ABSTRACT
Background
Parkinson's disease (PD) is best managed by neurologists, traditionally including frequent doctor–patient contact. Because of a rise in PD prevalence and associated healthcare costs, this personnel‐intensive care may not be future proof. Telemedicine tools for home monitoring have shown to reduce healthcare consumption in several chronic diseases and also seem promising for PD.
Objective
To explore whether telemonitoring can reduce outpatient healthcare consumption in PD.
Methods
We conducted a cohort study with 116 outpatients with PD who used the telemedicine tool “myParkinsoncoach.” The tool involved periodic monitoring, feedback, knowledge modules, and text message functionality. Retrospective data about PD‐related healthcare consumption in the year before and after introduction of the tool were retrieved from the hospital information system. Additional data about tool‐related activities performed by nursing staff were logged prospectively for 3 months.
Results
There was a 29% reduction in the number of outpatient visits (P < 0.001) in the year after introduction of the tool compared with the year before. A 39% reduction was seen in overall PD‐related healthcare costs (P = 0.001). Similar results were found for patients ≥70 years old. Nursing staff spent on average 15.5 minutes per patient a month on monitoring the tool and follow‐up activities.
Conclusions
Study results demonstrate a significant reduction in PD‐related healthcare consumption using telemonitoring. Notably, these results were also found in elderly patients. Further research is needed to confirm these findings, preferably taking a broader perspective on healthcare consumption and within a larger, multicenter and prospective setup.
Keywords: telemedicine, home monitoring, Parkinson's disease, healthcare consumption
Parkinson's disease (PD) is a slowly progressive and chronic disease characterized by a range of motor and non‐motor symptoms that often fluctuate over time and between patients.1, 2 PD therefore requires proper monitoring and individualized treatment. Previous research in the United States has shown that management of patients by a neurologist, especially a movement disorder specialist, reduces PD‐related hospitalization, placement in a nursing home, and even death.3, 4 The guidelines on PD by both the British National Institute of Health and Care Excellence and the Dutch Federation of Medical Specialists recommend outpatient visits with a movement disorder specialist between 1 and 8 times a year based on disease severity and progression.5, 6 In the Netherlands, most patients with PD have scheduled outpatient visits with their neurologist or specialized nurse 3 to 4 times a year (reliable data are lacking). However, in many countries, access to proper treatment for patients with PD is poor, and frequent doctor–patient contact is not attainable.7 In the face of the increasing prevalence of PD8 and rising healthcare costs,9 a system of frequent outpatient visits with a movement disorder specialist is likely not even sustainable in high‐income countries.
As stated previously in Movement Disorders,10, 11 a possible solution to this problem lies in the use of telemedicine (also referred to as “telehealth” or “eHealth”), the remote diagnosis and treatment of patients by means of telecommunications technology.12 Telemedicine exists in a wide variety of forms such as, but not limited to, web portals, health sensors or wearables, mobile applications, video conferencing, home automation, or robotics. In this article, we focus on systems for long‐term home monitoring of patients, further called “telemonitoring.”
In recent years, telemonitoring has shown to reduce healthcare consumption in several chronic diseases such as inflammatory bowel disease, chronic heart failure, and chronic obstructive pulmonary disease.13, 14, 15 The proposed mechanism by which telemonitoring can lead to a reduction in healthcare costs is 3‐fold. First, early observation of deterioration gives the opportunity to evaluate and optimize treatment before problems become worse. For example, detecting starting symptoms of delusion and suspicion may prompt changes in medication, possibly preventing a florid psychosis which often necessitates long‐term hospitalization. Second, telemonitoring helps to eliminate “needless” outpatient visits in patients that are stable, saving time for both patient, primary caregiver, and medical professional. Third, telemonitoring can give patients better insight into their disease, leading to patient empowerment and improved self‐management.16, 17, 18 Self‐management is in itself associated with improved health status and well‐being in patients with a chronic disease.19, 20
Up to now, PD research into telemedicine has focused mainly on the following 3 areas: wearable devices or test batteries to monitor PD symptoms,21, 22, 23, 24 substitution of outpatient visits by screen‐to‐screen virtual house calls25, 26, 27, 28 and administration of treatment via telephone or internet.29, 30 To our knowledge, there are no published articles that make use of a questionnaire‐based telemonitoring of patients.
We developed a telemonitoring tool called “myParkinsoncoach” (in Dutch “MijnParkinsoncoach”), a web‐based monitoring system for outpatients with PD.31 A previous study showed that implementation of this tool in the outpatient clinic was feasible and that patient satisfaction and experienced quality of care were high.32 In the current article, PD‐related healthcare consumption is compared in patients before and after the introduction of the tool. We hypothesized that their PD‐related healthcare consumption would decline, most notably the number of outpatient visits.
Methods
Study Design
We conducted a retrospective cohort study in a large, general teaching hospital in the Netherlands. We evaluated healthcare consumption comparing 1 year before and 1 year after patients started using “myParkinsoncoach.” Ethical approval for this study was obtained from the Medical Ethical Committee Zuyderland‐Zuyd (17‐N‐152).
Intervention
“MyParkinsoncoach” is a web‐based monitoring tool for patients with PD developed in close collaboration with both movement disorder specialists and specialized nurses as well as patients with PD. There are separate interfaces for patients and professionals.
The patient's interface includes 4 parts. First, a periodic, 40‐item questionnaire asks about patients' symptoms. This questionnaire is a reflection of the history that is normally taken in the consultation room, but essentially it can be interpreted as a patient‐reported outcome measure.33 The frequency of these questionnaires can be altered by the professional based on, for example, disease stability or recent therapeutic changes. Most patients complete a questionnaire once every 1 to 3 months. Second, questionnaire responses are converted to scores on 15 PD‐related domains, such as motor symptoms, activities of daily living, or psychosis. The scores on these domains are shown on a 1 to 5 Likert scale (no problems–very severe problems). Both the current score and the score's change compared with the previous questionnaire are visible (Fig. 1). Third, patients and healthcare providers can send each other text messages in case of, for example, quick questions, advice, or treatment changes. Fourth, there are interactive learning modules to improve patients' knowledge about PD. After login, notifications inform patients about unread messages, outstanding questionnaires, or activated learning modules.
FIG. 1.
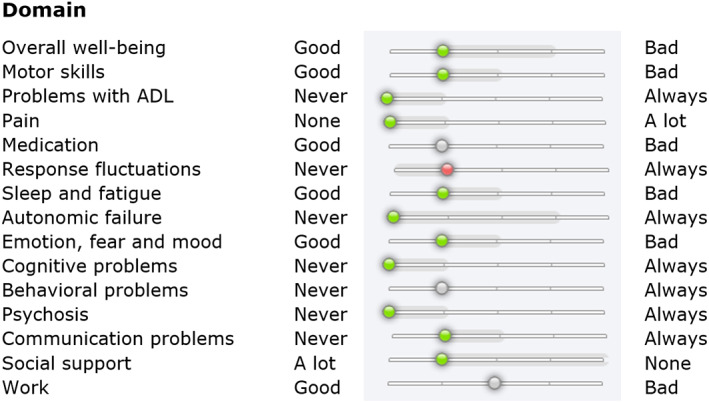
Overview of patient scores converted from a questionnaire. Example of a patient's score on the 15 Parkinson's disease–related domains. A leftward shift and a green dot indicate improvement in that domain compared with the previous questionnaire, a rightward shift and a red dot indicate deterioration. ADL, activities of daily living.
The professional's interface starts with an overview of all patients using the tool. A notification screen shows all newly completed questionnaires and unread text messages. Selecting a patient gives access to the messages and to the scores as converted from the questionnaires. Because improvement, stabilization, and worsening of the different aspects of PD are shown in different colors, both the separate domains and the overall situation of a patient can be ascertained at a glance.
On weekdays, a specialized nurse processes all new questionnaires and text messages. If necessary, based on their professional expertise, they send a text message or make a phone call themselves or schedule a phone call or an outpatient visit with the neurologist. In case of doubt or worsening of symptoms, the neurologist is always consulted. As a starting principle, patients do not have outpatient visits or scheduled phone calls when “myParkinsoncoach” does not give an indication of worsening of the disease. It is worth noting that the questionnaires and the messages that a patient sends via the tool are always assessed by a healthcare professional who knows the patient.
Usual care consists of scheduled outpatient visits several times a year, mostly based on the patient's stability of disease. In case of questions or problems between these visits, patients can call the outpatient clinic. The secretaries will then forward this message to the neurologist, who will decide on the course of action.
Participants
Patients who were treated for idiopathic PD at the outpatient clinic of a large teaching hospital in the Netherlands and were deemed eligible by their treating physician were asked to use “myParkinsoncoach.” If patients were not eligible or not willing, they continued with usual care. All patients who used the tool were included in this study. For the analysis, we excluded those patients who did not have data available on their PD‐related healthcare consumption 1 full year before and after introduction of the tool.
Data Collection
Data about outpatient visits, scheduled calls, emergency room visits, and hospital admission days were extracted from the hospital information system between January 1, 2013, and December 31, 2017. For each individual patient, a baseline and test period were determined based on the moment when that patient started to use “myParkinsoncoach.” Associated costs were calculated based on reference prices in the guidelines for health economic evaluations from “zorginstituut Nederland.”34 This is the government agency that supervises the quality and affordability of Dutch healthcare.35 The last available reference prices are from 2014. These have been adjusted for inflation to 2021. Data about questionnaires, learning modules, and text messages were logged on the server and collected to assess patients' adherence to the tool. All data were anonymized before analysis. Activities related to the tool were not registered in the hospital information system. Therefore, the specialized nurses prospectively kept a log of the number of activities conducted for all patients using the tool during a 3‐month period (from November 1, 2017, to January 31, 2018). This was converted to “time spent” through a fixed amount of minutes per activity. Analyzing the results of a questionnaire or replying to a message counted for 5 minutes and a phone call or consultation with the neurologist for 7 minutes. “Time spent” was thereafter converted to “costs” based on the guidelines for health economic evaluations mentioned previously.34
Outcome Measures
The primary outcome was change in the number of PD‐related outpatient visits between 1 year before and 1 year after patients started using “myParkinsoncoach.” The secondary outcomes were change in PD‐related scheduled telephone calls, emergency room visits, days hospitalized, and healthcare costs. We preplanned subgroup analyses based on patient age (≥70 years old vs. <70 years old) and compliance with the telemonitoring tool. Patients were considered “compliant” if they completed 3 or more questionnaires in a year.
Statistical Analysis
We calculated the average PD‐related healthcare consumption and related healthcare costs per patient in the year before and after they started using myParkinsoncoach. Due to the fact that each patient started using the tool at a different moment, the exact time period that was used in the analysis differs from individual to individual. Results before and after introduction of the tool were compared using Wilcoxon signed‐rank tests. Log data were analyzed with descriptive statistics and frequencies. Analyses were done using Celonis Process Mining version 4 (Celonis, Munich, Germany) and IBM (Armonk, NY) SPSS Statistics version 25.
Results
In total, 204 patients started to use “myParkinsoncoach.” A complete data set both 1 year before and after they started using the tool was obtained for 116 patients (see Fig. 2). Baseline characteristics are shown in Table 1. Noncompliant patients had a longer mean disease duration compared with compliant patients, and age differed between age‐based subgroups. Other baseline characteristics were similar between subgroups.
FIG. 2.
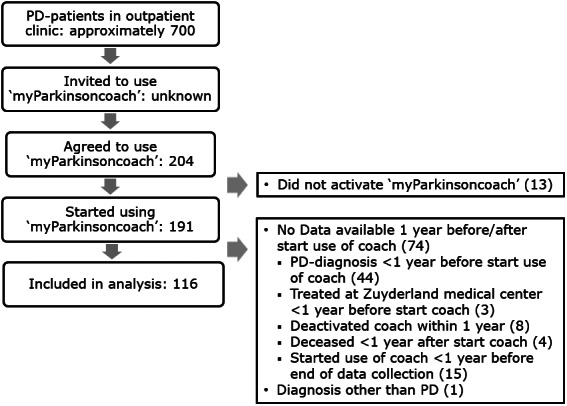
Patient's flow diagram. PD, Parkinson's disease.
TABLE 1.
Baseline characteristics
| Patient characteristics | Total Sample, N = 116 | Subgroup Age | Subgroup Compliance | ||
|---|---|---|---|---|---|
| <70 years, n = 78 | ≥70 years, n = 38 | ≥3 Questionnaires, n = 90 | <3 Questionnaires, n = 26 | ||
| Demographics | |||||
| Sex, n (%) | |||||
| Male | 70 (60.3) | 47 (60.3) | 23 (60.5) | 58 (64.4) | 12 (46.1) |
| Female | 46 (39.7) | 31 (39.7) | 15 (39.5) | 32 (35.6) | 14 (53.9) |
| Age | |||||
| Mean in years (SD) | 66.2 (8.25) | 62.0 (6.27)a | 74.8 (3.96)a | 66.3 (7.84) | 65.7 (9.72) |
| Range in years | 41–83 | 41–69 | 70–83 | 41–81 | 42–83 |
| Medical | |||||
| Time since diagnosis | |||||
| Mean in years (SD) | 6.5 (5.11) | 6.2 (5.29) | 7.2 (4.69) | 5.8 (4.53)a | 8.8 (6.28)a |
| Range in years | 1.0–25.2 | 1.0–25.2 | 1.0–18.5 | 1.0–23.5 | 1.2–25.2 |
| Use of advanced therapies | |||||
| Any, n (%) | 18 (15.5) | 12 (15.4) | 6 (15.8) | 12 (13.3) | 6 (23.1) |
| Apomorfine, n | 4 | 3 | 1 | 3 | 1 |
| DBS, n | 2 | 2 | 0 | 2 | 0 |
| LCIS, n | 12 | 7 | 5 | 7 | 5 |
Significant (P < 0.05) difference between subgroups.
SD, standard deviation; DBS, deep brain stimulation; LCIG, levodopa‐carbidopa intestinal gel.
Primary Outcome
There was a 29% reduction in the number of outpatient visits in the year after introduction of the tool compared with the year before (see Table 2). In 27 patients (23%), the number of outpatient visits increased in the year after introduction of the tool, 26 patients (22%) showed no difference, and in 63 patients (54%) there was a reduction. Comparable outcomes were found in patients aged 70 years or older (−35%) and in the patients compliant with the tool (−32%), but not in the noncompliant group.
TABLE 2.
Primary and secondary outcomes
| Outcome measures | Number of Patients | Before Start Tool, Median (Range) | After Start Tool, Median (Range) | P Value |
|---|---|---|---|---|
| Outpatient visits | ||||
| Total sample | 116 | 2 (0–8) | 1 (1–6) | <0.001a |
| <70 years of age | 78 | 2 (0–8) | 1 (0–5) | 0.002b |
| ≥70 years of age | 38 | 2 (0–5) | 1 (0–6) | 0.009b |
| Compliant | 90 | 2 (0–8) | 1 (0–6) | <0.001a |
| Noncompliant | 26 | 2 (1–5) | 1 (0–5) | 0.198 |
| Scheduled calls | ||||
| Total sample | 116 | 0 (0–2) | 0 (0–8) | 0.065 |
| <70 years of age | 78 | 0 (0–2) | 0 (0–8) | 0.048c |
| ≥70 years of age | 38 | 0 (0–2) | 0 (0–3) | 0.783 |
| Compliant | 90 | 0 (0–2) | 0 (0–8) | 0.147 |
| Noncompliant | 26 | 0 (0–1) | 0 (0–3) | 0.234 |
| Emergency room visits | ||||
| Total sample | 116 | 0 (0–1) | 0 (0–1) | 0.564 |
| <70 years of age | 78 | 0 (0–1) | 0 (0–1) | 1.000 |
| ≥70 years of age | 38 | 0 (0–0) | 0 (0–1) | 0.317 |
| Compliant | 90 | 0 (0–1) | 0 (0–0) | 0.317 |
| Noncompliant | 26 | 0 (0–0) | 0 (0–1) | 0.157 |
| Days hospitalized | ||||
| Total sample | 116 | 0 (0–30) | 0 (0–22) | 0.308 |
| <70 years of age | 78 | 0 (0–30) | 0 (0–22) | 0.441 |
| ≥70 years of age | 38 | 0 (0–11) | 0 (0–6) | 0.285 |
| Compliant | 90 | 0 (0–11) | 0 (0–17) | 0.207 |
| Noncompliant | 26 | 0 (0–30) | 0 (0–22) | 0.715 |
| Costs per patientd | Mean (range) | Mean (range) | ||
| Total sample | 116 | €619.17 (€0–€12,345) | €376.64 (€0–€8888) | 0.001b |
| $745.23 ($0–$14,862.76) | $453.32 ($0–$10,700.71) | |||
| <70 years of age | 78 | €698.31 (€0–€12,345) | €443.11 (€0–€8888) | 0.011c |
| $840.48 ($0–$14,862.76) | $533.32 ($0–$10,700.71) | |||
| ≥70 years of age | 38 | €456.75 (€0–€4741) | €240.22 (€0–€2926) | 0.021c |
| $549.74 ($0–$5707.93) | $289.13 ($0–$3522.76) | |||
| Compliant | 90 | €477.03 (€0–€4741) | €272.42 (€0–€7210) | <0.001a |
| $574,15 ($0–$5707.93) | $327,88 ($0–$8680.48) | |||
| Noncompliant | 26 | €1111.19 (€99–€12,345) | €737.42 (€0–€8888) | 0.497 |
| $1337.42 ($119.19–$14,862.76) | $887.56 ($0–$10,700.71) |
aP < 0.001; b P < 0.01; c P < 0.05.
Excluding costs pertaining to the monitoring of “myParkinsoncoach.”
Secondary Outcomes
We found a significant reduction in total PD‐related healthcare costs comparing the year before and after introduction of the tool. This reduction was seen in the total sample (−39%) and in the subgroups <70 years (−37%), ≥70 years (−47%), and compliant patients (−43%), but not in the noncompliant subgroup (see Table 2). All groups showed a trend toward a reduction in the number of days hospitalized; however, this was not statistically significant in any group. An increase in the number of scheduled calls was seen in all analyses, although this was only statistically significant in the subgroup <70 years old.
Activities Performed and Time Spent by Nursing Staff Related to “myParkinsoncoach”
The results of our prospective analysis are shown in Table 3. Monitoring and follow‐up activities cost on average 15.5 minutes per patient per month. Most time was spent reading and replying to messages (9.4 minutes), followed by analyzing questionnaires. This time investment corresponds to salary costs of €140.38 ($168.96) per patient per year for the nurse.
TABLE 3.
Activities performed and time spent monitoring “myParkinsoncoach”
| Time period | Questionnaires | Messages | Phone calls | Consultation | Total |
|---|---|---|---|---|---|
| November 2017 | |||||
| Total number | 95 | 325 | 70 | 42 | – |
| Mean number per patient | 0.53 | 1.80 | 0.39 | 0.23 | – |
| Mean time per patient, minutes | 2.64 | 9.02 | 2.70 | 1.63 | 16.02 |
| December 2017 | |||||
| Total number | 160 | 320 | 21 | 14 | – |
| Mean number per patient | 0.95 | 1.90 | 0.13 | 0.08 | – |
| Mean time per patient, minutes | 4.76 | 9.52 | 0.88 | 0.58 | 15.74 |
| January 2018 | |||||
| Total number | 105 | 325 | 28 | 21 | – |
| Mean number per patient | 0.62 | 1.92 | 0.17 | 0.12 | – |
| Mean time per patient, minutes | 3.11 | 9.62 | 1.16 | 0.87 | 14.75 |
| Total | |||||
| Mean time per patient, minutes | 3.50 | 9.39 | 1.59 | 1.03 | 15.51 |
Discussion
To our knowledge, this is the first study evaluating the impact of telemonitoring by periodic online questionnaires on healthcare consumption in PD.
The results show a significant 29% reduction in the average number of outpatient visits in the first year after introduction of “myParkinsoncoach.” The same was also found in the subgroup of patients ≥70 years old. In some patients, there was an increase in the number of outpatient visits. We believe that this may have been attributed to “myParkinsoncoach” picking up on early signs of deterioration necessitating increased doctor–patient contact and that it has possibly prevented emergency room visits or hospitalization.
Secondary outcome measures showed a non‐significant increase in the number of scheduled phone calls. It is likely that the introduction of the tool led to a partial shift from regular outpatient visits to telephone consultations. In addition, there was a significant reduction in overall PD‐related healthcare costs caused mainly by a reduction in the number of hospital admission days as well as the number of outpatient visits. Prospectively it was assessed that time spent monitoring the tool takes on average 15.5 minutes per patient per month.
The aforementioned findings of telemonitoring leading to a reduction in healthcare consumption in PD correspond to earlier studies in patients with other chronic diseases.13, 14, 15 The fact that these results were not related to the age of the patients is also in line with previous research showing that, contrary to popular belief, telemedicine tools are also applicable in the care for elderly patients.36, 37
Others have investigated the use of screen‐to‐screen contact either replacing or in addition to outpatient visits in PD. One of these studies showed a trend toward a reduction in the number of outpatient visits, but most were not set up to analyze change in healthcare consumption.25 Reports generally showed an economic benefit based on a reduction in travel time and associated costs. Patient satisfaction and willingness to use this type of care were high to very high. No differences in quality of care were found with screen‐to‐screen visits in comparison with regular outpatient visits.25, 27, 28 High levels of patient satisfaction and experienced quality of care were also seen with the use of “myParkinsoncoach” in a previous study.32
In addition, a large number of studies have explored the usefulness of wearable devices in monitoring symptoms in PD.21, 22, 23, 24 These generally focus on the reliability of such devices and on quality of care. One study taking into consideration healthcare cost found the use of wearable devices to be cost‐effective for improvement of functional status, motor severity, and motor complications.22 We did not find any study analyzing the use of wearable devices in relation to healthcare consumption.
There are several limitations concerning this study. First, no control group was included in the analyses, making it difficult to attribute the results that were found completely to the tool. Nevertheless, the fact that no significant changes in healthcare consumption were found in the noncompliant subgroup makes it more likely that the effects found were at least partly caused by the tool.
Second, possible selection bias could have influenced results because not all patients at the clinic were invited to use “myParkinsoncoach,” only those deemed eligible by their neurologist. Hence our sample was on average younger than most patients with PD and did not contain people with severe cognitive or psychiatric disorders nor patients living in a nursing home facility. However, the study population does match the patients that would use such a tool in practice and the outcomes are therefore likely to reflect its real‐world effects. Furthermore, the subgroup analysis in elderly patients revealed that for those ≥70 years old who were interested in using telemonitoring, the effect was similar to the overall population.
Third, data were collected in one hospital with most patients being treated by a single movement disorder specialist, in close collaboration with PD nurses, possibly limiting the generalizability of the results. As mentioned previously, most patients with PD in the Netherlands have outpatient visits with their neurologist every 3 to 4 months, whereas this study found a median of 2 visits a year before the introduction of “myParkinsoncoach.” This indicates a preexisting restrictive attitude toward the regular planning of outpatient visits. A larger rather than smaller reduction in outpatient visits may be attainable in most hospitals compared with the 29% found in this study.
In addition, it is self‐evident that our results are only viable in settings where internet access from home is readily available for patients with PD.
Despite these limitations, the results found in this study indicate that telemonitoring can have a place in reducing healthcare consumption in PD. Because only in‐hospital activities registered with PD as the primary diagnosis were taken into account, it is likely that the true economic impact is even larger than this study shows. For example, patients with PD often visit the emergency room or are admitted to the hospital because of complications such as (aspiration) pneumonia or falls leading to fractures or head trauma. Better disease management with telemonitoring may lead to a reduction in these complications and thus in additional cost reductions. Furthermore, patients admitted to the hospital because of PD often need rehabilitation in a nursing home or other facility before being able to return home. A decrease in hospital admissions would also mean less admissions to rehabilitation facilities. In addition, a reduction in outpatient visits will also lead to indirect cost reductions outside of healthcare. Patients and/or caregivers do not need to take time of work to attend the outpatient clinic, it eliminates travel time and costs, and reduces the amount of parking lots, waiting rooms, and clinic space needed at hospitals. In several studies that compared screen‐to‐screen doctor–patient contact with outpatient visits in PD, patients and caregivers stated that they found the reduction in travel time and costs to be the largest advantage of telemedicine.25, 26
Future research on this subject is needed and should focus on validating current results, particularly in a larger, prospective, and multicenter setups. Outcome measures related to the quality of care should be included as well as a broader perspective on healthcare costs. Long‐term follow‐up is necessary to ascertain the sustainability of care with limited face‐to‐face doctor–patient contact over time. Moreover, research should reveal whether telemonitoring and proactive disease management do indeed lead to a reduction in hospital admissions.
Considering the expected rise in the incidence of PD mentioned previously, we believe it is of great importance that implementation of telemonitoring and other types of telemedicine in standard care will be prioritized in the upcoming years. This requires a culture change to make telemedicine standard care in the minds of both patients and healthcare professionals. Nevertheless, implementation will also be influenced greatly by whether and how agreements concerning the financing of telemedicine are made. Thus far, movement disorder specialists using telemedicine often report that the expansion of this type of care is limited by inconsistencies in reimbursement or by not being reimbursed at all.28, 38 The current COVID‐19 pandemic has shown many people the benefits of telemedicine and might be a catalyst in prompting all parties concerned to facilitate its implementation.39
To optimize the care for patients with PD, telemonitoring systems, including “myParkinsoncoach,” should further be expanded. Based on experiences of healthcare professionals and patients, useful additions include, for example, combining questionnaire‐based data with data from wearables to give a better view of a patient's symptoms and effect of therapy, notifications or other reminders for patients about medication intake to promote compliance, and modules to allow for the exchange of information between different healthcare professionals and other caregivers to align treatment. In addition, to further personalize care, it would be useful to have individualized questionnaires based on patient characteristics such as age, disease stage, type of therapy, or admission to a nursing home facility. These points for improvement are in line with the roadmap for implementation of mobile technologies for patient‐centered outcome measurements as proposed by the Movement Disorder Society Task Force on Technology.40
Author Roles
(1) Research Project: A. Conception, B. Organization, C. Execution; (2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique; (3) Manuscript Preparation: A. Writing of the First Draft, B. Review and Critique.
A.W.: 1A, 2A, 2B, 3A
L.H.: 1A, 1B, 1C, 2A, 2C, 3B
G.T.: 1A, 2A, 3B
Disclosures
Ethical Compliance Statement
Ethical approval for this study was obtained from the Medical Ethical Committee Zuyderland‐Zuyd (17‐N‐152). Informed patient consent was not necessary for this work. We confirm that all authors have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Funding Sources and Conflicts of Interest
This study was conducted within the context of the eHealth living lab, an initiative of Zuyderland Medical Centre and healthcare insurance company CZ, in which promising eHealth solutions—screen to screen contact, telemonitoring applications and decision support tools—are implemented and evaluated. More information can be found at https://www.zuyderland.nl/nieuws/zuyderland-living-lab-voor-e-health-cz-actuele-pilots/. Neither CZ nor anyone in the hospital management staff was involved in the data collection, analysis, or writing of this article. The authors declare that there are no conflicts of interest relevant to this work.
Financial Disclosures for the Previous 12 months
Anke Wijers is employed by Zuyderland Medical Centre. Laura Hochstenbach is employed by Zuyd University of Applied Sciences. Gerrit Tissingh declares that he has no additional disclosures to report.
Acknowledgments
MyParkinsoncoach was developed by Sananet BV, a company experienced in designing comparable monitoring systems for patients with chronic obstructive pulmonary disease, hearth failure, and diabetes in close collaboration with neurologists specialized in movement disorders, Parkinson's disease nurses, and patients from Zuyderland Medical Centre. The authors thank Nicole Soleil and Jerney Groenendal for their efforts regarding data collection and Rob van de Coevering, Zuiver Information and Communication Technology (ICT) (http://www.zuiverict.nl/en/homepage) for his help in data preparation and analysis.
Relevant disclosures and conflicts of interest are listed at the end of this article.
References
- 1.Clarke CE. Parkinson's disease. BMJ 2007;335:441–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Poewe W, Seppi K, Tanner CM, et al. Parkinson disease. Nat Rev Dis Primers 2017;3:17013. [DOI] [PubMed] [Google Scholar]
- 3.Willis AW, Schootman M, Tran R, et al. Neurologist‐associated reduction in PD‐related hospitalisations and health care expenditures. Neurology 2012;79:1774–1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Willis AW, Schootman M, Evanoff BA, Perlmutter JS, Racetta BA. Neurologist care in Parkinson disease: a utilization, outcomes, and survival study. Neurology 2011;77:851–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Collaborating Centre for Chronic Conditions . Parkinson's Disease: National Clinical Guideline for Diagnosis and Management in Primary and Secondary Care. London, UK: Royal College of Physicians; 2006. National Institute of Health and Care Excellence Clinical Guideline No. 35. [Google Scholar]
- 6.Guideline Committee of the Dutch Association for Neurology (NVN) Guideline on Parkinson's disease by the Dutch Federation of Medical Specialist, section on optimal follow‐up frequency. https://richtlijnendatabase.nl/richtlijn/ziekte_van_parkinson/startpagina_ziekte_van_parkinson.html. Accessed on 05 June 2021.
- 7.Dorsey ER, Willis AW. Caring for the majority. Mov Disord 2013;28:261–262. [DOI] [PubMed] [Google Scholar]
- 8.Dorsey ER, Elbaz A, Nichols E, et al. Global, regional, and national burden of Parkinson's disease, 1990–2016: a systematic analysis for the global burden of disease study 2016. Lancet Neurol 2018;17(11):939–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kowal SL, Dall TM, Chakrabarti R, Storm MV, Jain A. The current and projected economic burden of Parkinson's disease in the United States. Mov Disord 2013;28:311–318. [DOI] [PubMed] [Google Scholar]
- 10.Dorsey ER, Vlaanderen FP, Engelen LJ, et al. Moving Parkinson care to the home. Mov Disord 2016;31(9):1258–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Achey M, Aldred JL, Aljehani N, et al. The past, present, and future of telemedicine for Parkinson's disease. Mov Disord 2014;29(7):871–883. [DOI] [PubMed] [Google Scholar]
- 12.The Oxford English Dictionary. https://www.oed.com/. Accessed on 15 May 2021.
- 13.de Jong MJ, van der Meulen‐de Jong AE, Romberg‐Camps MJ, et al. Telemedicine for management of inflammatory bowel disease (myIBDcoach): a pragmatic, multicentre, randomised controlled trial. Lancet 2017;390(10098):959–968. [DOI] [PubMed] [Google Scholar]
- 14.de la Torre DI, Garcia‐Zapirain B, Méndez‐Zorrilla A, López‐Coronado M. Monitoring and follow‐up of chronic heart failure: a literature review of eHealth applications and systems. J Med Syst 2016;40(7):179. [DOI] [PubMed] [Google Scholar]
- 15.Jonkman NH, Westland H, Trappenburg JC, et al. Do self‐management interventions in COPD patients work and which patients benefit most? An individual patient data meta‐analysis. Int J Chron Obstruct Pulmon Dis 2016;11:2063–2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilde MH, Garvin S. A concept analysis of self‐monitoring. J Adv Nurs 2007;57:339–350. [DOI] [PubMed] [Google Scholar]
- 17.Qian W, Lam TT‐N, Lam HHW, Li C‐K, Cheung YT. Telehealth interventions for improving self‐management in patients with hemophilia: scoping review of clinical studies. J Med Internet Res 2019;21(7):e12340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jalil S, Myers T, Atkinson I. A meta‐synthesis of behavioral outcomes from telemedicine clinical trials for type 2 diabetes and the clinical user‐experience evaluation (CUE). J Med Syst 2015;39(3):28. [DOI] [PubMed] [Google Scholar]
- 19.Lorig KR, Holman H. Self‐management education: history, definition, outcomes, and mechanisms. Ann Behav Med 2003;26:1–7. [DOI] [PubMed] [Google Scholar]
- 20.Barlow J, Wright C, Sheasby J, Turner A, Hainsworth J. Self‐management approaches for people with chronic conditions: a review. Patient Educ Couns 2002;48:177–187. [DOI] [PubMed] [Google Scholar]
- 21.Silva de Lima AL, Evers LJW, Hahn T, et al. Freezing of gait and fall detection in Parkinson's disease using wearable sensors: a systematic review. J Neurol 2017;264(8):1642–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cubo E, Mariscal N, Solano B, et al. Prospective study on cost‐effectiveness of home‐based motor assessment in Parkinson's disease. J Telemed Telecare 2017;23(2):328–338. [DOI] [PubMed] [Google Scholar]
- 23.Silva de Lima AL, Smits T, Darweesh SKL, et al. Home‐based monitoring of falls using wearable sensors in Parkinson's disease. Mov Disord 2020;35(1):109–115. 10.1002/mds.27830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gatsios D, Antonini A, Gentile G, et al. Feasibility and utility of mHealth for the remote monitoring of Parkinson disease: ancillary study of the PD_manager randomized controlled trial. JMIR Mhealth Uhealth 2020;8(6):e16414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beck CA, Beran DB, Biglan KM, et al. National randomized controlled trial of virtual house calls for Parkinson disease. Neurology 2017;89(11):1152–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dorsey ER, Venkataraman V, Grana MJ. Randomized controlled clinical trial of "virtual house calls" for Parkinson disease. JAMA Neurol 2013;70(5):565–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schneider RB, Biglan KM. The promise of telemedicine for chronic neurological disorders: the example of Parkinson's disease. Lancet Neurol 2017;16:541–551. [DOI] [PubMed] [Google Scholar]
- 28.Barbour PJ, Arroyo J, High S, Fichera LB, Staska‐Pier MM, McMahon MK. Telehealth for patients with Parkinsons's disease: delivering efficient and sustainable long‐term care. Hosp Pract 2016;44(2):92–97. [DOI] [PubMed] [Google Scholar]
- 29.Lei C, Sunzi K, Dai F, et al. Effects of virtual reality rehabilitation training on gait and balance in patients with Parkinson's disease: a systematic review. PLoS One 2019;14(11):e0224819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Swalwell C, Pachana NA, Dissanayaka NN. Remote delivery of psychological interventions for Parkinson's disease. Int Psychogeriatr 2018;30(12):1783–1795. [DOI] [PubMed] [Google Scholar]
- 31.Sananet BV. Mijn parkinsoncoach. https://www.sananet.nl/sanacoach-parkinson/. Accessed on 20 June 2020.
- 32.Jie L‐J, Lie A, Hoff J, Tissingh G. Telemonitoring verbetert parkinsonzorg. Medisch Contact 2016;14:21–24. [Google Scholar]
- 33.Nelson EC, Eftimovska E, Lind C, Hager A, Wasson JH, Lindblad S. Patient reported outcome measures in practice. BMJ 2015;350:g7818. [DOI] [PubMed] [Google Scholar]
- 34.Hakkaart‐van Roijen L, van der Linden N, Bouwmans C, Kanters T, Tan SS. Methodologie van kostenonderzoek en referentieprijzen voor economische evaluaties in de gezondheidszorg. Rotterdam: Institute for Medical Technology Assessment Erasmus Universiteit; 2016. [Google Scholar]
- 35.Zorginstituut Nederland. Zorginstituut Nederland, over ons. https://www.zorginstituutnederland.nl/over-ons. Accessed on 08 June 2021.
- 36.Brignell M, Wootton R, Gray L. The application of telemedicine to geriatric medicine. Age Ageing 2007;36(4):369–374. [DOI] [PubMed] [Google Scholar]
- 37.Gentry MT, Lapid MI, Rummans TA. Geriatric telepsychiatry: systematic review and policy considerations. Am J Geriatr Psychiatry 2019;27(2):109–127. [DOI] [PubMed] [Google Scholar]
- 38.Hassan A, Dorsey ER, Goetz CG, et al. Telemedicine use for movement disorders: a global survey. Telemed J E Health 2018;24(12):979–992. [DOI] [PubMed] [Google Scholar]
- 39.Bloem BR, Dorsey ER, Okun MS. The coronavirus disease 2019 crisis as catalyst for telemedicine for chronic neurological disorders. JAMA Neurol 2020;77:927–928. [DOI] [PubMed] [Google Scholar]
- 40.Espay AJ, Hausdorff JM, Sánchez‐Ferro Á, et al. A roadmap for implementation of patient‐centered digital outcome measures in Parkinson's disease obtained using mobile health technologies. Mov Disord 2019;34(5):657–663. [DOI] [PMC free article] [PubMed] [Google Scholar]


