ABSTRACT
Background
Movement disorders affecting the trunk remain a diagnostic challenge even for experienced clinicians. However, despite being common and debilitating, truncal movement disorders are rarely discussed and poorly reviewed in the medical literature.
Objectives
To review common movement disorders affecting the trunk and provide an approach for clinicians based on the truncal region involved (shoulder, chest, diaphragm, abdomen, pelvis, and axial disorders). For each disorder, clinical presentation, etiologic differential diagnosis, and “clinical clues” are discussed.
Conclusion
This review provides a clinically focused, practical approach to truncal movement disorders, which will be helpful for physicians in everyday practice.
Keywords: dystonia, myoclonus, axial, trunk, movement disorders
Despite being common, debilitating, and sometimes critical in deciphering disease etiology, truncal movement disorders are rarely discussed and poorly reviewed in the medical literature. The trunk makes up over 35% of body surface area, yet it is seldom formally examined in clinical movement disorder practice. Even when abnormal trunk movements are spotted, their significance may be unclear. In this review, we provide a practical, clinically focused approach to movement disorders of the trunk. We divide our discussion into a number of different sections, based on the involved truncal region, an approach that we find most useful in clinical practice (see Fig. 1). Common and important differential diagnoses are discussed in the text, whereas a more exhaustive list of diagnostic considerations is given in the Supporting Information Tables.
FIG. 1.
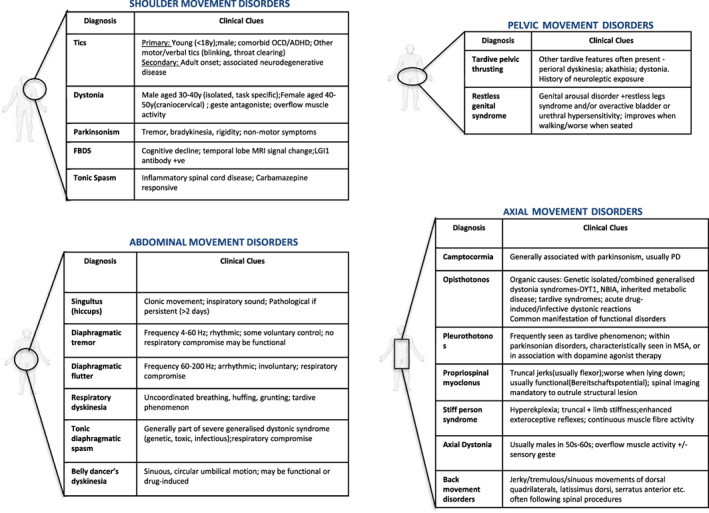
Summary of common truncal movement disorders and clinical clues suggesting the diagnosis.
Shoulder Movement Disorders
Motor tics rank high on the differential diagnosis of hyperkinetic shoulder movements, particularly in children or young adults. They may be simple (affecting single muscle groups, eg, causing intermittent elevation) or complex (resembling purposeful, goal‐directed behavior)1 and generally manifest along a rostro‐caudal gradient (preferentially involving the face, neck, and shoulders). Patients frequently have neuropsychiatric comorbidities such as attention deficit‐hyperactivity disorder (ADHD), obsessive compulsive disorders (OCD), and autism spectrum disorders (ASD).2 Motor tics may exhibit suppressibility, unpleasant premonitory urges, and relief of unpleasant sensations on the performance of the tic. These features, along with identification of other motor (eye blinks, ocular, facial movements) or vocal (sniffing, throat clearing) tics, help to seal the diagnosis.2 New onset of tics in adulthood is unusual and requires exclusion of secondary causes, particularly structural brain disease, neurodegenerative disorders (eg, Huntington's disease, neuro‐acanthocytosis, Wilson's disease, and brain iron accumulation disorders), and illicit drug use.2
Dystonia accounts for a large proportion of hyperkinetic shoulder movements in mid‐life, where involvement of shoulder elevators such as the levator scapulae or trapezius, generally as part of a craniocervical dystonic syndrome, can produce either tonic or phasic shoulder movements. Clinical clues include patient demographics (isolated craniocervical dystonia being most common in females in the fifth and sixth decades of life), hypertrophy of involved muscles, the presence of a geste antagoniste, and associated dystonia in contiguous body regions (generally the neck).3 Isolated shoulder dystonia is rare, mainly occurring as a task‐specific phenomenon following overuse4 or after a local trauma.5 Task‐specific shoulder dystonia tends to affect young males (often in their 30s), and it is described primarily in highly skilled sports people (eg, golfers, billiard, and petanque players) and musicians, but can occur in other instances following repetitive upper‐limb activities.5 Fixed dystonic shoulder posturing should raise the question of functional dystonia.
Asymmetric parkinsonism, most commonly idiopathic Parkinson's disease (PD), is the principal cause of shoulder hypokinesia. This manifests as slowed shoulder elevation on the affected side (reduced shoulder shrug to command) and is usually accompanied by other cardinal motor features (tremor, bradykinesia, and rigidity).6 Conversely, PD patients treated with levodopa can also develop hyperkinetic levodopa‐induced dyskinesia affecting the shoulder region, particularly as “peak‐dose” phenomena. However, such movements are rarely isolated to the shoulder, and the diagnosis is usually evident from the history.
Although rare, two further hyperkinetic shoulder movement disorders are worth mentioning because they are both associated with treatable neurological disorders.
Faciobrachial dystonic seizures (FBDS) are semiologically distinct, immune‐mediated focal seizure events characteristically encountered in leucine‐rich glioma inactivated‐1(LG1)‐antibody associated limbic encephalitis.7 Typically manifesting as brief (<3 seconds), frequent (median, >50/day), coordinated contractions of the face, shoulder, and less frequently leg (although they can involve the upper limb only). Their presence often precedes the development of other disease features (confusion, amnesia, seizures, hallucinations, sleep disturbances, and hyponatremia). Early treatment with immunotherapy is critical to achieve seizure control (FBDS respond poorly to antiepileptic agents) and optimize these patients' outcomes.7
Tonic spasms (TS) are brief (seconds), paroxysmal dystonic‐like spasms, which occur in the setting of spinal inflammatory lesions, most commonly multiple sclerosis (MS),8 but also others like neuromyelitis optica‐spectrum disorders (NMOSD).9 The upper limbs are most commonly affected, reflecting predilection for cervical cord involvement in MS. In contrast to primary dystonia, TS are painful and often exhibit a striking response to carbamazepine.8
Chest Movement Disorders
Isolated thoracic movement disorders are incredibly rare. The pectoral muscles can be involved in neuromyotonia, a peripheral nerve hyperexcitability syndrome producing continuous rippling muscle activity, classically associated with anti‐contactin‐associated protein 2 (CASPR2) antibodies.10, 11, 12 Neuromyotonia—the presence of which can be confirmed on electromyography (EMG)—may be isolated or part of either Isaac's syndrome (associated with muscle stiffness and hyporeflexia)11 or Morvan's syndrome (associated with dysautonomia and headache and encephalopathy).11
Neuromyotonia must not be confused with fasciculations, which can be benign, but may reflect denervation and, if seen in the pectoral region, especially alongside muscle wasting, should raise suspicion of an anterior horn cell disease. They can be differentiated both on neurophysiological grounds and clinically, with fasciculations being brief, random, and fleeting muscle twitches in contrast to the continuous rippling seen in neuromyotonia.
Stimulus‐sensitive chest muscle jerks might occur in stiff person syndrome (SPS) and progressive encephalomyelitis with rigidity and myoclonus (PERM).13 Stiffness, slow voluntary movements, muscle hypertrophy, and lumbar hyperlordosis associated with continuous motor unit activity on EMG of paraspinal muscles suggest SPS. In contrast, severe stiffness accompanied by ataxia, dysautonomia, and psychiatric features are seen in PERM.13
Diaphragmatic Movement Disorders
Diaphragmatic movement disorders can be broadly divided into tonic, clonic, and dyskinetic movements and often present with unusual abdominal movements or respiratory issues (see Table S3). The diagnostic work‐up for diaphragmatic movement disorders should include abdominal examination at rest (when lying, sitting, or standing), during specific tasks (eg, finger tapping), and with deep breathing, as deep inspiration and tasks may interrupt diaphragmatic tremor, therefore, giving a clue to the diagnosis.14 Real‐time evaluation of both hemidiaphragms using diaphragmatic videofluoroscopy, EMG, and/or ultrasonography is also suggested.14 Alongside this, features of functional (distractibility, suggestibility, entrainment) or tardive (exposures to dopamine receptor blocking drugs, akathisia, stereotypies, oro‐bucco‐lingual dyskinesia) movement disorders should be sought.
Tonic diaphragmatic spasm is rare and usually seen in the setting of specific toxidromes—in particular strychnine poisoning—or infections such as tetanus and furious rabies.15, 16, 17 In these conditions, spasms often begin in the jaw (trismus), face (risus sardonicus), and neck, spreading later to the chest, back, and abdomen (often producing striking opisthotonus). Severe, sometimes life‐threatening laryngeal and/or diaphragm spasm may ensue. Diaphragmatic spasms may also be encountered in patients with generalized dystonia syndromes (eg, dystonia‐torsin family 1 member A‐DYT‐TOR1A‐) in whom dystonic storms can be triggered by factors such as medication changes, infections, surgical procedures, or sudden discontinuation of deep brain stimulation.18
The most common clonic diaphragmatic movement, singultus (hiccup), is a universal experience. It results from disruption within the hiccup reflex arc, comprising afferent (vagus nerve), central (upper spinal cord and brainstem), and efferent (phrenic nerve) limbs. Hiccups can be triggered by irritation at any of these points. Although most often a benign, self‐terminating condition, hiccups can occasionally become persistent (>2 days) or intractable (>1 month); persistent singultus should be investigated to rule out secondary causes. The list of potential culprits is vast.19 Important differential diagnoses not to miss include brainstem (especially area postrema) vascular or inflammatory lesions, electrolyte disturbances, or cardiac diseases.19 Chronic neurological and gastrointestinal disorders also predispose to pathologic singultus. For instance, 20% of patients with PD report recurrent hiccups; interestingly, dopaminergic transmission is involved in the “hiccup arc,” perhaps explaining this phenomenon and the occasional reports of hiccups induced by dopaminergic agonists.19
Other rare clonic diaphragmatic movements include diaphragmatic tremor and diaphragmatic flutter. Both manifest with unusual abdominal movements. They are differentiated from singultus by their high frequency—4–60 Hz (tremor) and 60–200 Hz (flutter)—and absence of inspiratory sound. Espay et al.14 define diaphragmatic tremor as persistent, synchronous, rhythmic diaphragmatic contraction under some voluntary control, mainly without respiratory compromise or functional disability. In contrast, diaphragmatic flutter is an involuntary, uni‐ or bi‐diaphragmatic myoclonic diaphragmatic contraction, which produces semi‐rhythmic jerking of the abdominal wall and often associates with respiratory distress or abdominal wall pain.20, 21 Diaphragmatic tremor may sometimes have a tardive or functional etiology.20 In contrast, diaphragmatic flutter often results from brainstem lesions, mainly vascular, involving the dentate‐rubro‐olivary connections.21
Respiratory dyskinesia is usually a tardive phenomenon manifesting as dyspnea, uncoordinated breathing patterns, huffing, grunting, or forced inspiration against a closed glottis.22 A history of dopamine receptor blocking drug use, as well as other features compatible with tardive syndromes, suggest the diagnosis. Diaphragmatic dyskinesia can also be observed as a levodopa‐induced phenomenon in patients with PD. In this instance, a clear temporal association with levodopa intake is useful in making the diagnosis.23
Abdominal Movement Disorders
Choreic abdominal movements, known as “Belly dancer's dyskinesia” (BDD), refer to undulating rhythmical movements of the abdominal wall causing distressing circular rotatory umbilical motion.21 These abdominal movements, which can be transient or continuous, may superficially resemble those seen with diaphragmatic disorders, but differ from these in that they are generated from the abdominal wall proper and have a sinuous, flowing nature as opposed to the jerky movements triggered by diaphragmatic contractions.21 Half of BDD cases follow local trauma or surgical procedures on the abdomen, often without any other abnormalities on examination. Functional disorders should be considered in these patients,24 especially if movements disappear during sleep. Drug‐induced BDD has also been reported most commonly either as tardive events or levodopa‐induced phenomena in patients with PD.25 Rarely, abdominal myoclonus may be observed, generally secondary to propriospinal myoclonus (see below), or extremely rarely, because of epileptic events (abdominal motor seizures)26 (see Table S4). The abdomen may also be involved in stiff‐person spectrum disorders, producing board‐like abdominal rigidity, usually alongside other characteristic features of these disorders (see below).
Pelvic Movement Disorders
Pelvic movement disorders are rare, but if present, are almost commonly the result of dopamine receptor blocking drug administration. Tardive syndromes may comprise various stereotypies including pelvic thrusting or rocking, often alongside other tardive phenomena.27 Equally, acute drug‐induced dystonic reactions can involve the pelvis. These “tortipelvic crises” produce acute involuntary contractions of the abdominal wall, hip, and pelvic muscles.28 Functional pelvic movement disorders should also be considered particularly in the setting of thrusting/copulatory movements.29 Finally, restless genital syndrome is an unusual syndrome of intrusive and unwanted persistent genital or clitoral arousal in the absence of sexual desire. It is diagnosed when a person meets the criteria for genital arousal disorder alongside either restless legs syndrome (RLS) and/or overactive bladder or urethral hypersensitivity. It worsens while sitting, improves while walking, and seems related to small‐fiber sensory neuropathy of the dorsal nerve of the clitoris. In some cases, just like RLS, it may respond to dopamine agonist (DA) therapy.30
Axial Truncal Movement Disorders
Most axial movement disorders are ones of tonic postural abnormality, with one important exception‐propriospinal myoclonus.
Postural disorders are classified according to the dominant direction of movement into camptocormia (from the Greek, meaning “to bend”, κάμπτω, kamptō, and “trunk”, κόρμος, kormos), opisthotonus (from the Greek, “tension”,Τάση, tonos, coming from “behind”, όπιστο, opistho) and pleurothotonus or Pisa syndrome (also from the Greek, “tension”, Τάση, thotonus, coming from “sideways”, πλευρόϑεν, pleuro).
Important features to assess during their evaluation include: (1) current and previous medication exposure—the development of camptocormia or Pisa syndrome in patients with PD may be associated with DA therapy;31 moreover, abnormal truncal postures are frequent manifestations of tardive syndromes; (2) whether the abnormal movement is fixed or mobile‐ fixed postural abnormality may point to a structural spinal disease or a functional etiology; and (3) triggering and relieving factors: camptocormic posturing should improve significantly on lying down. In contrast, spinal deformities (vertebral fractures, ankylosing spondylitis, and acquired degenerative spondyloarthropathy), and functional movements usually persist. Overflow muscle activity, for example, during ambulation, may point to a dystonic disorder.
Presence of other neurological signs, particularly parkinsonism, muscle weakness and/or atrophy (suggesting), and hyperekplexia., suggests other neurological disorders (eg. PD, anterior horn cell diseases or myopathy).32
Camptocormia
Camptocormia describes a marked (>45°) forward flexion of the thoracolumbar spine in the sagittal plane when standing or walking, which improves with supine positioning.31 It is generally encountered as a part of neurodegenerative disorders, dystonic syndrome, or muscular disorders.33, 34
The commonest cause of camptocormia is PD, wherein the risk may be influenced by sex, disease duration, previous DA exposure, and ethnicity.35 Camptocormia may also occur in atypical parkinsonian and other neurodegenerative disorders.36
Isolated axial dystonic camptocormia is rare. It most commonly affects men in their fifth decade, with forward flexion being the dominant phenotype. Clues to a primary dystonic etiology include overflow muscle activity (causing worsening with action, eg, walking or exercising) and the presence of a sensory geste.37 Most cases are refractory to oral pharmacotherapies, but a response to botulinum toxin or deep brain stimulation may be seen.38
Paraspinal muscle weakness secondary to inherited/acquired myopathies, anterior horn cell disease, or neuromuscular junction disorders may also produce camptocormia. Compensatory movements to overcome truncal weakness in such instances have been misconstrued as sensory tricks, leading to misdiagnoses as axial dystonia.39 Clinical clues include weakness of other muscles, difficulty extending the trunk when prone, difficulty performing a “sit up”, or family history of muscle disease. Spinal imaging, paraspinal EMG, and occasionally muscle biopsy may be necessary where there is diagnostic uncertainty.32
Structural and degenerative spinal diseases may produce forward truncal flexion, although, in contrast to “true” camptocormia, this will not improve with supine positioning.32 (See expanded differential diagnosis on Table S4).
Opisthotonus
Opisthotonus is a critical clue in movement disorder practice, because it carries a limited differential diagnosis that can be further distilled by considering the timing of onset, predominant spinal segment affected, and presence of other neurological and/or systemic signs.
Acute onset (over hours/days) should raise concern for infectious, drug‐induced, and functional etiologies. Tetanus and rabies are the most common infectious causes. In tetanus, jaw and neck spasms are often the first symptoms, but progression to global back spasms resulting in a fixed back‐arching often occurs.15 In rabies, the spasms are intermittent and provoked by water ingestion.17 Drug‐induced acute dystonic reactions most commonly occur in young men following the administration of dopamine antagonists. Risk factors include previous acute dystonic reactions and recent cocaine use. In addition to truncal posturing, oculogyric crises are also characteristic. “Awake convulsions,” characterized by episodic muscle spasms without loss of consciousness, occur within 15–30 minutes of strychnine exposure.18
A more subacute/chronic evolution over weeks or months suggests neurodegenerative, autoimmune, or tardive etiologies.40 The predominant location of the posturing may give a further clue. The predominant involvement of the upper back and neck (retrocollis) often occurs in tardive dystonia.41 Conversely, SPS predominantly affects the lumbar segments, leading to inability to bend forward and touch the toes and an exaggerated lumbar crease. In contrast to dystonia, SPS postures are fixed, do not improve on recumbency, do not demonstrate sensory gestes, and have other associated features, including hyperekplexia and “freezing‐like episodes” while walking.41 Additionally, neurophysiologic testing shows continuous motor unit activity and enhanced exteroceptive reflexes; anti‐glutamic acid decarboxylase (anti‐GAD) or anti‐glycine antibodies are frequently detected.42
Opisthotonic posturing is a classic manifestation of disorders of neurodegeneration with brain iron accumulation (NBIA), but can also be encountered in other isolated and combined dystonia syndromes. Additional oromandibular dystonia, pyramidal signs, and cognitive decline are important red flags for NBIA, especially pantothenate kinase 2 (PANK2) and phospholipase A2 group VI (PLA2G6).40
Pleurothotonus
Pleurothotonus or Pisa Syndrome is defined as >10° lateral spinal flexion on standing, relieved almost completely by passive mobilization or supine positioning, especially common in multiple system atrophy (MSA).31, 43 Approximately 10% of PD patients will also develop Pisa syndrome, with DA use being a risk factor. Tardive pleurothotonus is commonly described, and rarely can it be an isolated phenomenon without discernible cause.34
Notably, all 3 postural truncal abnormalities, although classically opisthotonus, can be a manifestation of functional neurological disease. In this case, it often appears suddenly, sometimes triggered by physical and/or emotional events, demonstrates variability in phenomenology, distractibility,44, 45 and is more likely to be fixed than mobile.45
Propriospinal Myoclonus
Propriospinal myoclonus is a peculiar disorder characterized by painless, arrhythmic, usually flexor (but occasionally extensor) jerks, which begin axially, usually in thoracic spinal segments, and propagate rostro‐caudally to the neck, hips, and knees.46 Approximately half of the cases will demonstrate some position dependency, with the usual pattern being worsening of jerks when lying down or sitting.28, 46 Jerks may be provoked by tactile stimuli on the chest or abdomen, by eliciting tendon reflexes, but generally not in response to auditory stimuli (the latter being more suggestive of brainstem myoclonus). Middle‐age men are most commonly affected, and the majority of cases have a functional etiology. The rare organic cases result from spinal cord pathology (most commonly thoracic disc herniations), and spinal imaging is, therefore, recommended in all cases. Acute onset, distractibility, variability, spread of jerks to the face, disappearance during sleep, and the presence of bereitschaftpotentials (BP) on jerk‐locked back‐averaging of electroencephalography (EEG)–EMG recordings are further clues to a functional etiology.46
Movement Disorders of the Back
Rarely, movement disorders can be restricted to other regions of the back. These may occur following spinal instrumentation or peripheral nerve trauma and assume diverse phenotypes, including “dancing dorsal quadrilaterals,” peri‐scapular movements, and various jerky, sinuous or tremulous movements of serratus anterior, latissimus dorsi, or trapezius following local nerve injuries.47, 48, 49
Conclusion
Truncal movement disorders are a neglected area of movement disorder practice, which has received little traction in the field. Yet, a systematic approach to their evaluation, focusing on movement disorder phenomenology, the portion of the trunk affected, and the presence or absence of other diagnostic “clues” (Tables S1–S4), can rapidly narrow the differential diagnosis. Age of onset is always an important consideration. Indeed, tics and inherited metabolic disorders are particularly prevalent in young individuals, whereas others are primarily of older age‐ eg. degenerative diseases, tardive syndromes. The tempo of progression is also important to ascertain. Acute or subacute onset suggests infectious, toxic, autoimmune, or sometimes functional etiologies, whereas slow progression suggests degenerative disorders. Tardive etiologies enter the differential diagnosis of many abnormal truncal movements; a history of exposure to dopamine receptor blocking medications, either through prescription, poisoning, traditional or herbal medicines, must always be sought. Similarly, functional movement disorders should always be kept in mind, especially when other suggestive clues (distractibility, entrainability, history of joint hypermobility, and/or postural orthostatic‐tachycardia syndrome) are present when this is the most common cause of the syndrome, for example, propriospinal myoclonus, or when the presentation is incongruent with organic diseases. Such clinical approaches will help clinicians rapidly narrow diagnostic possibilities and apply diagnostic work‐up, and in some cases, treatment, accordingly.
Author Roles
(1) Research project: A. Conception, B. Organization, C. Execution; (2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique; (3) Manuscript Preparation: A. Writing of the First Draft, B. Review and Critique.
F.C.: 1A, 1B, 1C, 3A
V.C.: 1A, 1B, 1C, 3A
C.G.: 1A, 1B, 1C, 3A
E.M.: 1A, 1B, 1C, 3B
K.P.B.: 1A, 1B, 1C, 3B
Disclosures
Ethical Compliance Statement
The authors confirm that the approval of an institutional review board was not required for this work. Informed patient consent was not necessary for this work. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Funding Sources and Conflicts of Interest
No specific funding was received for this work. The authors declare that there are no conflicts of interest relevant to this work.
Financial Disclosures for the Previous 12 Months
F.C. reports no disclosures. V.C. reports no disclosures. C.G. reports no disclosures. K.P.B. holds research grants from EU Horizon 2020 and has received honoraria to speak at meetings or attend advisory boards from Ipsen, Cavion, Allergan, Teva Lundbeck, and Bial pharmaceutical companies. He also receives royalties from Oxford University Press and a stipend for Movement Disorders Clinical Practice (MDCP) editorship. E.M. is supported by the Edmond J. Safra Foundation and the National Institute for Health Research University College London Hospitals Biomedical Research Centre.
Supporting information
Table S1. Disorders causing hyperkinetic shoulder movements
Table S2. Differential diagnosis in thoracic movement disorders
Table S3. Differential diagnosis of diaphragmatic, abdominal, and pelvic movement disorders
Table S4. Differential diagnosis of axial truncal movement disorders
Relevant disclosures and conflicts of interest are listed at the end of this article.
References
- 1.Cath DC, Hedderly T, Ludolph AG, Stern JS, Murphy T, Hartmann A, et al. European clinical guidelines for Tourette syndrome and other tic disorders. Part I: assessment. Eur Child Adolesc Psychiatry 2011;20(4):155–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ganos C, Martino D. Tics and Tourette syndrome. Neurol Clin 2015;33(1):115–136. [DOI] [PubMed] [Google Scholar]
- 3.Phukan J, Albanese A, Gasser T, Warner T. Primary dystonia and dystonia‐plus syndromes: clinical characteristics, diagnosis, and pathogenesis. Lancet Neurol 2011;10(12):1074–1085. [DOI] [PubMed] [Google Scholar]
- 4.Lagueny A, Burbaud P, Dubos JL, Le Masson G, Guelh D, Macia F, et al. Freezing of shoulder flexion impeding boule throwing: a form of task‐specific focal dystonia in petanque players. Mov Disord 2002;17(5):1092–1095. [DOI] [PubMed] [Google Scholar]
- 5.Wright RA, Ahlskog JE. Focal shoulder‐elevation dystonia. Mov Disord 2000;15(4):709–713. [DOI] [PubMed] [Google Scholar]
- 6.Riley D, Lang AE, Blair RD, Birnbaum A, Reid B. Frozen shoulder and other shoulder disturbances in Parkinson's disease. J Neurol Neurosurg Psychiatry 1989;52(1):63–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Irani SR, Michell AW, Lang B, et al. Faciobrachial dystonic seizures precede Lgi1 antibody limbic encephalitis. Ann Neurol 2011;69(5):892–900. [DOI] [PubMed] [Google Scholar]
- 8.Ostermann PO, Westerberg CE. Paroxysmal attacks in multiple sclerosis. Brain 1975;98(2):189–202. [DOI] [PubMed] [Google Scholar]
- 9.Kim SM, Go MJ, Sung JJ, Park KS, Lee KW. Painful tonic spasm in neuromyelitis optica: incidence, diagnostic utility, and clinical characteristics. Arch Neurol 2012;69(8):1026–1031. [DOI] [PubMed] [Google Scholar]
- 10.Maddison P. Neuromyotonia. Clin Neurophysiol 2006;117(10):2118–2127. [DOI] [PubMed] [Google Scholar]
- 11.Ahmed A, Simmons Z. Isaacs syndrome: a review. Muscle Nerve 2015;52(1):5–12. [DOI] [PubMed] [Google Scholar]
- 12.Gutmann L, Libell D, Gutmann L. When is myokymia neuromyotonia? Muscle Nerve 2001;24(2):151–153. [DOI] [PubMed] [Google Scholar]
- 13.Duddy ME, Baker MR. Stiff person syndrome. Front Neurol Neurosci 2009;26:147–165. [DOI] [PubMed] [Google Scholar]
- 14.Espay AJ, Fox SH, Marras C, Lang AE, Chen R. Isolated diaphragmatic tremor. Neurology 2007;69(7):689–692. [DOI] [PubMed] [Google Scholar]
- 15.Yen LM, Thwaites CL. Tetanus. Lancet 2019;393(10181):1657–1668. [DOI] [PubMed] [Google Scholar]
- 16.Otter J, D'Orazio JL. Strychnine Toxicity. [Internet]. Treasure Island, FL: StatPearls Publishing; 2019. [PubMed] [Google Scholar]
- 17.Hemachudha T, Ugolini G, Wacharapluesadee S, Sungkarat W, Shuangshoti S, Laothamatas J. Human rabies: neuropathogenesis, diagnosis, and management. Lancet Neurol 2013;12(5):498–513. [DOI] [PubMed] [Google Scholar]
- 18.Termsarasab P, Frucht SJ. Dystonic storm: a practical clinical and video review. J Clin Mov Disord 2017;4:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steger M, Schneemann M, Fox M. Systemic review: the pathogenesis and pharmacological treatment of hiccups. Aliment Pharmacol Ther 2015;42(9):1037–1050. [DOI] [PubMed] [Google Scholar]
- 20.Gupta HV. Teaching video neuroimages: tardive diaphragmatic tremor. Neurology 2020;94(6):e656. [DOI] [PubMed] [Google Scholar]
- 21.Iliceto G, Thompson PD, Day BL, Rothwell JC, Lees AJ, Marsden CD. Diaphragmatic flutter, the moving umbilicus syndrome, and "belly dancer's" dyskinesia. Mov Disord 1990;5(1):15–22. [DOI] [PubMed] [Google Scholar]
- 22.Savitt D, Jankovic J. Tardive syndromes. J Neurol Sci 2018;389:35–42. [DOI] [PubMed] [Google Scholar]
- 23.Xie T, Guan R, Staisch J, Towle VL, Warnke P. Respiratory dyskinesia in a patient with Parkinson disease successfully treated with STN DBS. Neurology 2015;85(5):479–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cho HJ, Panyakaew P, Srivanitchapoom P, Hallett M. A case of functional belly dancer's dyskinesia. Mov Disord Clin Pract 2015;3(3):306–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carecchio M, Collini A, Comi C, Cantello R, Bhatia KP, Monaco F. Levodopa‐induced belly dancer's dyskinesias in Parkinson's disease: report of one case. Mov Disord 2010;25(11):1760–1762. [DOI] [PubMed] [Google Scholar]
- 26.Lizarraga KJ, Serrano EA, Tornes L, Kanner AM, Lang AE. Isolated abdominal motor seizures of mesial parietal origin: epileptic belly dancing? Mov Disord Clin Pract 2019;6(5):396–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mulroy E, Balint B, Bhatia KP. Tardive syndromes. Pract Neurol 2020;20(5):368–376. [DOI] [PubMed] [Google Scholar]
- 28.Lewis K, O'Day CS. Dystonic Reactions. Treasure Island, FL: StatPearls Publishing; 2020. [PubMed] [Google Scholar]
- 29.Hopp JL. Nonepileptic episodic events. Continuum 2019;25(2):492–507. [DOI] [PubMed] [Google Scholar]
- 30.Sforza E, Hupin D, Roche F. Restless genital syndrome: differential diagnosis and treatment with pramipexole. J Clin Sleep Med 2017;13(9):1109–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barone P, Santangelo G, Amboni M, Pellecchia MT, Vitale C. Pisa syndrome in Parkinson's disease and parkinsonism: clinical features, pathophysiology, and treatment. Lancet Neurol 2016;15(10):1063–1074. [DOI] [PubMed] [Google Scholar]
- 32.Margraf NG, Wrede A, Deuschl G, Schulz‐Schaeffer WJ. Pathophysiological concepts and treatment of camptocormia. J Parkinsons Dis 2016;6(3):485–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Srivanitchapoom P, Hallett M. Camptocormia in Parkinson's disease: definition, epidemiology, pathogenesis and treatment modalities. J Neurol Neurosurg Psychiatry 2016;87(1):75–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bhatia KP, Quinn NP, Marsden CD. Clinical features and natural history of axial predominant adult onset primary dystonia. J Neurol Neurosurg Psychiatry 1997;63(6):788–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jankovic J, Tintner R. Dystonia and parkinsonism. Parkinsonism Relat Disord 2001;8(2):109–121. [DOI] [PubMed] [Google Scholar]
- 36.Boesch SM, Wenning GK, Ransmayr G, Poewe W. Dystonia in multiple system atrophy. J Neurol Neurosurg Psychiatry 2002;72(3):300–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ehrlich DJ, Frucht SJ. The phenomenology and treatment of idiopathic adult‐onset truncal dystonia: a retrospective review. J Clin Mov Disord 2016;3:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lizarraga KJ, Fasano A. Effects of deep brain stimulation on postural trunk deformities: a systematic review. Mov Disord Clin Pract 2019;6(8):627–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Doherty KM, Noyce AJ, Silveira‐Moriyama L, Nisbet A, Quinn N, Lees AJ. Familial camptocormia: from dystonia to myopathy. J Neurol Neurosurg Psychiatry 2012;83(3):350–351. [DOI] [PubMed] [Google Scholar]
- 40.Stamelou M, Lai SC, Aggarwal A, et al. Dystonic opisthotonus: a “red flag” for neurodegeneration with brain iron accumulation syndromes? Mov Disord 2013;28(10):1325–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Balint B, Meinck HM, Bhatia KP. Axial dystonia mimicking stiff person syndrome. Mov Disord Clin Pract 2016;3(2):176–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Balint B, Meinck HM. Pragmatic treatment of stiff person spectrum disorders. Mov Disord Clin Pract 2018;5(4):394–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lehmann Urban D, Motlagh Scholle L, Alt K, Ludolph AC, Rosenbohm A. Camptocormia as a novel phenotype in a heterozygous POLG2 mutation. Diagnostics 2020;10(2):68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ganos C, Edwards MJ, Bhatia KP. The phenomenology of functional (psychogenic) dystonia. Mov Disord Clin Pract 2014;1(1):36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Espay AJ, Lang AE. Phenotype‐specific diagnosis of functional (psychogenic) movement disorders. Curr Neurol Neurosci Rep 2015;15(6):32. [DOI] [PubMed] [Google Scholar]
- 46.van der Salm SM, Erro R, Cordivari C, Edwards MJ, Koelman JH, van den Ende T, et al. Propriospinal myoclonus: clinical reappraisal and review of literature. Neurology 2014;83(20):1862–1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Caviness JN, Gabellini A, Kneebone CS, Thompson PD, Lees AJ, Marsden CD. Unusual focal dyskinesias: the ears, the shoulders, the back, and the abdomen. Mov Disord 1994;9(5):531–538. [DOI] [PubMed] [Google Scholar]
- 48.Lizarraga KJ, Thompson PD, Moore HP, Mizraji G, Gershanik OS, Singer C, Lang AE. Dancing dorsal quadrilaterals: a novel peripherally induced movement disorder. JAMA Neurol 2019;76(3):351–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Glocker FX, Deuschl G, Volk B, Hasse J, Lücking CH. Bilateral myoclonus of the trapezius muscles after distal lesion of an accessory nerve. Mov Disord 1996;11(5):571–575. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Disorders causing hyperkinetic shoulder movements
Table S2. Differential diagnosis in thoracic movement disorders
Table S3. Differential diagnosis of diaphragmatic, abdominal, and pelvic movement disorders
Table S4. Differential diagnosis of axial truncal movement disorders


