Abstract
We have developed an agar-based methodology for testing susceptibilities of Candida spp. to the new antifungal agent MK-0991, a glucan synthase inhibitor. Results obtained with this method correlated well with the results obtained by the National Committee for Clinical Laboratory Standards M27-A broth microdilution reference method. However, as noted with prior comparisons of broth- and agar-based systems, some isolates yielded inhibition zones which were not consistent with the MICs obtained for them. Understanding the implications of these differences will require testing in an in vivo system.
New approaches for testing the susceptibilities of fungi continue to be developed. Agar-based methods are attractive because of their simplicity and low cost (2). In addition, they may provide an enhanced detection of resistance, as is the case with the amphotericin B susceptibilities of Candida spp. (9) and Cryptococcus neoformans (6). In this study, we have explored the use of an agar-based methodology for testing the susceptibilities of Candida spp. to the new water-soluble pneumocandin, MK-0991. This agent has fungicidal activity in vitro (3) and potent activity in animal models against disseminated candidiasis and aspergillosis (1) and has been shown to enhance the activities of amphotericin B and fluconazole against C. neoformans in vitro (4).
(This work was presented in part at the 97th General Meeting of the American Society for Microbiology in 1997 [7a].)
A collection of 94 isolates belonging to three different genera was used in this study (Table 1). The collection also included two isolates of C. neoformans which were used as controls for resistance because of the known lack of susceptibility of these organisms to this class of drugs both in vitro (3) and in vivo (1). All the organisms were kept at −70°C and were passaged at least twice on Sabouraud dextrose agar at 35°C prior to being tested.
TABLE 1.
Susceptibility data obtained with the two methodologiesa
| Incubation time (h) | Species | No. of isolates | Inhibition zone (mm)
|
MIC (μg/ml)
|
||
|---|---|---|---|---|---|---|
| Mean | Range | Geo mean | Range | |||
| 24 | C. albicans | 45 | 15.9 | 14–19 | 0.44 | 0.25–4 |
| C. tropicalis | 10 | 15.2 | 13.5–17 | 0.8 | 0.5–2 | |
| C. glabrata | 10 | 16.9 | 15–18 | 1.2 | 0.5–2 | |
| C. parapsilosis | 10 | 13.2 | 9.5–16 | 2 | 0.5–>4 | |
| C. krusei | 9 | 14.6 | 12–17 | 2 | 2 | |
| C. lusitaniae | 6 | 12.7 | 11–14 | 2.6 | 1–4 | |
| C. lipolytica | 1 | 12.5 | 2 | |||
| S. cerevisiae | 1 | 13 | 2 | |||
| C. neoformans | 2 | No zone | No zone | >4 | >4 | |
| 48 | C. albicans | 45 | 15.6 | 14–19 | 0.47 | 0.25–4 |
| C. tropicalis | 10 | 14.9 | 13.5–17 | 1.1 | 0.5–4 | |
| C. glabrata | 10 | 16.9 | 15–18 | 1.7 | 0.5–2 | |
| C. parapsilosis | 10 | 12 | 9.5–16 | 2 | 2–>4 | |
| C. krusei | 9 | 14.1 | 12–17 | 2 | 2–>4 | |
| C. lusitaniae | 6 | 11.8 | 11–14 | 2.6 | 2–4 | |
| C. lipolytica | 1 | 12.5 | 2 | |||
| S. cerevisiae | 1 | 13 | 2 | |||
| C. neoformans | 2 | No zone | No zone | >4 | >4 | |
Geo mean, geometric mean MIC. No zone, no inhibition zone was seen.
A single lot of RPMI 1640 medium (lot 85H46331; Sigma Chemicals Co., St. Louis, Mo.) was used throughout the study. This culture medium was buffered with 3-(N-morpholino)propanesulfonic acid (MOPS) (lot 75H5734; Sigma Chemicals Co.) to achieve a final concentration of 0.165 M, and its pH was adjusted to 7.0. The final glucose concentration of the RPMI 1640 medium was 0.2% (2 g/liter). For the agar tests, a double-strength RPMI 1640 medium was supplemented with glucose to achieve a final concentration of 4% (40 g/liter). The pH was adjusted to 7.0, and the culture medium was then mixed with an equal volume of 30 g of agar per liter in sterile distilled water (Bacto Agar; Difco Laboratories, Detroit, Mich.). The final medium in agar thus contained 15 g of agar per liter and 20 g of glucose per liter.
Susceptibility determination was carried out two different ways. For the broth tests, the microdilution adaptation of M27-A (7) was performed by using twofold dilutions of MK-0991 at concentrations ranging from 0.0078 to 4 μg/ml. MICs were determined by measuring the optical density at 530 nm with a plate reader (model EL-310; BIO-TEK, Burlington, Vt.) after both 24 and 48 h of incubation at 35°C. The plates were agitated prior to being read, and the MIC was the lowest concentration of MK-0991 which completely inhibited fungal growth. For the agar-based methods, a diffusion disk method was used. A 0.5 McFarland suspension of each isolate (prepared per the M27-A protocol) was swabbed in three directions on RPMI 1640 medium–2% glucose agar plates. These inoculated plates were left to dry for at least 20 min, after which BBL blank paper disks (6.3-mm diameter; Becton Dickinson Microbiology Systems, Cockeysville, Md.) previously saturated with 25 μl of a solution containing 100 μg of MK-0991 per ml were placed on the plates. The final concentration was 2.5 μg/disk. This concentration was selected based on preliminary tests and yielded the best range of zone diameters of all the concentrations tested (50, 100, and 200 μg/ml). Zone diameters (in millimeters) for the zone of complete inhibition were determined after 24 and 48 h of incubation at 35°C and compared with MICs determined by the National Committee for Clinical Laboratory Standards M27-A microdilution method. Under these conditions, good growth was obtained for all tested isolates. Zone edges were sharply defined and easily determined.
Table 1 shows the activity of the compound against the 94 isolates sorted by species. A tendency towards slightly smaller zones and increased MICs was apparent after 48 h of incubation, but results after 24 and 48 h of incubation were otherwise qualitatively similar, as were the results obtained upon repeat testing of a subset of the isolates (data not shown). Consistent with previous reports (1, 3), the two C. neoformans isolates were not inhibited by MK-0991 as manifested by MICs of >4 μg/ml and no zone of inhibition.
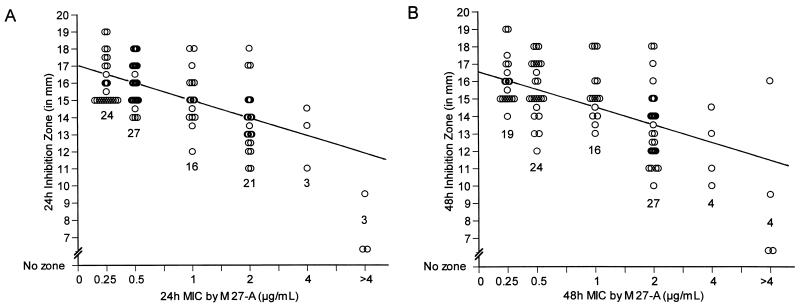
Figure 1 shows the isolate-by-isolate relationship between log10 MIC and the inhibition zones produced on agar after 24 and 48 h of incubation, respectively. For most organisms tested, the more susceptible the organism was by M27-A, the greater the inhibition zone that was produced on agar. Thus, for the isolates that had the smallest zone diameters M27-A broth MICs were always elevated compared with the median MIC. However, the converse was not always true: we noted a few isolates for which MICs were elevated that did not have small inhibition zones after either 24 or 48 h of incubation. These include one isolate of Candida glabrata for which the MIC was 2 μg/ml (24 and 48 h) but with a zone of 18 mm (24 and 48 h), one isolate of Candida krusei for which the MIC was 2 μg/ml (24 and 48 h) but with zones of 17 mm (24 h) and 16 mm (48 h), and one isolate of C. krusei for which the MICs were 2 μg/ml (24 h) and >4 μg/ml (48 h) but with zones of 17 mm (24 h) and 16 mm (48 h). In addition, for two other isolates of C. glabrata the 24-h-incubation MIC was 1 μg/ml and the 48-h-incubation MIC was 2 μg/ml, but zone diameters remained stable at 17 to 18 mm at both time points.
FIG. 1.
Correlation of broth method-determined MICs with agar inhibition zones. MK-0991 susceptibility values for 94 isolates obtained by the broth microdilution M27-A methodology versus the corresponding inhibition zones on agar are shown at 24 h (A) and 48 h (B). The line of best fit is shown, and the regression statistics are as follows: y = 14.77 − 2.96 log10 [MIC] (r = 0.59) at 24 h and y = 14.59 − 2.83 log10 [MIC] (r = 0.53) at 48 h. Shown also is the number of isolates at each MIC.
To our knowledge, this is the first report on MK-0991 to demonstrate a correlation between the in vitro susceptibility data from a broth microdilution technique and an agar-based methodology. For most isolates, zone diameters for MK-0991 on RPMI 1640 medium–2% glucose agar rose predictably as M27-A-determined MICs fell. In agreement with the work of others (5, 8), we observed that MICs varied according to species and that two distinct groups of species could be identified. The first group contained Candida albicans, Candida tropicalis, and C. glabrata and was more susceptible than the group containing Candida lusitaniae, C. krusei, and Candida parapsilosis. When analyzed with the agar zone diameter data, this same susceptibility gradient was again evident. This general pattern was seen with both the 24- and the 48-h-incubation data. MICs were consistently the highest for the C. lusitaniae isolates (geometric mean, 2.6 μg/ml at both 24 and 48 h), and these isolates had the smallest inhibition zones on agar (12.7 and 11.8 mm after 24 and 48 h, respectively), suggesting the possibility of a strain-specific reduced susceptibility to MK-0991. For one isolate each of Candida lipolytica and Saccharomyces cerevisiae MICs were also elevated and zone diameters were small, but additional isolates of these species need to be tested before any conclusions can be drawn.
In summary, we found that an MK-0991 MIC range of 0.25 to 1 μg/ml comprised 71 and 63% of all the isolates tested after 24 and 48 h, respectively. Bartizal et al. (3) recently reported a similar range for a collection of isolates which included six of the seven Candida species we present here. The range of the inhibition zones produced for this MIC range was 12 to 19 mm for both 24 and 48 h. Based on the distribution of MICs for the entire study population, isolates for which MICs and zone diameters were in this range should be judged as susceptible to MK-0991. The clinical implications of smaller zone diameters and larger MICs will require further investigation, as will the disparity between these two measures for some isolates.
Acknowledgments
This work was supported in part by a grant to M. Lozano-Chiu from the Dirección General de Investigación Científica y Enseñanza Superior of Spain.
REFERENCES
- 1.Abruzzo G K, Flattery A M, Gill C J, Kong L, Smith J G, Pikounis V B, Balkovec J M, Bouffard A F, Dropinski J F, Rosen H, Kropp H, Bartizal K. Evaluation of the echinocandin antifungal MK-0991 (L-743,872): efficacies in mouse models of disseminated aspergillosis, candidiasis, and cryptococcosis. Antimicrob Agents Chemother. 1997;41:2333–2338. doi: 10.1128/aac.41.11.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barry A L, Brown S D. Fluconazole disk diffusion procedure for determining susceptibility of Candida species. J Clin Microbiol. 1996;34:2154–2157. doi: 10.1128/jcm.34.9.2154-2157.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartizal K, Gill C J, Abruzzo G K, Flattery A M, Kong L, Scott P M, Smith J G, Leighton C E, Bouffard A, Dropinski J F, Balkovec J. In vitro preclinical evaluation studies with the echinocandin antifungal MK-0991 (L-743,872) Antimicrob Agents Chemother. 1997;41:2326–2332. doi: 10.1128/aac.41.11.2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Franzot S P, Casadevall A. Pneumocandin L-743,872 enhances the activities of amphotericin B and fluconazole against Cryptococcus neoformans in vitro. Antimicrob Agents Chemother. 1997;41:331–336. doi: 10.1128/aac.41.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krishnarao T V, Galgiani J N. Comparison of the in vitro activities of the echinocandin LY303366, the pneumocandin MK-0991, and fluconazole against Candida species and Cryptococcus neoformans. Antimicrob Agents Chemother. 1997;41:1957–1960. doi: 10.1128/aac.41.9.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lozano-Chiu M, Paetznick V L, Ghannoum M A, Rex J H. Detection of resistance to amphotericin B among Cryptococcus neoformans clinical isolates: performances of three different media assessed by using E-test and National Committee for Clinical Laboratory Standards M27-A methodologies. J Clin Microbiol. 1998;36:2817–2822. doi: 10.1128/jcm.36.10.2817-2822.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard M27-A. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 7a.Nelson P W, Lozano-Chiu M, Rex J H. Program and abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1997. A disk diffusion method for determining the susceptibility of Candida spp. to L-743,872, abstr. F-79. [Google Scholar]
- 8.Vazquez J A, Lynch M, Boikov D, Sobel J D. In vitro activity of a new pneumocandin antifungal, L-743,872, against azole-susceptible and -resistant Candida species. Antimicrob Agents Chemother. 1997;41:1612–1614. doi: 10.1128/aac.41.7.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wanger A, Mills K, Nelson P W, Rex J H. Comparison of Etest and National Committee for Clinical Laboratory Standards broth macrodilution method for antifungal susceptibility testing: enhanced ability to detect amphotericin B-resistant Candida isolates. Antimicrob Agents Chemother. 1995;39:2520–2522. doi: 10.1128/aac.39.11.2520. [DOI] [PMC free article] [PubMed] [Google Scholar]