Abstract
Pediatric low-grade gliomas (pLGGs) are the most common brain tumor in children and are associated with lifelong clinical morbidity. Relative to their high-grade adult counterparts or other malignant childhood brain tumors, there is a paucity of authenticated preclinical models for these pLGGs and an incomplete understanding of their molecular and cellular pathogenesis. While large-scale genomic profiling efforts have identified the majority of pathogenic driver mutations, which converge on the MAPK/ERK signaling pathway, it is now appreciated that these events may not be sufficient by themselves for gliomagenesis and clinical progression. In light of the recent World Health Organization reclassification of pLGGs, and pilocytic astrocytoma (PA), in particular, we review our current understanding of these pediatric brain tumors, provide a conceptual framework for future mechanistic studies, and outline the challenges and pressing needs for the pLGG clinical and research communities.
Keywords: BRAF, cellular senescence, low-grade glioma, MEK, neurofibromatosis type 1, pediatric brain tumor, pilocytic astrocytoma, tumor microenvironment
Brain tumors are one of the most common solid tumors in children and adults, accounting for significant morbidity and mortality across the lifespan.1 In children, the majority of brain tumors are low-grade glial (astrocytic) neoplasms (World Health Organization [WHO] grade 1 and 2 astrocytomas), whereas in adults, malignant gliomas (WHO grade 3 and 4 astrocytomas) predominate.2 Because of their poor prognosis, the vast majority of glioma research has focused on adult glioblastoma, and more recently, pediatric diffuse intrinsic pontine glioma (DIPG) and other tumors with histone-3 (H3)-K27M mutations. While the molecular landscape of pediatric low-grade gliomas (pLGGs) has been described, less is known about the pathobiology of low-grade gliomas, and few preclinical models exist to enable effective clinical translation. Moreover, these pediatric gliomas differ dramatically from their adult malignant counterparts in terms of age of onset, probable cells of origin, brain locations, histological spectrum, clinical progression, genetic alterations, and stromal (tumor microenvironment [TME]) dependence. In this review, we assembled a diverse team of subject matter experts to summarize our current understanding of these unique low-grade tumors and present challenges for future research and clinical application.
Epidemiology
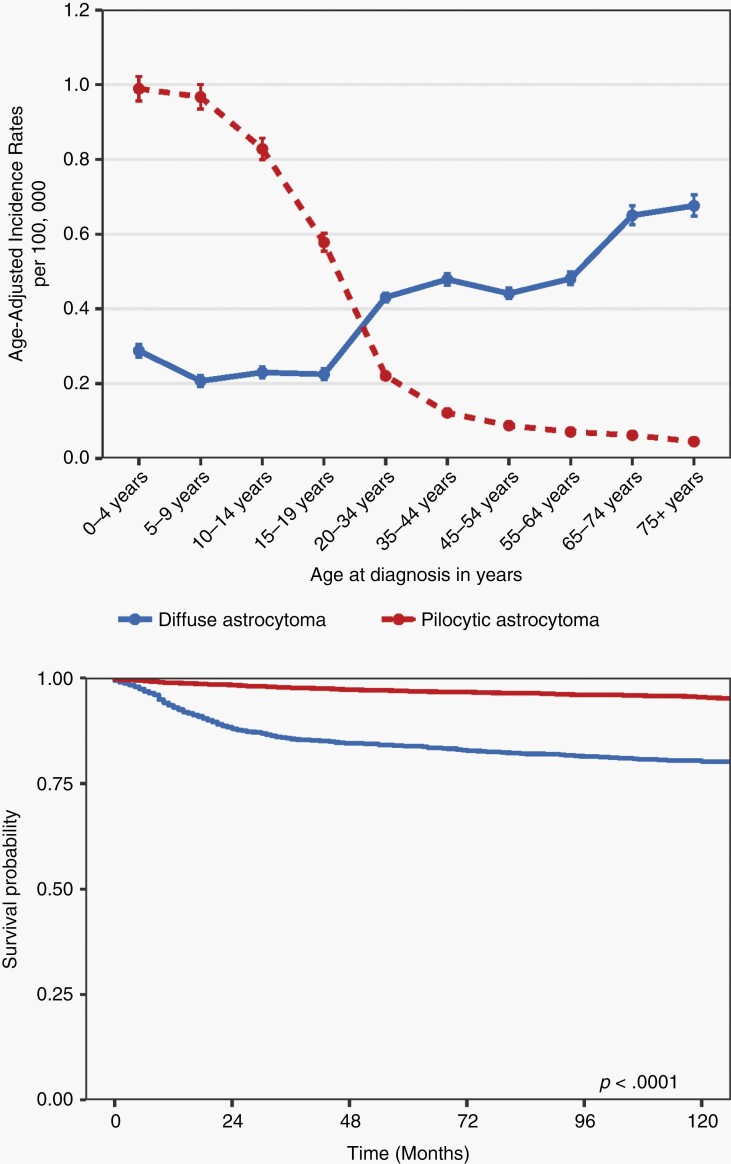
While we now appreciate that there are a diverse number of molecularly- and histopathologically-distinct types of low-grade gliomas (see below), current epidemiologic data are only available for grade 1 (PA) and grade 2 diffuse astrocytoma (DA). In the United States from 2000 to 2017, there were 12 403 patients reported with PA and 3432 patients with grade 2 DA among children and adolescents 19 years of age or younger (Supplementary Tables 1 and 2).3–5 The overall incidence rate for PA (age-adjusted annual incidence rate [AAAIR]: 0.839 [95% CI: 0.824-0.854]) was higher compared to DA (AAAIR: 0.231 [95% CI: 0.223-0.239]) (Supplementary Table 2). As such, the incidence of PA was highest in patients 0-4 years of age (AAIR: 0.989 [95% CI: 0.956-1.022]), and lowest for patients >19 years of age (AAIR: 0.578 [95% CI: 0.554-0.602]) (Figure 1A). In contrast, the incidence of DA was lowest in patients 0-4 years of age (AAIR: 0.284 [95% CI: 0.267-0.302]) and increases into adulthood. The overall incidence rate of both PA and DA was slightly higher in males relative to females (AAAIR: 0.856 in males vs 0.821 in females for PA and AAAIR: 0.247 in males vs 0.215 in females for DA) (Supplementary Table 1). While a greater proportion of PAs was found within cerebellum (33.1%) compared to any other site, no such spatial predominance was seen with DA.
Fig. 1.
Epidemiology of pediatric low-grade gliomas. (A) Average annual age-adjusted incidence rates with 95% confidence intervals for individuals aged 19 years or younger with pilocytic astrocytoma (PA; red) vs diffuse astrocytoma (DA; blue) by age at initial diagnosis. Rates are per 100 000 and age-adjusted to the 2000 US Standard Population (19 age groups—Census P25-1130) standard; confidence intervals (Tiwari mod) are 95% for rates. CBTRUS: data provided by CDC’s National Program of Cancer Registries and NCI’s Surveillance, Epidemiology and End Results Program, 2000-2017. (B) Kaplan-Meier overall survival curve for PA (red) vs DA (blue) in individuals aged 19 years or younger (log-rank P value). National Program of Cancer Registries, 2001-2016.
In general, patients with PA had significantly better overall survival (OS) relative to those with DA (log-rank P < .0001) (Figure 1B), where the overall age-adjusted mortality rate (AAMR) for PA (0.025 [95% CI: 0.020-0.030]) was higher than observed for DA (0.006 [95% CI: 0.004-0.008]).6 Of note, the AAMR was highest in children 15-19 years of age with PA (AAMR: 0.026 [95% CI: 0.0217-0.038]) (Supplementary Table 3).
Histopathology
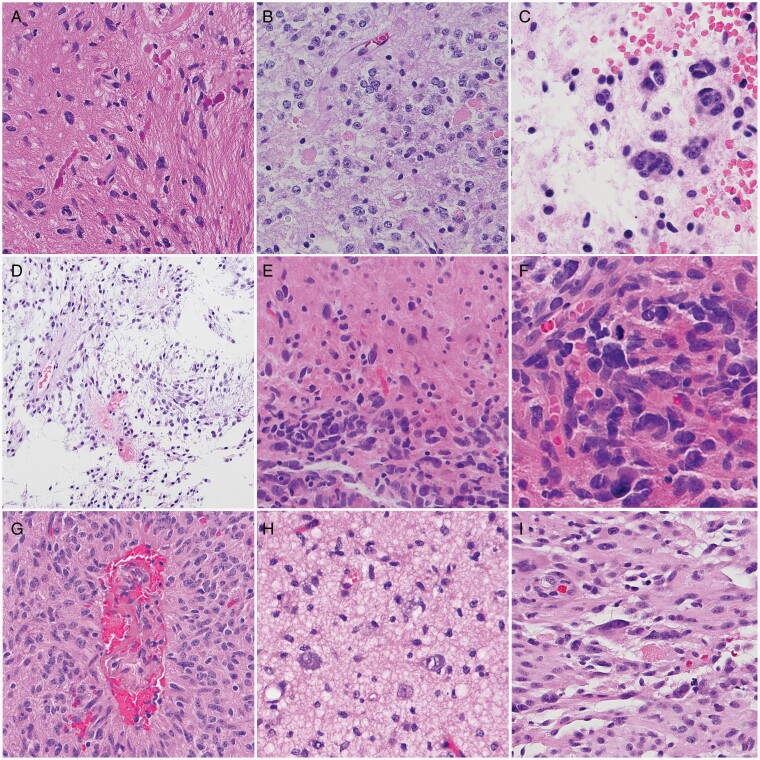
pLGGs comprise a diverse collection of tumors. Pilocytic astrocytoma (PA), pilomyxoid astrocytoma (PMA), and pleomorphic xanthoastrocytoma (PXA) are typically relatively circumscribed, whereas DA, angiocentric glioma, and polymorphous low-grade neuroepithelial tumor of the young (PLNTY) show more infiltrative growth patterns (Figure 2). However, infiltration can occur in both PA and PXA.
Fig. 2.
Histopathology of pilocytic astrocytomas (PA) and other related pediatric low-grade gliomas. PAs are typically biphasic tumors containing piloid areas with (A) Rosenthal fibers and (B) oligodendrocyte-like areas with eosinophilic granular bodies. (C) Multinucleated cells in a loose myxoid stroma are found in a subset of cases. (D) In contrast, the pilomyxoid astrocytoma subtype has a monomorphous appearance embedded within a myxoid background and frequent perivascular aggregates. (E) Histologic features of anaplasia in PA may be recognizable as cellular aggregates in an otherwise conventional PA, (F) with increased mitotic activity. Subsets of pediatric low-grade astrocytomas with distinct alterations include (G) angiocentric glioma (with MYB-QKI fusions), (H) MYB-altered diffuse astrocytoma, and (I) pleomorphic xanthoastrocytoma, which frequently harbors BRAFV600E mutations. Magnification, ×400 (A, B, C, E, H, I), ×600 (F), ×200 (D, G).
Pilocytic Astrocytoma
At the histologic level, PAs show a predominantly compact growth pattern, although more infiltrative regions can also be identified, particularly in the cerebellum and optic nerve. Classic PAs exhibit a biphasic pattern, with dense fibrillar areas rich in “piloid” cells with long, thin processes, as well as Rosenthal fibers, alternating with microcystic regions, where oligodendrocyte-like cells may predominate. In some cases, however, only piloid- or oligodendrocyte-rich regions are encountered. While mitotic figures are generally rare, vascular proliferation is common, but does not have the same association with poor prognosis as seen in diffuse infiltrating astrocytomas. Non-palisading necrosis occurs in a subset of cases, but is not considered a prognostic feature, and all PAs are grade 1 tumors. Additional microscopic features can include myxoid or degenerative changes, calcifications, hyalinized vessels, and hemorrhages.
Pilomyxoid Astrocytoma Subtype
PMA is a subtype of PA, clinically characterized by a predilection for young children, suprachiasmatic locations, and a higher propensity for cerebrospinal fluid dissemination.7,8 Histologically, PMAs have a monomorphous, rather than biphasic, appearance, myxoid stroma, perivascular arrangements of cells, and a lack of Rosenthal fibers and eosinophilic granular bodies. Despite some clinical and pathologic differences, the presence of shared genetic drivers, a co-occurrence of PA and pilomyxoid patterns in some instances, as well as the phenomenon of “maturation” where a PMA develops more overt PA morphology over time,9 suggest a shared origin between conventional PA and PMA. These features also raise the possibility that, in some cases, PMA may serve as a precursor to PA.
Pilocytic Astrocytoma With Histological Features of Anaplasia
PAs, in general, maintain their low-grade histology over time, even after multiple recurrences. However, a rare subset of PA, at presentation or progression, contains areas of brisk mitotic activity, with or without necrosis, leading to the designation of PA with histological anaplasia or anaplastic pilocytic astrocytoma (APA). These tumors are associated with an increased frequency of CDKN2A/B, NF1, and ATRX mutations.10,11 These changes are almost always encountered in PAs arising in adults.12
Pleomorphic Xanthoastrocytoma
These grade 2 compact gliomas are typically supratentorial in location and often involve the leptomeninges. PXAs are comprised of astrocytic cells, which can be spindled, epithelioid, pleomorphic/multinucleated, or lipidized. Eosinophilic granular bodies and reticulin deposition are frequently encountered microscopically, and BRAFV600E mutation, other MAPK/ERK (mitogen-activated protein kinase/extracellular signal-regulated kinase) pathway gene alterations, and homozygous CDKN2A or CDKN2B deletions represent common molecular alterations. Anaplastic PXA (WHO grade 3) harbor increased mitotic activity, with or without other adverse histologic features (e.g., microvascular proliferation and necrosis).
Diffuse Pediatric Low-Grade Gliomas
Many infiltrative low-grade gliomas encountered in children are histopathologically bland, with mildly atypical astrocytic or oligodendroglial cells diffusely invading brain parenchyma and modestly raising cellular density. Most of these diffuse LGGs are characterized genetically by a pathogenic alteration in genes that code for MAPK/ERK pathway proteins.13,14 Some have changes involving the MYB or MYBL1 locus, and these molecular features have been associated with tumors designated isomorphic diffuse glioma (IDG).15 Another rare subtype with distinctive features is the PLNTY, characterized clinically by a strong association with seizures and microscopically by oligodendroglioma-like regions, calcification, and CD34 immunoreactivity; these tumors also frequently have MAPK/ERK pathway-activating genetic abnormalities.16 Finally, angiocentric glioma is an infiltrative tumor composed mainly of bland, bipolar cells with thin processes, which aggregate in perivascular spaces.17 Angiocentric gliomas almost always have a MYB-QKI gene fusion or another MYB gene alteration.18
Since the spectrum of distinct molecular subtypes in pediatric LGG is so diverse, it has been difficult to create a comprehensive classification of these tumors, which integrates histology and molecular alterations. Several classifications have been proposed, based on the underlying BRAF alteration,19 by integrating age, histology, and molecular alterations,14 or by combining clinical parameters, histology, and molecular analysis to stratify patients into low-, intermediate-, and high-risk groups.13 However, considering the increasing number of reports describing novel molecular entities and improvements in diagnostic resolution, a consensus classification has yet to be reached. For this reason, we will mainly focus on the most common subgroup of PA harboring MAPK/ERK pathway alterations.
Molecular Genetics
Recent advances in molecular diagnostics have had a lasting impact on defining LGG diagnostic entities. These technical advancements, coupled with the greater availability and diminishing costs of novel diagnostic methods, allow for more definitive classification of clinically relevant diagnostic categories.2 Moreover, DNA methylation-based classification of brain tumors has similarly improved brain tumor diagnostics.20,21 In this manner, the use of DNA methylation arrays and a reference dataset (https://www.molecularneuropathology.org/mnp), currently consisting of over 2800 brain tumors, accurately identifies over 80 distinct brain tumor subtypes. Applying this DNA methylation fingerprint also permits confirmation of the original histopathologic assignment, as well as the establishment of new diagnoses in 12% of cases.21 Additionally, this method can be used for DNA methylation-based identification of potential new brain tumor entities. Finally, DNA methylation array fingerprinting can be used to derive copy number profiles,20 which can be used to reliably call KIAA1549-BRAF fusions.22 These fusions can also be identified by RNA sequencing, even using formalin-fixed paraffin-embedded (FFPE) specimens.23
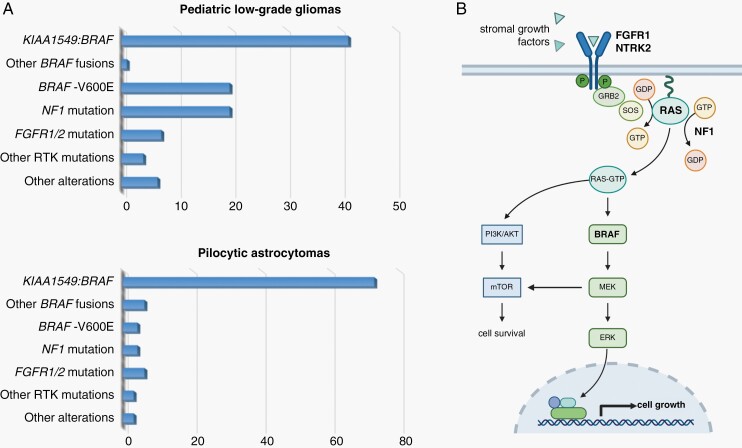
The majority of pLGGs show a broad range of molecular alterations converging on the MAPK/ERK signaling pathway.13,24,25 As such, nearly 100% of PAs harbor an alteration that affects MAPK/ERK pathway regulation,26 where BRAF kinase alterations are considered to be the hallmark of this class of brain tumors (Figure 3A). The most frequent BRAF alteration involves a genomic rearrangement that results in a fusion between the KIAA1549 and BRAF genes (70% of PAs25,27), followed by inactivating Neurofibromatosis type 1 (NF1) alterations and oncogenic BRAFV600E mutations.26,28 Each of these alterations results in increased MAPK/ERK activation, either through loss of RAS suppression or elevated MEK/ERK activation. Less frequent alterations include other BRAF fusions, FGFR1 mutations or fusions, NTRK2 fusions, and oncogenic KRAS mutations.13,26 Importantly, the common abnormality involving increased MAPK/ERK pathway activation makes PA a single pathway disease, ideally suited for therapeutic targeting (Figure 3B).
Fig. 3.
Mutational spectrum in pediatric low-grade gliomas. (A) Distribution of molecular alterations in pediatric low-grade gliomas (top13) and pilocytic astrocytomas (bottom26). X-axis denotes the percentage of tumors with identified mutations in each cohort (N = 400, pLGGs; N = 96, PAs). (B) Graphic illustration of the MAPK/ERK signaling pathway, highlighting the genetic/genomic alterations commonly observed in these tumors that increase MEK signaling (bold; involving the FGFR1, NTRK2, RAS, BRAF, and NF1 genes). Created with BioRender.com. Abbreviations: MAPK/ERK, mitogen-activated protein kinase/extracellular signal-regulated kinase; PAs, pilocytic astrocytomas; pLGGs, pediatric low-grade gliomas.
With the advent of molecular diagnosis, some prognostic information is now available. As such, individuals with genomic rearrangements (e.g., BRAF fusion) tend to have more favorable progression-free survival (PFS) and OS rates relative to those whose tumors harbor single-nucleotide variants (SNVs).13 The presence of a BRAFV600E mutation seems to confer a poorer prognosis compared to tumors with wild-type BRAF.13 However, this is not independent of additional contributing factors, such as degree of surgical resection and the presence of a CDKN2A deletion. When combined (incompletely resected pLGGs with BRAFV600E mutation and CDKN2A deletion), these factors are associated with a higher risk of clinical progression.29 Finally, midline gliomas with H3-K27M mutations are associated with anaplastic features,10 confer a poor prognosis and a high risk of tumor progression.13 Those rare instances of H3-K27M mutations with concurrent BRAFV600E mutations (which seem to be mostly ganglioglioma) may behave in a less aggressive manner than their more high-grade counterparts.30
Microenvironmental Support of pLGG Growth
A particular feature of pLGGs is a heavy dependence on the local TME. While the TME constitutes a major cellular component of pLGGs, reports on immune cell composition differ. In both sporadic and NF1-PA, microglia/macrophages comprise 30%-50% of the cells in the tumor,31,32 while T-cell content is more variable.33
Importantly, the communication between the neoplastic cells and components of the TME is bidirectional, as has been shown in murine models of pLGG. As such, cerebellar neural stem cells expressing human KIAA1549:BRAF fail to form tumors following implantation into the cerebella of mice lacking the microglia chemotactic receptor (Ccr2),34 whereas cancer stem cells from Nf1-mutant mice with optic gliomas do not form tumors after injection into the brainstems of mice lacking either T cells35 or microglia/T-cell chemotactic receptors (Cx3cr1, Ccr2).36 In Nf1 optic glioma mice, optic glioma stem cells elaborate chemokines (Ccl2, Cx3cl1, Ccl12) that differentially attract T cells and microglia37 to establish a paracrine circuit in which CD8+ T cells produce the cytokine Ccl4, to induce microglial Ccl5 expression necessary for optic glioma stem cell and tumor growth.38
In addition to providing trophic support, the TME can also contribute to negative regulation of PA growth through the induction of oncogene-induced senescence (OIS), resulting from MAPK/ERK hyperactivation. Support for OIS derives from studies in which ectopic expression of oncogenic RAS in primary human or rodent cells results in a permanent G1 arrest, and the accumulation of p53 and p16, indistinguishable from cellular senescence.39 Since NF1-PAs harbor increased RAS activity, NF1 loss could induce senescence, as seen in melanocytes and patient-derived café-au-lait macules (CALMs).40 Similarly, sustained activation of wild-type or mutant BRAF in astrocyte cell lines in vitro induces OIS,41 whereas oncogenic BRAFV600E expression in neural stem cells leads to growth arrest and OIS induction.42 While OIS results in growth arrest, it also induces the expression of a senescence-associated secretory phenotype (SASP), comprising a complex inflammatory network in models of fusion BRAF-driven PA,43 which further sustains OIS in an auto- and paracrine manner.44 The induction of this OIS/SASP circuit favors growth arrest and, potentially, lack of pLGG progression or partial tumor regression.
Current Therapies for PA
In general, individuals harboring PAs have a very good prognosis, with a 10-year OS of 94% and a 10-year event-free survival (EFS) of 44%.45 These numbers highlight the principle that PAs represent a chronic lifelong disease with multiple potential recurrences. For this reason, there is a lower risk tolerance on the parts of families and physicians for treatments with significant therapy-associated morbidity. As such, surgical operations, whenever feasible, should be limited to “maximum safe resections,” and a “watch and wait” approach without adjuvant therapy should be adopted after a complete resection or when only a small amount of residual tumor is present. Unfortunately, future tumor growth is highly variable among patients with the same histologic tumor subtype, and as many as one-third of individuals with partially resected cerebellar astrocytomas show no sign of progression.46 In contrast, once patients progress after first-line adjuvant treatment, they show an increased risk of further tumor progression, where the risk factors include young age (<1 year of age at the time of initial treatment), tumor dissemination, and early progression following therapy (<18 months after the start of chemotherapy).47
The current standard-of-care therapeutic options consist of systemic chemotherapy and, secondly, radiotherapy (RT). Standard chemotherapy typically entails the use of carboplatin and vincristine or vinblastine monotherapy.48,49 RT, if necessary, should be only given at a later age to minimize neurocognitive sequelae.48 For patients with NF1, RT is typically avoided, due to the elevated risk of secondary malignancy50 or moya-moya syndrome.51 Adjuvant therapy is typically only recommended for inoperable, symptomatic, or progressive tumors that cannot be re-resected.48
However, in light of the common hyperactivation of the MAPK/ERK pathway in pLGGs, targeted therapies focusing on MEK inhibition (MEKi) have been at the forefront of clinical therapeutic trials, both for NF1- and BRAF-altered pLGGs in general, and for PAs, in particular. As such, the MEKi selumetinib has been investigated in phase I and II trials,52–54 with promising clinical and radiographic responses. Similarly, the phase II TRAM-01 trial is currently investigating the MEKi trametinib for recurrent NF1-associated and BRAF-fusion pLGGs.55 Another trial (NCT03871257) is investigating the MEKi selumetinib relative to carboplatin/vincristine in a front-line setting for NF1-mutant pLGG. The LOGGIC European trial will be the first prospective randomized 3-arm clinical study for newly diagnosed BRAF-fusion–positive pLGGs, specifically designed to compare the MEKi trametinib to carboplatin/vincristine combination therapy and vinblastine monotherapy as first-line treatment for these tumors. Finally, based on fast, robust, and durable responses in BRAFV600E-mutant pLGG,56 a prospective randomized phase II study of the BRAF inhibitor dabrafenib and the MEK inhibitor (MEKi) trametinib as first-line therapy (NCT02684058) for BRAFV600E-mutant pLGG is currently underway.
To date, there have been limited efforts to target the TME or the senescence phenotype in pLGGs. While murine studies demonstrate the dramatic impact of silencing stromal cell types and paracrine factors on Nf1 optic glioma formation and growth,37,57 these agents have not entered human clinical trials. In addition, activating the immune system to change the interactions between T cells and microglia relevant to glioma growth has begun to gain some traction. Similarly, the application of drugs that modulate senescence (senolytics) and promote OIS might also have utility in the future treatment of pLGGs.43,58
PAs Are Developmental Disorders
In contrast to conflicting studies using mouse models of glioblastoma,59–66 there appear to be a limited number of cell types that could serve as the potential cellular origins for low-grade glioma. Using experimental murine systems to model low-grade gliomas arising in response to Nf1 inactivation or BRAF fusion (KIAA1549:BRAF), tumors form from progenitor cells, rather than mature astrocytes.67,68 Specifically, in the case of Nf1 optic gliomas, tumors form from BLBP+, GFAP+, CD133+, SOX2+, Nestin+ progenitors (neural stem cells) or Olig2+ progenitors, but not from NG2+ glia or astrocytes.69–72 The use of mice with an inducible Cre recombinase further helped to refine the temporal window for tumor development, which is largely restricted to embryonic development.69 Similarly, in experimental mouse models of sporadic PA, implantation of fusion BRAF-expressing neural progenitors, but not BRAF-expressing astrocytes, led to glioma-like lesion formation in vivo, whereas fusion BRAF expression in BLBP+ progenitors, but not in NG2+ or GFAP+ glia, resulted in greater proliferation and gliogenesis in the cerebellum in vitro and in vivo.68,73 These studies parallel observations made in their human counterparts, in which pediatric74 and adult infratentorial75 PAs likely arise from neural progenitor cells early in life.
In addition to the cell of origin and the timing of their acquired driver mutation (developmental age), there are also regional differences in the response to these LGG mutations: neural progenitors from the brainstem and third ventricle exhibit increased proliferation and glial differentiation in response to Nf1 gene inactivation or KIAA1549-BRAF expression, whereas those from the lateral ventricles or cortex do not.67,68,76 Taken together, these observations reveal both spatial and temporal restrictions on the patterning of low-grade gliomas, analogous to the constraints that govern normal brain development.
Proposed Conceptual Framework for PA Pathogenesis
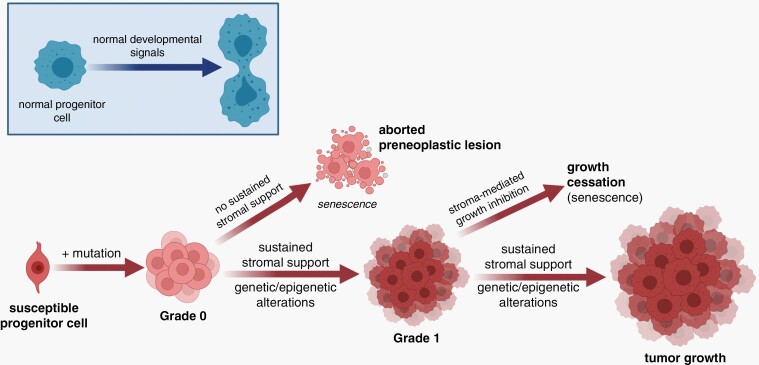
Based on clinical observations and preclinical modeling, it is likely that many PA-associated driver mutations create an indolent preneoplastic state (Figure 4). In this regard, we envision pediatric LGG formation to require that the causative genetic alterations occur in a susceptible cell (LGG cell of origin) during a specified developmental window. While this event is necessary for tumorigenesis, it is likely not sufficient. As such, human PA tumors do not grow well in vitro or as explants in immunocompromised mice in vivo, requiring specialized tissue culture conditions and growth factors.42,77 In this regard, telomere maintenance,78 as well as the induction of OIS41 and the production of inflammatory cytokines of the SASP,43 may play roles in mediating this premature growth arrest in vitro and in vivo.
Fig. 4.
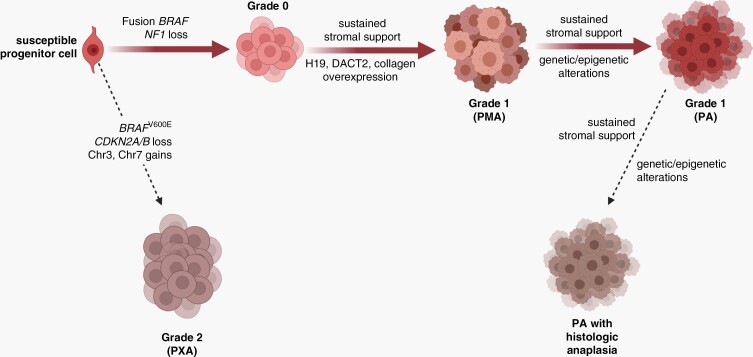
Proposed model of PA pathogenesis. Similar to normal brain development where progenitor cells receive developmental signals from their environment (blue inset), pilocytic astrocytomas arise from susceptible progenitor cells following the acquisition of a genetic alteration (e.g., NF1 loss, FGFR1 mutation, KIAA1549:BRAF fusion), which creates a preneoplastic state (grade 0). These lesions in the setting of inhibitory growth signals from the tumor microenvironment undergo senescence or death and do not form gliomas. However, in the setting of sustained stromal support, they evolve into grade 1 tumors whose continued growth is dictated by ongoing microenvironment growth factors and conditions. Alternatively, or in concert with stromal paracrine factor support, additional genetic (microRNA) or epigenetic events could facilitate continued tumor growth and progression. Created with BioRender.com. Abbreviations: NF1, neurofibromatosis type 1; PA, pilocytic astrocytoma.
Progression from a grade 0 preneoplastic state requires supportive growth signals from macrophage-like cells in the local TME. The importance of these monocytes to murine low-grade glioma formation and growth is evidenced by numerous studies, in which KIAA1549-BRAF-driven glioma-like lesions and Nf1 optic gliomas do not form in the absence of functional microglia34,37,38,79–81 or T cells.36,38,57
In addition to these growth regulatory signals, transcriptional or genomic changes, including the expression of specific microRNA molecules, or the development of epigenetic alterations may also promote PA tumor survival and progression. Specific microRNAs are differentially expressed in PA and other pLGGs: for example, miR-125b overexpression in PA cells leads to decreased growth, apoptosis, and/or senescence,82 whereas other miRNAs establish global gene expression changes,83–84 some of which affect mitogenic signaling,85 as well as tumor growth and migration.82,86
While epigenetic features have been essential to the identification of novel diagnostic glioma subgroups, and IDH1/2 and H3K27 mutations are critical for high-grade glioma biology,87–89 much less is known about the role of epigenetic events in pLGG pathogenesis. In this regard, H3K27 mutations are associated with a greater risk of clinical progression in a small subset of PAs, whereas differential methylation leads to altered expression of key neural developmental genes, including NR2E1, EN2, and the polycomb repressor complex 2 (PRC2).90 Future research will be required to define how these epigenetic and gene expression network changes influence PA pathobiology.
Taken together, these findings support a model in which stromal support is an obligate step in tumor formation and progression, and may explain both uncommon clinical observations in patients and experimental studies in vitro. First, while not commonplace, some PAs regress without therapy, particularly those encountered in children with NF1.91–93 Second, a significant portion of NF1-PAs do not continue to grow or cause clinical signs or symptoms.94–96 For these reasons, it is likely that the absence of proper stromal support induces the demise of the tumor cells (aborted preneoplastic lesion [APL]).
Integrating the known clinical evolution of PA-like tumors and associated genomic/genetic alterations, we also propose unique developmental stages during which PAs might have the potential to form more aggressive variants (PXA; Figure 5), although progression is much less likely to occur in pLGGs than in their biologically distinct adult glioma counterparts. In this model, which will likely evolve as these subtypes are molecularly characterized in more detail, PMA and PA tumors are likely on the spectrum of grade 1 astrocytic tumors, in which driver events (KIAA1549:BRAF fusion, NF1 loss) cooperate with genetic/epigenetic alterations and stromal paracrine factors to facilitate tumor growth. Higher grade tumors likely arise either directly from susceptible progenitors or grade 0 lesions following the acquisition of chromosomal gains/losses and additional genetic alterations. Similarly, PAs with histologic anaplasia evolves from grade 1 PAs as a result of specific genetic/epigenetic changes that largely remain to be identified, although some molecular alterations associated with anaplasia have been reported.10 This proposed hierarchical evolution of PA-like tumors will require detailed study using spatially- and temporally resolved single-cell DNA and RNA sequencing.
Fig. 5.
Proposed model of pilocytic astrocytoma (PA) progression. The spectrum of PA-like tumors are hypothesized to arise from similar cells of origin (susceptible progenitor cells), whose further evolution is dictated by the specific genetic/genomic alterations and stromal support. While formation and continued growth of classic PAs are heavily dependent on microenvironment conditions (e.g., stromal growth factors) and/or genetic (microRNA) or epigenetic alterations, the acquisition of additional genetic/genomic aberrations facilitates the formation of tumors with less stromal dependence and more clinically aggressive behavior. In this regard, pleomorphic xanthoastrocytoma (PXA) harbor chromosomal gains, CDKN2A/B locus loss, and oncogenic BRAF mutations. Additionally, PAs may arise from pilomyxoid astrocytomas (PMA) that develop in response to the overexpression of specific genes (i.e., H19, DACT2, and collagen) important for tumor progression. Lastly, sustained stromal support and further genetic/epigenetic alterations are likely responsible for the progression of PAs to PAs with histologic anaplasia. Created with BioRender.com.
Unresolved Questions and Future Directions
The unique properties of pLGGs and their clear dependence on cells and signals in the local microenvironment present challenges for both researchers and clinicians. In this regard, their slow growth rates in young children whose brains are still developing require that the treatments are effective at halting further tumor progression and neurologic decline (e.g., vision loss, weakness), while not creating long-term neurocognitive sequelae. As such, the management of pediatric LGGs necessitates a multidisciplinary team of experts who work seamlessly together to balance anti-tumoral therapeutic gains with potential adverse consequences on the developing brain, considering pLGG as a chronic disease. In addition, with the discovery of new therapeutic targets through advanced preclinical modeling and improved molecular classifications of these tumors, treatments will have to be optimized in children to define the most efficacious dosing and delivery schedules, as well as the identification of prognostic factors for clinical progression.
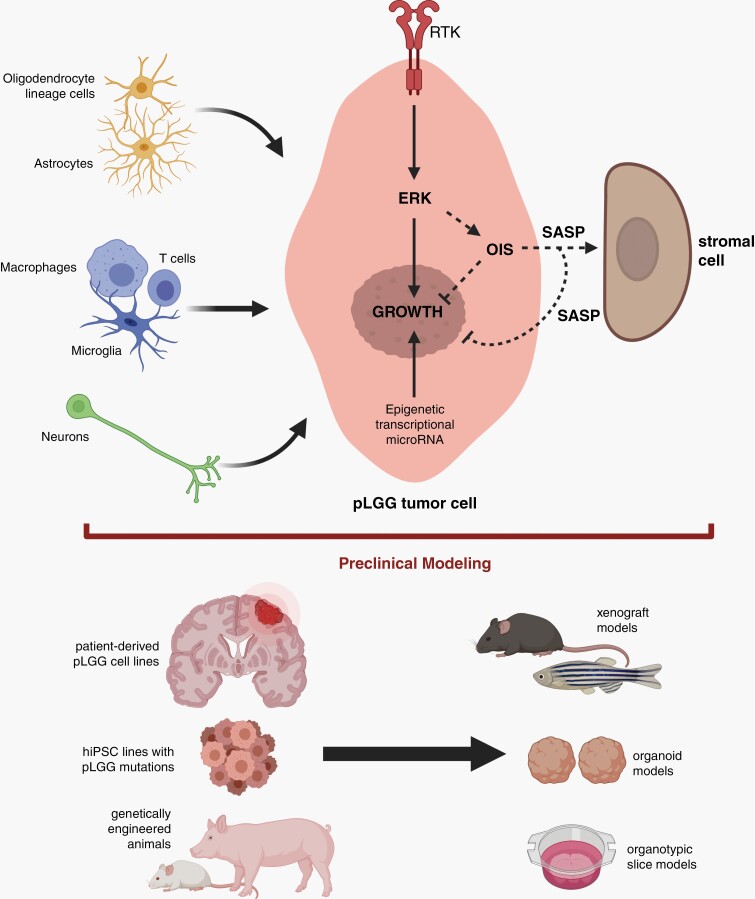
Similarly, given the complexity of these pediatric brain tumors, cross-informative groups of scientists, including cancer biologists, neuroscientists, and immunologists, are required for meaningful progress in the field (Figure 6). First, these teams will need to collaborate to define the cellular and molecular determinants that support and limit tumor growth, including a focus on the immune composition of pLGGs and a detailed analysis of stromal pro- and anti-neoplastic properties. In addition to immune-like cell elements (macrophages, microglia, T cells), other glial cells (astrocytes, oligodendrocyte lineage cells) and neurons may also contribute to pLGG pathobiology.97 Second, understanding how the microenvironment adapts and evolves in the setting of chemotherapy or molecularly-targeted treatments is critical. Third, more work is necessary to elucidate how immune cell paracrine factors impact on the tumor cell ERK signaling axis, activated by the majority of pLGG driver mutations, to favor neoplastic cell expansion. Fourth, additional studies on microenvironment-tumor cell senescence (OIS/SASP) circuitry induced by growth-promoting MAPK hyperactivation, will yield new insights into the age- and context-dependent factors that induce pLGG growth arrest, which, in turn, could result in the discovery of alternative therapeutic strategies for these childhood brain tumors. Fifth, since pLGGs form with limited genetic alterations at the genomic level, it is necessary to consider the possibility that pLGG biology is also influenced by epigenetic, transcriptional, and miRNA expression changes, which amplify the effects of stroma-driven MEK hyperactivation. Sixth, with a more detailed understanding of basic cellular and molecular underpinnings of pLGG, it will become increasingly important to develop further authenticated preclinical models that capture the tumor cell-intrinsic and microenvironmental (cell-extrinsic) conditions inherent in the human disease. Current efforts are focused on optimizing genetically engineered mouse and swine models coupled with patient tumor- and human-induced pluripotent stem-derived models (e.g., organoids) in vitro and in various organisms (e.g., zebrafish, mice) in vivo. With these informative second-generation platforms, it is highly likely that improved therapies and risk assessments will emerge that leverage our growing appreciation of pLGG ecosystem dynamics.
Fig. 6.
Future directions for pediatric low-grade gliomas (pLGG) study. Additional research is required to explore the interactions between stromal cells (oligodendrocyte lineage cells, astrocytes, immune-like cells, neurons) and pLGG tumor cells, especially as they relate to ERK-mediated growth regulation. Moreover, the determinants that regulate oncogene-induced senescence (OIS) and the senescence-associated secretory phenotype (SASP) will provide key insights into how growth cessation is controlled. Similarly, the role of epigenetic, transcriptional, and microRNA expression changes within pLGG cells on ERK-regulated cell growth necessitates further investigation. With a greater appreciation of the pLGG tumor microenvironment circuitry, more refined preclinical models will emerge that leverage patient-derived pLGG cell lines, hiPSC cellular engineering, and genetically engineered animals. Created with BioRender.com.
Supplementary Material
Funding
D.H.G. is supported by a Research Program Award grant from the National Institutes of Health (1-R35-NS07211-01) and Schnuck Markets, Inc. T.M. is supported by The Brain Tumor Charity (TBTC, The Everest Centre for Low-Grade Pediatric Brain Tumours; GN-000382) and the DKTK German Cancer Consortium (Joint Funding Next Gen LOGGIC). C.G.E. and F.J.R. acknowledge Imagine an Answer to Kids Brain Cancer, Lauren’s First and Goal, and the Stick it to Brain Tumors Annual Women’s Ice Hockey Tournament. CBTRUS data were provided for analysis through an agreement with the Centers for Disease Control and Prevention (CDC) National Program of Cancer Registries, and contained data from research data files of the National Cancer Institute (NCI) Surveillance, Epidemiology, and End Results Program and the National Center for Health Statistics National Vital Statistics System. CBTRUS acknowledges and appreciates these contributions to this report and to cancer surveillance in general. Funding for CBTRUS was provided by the CDC (contract 75D30119C06056), the American Brain Tumor Association, The Sontag Foundation, Novocure, the Musella Foundation, National Brain Tumor Society, the Pediatric Brain Tumor Foundation, the Uncle Kory Foundation, the Zelda Dorin Tetenbaum Memorial Fund, as well as private and in-kind donations. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the CDC or the NCI.
Conflict of interest statement. D.H.G. has a licensing agreement with the Tuberous Sclerosis Alliance (GFAP-Cre mice). T.M. received research funding from BioMed Valley Discoveries for an unrelated project (preclinical drug development). The other authors have no relevant conflicts of interest to disclose.
Authorship statement. Writing and Figures—C.G.E., F.J.R., J.S.B.-S., N.P., T.M., and D.H.G.; CBTRUS analysis—N.P. and J.S.B.-S.; Final editing and submission—D.H.G.
References
- 1.Patil N, Kelly ME, Yeboa DN, et al. Epidemiology of Brainstem High-Grade Gliomas in Children and Adolescents in the United States, 2000-2017. Neuro Oncol. 2020;23(6):990–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–820. [DOI] [PubMed] [Google Scholar]
- 3.Ostrom QT, Patil N, Cioffi G, Waite K, Kruchko C, Barnholtz-Sloan JS. CBTRUS Statistical Report: primary brain and other central nervous system tumors diagnosed in the United States in 2013-2017. Neuro Oncol. 2020;22(12 Suppl 2):iv1–iv96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.NPCR and SEER. National Program of Cancer Registries and Surveillance Epidemiology, and End Results Program. SEER*Stat Database: NPCR and SEER Incidence – U.S. Cancer Statistics 2001–2016 Public Use Research Database, 2019 submission (2001–2016), United States Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute. Released June 2020; Available at www.cdc.gov/cancer/uscs/public-use. Accessed May 18, 2021.
- 5.SEER. Surveillance Epidemiology, and End Results Program SEER*Stat Database: Incidence - SEER Research Data, 9 Registries, Nov 2019 Sub (1975-2017) - Linked To County Attributes - Time Dependent (1990-2017) Income/Rurality, 1969-2017 Counties, National Cancer Institute, DCCPS, Surveillance Research Program. Available at www.seer.cancer.gov. Accessed May 18, 2021.
- 6.SEER. Surveillance Epidemiology, and End Results Program. SEER*Stat Database: Incidence-Based Mortality - SEER Research Data, 18 Registries, Nov 2019 Sub (2000-2017) - Linked To County Attributes - Time Dependent (1990-2017) Income/Rurality, 1969-2018 Counties, National Cancer Institute, DCCPS, Surveillance Research Program. Available at www.seer.cancer.gov. Accessed May 18, 2021.
- 7.Tihan T, Fisher PG, Kepner JL, et al. Pediatric astrocytomas with monomorphous pilomyxoid features and a less favorable outcome. J Neuropathol Exp Neurol. 1999;58(10):1061–1068. [DOI] [PubMed] [Google Scholar]
- 8.Fernandez C, Figarella-Branger D, Girard N, et al. Pilocytic astrocytomas in children: prognostic factors–a retrospective study of 80 cases. Neurosurgery. 2003;53(3):544–53; discussion 554. [DOI] [PubMed] [Google Scholar]
- 9.Johnson MW, Eberhart CG, Perry A, et al. Spectrum of pilomyxoid astrocytomas: intermediate pilomyxoid tumors. Am J Surg Pathol. 2010;34(12):1783–1791. [DOI] [PubMed] [Google Scholar]
- 10.Rodriguez FJ, Brosnan-Cashman JA, Allen SJ, et al. Alternative lengthening of telomeres, ATRX loss and H3-K27M mutations in histologically defined pilocytic astrocytoma with anaplasia. Brain Pathol. 2019;29(1):126–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reinhardt A, Stichel D, Schrimpf D, et al. Anaplastic astrocytoma with piloid features, a novel molecular class of IDH wildtype glioma with recurrent MAPK pathway, CDKN2A/B and ATRX alterations. Acta Neuropathol. 2018;136(2):273–291. [DOI] [PubMed] [Google Scholar]
- 12.Gareton A, Tauziède-Espariat A, Dangouloff-Ros V, et al. The histomolecular criteria established for adult anaplastic pilocytic astrocytoma are not applicable to the pediatric population. Acta Neuropathol. 2020;139(2):287–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ryall S, Zapotocky M, Fukuoka K, et al. Integrated molecular and clinical analysis of 1,000 pediatric low-grade gliomas. Cancer Cell. 2020;37(4):569-583.e565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones DTW, Bandopadhayay P, Jabado N. The power of human cancer genetics as revealed by low-grade gliomas. Annu Rev Genet. 2019;53:483–503. [DOI] [PubMed] [Google Scholar]
- 15.Wefers AK, Stichel D, Schrimpf D, et al. Isomorphic diffuse glioma is a morphologically and molecularly distinct tumour entity with recurrent gene fusions of MYBL1 or MYB and a benign disease course. Acta Neuropathol. 2020;139(1):193–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huse JT, Snuderl M, Jones DT, et al. Polymorphous low-grade neuroepithelial tumor of the young (PLNTY): an epileptogenic neoplasm with oligodendroglioma-like components, aberrant CD34 expression, and genetic alterations involving the MAP kinase pathway. Acta Neuropathol. 2017;133(3):417–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brat DJ, Scheithauer BW, Fuller GN, Tihan T. Newly codified glial neoplasms of the 2007 WHO Classification of Tumours of the Central Nervous System: angiocentric glioma, pilomyxoid astrocytoma and pituicytoma. Brain Pathol. 2007;17(3):319–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bandopadhayay P, Ramkissoon LA, Jain P, et al. MYB-QKI rearrangements in angiocentric glioma drive tumorigenicity through a tripartite mechanism. Nat Genet. 2016;48(3):273–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones DTW, Kieran MW, Bouffet E, et al. Pediatric low-grade gliomas: next biologically driven steps. Neuro Oncol. 2018;20(2):160–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hovestadt V, Remke M, Kool M, et al. Robust molecular subgrouping and copy-number profiling of medulloblastoma from small amounts of archival tumour material using high-density DNA methylation arrays. Acta Neuropathol. 2013;125(6):913–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Capper D, Jones DTW, Sill M, et al. DNA methylation-based classification of central nervous system tumours. Nature. 2018;555(7697):469–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stichel D, Schrimpf D, Sievers P, et al. Accurate calling of KIAA1549-BRAF fusions from DNA of human brain tumours using methylation array-based copy number and gene panel sequencing data. Neuropathol Appl Neurobiol. 2021;47(3):406–414. [DOI] [PubMed] [Google Scholar]
- 23.Stichel D, Schrimpf D, Casalini B, et al. Routine RNA sequencing of formalin-fixed paraffin-embedded specimens in neuropathology diagnostics identifies diagnostically and therapeutically relevant gene fusions. Acta Neuropathol. 2019;138(5):827–835. [DOI] [PubMed] [Google Scholar]
- 24.Zhang J, Wu G, Miller CP, et al. ; St. Jude Children’s Research Hospital–Washington University Pediatric Cancer Genome Project . Whole-genome sequencing identifies genetic alterations in pediatric low-grade gliomas. Nat Genet. 2013;45(6):602–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones DT, Kocialkowski S, Liu L, et al. Tandem duplication producing a novel oncogenic BRAF fusion gene defines the majority of pilocytic astrocytomas. Cancer Res. 2008;68(21):8673–8677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones DT, Hutter B, Jäger N, et al. ; International Cancer Genome Consortium PedBrain Tumor Project . Recurrent somatic alterations of FGFR1 and NTRK2 in pilocytic astrocytoma. Nat Genet. 2013;45(8):927–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pfister S, Janzarik WG, Remke M, et al. BRAF gene duplication constitutes a mechanism of MAPK pathway activation in low-grade astrocytomas. J Clin Invest. 2008;118(5):1739–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Collins VP, Jones DT, Giannini C. Pilocytic astrocytoma: pathology, molecular mechanisms and markers. Acta Neuropathol. 2015;129(6):775–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jones DTW, Witt O, Pfister SM. BRAF V600E status alone is not sufficient as a prognostic biomarker in pediatric low-grade glioma. J Clin Oncol. 2018;36(1):96. [DOI] [PubMed] [Google Scholar]
- 30.Pagès M, Beccaria K, Boddaert N, et al. Co-occurrence of histone H3 K27M and BRAF V600E mutations in paediatric midline grade I ganglioglioma. Brain Pathol. 2018;28(1):103–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simmons GW, Pong WW, Emnett RJ, et al. Neurofibromatosis-1 heterozygosity increases microglia in a spatially and temporally restricted pattern relevant to mouse optic glioma formation and growth. J Neuropathol Exp Neurol. 2011;70(1):51–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gutmann DH, McLellan MD, Hussain I, et al. Somatic neurofibromatosis type 1 (NF1) inactivation characterizes NF1-associated pilocytic astrocytoma. Genome Res. 2013;23(3):431–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Robinson MH, Vasquez J, Kaushal A, et al. Subtype and grade-dependent spatial heterogeneity of T-cell infiltration in pediatric glioma. J Immunother Cancer. 2020;8(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen R, Keoni C, Waker CA, Lober RM, Chen YH, Gutmann DH. KIAA1549-BRAF expression establishes a permissive tumor microenvironment through NFκB-Mediated CCL2 Production. Neoplasia. 2019;21(1):52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen YH, McGowan LD, Cimino PJ, et al. Mouse low-grade gliomas contain cancer stem cells with unique molecular and functional properties. Cell Rep. 2015;10(11):1899–1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pan Y, Xiong M, Chen R, et al. Athymic mice reveal a requirement for T-cell-microglia interactions in establishing a microenvironment supportive of Nf1 low-grade glioma growth. Genes Dev. 2018;32(7-8):491–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guo X, Pan Y, Gutmann DH. Genetic and genomic alterations differentially dictate low-grade glioma growth through cancer stem cell-specific chemokine recruitment of T cells and microglia. Neuro Oncol. 2019;21(10):1250–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guo X, Pan Y, Xiong M, et al. Midkine activation of CD8+ T cells establishes a neuron-immune-cancer axis responsible for low-grade glioma growth. Nat Commun. 2020;11(1):2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell. 1997;88(5):593–602. [DOI] [PubMed] [Google Scholar]
- 40.Larribere L, Wu H, Novak D, et al. NF1 loss induces senescence during human melanocyte differentiation in an iPSC-based model. Pigment Cell Melanoma Res. 2015;28(4):407–416. [DOI] [PubMed] [Google Scholar]
- 41.Jacob K, Quang-Khuong DA, Jones DT, et al. Genetic aberrations leading to MAPK pathway activation mediate oncogene-induced senescence in sporadic pilocytic astrocytomas. Clin Cancer Res. 2011;17(14):4650–4660. [DOI] [PubMed] [Google Scholar]
- 42.Raabe EH, Lim KS, Kim JM, et al. BRAF activation induces transformation and then senescence in human neural stem cells: a pilocytic astrocytoma model. Clin Cancer Res. 2011;17(11):3590–3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Buhl JL, Selt F, Hielscher T, et al. The senescence-associated secretory phenotype mediates oncogene-induced senescence in pediatric pilocytic astrocytoma. Clin Cancer Res. 2019;25(6):1851–1866. [DOI] [PubMed] [Google Scholar]
- 44.Coppé JP, Patil CK, Rodier F, et al. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008;6(12):2853–2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gnekow AK, Falkenstein F, von Hornstein S, et al. Long-term follow-up of the multicenter, multidisciplinary treatment study HIT-LGG-1996 for low-grade glioma in children and adolescents of the German Speaking Society of Pediatric Oncology and Hematology. Neuro Oncol. 2012;14(10):1265–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Palma L, Celli P, Mariottini A. Long-term follow-up of childhood cerebellar astrocytomas after incomplete resection with particular reference to arrested growth or spontaneous tumour regression. Acta Neurochir (Wien). 2004;146(6):581–8; discussion 588. [DOI] [PubMed] [Google Scholar]
- 47.Kandels D, Pietsch T, Bison B, et al. Loss of efficacy of subsequent nonsurgical therapy after primary treatment failure in pediatric low-grade glioma patients-Report from the German SIOP-LGG 2004 cohort. Int J Cancer. 2020;147(12):3471–3489. [DOI] [PubMed] [Google Scholar]
- 48.Gnekow AK, Kandels D, Tilburg CV, et al. SIOP-E-BTG and GPOH guidelines for diagnosis and treatment of children and adolescents with low grade glioma. Klin Padiatr. 2019;231(3):107–135. [DOI] [PubMed] [Google Scholar]
- 49.Lassaletta A, Scheinemann K, Zelcer SM, et al. Phase II weekly vinblastine for chemotherapy-naïve children with progressive low-grade glioma: a Canadian Pediatric Brain Tumor Consortium Study. J Clin Oncol. 2016;34(29):3537–3543. [DOI] [PubMed] [Google Scholar]
- 50.Sharif S, Ferner R, Birch JM, et al. Second primary tumors in neurofibromatosis 1 patients treated for optic glioma: substantial risks after radiotherapy. J Clin Oncol. 2006;24(16):2570–2575. [DOI] [PubMed] [Google Scholar]
- 51.Ullrich NJ, Robertson R, Kinnamon DD, et al. Moyamoya following cranial irradiation for primary brain tumors in children. Neurology. 2007;68(12):932–938. [DOI] [PubMed] [Google Scholar]
- 52.Banerjee A, Jakacki RI, Onar-Thomas A, et al. A phase I trial of the MEK inhibitor selumetinib (AZD6244) in pediatric patients with recurrent or refractory low-grade glioma: a Pediatric Brain Tumor Consortium (PBTC) study. Neuro Oncol. 2017;19(8):1135–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fangusaro J, Onar-Thomas A, Young Poussaint T, et al. Selumetinib in paediatric patients with BRAF-aberrant or neurofibromatosis type 1-associated recurrent, refractory, or progressive low-grade glioma: a multicentre, phase 2 trial. Lancet Oncol. 2019;20(7):1011–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fangusaro J, Onar-Thomas A, Poussaint TY, et al. A phase 2 trial of selumetinib in children with recurrent optic pathway and hypothalamic low-grade glioma without NF1: a Pediatric Brain Tumor Consortium Study. Neuro Oncol. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Perreault S, Larouche V, Tabori U, et al. A phase 2 study of trametinib for patients with pediatric glioma or plexiform neurofibroma with refractory tumor and activation of the MAPK/ERK pathway: TRAM-01. BMC Cancer. 2019;19(1):1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nobre L, Zapotocky M, Ramaswamy V, et al. Outcomes of BRAF V600E pediatric gliomas treated with targeted BRAF inhibition. JCO Precision Oncology. 2020;4PO:19.00298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Solga AC, Pong WW, Kim KY, et al. RNA sequencing of tumor-associated microglia reveals Ccl5 as a stromal chemokine critical for neurofibromatosis-1 glioma growth. Neoplasia. 2015;17(10):776–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Short S, Fielder E, Miwa S, von Zglinicki T. Senolytics and senostatics as adjuvant tumour therapy. EBioMedicine. 2019;41:683–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee JH, Lee JE, Kahng JY, et al. Human glioblastoma arises from subventricular zone cells with low-level driver mutations. Nature. 2018;560(7717):243–247. [DOI] [PubMed] [Google Scholar]
- 60.Alcantara Llaguno SR, Wang Z, Sun D, et al. Adult lineage-restricted CNS progenitors specify distinct glioblastoma subtypes. Cancer Cell. 2015;28(4):429–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hambardzumyan D, Cheng YK, Haeno H, Holland EC, Michor F. The probable cell of origin of NF1- and PDGF-driven glioblastomas. PLoS One. 2011;6(9):e24454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Uhrbom L, Dai C, Celestino JC, Rosenblum MK, Fuller GN, Holland EC. Ink4a-Arf loss cooperates with KRas activation in astrocytes and neural progenitors to generate glioblastomas of various morphologies depending on activated Akt. Cancer Res. 2002;62(19):5551–5558. [PubMed] [Google Scholar]
- 63.Sugiarto S, Persson AI, Munoz EG, et al. Asymmetry-defective oligodendrocyte progenitors are glioma precursors. Cancer Cell. 2011;20(3):328–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Persson AI, Petritsch C, Swartling FJ, et al. Non-stem cell origin for oligodendroglioma. Cancer Cell. 2010;18(6):669–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu C, Sage JC, Miller MR, et al. Mosaic analysis with double markers reveals tumor cell of origin in glioma. Cell. 2011;146(2):209–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Friedmann-Morvinski D, Bushong EA, Ke E, et al. Dedifferentiation of neurons and astrocytes by oncogenes can induce gliomas in mice. Science. 2012;338(6110):1080–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee DY, Gianino SM, Gutmann DH. Innate neural stem cell heterogeneity determines the patterning of glioma formation in children. Cancer Cell. 2012;22(1):131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kaul A, Chen YH, Emnett RJ, Dahiya S, Gutmann DH. Pediatric glioma-associated KIAA1549:BRAF expression regulates neuroglial cell growth in a cell type-specific and mTOR-dependent manner. Genes Dev. 2012;26(23):2561–2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Solga AC, Toonen JA, Pan Y, et al. The cell of origin dictates the temporal course of neurofibromatosis-1 (Nf1) low-grade glioma formation. Oncotarget. 2017;8(29):47206–47215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Brossier NM, Thondapu S, Cobb OM, Dahiya S, Gutmann DH. Temporal, spatial, and genetic constraints contribute to the patterning and penetrance of murine neurofibromatosis-1 optic glioma. Neuro Oncol. 2021;23(4):625–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Solga AC, Gianino SM, Gutmann DH. NG2-cells are not the cell of origin for murine neurofibromatosis-1 (Nf1) optic glioma. Oncogene. 2014;33(3):289–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hegedus B, Dasgupta B, Shin JE, et al. Neurofibromatosis-1 regulates neuronal and glial cell differentiation from neuroglial progenitors in vivo by both cAMP- and Ras-dependent mechanisms. Cell Stem Cell. 2007;1(4):443–457. [DOI] [PubMed] [Google Scholar]
- 73.Kaul A, Chen YH, Emnett RJ, Gianino SM, Gutmann DH. Conditional KIAA1549:BRAF mice reveal brain region- and cell type-specific effects. Genesis. 2013;51(10):708–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Reitman ZJ, Paolella BR, Bergthold G, et al. Mitogenic and progenitor gene programmes in single pilocytic astrocytoma cells. Nat Commun. 2019;10(1):3731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Voronina N, Aichmüller C, Kolb T, et al. The age of adult pilocytic astrocytoma cells. Oncogene. 2021;40(16):2830–2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lee DY, Yeh TH, Emnett RJ, White CR, Gutmann DH. Neurofibromatosis-1 regulates neuroglial progenitor proliferation and glial differentiation in a brain region-specific manner. Genes Dev. 2010;24(20):2317–2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yuan M, White D, Resar L, et al. Conditional reprogramming culture conditions facilitate growth of lower-grade glioma models. Neuro Oncol. 2021;23(5):770–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tabori U, Vukovic B, Zielenska M, et al. The role of telomere maintenance in the spontaneous growth arrest of pediatric low-grade gliomas. Neoplasia. 2006;8(2):136–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pong WW, Higer SB, Gianino SM, Emnett RJ, Gutmann DH. Reduced microglial CX3CR1 expression delays neurofibromatosis-1 glioma formation. Ann Neurol. 2013;73(2):303–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Daginakatte GC, Gianino SM, Zhao NW, Parsadanian AS, Gutmann DH. Increased c-Jun-NH2-kinase signaling in neurofibromatosis-1 heterozygous microglia drives microglia activation and promotes optic glioma proliferation. Cancer Res. 2008;68(24):10358–10366. [DOI] [PubMed] [Google Scholar]
- 81.Daginakatte GC, Gutmann DH. Neurofibromatosis-1 (Nf1) heterozygous brain microglia elaborate paracrine factors that promote Nf1-deficient astrocyte and glioma growth. Hum Mol Genet. 2007;16(9):1098–1112. [DOI] [PubMed] [Google Scholar]
- 82.Yuan M, Da Silva ACAL, Arnold A, et al. MicroRNA (miR) 125b regulates cell growth and invasion in pediatric low grade glioma. Sci Rep. 2018;8(1):12506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Darrigo Júnior LG, Lira RCP, Fedatto PF, et al. MicroRNA profile of pediatric pilocytic astrocytomas identifies two tumor-specific signatures when compared to non-neoplastic white matter. J Neurooncol. 2019;141(2):373–382. [DOI] [PubMed] [Google Scholar]
- 84.Ho CY, Bar E, Giannini C, et al. MicroRNA profiling in pediatric pilocytic astrocytoma reveals biologically relevant targets, including PBX3, NFIB, and METAP2. Neuro Oncol. 2013;15(1):69–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jones TA, Jeyapalan JN, Forshew T, et al. Molecular analysis of pediatric brain tumors identifies microRNAs in pilocytic astrocytomas that target the MAPK and NF-κB pathways. Acta Neuropathol Commun. 2015;3:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nix JS, Yuan M, Imada EL, et al. Global microRNA profiling identified miR-10b-5p as a regulator of neurofibromatosis 1 (NF1)-glioma migration. Neuropathol Appl Neurobiol. 2021;47(1):96–107. [DOI] [PubMed] [Google Scholar]
- 87.Mackay A, Burford A, Carvalho D, et al. Integrated molecular meta-analysis of 1,000 pediatric high-grade and diffuse intrinsic pontine glioma. Cancer Cell. 2017;32(4):520–537.e525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Larson JD, Kasper LH, Paugh BS, et al. Histone H3.3 K27M accelerates spontaneous brainstem glioma and drives restricted changes in bivalent gene expression. Cancer Cell. 2019;35(1):140–155.e147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Haag D, Mack N, Benites Goncalves da Silva P, et al. H3.3-K27M drives neural stem cell-specific gliomagenesis in a human iPSC-derived model. Cancer Cell. 2021;39(3):407–422.e413. [DOI] [PubMed] [Google Scholar]
- 90.Lambert SR, Witt H, Hovestadt V, et al. Differential expression and methylation of brain developmental genes define location-specific subsets of pilocytic astrocytoma. Acta Neuropathol. 2013;126(2):291–301. [DOI] [PubMed] [Google Scholar]
- 91.Parsa CF, Hoyt CS, Lesser RL, et al. Spontaneous regression of optic gliomas: thirteen cases documented by serial neuroimaging. Archives of ophthalmology (Chicago, Ill. : 1960). 2001;119(4):516–529. [DOI] [PubMed] [Google Scholar]
- 92.Schmandt SM, Packer RJ, Vezina LG, Jane J. Spontaneous regression of low-grade astrocytomas in childhood. Pediatr Neurosurg. 2000;32(3):132–136. [DOI] [PubMed] [Google Scholar]
- 93.Piccirilli M, Lenzi J, Delfinis C, Trasimeni G, Salvati M, Raco A. Spontaneous regression of optic pathways gliomas in three patients with neurofibromatosis type I and critical review of the literature. Childs Nerv Syst. 2006;22(10):1332–1337. [DOI] [PubMed] [Google Scholar]
- 94.Fisher MJ, Loguidice M, Gutmann DH, et al. Visual outcomes in children with neurofibromatosis type 1-associated optic pathway glioma following chemotherapy: a multicenter retrospective analysis. Neuro Oncol. 2012;14(6):790–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kinori M, Armarnik S, Listernick R, Charrow J, Zeid JL. Neurofibromatosis type 1-associated optic pathway glioma in children: a follow-up of 10 years or more. Am J Ophthalmol. 2021;221:91–96. [DOI] [PubMed] [Google Scholar]
- 96.Listernick R, Charrow J, Greenwald M, Mets M. Natural history of optic pathway tumors in children with neurofibromatosis type 1: a longitudinal study. J Pediatr. 1994;125(1):63–66. [DOI] [PubMed] [Google Scholar]
- 97.Pan Y, Hysinger JD, Barron T, et al. NF1 mutation drives neuronal activity-dependent initiation of optic glioma. Nature. 2021;594(7862):277–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.