ABSTRACT
Background
Levodopa‐carbidopa intestinal gel (LCIG) is an established treatment for improving motor and some non‐motor symptoms (NMS) in patients with advanced Parkinson's disease (PD). Prospective long‐term data in routine clinical practice are limited.
Objective
Assess LCIG effectiveness and safety in patients with advanced PD after 12 months during real‐world routine clinical practice.
Methods
Duodopa/Duopa in patients with advanced Parkinson's disease—a global observational study evaluating long‐term effectiveness (DUOGLOBE) (NCT02611713) is an ongoing, prospective, multinational, observational study of LCIG‐naïve patients treated as part of routine clinical practice; 3 years of follow‐up are planned. The primary outcome is the change in patient‐reported off time. Other assessments include the Unified Dyskinesia Rating Scale (UDysRS), Non‐Motor Symptoms Scale (NMSS), Parkinson's Disease Sleep scale (PDSS‐2), Epworth Sleepiness Scale (ESS), health‐related quality of life (HR‐QoL), caregiver burden, and serious adverse events (SAEs). Outcomes from baseline to month (M) 12 are presented.
Results
In this 12‐month follow‐up, patients (N = 195) had baseline characteristics similar to other LCIG studies. Significant improvements (mean change to M12) were observed in off time (−3.9 ± 3.6 hr/day, P < 0.001), dyskinesia assessed using the UDysRS (−9.6 ± 22.5, P < 0.001), NMSS (−23.1 ± 41.4, P < 0.001), sleep and sleepiness symptoms on the PDSS‐2 (−6.5 ± 12.2, P < 0.001) and ESS (−1.0 ± 5.7, P < 0.05), HR‐QoL (−9.0 ± 21.6, P < 0.001), and caregiver burden (−1.9 ± 6.7, P = 0.008). Overall, 40.5% (n = 79) of patients experienced SAEs; fall (n = 6; 3.1%) and urinary tract infection (n = 6; 3.1%) were SAEs reported in ≥3% of patients.
Conclusions
These 12‐month outcome data show sustained, long‐term improvements and support the real‐world effectiveness of LCIG in patients with advanced PD. Safety was consistent with previous studies.
Keywords: DUOGLOBE, Parkinson's disease, levodopa‐carbidopa intestinal gel, dyskinesia, real‐world data
In most cases, standard oral therapy to treat Parkinson's disease (PD) is based on the use of levodopa, either alone or together with other medications. As PD progresses, many patients experience symptoms that are no longer adequately controlled by oral dopaminergic therapy. Mechanistically, contributors to these fluctuations are oscillations in plasma levodopa concentrations because of levodopa's short half‐life, erratic gastric emptying, and neural plasticity leading to a narrowed therapeutic window.1, 2, 3 Fluctuations in non‐motor symptoms(NMS) such as fatigue, anxiety, and autonomic dysfunction may also occur.4 Furthermore, both motor and NMS can produce substantial disability and adversely impact patient health‐related quality of life (HR‐QoL) and caregiver burden.5, 6, 7, 8
Continuous intrajejunal administration of levodopa‐carbidopa intestinal gel (LCIG) reduces variability in levodopa plasma levels9 and produces stable synaptic dopamine as measured by raclopride displacement positron emission tomography scans.10 Furthermore, controlled clinical trials have demonstrated that LCIG therapy reduces off time, increases on time without troublesome dyskinesia, and improves patients' HR‐QoL.11, 12, 13, 14 Several larger observational studies have confirmed the beneficial effects of LCIG on both motor and NMS and patient HR‐QoL in clinical practice, although these studies were either not prospective (included non‐naïve LCIG patients), single‐centered, and/or lacked United States (US) study sites.15, 16, 17, 18, 19
Here, we present data from a large, long‐term study of LCIG safety and efficacy in a multicenter, multinational cohort of patients with advanced PD. This study examines outcomes in patients treated according to local regulatory requirements and routine practice protocols across a diverse group of countries and practice settings. The design incorporates examination of the full spectrum of motor and NMS, including sleep, HR‐QoL, and caregiver burden. There is also a special focus on dyskinesia: most previous studies have used the Unified Parkinson's Disease Rating Scale (UPDRS) Part IV or Hauser diary data, which have limited sensitivity to change and ability to quantitate dyskinesia symptoms. We used the recently developed and validated Unified Dyskinesia Rating Scale (UDysRS), a scale with established metrics that can measure all aspects of dyskinesia.20 The duration of treatment in the Duodopa/Duopa in patients with advanced Parkinson's disease—a global observational study evaluating long‐term effectiveness (DUOGLOBE) study is planned for 3 years. Here, we present results from the first 12 months of LCIG therapy.
Methods
Study Design
DUOGLOBE is a global, multicenter, single‐arm, non‐interventional, post‐marketing observational study (NCT02611713) conducted at 55 specialized movement disorder centers in 10 countries (Australia, Belgium, Hungary, Israel, Italy, Romania, Slovenia, Spain, United Kingdom, and the US). The first patient was enrolled in December 2015; a total of 212 patients were screened and 195 patients were enrolled and are included in this analysis (Fig. 1).
FIG. 1.
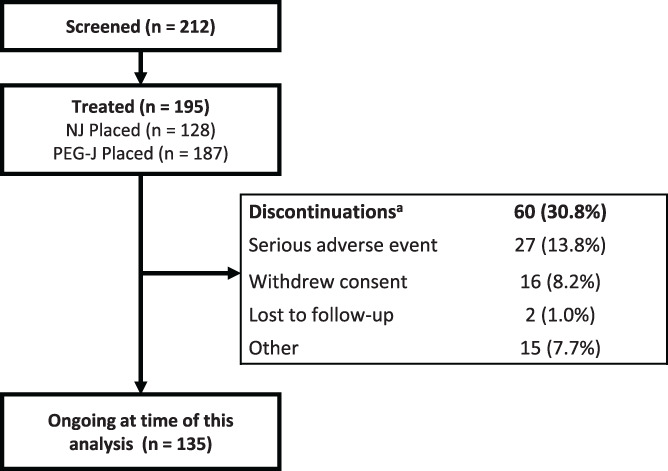
Patient disposition. aAfter premature discontinuation, 13 patients continued LCIG treatment outside the study. LCIG, levodopa‐carbidopa intestinal gel; NJ, nasojejunal; PEG‐J, percutaneous endoscopic gastrostomy with jejunal extension.
The protocol, patient information, and informed consent requirements were approved in all countries by national and/or local independent ethics committees and health authorities according to the applicable national regulatory requirements.
Patients
Patients were selected for LCIG treatment by physicians at each site according to the local label, reimbursement criteria, and customary local clinical practice. Key eligibility criteria (beyond those listed on the product label) included no previous exposure to LCIG and a Mini‐Mental State Examination (MMSE) score ≥24. Patients could not have had a previous surgery for PD including but not limited to deep brain stimulation or cell transplantation (this criterion was removed for US sites). Subcutaneous apomorphine infusion was not permitted during the study, and, if used, required a 4‐week wash‐out period. Treatment with LCIG (levodopa [20 mg/mL] and carbidopa [5 mg/mL]) was delivered via a percutaneous endoscopic gastrostomy with jejunal extension tube (PEG‐J) using a portable pump (CADD‐Legacy, Smiths Medical ASD, Minneapolis, MN). The daily duration of the therapy was determined by local practice and individual patient need. LCIG dosage was individually optimized for clinical response and concomitant use of other PD medications was permitted at the discretion of the treating clinician. A nasojejunal (NJ) test phase, as described in the prescribing label, was optional, unless recommended by the treating clinician.
Planned enrollment was ~200 patients based on assumptions of 60% completion and mean ± standard deviation (SD) decrease from baseline in off time 4 ± 4 hr at month (M) 36. With these assumptions, the distance from the lower limit of the 95% confidence interval (CI) to the mean decrease would be 0.72 hr (ie, the half width of the 95% CI of the mean decrease from baseline would be 0.72 hr).
Assessments included those conducted at baseline (BL, defined as the last non‐missing clinical data on or before the date of LCIG delivery via first device placement) and then at follow‐up visits at M3, M6, and M12 (Fig. S1). For patients who underwent the optional temporary NJ phase and transitioned to PEG‐J, data at day 1 (D1) were also collected; however, D1 data were not collected for patients who initiated treatment directly via PEG‐J without NJ (Fig. S1).
The primary efficacy assessment for this analysis was the change in the number of hours spent in off time as reported by the patient on the day before the clinical visit (BL) compared with the same measure at M12. Additional efficacy measurements included the mean change from BL in the following: UPDRS part II (activities of daily living [ADL]), part III (motor examination), part IV modified item 32 (dyskinesia duration), item 33 (dyskinesia‐related disability), item 34 (dyskinesia‐related pain), item 35 (early morning dystonia), and modified item 39 (off time); UDysRS total score (dyskinesia signs and symptoms) and subdomain scores; NMS Scale (NMSS) total score and subdomain scores; PD Sleep Scale (PDSS)‐2 total score (sleep quality) and Epworth Sleepiness Scale (ESS) total score (daytime somnolence); 8‐item Parkinson's Disease Questionnaire (PDQ‐8) summary index (HR‐QoL); and Modified Caregiver Strain Index (MCSI) total score (caregiver burden). Significance was determined using a 1‐sample t test compared with BL efficacy assessments. In this observational study, serious adverse events (SAEs), product complaints, and pregnancies were recorded. Adverse events (AEs) not meeting the criteria for SAEs were not recorded. All patients who received any infusion of LCIG (either via temporary NJ or long‐term PEG‐J were included in the safety analysis population (N = 195). The full analysis set includes all patients in the safety population who have a BL effectiveness evaluation and at least 1 post‐BL effectiveness assessment during PEG‐J visits.
Post Hoc Analyses
Additional post hoc analyses of subgroups were also conducted. Off time and HR‐QoL were assessed in patients stratified by baseline age (<65 and ≥65 years of age), sex (male and female), and PD duration (<10 and ≥10 years). Comparison with BL values at indicated time points were determined using a repeated measures model that included country visit BL subgroup, the BL*visit, and the subgroup*visit interaction. Additionally, patients (n = 188) were stratified by their BL Hoehn and Yahr (H&Y) scale scores (<3 and ≥3) in the on state and were analyzed for off time, dyskinesia signs and symptoms (UDysRS), axial symptoms (UPDRS part III: sum of items 18 [speech], 22 [rigidity], and 27–30 [arising from chair, posture, gait, and postural stability]), NMS (NMSS total score), and HR‐QoL (PDQ‐8 summary index). Additionally, BL demographics and disease characteristics were assessed in patients who did and did not complete 12 months of LCIG therapy (12‐month completer vs. 12‐month non‐completer).
Results
Patient Disposition
Demographics, medical histories, PD characteristics, and BL assessments of motor, NMS, HR‐QoL, and caregiver burden of the 195 patients included in the analysis are summarized in Table 1. The temporary NJ test period occurred in 128 patients (no US patients [n = 39] participated in the optional NJ test period) with 120 patients continuing to PEG‐J placement after the NJ test period. The average duration of NJ exposure was 6.7 ± 5.3 days; in total, 187 patients underwent PEG‐J placement (Fig. 1). In patients with initial tubing type data available, 20.3% (n/N = 36/177) had at least 1 PEG‐J tube replacement (initial tubing type: AbbVie [21.1%, n/N = 31/147] and non‐AbbVie tubing [16.7%, n/N = 5/30]). At the time of data lock for this analysis, the mean ± SD LCIG treatment duration was 494.5 ± 249.9 days, with a median daily duration of LCIG infusion of 16 hr from D1 through M12; 7 patients were receiving stable 24‐hr LCIG therapy until their last follow‐up visit. For all patients, the mean ± SD daily LCIG dose was 1241.2 ± 501.6 mg/day at D1 and remained relatively stable through M12 (1356.9 ± 504.2 mg/day). Of 195 patients, 60 (30.8%) discontinued the study prematurely. Primary reasons for premature discontinuation included SAEs (13.8%), withdrawal of consent (8.2%), lost to follow‐up (1.0%), and other (7.7%) (Fig. 1). Of the 60 patients who discontinued the study, 13 continued LCIG treatment outside the study.
TABLE 1.
Baseline patient demographics and disease characteristics
| N = 195 | |
|---|---|
| Demographics | |
| Sex, n (%) | |
| Male | 120 (61.5%) |
| Female | 75 (38.5) |
| Age, years, mean ± SD | 70.2 ± 8.2 |
| <65 years, n (%) | 44 (22.6) |
| 65–75 years, n (%) | 95 (48.7) |
| >75 years, n (%) | 56 (28.7) |
| BMI, kg/m2, mean ± SD | 25.9 ± 4.1 |
| <25, n (%) | 83 (45.6) |
| ≥25, n (%) | 99 (54.4) |
| Medical history | |
| PD duration, years, mean ± SD | 11.2 ± 4.8 |
| <10 years, n (%) | 94 (48.5) |
| ≥10 years, n (%) | 100 (51.5) |
| Time to LCIG initiation from | |
| PD symptoms, years, mean ± SD | 12.2 ± 5.0 |
| Start of motor fluctuations, years, mean ± SD | 5.6 ± 4.7 |
| MMSE total scorea, mean ± SD | 27.7 ± 2.2 |
| Hoehn and Yahr, mean ± SD | |
| During on | 3.0 ± 0.8 |
| During off | 3.6 ± 0.8 |
| PD symptoms, HR‐QoL, and caregiver burden measures at baseline | |
| Off time, hr/day, mean ± SD | 6.0 ± 3.4 |
| UPDRS Part II (activities of daily living), mean ± SD | 14.8 ± 7.8 |
| UPDRS Part III (motor examination), mean ± SD | 27.6 ± 13.2 |
| UPDRS Part IV, mean ± SD | |
| Item 32 (duration of dyskinesia) | 1.5 ± 1.2 |
| Item 39 (duration of off time) | 1.9 ± 0.8 |
| UDysRS total score, mean ± SD | 33.7 ± 21.1 |
| NMSS total score, mean ± SD | 87.9 ± 51.3 |
| PDSS‐2 total score (sleep quality), mean ± SD | 26.6 ± 11.7 |
| ESS total score (daytime sleepiness), mean ± SD | 9.8 ± 5.3 |
| PDQ‐8 summary index (HR‐QoL), mean ± SD | 45.1 ± 18.1 |
| MCSI total score (caregiver burden), mean ± SD | 10.9 ± 6.4 |
Patient MMSE total score at baseline must be 24 for inclusion.
BMI, body mass index; ESS, Epworth Sleepiness Scale; HR‐QoL, health‐related quality of life; LCIG, levodopa‐carbidopa intestinal gel; MCSI, Modified Caregiver Strain Index; MMSE, Mini‐Mental State Examination; NMSS, Non‐Motor Symptoms Scale; PD, Parkinson's disease; PDSS‐2, Parkinson's Disease Sleep Scale‐2; PDQ‐8, 8‐item Parkinson's Disease Questionnaire; UDysRS, Unified Dyskinesia Rating Scale, UPDRS, Unified Parkinson's Disease Rating Scale.
Motor Symptoms
The mean ± SD daily hours spent in the off state compared with BL (the day before the clinical visit) significantly decreased at D1 and was maintained through M12 (−3.9 ± 3.6 hr/day, P < 0.001; Fig. 2A). Reductions in off time were independent of patient age, sex, and PD duration (Fig. S2A‐C).
FIG. 2.
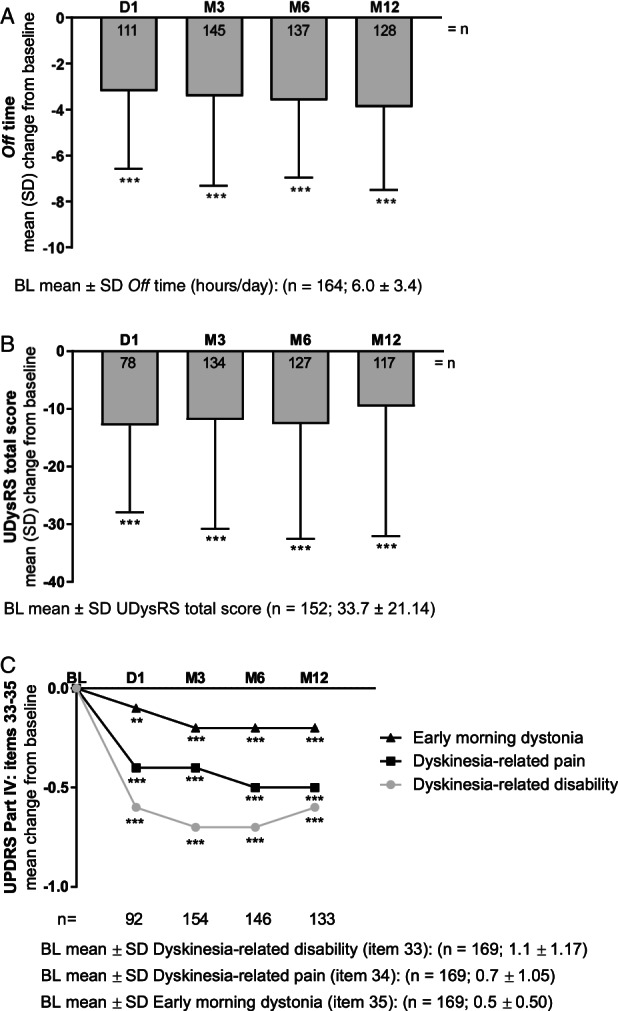
LCIG efficacy on motor symptoms as measured by change from baseline. P values indicate significant difference compared with baseline using a 1‐sample t test: P ≤ 0.001 (***) and P ≤ 0.01 (**). BL, baseline; D, day; LCIG, levodopa‐carbidopa intestinal gel; M, month; UDysRS, Unified Dyskinesia Rating Scale; UPDRS, Unified Parkinson's Disease Rating Scale.
UDysRS total scores significantly improved at D1 (−12.9 ± 15.0, P < 0.001), and improvements were maintained through M12 (−9.6 ± 22.5, P < 0.001; Fig. 2B). Additionally, 3 of 4 UDysRS subdomains significantly improved from D1 to M12: part 1 (ON‐dyskinesia: −4.7 ± 11.8, P < 0.001), part 2 (OFF‐dystonia: −3.0 ± 4.6, P < 0.001), part 4 (disability: −1.3 ± 4.5, P < 0.01), as well as the composite historical (−7.7 ± 13.5, P < 0.001) and objective scores (−2.3 ± 11.1, P < 0.05; Table 2). Significant improvements were also observed in the UDysRS impairment subdomain from D1 to M6 with a numerical reduction at M12 (Table 2). Supporting evidence for reduction of dyskinesia was observed for patient‐reported dyskinesia duration at BL and M12 (4.1 ± 3.7 to 3.7 ± 4.0 hours/day, P = 0.229), and using the UPDRS scale; including reductions from BL to M12 in dyskinesia‐related disability (1.1 ± 1.17 to 0.6 ± 0.84 [P < 0.001]; Fig. 2C), dyskinesia‐related pain (0.7 ± 1.05 at BL to 0.2 ± 0.49 at M12 [P < 0.001]; Fig. 2C); and item 35 (early morning dystonia; 0.5 ± 0.50 at BL to 0.3 ± 0.46 at M12 [P < 0.001]; Fig. 2C). UPDRS part II (ADL) total scores were not significantly changed from BL; UPDRS part III (motor examination) were significantly reduced at D1 (P = 0.004) and M3 (P = 0.001), with numerical reductions at M6 and M12 (Table 2). Compared with BL, both item 20 (on state‐resting tremor) and 21 (on state‐action tremor) significantly improved at M12 (P = 0.01 and P < 0.05, respectively; Table 2).
TABLE 2.
Change from baseline in the mean UPDRS II, UPDRS III, UDysRS subdomain, and NMSS subdomain scores
| Visit | |||||
|---|---|---|---|---|---|
| Baseline | D1a | M3 | M6 | M12 | |
| UPDRS II, mean ± SD |
n = 173 14.8 ± 7.8 |
n = 92 −1.3 ± 6.6 |
n = 154 −0.4 ± 6.7 |
n = 149 0.0 ± 7.4 |
n = 137 0.7 ± 7.7 |
| UPDRS III, mean ± SD |
n = 171 27.6 ± 13.2 |
n = 91 −2.7 ± 8.7** |
n = 156 −2.5 ± 9.6** |
n = 147 −1.4 ± 10.6 |
n = 135 −0.6 ± 10.4 |
| Item 20: ON state‐resting tremor |
n = 172 0.9 ± 2.2 |
n = 91 −0.1 ± 1.3 |
n = 156 −0.3 ± 1.6* |
n = 148 −0.3 ± 1.8 |
n = 136 −0.3 ± 1.5** |
| Item 21: ON state‐action tremor |
n = 172 1.0 ± 1.4 |
n = 91 −0.1 ± 1.2 |
n = 156 −0.3 ± 1.3** |
n = 148 −0.3 ± 1.3** |
n = 136 −0.3 ± 1.3* |
| UDysRS subdomains, mean ± SD | |||||
| Total score |
n = 152 33.7 ± 21.1 |
n = 78 −12.9 ± 15.0*** |
n = 134 −11.9 ± 18.9*** |
n = 127 −12.6 ± 20.0*** |
n = 117 −9.6 ± 22.5*** |
| Part 1: ON‐dyskinesia |
n = 153 14.6 ± 11.0 |
n = 79 −5.4 ± 8.1*** |
n = 136 −5.3 ± 10.3*** |
n = 129 −5.7 ± 10.8*** |
n = 119 −4.7 ± 11.8*** |
| Part 2: OFF‐dystonia |
n = 154 6.9 ± 4.8 |
n = 80 −3.3 ± 4.0*** |
n = 137 −3.2 ± 4.3*** |
n = 130 −3.3 ± 4.5*** |
n = 121 −3.0 ± 4.6*** |
| Part 3: impairment |
n = 154 7.4 ± 6.5 |
n = 80 −2.2 ± 4.3*** |
n = 137 −2.2 ± 6.1*** |
n = 129 −2.2 ± 6.7*** |
n = 120 −1.0 ± 7.4 |
| Part 4: disability |
n = 152 4.9 ± 3.8 |
n = 78 −1.6 ± 2.8*** |
n = 135 −1.5 ± 3.5*** |
n = 127 −1.8 ± 3.9*** |
n = 118 −1.3 ± 4.5** |
| Historical score |
n = 153 21.5 ± 13.1 |
n = 79 −8.8 ± 10.0*** |
n = 136 −8.5 ± 12.1*** |
n = 129 −9.0 ± 12.1*** |
n = 119 −7.7 ± 13.5*** |
| Objective score |
n = 152 12.2 ± 9.8 |
n = 78 −3.8 ± 6.9*** |
n = 135 −3.6 ± 9.0*** |
n = 127 −3.9 ± 10.1*** |
n = 117 −2.3 ± 11.1* |
| NMSS subdomains, mean ± SD | |||||
| Total score |
n = 162 87.9 ± 51.3 |
n = 89 −26.7 ± 42.0*** |
n = 141 −29.6 ± 37.7*** |
n = 137 −27.9 ± 42.7*** |
n = 123 −23.1 ± 41.4*** |
| Domain 1: cardiovascular including falls |
n = 169 3.1 ± 4.4 |
n = 92 −1.2 ± 3.9** |
n = 153 −1.1 ± 4.2** |
n = 144 −1.0 ± 4.7** |
n = 131 −0.8 ± 4.1* |
| Domain 2: sleep/fatigue |
n = 169 16.3 ± 10.5 |
n = 93 −6.5 ± 10.2*** |
n = 153 −5.9 ± 11.0*** |
n = 144 −5.5 ± 10.3*** |
n = 131 −4.8 ± 11.6*** |
| Domain 3: mood/cognition |
n = 169 15.7 ± 15.9 |
n = 93 −5.3 ± 14.2*** |
n = 153 −5.9 ± 13.4*** |
n = 143 −4.7 ± 14.7*** |
n = 131 −4.4 ± 13.9*** |
| Domain 4: perceptual problems/hallucinations |
n = 169 2.9 ± 5.3 |
n = 92 −1.5 ± 4.7** |
n = 153 −1.4 ± 4.7*** |
n = 144 −1.0 ± 5.5* |
n = 131 −0.4 ± 4.9 |
| Domain 5: attention/memory |
n = 172 8.2 ± 8.6 |
n = 113 −2.0 ± 6.4** |
n = 153 −2.6 ± 7.0*** |
n = 149 −2.9 ± 7.9*** |
n = 135 −0.9 ± 7.3 |
| Domain 6: gastrointestinal tract |
n = 171 9.5 ± 8.2 |
n = 113 −2.4 ± 6.1*** |
n = 151 −3.7 ± 6.2*** |
n = 147 −4.0 ± 7.5*** |
n = 134 −3.2 ± 7.7*** |
| Domain 7: urinary |
n = 170 14.3 ± 11.0 |
n = 114 −1.8 ± 6.7** |
n = 149 −2.7 ± 9.2*** |
n = 146 −2.5 ± 9.5** |
n = 133 −1.6 ± 10.5 |
| Domain 8: sexual function |
n = 171 5.0 ± 7.1 |
n = 113 −0.6 ± 4.1 |
n = 150 −0.6 ± 5.5 |
n = 147 −0.4 ± 6.3 |
n = 130 −1.0 ± 6.3 |
| Domain 9: miscellaneous |
n = 171 12.6 ± 9.7 |
n = 112 −4.1 ± 8.5*** |
n = 151 −4.7 ± 8.3*** |
n = 147 −5.8 ± 9.6*** |
n = 134 −4.4 ± 9.2*** |
Patient numbers are lower at D1 as no efficacy assessments were collected in patients without the optional NJ phase.
D, day; M, month; NJ, nasojejunal; NMSS, Non‐Motor Symptoms Scale; UDysRS, Unified Dyskinesia Rating Scale; UPDRS, Unified Parkinson's Disease Rating Scale.
Values compared with baseline with paired t test, P < 0.001 (***), P < 0.01 (**), and P < 0.05 (*).
Non‐Motor Symptoms
LCIG significantly improved NMSS total scores through M12 (mean ± SD change from BL: −23.1 ± 41.4 [P < 0.001]; Fig. 3A). Additionally, 5 of 9 NMSS subdomains were significantly improved at M12: cardiovascular including falls (−0.8 ± 4.1 [P = 0.028]), sleep/fatigue (−4.8 ± 11.6 [P < 0.001]), mood/cognition (−4.4 ± 13.9 [P < 0.001]), gastrointestinal tract (−3.2 ± 7.7 [P < 0.001]), and miscellaneous (−4.4 ± 9.2 [P < 0.001]; Table 2). In accordance with the NMSS sleep/fatigue domain, patients receiving LCIG reported significantly improved sleep quality (mean ± SD change from BL in PDSS‐2 total score: −6.5 ± 12.2 [P < 0.001]; Fig. 3B) and daytime somnolence (mean ± SD change from BL in ESS total score: −1.0 ± 5.7 [P = 0.042] Fig. 3C) through M12.
FIG. 3.
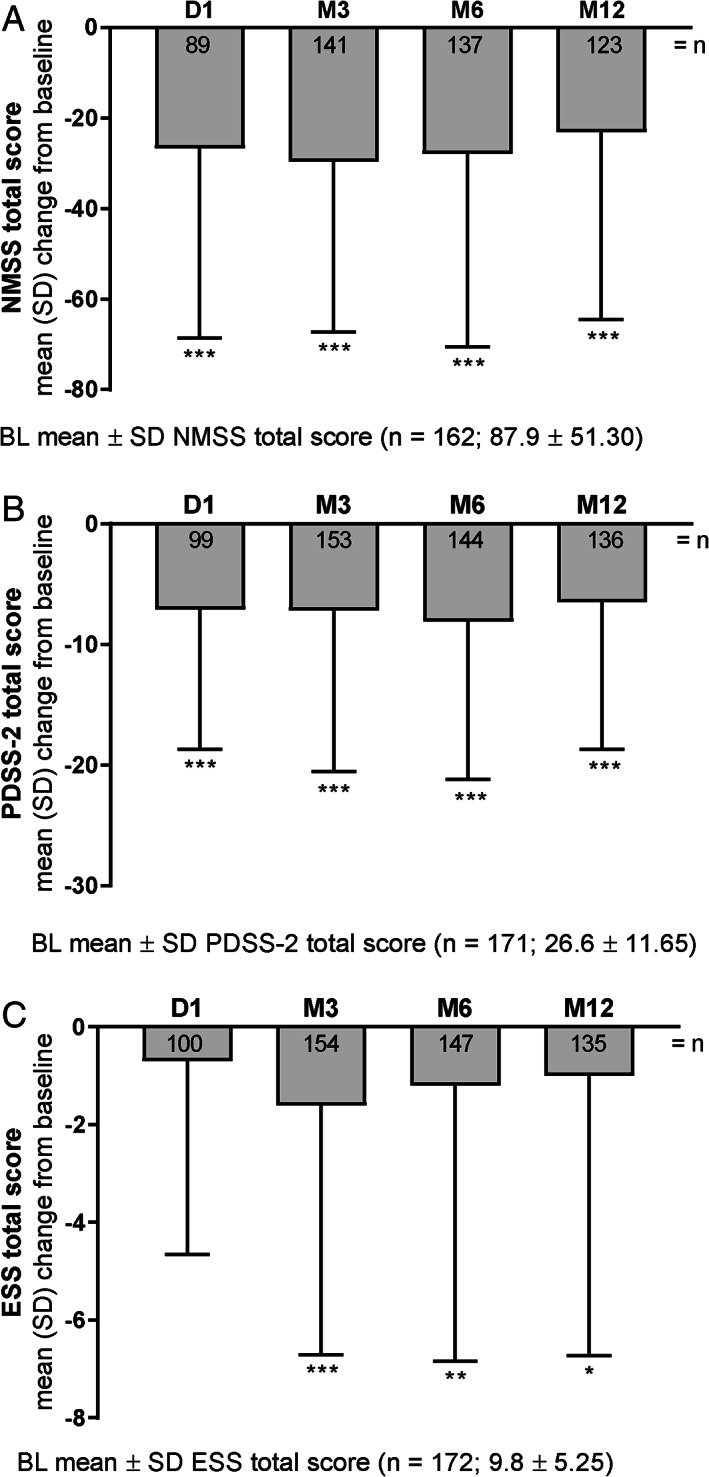
LCIG efficacy on non‐motor symptoms as measured by change from baseline. P values indicate significant difference compared with baseline using a 1‐sample t test: P ≤ 0.001 (***), P ≤ 0.01 (**), and P ≤ 0.05 (*). BL, baseline; D, day; ESS, Epworth Sleepiness Scale; LCIG, levodopa‐carbidopa intestinal gel; M, month; NMSS, Non‐Motor Symptom Scale; PDSS‐2, Parkinson's Disease Sleep Scale‐2.
Patient HR‐QoL and Caregiver Burden
Patient HR‐QoL (mean ± SD change from BL in PDQ‐8 summary index) significantly improved from D1 to M12 (−9.0 ± 21.6 [P < 0.001]; Fig. 4A). The sustained and significant improvements in patient HR‐QoL were independent of patient age, sex, or PD duration from M3 to M12 (Fig. S4A‐C and Fig. S3E). Additionally, LCIG treatment significantly improved caregiver burden from M3 to M12 (mean ± SD change from BL in MCSI total score: −1.9 ± 6.7 [P = 0.008]; Fig. 4B).
FIG. 4.
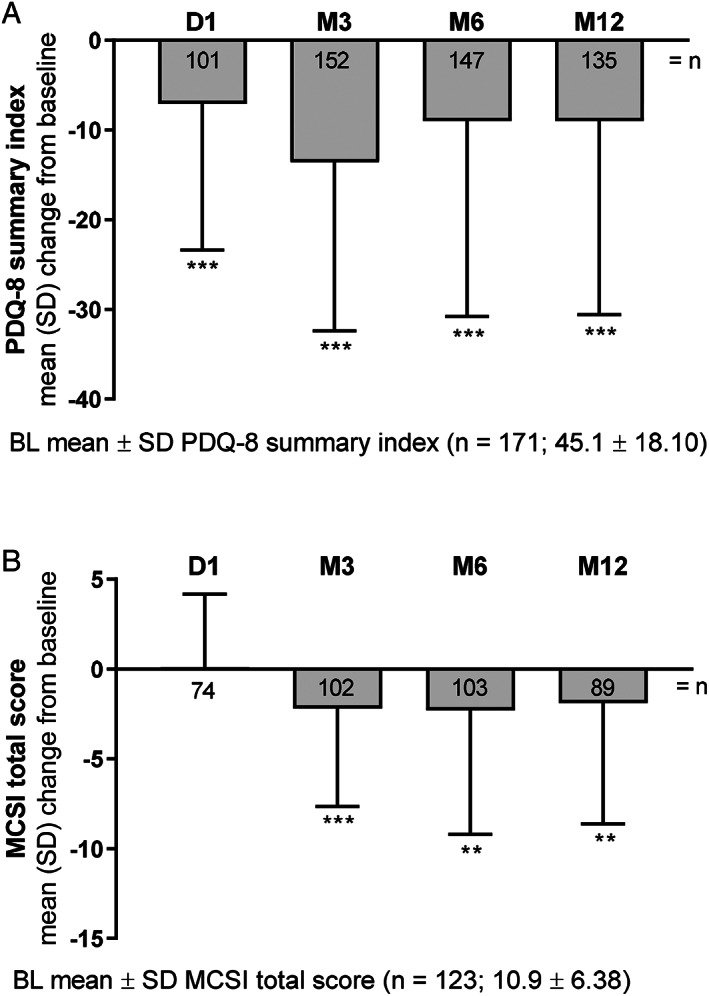
Patient HR‐QoL and caregiver burden with LCIG treatment as measured by change from baseline. P values indicate significant difference compared with baseline using a 1‐sample t test: P ≤ 0 .001 (***), and P ≤ 0.01 (**). BL, baseline; D, day; HR‐QoL, health‐related quality of life; LCIG, levodopa‐carbidopa intestinal gel; M, month; MCSI, Modified Caregiver Strain Index; PDQ‐8, 8‐item Parkinson's Disease Questionnaire.
Impact of Baseline Disease Severity
To assess the effect of baseline disease severity, a post hoc analysis of the effect of baseline H&Y stage was conducted, dividing the subjects into those with H&Y scores <3 and those with scores ≥3 (in the on state). Significant improvements in off time were observed independent of disease severity (H&Y scores: <3 and ≥3, mean ± SD change from BL, n = 41 and 86; −4.2 ± 3.6 and − 3.8 ± 3.7 hr/day, both P < 0.001; Fig. S3A) and dyskinesia signs and symptoms (UDysRS total scores: n = 45 and 72, −6.3 ± 19.7 and − 11.6 ± 23.9, P = 0.037 and P < 0.001, respectively; Fig. S3B). In the total population, axial symptoms significantly improved at D1 (mean ± SD change from BL −1.5 ± 4.4, P < 0.01) and M3 (−1.5 ± 4.6 [P < 0.001]) and numerically reduced at M6 (−0.7 ± 5.1) and M12 (−0.5 ± 4.6). The axial symptom scores at M12 increased significantly in the group with scores <3 (n = 47, 1.4 ± 4.4 [P = 0.040]) but significantly improved in the group with scores ≥3 (n = 86, −1.5 ± 4.3 [P = 0.002]; Fig. S3C). Independent of BL disease severity, significant improvements were observed in NMS (NMSS total scores: n = 42/81, −18.6 ± 42.3 and − 25.5 ± 41.1; both P < 0.01; Fig. S3D) and HR‐QoL (PDQ‐8 summary index: n = 46/88, −11.0 ± 3.2 and − 7.8 ± 2.3; both P < 0.01; Fig. S3E).
Completer Analysis
There were no clinically meaningful differences in BL demographics or disease characteristics in patients who completed 12 months of LCIG therapy (12‐month completers) and patients who discontinued before 12 months of LCIG therapy (12‐month non‐completers; Table 3).
TABLE 3.
Baseline demographics and disease characteristics of 12‐month completer versus non‐completers
| 12‐month completer (N = 148) | 12‐month non‐completer (N = 47) | |||
|---|---|---|---|---|
| n | % | n | % | |
| Sex | ||||
| Female | 53 | 35.8 | 22 | 46.8 |
| Male | 95 | 64.2 | 25 | 53.2 |
| n | mean ± SD | n | mean ± SD | |
| Age, years | 148 | 69.9 ± 8.0 | 47 | 71.3 ± 8.5 |
| BMI, kg/m2 | 140 | 25.9 ± 4.1 | 42 | 25.6 ± 4.1 |
| Weight, kg | 140 | 73.9 ± 14.2 | 42 | 69.7 ± 13.9 |
| Hoehn and Yahr stage | ||||
| On state | 143 | 3.0 ± 0.8 | 45 | 3.3 ± 0.8 |
| Off state | 141 | 3.6 ± 0.8 | 45 | 3.7 ± 0.7 |
| PD duration, years | 147 | 11.3 ± 4.8 | 47 | 11.0 ± 4.9 |
| MMSE score | 142 | 27.8 ± 1.8 | 45 | 27.4 ± 3.0 |
| Off time, hr/day | 137 | 5.9 ± 3.3 | 27 | 6.4 ± 3.8 |
| UDysRS total score | 126 | 33.8 ± 20.9 | 26 | 32.8 ± 23.0 |
| NMSS total score | 136 | 89.4 ± 52.0 | 28 | 82.6 ± 47.1 |
| PDQ‐8 summary index score | 143 | 45.4 ± 18.6 | 28 | 43.2 ± 15.5 |
| MCSI total score | 104 | 11.2 ± 6.4 | 19 | 9.2 ± 6.0 |
BMI, body mass index; MCSI, Modified Caregiver Strain Index; MMSE, Mini‐Mental State Examination; NMSS, Non‐Motor Symptoms Scale; PD, Parkinson's disease; PDQ‐8, 8‐item Parkinson's Disease Questionnaire; UDysRS, Unified Dyskinesia Rating Scale.
Safety
Overall, 79 (40.5%) patients experienced SAEs with 23 SAEs having a reasonable possibility of being related to LCIG and 29 (14.9%) patients discontinued LCIG because of an SAE (Table 4). SAEs that occurred in >2 patients are presented in Table 4. The most common SAEs were fall (n = 6), urinary tract infection (n = 6), hip fracture (n = 5), pneumonia (n = 5), and abdominal pain (n = 4) (Table 4). In the total population, mean weights were 72.9 kg at BL and 72.3 kg at M12; however, 56 patients (28.7%) experienced a ≥7% decrease in weight and 29 (14.9%) experienced a ≥7% increase in weight compared with BL measures. Three neuropathies were reported and were described by the investigators as chronic inflammatory demyelinating polyradiculoneuropathy (n = 1), polyneuropathy (n = 1), and sensory loss (n = 1) (Table 4). From initiation of data collection to the database lock for this analysis, 13 patients died, and 1 of these deaths (intestinal obstruction) in the investigator's judgment was deemed possibly related to LCIG (Table 4).
TABLE 4.
Safety summary
| Patients with | n (% of N = 195) | |
|---|---|---|
| Any severe AE | 46 (23.6) | |
| Any SAE | 79 (40.5) | |
| Any SAE with reasonable possibility of causal relationship with LCIG | 23 (11.8) | |
| Any AE leading to drug being withdrawn | 29 (14.9) | |
| Deaths | 13 (6.7) | |
| Deaths considered to be related to LCIG treatment by the study investigatora | 1 (0.5) | |
| SAEs | Treatment‐emergent SAEs (reasonable possibility) | |
|---|---|---|
| n (% of N = 195) | n (% of N = 195) | |
| Patient with any SAE | 79 (40.5) | 23 (11.8) |
| Treatment‐emergent SAE occurring in >1% of patients by MedDRA preferred term | ||
| Fall | 6 (3.1) | 2 (1.0) |
| Urinary tract infection | 6 (3.1) | 1 (0.5) |
| Hip fracture | 5 (2.6) | 0 |
| Pneumonia | 5 (2.6) | 0 |
| Abdominal pain | 4 (2.1) | 3 (1.5) |
| Device dislocation | 3 (1.5) | 2 (1.0) |
| Device occlusion | 3 (1.5) | 2 (1.0) |
| Femoral neck fracture | 3 (1.5) | 0 |
| Hallucination | 3 (1.5) | 2 (1.0) |
| Neuropathyb , c | 3 (1.5) | 0 |
| Pneumoperitoneum | 3 (1.5) | 2 (1.0) |
| Urosepsis | 3 (1.5) | 1 (0.5) |
Intestinal obstruction.
Chronic inflammatory demyelinating polyradiculoneuropathy (n = 1), polyneuropathy (n = 1), and sensory loss (n = 1).
All 3 neuropathies were reported by the investigator as not treatment related.
AE, adverse event; LCIG, levodopa‐carbidopa intestinal gel; MedDRA, Medical Dictionary for Regulatory Activities; SAE, serious adverse event.
Discussion
We present an analysis of the safety and efficacy of LCIG treatment in a large‐scale, long‐term, fully prospective observational study in patients with advanced PD. This study, the first to include US sites in a large observational cohort, was designed to replicate the clinical practice experience. Although practice settings were diverse, after 1 year of treatment, there were strong and consistent improvements in motor features, NMS, patient HR‐QoL, and caregiver burden.
The LCIG dose was relatively stable over 12 months of treatment, as seen previously.15 LCIG led to significant reductions in off time with ≥3 hours absolute reduction and ≥50% reduction at every study visit compared with measures at BL, independent of patient age, sex, or disease duration. This observation is consistent with other observational and controlled‐clinical trials of LCIG,11, 12, 14, 15 and are substantially greater than the 1‐hr off time reduction threshold for a clinically meaningful change.21
This is the first prospective, multinational study assessing dyskinesia signs and symptoms using the novel, validated, and highly sensitive UDysRS.20, 22 UDysRS total score improvements were maintained through 12 months of treatment (29% reduction at M12). Additionally, 3 of 4 UDysRS parts significantly improved: ON‐dyskinesia, OFF‐dystonia, disability, and composite historical and objective scores. Improvement in the impact of dyskinesia on everyday functioning as measured by parts 1 and 2 of the UDysRS exceeded values proposed as the minimal clinically significant change (2.1 and 1.8, respectively).23 Similar improvements in UDysRS scores were reported in a smaller open‐label study of Hungarian patients with advanced PD who were treated with LCIG for 12 months.22 This robust effect on dyskinesia was supported by corresponding improvements in UPDRS part IV. These observations support the idea that LCIG can be a useful clinical strategy, particularly in patients experiencing both off periods and dyskinesias.
Tremor is a troublesome motor feature that exhibits variable response to dopaminergic treatment.24, 25 Here, we observed significant improvement in both the on state‐resting and ‐action tremors with LCIG. Of note, most enrolled patients did not have severe tremor at baseline. Further studies of the effects of LCIG on tremor are warranted.
We also observed significant improvements in NMSS total scores through M12 that are consistent with previous clinical trials and observational studies,13, 15, 16, 17, 19 including studies from independent cohorts that assessed the effects of LCIG on NMSS.26, 27, 28 The magnitude of NMSS total score improvement (26% reduction at M12) with LCIG ranged from 23 to 30 points at all time points evaluated and were substantially greater than the 13.9‐point reduction considered the minimal clinically important difference (MCID) for this scale.29
Improvement in sleep was an important contributor to the overall improvement in total NMSS. Of note, most patients received 16‐hr per day LCIG infusion therapy through the study. Sleep disturbances are commonly reported in patients with PD,30, 31, 32 here, we show improvements on the sleep/fatigue subdomain of the NMSS (29% reduction at M12) similar to those seen in other LCIG observational studies.15, 16, 17 Smaller studies have shown improvements in sleep symptoms with LCIG treatment as assessed by the PDSS‐233, 34 and ESS33 and improvements in PDSS‐2 sleep quality scores through 12 months of LCIG treatment, all above the MCID threshold of −3.44 points.35 The sustained improvements of NMS with LCIG are important clinical outcomes because NMS treatment is considered a key unmet need36 and is articulated in the recent Movement Disorders Society evidence‐based task force on management of NMS in PD.
Beneficial effects of LCIG treatment were also observed in patient HR‐QoL. Improvement in PDQ‐8 summary index scores with LCIG exceeds the MCID threshold of −5.9,35, 37 independent of patient age, sex, or disease duration. These observations align with several studies indicating NMS are important contributors to patient HR‐QoL, although a direct relationship between NMS and HR‐QoL was not investigated here. In this study, using the MCSI, caregiver burden was significantly improved at M12. Multiple non‐disease‐specific scales have been used to assess the impact of LCIG treatment on caregiver burden, which could explain variable results reported in the literature.12, 38, 39, 40, 41
Our analysis of patients stratified by H&Y scores before LCIG treatment provides further evidence that, independent of disease severity, LCIG improves motor fluctuations including off time and dyskinesia. We also observed that in patients with higher disease severity at BL, axial symptoms significantly improved through M12. Another report found that axial symptoms in patients treated with LCIG remained relatively stable up to 1‐year follow‐up; however, that study included fewer patients and different stratification of patients by baseline H&Y scores.42 Here, we also provide a description of those patients who completed 12 months of LCIG treatment, although no clinically meaningful differences were observed, it will be important for further studies to assess whether any of these characteristics are predictive of a positive response to LCIG therapy.
Our observations on safety in this study are consistent with the established LCIG safety profile from controlled clinical trials and other observational studies.11, 12, 14, 15 SAEs were reported in 79 patients (40.5%) consisting of events typically encountered in this population. Weight variation and neuropathies have multifaceted manifestations, but have been potentially linked to the underlying pathological changes associated with PD, aging, and levodopa administration and dosage.43, 44, 45, 46, 47 We report a ≥7% decrease or a ≥7% increase in weight in 56 (28.7%) and 29 (14.9%) patients after 12 months of LCIG therapy, respectively, and 3 patients with symptoms of neuropathies reported as SAEs. However, as an observational study, the protocol did not mandate prospective laboratory or nerve conduction velocity assessments. From initiation of data collection to the database lock for this analysis, 13 deaths occurred with 1 deemed possibly related to LCIG, which is considered within the expected range in this age group. Overall, 60 patients (30.8%) prematurely discontinued the study; however, of note, 13 of these patients continued to receive LCIG therapy outside of the study. Overall, this AE profile is consistent with a procedure that is often administered to patients with advanced PD who may be frail and elderly.
The main limitation of this study is that it is open‐label, non‐comparative, and observational. We cannot exclude a component of placebo effect in the outcomes observed, but LCIG has previously been shown to be effective for off time in a randomized double‐blind study, and the magnitude of the changes in off time, dyskinesia, and HR‐QoL measures are large and unlikely because of placebo alone. This observational study relied on self‐reported off time for the day before the study visit. Although Hauser diaries are the gold standard for measuring off time,48 use of these diaries would have added non‐routine burden to patients, caregivers, and clinicians. We used single‐day retrospective reporting to avoid potential recall bias, and to reflect assessments of off time in routine clinical practice. The large number of patients included in the study should sufficiently offset individual daily fluctuations in off time. However, we note that subgroup analyses are inherently less precise, because they rely on smaller numbers of patients. Despite these limitations, the data presented here provide important real‐world information on LCIG in routine clinical practice.
LCIG treatment of patients with advanced PD in the diverse routine clinical care settings found in a variety of countries and practices consistently led to significant and sustained improvements in both motor features (including off time and dyskinesia) and non‐motor measures (including sleep). There was also consistent improvement in measures of patient HR‐QoL and caregiver burden. The safety events reported here were consistent with the previously established safety profile of LCIG as determined from controlled‐clinical trials and observational studies. Overall, these findings reinforce the effectiveness of LCIG in a real‐world setting and provide valuable new information regarding effects on dyskinesia and NMS. Continued observation of this cohort to 36 months of treatment, as planned, will provide further insight into how these features evolve over extended periods of treatment.
Author Roles
(1) Research Project: A. Conception, B. Organization, C. Execution; (2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique; (3) Manuscript: A. Writing of the First Draft, B. Review and Critique.
D.G.S.: 1A, 2A, 2C, 3A, 3B
J.A.: 1A, 2A, 2C, 3A, 3B
M.A.‐H.: 1A, 2A, 2C, 3A, 3B
P.B.: 1A, 2A, 2C, 3A, 3B
E.C.: 1A, 2A, 2C, 3A, 3B
T.L.D.: 1A, 2A, 2C, 3A, 3B
R.I.: 1A, 2A, 2C, 3A, 3B
N.K.: 1A, 1C, 2A, 2C, 3A, 3B
F.E.P.: 1A, 1C, 2A, 2C, 3A, 3B
M.S.S.: 1A, 2A, 2C, 3A, 3B
M.S.: 1A, 2A, 2C, 3A, 3B
L.B.: 1A, 2A, 2C, 3A, 3B
P.K.: 1A, 2A, 2C, 3A, 3B
W.Z.R.: 1A, 2A, 2B, 2C, 3A, 3B
K.R.C.: 1A, 2A, 2C, 3A, 3B
All authors approved this manuscript for publication.
Disclosures
Ethical Compliance Statement
The protocol, patient information, and informed consent were approved in all countries by national and/or local independent ethics committees and health authorities according to the applicable national regulatory requirements. All patients participating in the study provided written informed consent. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Funding Sources and Conflicts of Interest
AbbVie participated in the study design; study research; collection, analysis, and interpretation of data; and writing, reviewing, and approving this manuscript for submission. All authors had access to the data, participated in manuscript development and review, and agreed to submit this manuscript. AbbVie funded the research for this study and provided writing support for this manuscript. No honoraria or payments were made for authorship. Medical writing assistance, funded by AbbVie was provided by Justin Eddy, PhD, of AbbVie and Alicia Salinero, PhD, of JB Ashtin.
Financial Disclosure for the Previous 12 months
J.A. is a study investigator and has received honorarium from AbbVie, Allergan, Teva, US World Meds, Medtronic, and Abbott. He is an investigator in studies sponsored by AbbVie, Biogen, Acadia, Northwestern University, Neuroderm, Massachusetts General Hospital, and Astra Zeneca. He is also a scientific advisor for AbbVie. M.A.‐H. is a study investigator and consultant for AbbVie. P.B. is a study investigator and has received honoraria for consulting from AbbVie. K.R.C. is a study investigator and has received honorarium from UCB, AbbVie, Britannia, Mundipharma, Boehringer Ingelheim, and GSK Pharmaceuticals for lecturing at symposia. He has been a consultant for UCB, AbbVie, Britannia, Neuronova, and Mundipharma. He has received research funding from Parkinson's UK, NIHR, PDNMG, and educational grants from UCB, Britannia, AbbVie, GSK Pharmaceuticals, Boehringer Ingelheim, and Neuronova. He receives royalties from Oxford University Press and holds intellectual property rights for the King's Parkinson's Pain Scale and Parkinson's Disease Sleep Scale. E.C. is a study investigator and has received honoraria for consulting from AbbVie, and travel grants from Allergan and Boston Scientific. T.L.D. is a study investigator and has received grant/research support from the Parkinson Foundation and the Peterson Foundation for Parkinson's, and honoraria for consulting from AbbVie and Acadia Pharmaceuticals. R.I. is a study investigator and has received honorarium for consulting from AbbVie. N.K. is a study investigator and has received honorarium from UCB, AbbVie, Abbott, Boston Scientific, Medtronic, Krka, Teva, Boehringer Ingelheim, GSK Pharmaceuticals, and Sandoz for lecturing at symposia. He has been a consultant for AbbVie, Teva, and Krka. He has received research funding from the Hungarian National Research Development and Innovation Office, University of Pécs, Medtronic, and Abbott. M.S.S. is a study investigator. Since January 2019, he has received research support as a Principal Investigator from Sunovion Pharma, AbbVie, Boston Scientific Inc, Biogen MA Inc, Theravance Biopharma, The Michael J. Fox Foundation/Neuropoint Alliance, Impax Laboratories, and Sun Pharma. He has served as a consultant and/or scientific advisor for Boston Scientific and AbbVie. F.E.P. is a study investigator and a member of the faculty at the Sapienza University of Rome and is supported by University funds. He is an investigator in studies funded by AbbVie, Zambon, and the Italian Ministry of Health. He has a clinical practice and is compensated for this activity by the Sant'Andrea Hospital in Rome. In addition, since January 1, 2016, he has served as consultant for or received honoraria from AbbVie, Zambon, FB Health, and Lundbeck. M.S. is a study investigator and has received honoraria for lectures at symposia and consultant fees from Teva, AbbVie, Merck, Servier Pharma, AOP Orphan, Boehringer Ingelheim, Sanofi, Krka, and UCB Pharma. D.G.S. is a member of the faculty of the University of Alabama at Birmingham and is supported by endowment and University funds. He is an investigator in studies funded by AbbVie, the American Parkinson Disease Association, The Michael J. Fox Foundation for Parkinson Research, Alabama Department of Commerce, the Department of Defense, and NIH (grants P50NS108675, R25NS079188, and T32NS095775). He has a clinical practice and is compensated for these activities through the University of Alabama Health Services Foundation. In addition, since January 1, 2020 he has served as a consultant for or received honoraria from AbbVie, Sutter Health, the International Parkinson Disease and Movement Disorder Society, Theravance, McGraw Hill, and Sanofi‐Aventis. L.B., P.K., and W.Z.R. are employees of AbbVie and may hold AbbVie stock and/or stock options.
Data Sharing Statement
AbbVie is committed to responsible data sharing regarding the clinical trials we sponsor. This includes access to anonymized individual and trial‐level data (analysis data sets), as well as other information (eg, protocols and Clinical Study Reports), as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications. This clinical trial data can be requested by any qualified researchers who engage in rigorous, independent scientific research, and will be provided following review and approval of a research proposal and Statistical Analysis Plan (SAP) and execution of a Data Sharing Agreement (DSA). Data requests can be submitted at any time and the data will be accessible for 12 months, with possible extensions considered. For more information on the process, or to submit a request, visit the following link: https://www.abbvie.com/our-science/clinical-trials/clinical-trials-data-and-information-sharing/data-and-information-sharing-with-qualified-researchers.html.
Supporting information
Figure S1. Study schematic. aApproximately 14 days or as recommended by the physician according to local reimbursement guidelines. bEffectiveness data only for patients with prior NJ treatment phase. LCIG = levodopa‐carbidopa intestinal gel; M = month; NJ = nasojejunal; PD = Parkinson's disease; PEG‐J = percutaneous endoscopic gastrostomy with jejunal extension tube.
Figure S2. Mean (SE) change from baseline in off time by (A) age, (B) sex, and (C) disease duration at indicated time points. P values indicate significant difference compared with baseline using repeated measures model that includes country visit baseline subgroup, the baseline*visit, and the subgroup*visit interaction: P ≤ 0.001 (***). BL = baseline; LCIG = levodopa‐carbidopa intestinal gel; M = month.
Figure S3. Motor and non‐motor symptoms in patients treated with LCIG stratified by baseline H&Y score. Axial symptom scores are the sum of items 18, 22, 27–30 on UPDRS part III. P values indicate significant difference compared with baseline using: P ≤ 0.001 (***). BL = baseline; D = day; H&Y = Hoehn and Yahr; HR‐QoL = health‐related quality of life; LCIG = levodopa‐carbidopa intestinal gel; M = month; NMSS = Non‐Motor Symptom Scale; PDQ‐8 = 8‐item Parkinson's Disease Questionnaire; UDysRS = Unified Dyskinesia Rating Scale; UPDRS = Unified Parkinson's Disease Rating Scale.
Figure S4. Mean (SE) change in PDQ‐8 summary index scores by (A) age, (B) sex, and (C) disease duration at indicated time points. P values indicate significant difference compared with baseline using repeated measures model that includes country visit baseline subgroup, the baseline*visit, and the subgroup*visit interaction: P ≤ 0.001 (***), P ≤ 0.01 (**), and P ≤ 0.05 (*). BL = baseline; D = day; LCIG = levodopa‐carbidopa intestinal gel; M = month; PDQ‐8 = 8‐item Parkinson's Disease Questionnaire; SE = standard error.
References
- 1.Antonini A, Chaudhuri KR, Martinez‐Martin P, Odin P. Oral and infusion levodopa‐based strategies for managing motor complications in patients with Parkinson's disease. CNS Drugs 2010;24(2):119–129. [DOI] [PubMed] [Google Scholar]
- 2.Prescott IA, Dostrovsky JO, Moro E, Hodaie M, Lozano AM, Hutchison WD. Levodopa enhances synaptic plasticity in the substantia nigra pars reticulata of Parkinson's disease patients. Brain 2009;132(Pt 2):309–318. [DOI] [PubMed] [Google Scholar]
- 3.Calabresi P, Standaert DG. Dystonia and levodopa‐induced dyskinesias in Parkinson's disease: Is there a connection? Neurobiol Dis 2019;132:104579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ray Chaudhuri K, Poewe W, Brooks D. Motor and nonmotor complications of levodopa: Phenomenology, risk factors, and imaging features. Mov Disord 2018;33(6):909–919. [DOI] [PubMed] [Google Scholar]
- 5.Daley DJ, Myint PK, Gray RJ, Deane KH. Systematic review on factors associated with medication non‐adherence in Parkinson's disease. Parkinsonism Relat Disord 2012;18(10):1053–1061. [DOI] [PubMed] [Google Scholar]
- 6.Davis KL, Edin HM, Allen JK. Prevalence and cost of medication nonadherence in Parkinson's disease: Evidence from administrative claims data. Mov Disord 2010;25(4):474–480. [DOI] [PubMed] [Google Scholar]
- 7.Malek N, Grosset DG. Medication adherence in patients with Parkinson's disease. CNS Drugs 2015;29(1):47–53. [DOI] [PubMed] [Google Scholar]
- 8.Shin JY, Habermann B, Pretzer‐Aboff I. Challenges and strategies of medication adherence in Parkinson's disease: A qualitative study. Geriatr Nurs 2015;36(3):192–196. [DOI] [PubMed] [Google Scholar]
- 9.Othman AA, Rosebraugh M, Chatamra K, Locke C, Dutta S. Levodopa‐carbidopa intestinal gel pharmacokinetics: Lower variability than oral levodopa‐carbidopa. J Parkinsons Dis 2017;7(2):275–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Politis M, Sauerbier A, Loane C, et al. Sustained striatal dopamine levels following intestinal levodopa infusions in Parkinson's disease patients. Mov Disord 2017;32(2):235–240. [DOI] [PubMed] [Google Scholar]
- 11.Olanow CW, Kieburtz K, Odin P, et al. Continuous intrajejunal infusion of levodopa‐carbidopa intestinal gel for patients with advanced Parkinson's disease: a randomised, controlled, double‐blind, double‐dummy study. Lancet Neurol 2014;13(2):141–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Slevin JT, Fernandez HH, Zadikoff C, et al. Long‐term safety and maintenance of efficacy of levodopa‐carbidopa intestinal gel: An open‐label extension of the double‐blind pivotal study in advanced Parkinson's disease patients. J Parkinsons Dis 2015;5(1):165–174. [DOI] [PubMed] [Google Scholar]
- 13.Standaert DG, Rodriguez RL, Slevin JT, et al. Effect of levodopa‐carbidopa intestinal gel on non‐motor symptoms in patients with advanced Parkinson's disease. Mov Disord Clin Pract 2017;4(6):829–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fernandez HH, Standaert DG, Hauser RA, et al. Levodopa‐carbidopa intestinal gel in advanced Parkinson's disease: final 12‐month, open‐label results. Mov Disord 2015;30(4):500–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Antonini A, Poewe W, Chaudhuri KR, et al. Levodopa‐carbidopa intestinal gel in advanced Parkinson's: Final results of the GLORIA registry. Parkinsonism Relat Disord 2017;45:13–20. [DOI] [PubMed] [Google Scholar]
- 16.Kruger R, Lingor P, Doskas T, et al. An observational study of the effect of levodopa‐carbidopa intestinal gel on activities of daily living and quality of life in advanced Parkinson's disease patients. Adv Ther 2017;34(7):1741–1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Poewe W, Bergmann L, Kukreja P, Robieson WZ, Antonini A. Levodopa‐carbidopa intestinal gel monotherapy: GLORIA registry demographics, efficacy, and safety. J Parkinsons Dis 2019;9(3):531–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lopiano L, Modugno N, Marano P, et al. Motor and non‐motor outcomes in patients with advanced Parkinson's disease treated with levodopa/carbidopa intestinal gel: Final results of the GREENFIELD observational study. J Neurol 2019;266(9):2164–2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ray Chaudhuri K, Antonini A, Robieson WZ, et al. Burden of non‐motor symptoms in Parkinson's disease patients predicts improvement in quality of life during treatment with levodopa‐carbidopa intestinal gel. Eur J Neurol 2019;26(4):581–e543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goetz CG, Nutt JG, Stebbins GT. The unified dyskinesia rating scale: Presentation and clinimetric profile. Mov Disord 2008;23(16):2398–2403. [DOI] [PubMed] [Google Scholar]
- 21.Hauser RA, Auinger P, Parkinson SG. Determination of minimal clinically important change in early and advanced Parkinson's disease. Mov Disord 2011;26(5):813–818. [DOI] [PubMed] [Google Scholar]
- 22.Juhasz A, Aschermann Z, Acs P, et al. Levodopa/carbidopa intestinal gel can improve both motor and non‐motor experiences of daily living in Parkinson's disease: An open‐label study. Parkinsonism Relat Disord 2017;37:79–86. [DOI] [PubMed] [Google Scholar]
- 23.Makkos A, Kovacs M, Pinter D, Janszky J, Kovacs N. Minimal clinically important difference for the historic parts of the unified dyskinesia rating scale. Parkinsonism Relat Disord 2019;58:79–82. [DOI] [PubMed] [Google Scholar]
- 24.Elble RJ. Tremor and dopamine agonists. Neurology 2002;58(4 Suppl 1):S57–S62. [DOI] [PubMed] [Google Scholar]
- 25.Sung YH, Chung SJ, Kim SR, Lee MC. Factors predicting response to dopaminergic treatment for resting tremor of Parkinson's disease. Mov Disord 2008;23(1):137–140. [DOI] [PubMed] [Google Scholar]
- 26.Dafsari HS, Martinez‐Martin P, Rizos A, et al. EuroInf 2: Subthalamic stimulation, apomorphine, and levodopa infusion in Parkinson's disease. Mov Disord 2019;34(3):353–365. [DOI] [PubMed] [Google Scholar]
- 27.Dafsari HS, Reddy P, Herchenbach C, et al. Beneficial effects of bilateral subthalamic stimulation on non‐motor symptoms in Parkinson's disease. Brain Stimul 2016;9(1):78–85. [DOI] [PubMed] [Google Scholar]
- 28.Martinez‐Martin P, Reddy P, Katzenschlager R, et al. EuroInf: A multicenter comparative observational study of apomorphine and levodopa infusion in Parkinson's disease. Mov Disord 2015;30(4):510–516. [DOI] [PubMed] [Google Scholar]
- 29.Martinez‐Martin P, Rodriguez‐Blazquez C, Abe K, et al. International study on the psychometric attributes of the non‐motor symptoms scale in Parkinson disease. Neurology 2009;73(19):1584–1591. [DOI] [PubMed] [Google Scholar]
- 30.Bartolomei L, Pastore A, Meligrana L, et al. Relevance of sleep quality on caregiver burden in Parkinson's disease. Neurol Sci 2018;39(5):835–839. [DOI] [PubMed] [Google Scholar]
- 31.Pal PK, Thennarasu K, Fleming J, Schulzer M, Brown T, Calne SM. Nocturnal sleep disturbances and daytime dysfunction in patients with Parkinson's disease and in their caregivers. Parkinsonism Relat Disord 2004;10(3):157–168. [DOI] [PubMed] [Google Scholar]
- 32.Scaravilli T, Gasparoli E, Rinaldi F, Polesello G, Bracco F. Health‐related quality of life and sleep disorders in Parkinson's disease. Neurol Sci 2003;24(3):209–210. [DOI] [PubMed] [Google Scholar]
- 33.Zibetti M, Rizzone M, Merola A, et al. Sleep improvement with levodopa/carbidopa intestinal gel infusion in Parkinson disease. Acta Neurol Scand 2013;127(5):e28–e32. [DOI] [PubMed] [Google Scholar]
- 34.Zibetti M, Romagnolo A, Merola A, et al. A polysomnographic study in parkinsonian patients treated with intestinal levodopa infusion. J Neurol 2017;264(6):1085–1090. [DOI] [PubMed] [Google Scholar]
- 35.Horvath K, Aschermann Z, Acs P, et al. Minimal clinically important difference on Parkinson's disease sleep scale 2nd version. Parkinsons Dis 2015;2015:970534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seppi K, Ray Chaudhuri K, Coelho M, et al. Update on treatments for nonmotor symptoms of Parkinson's disease‐an evidence‐based medicine review. Mov Disord 2019;34(2):180–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Horvath K, Aschermann Z, Kovacs M, et al. Changes in quality of life in Parkinson's disease: How large must they be to be relevant? Neuroepidemiology 2017;48(1–2):1–8. [DOI] [PubMed] [Google Scholar]
- 38.Caceres‐Redondo MT, Carrillo F, Lama MJ, et al. Long‐term levodopa/carbidopa intestinal gel in advanced Parkinson's disease. J Neurol 2014;261(3):561–569. [DOI] [PubMed] [Google Scholar]
- 39.Ciurleo R, Corallo F, Bonanno L, et al. Assessment of Duodopa® effects on quality of life of patients with advanced Parkinson's disease and their caregivers. J Neurol 2018;265(9):2005–2014. [DOI] [PubMed] [Google Scholar]
- 40.De Fabregues O, Dot J, Abu‐Suboh M, et al. Long‐term safety and effectiveness of levodopa‐carbidopa intestinal gel infusion. Brain Behav 2017;7(8):e00758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Santos‐Garcia D, Anon MJ, Fuster‐Sanjurjo L, de la Fuente‐Fernandez R. Duodenal levodopa/carbidopa infusion therapy in patients with advanced Parkinson's disease leads to improvement in caregivers' stress and burden. Eur J Neurol 2012;19(9):1261–1265. [DOI] [PubMed] [Google Scholar]
- 42.Fabbri M, Pongmala C, Artusi CA, et al. Long‐term effect of levodopa‐carbidopa intestinal gel on axial signs in Parkinson's disease. Acta Neurol Scand 2019;140(2):157–161. [DOI] [PubMed] [Google Scholar]
- 43.Akbar U, He Y, Dai Y, et al. Weight loss and impact on quality of life in Parkinson's disease. PLoS One 2015;10(5):e0124541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fabbri M, Zibetti M, Beccaria L, et al. Levodopa/carbidopa intestinal gel infusion and weight loss in Parkinson's disease. Eur J Neurol 2019;26(3):490–496. [DOI] [PubMed] [Google Scholar]
- 45.Jugel C, Ehlen F, Taskin B, Marzinzik F, Muller T, Klostermann F. Neuropathy in Parkinson's disease patients with intestinal levodopa infusion versus oral drugs. PLoS One. 2013;8(6):e66639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Santos‐Garcia D, de la Fuente‐Fernandez R, Valldeoriola F, et al. Polyneuropathy while on duodenal levodopa infusion in Parkinson's disease patients: We must be alert. J Neurol 2012;259(8):1668–1672. [DOI] [PubMed] [Google Scholar]
- 47.Wills AM, Li R, Perez A, Ren X, Boyd J, Investigators NN‐P. Predictors of weight loss in early treated Parkinson's disease from the NET‐PD LS‐1 cohort. J Neurol 2017;264(8):1746–1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hauser RA, Friedlander J, Zesiewicz TA, et al. A home diary to assess functional status in patients with Parkinson's disease with motor fluctuations and dyskinesia. Clin Neuropharmacol 2000;23(2):75–81. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Study schematic. aApproximately 14 days or as recommended by the physician according to local reimbursement guidelines. bEffectiveness data only for patients with prior NJ treatment phase. LCIG = levodopa‐carbidopa intestinal gel; M = month; NJ = nasojejunal; PD = Parkinson's disease; PEG‐J = percutaneous endoscopic gastrostomy with jejunal extension tube.
Figure S2. Mean (SE) change from baseline in off time by (A) age, (B) sex, and (C) disease duration at indicated time points. P values indicate significant difference compared with baseline using repeated measures model that includes country visit baseline subgroup, the baseline*visit, and the subgroup*visit interaction: P ≤ 0.001 (***). BL = baseline; LCIG = levodopa‐carbidopa intestinal gel; M = month.
Figure S3. Motor and non‐motor symptoms in patients treated with LCIG stratified by baseline H&Y score. Axial symptom scores are the sum of items 18, 22, 27–30 on UPDRS part III. P values indicate significant difference compared with baseline using: P ≤ 0.001 (***). BL = baseline; D = day; H&Y = Hoehn and Yahr; HR‐QoL = health‐related quality of life; LCIG = levodopa‐carbidopa intestinal gel; M = month; NMSS = Non‐Motor Symptom Scale; PDQ‐8 = 8‐item Parkinson's Disease Questionnaire; UDysRS = Unified Dyskinesia Rating Scale; UPDRS = Unified Parkinson's Disease Rating Scale.
Figure S4. Mean (SE) change in PDQ‐8 summary index scores by (A) age, (B) sex, and (C) disease duration at indicated time points. P values indicate significant difference compared with baseline using repeated measures model that includes country visit baseline subgroup, the baseline*visit, and the subgroup*visit interaction: P ≤ 0.001 (***), P ≤ 0.01 (**), and P ≤ 0.05 (*). BL = baseline; D = day; LCIG = levodopa‐carbidopa intestinal gel; M = month; PDQ‐8 = 8‐item Parkinson's Disease Questionnaire; SE = standard error.


