Abstract
Background
Pediatric low-grade gliomas (pLGGs) are the most common childhood brain tumor. Progression-free survival (PFS) is much lower than overall survival, emphasizing the need for alternative treatments. Sporadic (without neurofibromatosis type 1) optic pathway and hypothalamic gliomas (OPHGs) are often multiply recurrent and cause significant visual deficits. Recently, there has been a prioritization of functional outcomes.
Methods
We present results from children with recurrent/progressive OPHGs treated on a PBTC (Pediatric Brain Tumor Consortium) phase II trial evaluating efficacy of selumetinib (AZD6244, ARRY-142886) a MEK-1/2 inhibitor. Stratum 4 of PBTC-029 included patients with sporadic recurrent/progressive OPHGs treated with selumetinib at the recommended phase II dose (25mg/m2/dose BID) for a maximum of 26 courses.
Results
Twenty-five eligible and evaluable patients were enrolled with a median of 4 (1-11) previous therapies. Six of 25 (24%) had partial response, 14/25 (56%) had stable disease, and 5 (20%) had progressive disease while on treatment. The median treatment courses were 26 (2-26); 14/25 patients completed all 26 courses. Two-year PFS was 78 ± 8.5%. Nineteen of 25 patients were evaluable for visual acuity which improved in 4/19 patients (21%), was stable in 13/19 (68%), and worsened in 2/19 (11%). Five of 19 patients (26%) had improved visual fields and 14/19 (74%) were stable. The most common toxicities were grade 1/2 CPK elevation, anemia, diarrhea, headache, nausea/emesis, fatigue, AST and ALT increase, hypoalbuminemia, and rash.
Conclusions
Selumetinib was tolerable and led to responses and prolonged disease stability in children with recurrent/progressive OPHGs based upon radiographic response, PFS, and visual outcomes.
Keywords: hypothalamic glioma, MEK-1/2, optic pathway glioma, pediatric low-grade glioma, selumetinib
Key Points.
Selumetinib led to imaging responses and stability in heavily pre-treated and recurrent OPHGs.
Selumetinib led to stable and improved vision in recurrent OPHGs.
Selumetinib’s toxicity in recurrent OPHGs was tolerable and similar to previous reports.
Importance of the Study.
This study highlights that selumetinib led to both radiographic responses and stable disease as well as visual stability and improvement in patients with recurrent and progressive, sporadic (non-NF1-associated) optic pathway and hypothalamic gliomas (OPHGs). These data provide a treatment alternative for children with multiply recurrent and progressive OPHGs. Based on these preliminary data, patients with sporadic OPHGs are eligible for enrollment on a randomized, prospective, and multi-institutional Children’s Oncology Group (COG) phase III study for children with newly diagnosed and previously untreated pLGG comparing the standard chemotherapy regimen, carboplatin and vincristine, to selumetinib (NCT04166409). Finally, preliminary visual outcome data herein prompted more detailed evaluations of visual acuity using well-defined standardized approaches in the ongoing COG trial.
Pediatric low-grade gliomas (pLGG) are the most common central nervous system (CNS) tumors in children, representing 40%-50% of all pediatric CNS tumors.1 pLGG most frequently arise in the optic pathway and hypothalamic region (40%), followed by the cerebellum (25%), cerebral hemispheres (17%), and brainstem (9%).2,3 In addition to common morbidities such as motor deficits, neurocognitive delay, and poor quality of life, children with optic pathway hypothalamic gliomas (OPHGs), in particular, often suffer from additional morbidities such as endocrine dysfunction and visual disturbance due to tumor location.
Sporadic OPHGs can present throughout childhood, and it is estimated that over 90% will eventually require some type of therapeutic intervention.4 This is in contrast to neurofibromatosis type 1 (NF1)-associated OPHGs that most often present in the first 5-6 years of life and often do not require treatment.5,6 NF1 is a genetic predisposition disorder caused by loss-of-function alterations in NF1, a negative regulator of the MAPK pathway, and approximately 15%-20% of patients with NF1 will develop pLGG, most commonly within the optic pathway.6 Most studies show that the progression-free survival (PFS) and response to classic chemotherapy are superior in children with NF1-associated pLGG versus those with sporadic pLGG.7,8 Sporadic OPHGs are more likely to progress radiographically, present with clinical symptoms, and cause visual impairment compared to NF1-associated OPHGs.9,10 The pathophysiology behind these differences, despite the same histology and location, is yet unclear and assumed to be associated with the NF1 mutation.
Complete surgical resection without significant morbidity is typically not feasible in children with OPHGs, and therefore, most children will be treated with chemotherapy. Standard first-line chemotherapy combinations have not historically varied based upon specific pLGG histology, location, or molecular aberration. The commonly accepted first-line chemotherapies include combinations of carboplatin and vincristine (CV); combinations of thioguanine, procarbazine, lomustine, and vincristine (TPCV); and vinblastine monotherapy.11,12 In the Children’s Oncology Group (COG), pLGG study for previously untreated pLGG in all locations, CCG A9952, which compared CV to TPCV, the 5-year overall survival (OS) for the 274 children without NF1, was excellent at 86% ± 2%, however, the same study revealed a 5-year event-free survival of only 45 ± 3%, suggesting that alternative effective therapies are needed. In the CCG A9952 study, approximately 50% of the non-NF1 patients had OPHGs.
At recurrence and progression, OPHGs are typically treated the same as recurrent pLGG in other locations with a variety of chemotherapy options. The most common second-line and beyond treatments include the following: carboplatin monotherapy, vinblastine monotherapy, and combination of bevacizumab and irinotecan.13–15 Despite these other therapies, many children will continue to have multiply recurrent disease throughout childhood. Also, although radiotherapy is an effective treatment strategy, it increases the risks of secondary malignancies, ototoxicity, endocrinopathies, and neurocognitive decline.16 For these reasons, radiation therapy is usually avoided in young children with pLGG, especially given their good OS and the goal of minimizing morbidity.17
A greater understanding of the genetic landscape of pLGG and the development of novel drugs that target some of these molecular aberrations have heralded a new focus upon treatments that are molecularly driven, with a goal to minimize toxicity and maximize survival and functional outcomes.18 It is now well understood that abnormal MAPK pathway activation is the most frequent genetic aberration seen in pLGG, most commonly resulting from activation of the BRAF oncogene.18–20 The two most common aberrations seen are a tandem duplication resulting in a KIAA1549-BRAF fusion and an activating point mutation, BRAFV600E. The KIAA1549-BRAF fusion can be identified in over 80% of non-NF1 pilocytic astrocytomas (PA), the most common histology among all pLGG, whereas BRAFV600E mutations are identified in only about 10%-20% of all non-NF1 pLGG.18,20 Historically, many OPHGs are not biopsied, so the data detailing the presence or absence of these aberrations in tumors in these locations is lacking, however, the literature to date suggests the common BRAF fusion and other abnormalities leading to MAPK activation are frequently seen in optic nerve gliomas.21 This wealth of new data on the molecular landscape of pLGG has led to prospective clinical trials testing agents that target the MAPK pathway.8,22,23 There are also numerous case reports depicting the efficacy of these agents treating children outside of a clinical study, including children with OPHGs.24–26
The Pediatric Brain Tumor Consortium (PBTC) has previously reported the results of our phase I study (PBTC-029), and outcomes from stratum 1 (PA with BRAF fusion or mutation) and stratum 3 (NF1-associated LGG) of the phase II study (PBTC-029B) utilizing selumetinib, a MEK-1/2 inhibitor for the treatment of recurrent and progressive pLGG.8 Here, we report the outcomes of PBTC-029B, stratum 4, which enrolled patients with sporadic (not associated with NF1) recurrent and progressive OPHGs.
Materials and Methods
Study Design
PBTC-029B is a multicenter, phase II trial developed and performed by the PBTC in collaboration with the National Cancer Institute’s Cancer Therapy Evaluation Program (CTEP), which sponsored and funded the study. Eligible patients were enrolled to six unique strata based upon histology, tumor location, NF1 status, and BRAF aberration status (the presence/absence of either KIAA1549-BRAF fusion or BRAFV600E mutation). Description of all strata and the specific results from strata 1 and 3 have been previously reported.8 Herein, we report findings from stratum 4, which enrolled patients with sporadic OPHGs. The difference between the previously reported stratum 1 (PA with either BRAFV600E mutation or KIAA1549-BRAF fusion) and the current stratum 4 is based upon both a known histology and a known BRAF positivity, either the BRAFV600E mutation or the BRAF KIAA1549 fusion. Stratum 1 was location agnostic, but to be eligible, patients must have had a confirmed PA on histology and must have screened positive for 1 of the 2 BRAF aberrations. In contrast, only patients with OPHGs could be enrolled on the current stratum 4 with any pLGG histology, and tissue was not required for screening for BRAF status, so in many patients, this was unknown. In truth, our understanding of the pLGG molecular landscape and its relationship to tumor location has evolved during the lifetime of the study. When the trial began, it was yet unclear if tumors behaved differently based on their histology, molecular aberration, or tumor location, so the trial separated tumor into unique strata based on these characteristics.
Eligibility
Patients aged 3-21 years with recurrent or progressive sporadic OPHGs who failed at least one standard therapy other than surgery, including chemotherapy or radiotherapy, were eligible for inclusion in stratum 4. The last dose of known myelosuppressive chemotherapy must have been given at least 3 weeks before study registration; 6 weeks before if it was a nitrosourea. The last dose of any biological agent must have been given at least 7 days before study registration. For biological agents with a long half-life (>2 days) and for all monoclonal antibodies, at least three half-lives must have elapsed before registration. Patients must have had their last fraction of local irradiation to the target tumor at least 12 months before registration. Patients with prior BRAF or MEK inhibitor treatment were excluded. Enrollment required documentation of progressive or recurrent disease. A Lansky or Karnofsky functional performance score equal or greater to 60 was required, and patients with any clinically significant unrelated systemic illness likely to interfere with the study procedures or results were excluded. All neurological deficits were required to be stable for at least 7 days before registration. Patients with uncontrolled seizures were excluded. The study required that a patient swallow capsules whole. Patients were required to have adequate complete blood counts, liver and renal function, left ventricular ejection fraction of at least 55%, and QTc interval less than 450 ms. Bi-dimensional measurable disease was required.
Treatment and Dosing Schedule
Selumetinib was supplied as capsules administered orally at a dose of 25 mg/m2/dose twice daily, the recommended phase II dose.22 Patient adherence was measured utilizing patient diaries and pill counts, both evaluated at the end of each course. Each course was 28 days, and the dosing was continuous without planned breaks between courses. Patients could receive up to a maximum of 26 courses (approximately 2 years) in the absence of unacceptable toxicity or tumor progression.
A maximum of two dose reductions were allowed; dose re-escalation was not permitted. Dose-modifying toxicities included any adverse event at least possibly related to selumetinib that resulted in a delay of treatment of longer than 7 days; any grade 3 non-hematologic toxicity with the exceptions of grade 3 nausea and vomiting for fewer than 5 days, grade 3 elevated alanine aminotransferase (ALT) and aspartate aminotransferase (AST) that returned to required eligibility levels within 7 days of stopping drug, grade 3 fever or infection for fewer than 5 days, grade 3 electrolyte abnormalities that responded to oral supplementation, and grade 3 asymptomatic elevated creatine phosphokinase (CPK). Other non-hematologic dose-modifying toxicities included any grade 4 non-hematologic toxicity and any grade 2 non-hematologic toxicity that persisted for more than 7 days and was thought medically significant or intolerable by the patient or physician to warrant treatment interruption, dose reduction, or both. Hematologic dose-modifying toxicity included any grade 4 toxicity (with the exception of lymphopenia) and grade 3 thrombocytopenia associated with bleeding. Toxicities were graded according to the Common Terminology Criteria for Adverse Events version 4.0.
Required Observations and MRI Response Criteria
The required follow-up assessments included physical examination, laboratory evaluations, ophthalmological examination, and MRI. MRIs were performed every 8 weeks during the first year and every 12 weeks thereafter. Central radiological review conducted at the PBTC Neuroimaging Center was performed in patients whose tumor achieved a radiographic response (complete response or partial response [PR]) as assessed by the local institution. All responses were based upon two-dimensional perpendicular measurements of the target lesions on MR images. As described in our previous publications of phase I and II results, initial imaging response definitions were based more heavily upon enhancement seen after administration of gadolinium rather than on T2 and Fluid-attenuated inversion recovery (FLAIR) sequences.22 Since modern consensus has moved away from enhancement as a primary measure of evaluating response in pLGG, the protocol was amended to incorporate the response criteria based more heavily upon T2 and FLAIR and as previously reported.8 All response assessments obtained prior to the amendment were re-reviewed by site neuroradiologists, and the results reported here are based on these revised assessments. Laboratory assessments, including complete blood count, full chemistry panel, liver function tests, and CPK, were done every 4 weeks. Echocardiograms were performed every 12 weeks.
Outcome Measures
The primary endpoint was to determine the proportion of patients who achieved an objective response (PR or complete response) sustained for at least 8 weeks. Secondary endpoints included PFS (defined as the time from treatment initiation until disease progression or death from any cause or time to last follow-up for patients without these events); associations between BRAF aberrations and treatment response and PFS in patients for whom these data were available; MAPK aberrations by a combination of whole-exome and RNA sequencing in patients with these data; and description of the inter-patient and intra-patient variability in the pharmacokinetics of selumetinib.
Assessment of Visual Acuity (VA) and Visual Fields (VF)
VA assessment was required in all patients on stratum 4 on the same schedule as MRI; at baseline, every 8 weeks during the first year and every 12 weeks thereafter. The specific assessment tool utilized was at the discretion of the treating Oncologist and Ophthalmologist, however, all centers performed Snellen VA testing and Goldmann perimetry testing for assessment of VA and VF at a minimum. VA response was determined by a comparison of the VA at baseline to 1 year on therapy. Improvement in vision was defined as a ≥0.2 logarithm of the minimum angle of resolution (logMAR) improvement in VA; stable vision was defined as neither ≥0.2 logMAR improvement nor worsening in VA; and worsening vision was defined as ≥0.2 logMAR worsening in VA. These are the same definitions of VA response established by the Response Evaluation in Neurofibromatosis and Schwannomatosis Visual Outcomes Committee and later adopted by the pLGG Response Assessment of Pediatric Neuro-Oncology (RAPNO) international consensus panel.27,28 Comparisons of Goldmann perimetry testing at baseline and 1 year for VF were subjective and based upon the local Ophthalmologist’s interpretation as improved, stable, or worsening.
Pharmacokinetics
Pharmacokinetic studies evaluated the disposition of selumetinib and its active metabolite N-desmethyl-selumetinib on day 1 of course 1 in consenting patients. Blood samples were collected before a selumetinib dose and 1, 3, and 8 ± 1-h post-dose. A non-compartmental approach was used to determine the maximum concentration (Cmax), time to maximum concentration (Tmax), area under the plasma concentration-time curve from 0 to 8 h (AUC0-8), and the apparent oral clearance.
Tumor Tissue Screening and Biologic Correlates
Tumor BRAF testing for BRAFV600E mutation and KIAA1549-BRAF fusion was not required for enrollment onto stratum 4, but they were performed in patients with available tissue who agreed to screening consent. These tests were performed as previously described.8
Statistical Design and Data Analysis
All data were analyzed by the PBTC Operations Biostatistics and Data Management Core. The protocol was approved by CTEP as well as each local site’s institutional review board. All patients or legal guardians provided written, informed consent, and assent when applicable based on local institutional guidelines. Identical Simon’s Minimax designs were used to determine the sample size for each stratum; an unacceptable response proportion was 10% and a desirable response proportion was 30%, leading to a sample size of 25 with 10% type 1 error and 90% power. Interim analyses were planned after 16 patients; at least two responses were required to expand beyond the first stage to a total accrual of 25 patients. Five or more responses were required to declare the treatment promising. Responses had to be sustained for at least 8 weeks to be counted.
The initial study design used the first 10 cycles of therapy as the response evaluation window, thus only responses during this interval were counted toward the primary success criterion. However, at the time of the interim analysis, based on the observed disease stabilization rate as well as efficacy in the other strata, the response evaluation window was extended to include the entire duration of treatment (26 cycles, approximately 2 years) following discussions with the PBTC Data Safety Monitoring Board (DSMB) and CTEP.
All eligible patients who started therapy were evaluable for efficacy and toxicity analyses. The Kaplan-Meier method was used for PFS calculations. Log-log transformation was applied to the survival function to obtain confidence intervals. Logistic regression was used to investigate possible associations of response with age, gender, and baseline tumor size. All statistical analyses were done in SAS (version 9.4). Also, as an exploratory, unplanned analysis, log-rank tests were used to compare the PFS distribution observed in this stratum to matched cohorts from PBTC-029, stratum 3 (NF1-associated pLGG) as well as from PBTC-022 (bevacizumab and irinotecan), stratum E for recurrent pLGG.8,14
Results
Patient Characteristics
Table 1 shows the patients’ tumor and demographic information. Between July 17, 2013 and May 31, 2017, 25 eligible and evaluable patients were enrolled onto stratum 4. The median age at study entry was 9.4 years (3.7-17.6); 13/25 (52%) patients were female. Among the 25 patients, 16 (64%) patients had tumors in the optic pathway, 7 (28%) patients had tumors in the hypothalamus, and 2 (8%) had tumors in the thalamus that involved the hypothalamus. The distinction between optic pathway/chiasm and hypothalamic location was made by the local treating institution and was not centrally reviewed. All 25 patients had previous chemotherapy, 3/25 patients had received previous radiotherapy, and 19/25 had a previous surgical resection or biopsy. The median number of previous therapies was 4 (1-11). The pathology was known from previous or recent surgeries in 19 of 25 patients; 18/19 (94.7%) were PA and 1/19 (5.3%) was a ganglioglioma. The 6 patients without a known tissue histology were generically classified as glioma not otherwise specified (NOS) (Table 1). Only 6/25 patients were screened on study for 3′ BRAF tandem duplication leading to KIAA1549-BRAF fusion and for the BRAFV600E mutation. Three of 6 (50%) were positive for the 3′ BRAF duplication, 2/6 (33%) were negative, and 1/6 (17%) had inadequate tissue for a successful test. All 6 (100%) were negative for the BRAFV600E mutation.
Table 1.
Patient and Tumor Demographic Data
| N = 25 | ||
|---|---|---|
| Age | ||
| Median = 9.4 years | Range (3.7-17.6) | |
| Sex | ||
| Female | 13 (52%) | |
| Male | 12 (48%) | |
| Race | ||
| Asian | 2 (8%) | |
| Black | 4 (16%) | |
| White | 18 (72%) | |
| Unknown | 1 (4%) | |
| Tumor location | ||
| Hypothalamus | 7 (28%) | |
| Optic pathway | 16 (64%) | |
| Thalamic and involving the hypothalamus | 2 (8%) | |
| Pathology | ||
| Ganglioglioma | 1 (4%) | |
| Glioma (NOS) | 6 (24%) | |
| Pilocytic astrocytoma | 18 (72%) | |
| Previous therapy | ||
| Median number (range) | 4 (1-11) | |
| Chemotherapy | 25 (100%) | |
| Surgery | 19 (76%) | |
| Radiation | 3 (12%) |
At the time of data freeze for this manuscript (July 23, 2020), all patients in this stratum were off treatment.
Responses and Survival
As per the original Simon Stage 2 design, 16 patients were initially enrolled and evaluated for response. At the time of the interim analysis, the response definition still required that the responses occur within the first 10 cycles to be counted. Based on this criterion, among the first 16 patients, there was only 1 sustained PR reported by the treating institution. Therefore, per the original design, the number of responses was not sufficient to continue onto Simon Stage 2 which required a minimum of 2 centrally confirmed sustained responses that occurred within the first 10 cycles of therapy. However, 3 additional patients with SD at course 10 achieved greater than 50% tumor reduction on imaging as assessed locally which met the radiographic criteria for a PR at later courses (course 21, 22, and 26). The 2-year PFS estimate for these 16 patients was 64 ± 13%.
The PBTC DSMB and CTEP, as the study sponsor, carefully reviewed all of the data, including radiographic responses, PFS, and the waterfall plot on these first 16 patients as well as the efficacy data available from the other strata at that time and judged these data as promising. This was based on the late responses seen after cycle 10, prolonged SD in the majority of patients in this stratum, the high number of responses and stable disease seen in the other cohorts, and early PFS that was thought to be promising compared to historical cohorts of recurrent OPHG patients. Therefore, the protocol was amended and approved by all regulatory groups (PBTC Scientific Committee, the PBTC DSMB and CTEP) to expand accrual to 25 patients in an effort to provide patients with a potentially beneficial treatment and evaluate further responses, toxicities, and survival in a descriptive fashion. Shortly thereafter, the amendment counting response at any point (not just within the first 10 cycles) as part of the primary aim was also approved.
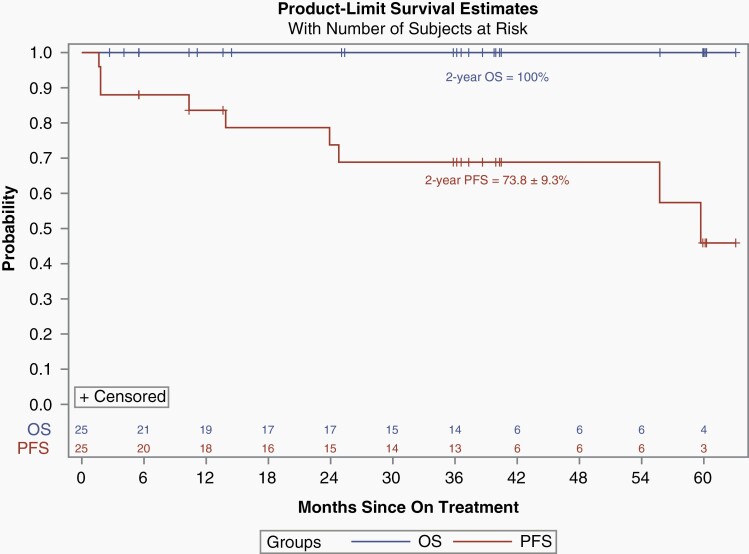
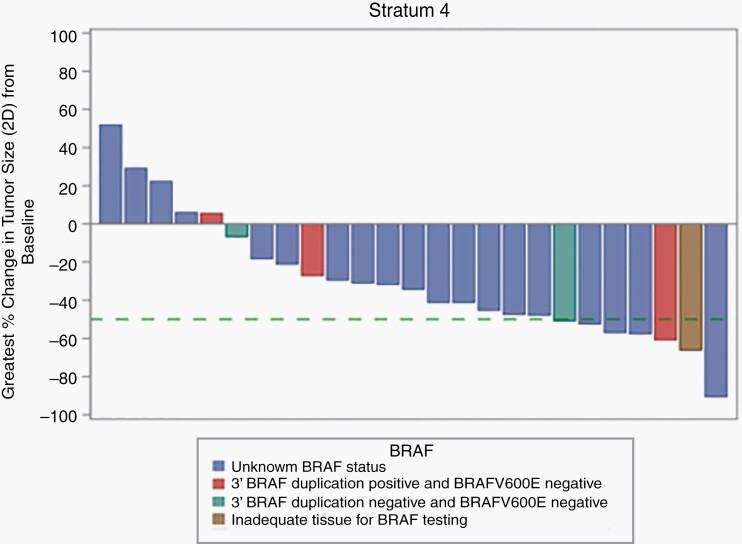
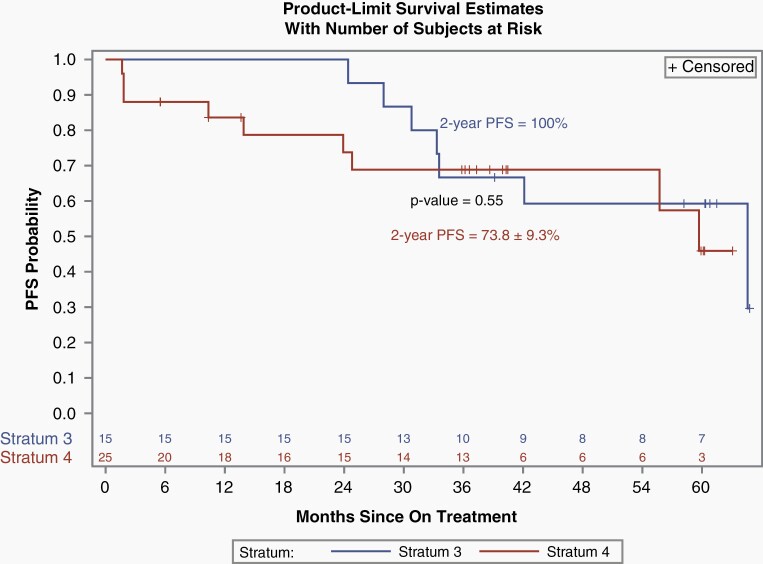
With the amendment that defined response at any time point, among all 25 eligible and evaluable patients enrolled, 6/25 (24%) patients had a PR, 14/25 (56%) had SD, and 5/25 (20%) had progressive disease (PD) while on treatment as assessed by the local enrolling institutions. Median time to response was 19.7 months (3.7-24.3). Four of the 6 PRs were confirmed by the PBTC Neuroimaging Center upon central review. The 2 PRs that were not confirmed had shrinkage of 23% and 49%, respectively, upon central review which did not meet the 50% threshold necessary to qualify as a centrally reviewed PR. Of the 6 local PRs, 2/6 had BRAF screening performed; one of the patients had a 3′ BRAF duplication which leads to the KIAA1549-BRAF fusion and tested negative for the BRAFV600E mutation, and the second patient had inadequate tissue for 3′ BRAF duplication testing and tested negative for the BRAFV600E. Data were too limited to make any statistical assessment on the relationship between BRAF aberration status and response or survival. The median number of treatment courses received in the study was 26 (2-26) and 14/25 (56%) of patients received all 26 courses of therapy. Among the patients who did not receive all 26 course, 4 developed PD (one additional PD during course 26), 3 discontinued therapy due to patient or physician choice, and 4 patients came off study due to adverse events. The 2-year PFS and OS of all 25 eligible and evaluable patients were 73.8 ± 9.3% and 100%, respectively (Figure 1). Figure 2 depicts the waterfall plot of the maximum percentage change associated with smallest tumor size recorded in two dimensions as assessed by T2-FLAIR images at the local institution, and BRAF status when available. This reveals that most patients had some shrinkage of tumor, though, often less than the 50% reduction necessary to qualify as a PR. There was no statistical association between response and age, sex, or tumor size at baseline. A separate post-hoc analysis compared the PFS of the patients with NF1-associated optic pathway and hypothalamic tumors enrolled on stratum 3 (n = 15) to all of the non-NF1 patients enrolled on this current stratum 4 (n = 25) and revealed no statistical differences (Figure 3).
Fig. 1.
Kaplan-Meier estimates of PFS (red) and OS (blue) for all eligible and evaluable patients (n = 25). Abbreviations: OS, overall survival; PFS, progression-free survival.
Fig. 2.
Waterfall plot depicting the maximum percentage change in 2D tumor size evaluated on T2-FLAIR imaging by the local institution. The green dashed line represents the 50% reduction required to qualify as a PR. Abbreviation: PR, partial response.
Fig. 3.
Kaplan-Meir comparison of PFS for those patients enrolled on PBTC-029, stratum 3 (NF1-associated), with optic pathway and hypothalamic tumors (n = 15) vs those enrolled on PBTC-029, stratum 4 (non-NF OPHG; n = 25). Abbreviations: NF1, neurofibromatosis type 1; OPHG, optic pathway and hypothalamic glioma; PBTC, Pediatric Brain Tumor Consortium; PFS, progression-free survival.
Nine of 25 patients have experienced PD, 5 while on treatment, and 4 after stopping treatment. Among the 5 patients who experienced PD while on treatment, the median time to PD was 1.8 months (1.7-23.9). Among the 4 patients who have progressed since stopping therapy, the median time off-treatment to PD was 16.0 months (0.7-35.4). Sixteen of 25 (64%) patients remain progression-free at the time of last follow-up. Among these 16 patients who remain progression-free, the median time from starting therapy to last follow-up is 38 months (5.5-63), and the median time since the end of therapy to last follow-up is 15.7 months (0-38.8).
Visual Outcomes
Nineteen of 25 patients had baseline VA and VF deficits and underwent VA assessments utilizing Snellen charts and had VF assessed by Goldmann confrontation at both baseline and at 1 year on therapy. The remaining 6 patients either progressed prior to the visual assessments at 1 year or did not have both the baseline and 1-year timepoints performed. VA improved in 4/19 patients (21%), was stable in 13/19 (68%), and worsened in 2/19 (11%). Of the 2 patients with worsening VA, one had stable disease and one had progressive disease on imaging. Five of 19 patients (26%) had improved VF and 14/19 (74%) had stable VF.
Toxicity
The most common attributable toxicities included grade 1 and 2 CPK elevation, anemia, diarrhea, headache, nausea, emesis, fatigue, AST increase, ALT increase, hypoalbuminemia, and rash, all similar to what has been previously reported.8,22 Rare grade 3 toxicities included CPK elevation, emesis, weight gain, hyponatremia, abdominal pain, diarrhea, mucositis, limb edema, lymphopenia, weight loss, anorexia, maculopapular rash, and skin ulceration (Table 2). Four patients on the current trial developed hyponatremia, which has been reported with other MEK inhibitors; none of these patients had baseline diabetes insipidus or panhypopituitarism.29,30 There were three specific grade 4 toxicities reported among the 25 patients; one “optic nerve disorder,” one serum amylase increased, and three CPK elevations (Table 2). Patients who developed grade 4 CPK elevations did not have any associated renal impairment or any subjective complaints of muscle pain documented. Holding drug and reducing dose as per protocol was successful in resolving these grade 4 toxicities. No patient came off study due to CPK elevation. Upon further scrutiny, the grade 4 “optic nerve disorder” was the development of VA worsening that occurred 14 days after selumetinib was stopped and thought most likely due to progressive disease. This decreased to a grade 3 toxicity 2 days after the initial grade 4 was reported. The treating physician felt attribution to selumetinib was at least possible. Eleven patients had dose reductions due to toxicities, 6 patients had one dose reduction, and 5 patients had 2 dose reductions (Table 3). Only four patients discontinued selumetinib due to toxicity including a grade 3 weight loss, a grade 2 retinal detachment, a grade 3 amylase, and one patient with both a grade 3 emesis and “visual changes.”
Table 2.
Grade 3 and 4 Attributable Toxicities. Number of Events (Number of Patients)
| Toxicity | Grade 3 | Grade 4 |
|---|---|---|
| CPK elevation | 5 (5) | 3 (3) |
| Emesis | 2 (2) | |
| Weight gain | 2 (2) | |
| Hyponatremia | 2 (2) | |
| Abdominal pain | 1 (1) | |
| Diarrhea | 1 (1) | |
| Mucositis | 1 (1) | |
| Limb edema | 1 (1) | |
| Lymphopenia | 1 (1) | |
| Weight loss | 1 (1) | |
| Anorexia | 1 (1) | |
| Maculopapular rash | 1 (1) | |
| Skin ulceration | 1 (1) | |
| Serum amylase increased | 1(1) | |
| Optic nerve disorder | 1 (1) |
Abbreviation: CPK, creatine phosphokinase.
Table 3.
Dose Reductions
| Patient | Assigned Selumetinib Dose (mg/m2/Dose BID) | Course when the Reduction Occurred | Reduced Selumetinib Dose (mg/m2/Dose BID) | Reason for Dose Reduction |
|---|---|---|---|---|
| 1 | 25 | 7 | 20 | Grade 4 CPK elevation |
| 25 | 9 | 15 | Grade 3 CPK elevation | |
| 2 | 25 | 3 | 20 | Grade 3 CPK elevation |
| 3 | 25 | 1 | 20 | Grade 3 hyponatremia |
| 25 | 23 | 15 | Grade 3 CPK elevation | |
| 4 | 25 | 2 | 20 | Grade 3 mucositis |
| 25 | 9 | 15 | Grade 3 CPK elevation | |
| 5 | 25 | 2 | 20 | Grade 4 CPK elevation |
| 25 | 6 | 15 | Grade 3 CPK elevation | |
| 6 | 25 | 6 | 20 | Grade 2 intolerable acneiform rash |
| 7 | 25 | 1 | 20 | Grade 3 abdominal pain |
| 25 | 13 | 15 | Grade 3 skin ulceration | |
| 8 | 25 | 2 | 20 | Grade 4 CPK elevation |
| 9 | 25 | 13 | 20 | Grade3 skin rash |
| 10 | 25 | 2 | 20 | Grade 2 intolerable rash |
| 11 | 25 | 2 | 20 | Ejection fraction decrease requiring drug hold for >7 days |
Abbreviation: CPK, creatine phosphokinase.
Pharmacokinetics
Optional pharmacokinetic studies were performed in 7 of 25 patients enrolled on stratum 4. Median (range) Cmax was 1296 nM (range 813-2971), Tmax was 2.0 h (1.0-3.0), and AUC0-8 was 4148 h·nM (3859-7424). Median apparent oral selumetinib clearance was 13.0 L/h (5.65-19.5), and median AUC0-8 for the N-desmethyl-selumetinib was 333 h·nM (213-410). Selumetinib apparent oral clearance tended to linearly increase with age (Spearman’s correlation coefficient; r = 0.71; P = 0.13).
Discussion
Despite not meeting the initial Simon Stage 1 criterion for expansion, an amendment allowing for additional accrual was approved given the observed prolonged disease stability, encouraging PFS, tumor shrinkage noted in the first 16 patients, and the promising efficacy data from other strata in this trial. This was a decision recommended by the PBTC DSMB and approved by CTEP as well as PBTC scientific leadership in an effort to provide potential beneficial treatment to patients and document response, PFS, and visual outcomes in a descriptive fashion. The observed outcome measures in this cohort compare favorably to historic trials of children with recurrent OPHG.13,14,31
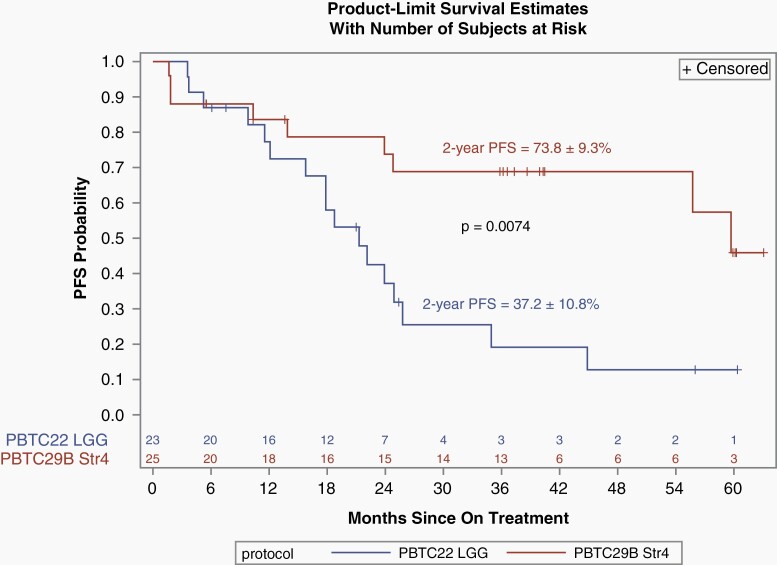
A phase II study of bevacizumab and irinotecan conducted by the PBTC enrolled 35 eligible and evaluable children with recurrent pLGG in any location (PBTC-022, stratum E). There were 2 PRs (5.7%) and the 2-year PFS for all patients was 47.8 ± 9.34%.14 Of the 35 eligible and evaluable patients, 12 were optic tract tumors, 8 were hypothalamic, and 3 were thalamic. To match PBTC-29B stratum 4, these 23 patients were included in exploratory survival analyses and comparison. The 2-year PFS was 37.20% (±10.83) for the included PBTC-022, stratum E patients and 73.76% (±9.31) for PBTC-29B, stratum 4, respectively, which was a statistically significant difference (P = 0.0074) (Figure 4). There was no statistical difference noted in OS. Also, there was no statistical difference between the median age at time of enrollment on the 2 trials (8.23 years [range 0.62-17.63] on PBTC-022 vs 9.43 years [range 3.69-17.61] on the current study). Although the numbers are small and this was a non-randomized and a post-hoc comparison, these data suggest that selumetinib may be more effective than bevacizumab and irinotecan at treating multiply recurrent and previously treated OPHGs as measured by PFS outcomes. Visual outcome measures were not part of the PBTC-022 study.
Fig. 4.
Kaplan-Meir comparison of PFS between PBTC-022, stratum E (limited to the 23 patients with OPHG and thalamic tumors; n = 23) to PBTC-029, stratum 4 (all eligible and evaluable patients; n = 25). Abbreviations: OPHG, optic pathway and hypothalamic glioma, PBTC, Pediatric Brain Tumor Consortium, PFS, progression-free survival.
Another phase II study of vinblastine monotherapy for children with recurrent pLGG included 34/41 (83%) patients with hypothalamic/chiasmatic tumors. There was a 36% response rate, however, these also included “minor responses,” which was defined as shrinkage between 25% and 49%, which would be considered SD in the current study. The 5-year PFS for patients with tumors in all locations was 42.3 ± 7.2%.13 Finally, a Pediatric Oncology Group phase II study evaluated carboplatin monotherapy specifically in children ≤5 years old with recurrent optic pathway tumors. Among the 50 eligible children enrolled, there were 2 PRs (4%), and 37 (74%) had SD with a 5-year event-free survival of 51.7 ± 12.7%.31 In the current trial, selumetinib treatment led to 4 centrally confirmed, sustained PRs (16%) and prolonged stability (PR or SD) in 21/25 (84%) of children with a 2-year PFS of 78 ± 8.5%. These data suggest that selumetinib is effective at treating recurrent and progressive OPHGs as measured by PFS outcomes, especially given the historic refractory nature of disease in this population. We recognize, however, that a direct comparison to historic trials is difficult and such comparisons should be interpreted with caution given the evolving patient management practices, varying tumor locations, age differences, inclusion of children with NF1, imaging technique differences, response measure differences, and lack of visual outcome data.
An exploratory PFS comparison between the OPHG subset of stratum 3 (NF1-associated pLGG) and the OPHG patients enrolled on stratum 4 (sporadic) of PBTC-029 did not reveal statistically different outcomes (Figure 3), however, there were many more centrally confirmed PRs among those patients with NF1-associated tumors.5 In general, the literature suggests that NF1-associated pLGG have superior response and survival outcomes compared to sporadic OPHG.7 Although our data seem promising, we recognize that the numbers are small, and the analysis is post-hoc, so it is difficult to make any definitive conclusions about whether the lack of difference in PFS is meaningful. Analyses in larger prospective studies using molecularly targeted agents, such as selumetinib, may further clarify these findings.
A limitation of the current data is that tumor location was designated by the local institution and not centrally verified. This is particularly important because it is often difficult to distinguish chiasmatic optic pathway tumors from hypothalamic tumors. It is possible that this could impact outcomes, however, in the current study, this distinction was not standardized across institutions and these tumors were all considered together for the purpose of survival and response assessments. In the current study, there was no distinction made based on specific location within the optic pathway (optic nerve, optic chiasm, optic tracts, or optic radiations), which has shown prognostic value in a previous study.32 Also, since the standard assessments of VA evolved over the study lifetime, currently accepted standard measures of VA such as Teller Acuity Cards (TAC) and HOTV were not utilized. Other limitations included the small number of patients, the lack of molecular testing which precluded statistical analyses, and the somewhat short follow-up period. Finally, patients were required to swallow capsules; and therefore, very young children less than 3 years old were excluded which may have resulted in a selection bias.
Sixty-four percent of patients (16/25) remained progression-free at the time of data freeze for this publication. Among these patients, the median time from starting therapy to last follow-up is 38 months (5.5-63) and the median time since stopping therapy is 15.7 months (0-38.8). These data suggest that prolonged PFS can be achieved in some patients with recurrent and progressive non-NF1 OPHGs even after selumetinib therapy is completed. There was a group of patients (n = 5) with progressive disease (20%) despite selumetinib therapy at a median of 1.8 months (1.7-23.9). It is unclear if these tumors harbored unique unidentified molecular aberrations which may have contributed to their resistance and more aggressive clinical behavior.
The visual outcomes seen in the current trial also compare favorably to historic reports of visual outcomes in patients with OPHG treated with chemotherapy. However, a direct comparison is difficult as there are limited available data, particularly for sporadic OPHG. In 2010, a systematic review and meta-analysis of the literature, which included 174 patients, reported visual outcomes in children with optic pathway tumors after receiving chemotherapy. Approximately, a third of patients had NF1. Twenty-five of 174 (14.4%) had improved vision, and 82/174 (47.1%) had stable vision. The authors concluded that in most children with optic pathway tumors, chemotherapy does not improve vision.33 Another retrospective study evaluating visual outcomes of sporadic optic pathway gliomas at final follow-up (median follow-up = 5.2 years) and reported that among the 54 patients who received chemotherapy, 40/54 (74%) had evidence of progression necessitating a change in therapy. Twenty-four percent progressed with visual changes alone, 54% had MRI progression alone, and another 22% had both vision and MRI changes. Taken together, 46% of the patients had a component of visual worsening, and the majority of patients had significant long-term visual impairment.4 Finally, an International Society of Pediatric Oncology (SIOP) multicenter, prospective cohort study reported VA outcomes among 155 children (both with and without NF1) with optic pathway tumors treated with chemotherapy (CV or CV + etoposide). In patients with sporadic optic pathway glioma, VA improved in 18%, was stable in 43%, and worsened in 39%.34 In the current study, VA improved in 21%, was stable in 68%, and worsened in 11%; 26% had improved VF and 74% had stable VF.
The common side effects of the classic chemotherapeutic agents used in many historic trials include myelosuppression, fever with neutropenia, allergic reactions, peripheral neuropathy, constipation, secondary malignancies, infertility, hypertension, fatigue, epistaxis, hypertension, and proteinuria.7,11,14 The toxicity profile of selumetinib as previously reported and seen in the current trial is well tolerated with very few grade 3/4 toxicities.8,22 Also, selumetinib is an oral agent that does not require intravenous access or a central line and requires less frequent clinic visits. On the current protocol, patients were seen monthly, whereas most classic chemotherapy regimens require weekly or every other week clinic visits.11,12,14 Marrow suppression was infrequent and only grade 1 or 2 when it occurred, and no patients required blood or platelet transfusions. Neutropenia was rare and only grade 1 or 2 with no reports of neutropenic fever or infections associated with neutropenia. Skin rash is commonly seen in patients on selumetinib as seen in the current strata and as previously reported.8,22 This toxicity can be more severe in some patients, though with aggressive skin management, dose interruptions, and dose reductions, it usually improves. Although 11/25 (44%) of patients required dose reductions due to toxicity, the majority were able to continue on therapy. Also, dose reductions did not appear to impact responses which suggest that the effective dose may be lower than the RP2D.
We hypothesize that these selumetinib toxicities, fewer clinic visits, and the absence of a central line/intravenous access would impact favorably on a patient’s quality of life as compared to standard chemotherapy. However, quality of life was not evaluated in the current study. A mentioned previously, one potential limiting factor of selumetinib is that, currently, patients must be able to swallow capsules whole which is often difficult in young children, those with swallowing dysfunction or developmental delays. A more “child-friendly” formulation of selumetinib is currently under development. Also, the late effects of selumetinib are yet unknown. It will be important to follow patients who have received selumetinib long term to document any late adverse events.
Not only do the results of our study provide support for an alternative treatment option for patients with recurrent OPHGs, based on imaging and visual outcomes, but the data herein also led to the inclusion of patients with sporadic OPHG on the current COG large prospective phase III clinical trial for children with non-NF1, non-BRAFV600E, newly diagnosed or previously untreated pLGG (NCT04166409). This study will compare selumetinib to CV in a randomized fashion, evaluating PFS, visual outcomes, neuropsychological outcomes, and quality of life measures. Tissue will be required, even for patients with sporadic OPHG, to assess for MAPK aberrations, and if confirmed, determine whether specific aberrations correlate with response, vision, and survival outcomes. The outcomes of this ongoing COG study could impact the future accepted standard of care in treating children with pLGG.
Acknowledgments
Special thanks to Drs David T. Jones and Stefan M. Pfister at the Hopp Children’s Cancer Center Heidelberg (KiTZ) and German Cancer Research Center (DKFZ), Heidelberg, Germany, for completing the detailed molecular analyses of tumor samples for children enrolled on other strata of the PBTC-029 protocol.
Conflict of interest statement. J.F. was compensated for participation on an Advisory Board for QED Therapeutics. I.Q. was compensated for serving as a consultant on a DSMB for SpringWorks. S. Goldman is on speakers Bureau for Koselugo and AZD in 2020. I.J.D. is a consultant or on an advisory board for Apexigen (uncompensated), Astra Zeneca, Bristol-Myers Squibb, Fennec, QED, and Roche. A.O.T. was compensated for participation on advisory boards for Lilly and Roche. All other authors report no COI with this work.
Authorship statement. Study development: J.F., A.O.T., T.Y.P., A.B., I.J.D., and M.F.; Critical review and approval of the final manuscript: all authors; Enrollment and monitoring of patients: J.F., A.B., S. Gururangan, L.B.K., S. Goldman, I.Q., P.B., I.J.D., and M.F.; Critical review of radiologic assessment: T.Y.P., G.V., C.B., Z.P., and J.Y.J.; Pharmacokinetic study design, methods, and testing: O.C. and C.F.S.; Data analyses and interpretation: J.F., A.O.T., and S.W.; Drafting of the manuscript. J.F. and A.O.T.
Trial registration. NCT01089101.
Funding
This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748 and UM1CA081457. Funding was also provided by the National Cancer Institute Cancer Therapy Evaluation Program and the American Lebanese Syrian Associated Charities and AstraZeneca.
References
- 1.Ostrom QT, Cioffi G, Gittleman H, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2012–2016. Neuro Oncol. 2019;21(Supplement_5):v1–v100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Blank P, Bandopadhayay P, Haas-Kogan D, Fouladi M, Fangusaro J. Management of pediatric low-grade glioma. Curr Opin Pediatr. 2019;31(1):21–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sturm D, Pfister SM, Jones DTW. Pediatric gliomas: current concepts on diagnosis, biology, and clinical management. J Clin Oncol. 2017;35(21):2370–2377. [DOI] [PubMed] [Google Scholar]
- 4.Wan MJ, Ullrich NJ, Manley PE, Kieran MW, Goumnerova LC, Heidary G. Long-term visual outcomes of optic pathway gliomas in pediatric patients without neurofibromatosis type 1. J Neurooncol. 2016;129(1):173–178. [DOI] [PubMed] [Google Scholar]
- 5.Parsa CF, Hoyt CS, Lesser RL, et al. Spontaneous regression of optic gliomas: thirteen cases documented by serial neuroimaging. Arch Ophthalmol. 2001;119(4):516–529. [DOI] [PubMed] [Google Scholar]
- 6.Packer RJ, Iavarone A, Jones DTW, et al. Implications of new understandings of gliomas in children and adults with NF1: report of a consensus conference. Neuro Oncol. 2020;22(6):773–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ater JL, Xia C, Mazewski CM, et al. Nonrandomized comparison of neurofibromatosis type 1 and non-neurofibromatosis type 1 children who received carboplatin and vincristine for progressive low-grade glioma: a report from the Children’s Oncology Group. Cancer. 2016;122(12):1928–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fangusaro J, Onar-Thomas A, Young Poussaint T, et al. Selumetinib in paediatric patients with BRAF-aberrant or neurofibromatosis type 1-associated recurrent, refractory, or progressive low-grade glioma: a multicentre, phase 2 trial. Lancet Oncol. 2019;20(7):1011–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Janss AJ, Grundy R, Cnaan A, et al. Optic pathway and hypothalamic/chiasmatic gliomas in children younger than age 5 years with a 6-year follow-up. Cancer. 1995;75(4):1051–1059. [DOI] [PubMed] [Google Scholar]
- 10.Czyzyk E, Jóźwiak S, Roszkowski M, Schwartz RA. Optic pathway gliomas in children with and without neurofibromatosis 1. J Child Neurol. 2003;18(7):471–478. [DOI] [PubMed] [Google Scholar]
- 11.Ater JL, Zhou T, Holmes E, et al. Randomized study of two chemotherapy regimens for treatment of low-grade glioma in young children: a report from the Children’s Oncology Group. J Clin Oncol. 2012;30(21):2641–2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lassaletta A, Scheinemann K, Zelcer SM, et al. Phase II weekly vinblastine for chemotherapy-naïve children with progressive low-grade glioma: a Canadian Pediatric Brain Tumor Consortium study. J Clin Oncol. 2016;34(29):3537–3543. [DOI] [PubMed] [Google Scholar]
- 13.Bouffet E, Jakacki R, Goldman S, et al. Phase II study of weekly vinblastine in recurrent or refractory pediatric low-grade glioma. J Clin Oncol. 2012;30(12):1358–1363. [DOI] [PubMed] [Google Scholar]
- 14.Gururangan S, Fangusaro J, Poussaint TY, et al. Efficacy of bevacizumab plus irinotecan in children with recurrent low-grade gliomas – a Pediatric Brain Tumor Consortium study. Neuro Oncol. 2014;16(2):310–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gururangan S, Cavazos CM, Ashley D, et al. Phase II study of carboplatin in children with progressive low-grade gliomas. J Clin Oncol. 2002;20(13):2951–2958. [DOI] [PubMed] [Google Scholar]
- 16.Roddy E, Mueller S. Late effects of treatment of pediatric central nervous system tumors. J Child Neurol. 2016;31(2):237–254. [DOI] [PubMed] [Google Scholar]
- 17.Bandopadhayay P, Bergthold G, London WB, et al. Long-term outcome of 4,040 children diagnosed with pediatric low-grade gliomas: an analysis of the Surveillance Epidemiology and End Results (SEER) database. Pediatr Blood Cancer. 2014;61(7):1173–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Packer RJ, Pfister S, Bouffet E, et al. Pediatric low-grade gliomas: implications of the biologic era. Neuro Oncol. 2017;19(6):750–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ryall S, Zapotocky M, Fukuoka K, et al. Integrated molecular and clinical analysis of 1,000 pediatric low-grade gliomas. Cancer Cell. 2020;37(4):569–583.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones DTW, Kieran MW, Bouffet E, et al. Pediatric low-grade gliomas: next biologically driven steps. Neuro Oncol. 2018;20(2):160–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodriguez FJ, Ligon AH, Horkayne-Szakaly I, et al. BRAF duplications and MAPK pathway activation are frequent in gliomas of the optic nerve proper. J Neuropathol Exp Neurol. 2012;71(9):789–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Banerjee A, Jakacki RI, Onar-Thomas A, et al. A phase I trial of the MEK inhibitor selumetinib (AZD6244) in pediatric patients with recurrent or refractory low-grade glioma: a Pediatric Brain Tumor Consortium (PBTC) study. Neuro Oncol. 2017;19(8):1135–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perreault S, Larouche V, Tabori U, et al. A phase 2 study of trametinib for patients with pediatric glioma or plexiform neurofibroma with refractory tumor and activation of the MAPK/ERK pathway: TRAM-01. BMC Cancer. 2019;19(1):1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller C, Guillaume D, Dusenbery K, Clark HB, Moertel C. Report of effective trametinib therapy in 2 children with progressive hypothalamic optic pathway pilocytic astrocytoma: documentation of volumetric response. J Neurosurg Pediatr. 2017;19(3):319–324. [DOI] [PubMed] [Google Scholar]
- 25.Brown NF, Carter T, Kitchen N, Mulholland P. Dabrafenib and trametinib in BRAFV600E mutated glioma. CNS Oncol. 2017;6(4):291–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manoharan N, Choi J, Chordas C, et al. Trametinib for the treatment of recurrent/progressive pediatric low-grade glioma. J Neurooncol. 2020;149(2):253–262. [DOI] [PubMed] [Google Scholar]
- 27.Fangusaro J, Witt O, Hernáiz Driever P, et al. Response assessment in paediatric low-grade glioma: recommendations from the Response Assessment in Pediatric Neuro-Oncology (RAPNO) working group. Lancet Oncol. 2020;21(6):e305–e316. [DOI] [PubMed] [Google Scholar]
- 28.Fisher MJ, Avery RA, Allen JC, et al. Functional outcome measures for NF1-associated optic pathway glioma clinical trials. Neurology. 2013;81(21 Suppl 1):S15–S24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lazow MA, Lawson SA, Salloum R, et al. Trametinib-associated hyponatremia in a child with low-grade glioma is not seen following treatment with alternative MEK inhibitor. J Pediatr Hematol Oncol. 2020. [DOI] [PubMed] [Google Scholar]
- 30.Egan G, Hamilton J, McKeown T, et al. Trametinib toxicities in patients with low-grade gliomas and diabetes insipidus: related findings? J Pediatr Hematol Oncol. 2020;42(4):e248–e250. [DOI] [PubMed] [Google Scholar]
- 31.Mahoney DH Jr, Cohen ME, Friedman HS, et al. Carboplatin is effective therapy for young children with progressive optic pathway tumors: a Pediatric Oncology Group phase II study. Neuro Oncol. 2000;2(4):213–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tow SL, Chandela S, Miller NR, Avellino AM. Long-term outcome in children with gliomas of the anterior visual pathway. Pediatr Neurol. 2003;28(4):262–270. [DOI] [PubMed] [Google Scholar]
- 33.Moreno L, Bautista F, Ashley S, Duncan C, Zacharoulis S. Does chemotherapy affect the visual outcome in children with optic pathway glioma? A systematic review of the evidence. Eur J Cancer. 2010;46(12):2253–2259. [DOI] [PubMed] [Google Scholar]
- 34.Falzon K, Drimtzias E, Picton S, Simmons I. Visual outcomes after chemotherapy for optic pathway glioma in children with and without neurofibromatosis type 1: results of the International Society of Paediatric Oncology (SIOP) low-grade glioma 2004 trial UK cohort. Br J Ophthalmol. 2018;102(10):1367–1371. [DOI] [PubMed] [Google Scholar]