ABSTRACT
Background
The Movement Disorder Society revision of the Unified Parkinson's Disease Rating Scale (MDS‐UPDRS) was designed to be more sensitive to mild motor severity than the Unified Parkinson's Disease Rating Scale (UPDRS).
Objective
To test whether MDS‐UPDRS Part III items provide increased sensitivity to mild motor severity when compared to the same items of the UPDRS in de novo PD patients.
Method
Using a sample of 129 de novo PD patients assessed at one time point simultaneously with both scales, we compared the scale's scores on the 17 items measuring the same motor function. The scaling anchors for the MDS‐UPDRS were Slight, Mild, Moderate and Severe, and for the UPDRS were Mild, Moderate, Severe and Marked. Using Classical Test Theory (CTT) we compared the distributions of the scaling anchors from the individual items. Using Item Response Theory (IRT), we examined the sensitivity of the scaling anchors from each scale to the latent‐trait measurement of overall parkinsonian motor severity.
Results
There was 2193 observations of individual scaling anchors from the 17 items in both scales. The CTT approach revealed frequent floor effects with only the item assessing Gait demonstrating a significance difference in the scaling distribution between the scales (P = 0.005). The IRT analyses revealed similar levels of sensitivity to the latent trait of PD motor function.
Conclusion
These results do not support increased sensitivity of MDS‐UPDRS over the UPDRS for assessing mild motor severity in de novo PD patients, with significant difference in the scaling only for the item assessing gait.
Keywords: Parkinson's disease, clinimetrics, outcome measures, severity of illness index
Accurate measurements of the progression of Parkinson's disease (PD) symptom severity and therapeutic response are necessary for the development of disease‐modifying and symptomatic therapies.1, 2, 3 The Unified Parkinson's Disease Rating Scale (UPDRS) was published in 19874 and was the most widely used scale to measure motor outcomes in PD patients.4, 5 This scale was revised by the International Parkinson and Movement Disorder Society (MDS) in 2008.6 Since that time, the MDS‐UPDRS has been the most widely used scale in various settings of clinical and research practices.7
The MDS‐UPDRS was developed with the aim of retaining the strengths of the original scale, but addressing limitations identified in the original version.8 One such limitation was a perceived insensitivity to very mild motor manifestations. It was felt that a new scaling anchor was needed to separate no motor impairment from mild motor impairment.9 For example, while the original UPDRS uses the scaling anchors “1‐ Mild, 2‐Moderate, 3‐ Severe, and 4‐ Marked,” the newer MDS‐UPDRS uses the scaling anchors “1‐ Slight, 2‐ Mild, 3‐ Moderate and 4‐ Severe.” The addition of the “1 – Slight” anchor in the MDS‐UPDRS was introduced to better detect very mild motor impairment and differentiate it from mild motor impairment.10 These differences are described in detail by Goetz et al. (2007).8, 10
Since the first clinimetric results supporting the validity of the MDS‐UPDRS were published,6 several studies have been conducted in early PD patients analyzing data acquired from one scale or other.11, 12, 13, 14 With that, some valid calibration methods have been used to compare the results from these instruments. Methods developed to convert the total score and sum of domains of the scales have been reported in the literature, as well as methods to convert individual items shared by the two versions of the scale.15, 16, 17 To date, however, no study has compared the accuracy of the scaling parameters of the motor assessment of both versions of the scale, acquired simultaneously in the same sample of de novo PD patients. This led us to hypothesize that the MDS‐UPDRS Part III (Motor Examination) may provide a more sensitive measure of early motor signs when compared to the motor portion of UPDRS in a cohort of de novo PD patients.
To prove this hypothesis, this study aimed to explore whether the MDS‐UPDRS Part III (Motor Examination) provides a more sensitive measure of very early motor signs when compared to the same part of the UPDRS in a cohort of de novo PD patients.
Methods
We analyzed data from the Study in PD of Exercise (SPARX), a multicenter study composed of a cohort of PD patients recruited for a Phase II, randomized, controlled, single‐blinded, longitudinal, three‐treatment arm clinical trial (NCT01506479). The aims and methods of the SPARX study have been published elsewhere,18 as well as its results.19, 20 During data collection, both scales were applied to each patient simultaneously by the same rater and an order for filling out the scales was not predefined.
Data and Sample
From the screened cohort of 153 subjects of the SPARX dataset with de novo PD, we limited the sample to PD subjects with complete data from the UPDRS and the MDS‐UPDRS Part III (Motor examination) at baseline, totaling 129 subjects. Unlike the original study, that evaluated 128 patients divided into three groups and different follow‐up periods, we evaluated 129 patients composing the database during the baseline. Most of them were men (57.4%), Caucasian (89.9%), right‐handed (86.8%), with a mean age 63.7 ± 9.3 (range 39–80), and median Hoehn & Yahr stage 2 (76.7%)21 (ranging from 0 to 3, where only 2.3% of the patients were classified as 3).
Data Modeling
We modeled the data so that it was possible to compare the scaling and response options of the two versions of the scale. We applied the method outlined by Goetz et al.6 for the cross‐mapping of items, scaling anchors and scores from the original UPDRS to the MDS‐UPDRS (see Supplemental S1). Considering the 27 items of the UPDRS Part III and 33 of the MDS‐UPDRS Part III, there are 20 items that can be compared across the two scales because these items measure the same motor function. For all those items, the scaling anchor for the score 0 are equivalent in both versions of the scale (meaning Normal). For three items (Facial Expression, Arising from a Chair, and Body Bradykinesia), the scaling anchors and scores are equivalent in the two versions. However, for the remaining 17 items (which are the focus of our analysis), the description of impairment associated with the anchors and scores do not match between the two scales. The specific cross‐mapping between the items of each scale can be seen in Table 1.
TABLE 1.
Cross‐mapping of items from UPDRS to MDS‐UPDRS
| Item | Cross‐mapping from UPDRS → MDS‐UPDRS |
|---|---|
| Postural Stability | 1 → 1 or 2; 2 → 3; 3 → 4 |
| Finger Tapping right/left, Hands Movement right/left, Pronation Supination right/left, Leg Agility right/left | 1 → 1 or 2; 2 → 2 or 3 |
| Posture | 2 → 2 or 3; 3 → 4 |
| Speech, Gait | 3 → 3 or 4 |
| Rigidity neck, Rigidity right/left upper extremity, Rigidity right/left lower extremity |
3 → 2; 4 → 3 4 rating on the MDS‐UPDRS is not captured by the original UPDRS |
The description of impairment associated with the anchors and scores of the 17 items and its equivalence across the two versions of the scale can be accessed in the Supplemental S2.
Statistical Analysis
We analyzed the number of observations of each response option from the sample of 129 PD subjects assessed by the 17 items with differing scaling. We applied assessment methods from Classic Test Theory (CTT) and Item Response Theory (IRT) to investigate potential sensitivity differences of the items.
CTT Approach
Using SPSS® Statistics version 26, we first assessed the occurrence of floor effects for each item in the two scales. We then applied two nonparametric tests to assess the potential for differential scaling effects: 1‐ Kolmogorov Smirnov test (KS, two sampled test) to verify the equality of distributions of the response options in both versions of the scale; and 2‐ Kendall rank correlation coefficient (K‐b) to measure the classification correlation, that is, the similarity of the data ordering when classified by each version of the scale.
IRT Approach
Using the multidimensional IRT mirt R statistical program (R Foundation for Statistical Computing, Vienna, Austria), we applied the graded‐response IRT model to determine the sensitivity of the items to the latent‐trait measurement of overall parkinsonian motor severity, termed theta, by assessing the association of a response for a person with given trait level.22
The strength of these relationships was determined by the discrimination parameter and the test information parameter. The discrimination parameter corresponds to the inverse of the residual variability of the item, where higher discrimination value means that the item is more powerful for determining the individual's overall parkinsonian motor severity. The magnitude of the discrimination parameter import can be judged using the following criteria: none = 0; very low = 0.01 to 0.34; low = 0.35 to 0.64; moderate = 0.65 to 1.34; high = 1.35 to 1.69; very high = >1.70; and perfect = +∞. 23 We computed the item information for both scales by plotting the area within the test over a definite integral theta range of −9 to 9 standard deviations. We calculate the test information based on the amount of information converted into a standard error of estimation (SE).
Results
There were a total of 2193 responses from 129 subjects assessed by the 17 items measuring the same motor function in both scales, but with different anchors and scores. Of the total, most observations of response options, with scaling anchors differing between the scales, were represented by the score 1 (19.2%). There was a sparse representation of scores of 3 and 4 across the two scales (Table 2). The average total score for Part III on UPDRS was 10.8 (SD 5.4) and 11.3 (SD 5.5) for the MDS‐UPDRS.
TABLE 2.
Distribution of observations of response options of the 17 items with scaling anchors differing between the scales (sample = 129, observations = 2193)
| Scoresa | UPDRS | MDS‐UPDRS | |
|---|---|---|---|
| nb (%) | nb (%) | Items with different scaling anchors between the scales | |
| 1 | 420 (19.2) | 421 (19.2) | Finger Tappingc, Hands Movementc, Pronation Supinationc, Leg Agilityc, Postural Stability |
| 2 | 166 (7.6) | 177 (8.1) | Finger Tappingc, Hands Movementc, Pronation Supinationc, Leg Agilityc, Postural Stability, Posture |
| 3 | 3 (0.1) | 6 (0.3) | Speech, Rigidityc, Gait, Postural Stability, Posture |
| 4 | 0 (0) | 0 (0) | Rigidityc |
| Total (n = 2193, 100%) | 639 (29.1) | 652 (29.7) |
The scaling anchor for the score 0 is equivalent in both scales (0 = Normal).
Number and the percentage of observations from the UPDRS/MDS‐UPDRS items, with different scaling anchors between the scales, mapped in each category of score.
Items assessing different parts and/or sides of the body (eg, arms/leg/neck, left/right).
CTT Approach
A high floor effect (>15%) was evidenced for 16 items, resulting in a substantial percentage of subjects rated as “Normal” (UPDRS: 51.7%, MDS‐UPDRS: 50.2%) (Table 3).
TABLE 3.
Heatmap of items and scores correlation after conceptual cross‐mapping from the original UPDRS to the MDS‐UPDRS (n = 129 patients)
| Version of the scale | UPDRS | P‐value | MDS‐UPDRS | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Score Gradation/Items | 0 | 1 | 2 | 3 | 4 | K‐b | KS | 0 | 1 | 2 | 3 | 4 |
| Postural Stability | 89.1 | 10.9 | 0 | 0 | 0 | 0.856 | 1 | 89.9 | 9.3 | 0.8 | 0 | 0 |
| Rigidity RLE | 83.7 | 14.7 | 1.6 | 0 | 0 | 0.87 | 1 | 82.9 | 15.5 | 1.6 | 0 | 0 |
| Rigidity LLE | 82.2 | 10.9 | 7 | 0 | 0 | 0.833 | 1 | 82.9 | 11.6 | 5.4 | 0 | 0 |
| Gait | 73.6 | 25.6 | 0.8 | 0 | 0 | 0.005 | 0.066 | 57.4 | 41.1 | 1.6 | 0 | 0 |
| Leg Agility Right | 71.3 | 26.4 | 2.3 | 0 | 0 | 0.546 | 1 | 68.2 | 27.9 | 3.9 | 0 | 0 |
| Rigidity Neck | 61.2 | 32.6 | 6.2 | 0 | 0 | 0.884 | 1 | 61.2 | 30.2 | 8.5 | 0 | 0 |
| Posture | 58.9 | 38.8 | 2.3 | 0 | 0 | 0.918 | 1 | 58.9 | 37.2 | 3.9 | 0 | 0 |
| Leg Agility Left | 55.8 | 36.4 | 7 | 0.8 | 0 | 0.918 | 1 | 55 | 37.2 | 7.8 | 0 | 0 |
| Rapid Alternating Movements of Hands RH | 48.8 | 38.8 | 10.9 | 1.6 | 0 | 0.895 | 1 | 34.1 | 42.6 | 20.9 | 2.3 | 0 |
| Speech | 45 | 46.5 | 8.5 | 0 | 0 | 0.913 | 1 | 44.2 | 47.3 | 8.5 | 0 | 0 |
| Hand Movements Right | 41.9 | 44.2 | 13.2 | 0.8 | 0 | 0.826 | 1 | 40.3 | 45.7 | 12.4 | 1.6 | 0 |
| Rapid Alternating Movements of Hands LH | 40.3 | 40.3 | 15.5 | 3.9 | 0 | 0.509 | 1 | 36.4 | 41.9 | 17.8 | 3.9 | 0 |
| Hand Movements Left | 33.3 | 43.4 | 20.9 | 2.3 | 0 | 0.929 | 1 | 48.8 | 37.2 | 11.6 | 2.3 | 0 |
| Finger Taps Left | 29.5 | 35.7 | 31.8 | 3.1 | 0 | 0.761 | 1 | 27.9 | 36.4 | 31 | 4.7 | 0 |
| Rigidity LUE | 29.5 | 34.9 | 34.1 | 1.6 | 0 | 0.914 | 1 | 30.2 | 35.7 | 30.2 | 3.9 | 0 |
| Finger Taps Right | 23.3 | 49.6 | 24.8 | 2.3 | 0 | 0.73 | 1 | 22.5 | 48.1 | 27.1 | 2.3 | 0 |
| Rigidity RUE | 11.6 | 45.7 | 41.9 | 0.8 | 0 | 0.756 | 1 | 11.6 | 43.4 | 44.2 | 0.8 | 0 |
| Total (n = 2193, 100%) | 1134(51.7%) | 742(33.8%) | 295(13.5%) | 0(0%) | 0(0%) | 1100(50.2%) | 759(34.6%) | 306(14%) | 28(1.3%) | 0(0%) | ||
Each cell in the table shows the percentage of patients rated at the given score (column) for the given item (raw). Darker fill colors indicate higher percentages. K‐b, Kendall's tau‐b; KS, Kolmogorov Smirnov; RLE, right lower extremity; LLE, left lower extremity; RH, right hand; LH, left hand; LUE, left upper extremity; RUE, right upper extremity.
Comparing the distribution of scaling anchors for each item between the two scales demonstrated that the item assessing gait was the only one that demonstrated a significant difference (P = 0.005, for K‐b), represented by patients who had a score of 0 (normal) in the UPDRS and who were classified as 1 (Slight: Independent walking with minor gait impairment) with the MDS‐UPDRS. Analyzing the equality of distributions of the response options (measured by KS) revealed no significant difference between the items assessed by both versions of the scales (Table 3).
IRT Approach
The graded response model for the 17 items of Part III required for the UPDRS 106 iterations (goodness‐of‐fit: Log‐Lik: −1713.154, Max‐Change: 0.00009) and for the MDS‐UPDRS 114 iterations (goodness‐of‐fit: Log‐Lik: −1852.484, Max‐Change: 0.00010).
The discrimination parameters are presented in Table 4. The discrimination parameters were “very high” for items of both scales measuring Rigidity Neck. The parameters were “moderate” for items of both scales measuring Speech, Posture and Rigidity of Right Upper Extremity.
TABLE 4.
Discrimination and item location parameters for the 17 items with scaling anchors differing between the scales (sample = 129, observations = 2193)
| UPDRS | MDS‐UPDRS | Scale that best discriminates the item | ||
|---|---|---|---|---|
| Items | Discrim | Items | Discrim | |
| Rigidity LLE | 4.765VH | Rigidity LLE | 0.981M | UPDRS |
| Rigidity LUE | 3.206VH | Rigidity LUE | 0.518L | UPDRS |
| Rigidity Neck | 1.733VH | Rigidity Neck | 2.163VH | Both equivalent |
| Finger Taps Left | 1.528H | Finger Tapping Left | 0.431L | UPDRS |
| Rapid Alternate Movement of Hands LH | 1.526H | Pronation Supination LH | 0.332VL | UPDRS |
| Gait | 1.447H | Gait | 0.77M | UPDRS |
| Rigidity RLE | 1.425H | Rigidity RLE | 1.919VH | MDS‐UPDRS |
| Leg Agility Left | 0.912M | Leg Agility Left | 0.262VL | UPDRS |
| Stability | 0.788M | Postural Stability | 0.555L | UPDRS |
| Speech | 0.696M | Speech | 0.677M | Both equivalent |
| Posture | 0.669M | Posture | 1.198M | Both equivalent |
| Rigidity RUE | 0.652M | Rigidity RUE | 1.199M | Both equivalent |
| Leg Agility Right | 0.587L | Leg Agility Right | 1.181M | MDS‐UPDRS |
| Hands Movement Left | 0.526L | Hands Movement Left | −0.077VL | UPDRS |
| Rapid Alternate Movement of Hands RH | 0.466L | Pronation Supination RH | 1.76VH | MDS‐UPDRS |
| Finger Taps Right | 0.458L | Finger Tapping Right | 2.2VH | MDS‐UPDRS |
| Hands Movement Right | 0.022VL | Hands Movement Right | 1.517H | MDS‐UPDRS |
VL, 0.01 to 0.34; L, 0.35 to 0.64; M, 0.65 to 1.34; H, 1.35 to 1.69; VH, >1.70; RLE, right lower extremity; LLE, left lower extremity; RH, right hand; LH, left hand; LUE, left upper extremity; RUE, right upper extremity.
The UPDRS demonstrated a stronger relationship to the individual's parkinsonian motor severity latent trait for eight items, most of them assessing the left side of the body: Rigidity Left Lower and Upper Extremities, Finger Taps Left, Rapid Alternating Movement of Left Hands, Gait, Leg Agility Left, Stability, Hands Movement Left. The MDS‐UPDRS demonstrated a stronger relationship for the remaining five items, all of them assessing the right side of the body: Rigidity Right Lower Extremity, Leg Agility Right, Pronation Supination Right Hand, Finger Tapping Right, and Hands Movement Right.
The information parameter (info) was slightly higher (1.4%) for the UPDRS (info: 36.68995) compared to the MDS‐UPDRS (info: 36.18238), with equal standard errors (SE) estimations and reliability between the two versions (SE of 5.6; reliability of 0.97) (Fig. 1).
FIG. 1.
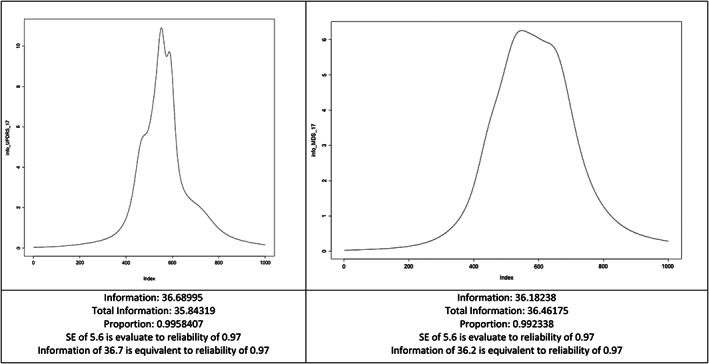
Test information of the 17 items with scaling anchors differing between the scales (bounds from −9 to 9). SE, Standard Error of Estimation.
Discussion
The clinical and scientific advances achieved in more than a decade of using the MDS‐UPDRS are undeniable, being this is the preferred modern tool for measuring PD deficiency.
Since its launch in 2008, the MDS‐UPDRS has been used in disease modification trials, which traditionally recruit cohorts of PD patients in the early stages of the disease. In some of these cohorts, outcomes are measured using data derived from the two scales, while in others, outcomes are derived from one or the other. Through these cohorts, it has been possible to characterize the worsening of PD symptoms in variable combinations and rates of progression, estimating the rate of motor and non‐motor clinical progression,11 calculating the impact of laterality on disease progression,12 analyzing the potentially different phenotypic types of PD patients,13 and investigating whether the baseline PD subtypes behave as clinical predictors of the rate of progression.14 When reviewing the results of these studies, the greatest challenge is to understand how these results could be compared when analyzed together, across the two versions of the scale, to improve the efficiency in the analysis of the PD trials.
In order to address this challenge, Gottipati et al.16 employed an IRT approach, based on the Rasch measurement theory (unidimensional model), to combine longitudinal and baseline information from the two versions of the scale in a cohort composed of de novo PD patients. With this model, they successfully estimated, for matching items and non‐matching items, the parameters that reflect the agreement between the items of the scales. They found that the mapping was more valid for individuals with early as opposed to advanced PD.16 Similar findings have been observed both in heterogeneous PD populations6 and in the de novo PD population,13 although in this last population they did not use the graded response model of IRT, a model that best fits the analysis of polytomous categories.
Although our results are unable to confirm the hypothesis of this study, we can add important information to the measurement properties of MDS‐UPDRS Part III, which have not been captured in previous clinimetric analyses in patients with early‐stage PD.6, 15, 17
Through the CTT approach, we demonstrated that, even for the scaling‐gradation scores of items not matching in the cross‐mapping, a true correlation was found between the distribution of response options in both versions of the scale, with similarity in the ordering of the data and prevalence of subjects rated as “Normal.” This high floor effect suggests that the items from both scales mostly reflect levels of severity of motor signs that are not experienced by de novo PD patients.
Only the item measuring gait showed significant differences in ordering of scores between the two versions. The major difference was represented mainly by subjects who had a score of 0 (normal) in the UPDRS and who were mainly classified as 1 (Slight: Independent walking with minor gait impairment) on the MDS‐UPDRS. According to the calibration method, the scaling‐gradation score 1 on the UPDRS (1 = Walks slowly, may shuffle with short steps, but no festination (hastening steps) or propulsion) has the same conceptual meaning on the MDS‐UPDRS.6 This significant difference may have occurred because, although there is a true conceptual comparison of the scaling‐gradation score 1 between the two scales, the description of the item's anchor in the MDS‐UPDRS better captures the very mild gait abnormality in de novo PD patients. Despite this, the IRT analysis showed that the item “Gait” has better discrimination parameter in UPDRS (High: 1.447) when compared to the same item in MDS‐UPDRS (Moderate: 0.77).
The IRT graded response model tested in this study also showed that, in general, the scales are equivalent and highly reliable for measuring the severity of motor symptoms in de novo PD patients. Our analysis demonstrated empirically that the items in Part III reflect a different clinical hierarchy between both scales. While the UPDRS demonstrated a stronger relationship to parkinsonian motor severity latent trait for items assessing the left side of the body, the MDS‐UPDRS demonstrated a stronger relationship for items assessing the right side of the body, the dominant side of this sample (composed of 87.6% right‐handed). The finding of closer association between motor severity and UPDRS scores of items assessing left side was previously found also by Martinez‐Martin et al. (1994).24 At that time, the investigators had no evidence to explain this association. In this study, we would empirically say that this relationship can be attributed to the fact that, in the MDS‐UPDRS the raters tend to test the motor signs on the right side of the patient's body before testing the left side, following the order of the instructions explicit in the MDS‐UPDRS, but not explicit in the UPDRS. However, further research is needed to prove this hypothesis.
In conclusion, we found that the calibration methodology between two versions of the scale does not reflect increased sensitivity of the MDS‐UPDRS, over the UPDRS, when applied to a cohort of de novo PD patients. It is unclear if we would find the same results in more advanced PD, and this remains an area of investigation. Additionally, our results show that both scales provide sensitivity to mild disease in de novo PD patients, with an empirical clinical hierarchy between scales.
Conclusions
The MDS‐UPDRS was designed to be more sensitive to mild motor severity than the original UPDRS. This was expected even for items shared by both scales, due to the re‐conceptualization of its scaling of the anchors (eg, adding the anchor “Slight” between “None” and “Mild”). If it had been successful, such re‐scaling should have resulted in an incompatibility of the anchor distributions between the two scales, as they were applied at the same time in the same sample of de novo PD patients, which was not seen in this study. A possible limitation to be considered is the effect on the order of administration of the scales, since, according to the design no order was predefined and the score in the first scale could have influenced the second score. That bias may have occurred if the evaluator was scoring her/his answers on paper side by side rather than rating one scale in entirety and then the second scale in entirety. If the physicians filled them in simultaneously, the results might have been biased towards the absence of difference. The same might also have happened if the scales would have been filled one immediately after the other. Therefore, studies with more appropriate designs are needed to assess mild disease sensitivity of MDS‐UPDRS.
The combined clinimetric results of our study do not support the increased sensitivity superiority of MDS‐UPDRS to very mild motor manifestations in de novo PD patients. Both scales are powerful tools that allow obtaining information from this population, therefore, maximizing the possibility of combined analysis of data collected in clinical trials of de novo PD patients. This finding offers a range of possibilities for further studies, as new developments in the specific area of the early stages of clinical PD evaluation are still needed.
Author Roles
(1) Research project: A. Conception, B. Organization, C. Execution; (2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique; (3) Manuscript Preparation: A. Writing of the first draft, B. Review and Critique.
M.H.S.T.: 1A, 1B, 1C, 2A, 2B, 2C, 3A
G.T.S.: 1A, 1B, 1C, 2A, 2C, 3B
C.C.: 1A, 1B, 2C, 3B
C.G.P.: 3B
D.A.H.: 1A, 1B, 2C, 3B
Disclosures
Ethical Compliance Statement
This is a study of secondary data analysis and according to the original study Clinical (Trials.gov Identifier NCT01506479), the protocol was approved by the ethics committee at e University of Colorado–Anschutz. Medical Campus; the University of Illinois at Chicago, the Rush University Medical Center; and the University of Pittsburgh. At each participating site, the patients provided written informed consent. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Funding Sources and Conflicts of Interest
No specific funding was received for this work.
and the authors declare that there are no conflicts of interest relevant to this work.
Financial Disclosures for the Previous 12 Months
MHST: Received compensation from the Section of Movement Disorders at Rush University Medical Center from contactor/consultant services associated with the project “Secondary analysis of the SPARX dataset.” GTS: Reports consulting and advisory board membership with honoraria from: Acadia, Pharmaceuticals, Adamas Pharmaceuticals, Inc., Biogen, Inc., Ceregene, Inc., CHDI Management, Inc., Cleveland Clinic Foundation, Ingenix Pharmaceutical Services (i3 Research), MedGenesis Therapeutix, Inc., Neurocrine Biosciences, Inc., Pfizer, Inc., Tools‐4‐Patients, Ultragenyx, Inc., and the Sunshine Care Foundation. GTS received grants and research from: National Institutes of Health, Department of Defense, Michael J. Fox Foundation for Parkinson's Research, Dystonia Coalition, CHDI, Cleveland Clinic Foundation, International Parkinson and Movement Disorder Society, and CBD Solutions. GTS reports honoraria from: International Parkinson and Movement Disorder Society, American Academy of Neurology, Michael J. Fox Foundation for Parkinson's Research, Food and Drug Administration, National Institutes of Health, and the Alzheimer's Association. GTS received salary from Rush University Medical Center. CC: Serves on the editorial board of Clinical Neuropharmacology and Sleep Medicine. She receives compensation/honoraria for services as a consultant or an advisory committee member: Acorda Therapeutics, Allergan, Inc; Lundbeck Ltd.; Merz Pharmaceuticals;Acadia Pharmaceuticals; Ipsen Pharmaceuticals, Jazz Pharmaceuticals, Neurocrine Biosciences Inc., Revance Therapeutic, Sunovion., EON Biopharma. She receives royalties from Cambridge, Wolters Kluwer. CGP: Receive research funding from National Institutes of Health, Patient Centered Outcomes Research Institute, and the Department of Defense. DAH: Research support from the Parkinson's Foundation, CHDI, Michael J. Fox Foundation, Anti‐Aging Foundation, Uniqure, Biohaven, Neurocrine, Fujifilm; Editorial support from the American Academy of Neurology.
Supporting information
Supplemental S1. Conceptual cross‐mapping of items and scaling anchors from the original UPDRS to the MDS‐UPDRS.
Supplemental S2. Description of impairment associated with the anchors and scores that can either be equivalent across the scales, or that can differ between the two versions (n = 17 items).
Supplemental Text S1. Members of the SPARX Study Group.
Acknowledgments
We thank all the members of the SPARX Study Group: Margaret L. Schenkman, PhD, Wendy M. Kohrt, PhD, Anthony Delitto, PhD, Deborah A. Josbeno PhD, Cory L. Christiansen, PhD, Brian D. Berman, MD, Benzi M. Kluger, MD, Edward L. Melanson, PhD, Samay Jain, MD, Julie A. Robichaud, PhD, Cynthia Poon, PhD, Daniel M. Corcos, PhD. Please see Supplemental Text S1 for more details.
Members of the SPARX Study Group are listed in the Acknowledgments.
Contributor Information
Michelle H.S. Tosin, Email: michelle_tosin@rush.edu.
the SPARX Study Group:
Margaret L. Schenkman, Wendy M. Kohrt, Anthony Delitto, Deborah A. Josbeno, Cory L. Christiansen, Brian D. Berman, Benzi M. Kluger, Edward L. Melanson, Samay Jain, Julie A. Robichaud, Cynthia Poon, and Daniel M. Corcos
References
- 1.Pires AO, Teixeira FG, Mendes‐Pinheiro B, Serra SC, Sousa N, Salgado AJ. Old and new challenges in Parkinson's disease therapeutics. Prog Neurobiol 2017;156:69–89. [DOI] [PubMed] [Google Scholar]
- 2.Espay AJ, Brundin P, Lang AE. Precision medicine for disease modification in Parkinson disease. Nat Rev Neurol 2017;13(2):119–126. [DOI] [PubMed] [Google Scholar]
- 3.Paolini Paoletti F, Gaetani L, Parnetti L. The challenge of disease‐modifying therapies in Parkinson's disease: role of CSF biomarkers. Biomolecules 2020;10(2):335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fahn S, Elton R, MotUD C. Unified Parkinson's disesae rating scale. In: Fahn S, Marsden C, Calne D, Goldstein M, eds. Recent Development in Parkinson's Disease. Florhan Park, NJ: Macmillan Health Care Information; 1987:153–164. [Google Scholar]
- 5.de Tosin MHS, Goetz CG, Luo S, Choi D, Stebbins GT. Item response theory analysis of the MDS‐UPDRS III motor examination: tremor vs nontremor items. Mov Disord 2020;35(9):1587–1595. [DOI] [PubMed] [Google Scholar]
- 6.Goetz CG, Tilley BC, Shaftman SR, et al. Movement Disorder Society‐sponsored revision of the unified Parkinson's disease rating scale (MDS‐UPDRS): scale presentation and clinimetric testing results. Mov Disord 2008;23(15):2129–2170. [DOI] [PubMed] [Google Scholar]
- 7.Rajan R, Brennan L, Bloem BR, et al. Integrated Care in Parkinson's disease: a systematic review and meta‐analysis. Mov Disord 2020;35(9):1509–1531. [DOI] [PubMed] [Google Scholar]
- 8.Goetz CG, Fahn S, Martinez‐Martin P, et al. Movement Disorder Society‐sponsored revision of the unified Parkinson's disease rating scale (MDS‐UPDRS): process, format, and clinimetric testing plan. Mov Disord 2007;22(1):41–47. [DOI] [PubMed] [Google Scholar]
- 9.Goetz CG. The unified Parkinson's disease rating scale (UPDRS): status and recommendations. Mov Disord 2003;18(7):738–750. [DOI] [PubMed] [Google Scholar]
- 10.Goetz CG. Unified Parkinson's disease rating scale (UPDRS) and Movement Disorder Society revision of the UPDRS (MDS‐UPDRS). In: Sampaio C, Goetz CG, Schrag A‐E, eds. Rating Scales in Parkinson's Disease: Clinical Practice and Research: Clinical Practice and Research. New York, NY: Oxford University Press; 2012:62–83. [Google Scholar]
- 11.Holden SK, Finseth T, Sillau SH, Berman BD. Progression of MDS‐UPDRS scores over five years in de novo Parkinson disease from the Parkinson's progression markers initiative cohort. Mov Disord Clin Pract 2018;5(1):47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baumann CR, Held U, Valko PO, Wienecke M, Waldvogel D. Body side and predominant motor features at the onset of Parkinson's disease are linked to motor and nonmotor progression. Mov Disord 2014;29(2):207–213. [DOI] [PubMed] [Google Scholar]
- 13.Regnault A, Boroojerdi B, Meunier J, Bani M, Morel T, Cano S. Does the MDS‐UPDRS provide the precision to assess progression in early Parkinson's disease? Learnings from the Parkinson's progression marker initiative cohort. J Neurol 2019;266(8):1927–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vu TC, Nutt JG, Holford NHG. Progression of motor and nonmotor features of Parkinson's disease and their response to treatment. Br J Clin Pharmacol 2012;74(2):267–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goetz CG, Stebbins GT, Tilley BC. Calibration of unified Parkinson's disease rating scale scores to Movement Disorder Society‐unified Parkinson's disease rating scale scores. Mov Disord 2012;27(10):1239–1242. [DOI] [PubMed] [Google Scholar]
- 16.Gottipati G, Berges AC, Yang S, Chen C, Karlsson MO, Plan EL. Item response model adaptation for analyzing data from different versions of Parkinson's disease rating scales. Pharm Res 2019;36(9):135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Merello M, Gerschcovich ER, Ballesteros D, Cerquetti D. Correlation between the movement disorders society unified Parkinson's disease rating scale (MDS‐UPDRS) and the unified Parkinson's disease rating scale (UPDRS) during l‐dopa acute challenge. Parkinsonism Relat Disord 2011;17(9):705–707. [DOI] [PubMed] [Google Scholar]
- 18.Moore CG, Schenkman M, Kohrt WM, Delitto A, Hall DA, Corcos D. Study in Parkinson disease of exercise (SPARX): translating high‐intensity exercise from animals to humans. Contemp Clin Trials 2013;36(1):90–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schenkman M, Moore CG, Kohrt WM, et al. Effect of high‐intensity treadmill exercise on motor symptoms in patients with de novo Parkinson disease. JAMA Neurol 2018;75(2):219–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hall DA, Moore C, Comella C. Recruitment of patients with de novo Parkinson disease: successful strategies in a randomized exercise clinical trial. Trials 2018;19(1):630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology 1967;17:427–442. [DOI] [PubMed] [Google Scholar]
- 22.R CoreTeam . R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing; 2020. [Google Scholar]
- 23.Fabozzi FJ, Focardi SM, Rachev ST, Arshanapalli BG, Hoechstoetter M. The Basics of Financial Econometrics: Tools, Concepts, and Asset Management Applications. Hoboken, NJ: John Wiley & Sons, Inc.; 2014:448. [Google Scholar]
- 24.Martínez‐Martín P, Gil‐Nagel A, Gracia LM, Gómez JB, Martínez‐Sarriés J, Bermejo F. Unified Parkinson's disease rating scale characteristics and structure. Mov Disord 1994;9(1):76–83. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental S1. Conceptual cross‐mapping of items and scaling anchors from the original UPDRS to the MDS‐UPDRS.
Supplemental S2. Description of impairment associated with the anchors and scores that can either be equivalent across the scales, or that can differ between the two versions (n = 17 items).
Supplemental Text S1. Members of the SPARX Study Group.


