Abstract
Routine genomic surveillance on samples from COVID-19 patients collected in Poland during summer 2021 revealed the emergence of a SARS-CoV-2 Delta variant with a large 872 nt deletion. This change, confirmed by Sanger and deep sequencing, causes complete loss of ORF7a, ORF7b, and ORF8 genes. The index case carrying the deletion is unknown. The standard pipeline for sequencing may mask this deletion with a long stretch of N’s. Effects of this deletion on phenotype or immune evasion needs further study.
Keywords: SARS-CoV-2, variant, deletion, pandemic, detection
Between 1 July and 16 August 2021, 316 severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) genomes from 13 of 16 Polish voivodships from isolates obtained during routine surveillance activities, were deposited by our group in the GISAID database [1]. Of these, 31 sequences, all of which belonged to the SARS-CoV-2 variant of concern (VOC) Delta (Phylogenetic Assignment of Named Global Outbreak (Pango) lineage designation B.1.617.2) AY.4 sublineage designation, showed an unusual pattern i.e. a long stretch of N’s, indicating a weak or undetermined nucleotide signal, in the region corresponding to the open reading frame (ORF)7a, ORF7b and ORF8 genes in the reference SARS-CoV-2 genome (GenBank NC_045512).
Most of these unique sequences (27/31) originated from coronavirus disease (COVID-19) patient samples collected in the Malopolska voivodship in southern Poland, between 17 July and 11 August 2021. Our aim was to investigate the emergence of the unusual sequence pattern in SARS-CoV-2 sequences from Poland.
Clinical presentation
Clinical data were available for 24 of the 31 patients with atypical Delta VOC sequences. COVID-19 was suspected in all patients, since they showed clinical symptoms compatible with the disease; all were confirmed by a SARS-CoV-2 RT-qPCR diagnostic test, referred by a general practitioner. The median age was 31 years (range: 4 months–84 years), with an equal number of females (n = 12) and males (n = 12). Symptoms included fever (n = 23), cough (n = 15), headache (n = 10), weakness (n = 10), muscle pain (n = 10), runny nose (n = 7), loss of smell (n = 3), loss of taste (n = 2), sore throat (n = 1), diarrhoea (n = 1), dyspnoea (n = 1) and breathing difficulties (n = 1). Seven patients were hospitalised, and four needed oxygen supplementation. Fifteen of the 24 patients were not vaccinated or partially vaccinated (one dose, various mRNA and non-mRNA vaccines) against SARS-CoV-2; of the seven hospitalised patients, five were not vaccinated. None of the patients travelled abroad before the COVID-19 symptoms developed.
PCR and sequencing analysis
For sample collection, nasopharyngeal swab samples were taken according to the WHO guidelines and in line with the protocols approved by the Polish Ministry of Health [2]. RNA was extracted by the various laboratories, accordingly to their validated, routinely performed methods used for SARS-CoV-2 diagnostic. Extracted RNA was selected randomly, was frozen at −80°C and sent from collection to sequencing laboratories within 2 weeks. The sequencing was performed with Oxford Nanopore Technologies (ONT) platform (Oxford, United Kingdom) using the ARTIC amplicon v3 and Midnight whole genome sequencing protocols [3-5]. Sequencing results showed a long stretch of N’s within the region covering the ORF7a, ORF7b and ORF8 genes. Since the technique is based on the targeted amplification, this observed artefact i.e. a long stretch of N’s, was suspected to be the result of a short insertion–deletion mutation (indel) or single nucleotide polymorphism (SNP) within the primer target sites. We selected 14 specimens for further genetic analyses. To exclude an unspecific primer binding event, we tested the primer pair (e.g. 90_Left and 93_Right primers, which was used for the ARTICv3 protocol and overlapped the region of interest) for SARS-CoV-2 genome amplification (Table 1).
Table 1. Primer pairs used for amplification and Sanger sequencing of selected SARS-CoV-2 specimens, Poland, 17 July–11 August 2021 .
| Pair | Sequencing protocol | Primer name | Primer sequence (5’ to 3’) |
|---|---|---|---|
| Pair 1 | ARTICv3 | 90_Left | ACACAGACCATTCCAGTAGCAGT |
| 93_Right | AGGTCTTCCTTGCCATGTTGAG | ||
| Pair 2 | Midnight | 27_Left | TGGATCACCGGTGGAATTGCTA |
| 28_Right | GTTTGGCCTTGTTGTTGTTGGC |
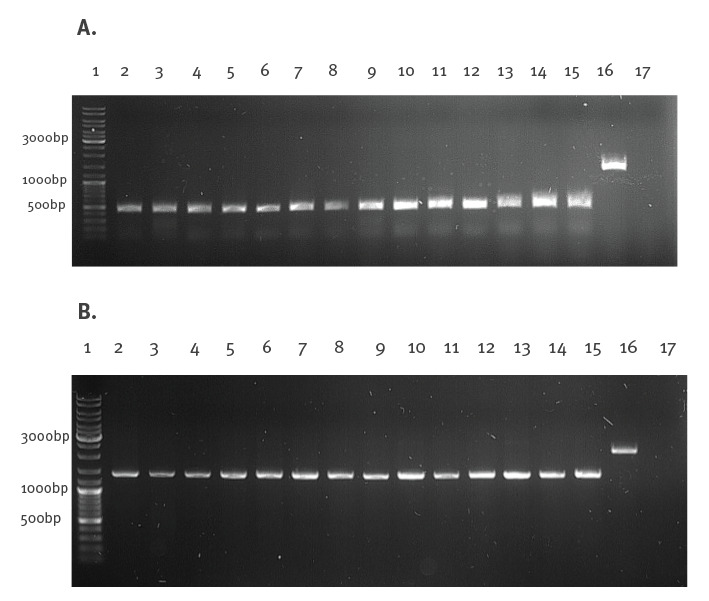
Agarose gel electrophoresis showed that the amplification products obtained from the ARTICv3 protocol primers [4,5] were ca 500 bp, rather than the expected size of 1,328 bp (Figure 1).
Figure 1.
Amplified PCR fragments from isolates expressing a unique SARS-CoV-2 Delta variant sequence, Poland, 17 July–11 August 2021 (n = 14)
SARS-CoV-2: severe acute respiratory syndrome coronavirus 2.
PCR was performed with (A) ARTICv3 primers (90_Left, 93_Right) [4,5] and (B) Midnight primers (27_Left, 28_Right) [3] and analysed with agarose gel electrophoresis. Isolates with unique SARS-CoV-2 Delta variant sequences (lanes 2–15) reveal a band at ca 500 bp (A) and at ca 1400 bp (B) rather than the expected size of 1,328 bp (lane 16, A) or 2,265 bp (lane 16, B). Lane 1: GeneRuler DNA ladder mix (Thermo Fisher Scientific); Lane 2: sample hCoV-19/Poland/PL_P3804/2021; Lane 3: sample hCoV-19/Poland/PL_P3711/2021; Lane 4: sample hCoV-19/Poland/PL_P3699/2021; Lane 5: sample hCoV-19/Poland/PL_P3705/2021; Lane 6: sample hCoV-19/Poland/PL_P3687/2021; Lane 7: sample hCoV-19/Poland/PL_P3686/2021; Lane 8: sample hCoV-19/Poland/PL_P3695/2021; Lane 9: sample hCoV-19/Poland/PL_P3696/2021; Lane 10: sample hCoV-19/Poland/PL_P3688/2021; Lane 11: sample hCoV-19/Poland/PL_P3693/2021; Lane 12: sample hCoV-19/Poland/PL_P3704/2021; Lane 13: sample hCoV-19/Poland/PL_P3794/2021; Lane 14: sample hCoV-19/Poland/PL_P3710/2021; Lane 15: sample hCoV-19/Poland/PL_P3709/2021; Lane 16: SARS-CoV-2 Delta AY.4 control sample, hCoV-19/Poland/PL_P3707/2021; Lane 17: negative control.
This unexpected result suggested a large ca 850 nt deletion in these isolates. In order to exclude mispriming events, the second pair of primers originating from the Midnight protocol [3] (e.g. 27_Left, 28_Right) (Table 1)(Figure 1) was used to confirm the deletion. Similar to the ARTICv3 primer pair, the products of the second primer set were ca 850 nt shorter than the expected 2,265 bp.
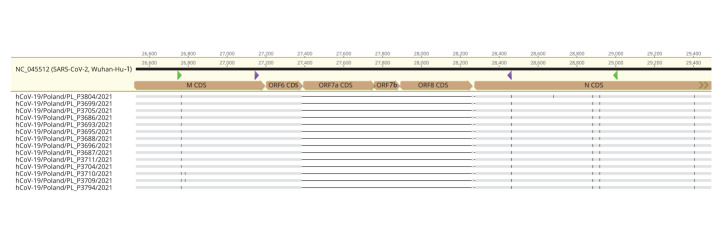
The obtained amplicons were subjected to in-house ONT sequencing using native barcoding for the whole amplicon. In parallel, the products obtained in the first round of RT-PCR were subjected to Sanger sequencing. Both methods confirmed a deletion of 872 nt, which stretches between nucleotide positions 27,385 and 28,256 of the reference genome. This sizable alteration causes a complete loss of ORF7a, ORF7b, and ORF8 genes (Figure 2). The obtained sequences were deposited in the GISAID database under the following accession numbers: hCoV-19/Poland/PL_P3686/2021; hCoV-19/Poland/PL_P3688/2021; hCoV-19/Poland/PL_P3693/2021; hCoV-19/Poland/PL_P3695/2021; hCoV-19/Poland/PL_P3696/2021; hCoV-19/Poland/PL_P3699/2021; hCoV-19/Poland/PL_P3704/2021; hCoV-19/Poland/PL_P3705/2021; hCoV-19/Poland/PL_P3709/2021; hCoV-19/Poland/PL_P3711/2021; hCoV-19/Poland/PL_P3794/2021; hCoV-19/Poland/PL_P3804/2021; hCoV-19/Poland/PL_P3687/2021; hCoV-19/Poland/PL_P3710/2021.
Figure 2.
Alignment of the consensus sequences of isolates lacking the 872 nt region to the reference SARS-CoV-2 genomic sequence, Poland, 17 July–11 August 2021 (n = 14)
CDS: coding sequence; ORF: open reading frame; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2.
The consensus sequences were combined based on Sanger and Oxford Nanopore Technology sequencing. The triangles marked within the reference sequence (yellow-shaded in the top) represent the primer binding sites of the two primer pairs: purple arrows (90_L and 93_R; ARTICv3 protocol [4,5]); green arrows (27_L and 28_R; Midnight protocol [3]).
Rapid barcoding error exclusion
Such methods of high-throughput whole genome sequencing like ONT of SARS-CoV-2 are usually performed in a 96-well plate format using the barcoding approach, which is time- and cost-effective, but may lead to sporadic misclassification of some reads between the samples. This mistake could be wrongly reported as ‘quasi-species’. To exclude artefacts related to the barcoding procedure and to detect SARS-CoV-2 quasi-species, selected samples were run individually on a single flow cell. Moreover, during our analyses, we used a highly accurate ONT Guppy 5.0.11 super high accuracy (SUP) base-calling algorithm supported by detecting a barcode from both ends. None of the reads were mapped to the deleted region, which supported the hypothesis that isolates containing the 872 nt deletion are not mixed with the wild-type virus, thus representing a pure population of these new Delta AY.4 isolates with the 872 nt deletion.
Viral load
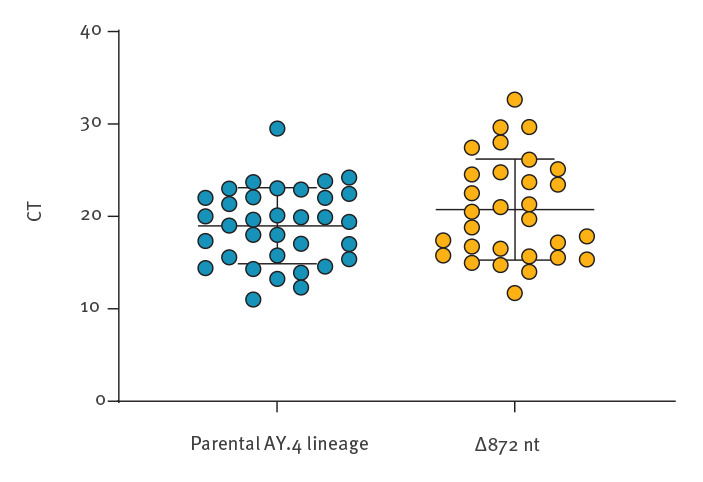
The viral load, expressed as a mean cycle threshold (CT) value obtained during diagnostic RT-qPCR, was available for 30 samples for three different commonly used gene targets (E: envelope gene, N: nucleoprotein gene, ORF1a: ORF1a gene). To assess the potential influence of the 872 nt deletion on the virus transmissibility, the CT was compared with selected other Delta variant AY.4 lineage samples (n = 34) confirmed in Poland between 15 July and 16 August 2021 (Figure 3). The viral loads did not vary between the groups, and thus it may be assumed that these Delta AY.4 isolates with the 872 nt deletion show similar transmissibility to the parental AY.4 lineage.
Figure 3.
Comparison of viral load between the SARS-CoV-2 Delta AY.4 lineage (n = 34) and the Delta AY.4 isolates with the 872 nt deletion (n = 30), Poland, 17 July–11 August 2021
CT: cycle threshold; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2.
Each point refers to single sample, for which the CT value was calculated as a mean CT obtained from three target genes (E, N, ORF1a) obtained during routine diagnostic RT-qPCR. CT values are compared between SARS-CoV-2 AY.4 samples (n = 34) and Delta AY.4 isolates with the 872 nt deletion (n = 30). Error bars indicate standard deviation of the mean. The difference is statistically insignificant (p > 0.05, unpaired t-test).
Ethical statement
No ethical approval was required for this study as samples were collected for routine surveillance.
Discussion and conclusions
SARS-CoV-2 VOCs have appeared in Poland in a pattern similar to that observed in other European countries. From July 2021, the Delta VOC has been the dominant circulating variant. Similar to other RNA viruses, the SARS-CoV-2 genome evolves quite rapidly, indicated by a 1.1 × 103 substitution/site/year mutation rate [6]. Numerous changes within SARS-CoV-2 genomes are continuously reported, from minor SNPs to extensive deletions, which have also been reported for other coronaviruses e.g. Middle East respiratory syndrome coronavirus (MERS-CoV) [7]. Regardless of size, all variations may impair the protein structure and function, which could cause changes in the virus phenotype, infectivity, infectiousness, illness severity, or host immune response. The detected 872 nt deletion leads to complete loss of three accessory proteins encoded by the ORF7a, ORF7b, and ORF8 genes.
The gene encoding accessory protein ORF8 shows high variability, and multiple alterations including SNPs, short indels causing frameshifts and partial or complete gene deletions have been reported to date [8-10]. In recombinant SARS-CoV-1, the truncation of ORF8 led to gradual virus attenuation in vitro [7]. In SARS-CoV-2, the accessory protein encoded by ORF8 has a function related to evasion of the host adaptive immune response via downregulation of MHC-1 (major histocompatibility complex) [11]. Moreover, ORF8 modulates the host’s interferon-mediated antiviral response [12]. However, the importance of ORF8 in vivo remains unresolved. While recombinant ΔORF8 SARS-CoV-2 viruses produced smaller plaques than the wild type (WT) in vitro, the viral growth kinetics remained unchanged [10,13]. Furthermore, human angiotensin converting enzyme 2 (hACE2)-transgenic mice infected with ΔORF8 recombinants showed similar pathological lesions and mortality to the WT strain, suggesting that ORF8 does not contribute to virus pathogenicity [13] An observational human cohort study revealed that individuals infected with SARS-CoV-2 lacking a functional ORF8 gene was associated with lower odds of developing hypoxia requiring supplemental oxygen [14].
The deletions within the ORF7a and ORF7b region have also previously been reported [15-18]. ORF7a activates the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kappa B) pathway and induces proinflammatory cytokine expression [19,20]. The function of ORF7b is largely unknown, but a recent study has shown that the protein activates the type-I interferon (IFN) signalling pathway and promotes expression of IFN-beta, interleukin (IL)-6, and tumor necrosis factor (TNF)-alpha, which induce apoptosis [21]. Viruses lacking these genes also produced smaller plaques than WT viruses in vitro, but their growth kinetics were similar to the parental strain [13]. Based on studies in animal models, the proteins have a minor impact on pathological lesions and disease outcomes [13].
This is the first study, to our knowledge, confirming the emergence of such a large deletion within the SARS-CoV-2 genome, causing the complete loss of three consecutive ORF sequences, e.g. ORF7a, ORF7b, and ORF8. The clinical data obtained from 25 infected individuals suggest a typical course of COVID-19, with mild to moderate symptoms. The origin of these Delta AY.4 isolates with the 872 nt deletion remains unknown, but their detection in a short period of time with high frequency in the Malopolska voivodship suggests that they may be maintained in the population. Based on GISAID data, during recent weeks (September 2021), more than 70 similar sequences containing a stretch of N’s within the region corresponding to ORF7a, ORF 7b and ORF 8 were confirmed in other areas in Poland, including Mazowieckie, Pomorskie and Swietokrzyskie voivodships. These sequences, similar to those presented here, could also contain the 872 nt deletion and thus, detection could be missed because of the bioinformatic algorithm leading to a long stretch of N’s at the position of the deleted genes. However, a re-analysis of raw sequencing data or Sanger sequencing is needed to confirm the deletion in these samples. Considering the potential spread of a virus strain similar to these Delta AY.4 isolates with the 872 nt deletion to the other regions in Poland, it may be hypothesised that its transmissibility has not been compromised. Unfortunately, the original swab samples were unavailable and the virus isolation was impossible at this point, but will be a subject for the future studies. The monitoring of this 872 nt deletion in clinical samples and the publicly available databases should be continued.
Acknowledgements
We would like to thank Provincial Sanitary and Epidemiological Station in Krakow for their excellent cooperation and clinical data disclosure.
Funding statement: This work was supported by the subsidy from the Polish Ministry of Science and Higher Education for the research on the SARS-CoV-2 and the grant 2020/ABM/COVID19/UJ from the Polish Agency of Biomedical Sciences to KP. LR was supported by the Ministry of Science and Higher Education Decision No. 54/WFSN/2020. “Co-infections with SARS-CoV-2, Database of COVID-19 Accompanying Infections”.
Conflict of interest: None declared.
Authors’ contributions: Study design: NMP, LR, KP. Data analysis: NMP, LR, KP. Experimental procedures: NMP, LR, TG, GN, MK, WW, PS, MK, KG, NDM, JG, APM, BS, KBS, JS, MG, PL. Manuscript preparation: NMP, LR, KP. Manuscript revision: all authors.
References
- 1.Global Initiative on Sharing All Influenza Data (GISAID). Munich: GISAID. [Accessed: 15 Sep 2021]. Available from: https://www.gisaid.org
- 2.World Health Organization (WHO). Laboratory testing for coronavirus disease (COVID-19) in suspected human cases: interim guidance, 19 March 2020. Geneva: WHO; 2020. Available from: https://apps.who.int/iris/handle/10665/331501
- 3.Freed N, Silander O. SARS-CoV2 genome sequencing protocol (1200bp amplicon "midnight" primer set, using Nanopore Rapid kit). protocols.io. 2021. 10.17504/protocols.io.bwyppfvn [DOI]
- 4.Quick J. nCoV-2019 sequencing protocol v3 (LoCost). protocols.io. 2020. Available from: https://protocols.io/view/ncov-2019-sequencing-protocol-v3-locost-bh42j8ye
- 5.Tyson J, James P, Stoddart D, Sparks N, Wickenhagen A, Hall G, et al. Improvements to the ARTIC multiplex PCR method for SARS-CoV-2 genome sequencing using nanopore. bioRxiv 2020.09.04.283077. 10.1101/2020.09.04.283077 [DOI]
- 6.Duchene S, Featherstone L, Haritopoulou-Sinanidou M, Rambaut A, Lemey P, Baele G. Temporal signal and the phylodynamic threshold of SARS-CoV-2. Virus Evol. 2020;6(2):veaa061. 10.1093/ve/veaa061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muth D, Corman VM, Roth H, Binger T, Dijkman R, Gottula LT, et al. Attenuation of replication by a 29 nucleotide deletion in SARS-coronavirus acquired during the early stages of human-to-human transmission. Sci Rep. 2018;8(1):15177. 10.1038/s41598-018-33487-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pancer K, Milewska A, Owczarek K, Dabrowska A, Kowalski M, Łabaj PP, et al. The SARS-CoV-2 ORF10 is not essential in vitro or in vivo in humans. PLoS Pathog. 2020;16(12):e1008959. 10.1371/journal.ppat.1008959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pereira F. Evolutionary dynamics of the SARS-CoV-2 ORF8 accessory gene. Infect Genet Evol. 2020;85:104525. 10.1016/j.meegid.2020.104525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gamage AM, Tan KS, Chan WOY, Liu J, Tan CW, Ong YK, et al. Infection of human nasal epithelial cells with SARS-CoV-2 and a 382-nt deletion isolate lacking ORF8 reveals similar viral kinetics and host transcriptional profiles. PLoS Pathog. 2020;16(12):e1009130. 10.1371/journal.ppat.1009130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Y, Chen Y, Li Y, Huang F, Luo B, Yuan Y, et al. The ORF8 protein of SARS-CoV-2 mediates immune evasion through down-regulating MHC-Ι. Proc Natl Acad Sci USA. 2021;118(23):e2024202118. 10.1073/pnas.2024202118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li J-Y, Liao C-H, Wang Q, Tan Y-J, Luo R, Qiu Y, et al. The ORF6, ORF8 and nucleocapsid proteins of SARS-CoV-2 inhibit type I interferon signaling pathway. Virus Res. 2020;286:198074. 10.1016/j.virusres.2020.198074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Silvas JA, Vasquez DM, Park J-G, Chiem K, Allué-Guardia A, Garcia-Vilanova A, et al. Contribution of SARS-CoV-2 accessory proteins to viral pathogenicity in K18 human ACE2 transgenic mice. J Virol. 2021;95(17):e0040221. 10.1128/JVI.00402-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Young BE, Fong SW, Chan YH, Mak TM, Ang LW, Anderson DE, et al. Effects of a major deletion in the SARS-CoV-2 genome on the severity of infection and the inflammatory response: an observational cohort study. Lancet. 2020;396(10251):603-11. 10.1016/S0140-6736(20)31757-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Panzera Y, Ramos N, Frabasile S, Calleros L, Marandino A, Tomás G, et al. A deletion in SARS-CoV-2 ORF7 identified in COVID-19 outbreak in Uruguay. Transbound Emerg Dis. 2021;10.1111. 10.1111/tbed.14002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tse H, Wong SC-Y, Ip K-F, Cheng VC-C, To KK-W, Lung DC, et al. Genome sequences of three SARS-CoV-2 ORF7a deletion variants obtained from patients in Hong Kong. Microbiol Resour Announc. 2021;10(15):7-9. 10.1128/MRA.00251-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Addetia A, Xie H, Roychoudhury P, Shrestha L, Loprieno M, Huang M-L, et al. Identification of multiple large deletions in ORF7a resulting in in-frame gene fusions in clinical SARS-CoV-2 isolates. J Clin Virol. 2020;129:104523. 10.1016/j.jcv.2020.104523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Joonlasak K, Batty EM, Kochakarn T, Panthan B, Kümpornsin K, Jiaranai P, et al. Genomic surveillance of SARS-CoV-2 in Thailand reveals mixed imported populations, a local lineage expansion and a virus with truncated ORF7a. Virus Res. 2021;292:198233. 10.1016/j.virusres.2020.198233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Su CM, Wang L, Yoo D. Activation of NF-κB and induction of proinflammatory cytokine expressions mediated by ORF7a protein of SARS-CoV-2. Sci Rep. 2021;11(1):13464. 10.1038/s41598-021-92941-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou Z, Huang C, Zhou Z, Huang Z, Su L, Kang S, Chen X, Chen Q, He S, Rong X, Xiao F, Chen J, Chen S. Structural insight reveals SARS-CoV-2 ORF7a as an immunomodulating factor for human CD14+ monocytes. iScience. 2021;24(3):102187. 10.1016/j.isci.2021.102187 [DOI] [PMC free article] [PubMed]
- 21.Yang R, Zhao Q, Rao J, Zeng F, Yuan S, Ji M, et al. SARS-CoV-2 Accessory protein ORF7b mediates Tumor Necrosis Factor-α-induced apoptosis in cells. Front Microbiol. 2021;12:654709. 10.3389/fmicb.2021.654709 [DOI] [PMC free article] [PubMed] [Google Scholar]