioblastomas were more frequently reported in 2010-2014 than in 2000-2004 and 2005-2009, we found a concurrent decline in the proportion of unspecified astrocytomas (eg, Croatia, Poland, Portugal, and the United Kingdom) or the proportion of diffuse and anaplastic astrocytomas (eg, Belgium, France, the Netherlands, and Korea). These findings may suggest improved quality in cancer registration but also a refinement in the pathology workup of astrocytic tumors enabling identification of clinically aggressive subtypes.
We combined diffuse astrocytoma and anaplastic astrocytoma in adults into a single group. Sub-optimal reproducibility of the pathological diagnosis of glioma has been clearly established, with an estimated 20%-30% of gliomas re-classified at the independent review.22,23 Mutations in the isocitrate dehydrogenase (IDH) gene 1 or 2 were recognized to be a genetic hallmark of glioblastoma in 2008.24 These mutations were later found to characterize 70%-80% of WHO grade II and III gliomas.25 Tumors harboring an IDH mutation have a more favorable outcome.26,27 Grade II or III gliomas with the same genetic profile have a similar clinical behavior, regardless of the pathological grading.23
The definition “astrocytoma NOS” was used in previous studies for ill-defined astrocytic tumors which could not be assigned a more precise descriptor (eg, glioblastoma).13,14 “Astrocytoma NOS,” however, is a standalone definition in ICD-O-3, rather than a category for astrocytic tumors that could not be otherwise specified, and it shares the same morphology code with “diffuse astrocytoma” (WHO grade II).1,17 In 2005-2009, the proportion of brain tumors that were coded as astrocytoma NOS varied widely between countries, suggesting different practices and interpretations among cancer registries. The quality of cancer registration, however, seemed to improve during 2000-2014, because the use of “astrocytoma NOS” fell substantially in several countries (eg, from 41% to 23% in Ecuador or from 29% to 5% in Thailand). We supposed that the recording of grade (rule G in ICD-O) was accurate. For a given record, if the grade (sixth digit of histology) was coded in the range 1-4, the use of the definition “astrocytoma NOS” was assumed to be a random error at coding level; if the grade was not available (coded to 9), we assumed that the tumor could not be defined more precisely. Such a strategy should control for randomly misclassified astrocytic tumors.
We included both histologically confirmed and histologically unconfirmed brain tumors, in line with previous studies.9,13,14 The diagnosis of a brain tumor may pose challenges due to anatomical constraints for safely performing biopsy or surgery, or the poor clinical condition of the patient. These hurdles may be more relevant for adults, who are frequently diagnosed at an advanced age. Brain tumors often show pathognomonic appearances at neuroimaging, potentially making a firm clinical diagnosis plausible.28,29 Nevertheless, the differential diagnosis between a solitary metastasis and a high-grade glioma may be challenging in clinical practice if advanced imaging techniques are not available.30 International coding guidelines currently restrict the use of a specific morphology code in the absence of histological verification to certain clinical situations (eg, neoplasms located in the brain stem). In all other cases, the morphology code for a tumor of unspecified morphology (ie, 8000-8005) should be preferred if the diagnosis cannot be histologically proven. However, with the refinement of neuroimaging, these guidelines may need to be updated.31
In these data, the proportion of histological verification for specified tumor subtypes was around 100% in children, while in adults it was slightly lower, but still in the range 90-100%. The higher proportion of histological confirmation in children than in adults may point to increased diagnostic intensity in children or to the existence of specialist pediatric cancer registries. Very high proportions of histological confirmation, however, may also suggest over-reliance on pathology records for cancer registration, or under-ascertainment of brain tumors.32 The selective recording of only histologically verified brain tumors may ultimately bias survival estimates upward, because patients receiving a biopsy or surgery are more likely to present in better clinical condition and because the availability of these procedures may reflect better access to treatment in general.
In this study, the proportion of tumors of unspecified histology (ICD-O-3 codes 8000-8005) was generally low, but some countries had high proportions, particularly in adults. Interestingly, in countries where the proportion of unspecified tumors was 30% or more, the quality of data was consistently poor across all participating sub-national registries (data not shown). In some countries (eg, Algeria, China), the proportion of tumors of unspecified histology was higher than the proportion of glioblastoma. These findings suggest that barriers to the accurate reporting of a brain tumor may intervene at all stages, including formulation and clinical recording of the diagnosis, data transmission, and data extraction for the cancer registry. If the accuracy of neuropathology reports is called into question, it is important to measure the effect on patient outcomes, which may be poorer if treatment is not appropriate for the specific histology. Furthermore, survival estimates for specific tumor subtypes are likely to be biased if the histology is fully known for only a subset of records, those estimates may not be robustly generalizable to the entire population of a given country or territory. The broad global variation in the proportion of tumors of unspecified histology calls for caution when interpreting the histology distribution itself, but also in interpreting the survival inequalities for individual brain tumor subtypes or for all brain tumors combined. Overall, we found a decline in the proportions of neoplasms of unspecified histology over the 15-year period 2000-2014, but increasing trends were observed in North America for both children and adults, although both the values and the changes were small. Surprisingly, in several countries, most or even 100% of brain tumors with an unspecified histology were reported as histologically confirmed. One would expect that histologically confirmed tumors could be assigned a specific morphology code. This suggests that the basis of diagnosis may be miscoded, so its use as an indicator of data quality requires caution. We did not exclude countries where the quality of brain tumor reporting was poor, because putting these countries in the context of a large international comparison is crucial to prompt action to improve cancer registration.
Our study provided insights into the data quality indicators that are relevant to reporting of brain tumors worldwide, namely the proportion of tumors of unspecified histology and the proportion of histological verification by brain tumor subtype. However, given the scale of the study, we could not explore other important quality measures, for instance whether multiple data sources were used to capture or validate a brain tumor diagnosis. Ascertainment of nonmalignant brain tumors is likely to be incomplete in several countries. While this finding may suggest poor access to care, it is more likely to reflect disparities in local health regulations, which we could not take into account. Other important indicators of data quality are the proportion of brain tumors registered only from a death certificate or detected at autopsy, or the proportion of patients lost to follow-up in countries using active follow-up, or, alternatively, the proportion of patients censored alive before 5 years from diagnosis where passive follow-up is in place. These indicators, however, are only relevant to the estimation of survival and will be analyzed in the future.
In CONCORD-3, we only collected data for tumors of the brain (ICD-O topography code C71). Diagnoses for histological subtypes in other parts of the CNS, such as germ cell tumors of the pineal gland or optic nerve gliomas, were excluded because they were likely to be misclassified or to represent a minority of the true population of patients for that subtype. For instance, germ cell tumors and optic nerve gliomas accounted for 4% and 6% of all childhood CNS tumors, respectively, in England, during 2001-2010.33 These tumors are uncommon, but they should ideally be included in future iterations of CONCORD.
In this study, the definition of the histology groupings, and the selection of the relevant ICD-O-3 morphology codes, was based on the WHO Classification of Central Nervous System Tumors, fourth edition (2007).1 In 2016, however, a revision of the WHO classification revolutionized the taxonomy of CNS tumors by defining tumor entities genetically, and prioritizing the molecular profile over the traditional WHO grading system.34 ICD-O-3 was updated accordingly. From 2018, the CBTRUS started collecting population-based data from 48 statewide cancer registries using the 2016 WHO categories.9 It may take a long time for other cancer registries worldwide to follow suit and for data to be used in survival analyses. However, in some countries, molecular assays may simply be unavailable. Notwithstanding these obstacles, international and continental associations of cancer registries should promote transition to the new neuropathology lexicon for data collection, paving the way for modern, informative international comparisons in brain tumor survival by histology.
In conclusion, this study population will be used for further global comparisons of brain tumor survival by histology. International disparities in survival can only be interpreted if a detailed analysis of the histology distribution in each cancer population is available. The quality of data is sub-optimal in several countries. Data from countries with low proportions of ill-defined tumors (ie, of unspecified histology or labeled as NOS) and in which the histology distribution is fairly reproducible over time, may be used to improve the comparability of survival estimates for all brain tumors combined. Standardization by histology, using proportionally weights based on a reliable histology distribution, is one possible approach.
In practice, hurdles to the collection of robust histology data can only be overcome if International Associations of Cancer Registries (IACR) and pathologists (IAP) can cooperate to promote harmonization of data collection. The Global Initiative for Cancer Registry Development (GICR),35 led by the International Agency for Research on Cancer and the Union for International Cancer Control (UICC), aims to help countries improve the quality of their population-based cancer data by training registry staff and strengthening local health information systems. Other strategies, relevant to both long-standing and more recent cancer registries, include audits at the local and national level on the quality of pathological diagnosis, as well as the quality and completeness of cancer registration. Ultimately, these initiatives would enable clinicians, policy-makers, and other stakeholders to use population-based data on the incidence and survival from brain tumors for public health purposes with greater confidence.
CONCORD Working Group
Africa—Algeria: S Bouzbid (Registre du Cancer d’Annaba); M Hamdi-Chérif, Z Zaidi (Registre du Cancer de Sétif); K Meguenni, D Regagba (Registre du Cancer Tlemcen); Mali: S Bayo, T Cheick Bougadari (Kankou Moussa University); Mauritius: S S Manraj (Mauritius National Cancer Registry); Morocco: K Bendahhou (Registre du Cancer du Grand Casablanca); Nigeria: A Ladipo, O J Ogunbiyi (Ibadan Cancer Registry); South Africa: T Ramaliba, N I M Somdyala (Eastern Cape Province Cancer Registry).
America (Central and South)—Argentina: M A Chaplin F Moreno (National Childhood Cancer Registry); G H Calabrano, S B Espinola (Chubut Cancer Registry); B Carballo Quintero, R Fita (Registro Provincial de Tumores de Córdoba); W D Laspada (Registro Provincial de Tumores de Mendoza); S G Ibañez (Population Registry of Cancer of the Province Tierra del Fuego); Brazil: C A Lima (Registro de Câncer de Base Populacional de Aracaju); A Mafra da Costa (Registro de Câncer de Base Populacional da Região de Barretos); P C F De Souza (Registro de Câncer de Base Populacional de Cuiabá); K Del Pino, C Laporte (Registro de Curitiba); M P Curado, J C de Oliveira (Registro de Goiânia); C L A Veneziano, D B Veneziano (Registro de Câncer de Base Populacional de Jaú); M R D O Latorre, L F Tanaka (Registro de Câncer de São Paulo); M S Rebelo, M O Santos (Instituto Nacional de Câncer, Rio de Janeiro); G Azevedo e Silva (University of Rio de Janeiro); Chile: J C Galaz (Registro Poblacional de Cáncer Region de Antofagasta); M Aparicio Aravena, J Sanhueza Monsalve (Registro Poblacional de Cáncer de la Provincia de Biobio; Registro Poblacional de Cáncer Provincia de Concepción); D A Herrmann, S Vargas (Registro Poblacional Region de Los Rios); Colombia: V M Herrera, C J Uribe (Registro Poblacional de Cáncer Area Metropolitana de Bucaramanga); L E Bravo, L S Garcia (Cali Cancer Registry); N E Arias-Ortiz, D Morantes (Registro Poblacional de Cáncer de Manizales); D M Jurado, M C Yépez Chamorro (Registro Poblacional de Cáncer del Municipio de Pasto); Costa Rica: S Delgado, M Ramirez (National Registry of Tumors, Costa Rica); Cuba: Y H Galán Alvarez, P Torres (Registro Nacional de Cáncer de Cuba); Ecuador: F Martínez-Reyes (Cuenca Tumor Registry); L Jaramillo, R Quinto (Guayaquil Cancer Registry); J Castillo (Loja Cancer Registry); M Mendoza (Manabí Cancer Registry); P Cueva, J G Yépez (Quito Cancer Registry); France: B Bhakkan, J Deloumeaux (Registre des cancers de la Guadeloupe); C Joachim, J Macni (General Cancer Registry of Martinique); Mexico: R Carrillo, J Shalkow Klincovstein (Centro Nacional para la Salud de la Infancia y la Adolescencia); R Rivera Gomez (Registro Poblacional de Cancer Region Fronteriza Norte de Mexico Zona Tijuana); Peru: P Perez, E Poquioma (Lima Metropolitan Cancer Registry); Puerto Rico: G Tortolero-Luna, D Zavala (Puerto Rico Central Cancer Registry); Uruguay: R Alonso, E Barrios (Registro Nacional de Cáncer).
America (North)—Canada: A Eckstrand, C Nikiforuk (Alberta Cancer Registry); R R Woods (British Columbia Cancer Registry); G Noonan, D Turner (Manitoba Cancer Registry); E Kumar, B Zhang (New Brunswick Provincial Cancer Registry); F R McCrate, S Ryan (Newfoundland & Labrador Cancer Registry); M MacIntyre, N Saint-Jacques (Nova Scotia Cancer Registry); A Anam, P De (Ontario Cancer Registry); C A McClure, K A Vriends (Prince Edward Island Cancer Registry); C Bertrand, J Latreille (Registre Québécois du Cancer); S Kozie, H Stuart-Panko (Saskatchewan Cancer Agency); United States: T Freeman, J T George (Alabama Statewide Cancer Registry); R M Avila, D K O’Brien (Alaska Cancer Registry); A Holt (Arkansas Central Cancer Registry); L Almon (Metropolitan Atlanta Registry); S Kwong, C Morris (California State Cancer Registry); R Rycroft (Colorado Central Cancer Registry); L Mueller, C E Phillips (Connecticut Tumor Registry); H Brown, B Cromartie (Delaware Cancer Registry); A G Schwartz, F Vigneau (Metropolitan Detroit Cancer Surveillance System); G M Levin, B Wohler (Florida Cancer Data System); R Bayakly (Georgia Cancer Registry); K C Ward (Georgia Cancer Registry; Metropolitan Atlanta Registry); S L Gomez, M McKinley (Greater Bay Area Cancer Registry); R Cress (Cancer Registry of Greater California); M D Green, K Miyagi (Hawaii Tumor Registry); C J Johnson (Cancer Data Registry of Idaho); L P Ruppert (Indiana State Cancer Registry); S Bentler, M E Charlton (State Health Registry of Iowa); B Huang, T C Tucker (Kentucky Cancer Registry); D Deapen, L Liu (Los Angeles Cancer Surveillance Program); M C Hsieh, X C Wu (Louisiana Tumor Registry); M Schwenn (Maine Cancer Registry); K Stern (Maryland Cancer Registry); S T Gershman, R C Knowlton (Massachusetts Cancer Registry); G Alverson, T Weaver (Michigan State Cancer Surveillance Program); S Bushhouse (Minnesota Cancer Surveillance System); D B Rogers (Mississippi Cancer Registry); J Jackson-Thompson (Missouri Cancer Registry and Research Center); D Lemons, H J Zimmerman (Montana Central Tumor Registry); M Hood, J Roberts-Johnson (Nebraska Cancer Registry); B Riddle, J R Rees (New Hampshire State Cancer Registry); K S Pawlish, A Stroup (New Jersey State Cancer Registry); C Key, C Wiggins (New Mexico Tumor Registry); A R Kahn, M J Schymura (New York State Cancer Registry); S Radhakrishnan, C Rao (North Carolina Central Cancer Registry); L K Giljahn, R M Slocumb (Ohio Cancer Incidence Surveillance System); A Feld (Oklahoma Central Cancer Registry); K G Aird, T Beran (Oregon State Cancer Registry); J J Rubertone, S J Slack (Pennsylvania Cancer Registry); J Oh (Rhode Island Cancer Registry); T A Janes, S M Schwartz (Seattle Cancer Surveillance System); S Chiodini, D M Hurley (South Carolina Central Cancer Registry); M A Whiteside (Tennessee Cancer Registry); S Rai, M A Williams (Texas Cancer Registry); K Herget, C Sweeney (Utah Cancer Registry); A T Johnson (Vermont Cancer Registry); M B Keitheri Cheteri, P Migliore Santiago (Washington State Cancer Registry); S E Blankenship, S Farley (West Virginia Cancer Registry); R Borchers, R Malicki (Wisconsin Department of Health Services); J Espinoza, J Grandpre (Wyoming Cancer Surveillance Program); H K Weir, R Wilson (Centers for Disease Control and Prevention); B K Edwards, A Mariotto (National Cancer Institute).
Asia—China: N Wang, L Yang (Beijing Cancer Registry); J S Chen, Y Zhou (Changle City Cancer Registry); Y T He, G H Song (Cixian Cancer Registry); X P Gu (Dafeng County Center for Disease Control and Prevention); D Mei, H J Mu (Dalian Centers for Disease Prevention and Control); H M Ge, T H Wu (Donghai County Center for Disease Prevention and Control); Y Y Li, D L Zhao (Feicheng County Cancer Registry); F Jin, J H Zhang (Ganyu Center for Disease Prevention and Control); F D Zhu (Guanyun Cancer Registry); Q Junhua, Y L Yang (Haimen Cancer Registry); C X Jiang (Haining City Cancer Registry); W Biao, J Wang (Jianhu Cancer Registry); Q L Li (Jiashan County Cancer Registry); H Yi, X Zhou (Jintan Cancer Registry); J Dong, W Li (Lianyungang Center for Disease Prevention and Control); F X Fu, S Z Liu (Linzhou Cancer Registry); J G Chen, J Zhu (Qidong County Cancer Registry); Y H Li, Y Q Lu (Sihui Cancer Registry); M Fan, S Q Huang (Taixing Cancer Registry); G P Guo, H Zhaolai (Cancer Institute of Yangzhong City); K Wei (Zhongshan City Cancer Registry); W-Q Chen, W Wei, H Zeng (The National Cancer Center); Cyprus: A V Demetriou (Cyprus Cancer Registry); Hong Kong: W K Mang, K C Ngan (Hong Kong Cancer Registry); India: A C Kataki, M Krishnatreya (Guwahati Cancer Registry); P A Jayalekshmi, P Sebastian (Karunagappally Cancer Registry); P S George, A Mathew (Trivandrum Cancer Registry); A Nandakumar (National Centre for Disease Informatics and Research); Iran: R Malekzadeh, G Roshandel (Golestan Population-Based Cancer Registry); Israel: L Keinan-Boker, B G Silverman (Israel National Cancer Registry); Japan: Y Koyanagi, H Ito (Aichi Cancer Registry); M Sato, F Tobori (Akita Prefectural Cancer Registry); I Nakata, N Teramoto (Ehime Prefectural Cancer Registry); M Hattori, Y Kaizaki (Fukui Cancer Registry); F Moki (Gunma Prefectural Cancer Registry); H Sugiyama, M Utada (Hiroshima Prefecture Cancer Registry); M Nishimura, K Yoshida (Hyogo Prefectural Cancer Registry); K Kurosawa, Y Nemoto (Ibaraki Prefectural Cancer Registry); H Narimatsu, M Sakaguchi (Kanagawa Cancer Registry); S Kanemura (Miyagi Prefectural Cancer Registry); M Naito, R Narisawa (Niigata Prefecture Cancer Registry); I Miyashiro, K Nakata (Osaka Cancer Registry); A Maeda, S Sato (Saga Prefectural Cancer Registry); I Oki (Tochigi Prefectural Cancer Registry); N Fukushima, A Shibata (Yamagata Prefectural Cancer Registry); K Iwasa, C Ono (Yamanashi Cancer Registry); T Matsuda (National Cancer Center); Jordan: O Nimri (Jordan National Cancer Registry); Korea: K W Jung, Y J Won (Korea Central Cancer Registry); Kuwait: E Alawadhi, A Elbasmi (Kuwait Cancer Registry); Malaysia: A Ab Manan (Malaysia National Cancer Registry); F Adam (Penang Cancer Registry); Mongolia: E Nansalmaa, U Tudev (Cancer Registry of Mongolia); C Ochir (Mongolian National University of Medical Sciences); Qatar: A M Al Khater, M M El Mistiri (Qatar Cancer Registry); Singapore: G H Lim, Y Y Teo (Singapore Cancer Registry); Taiwan: C J Chiang, W C Lee (Taiwan Cancer Registry); Thailand: R Buasom, S Sangrajrang (Bangkok Cancer Registry); K Suwanrungruang, P Vatanasapt (Khon Kaen Provincial Cancer Registry); K Daoprasert, D Pongnikorn (Lampang Cancer Registry; Lamphun Cancer Registry); A Leklob, S Sangkitipaiboon (Lopburi Cancer Registry); S L Geater, H Sriplung (Songkhla Cancer Registry); Turkey: O Ceylan, I Kög (Ankara Cancer Registry); O Dirican (Antalya Cancer Registry); T Köse (Bursa Cancer Registry); T Gurbuz (Edirne Cancer Registry); F E Karaşahin, D Turhan (Erzurum Cancer Registry Center); U Aktaş, Y Halat (Eskişehir Cancer Registry); S Eser, C I Yakut (Izmir Cancer Registry); M Altinisik, Y Cavusoglu (Samsun Cancer Registry); A Türkköylü, N Üçüncü (Trabzon Cancer Registry).
Europe—Austria: M Hackl (Austrian National Cancer Registry); Belarus: A A Zborovskaya (Belarus Childhood Cancer Subregistry); O V Aleinikova (Belarusian Research Center for Pediatric Oncology, Hematology and Immunology); Belgium: K Henau, L Van Eycken (Belgian Cancer Registry); Bulgaria: Z Valerianova, M R Yordanova (Bulgarian National Cancer Registry); Croatia: M Šekerija (Croatian National Cancer Registry); Czech Republic: L Dušek, M Zvolský (Czech National Cancer Registry); Denmark: L Steinrud Mørch, H Storm, C Wessel Skovlund (Danish Cancer Society); Estonia: K Innos, M Mägi (Estonian Cancer Registry); Finland: N Malila, K Seppä (Cancer Society of Finland); France: J Jégu, M Velten (Bas-Rhin General Cancer Registry); E Cornet, X Troussard (Registre Régional des Hémopathies Malignes de Basse Normandie); A M Bouvier (Registre Bourguignon des Cancers Digestifs); A V Guizard (Registre Général des Tumeurs du Calvados); V Bouvier, G Launoy (Registre des Tumeurs Digestives du Calvados); P Arveux (Breast cancers registry of Côte-d’Or France); M Maynadié, M Mounier (Hémopathies Malignes de Côte d’Or); A S Woronoff (Doubs and Belfort Territory General Cancer Registry); M Daoulas, M Robaszkiewicz (Finistère Cancer Registry); J Clavel, S Goujon (French National Registry of Childhood Hematopoietic Malignancies); B Lacour (National Registry of Childhood Solid Tumors); I Baldi, C Pouchieu (Gironde Registry of Primary Central Nervous System Tumors); B Amadeo, G Coureau (General Cancer Registry of Gironde Department); S Orazio (Registre des Hémopathies Malignes de la Gironde); A Monnereau (Registre des Hémopathies Malignes de la Gironde; French Network of Cancer Registries (FRANCIM)); P M Preux, F Rharbaoui (Registre Général des Cancers de Haute-Vienne); E Marrer (Haut-Rhin Cancer Registry); B Trétarre (Registre des Tumeurs de l’Hérault); M Colonna, P Delafosse (Registre du Cancer du Département de l’Isère); S Plouvier (Registre Général des Cancers de Lille et de sa Region); A Cowppli-Bony, F Molinié (Loire-Atlantique-Vendée Cancer Registry); S Bara (Manche Cancer Registry); O Ganry, B Lapôtre-Ledoux (Registre du Cancer de la Somme); P Grosclaude (Tarn Cancer Registry); N Bossard, Z Uhry (Hospices Civils de Lyon); J Estève (Université Claude Bernard, Lyon); Germany: R Stabenow, H Wilsdorf-Köhler (Common Cancer Registry of the Federal States); A Eberle, S Luttmann (Bremen Cancer Registry); I Löhden, A L Nennecke (Hamburg Cancer Registry); J Kieschke, E Sirri (Epidemiological Cancer Registry of Lower Saxony); C Justenhoven, S R Zeissig (Rhineland Palatinate Cancer Registry); B Holleczek (Saarland Cancer Registry); N Eisemann, A Katalinic (Schleswig-Holstein Cancer Registry); Gibraltar: R A Asquez, V Kumar (Gibraltar Cancer Registry); Greece: E Petridou (Nationwide Registry for Childhood Haematological Malignancies and Solid Tumors); Iceland: E J Ólafsdóttir, L Tryggvadóttir (Icelandic Cancer Registry, Icelandic Cancer Society); Ireland: K Clough-Gorr, P M Walsh (National Cancer Registry Ireland); H Sundseth (European Institute of Women’s Health); Italy: G Mazzoleni, F Vittadello (Registro Tumori Alto Adige); E Coviello, F Cuccaro (Registro Tumori Puglia—Sezione ASL BT); R Galasso (Registro Tumori di Basilicata); G Sampietro (Registro Tumori di Bergamo); A Giacomin (Piedmont Cancer Registry Provinces of Biella and Vercelli); M Magoni (Registro Tumori Dell’ASL Di Brescia); A Ardizzone (Registro Tumori Brindisi); A D’Argenzio (Caserta Cancer Registry); M Castaing, G Grosso (Integrated Cancer Registry of Catania-Messina-Siracusa-Enna); A M Lavecchia, A Sutera Sardo (Registro Tumori Catanzaro); G Gola (Registro Tumori della Provincia di Como); L Gatti, P Ricci (Registro Tumori Cremona; Registro Tumori Mantova); S Ferretti (Registro Tumori della Provincia di Ferrara); L Dal Maso, D Serraino (Registro Tumori del Friuli Venezia Giulia); M V Celesia, R A Filiberti (Registro Tumori Regione Liguria); F Pannozzo (Registro Tumori della Provincia di Latina); A Melcarne, F Quarta (Registro Tumori Della Provincia Di Lecce Sezione RTP); A Andreano, A G Russo (Registro Tumori Milano); G Carrozzi, C Cirilli (Registro Tumori della Provincia di Modena); L Cavalieri d’Oro, M Rognoni (Registro Tumori di Monza e Brianza); M Fusco, M F Vitale (Registro Tumori della ASL Napoli 3 Sud); M Usala (Nuoro Cancer Registry); R Cusimano, W Mazzucco (Registro Tumori di Palermo e Provincia); M Michiara, P Sgargi (Registro Tumori della Provincia di Parma); L Boschetti (Cancer Registry of the province of Pavia); G Chiaranda, P Seghini (Registro Tumori Piacenza); M M Maule, F Merletti (Piedmont Childhood Cancer Registry); R Tumino (Registro Tumori della Provincia di Ragusa); P Mancuso, M Vicentini (Registro Tumori Reggio Emilia); T Cassetti, R Sassatelli (Pancreas Tumor Registry of Reggio Emilia Province); F Falcini, S Giorgetti (Registro Tumori della Romagna); A L Caiazzo, R Cavallo (Registro Tumori Salerno); R Cesaraccio, D R Pirino (Registro Tumori della Provincia di Sassari); F Bella, A Madeddu (Registro Tumori Siracusa); A C Fanetti, S Maspero (Registro Tumori della Provincia di Sondrio); S Carone, A Mincuzzi (Registro Tumori Taranto); G Candela, T Scuderi (Registro Tumori Trapani); M A Gentilini, S Piffer (Registro Tumori Trento); S Rosso (Piedmont Cancer Registry); A Barchielli, A Caldarella (Registro Tumori della Regione Toscana); F Bianconi, F Stracci (Registro Tumori Umbro di Popolazione); P Contiero, G Tagliabue (Registro Tumori Lombardia, Provincia di Varese); M Rugge, M Zorzi (Registro Tumori Veneto); S Beggiato, A Brustolin (Registro Tumori Della Provincia Di Viterbo); G Gatta (Fondazione IRCCS Istituto Nazionale dei Tumori); M Rugge (Italian Association of Cancer Registries (AIRTUM)); R De Angelis (National Centre for Epidemiology); R Zanetti (International Association of Cancer Registries; Piedmont Cancer Registry); Latvia: A Maurina, M Oniščuka (Latvian Cancer Registry); Liechtenstein: M Mousavi (Liechtenstein); Lithuania: N Lipunova, I Vincerževskienė (Lithuanian Cancer Registry); Malta: D Agius, N Calleja (Malta National Cancer Registry); Netherlands: S Siesling, O Visser (Netherlands Cancer Registry, IKNL); Norway: S Larønningen, B Møller (The Cancer Registry of Norway); Poland: A Dyzmann-Sroka, M Trojanowski (Greater Poland Cancer Registry); S Góźdź (Holy Cross Cancer Registry); T Mierzwa (Kuiavian-Pomeranian Cancer Registry); L Molong, J Rachtan (Lesser Poland Cancer Registry); S Szewczyk (Łódź Cancer Registry); J Błaszczyk, K Kępska (Lower Silesian Cancer Registry); B Kościańska (Lublin Cancer Registry); R Amunicka, A Ostrowski (Lubush Cancer Registry); M Zwierko (Mazovian Cancer Registry); W Kaczmarek (Opole Cancer Registry); K M Maksimowicz, E Purwin-Porowska (Podlahian Cancer Registry); E Reca, J Wójcik-Tomaszewska (Pomeranian Cancer Registry); E Czajkowska, M Motnyk (Silesian Cancer Registry); M Grądalska-Lampart, A U Radziszewska (Subcarpathian Cancer Registry); A Gos (Varmian-Mazurian Cancer Registry); M Talerczyk, M Wyborska (West-Pomeranian Cancer Registry); J A Didkowska, U Wojciechowska (National Cancer Registry); M Bielska-Lasota (National Institute of Public Health, NIH); Portugal: G Forjaz de Lacerda, R A Rego (Registo Oncológico Regional dos Açores); B Carrito, A Pais (Registo Oncológico Regional do Centro); M J A T Bento, J R Rodrigues (Registo Oncológico Regional do Norte); A Mayer-da-Silva, A Miranda (Registo Oncólogico Regional do Sul); Romania: L M Blaga, D Coza (Cancer Institute I. Chiricuta); Russia: M Y Valkov (Arkhangelsk Regional Cancer Registry); L Gusenkova, O Lazarevich (Population Cancer Registry of the Republic of Karelia); O Prudnikova, D M Vjushkov (Omsk Regional Cancer Registry); A G Egorova, A E Orlov (Samara Cancer Regional Registry); L A Kudyakov, L V Pikalova (Population-Based Cancer Registry of Tomsk); Slovakia: J Adamcik, C Safaei Diba (National Cancer Registry of Slovakia); Slovenia: M Primic-Žakelj, V Zadnik (Cancer Registry of Republic of Slovenia); Spain: L Gil, A Lopez de Munain (Basque Country Cancer Registry); A A Herrera, D Rojas (Registro Poblacional de Cáncer de la Comunidad Autónoma de Canarias); R J Chillarón, A I M Navarro (Registro de Cáncer de Cuenca); R Marcos-Gragera, M L Vilardell Gil (Epidemiology Unit and Girona Cancer Registry); E Molina, M J Sánchez Perez (Granada Cancer Registry); P Franch Sureda, M Ramos Montserrat (Mallorca Cancer Registry); M D Chirlaque, C Navarro (Murcia Cancer Registry); E Ardanaz, M Guevara (Registro de Cáncer de Navarra, CIBERESP); A Cañete-Nieto, R Peris-Bonet (Registro Español de Tumores Infantiles); M Carulla, J Galceran (Tarragona Cancer Registry); F Almela, C Sabater (Comunitat Valenciana Childhood Cancer Registry); Sweden: S Khan, D Pettersson (Swedish Cancer Registry); P Dickman (Karolinska Institutet, Stockholm); Switzerland: K Staehelin, B Struchen (Basel Cancer Registry); C Egger Hayoz (Registre Fribourgeois des Tumeurs); C Bouchardy, R Schaffar (Geneva Cancer Registry); M Rössle (Cancer Registry Grisons and Glarus); S M Mousavi (Cancer Registry Grisons and Glarus; Cancer Registry of St Gallen-Appenzell); J L Bulliard, M Maspoli-Conconi (Registre Neuchâtelois et Jurassien des Tumeurs); C Herrmann (Cancer Registry of St Gallen-Appenzell); C E Kuehni, S M Redmond (Swiss Childhood Cancer Registry); A Bordoni, L Ortelli (Registro Tumori Canton Ticino); A Chiolero, I Konzelmann (Registre Valaisan des Tumeurs); K L Matthes, S Rohrmann (Cancer Registry Zürich and Zug); United Kingdom: J Broggio, J Rashbass (National Cancer Registration and Analysis Service England); D Fitzpatrick, A Gavin (Northern Ireland Cancer Registry); D I Clark, A J Deas (Scottish Cancer Registry); D W Huws (Welsh Cancer Intelligence & Surveillance Unit); C Allemani, M P Coleman, V Di Carlo, F Girardi, M Matz, P Minicozzi, L Montel, M Nikšić, N Ssenyonga, (London School of Hygiene & Tropical Medicine); R Stephens (National Cancer Research Institute, London); C Stiller (Public Health England).
Oceania—Australia: E Chalker, M Smith (Australian Capital Territory Cancer Registry); R Walton, H You (NSW Cancer Registry); S Qin Li, S Dugdale (Northern Territory of Australia Cancer Registry); J Moore, S Philpot (Queensland Cancer Registry); R Pfeiffer, H Thomas (South Australian Cancer Registry); B C Stokes, A Venn (Tasmanian Cancer Registry); H Farrugia, V Thursfield (Victorian Cancer Registry); J Dowling (Western Australian Cancer Registry); D Currow (Cancer Institute NSW); New Zealand: C Fowler, C Lewis (New Zealand Cancer Registry).
Funding
Dr F.G. MD, MPhil is supported by the Davidson and O’Gorman Fellowship from Children with Cancer UK.
Conflict of interest statement. All authors declare no conflicts of interest.
Authorship statement. Conception of the work: F.G., C.A., and M.P.C. Data analysis: F.G. Data acquisition and interpretation: all authors. Draft of the manuscript: F.G. Critical revision of the manuscript: all authors. Final approval of the manuscript: all authors. Agreement to be accountable for all aspects of the work: all authors.
Supplementary Material
Contributor Information
CONCORD Working Group:
S Bouzbid, M Hamdi-Chérif, Z Zaidi, K Meguenni, D Regagba, S Bayo, T Cheick Bougadari, S S Manraj, K Bendahhou, A Ladipo, O J Ogunbiyi, T Ramaliba, N I M Somdyala, M A Chaplin, F Moreno, G H Calabrano, S B Espinola, B Carballo Quintero, R Fita, W D Laspada, S G Ibañez, C A Lima, A Mafra da Costa, P C F De Souza, K Del Pino, C Laporte, M P Curado, J C de Oliveira, C L A Veneziano, D B Veneziano, M R D O Latorre, L F Tanaka, M S Rebelo, M O Santos, G Azevedo e Silva, J C Galaz, M Aparicio Aravena, J Sanhueza Monsalve, D A Herrmann, S Vargas, V M Herrera, C J Uribe, L E Bravo, L S Garcia, N E Arias-Ortiz, D Morantes, D M Jurado, M C Yépez Chamorro, S Delgado, M Ramirez, Y H Galán Alvarez, P Torres, F Martínez-Reyes, L Jaramillo, R Quinto, J Castillo, M Mendoza, P Cueva, J G Yépez, B Bhakkan, J Deloumeaux, C Joachim, J Macni, R Carrillo, J Shalkow Klincovstein, R Rivera Gomez, P Perez, E Poquioma, G Tortolero-Luna, D Zavala, R Alonso, E Barrios, A Eckstrand, C Nikiforuk, R R Woods, G Noonan, D Turner, E Kumar, B Zhang, F R McCrate, S Ryan, M MacIntyre, N Saint-Jacques, A Anam, P De, C A McClure, K A Vriends, C Bertrand, J Latreille, S Kozie, H Stuart-Panko, T Freeman, J T George, R M Avila, D K O’Brien, A Holt, L Almon, S Kwong, C Morris, R Rycroft, L Mueller, C E Phillips, H Brown, B Cromartie, A G Schwartz, F Vigneau, G M Levin, B Wohler, R Bayakly, K C Ward, S L Gomez, M McKinley, R Cress, M D Green, K Miyagi, C J Johnson, L P Ruppert, S Bentler, M E Charlton, B Huang, T C Tucker, D Deapen, L Liu, M C Hsieh, X C Wu, M Schwenn, K Stern, S T Gershman, R C Knowlton, G Alverson, T Weaver, S Bushhouse, D B Rogers, J Jackson-Thompson, D Lemons, H J Zimmerman, M Hood, J Roberts-Johnson, B Riddle, J R Rees, K S Pawlish, A Stroup, C Key, C Wiggins, A R Kahn, M J Schymura, S Radhakrishnan, C Rao, L K Giljahn, R M Slocumb, A Feld, K G Aird, T Beran, J J Rubertone, S J Slack, J Oh, T A Janes, S M Schwartz, S Chiodini, D M Hurley, M A Whiteside, S Rai, M A Williams, K Herget, C Sweeney, A T Johnson, M B Keitheri Cheteri, P Migliore Santiago, S E Blankenship, S Farley, R Borchers, R Malicki, J Espinoza, J Grandpre, H K Weir, R Wilson, B K Edwards, A Mariotto, N Wang, L Yang, J S Chen, Y Zhou, Y T He, G H Song, X P Gu, D Mei, H J Mu, H M Ge, T H Wu, Y Y Li, D L Zhao, F Jin, J H Zhang, F D Zhu, Q Junhua, Y L Yang, C X Jiang, W Biao, J Wang, Q L Li, H Yi, X Zhou, J Dong, W Li, F X Fu, S Z Liu, J G Chen, J Zhu, Y H Li, Y Q Lu, M Fan, S Q Huang, G P Guo, H Zhaolai, K Wei, W-Q Chen, W Wei, H Zeng, A V Demetriou, W K Mang, K C Ngan, A C Kataki, M Krishnatreya, P A Jayalekshmi, P Sebastian, P S George, A Mathew, A Nandakumar, R Malekzadeh, G Roshandel, L Keinan-Boker, B G Silverman, Y Koyanagi, H Ito, M Sato, F Tobori, I Nakata, N Teramoto, M Hattori, Y Kaizaki, F Moki, H Sugiyama, M Utada, M Nishimura, K Yoshida, K Kurosawa, Y Nemoto, H Narimatsu, M Sakaguchi, S Kanemura, M Naito, R Narisawa, I Miyashiro, K Nakata, A Maeda, S Sato, I Oki, N Fukushima, A Shibata, K Iwasa, C Ono, T Matsuda, O Nimri, K W Jung, Y J Won, E Alawadhi, A Elbasmi, A Ab Manan, F Adam, E Nansalmaa, U Tudev, C Ochir, A M Al Khater, M M El Mistiri, G H Lim, Y Y Teo, C J Chiang, W C Lee, R Buasom, S Sangrajrang, K Suwanrungruang, P Vatanasapt, K Daoprasert, D Pongnikorn, A Leklob, S Sangkitipaiboon, S L Geater, H Sriplung, O Ceylan, I Kög, O Dirican, T Köse, T Gurbuz, F E Karaşahin, D Turhan, U Aktaş, Y Halat, S Eser, C I Yakut, M Altinisik, Y Cavusoglu, A Türkköylü, N Üçüncü, M Hackl, A A Zborovskaya, O V Aleinikova, K Henau, L Van Eycken, Z Valerianova, M R Yordanova, M Šekerija, L Dušek, M Zvolský, L Steinrud Mørch, H Storm, C Wessel Skovlund, K Innos, M Mägi, N Malila, K Seppä, J Jégu, M Velten, E Cornet, X Troussard, A M Bouvier, A V Guizard, V Bouvier, G Launoy, P Arveux, M Maynadié, M Mounier, A S Woronoff, M Daoulas, M Robaszkiewicz, J Clavel, S Goujon, B Lacour, I Baldi, C Pouchieu, B Amadeo, G Coureau, S Orazio, A Monnereau, P M Preux, F Rharbaoui, E Marrer, B Trétarre, M Colonna, P Delafosse, S Plouvier, A Cowppli-Bony, F Molinié, S Bara, O Ganry, B Lapôtre-Ledoux, P Grosclaude, N Bossard, Z Uhry, J Estève, R Stabenow, H Wilsdorf-Köhler, A Eberle, S Luttmann, I Löhden, A L Nennecke, J Kieschke, E Sirri, C Justenhoven, S R Zeissig, B Holleczek, N Eisemann, A Katalinic, R A Asquez, V Kumar, E Petridou, E J Ólafsdóttir, L Tryggvadóttir, K Clough-Gorr, P M Walsh, H Sundseth, G Mazzoleni, F Vittadello, E Coviello, F Cuccaro, R Galasso, G Sampietro, A Giacomin, M Magoni, A Ardizzone, A D’Argenzio, M Castaing, G Grosso, A M Lavecchia, A Sutera Sardo, G Gola, L Gatti, P Ricci, S Ferretti, L Dal Maso, D Serraino, M V Celesia, R A Filiberti, F Pannozzo, A Melcarne, F Quarta, A Andreano, A G Russo, G Carrozzi, C Cirilli, L Cavalieri d’Oro, M Rognoni, M Fusco, M F Vitale, M Usala, R Cusimano, W Mazzucco, M Michiara, P Sgargi, L Boschetti, G Chiaranda, P Seghini, M M Maule, F Merletti, R Tumino, P Mancuso, M Vicentini, T Cassetti, R Sassatelli, F Falcini, S Giorgetti, A L Caiazzo, R Cavallo, R Cesaraccio, D R Pirino, F Bella, A Madeddu, A C Fanetti, S Maspero, S Carone, A Mincuzzi, G Candela, T Scuderi, M A Gentilini, S Piffer, S Rosso, A Barchielli, A Caldarella, F Bianconi, F Stracci, P Contiero, G Tagliabue, M Rugge, M Zorzi, S Beggiato, A Brustolin, G Gatta, M Rugge, R De Angelis, R Zanetti, A Maurina, M Oniščuka, M Mousavi, N Lipunova, I Vincerževskienė, D Agius, N Calleja, S Siesling, O Visser, S Larønningen, B Møller, A Dyzmann-Sroka, M Trojanowski, S Góźdź, T Mierzwa, L Molong, J Rachtan, S Szewczyk, J Błaszczyk, K Kępska, B Kościańska, R Amunicka, A Ostrowski, M Zwierko, W Kaczmarek, K M Maksimowicz, E Purwin-Porowska, E Reca, J Wójcik-Tomaszewska, E Czajkowska, M Motnyk, M Grądalska-Lampart, A U Radziszewska, A Gos, M Talerczyk, M Wyborska, J A Didkowska, U Wojciechowska, M Bielska-Lasota, G Forjaz de Lacerda, R A Rego, B Carrito, A Pais, M J A T Bento, J R Rodrigues, A Mayer-da-Silva, A Miranda, L M Blaga, D Coza, M Y Valkov, L Gusenkova, O Lazarevich, O Prudnikova, D M Vjushkov, A G Egorova, A E Orlov, L A Kudyakov, L V Pikalova, J Adamcik, C Safaei Diba, M Primic-Žakelj, V Zadnik, L Gil, A Lopez de Munain, A A Herrera, D Rojas, R J Chillarón, A I M Navarro, R Marcos-Gragera, M L Vilardell Gil, E Molina, M J Sánchez Perez, P Franch Sureda, M Ramos Montserrat, M D Chirlaque, C Navarro, E Ardanaz, M Guevara, A Cañete-Nieto, R Peris-Bonet, M Carulla, J Galceran, F Almela, C Sabater, S Khan, D Pettersson, P Dickman, K Staehelin, B Struchen, C Egger Hayoz, C Bouchardy, R Schaffar, M Rössle, S M Mousavi, J L Bulliard, M Maspoli-Conconi, C Herrmann, C E Kuehni, S M Redmond, A Bordoni, L Ortelli, A Chiolero, I Konzelmann, K L Matthes, S Rohrmann, J Broggio, J Rashbass, D Fitzpatrick, A Gavin, D I Clark, A J Deas, D W Huws, C Allemani, M P Coleman, V Di Carlo, F Girardi, M Matz, P Minicozzi, L Montel, M Nikšić, N Ssenyonga, R Stephens, C Stiller, E Chalker, M Smith, R Walton, H You, S Qin Li, S Dugdale, J Moore, S Philpot, R Pfeiffer, H Thomas, B C Stokes, A Venn, H Farrugia, V Thursfield, J Dowling, D Currow, C Fowler, and C Lewis
References
- 1.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, eds. WHO Classification of Tumours of the Central Nervous System. 4th ed. Geneva: WHO (World Health Organization); 2007. [Google Scholar]
- 2.International Agency for Research on Cancer. Global cancer observatory; Cancer Today; 2018. http://gco.iarc.fr/today/home. Accessed September 1, 2020.
- 3.Fidler MM, Gupta S, Soerjomataram I, Ferlay J, Steliarova-Foucher E, Bray F. Cancer incidence and mortality among young adults aged 20-39 years worldwide in 2012: a population-based study. Lancet Oncol. 2017;18(12):1579–1589. [DOI] [PubMed] [Google Scholar]
- 4.Bhakta N, Force LM, Allemani C, et al. Childhood cancer burden: a review of global estimates. Lancet Oncol. 2019;20(1):e42–e53. [DOI] [PubMed] [Google Scholar]
- 5.Rodriguez-Galindo C, Friedrich P, Alcasabas P, et al. Toward the cure of all children with cancer through collaborative efforts: pediatric oncology as a global challenge. J Clin Oncol. 2015;33(27):3065–3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steliarova-Foucher E, Stiller C, Lacour B, Kaatsch P. International classification of childhood cancer, third edition. Cancer. 2005;103(7):1457–1467. [DOI] [PubMed] [Google Scholar]
- 7.Barr RD, Holowaty EJ, Birch JM. Classification schemes for tumors diagnosed in adolescents and young adults. Cancer. 2006;106(7):1425–1430. [DOI] [PubMed] [Google Scholar]
- 8.Bray F, Colombet M, Mery L, et al. Cancer Incidence in Five Continents. Vol. XI. Lyon: IARC (International Agency for Research on Cancer); 2017. http://ci5.iarc.fr. Accessed September 1, 2020. [Google Scholar]
- 9.Ostrom QT, Gittleman H, Liao P, et al. CBTRUS Statistical Report: primary brain and other central nervous system tumors diagnosed in the United States in 2010-2014. Neuro Oncol. 2017;19(suppl_5):v1–v88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Information Network on Rare Cancers. Cancer list.http://www.rarecarenet.eu/rarecarenet/index.php/cancerlist. Accessed September 1, 2020.
- 11.Girardi F, Allemani C, Coleman MP. Worldwide trends in survival from common childhood brain tumors: a systematic review. J Glob Oncol. 2019;5:1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steliarova-Foucher E, Stiller C, Kaatsch P, et al. Geographical patterns and time trends of cancer incidence and survival among children and adolescents in Europe since the 1970s (the ACCISproject): an epidemiological study. Lancet. 2004;364(9451):2097–2105. [DOI] [PubMed] [Google Scholar]
- 13.Gatta G, Peris-Bonet R, Visser O, et al. Geographical variability in survival of European children with central nervous system tumours. Eur J Cancer. 2017;82:137–148. [DOI] [PubMed] [Google Scholar]
- 14.Visser O, Ardanaz E, Botta L, et al. ; EUROCARE-5 Working Group . Survival of adults with primary malignant brain tumours in Europe; results of the EUROCARE-5 study. Eur J Cancer. 2015;51(15):2231–2241. [DOI] [PubMed] [Google Scholar]
- 15.Allemani C, Matsuda T, Di Carlo V, et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391(10125):1023–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Allemani C, Harewood R, Johnson CJ, et al. Population-based cancer survival in the United States: data, quality control, and statistical methods. Cancer. 2017;123(Suppl 24):4982–4993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fritz A, Percy C, Jack A, et al. , eds. International Classification for Diseases in Oncology. 3rd ed., 1st rev. Geneva: WHO (World Health Organization); 2013. [Google Scholar]
- 18.Percy C, Van Holten V, Muir C, eds. International Classification of Diseases for Oncology. 2nd ed.Geneva: WHO (World Health Organization); 1990. [Google Scholar]
- 19.Surveillance, Epidemiology, and End Results Program. Non-malignant brain tumors.https://training.seer.cancer.gov/brain/non-malignant/. Accessed September 1, 2020.
- 20.Ostrom QT, de Blank PM, Kruchko C, et al. Alex’s lemonade stand foundation infant and childhood primary brain and central nervous system tumors diagnosed in the United States in 2007–2011. Neuro Oncol. 2015;16(Suppl 10):x1–x36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ostrom QT, Egan KM, Nabors LB, et al. Glioma risk associated with extent of estimated European genetic ancestry in African Americans and Hispanics. Int J Cancer. 2020;146(3):739–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van den Bent MJ. Interobserver variation of the histopathological diagnosis in clinical trials on glioma: a clinician’s perspective. Acta Neuropathol. 2010;120(3):297–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schiff D, Van den Bent M, Vogelbaum MA, et al. Recent developments and future directions in adult lower-grade gliomas: Society for Neuro-Oncology (SNO) and European Association of Neuro-Oncology (EANO) consensus. Neuro Oncol. 2019;21(7):837–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parsons DW, Jones S, Zhang X, et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321(5897):1807–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yan H, Parsons DW, Jin G, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360(8):765–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van den Bent MJ, Dubbink HJ, Marie Y, et al. IDH1 and IDH2 mutations are prognostic but not predictive for outcome in anaplastic oligodendroglial tumors: a report of the European Organization for Research and Treatment of Cancer Brain Tumor Group. Clin Cancer Res. 2010;16(5):1597–1604. [DOI] [PubMed] [Google Scholar]
- 27.van den Bent MJ, Smits M, Kros JM, Chang SM. Diffuse infiltrating oligodendroglioma and astrocytoma. J Clin Oncol. 2017;35(21):2394–2401. [DOI] [PubMed] [Google Scholar]
- 28.Omuro AM, Leite CC, Mokhtari K, Delattre JY. Pitfalls in the diagnosis of brain tumours. Lancet Neurol. 2006;5(11):937–948. [DOI] [PubMed] [Google Scholar]
- 29.Fuentes-Raspall R, Solans M, Roca-Barceló A, et al. Descriptive epidemiology of primary malignant and non-malignant central nervous tumors in Spain: results from the Girona Cancer Registry (1994-2013). Cancer Epidemiol. 2017;50(Pt A):1–8. [DOI] [PubMed] [Google Scholar]
- 30.Vallée A, Guillevin C, Wager M, Delwail V, Guillevin R, Vallée JN. Added value of spectroscopy to perfusion MRI in the differential diagnostic performance of common malignant brain tumors. AJNR Am J Neuroradiol. 2018;39(8):1423–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.European Network of Cancer Registries. Recommendations for coding basis of diagnosis.https://www.encr.eu/sites/default/files/pdf/braincns.pdf. Accessed September 1, 2020.
- 32.Parkin DM, Bray F. Evaluation of data quality in the cancer registry: principles and methods Part II. Completeness. Eur J Cancer. 2009;45(5):756–764. [DOI] [PubMed] [Google Scholar]
- 33.Stiller CA, Bayne AM, Chakrabarty A, Kenny T, Chumas P. Incidence of childhood CNS tumours in Britain and variation in rates by definition of malignant behaviour: population-based study. BMC Cancer. 2019;19(1):139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Louis DN, Perry A, Reifenberger G, et al. The 2016 World health organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–820. [DOI] [PubMed] [Google Scholar]
- 35.International Agency for Research on Cancer. Global Initiative for Cancer Registry Development; 2011. https://gicr.iarc.fr/results-and-evidence/. Accessed September 1, 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.