Aicardi‐Goutières is an inherited encephalopathy characterized by acquired microcephaly, basal‐ganglia calcification, leukodystrophy, cerebral atrophy, and CSF with chronic lymphocytosis and raised interferon‐alpha.1 The neuroimage is usually diagnostic, showing basal ganglia and subcortical calcifications on CT and abnormal white matter signal on MRI. The lesions tend to be stable and no treatment is available. A 19‐year‐old male presented a neurologic condition with pyramidal and extrapyramidal signs associated with cardiomyopathy, livedo, and chilblains that appeared after acute episodes of encephalopathy (age of 2 and 10) precipitated by meningitis vaccine and salmonellosis, followed by neurologic regression and posterior recovering. CT scan (Fig. 1) identified brainstem calcifications (almost confined to the pons), without evident basal ganglia, internal capsule, white matter or dentate nucleus calcifications (faint calcifications could be seen on right putamen). Brain MRI (Fig. 2) showed T2 and T2 FLAIR bilateral hyperintensity on the putamina with atrophy (striatal necrosis), with no white matter signal intensity alterations. The genetic study—WES based gene panel designed for Aicardi‐Goutières (including the analysis of MYORG gene)—identified two heterozygous, likely pathogenic, variants in ADAR1 (NM_001111.4:c.577C > G and c.3116A > G). Compound heterozygosity was confirmed by testing both parents, establishing the diagnosis of ADAR1‐related Aicardi‐Goutières syndrome (Fig. 3).
FIG 1.
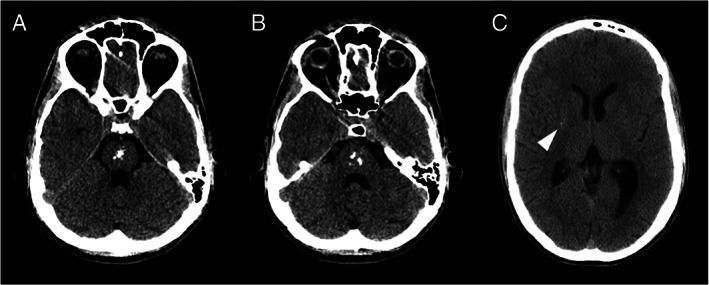
Axial CT scans showing multiple calcifications on the pons. There was a subtle calcification on right putamen (arrowhead). The remaining basal ganglia, internal capsule, white matter and dentate nucleus showed no calcification.
FIG 2.
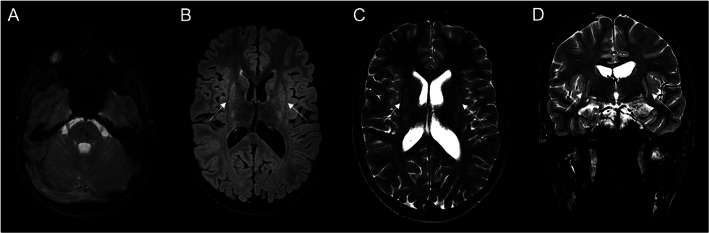
(A) Axial MRI T2*‐weighted image revealing hypointensity consistent with calcifications; (B) axial T2 FLAIR image and (C) axial T2 image showing symmetric hyperintensity in the putamina with associated atrophy; (D) coronal T2 image showing symmetric hyperintensity in the putamina with associated atrophy. No changes were present in the white matter.
FIG 3.
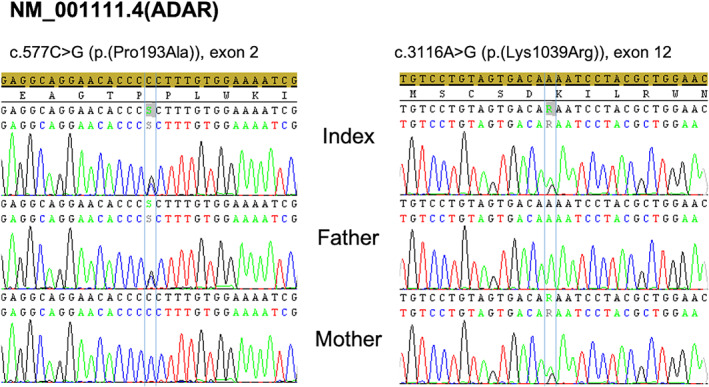
Electropherogram of the identified causative variants in the index and both parents. The c.577C > G (p.(Pro193Ala)) variant was paternally inherited, while the c.3116A > G (p.(Lys1039Arg)) was present in the mother.
The absence of white matter involvement is described in ADAR1 mutations and further help us to suggest the diagnosis based on image.1, 2
Author Roles
(1) Research Project: A. Conception, B. Organization, C. Execution; (2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique; (3) Manuscript: A. Writing of the First Draft, B. Review and Critique.
C.P.: 1A, 1C, 2A
A.F.B.: 3B
J.F.: 3B
M.M.: 1B, 3B
Disclosures
Ethical Compliance Statement
The authors confirm that the approval of an institutional review board was not required for this work. The informed consent was waived. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Funding Sources and Conflicts of Interest
No funding was used for this work. The authors declare no conflict of interest.
Financial Disclosures for the Previous 12 Months
No financial disclosures for the previous 12 months to declare.
Relevant disclosures and conflicts of interest are listed at the end of this article.
References
- 1.La Piana R, Uggetti C, Roncarolo F, et al. Neuroradiologic patterns and novel imaging findings in Aicardi‐Goutières syndrome. Neurology 2016;86(1):28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Livingston JH, Lin J‐P, Dale RC, Gill D, Brogan P, Munnich A, Crow YJ. A type I interferon signature identifies bilateral striatal necrosis due to mutations in ADAR1. J Med Genet 2013;51(2):76–82. [DOI] [PubMed] [Google Scholar]


