Abstract
Background
Chordoma is a rare mesenchymal malignancy, with a high recurrence rate and unclear tumorigenic mechanism. Genetic alterations, epigenetic regulators, and chromatin spatial organization play crucial roles in the initiation and progression of chordoma. In the current study, we aim to uncover the novel therapeutical targets for chordoma via using integrated multi-omics analysis.
Methods
The RNA-sequencing (RNA-seq), assay for transposable accessible chromatin by high-throughput sequencing (ATAC-seq), and Hi-C were performed between chordoma and human nucleus pulposus (HNP), along with imageological examination and clinical information. The expressions of identified targets were validated by clinical samples and their functions were further evaluated by cell and animal experiments via gene knockdown and inhibitors.
Results
The integrated multi-omics analysis revealed the important roles of bone microenvironment in chordoma tumorigenesis. By comparing the hierarchical structures, CA2 (carbonic anhydrase II) and THNSL2 (threonine synthase-like 2) were identified in the switched compartments, cell-specific boundaries, and loops. Additionally, CA2 was highly expressed in chordoma but barely found in HNP. The cell growth and migration of chordoma cells were dramatically suppressed via inhibition of CA2 either with genetic deletion or pharmaceutical treatment with Dorzolamide HCl. Furthermore, Dorzolamide HCl also regulated the bone microenvironment by blocking the osteoclast differentiation of bone marrow monocytes.
Conclusion
This study uncovers the roles of bone microenvironment in the chordoma tumorigenesis and identifies CA2 as a novel therapeutic target for chordoma. Besides, our findings suggest Dorzolamide HCl as a promising therapeutic option for chordoma.
Keywords: bone microenvironment, CA2, chordoma, Hi-C, multi-omics analysis
Key Points.
1. Bone microenvironment may contribute to chordoma tumorigenesis.
2. CA2 is a potential therapeutic target of chordoma.
3. Dorzolamide HCl serves as a promising therapeutic option for chordoma.
Importance of the Study.
With the largest sample validation, the comparative integrated multi-omics analysis of gene expression, open chromatin region, and chromatin spatial organization was generated via utilizing the chordoma and notochord-derived nucleus pulposus cells. The differentially hierarchical structures along with the observations in imageological examination and clinical information from external databases indicated the crucial roles of bone microenvironment in the tumorigenesis of chordoma. Furthermore, CA2 was found as a novel therapeutic target of chordoma based on clinical samples validation and functional experiments, suggesting that the inhibition of CA2 via Dorzolamide HCl may offer a therapeutic approach for the treatment of chordoma.
Chordoma is a rare mesenchymal malignancy and mainly occurs at axial skeleton, such as clivus and sacrococcyx.1 Therapeutically, it resists to conventional-dose radiotherapy and cytotoxic chemotherapy, thus surgery is regarded as the principal treatment.2 However, the complicated anatomical structures and relatively large tumor volume make resection with clean margins challenging, leading to a high rate of local relapse.3 In this regard, there is an urgent need to explore the underlying tumorigenic mechanism and identify novel therapeutic targets for chordoma.
Pathologically, chordoma derives from notochordal remnants within vertebral bodies, induced by incomplete squeezing at late embryonic stage.4 The tumorigenesis of chordoma involves several genetic alterations resulting in aberrant gene expressions, such as TBXT, CDKN2A, and LYST.5 Besides genetic alterations, epigenetic regulators and chromatin spatial organization are also crucial for gene regulation by regulating the accessibility of DNA to sequence-specific binding proteins and bringing distant promoters, enhancers and other cis-regulatory regions together, respectively,6,7 however, these data are still lacking in chordoma.
Here, we performed a comparative integrated analysis of RNA-sequencing (RNA-seq), assay for transposable accessible chromatin by high-throughput sequencing (ATAC-seq), and Hi-C between chordoma and notochord-derived nucleus pulposus, and further verified the expressions of identified targets by tissue microarray (TMA). Our findings indicated the potential roles of bone microenvironment and skeletal system development in chordoma tumorigenesis. In addition, as an identified differentially expressed gene (DEG) by comparing hierarchical structures, CA2 was highly expressed in chordoma and targeting CA2 by its inhibitor Dorzolamide HCl suppressed chordoma cell proliferation, migration, tumorigenicity, and osteoclast differentiation.
Materials and Methods
Ethics
This study was approved by the institutional review board of Shanghai General Hospital (approval number 2017KY092). The written informed consent for tissue and clinical data collection was signed by all patients or their legal guardians.
Cell Lines and Cell Culture
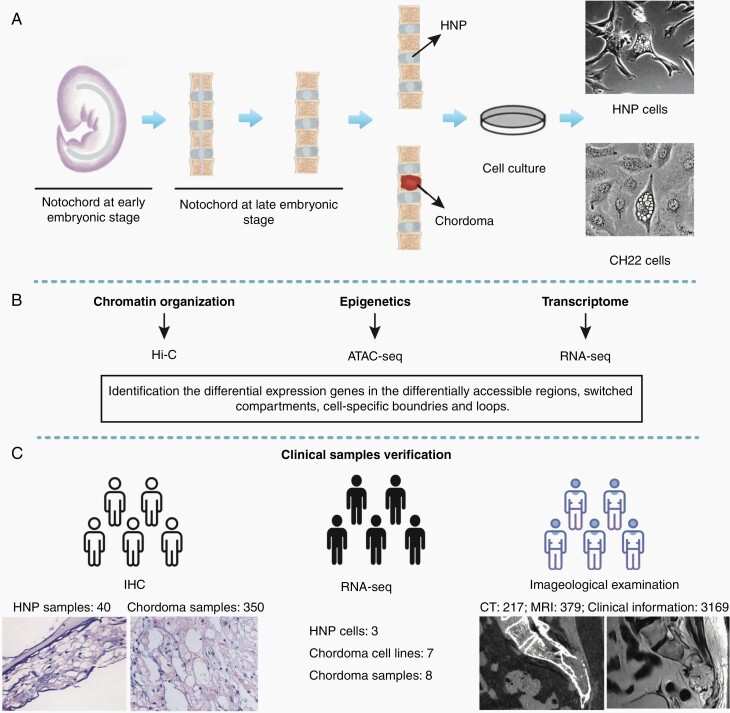
To develop a comparative integrated multi-omics map between chordoma and human nucleus pulposus (HNP), chordoma cell line CH22 and primary HNP cells were cultured (Figure 1A). HNP cells were primary culture cells from an aborted fetus with the gestational age of 24 weeks and maintained in Dulbecco’s modified Eagle’s medium-F12 (DMEM/F12; Invitrogen) supplemented with 10% fetal bovine serum (FBS; Gibco) and 1% penicillin/streptomycin (PS; Invitrogen). CH22 cells were obtained from the Chordoma Foundation and cultured in RPMI-1640 medium (Invitrogen) supplemented with 10% FBS and 1% PS.8
Fig. 1.
Project overview. (A) To develop a comparative integrated multi-omics map between chordoma and HNP, chordoma cell line CH22 and primary HNP cells were used. (B) To identify the candidate genes in chordoma tumorigenesis, we performed Hi-C, ATAC-seq, and RNA-seq. (C) To validate the identified targets and tumorigenic mechanism, we used tissue microarray, RNA-seq data, imageological examination, and clinical information of chordoma patients. Abbreviations: ATAC-seq, assay for transposable accessible chromatin by high-throughput sequencing; HNP, human nucleus pulposus; RNA-seq, RNA-sequencing.
The human chordoma cell line U-CH1 was purchased from the American Type Culture Collection (ATCC) and cultured in Iscove’s modified Dulbecco’s medium (IMDM; Invitrogen): RPMI-1640 medium (4:1) supplemented with 10% FBS, 1% PS, and 1% l-glutamine (Invitrogen). Bone marrow monocytes (BMMs) were isolated from C57/BL6 mice femurs and cultured in the minimum essential medium-alpha (α-MEM; Invitrogen) supplemented with 10% FBS and 1% PS.
Integrated Multi-Omics Analysis
The integrated multi-omics analysis included RNA-seq, ATAC-seq, and Hi-C data analysis (Figure 1B). They were performed on the Illumina platform and aligned to the human genome (GRCh38) using the BWA program (version: 0.7.10-r789).9 The edgeR v.3.24.3 algorithm was applied to obtain DEGs with a false discovery rate (FDR, Benjamini and Hochberg correction) <0.01 and |log fold-change (logFC)| > 1. Three biological replicates were used in RNA-seq experiments.
In ATAC-seq analysis, only unique mapped read was used for peak calling by MACS2 with q value <0.05. The ChIPseeker v1.1.16 was used for peak assignment annotation.10 Differentially accessible regions (DARs) calling was performed by DiffBind v2.10.0 package with FDR <0.05 and |logFC| > 1.11 The phastCons score, calculated by the multiple alignment algorithm (phastCons, version hg38, http://hgdownload.soe.ucsc.edu/goldenPath/hg38/phastCons100way/), was used to evaluate the conservation between HNP and CH22 cells.
In Hi-C data analysis, the corrected and normalized contact matrices were produced by HiC-Pro with 2 biological replicates.12 The copy number variations (CNV) were identified by Control-FREEC software and verified by HiCnv. We analyzed the compartments via HiTC v1.24.0. Positive or negative principal component (PC1) was assigned as A or B compartment, respectively.13 Topologically associating domain (TAD) calling was performed by TadLib.14 Dynamic TAD boundaries were defined according to Directionality Index (DI) delta scores of TAD boundaries between CH22 and HNP.15 Chromatin loops were found by adapted algorithm HICCUP method. A dynamic loop was defined with adjusted P < .01. When 1 anchor is located in promoter regions with the other in enhancer-like regions, a promoter-enhancer loop was defined. A high confidence promoter-enhancer loop was obtained by intersecting with ATAC-seq peaks.
Clinical Data and Chordoma Tissue Validation
Clinical data and chordoma tissue were further used to validate the findings (Figure 1C). The clinical information of chordoma patients was from 4 databases. (1) “Surveillance, Epidemiology, and End Results” (SEER) database: a total of 2060 patients with chordoma between 1975 and 2016 were selected. (2) The multicenter database with 586 chordoma patients 6 Chinese cancer centers (2000-2019). (3-4) The 2 chordoma patient support group (CPSG) in China, including 358 and 165 chordoma patients, respectively. The tumor location was retrieved from the 4 databases, and imageological data were obtained from electronic image management system or provided by patients. The chordoma and surrounding structures in imageological examinations were identified by oncologists and radiologists.
Chordoma TMA comprised 350 samples from 175 patients with histologically diagnosed chordoma. Paraffin-embedded HNP tissues (40 samples) were from 15 aborted fetuses. The chordoma TMA and HNP tissue sections were immunohistochemically stained for KRT8, KRT18, THNSL2, and CA2 using a kit from Dako (Copenhagen). Antibodies were presented in Supplementary Table 1. Nuclei were counterstained with hematoxylin (Vector Laboratories). Immunostaining on each slide was assessed by experienced pathologists with histochemistry score (H-score). H-score = Σpi(i + 1) where i is the intensity score and pi is the percentage of cells with that intensity.
Quantitative Real-Time PCR (qRT-PCR)
Total RNA was extracted using TRIzol reagent (Invitrogen) according to the manufacturer’s instructions, then column-purified and reverse-transcribed using PrimeScript 1st Strand cDNA Synthesis Kit (Takara). All qRT-PCR analyses were performed using Fast SYBR Green (Takara). Primers were presented in Supplementary Table 2.
Knockdown of CA2 by Small-Interfering RNA (siRNA) Transfection
The human CA2 siRNA was synthesized by GenePharma and CA2 knockdown in human chordoma cells was performed by siRNA transfection. A total of 2 × 105 chordoma cells per well were seeded in 12-well plates. The synthetic human CA2 siRNA (20 nmol/L) or nonspecific siRNA (20 nmol/L) were transfected using Lipofectamine RNAiMax Reagent (Invitrogen). The targeting sequences were described in Supplementary Table 3.
Cell Proliferation Assay and Clone Formation Assay
Cells were exposed to various concentrations of Dorzolamide HCl (Selleckchem) and CA2/nonspecific siRNA for respective time points as indicated. Cell proliferation ability was assessed by MTT assays. U-CH1, CH22 and BMMs were seeded in a 96-well plate (3 × 103 cells per well for U-CH1 and CH22; 6 × 103 cells per well for BMMs). Cells were harvested every 24 hours and MTT (Sigma-Aldrich) was added for 4 hours. The reactions were stopped by adding dimethyl sulfoxide (DMSO) solution for 20 minutes, and samples were measured at 490 nm.
For clone formation assays, U-CH1 and CH22 cells were seeded in a 6-well plate (500 cells per well) after transfection with CA2/nonspecific siRNA or different concentrations of Dorzolamide HCl for 48 hours. They were cultured for 2 weeks, fixed with 4% paraformaldehyde, stained with crystal violet (Sigma-Aldrich) and counted colonies.
Cell Migration Assay
Cell migration was analyzed using the transwell system (Corning). The 200 µL cell suspension (5 × 104 cells) with serum-free medium was obtained after transfection with CA2/nonspecific siRNA or different concentrations of Dorzolamide HCl for 48 hours. They were added to the upper transwell chambers and the lower chambers contained 800 µL of medium with 15% FBS. After incubation at 37°C for 24 hours, the migrated cells on the lower surface were fixed with 4% paraformaldehyde and stained with crystal violet.
In Vitro Mouse Osteoclastogenesis Assay
BMMs are incubated with complete α-MEM, 30 ng/mL macrophage colony-stimulating factor (M-CSF, R&D Systems), and 50 ng/mL receptor activator of nuclear factor-κB ligand (RANKL, R&D Systems) overnight in 96-well plates (8 × 103 cells/well) for 7 days. Then, cells were fixed with 4% paraformaldehyde and stained for tartrate-resistant acid phosphatase (TRAP) activity (Sigma-Aldrich). TRAP-positive multinucleated cells with 3 or more nuclei were imaged under light microscopy and counted as osteoclasts (in terms of area).
Tumor Xenografts
The animal experiment was administered according to the guidelines of the Institutional Animal Welfare and Ethics Committee. 5 × 106 CH22 cells in 50 µL of RPMI-1640 were injected into left tibias of male nude mice (5-6 weeks of age, 18-20 g, Shanghai SLAC Laboratory Animal Co., Ltd, China). The 2 groups (n = 10) were randomized and categorized: PBS (n = 5) and Dorzolamide HCl (n = 5). CH22-bearing mice were treated daily with Dorzolamide HCl (30 mg/kg/day) or PBS intraperitoneally on day 8 after tumor inoculation for 2 weeks.16 Tumor size was evaluated by micro-MRI under general anesthesia and analyzed with ITK-SNAP 3.6 software by 2 independent investigators. Tumor volume was calculated according to the formula V = ab2/2 (V represents volume; a represents length; b represents width). Then, mice were anesthetized and sacrificed by cervical dislocation.
Molecular Docking of CA2 and Dorzolamide
The structure of CA2 (PDB ID: 12CA) and Dorzolamide was used for docking with the program of AutoDock 4.0. Discovery Studio Visualizer was applied to analyze and visualize the generated model.
Statistical Analysis
All experimental data were presented as the mean ± SD and analyzed using SPSS Statistics (version 26.0). Statistical significance was determined by the Mann-Whitney U test and 2-tailed Student’s t test. The reported P values were adjusted to account for multiple comparisons. Significance was indicated by P < .05 and denoted in figures by *P < .05, **P < .01, or ***P < .001.
Results
The DEGs Between HNP and CH22 Cells
A comparative analysis of transcriptome between CH22 and HNP cells was performed, and a high correlation was detected between replicates within each cell type (r2 = 0.9937 and r2 = 0.9841). Compared with HNP, 3122 upregulated DEGs and 1810 downregulated DEGs were identified in CH22 (Supplementary File 1). The heatmap and volcano plot revealed significant DEGs, such as GDA, TBXT, CDKN2A, and CDKN2B (Figure 2A and B). The gene ontology (GO) analysis revealed that DEGs were associated with axon development and skeletal system development (Figure 2C). Additionally, PI3K-AKT and MAPK signaling pathways were enriched in chordoma tumorigenesis by Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis (Supplementary Figure 1A).
Fig. 2.
Data analysis of RNA-seq and ATAC-seq. The heatmap (A) and volcano plot (B) of DEGs between HNP and CH22; (C) GO analysis of the identified DEGs; (D) the volcano plot of DEGs in DARs between HNP and CH22; (E) GO analysis of DEGs in DARs. Abbreviations: ATAC-seq, assay for transposable accessible chromatin by high-throughput sequencing; DAR, differentially accessible region; DEGs, differentially expressed genes; GO, gene ontology; HNP, human nucleus pulposus; RNA-seq, RNA-sequencing.
The DARs Between HNP and CH22 Cells
The conservation profile illustrated the conservatism of key regulatory regions in HNP and CH22 (Supplementary Figure 1B). Compared with HNP, we identified 313 increased DARs and 1749 decreased DARs in CH22 (Supplementary File 2). Motif analysis for DARs revealed that CTCF motifs were strongly enriched in CH22, along with Nkx2-2, Tcfe2a_2, MED-1, and E2A (Supplementary Figure 1C). The DEGs in DARs were also identified, such as GDA, TBXT, and PAX1 (Figure 2D). The GO analysis revealed that genes in DARs were related to skeletal system development (Figure 2E). The KEGG enrichment analysis revealed that those genes were involved in PI3K-AKT signaling pathway and proteoglycans in cancer (Supplementary Figure 1D).
Chromosome Interaction and Location Difference Between HNP and CH22 Cells
A comparative analysis of Hi-C chromosome conformation capture sequencing between CH22 and HNP was performed and a total of 1.027 and 0.864 billion valid interaction pairs were obtained. The interaction type analysis revealed that the mean cis-to-trans ratio of HNP and CH22 was 1.815 and 0.69, respectively, suggesting a stronger inter-chromosome interaction in CH22 (Supplementary Figure 2A).
Chromosome interaction frequency (IR) reflects the relative position of genomic regions in cell nucleus, correlated with transcriptional activity.17 In IR heatmap of HNP, intense IR was observed in gene-rich, short chromosomes (chr16, 17, 19-22), especially between chr21 and chr22 (Supplementary Figure 2B). In CH22, IR between chr3 and chr15, chr5 and chr20, chr2 and chr4, chr9 and chr13, chr12 and chr16 was more intense and that among short chromosomes (chr16-22) was weaker, implying the alterant position of genomic regions (Supplementary Figure 2C). The IR intensity and DEGs/DARs were not significantly correlated (P = 1, Supplementary Figure 2D).
Structural Variants Between HNP and CH22 Cells
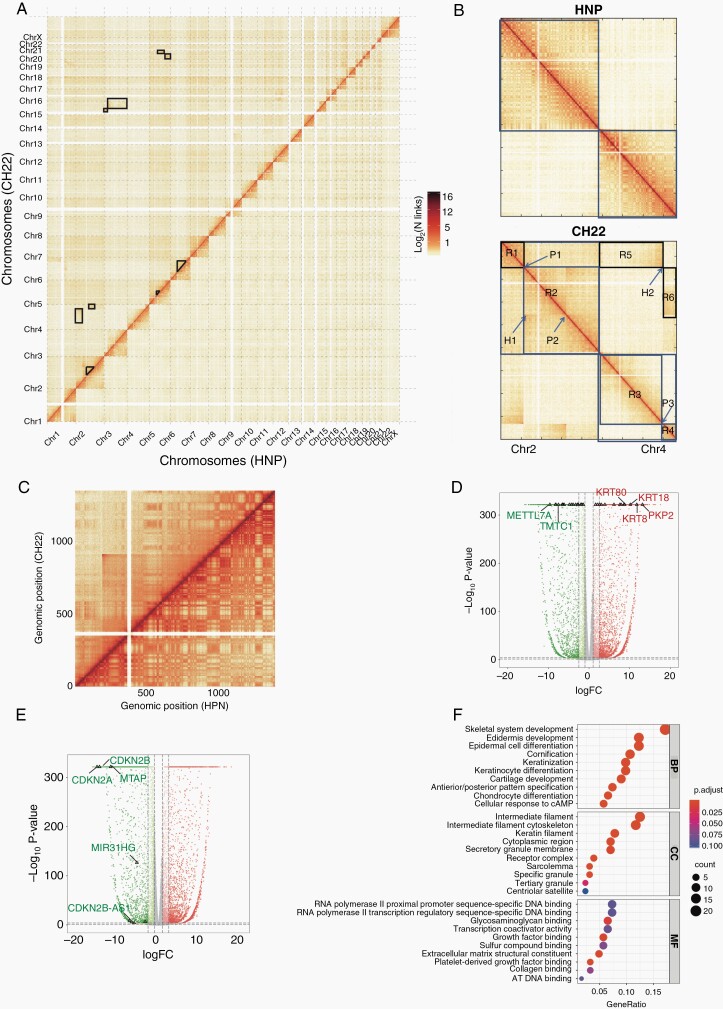
Compared with HNP, some large blocks of inter-chromosomal interactions were identified in CH22, indicating several translocation events in chordoma tumorigenesis, such as chr2 and chr4 (Figure 3A). To be more specific, 8 obvious structural variations regions were identified in CH22 (Supplementary Table 4). For example, 2 obvious interaction regions R5 (between Chr2 R1 and Chr4 R3) and R6 (between Chr2 R2 and Chr4 R4) were observed in Hi-C maps. The abrupt signal was occurred at position H2, indicating a reciprocal translocation between Chr2 and Chr4 at position P1(chr2: 55 540 000) and P3 (chr4: 156 520 000). A strong interaction signal (H1) between position P1 and P2 (chr2:160 550 000) suggested a large inversion region in Chr2 exactly close neighbor to the translocation region (Figure 3B). In addition, a more strongly intra-chromosomal interaction signal (CNV region) in chr12 (18 040 000-90 120 000 bp, Figure 3C, Supplementary Figure 2E) and a deletion region in chr9 (21 640 000-25 360 000 bp) were also found. In the CNV region, there were 187 DEGs including some generally reported chordoma-associated genes, such as KRT8 and KRT18 (Figure 3D, Supplementary File 3). Their high expressions of mRNA and protein in chordoma were further verified by clinical samples (Supplementary Figure 2F–I). In the deletion region, 9 DEGs were identified including tumor suppressor genes, such as CDKN2A and CDKN2B (Figure 3E). Based on GO and KEGG analysis, we found genes in the CNV region were associated with skeletal system development and proteoglycans in cancer (Figure 3F, Supplementary Figure 2J).
Fig. 3.
The structure variations of HNP and CH22. (A) The heatmap of the whole genome between HNP- and CH22-labeled black boxes for inter-chromosomal trans-locations; (B) the Hi-C maps between Chr2 and Chr4 for HNP (above) and CH22 (below); (C) the heatmap of Chr12 between HNP and CH22; the volcano plot of DEGs in CNV region (D) and deletion region (E) in CH22; (F) GO analysis of genes in the CNV region. Abbreviations: CNV, copy number variations; DEG, differentially expressed gene; GO, gene ontology; HNP, human nucleus pulposus.
The Switched Compartments Between HNP and CH22 Cells
At the megabase level, genomic regions with similar chromatin characteristics tend to interact with each other and constitute the compartment of internal interaction and external independence.18 Open and closed chromatin can be divided into 2 regions defined as compartment “A” and “B.” The genomic and epigenetic features of chordoma A/B compartments suggested that A compartments were associated with higher gene density, actively transcribed, and open chromatin, whereas B compartments had poor gene density, repressive transcribed, and closed chromatin (Supplementary Figure 3A–C).
During tumorigenesis, chromatin compartments underwent dynamic switches. Compared with HNP, switched compartments were found in every chromosome in CH22, especially in chr1, 9, 13-16, and 22 (Figure 4A). Throughout the chromosomes, 9.99% compartments from A in HNP transformed to B in CH22 and 6.06% from B in HNP transformed to A in CH22 (Supplementary Figure 3D). Predictably, the expressions of genes in switched compartments from A to B were significantly downregulated (P = 1.08 × e−74) and vice versa (P = 4.11 × e−47).
Fig. 4.
The identification of cell-specific compartment, TAD boundary, and loop. (A) The switched compartment in the whole genome; the numbers (B) and volcano plot (C) of DEGs in the switched compartments in the whole genome; the proportion (D) and volcano plot (E) of DEGs near cell-specific TAD boundaries; (F) the loop types in HNP and CH22; (G) the numbers of cell-specific loops; (H) the volcano plot of DEGs in the cell-specific loops; (I) the overlap DEGs located in the switched compartments, cell-specific TADs, and loops. Abbreviations: DEGs, differentially expressed genes; HNP, human nucleus pulposus; TAD, topologically associating domain.
We further labeled 441 DEGs with loci switched from compartment B to A (376 downregulated and 65 upregulated) and 341 DEGs with loci transformed from compartment A to B (38 downregulated and 303 upregulated, Supplementary File 4, Figure 4B and C). GO and KEGG analysis showed that they were related to skeletal system development, extracellular structure organization, and well-known oncogenic pathways, such as PI3K-AKT signaling and proteoglycan in cancer (Supplementary Figure 3E and F).
The Cell-Specific Boundaries of Topologically Associating Domains Between HNP and CH22 Cells
Compartments are composed of self-interacting domains (TADs), which remain stable across physiological conditions, cell types, and even species.19 In the process of tumorigenesis, the disrupted or newly formed TAD boundaries may lead to ectopic interactions between enhancers and promoters and ultimately regulate gene expression. We detected 6677 TADs in HNP and 4856 TADs in CH22. The enrichments of genes and chromosome-enriched open regions at TAD boundaries were more than those inside TADs (Supplementary Figure 4A and B).
We identified 1617 HNP-specific boundaries and 437 CH22-specific boundaries. According to the theory that genes residing at the cell-specific boundaries might be associated with tumorigenesis due to altered genomic structures, we identified 588 and 62 DEGs located in the HNP- and CH22-specific boundaries, including CA3, RAB17, and TRIB2 (Figure 4D and E, Supplementary File 5). GO and KEGG analysis revealed that these DEGs were related to extracellular matrix organization and cytokine-cytokine receptor interaction (Supplementary Figure 4C and D).
The Cell-Specific Loops Between HNP and CH22 Cells
In the gene regulation, enhancers activate promoters through chromatin loops.20 We detected 10 048 and 12 170 loops for HNP and CH22, with the median size of 100 000 bp (Supplementary Figure 5A). The loop number and positions at each chromosome were drawing in Supplementary Figure 5B. Generally, loops associated with enhancers could increase gene expression. We identified 3515 and 4187 promoter-enhancer loops in HNP and CH22 (Figure 4F). Among them, 2495 and 1732 were supported by ATAC-seq peaks, respectively. Additionally, we found more promoter loops and enhancer loops in CH22 than HNP (Supplementary Figure 5C).
The loops in 2 cell types were highly conserved and we identified 1226 HNP-specific loops and 3348 CH22-specific loops, respectively (Figure 4G). In HNP-specific loops, 45 CH22-upregulated genes and 41 CH22-downregulated genes were found, along with 75 CH22-upregulated genes and 129 CH22-downregulated genes in CH22-specific loops, such as GDA, EEF1A2, and ADAM12 (Figure 4H, Supplementary File 6). GO and KEGG analysis suggested that these DEGs were related to axon development, proteoglycans in cancer, and PI3K-AKT signaling pathway (Supplementary Figure 5D and E).
The Integrated Multi-Omics Analysis Between HNP and CH22 Cells
To explore the potential therapeutic targets, we investigated the overlapped DEGs located in the switched compartments, cell-specific TADs, and loops between CH22 and HNP cells. Two overlapped DEGs were identified (Figure 4I), namely carbonic anhydrase II (CA2) and threonine synthase-like 2 (THNSL2).
Based on the GO analysis and KEGG analysis, we found the important roles of skeletal system development in chordoma tumorigenesis. Besides, according to imageological data of 427 chordoma patients, we found that all chordoma were identified in bone structures, but not derived from HNP. The relationship among chordoma, bone structures, and HNP was described in Supplementary Figure 6A. In 4 external databases, bone was the most common location of chordoma, with the proportion of 73.98%, 79.18%, 86.31%, and 86.06%, respectively (Supplementary Figure 6B–E). In general, among 3169 chordoma patients, 2439 chordomas were located in bone, with 410 in cerebrospinal nervous system, 159 in nasopharynx and sphenoid sinus, 147 in soft tissue, 6 in thorax, and 8 in unknown primary site (Supplementary Table 5).
Clinical Samples Validation
The integrative maps of CA2 and THNSL2 are presented in Figure 5A and Supplementary Figure 7A. Their mRNA and protein expressions were further validated in clinical samples and cell lines. We found that both CA2 and THNSL2 were highly expressed in chordoma tissues and chordoma cell lines (CA2: P < .001, Figure 5B–D; THNSL2: P < .001, Supplementary Figure 7B–D; Supplementary Table 6).
Fig. 5.
Knockdown of CA2 by siRNA suppresses proliferation and migration in chordoma cell lines. (A) The differences of transcriptome, epigenetics, and hierarchical structures among genomic regions of CA2 between HNP and CH22. The mRNA expression (B), representative images of IHC (C), H-score, (D) of CA2 in HNP and chordoma; the mRNA (E) and protein expression levels (F) of CA2 after treatment with nonspecific siRNA or CA2 siRNA; (G) MTT assay showed a significant time-dependent inhibition of cell proliferation after CA2 siRNA treatment in U-CH1 cells; (H) representative images of chordoma cell colony formation after treatment with nonspecific siRNA or CA2 siRNA (left); clone formation assay showed the cell colony formation was significantly reduced after the treatment of nonspecific siRNA or CA2 siRNA in U-CH1 cells (right); (I) representative images of chordoma cell migration with the treatment of nonspecific siRNA or CA2 siRNA (left); the transwell invasion chamber assays showed that the migration ability of U-CH1 cells was markedly reduced after CA2 siRNA treatment for 48 hours (right). *P < .05, **P < .01, ***P < .001. Abbreviations: CA2, carbonic anhydrase II; HNP, human nucleus pulposus; IHC, immunohistochemistry; siRNA, small-interfering RNA.
CA2 Is Crucial for Chordoma Cell Growth and Motility
To explore the functional roles of CA2 in chordoma, we investigated the effect of CA2 knockdown on chordoma cell growth by siRNA in vitro. The downregulated expression of CA2 was found by qRT-PCR and Western blot (Figure 5E and F). Knockdown of CA2 significantly reduced the cell growth of U-CH1 in a time-dependent manner (Figure 5G). In addition, colonies and migration ability were significantly reduced in CA2 siRNA-treated U-CH1 cells (Figure 5H and I). These results indicate that CA2 is an important regulator of cell growth and migration in chordoma.
Dorzolamide HCl Is a Potential Therapeutic Option for Chordoma
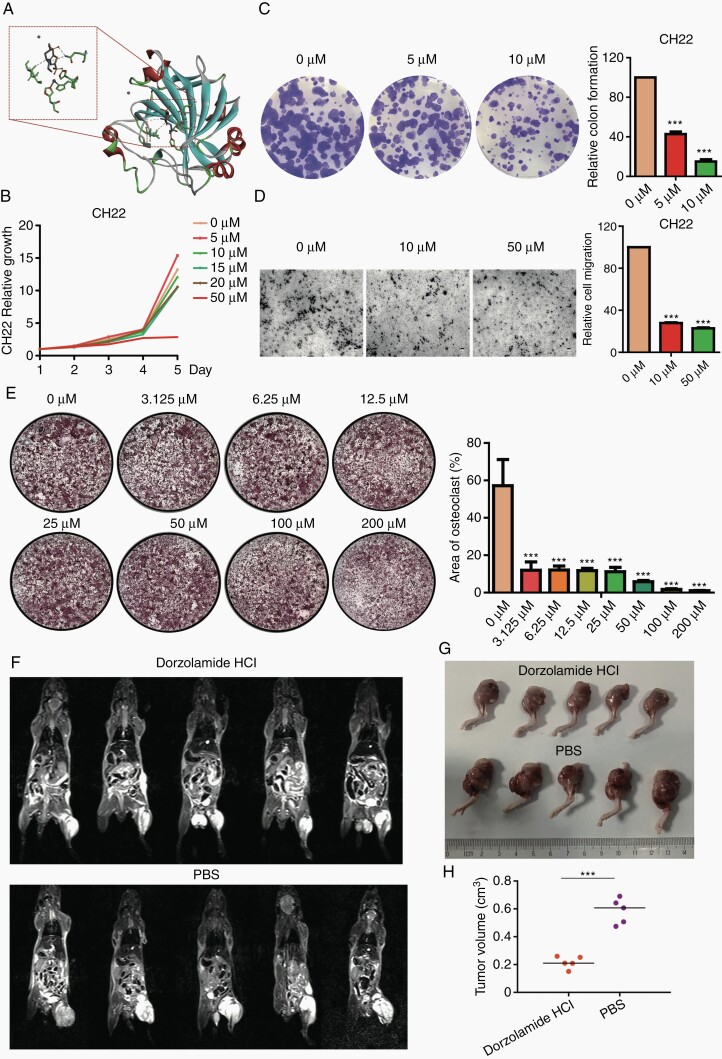
To further investigate the potential therapeutic option of chordoma, we selected 1 CA2 inhibitor (Dorzolamide HCl). By molecular docking, we found that Dorzolamide is located in the active site cavity of CA2 (Supplementary Figure 8A) and forms a very stable interaction network with the surrounding amino acid residues, such as hydrogen bonding with Thr-200 and Gln-92, which obviously inhibit the catalytic activity of CA2 (Figure 6A).
Fig. 6.
Dorzolamide HCl inhibits proliferation, migration, and tumorigenicity in chordoma cell lines and blocks osteoclastogenesis in BMMs. (A) The ribbon display mode of the overall structure of CA2 and Dorzolamide in docking conformation (right). α-helices were described in red, with β-sheets in cyan and β-turns in green; Dorzolamide was presented in stick; and Hg2+ and Zn2+ ion was presented in ball. Close-up view of active center and Dorzolamide was shown in left and key residues were in stick and labeled blue; (B) MTT assay showed a significant inhibition of cell proliferation after Dorzolamide HCl treatment in CH22 cells in a time- and dose-dependent manner; (C) representative images of CH22 colony formation after Dorzolamide HCl treatment (left); clone formation assay showed the cell colony formation was significantly reduced after Dorzolamide HCl treatment in chordoma cell lines (right); (D) representative images of CH22 cell migration after the treatment of Dorzolamide HCl (left); the transwell invasion chamber assays showed that the migration ability of chordoma cell lines was markedly reduced after Dorzolamide HCl treatment for 48 hours (right); (E) representative TRAP-stained images of BMM-derived osteoclasts stimulated with RANKL after indicated concentrations of Dorzolamide HCl treatment (left); the area of TRAP+ multinucleated osteoclasts indicated that Dorzolamide HCl dose-dependently inhibits RANKL-induced osteoclast differentiation (right). MRI scans (F), left legs (G) and tumor volumes (H) of Dorzolamide HCl- and PBS-treated CH22-bearing nude mice. *P < .05, **P < .01, ***P < .001. Abbreviations: BMM, bone marrow monocyte; CA2, carbonic anhydrase II; MRI, magnetic resonance imaging; PBS, phosphate-buffered saline; RANKL, receptor activator of nuclear factor-κB ligand; TRAP, tartrate-resistant acid phosphatase.
In the functional experiments, MTT assay showed that Dorzolamide HCl significantly reduced the cell proliferation of CH22 and U-CH1 at the dose of 50 µM in a time-dependent manner (Figure 6B, Supplementary Figure 8B). As such, it significantly suppressed the colonies at the concentration of 5 and 10 µM in CH22 and U-CH1 cells (Figure 6C, Supplementary Figure 8C). The migration ability of CH22 and U-CH1 cells was also markedly blocked by Dorzolamide HCl (10 µM, 50 µM; Figure 6D, Supplementary Figure 8D).
To determine the effects of Dorzolamide HCl on the regulation of bone microenvironment, we investigated the osteoclastogenesis by mouse BMMs stimulated with RANKL and M-CSF in vitro. BMMs were incubated with different concentrations of Dorzolamide HCl (0, 3.125, 6.25, 12.5, 25, 50, 100, 200 µM). RANKL-induced TRAP-positive multinucleated osteoclasts and Dorzolamide HCl abrogated this activation in a dose-dependent manner, with a markedly low number of TRAP-positive cells even at the dose of 3.125 µM (Figure 6E).
To further explore the therapeutic effects of Dorzolamide HCl in chordoma, we performed xenograft studies using CH22-bearing nude mice. The results revealed that Dorzolamide HCl significantly inhibited the chordoma formation after 2 weeks’ treatment (Figure 6F and G). Tumor volumes of mice in Dorzolamide HCl-treated group (mean tumor volume: 0.22 cm3) were significantly lower than those in PBS group (mean tumor volume: 0.58 cm3) at the end of the experiment (Figure 6H), while body weights were slightly decreased in Dorzolamide HCl-treated mice (Supplementary Figure 8E). Taken together, Dorzolamide HCl showed obvious inhibitory effects on chordoma cell proliferation, migration, tumorigenicity, and osteoclast differentiation.
Discussion
Nowadays, the therapeutic dilemma of chordoma makes oncologists constantly investigate the underlying mechanisms driving chordoma. Although some somatic changes have been analyzed for sporadic chordoma, the DNA accessibility and individual gene positioning during tumorigenesis are still elusive.5 In this comparative integrated multi-omics analysis, we observed obvious structural variations in 8 regions of CH22 and found significant associations between bone microenvironment and chordoma tumorigenesis. Additionally, we identified a novel therapeutic target (CA2), whose inhibitor Dorzolamide HCl may serve as a promising therapeutic option for chordoma.
As a primitive structural axial skeleton, the notochord promotes the development of surrounding tissues at early embryonic stage.21 Normally, the singular structure transforms into HNP within intervertebral discs at the late stages, whereas undifferentiated notochordal remnants may also reside within vertebral bodies and cancerate to chordoma.22 Based on GO analysis, skeletal system development and axon development were mainly enriched in chordoma, which is the primary notochord function in embryonic period.21 Therefore, chordoma still maintains the primary notochord function which postnatal HNP has lost.
By analyzing CT, MRI, and clinical information of chordoma patients, we identified bone as the overwhelming location of chordoma, and none of chordoma originated from HNP. Although both chordoma and HNP are derived from notochordal cells, their microenvironments are different (bone and annulus fibrosus), resulting in distinct cell fates.21 Generally, tumor microenvironment plays a crucial role in tumorigenesis and acts as a major predictor of tumor progression.23 Bone microenvironment, including osteocyte, osteoblast, and osteoclast, induces tumor cell proliferation and treatment resistance in primary bone tumors and supports disseminated tumor cell dormancy and reactivation in bone metastases.24 In line with the findings of imageological examination, the identified genes (CA2 and THNSL2) are related to bone microenvironment.25 Thus, we infer that chordoma tumorigenesis may be associated with bone microenvironment.
The CA family members are responsible for catalyzing the reversible hydration of CO2 to HCO3− and H+, contributing to various physiological functions.26 CA2, a mediator of hormones in stimulating bone resorption and osteoclast formation, appears at the early stage of osteoclast differentiation.27 Its mutation induces the syndrome of osteopetrosis, renal tubular acidosis, and cerebral calcification.28 In addition, CA2 has also been found in various tumors and linked to poor prognosis.29 This may be associated with the lactate transport in tumor microenvironment.30 In this study, we found that CA2 was a key gene in chordoma tumorigenesis. Targeting CA2 could inhibit chordoma cell proliferation and migration.
Due to the roles of CA family members in the tumorigenesis, many inhibitors targeting CAs have been synthesized.31,32 The new dibenzensulfonamides with the chemical structure of 4,4′-(5′-chloro-3′-methyl-5-aryl-3,4-dihydro-1′H,H-[3,4′-bipyrazole]-1′,2-diyl) were developed as novel anticancer drug candidates and induced inhibitory effects on CAs.31 Dorzolamide HCl, an inhibitor of CA2, is an important glaucoma medication.33 By molecular docking, we found a specific binding of Dorzolamide to CA2 which may induce the catalytic activity reduction of CA2.34 Moreover, Dorzolamide HCl treatment significantly blocked the chordoma cell proliferation, migration, tumorigenicity, and BMMs osteoclastogenesis in this study. Thus, we supposed that Dorzolamide HCl may offer a therapeutic approach for the treatment of chordoma.
Conclusions
This study provides a comparative integrated multi-omics analysis of gene expression, open chromatin region, and chromatin spatial organization generated for chordoma and HNP with the largest sample validation. Our data not only indicate the association between bone microenvironment and chordoma tumorigenesis but also identify a novel therapeutic target (CA2). In addition, the findings also suggest Dorzolamide HCl as a promising therapeutic option for chordoma.
Supplementary Material
Funding
This project was supported in part by the National Natural Science Foundation of China (No. 81702659, 81772856), Shanghai Rising-Star Program (No. 21QA1407500), Youth Fund of Shanghai Municipal Health Planning Commission (20174Y0117).
Authorship statement. T.M., R.H., and F.L. performed analysis of sequence data. T.M. and X.X. analyzed the CT/MRI. Z.C., J.L., and N.T. performed pathological analysis. Z.H., L.C., H.Y., and D.S. provided clinical practice to ensure the relevance of findings. T.M., J.G., Z.W., and J. J. performed the functional experiments. H.Y., W.Z., and D.S. directed and supervised the research. T.M. wrote the final manuscript. All authors have approved the final version of the manuscript.
Conflict of interest statement. The authors have declared that no conflict of interest exists.
Data Availability StatementSequencing data of Hi-C and ATAC-seq for HNP and CH22 cells, along with the RNA-seq of HNP, CH22 cells, and 8 primary chordoma samples are deposited at the Sequence Read Archive (SRA) database (PRJNA738543) . In addition, chordoma cell line sequencing data are available at www.chordomafoundation.org.
References
- 1.Chugh R, Tawbi H, Lucas DR, Biermann JS, Schuetze SM, Baker LH. Chordoma: the nonsarcoma primary bone tumor. Oncologist. 2007;12(11):1344–1350. [DOI] [PubMed] [Google Scholar]
- 2.Stacchiotti S, Sommer J; Chordoma Global Consensus Group . Building a global consensus approach to chordoma: a position paper from the medical and patient community. Lancet Oncol. 2015;16(2):e71–e83. [DOI] [PubMed] [Google Scholar]
- 3.Meng T, Yin H, Li B, et al. Clinical features and prognostic factors of patients with chordoma in the spine: a retrospective analysis of 153 patients in a single center. Neuro Oncol. 2015;17(5):725–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choi KS, Cohn MJ, Harfe BD. Identification of nucleus pulposus precursor cells and notochordal remnants in the mouse: implications for disk degeneration and chordoma formation. Dev Dyn. 2008;237(12):3953–3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tarpey PS, Behjati S, Young MD, et al. The driver landscape of sporadic chordoma. Nat Commun. 2017;8(1):890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buenrostro JD, Giresi PG, Zaba LC, Chang HY, Greenleaf WJ. Transposition of native chromatin for fast and sensitive epigenomic profiling of open chromatin, DNA-binding proteins and nucleosome position. Nat Methods. 2013;10(12):1213–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vian L, Pekowska A, Rao SSP, et al. The energetics and physiological impact of cohesin extrusion. Cell. 2018;173(5):1165–1178.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu X, Nielsen GP, Rosenberg AE, et al. Establishment and characterization of a novel chordoma cell line: CH22. J Orthop Res. 2012;30(10):1666–1673. [DOI] [PubMed] [Google Scholar]
- 9.Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25(14):1754–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu G, Wang LG, He QY. ChIPseeker: an R/Bioconductor package for ChIP peak annotation, comparison and visualization. Bioinformatics. 2015;31(14):2382–2383. [DOI] [PubMed] [Google Scholar]
- 11.Lesluyes T, Johnson J, Machanick P, Bailey TL. Differential motif enrichment analysis of paired ChIP-seq experiments. BMC Genomics. 2014;15:752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Servant N, Varoquaux N, Lajoie BR, et al. HiC-Pro: an optimized and flexible pipeline for Hi-C data processing. Genome Biol. 2015;16:259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Servant N, Lajoie BR, Nora EP, et al. HiTC: exploration of high-throughput ‘C’ experiments. Bioinformatics. 2012;28(21):2843–2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang XT, Cui W, Peng C. HiTAD: detecting the structural and functional hierarchies of topologically associating domains from chromatin interactions. Nucleic Acids Res. 2017;45(19):e163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bonev B, Mendelson Cohen N, Szabo Q, et al. Multiscale 3D genome rewiring during mouse neural development. Cell. 2017;171(3):557–572.e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ali BM, Zaitone SA, Shouman SA, Moustafa YM. Dorzolamide synergizes the antitumor activity of mitomycin C against Ehrlich’s carcinoma grown in mice: role of thioredoxin-interacting protein. Naunyn Schmiedebergs Arch Pharmacol. 2015;388(12):1271–1282. [DOI] [PubMed] [Google Scholar]
- 17.Bickmore WA, van Steensel B. Genome architecture: domain organization of interphase chromosomes. Cell. 2013;152(6):1270–1284. [DOI] [PubMed] [Google Scholar]
- 18.Lieberman-Aiden E, van Berkum NL, Williams L, et al. Comprehensive mapping of long-range interactions reveals folding principles of the human genome. Science. 2009;326(5950):289–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dixon JR, Selvaraj S, Yue F, et al. Topological domains in mammalian genomes identified by analysis of chromatin interactions. Nature. 2012;485(7398):376–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Visel A, Rubin EM, Pennacchio LA. Genomic views of distant-acting enhancers. Nature. 2009;461(7261):199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamada T, Pfaff SL, Edlund T, Jessell TM. Control of cell pattern in the neural tube: motor neuron induction by diffusible factors from notochord and floor plate. Cell. 1993;73(4):673–686. [DOI] [PubMed] [Google Scholar]
- 22.Lawson L, Harfe BD. Notochord to nucleus pulposus transition. Curr Osteoporos Rep. 2015;13(5):336–341. [DOI] [PubMed] [Google Scholar]
- 23.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. [DOI] [PubMed] [Google Scholar]
- 24.Croucher PI, McDonald MM, Martin TJ. Bone metastasis: the importance of the neighbourhood. Nat Rev Cancer. 2016;16(6):373–386. [DOI] [PubMed] [Google Scholar]
- 25.Rifas L, Weitzmann MN. A novel T cell cytokine, secreted osteoclastogenic factor of activated T cells, induces osteoclast formation in a RANKL-independent manner. Arthritis Rheum. 2009;60(11):3324–3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lomelino C, McKenna R. Carbonic anhydrase inhibitors: a review on the progress of patent literature (2011-2016). Expert Opin Ther Pat. 2016;26(8):947–956. [DOI] [PubMed] [Google Scholar]
- 27.Lehenkari P, Hentunen TA, Laitala-Leinonen T, Tuukkanen J, Väänänen HK. Carbonic anhydrase II plays a major role in osteoclast differentiation and bone resorption by effecting the steady state intracellular pH and Ca2+. Exp Cell Res. 1998;242(1):128–137. [DOI] [PubMed] [Google Scholar]
- 28.Sly WS, Hewett-Emmett D, Whyte MP, Yu YS, Tashian RE. Carbonic anhydrase II deficiency identified as the primary defect in the autosomal recessive syndrome of osteopetrosis with renal tubular acidosis and cerebral calcification. Proc Natl Acad Sci U S A. 1983;80(9):2752–2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haapasalo J, Nordfors K, Järvelä S, et al. Carbonic anhydrase II in the endothelium of glial tumors: a potential target for therapy. Neuro Oncol. 2007;9(3):308–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Annan DA, Maishi N, Soga T, et al. Carbonic anhydrase 2 (CAII) supports tumor blood endothelial cell survival under lactic acidosis in the tumor microenvironment. Cell Commun Signal. 2019;17(1):169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gul HI, Yamali C, Bulbuller M, et al. Anticancer effects of new dibenzenesulfonamides by inducing apoptosis and autophagy pathways and their carbonic anhydrase inhibitory effects on hCA I, hCA II, hCA IX, hCA XII isoenzymes. Bioorg Chem. 2018;78:290–297. [DOI] [PubMed] [Google Scholar]
- 32.Akocak S, Lolak N, Nocentini A, Karakoc G, Tufan A, Supuran CT. Synthesis and biological evaluation of novel aromatic and heterocyclic bis-sulfonamide Schiff bases as carbonic anhydrase I, II, VII and IX inhibitors. Bioorg Med Chem. 2017;25(12):3093–3097. [DOI] [PubMed] [Google Scholar]
- 33.Kouchak M, Mahmoodzadeh M, Farrahi F. Designing of a pH-triggered carbopol®/HPMC in situ gel for ocular delivery of dorzolamide HCl: in vitro, in vivo, and ex vivo evaluation. AAPS PharmSciTech. 2019;20(5):210. [DOI] [PubMed] [Google Scholar]
- 34.Bijari N, Ghobadi S, Mahdiuni H, Khodarahmi R, Ghadami SA. Spectroscopic and molecular modeling studies on binding of dorzolamide to bovine and human carbonic anhydrase II. Int J Biol Macromol. 2015;80:189–199. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.