ABSTRACT
Background
Dream content alterations in Parkinson's disease (PD) are associated with motor and cognitive dysfunction cross‐sectionally. Although recent studies suggest abnormal dream content in PD might also predict cognitive decline, the relationship between dream content and motor decline in PD remains unknown.
Objective
To investigate whether abnormal dream content in PD predicts both motor and cognitive decline.
Methods
Data were obtained from the Parkinson's Progression Markers Initiative cohort study. Patients were evaluated at baseline and at the 60‐month follow‐up, with validated clinical scales, including the REM Sleep Behavior Disorder Screening Questionnaire (RBDSQ), Montreal Cognitive Assessment (MoCA), and the Movement Disorder Society–Unified Parkinson's Disease Rating Scale Part III (MDS‐UPDRS III). Patients were dichotomized using RBDSQ item 2, which inquires whether they frequently experience aggression in their dreams. Regression analyses were used to assess whether frequent aggressive dreams at baseline predicted longitudinal changes in MDS‐UPDRS III and MoCA scores as well as progression to Hoehn and Yahr stage 3 (H&Y ≥ 3) and cognitive impairment.
Results
Of the patients, 58/224 (25.9%) reported frequent aggressive dreams at baseline. Aggressive dreams predicted a faster increase in MDS‐UPDRS III scores (β = 4.64; P = 0.007) and a faster decrease in MoCA scores (β = −1.49; P = 0.001). Furthermore, they conferred a 6‐fold and 2‐fold risk for progressing to H&Y ≥ 3 (odds ratio [OR] = 5.82; P = 0.005) and cognitive impairment (OR, 2.35; P = 0.023) within 60 months. These associations remained robust when adjusting for potential confounders.
Conclusions
This study demonstrates for the first time that frequent aggressive dreams in newly diagnosed PD may independently predict early motor and cognitive decline.
Keywords: Parkinson's disease, dreaming, PPMI, cognitive decline, REM sleep behavior disorder
Distressing dreams and nightmares are common, but underreported, nonmotor symptoms of Parkinson's disease (PD).1, 2, 3 They have usually been linked with rapid eye movement (REM) sleep behavior disorder (RBD)3, 4 or the introduction of dopaminergic therapy5; however, they can also occur independently.6 As many as 18% of patients with nondemented PD experience nightmares sufficiently frequent to be considered a clinical disorder.7 In PD dementia (PDD), this can be as high as 78%.8 Furthermore, unlike the presence of RBD, which becomes more prevalent in patients with PD over time,9 the prevalence of nightmares and distressing dreams in PD does not change significantly from baseline.9 This raises the possibility that altered dreaming in early PD could predict future cognitive decline.
Although early reports had speculated that dream alterations might represent the harbinger of dementia in PD,10 only recently has this been investigated in a systematic fashion. One recent cross‐sectional study found that altered dream content in PD, in particular, an increased frequency of physical aggression and animal characters, is associated with lower scores on tests of frontal lobe functions.6 Furthermore, distressing dreams in PD have been shown to correlate with lower scores on measures of global cognitive function.11
A total of 2 studies have evaluated the relationship between dream content and cognitive function in PD in a longitudinal rather than cross‐sectional design.12, 13 The first found intense, vivid, and frightening dreams in PD to independently predict a faster rate of decline in Mini Mental State Examination scores and an increased risk of dementia at the 2‐year follow‐up.12 A more recent study using systematically collected verbal dream reports found an increased frequency of negative emotions, but not animal characters or aggression, to predict a faster rate of decline in Montreal Cognitive Assessment (MoCA) scores, this time at the 4‐year follow‐up.13 However, these findings should still be considered preliminary given that both studies were limited by small sample sizes.
It has further been suggested that altered dream content in PD is associated with an increased severity of motor symptoms. Patients with more advanced PD, as indicated by higher Hoehn and Yahr (H&Y) stages, have a greater proportion of physical aggression and animal characters in their dreams14 as well as more frequent nightmares.15 However, it has yet to be investigated whether dream content alterations can predict subsequent motor progression in PD longitudinally.
The aim of the present study is to investigate whether dream content alterations at baseline can predict both motor and cognitive decline in a large cohort of patients with newly diagnosed, nondemented, and drug‐naive PD followed up for a period of 5 years.
Methods
Study Design
Data used in this study came from the Parkinson's Progression Marker Initiative (PPMI) database. PPMI is a multicenter, international, longitudinal cohort study that enrolled 423 participants with PD and 196 healthy controls at baseline and conducted repeated clinical assessments at yearly follow‐up visits for 5 years. Further study aims, methodology, and details of the included study assessments have been published elsewhere16 and are available on the PPMI website (https://www.ppmi-info.org/about-ppmi/study-goals/).
Participants
Inclusion criteria for the PD cohort included a diagnosis of PD for 2 years or less at screening, abnormal dopamine transporter imaging, not treated for PD, and no dementia as determined by the site investigator. For the present study, only data for participants who had complete 5‐year follow‐up data for continuous and categorical outcome variables as well as relevant covariates (described in 'Other Assessments') were included. In addition, patients had to be either H&Y stage 1 or 2 at baseline.
Demographics and Clinical Assessments
Demographics
Demographic details including age, sex, years of education, age at diagnosis, and disease duration (based on self‐reported first symptom onset) were collected at screening.
Dream Assessment
All patients completed the REM Sleep Behavior Disorder Screening Questionnaire (RBDSQ) at baseline.17 The RBDSQ comprises 10 questions exploring the patient's sleep behavior and dream phenomenology. The total score has a maximum of 13 points. Only binary responses (yes/no) were possible. For this study, item 2 of the RBDSQ, “My dreams frequently have an aggressive or action‐packed content,” was used to define aggressive dream content. Because vivid dreams have previously been associated with PD,1, 3 the responses for item 1,“I sometimes have very vivid dreams,” were extracted as an additional predictor variable. A history of dream enactment behaviors (DEBs) was identified by a positive response to 1 or more RBDSQ item probing DEBs (items 3, 6.1, 6.2, and 6.3).18, 19 This subscore has been shown to correspond more closely with polysomnography‐confirmed RBD in PD compared with standard RBDSQ cut‐off scores.18
Cognitive Assessments
The rate of cognitive decline was defined using change in MoCA scores, a scale for global cognitive function validated for use in PD, from baseline (t0) to the 5‐year follow‐up (t1). Change in MoCA scores were determined for each patient using the following calculation: score at t1 − score at t0.
Cognitive impairment was defined as scores on 2 or more neuropsychological tests below 1.5 standard deviations of normal, as per previous studies with the PPMI cohort,20 and consistent with the Movement Disorders Society (MDS) level I criteria for diagnosing PDD and PD with mild cognitive impairment (MCI).21 The following standardized neuropsychological instruments testing multiple cognitive domains were used: Symbol Digit Modalities Test (attention), Letter‐Number Sequencing Test (working memory), Semantic Fluency Test (verbal fluency), Hopkins Verbal Learning Test‐Revised (verbal episodic memory), and Benton Judgment of Line Orientation Test (visuospatial ability).
Motor Assessments
The rate of motor progression was defined using changes in Part III of the Movement Disorders Society–Unified Parkinson's Disease Rating Scale (MDS‐UPDRS III) from baseline (t0) to the 5‐year follow‐up (t1). The MDS‐UPDRS III comprehensively assesses the various motor symptoms of PD such as bradykinesia, tremor, and rigidity. Change in MDS‐UPDRS III total scores were determined for each patient using the following calculation: score at t1 − score at t0.
Motor deterioration was defined as change from H&Y stage 1 or 2 (mild PD) to H&Y ≥ 3 (moderate–severe PD). All motor assessments were performed in the off treatment state. H&Y stage 3 was chosen as this stage has previously been considered a relevant progression point in PD22 as it signifies the development of postural instability, reduced quality of life, higher MDS‐UPDRS III scores, and increased risk of mortality.22
Other Assessments
Because nightmares in PD and healthy adults have been shown to associate with mood disorders, excessive daytime sleepiness, and autonomic dysfunction,23, 24, 25 data for these symptoms at baseline were also collected, using the following validated scales: Geriatric Depression Scale 15‐item (GDS‐15), Spielberger's State–Trait Anxiety Inventory (STAI), Epworth Sleepiness Scale (ESS), and Scales for Outcomes in Parkinson's Disease–Autonomic (SCOPA‐AUT).
Statistical Analysis
Baseline and follow‐up data were compared between patients with and without frequent aggressive dreams using independent‐samples t‐tests, Mann–Whitney U tests, or Wilcoxon tests for normally and nonnormally distributed continuous variables as appropriate (Shapiro–Wilk test for normality). Chi‐square tests were used for categorical variables.
Univariate linear and logistic regression analyses were used to test the relationship between dream features (aggressive dream content, dream vividness, and DEBs) and (1) continuous dependent variables (absolute change in MDS‐UPDRS III and MoCA scores) and (ii) categorical dependent variables (progression to H&Y ≥ 3 and development of cognitive impairment). Regression analyses were then repeated in adjusted multivariate models with age, sex, education, disease duration, and baseline MDS‐UPDRS III as covariates.
A number of sensitivity analyses were subsequently performed to exclude alternative explanations, including repeating analyses for aggressive dream content after (1) further adjusting for MCI at baseline, (2) further adjusting for nonmotor symptoms that predicted motor and cognitive outcomes in univariate analyses, and (3) including a history of DEBs as an additional covariate.
A P value <0.05 was considered to be statistically significant. All statistical analyses were performed using SPSS version 26 (IBM Corp., Armonk, NY).
Results
Baseline Demographic and Clinical Characteristics
A total of 224/423 patients with PD in the original PPMI cohort were included in this analysis; 197 patients were excluded due to having less than 5 years of follow‐up (n = 108) or having incomplete year 5 outcome data (n = 89). In addition, 2 patients were excluded as they were H&Y stage 3 at baseline.
Of the final sample, 58/224 (25.9%) had frequent aggressive dreams at baseline. Baseline demographic and clinical characteristics of patients with (n = 58) and without (n = 166) aggressive dreams are presented in Table 1. The groups did not significantly differ at baseline for demographics, motor impairment, or cognitive function; however, patients with aggressive dreams had more severe autonomic symptoms, more frequently reported DEBs, and more often described their dreams as vivid. The symptom profile at follow‐up comparing patients with and without aggressive dreams at baseline is presented in Table 2.
TABLE 1.
Baseline demographics and clinical characteristics
| Characteristic | PD with aggressive dreams, n = 58 | PD without aggressive dreams, n = 166 | Statistic | P |
|---|---|---|---|---|
| Age, years | 60.5 (10.3) | 60.5 (9.6) | U = 4705 | 0.80 |
| Male sex, n (%) | 39 (67.2) | 111 (66.9) | χ2 = 0.003 | 0.96 |
| Education, years | 15.9 (2.8) | 15.5 (2.9) | U = 4432 | 0.36 |
| Disease duration, years | 2.1 (1.5) | 1.9 (1.5) | U = 4411 | 0.36 |
| Age at diagnosis, years | 59.9 (10.3) | 59.9 (9.6) | U = 4720 | 0.8 |
| Hoehn and Yahr stage, n (%) | χ2 = 2.51 | 0.11 | ||
| 1 | 28 (48.3) | 100 (60.2) | ||
| 2 | 30 (51.7) | 66 (38.8) | ||
| MDS‐UPDRS III total score | 21.6 (8.8) | 18.3 (7.4) | U = 3717 | 0.01 |
| DEBs, n (%) | 46 (79.3) | 80 (48.2) | χ2 = 16.91 | <0.001 |
| Vivid dreams, n (%) | 56 (96.6) | 80 (48.2) | χ2 = 42.14 | <0.001 |
| ESS score | 6.3 (3.4) | 5.2 (3.0) | U = 3881 | 0.03 |
| SCOPA‐AUT score | 12.4 (7.9) | 8.6 (5.4) | U = 3235 | <0.001 |
| MCI, n (%) | 11 (19) | 21 (12.7) | χ2 = 1.40 | 0.24 |
| MoCA score | 26.9 (2.5) | 27.1 (2.2) | U = 4603 | 0.61 |
| SFT score | 48.8 (12.4) | 49.6 (10.9) | t = 0.44 | 0.66 |
| SDMT score | 39.9 (9.3) | 43.7 (10.0) | U = 3875 | 0.03 |
| LNS score | 10.2 (2.1) | 10.8 (2.6) | U = 4078 | 0.08 |
| HVLT‐R, total recall | 24.2 (4.8) | 24.9 (5.0) | t = 1.0 | 0.32 |
| HVLT‐R, recognition | 11.3 (1.0) | 11.2 (1.1) | U = 4648 | 0.41 |
| JoLO score | 12.5 (2.6) | 13.1 (2.0) | U = 4334 | 0.25 |
| GDS‐15 score | 2.9 (2.6) | 2.0 (2.1) | U = 3936 | 0.04 |
| STAI total score | 68.0 (17.9) | 63.7 (16.9) | U = 4118 | 0.10 |
Bold indicates characteristics that significantly differed between PD groups at baseline after correcting for multiple comparisons (Bonferroni). Values are presented as mean (standard deviation) except for male sex, Hoehn and Yahr stage, DEBs, vivid dreams, and MCI. For MDS UPDRS‐III, ESS, SCOPA‐AUT, GDS‐15, and STAI, higher scores represent more severe symptoms. For MoCA, SFT, SDMT, LNS, HVLT‐R, and JoLO, lower scores represent worse cognitive function.
Abbreviations: PD, Parkinson's disease; MDS‐UPDRS III, Movement Disorder Society–Unified Parkinson's Disease Rating Scale Part III; DEBs, dream enactment behaviors; ESS, Epworth Sleepiness Scale; SCOPA‐AUT, Scales for Outcomes in Parkinson's Disease–Autonomic; MCI, mild cognitive impairment; MoCA, Montreal Cognitive Assessment; SFT, Semantic Fluency Test; SDMT, Symbol Digit Modalities Test; LNS, Letter‐Number Sequencing Test; HVLT‐R, Hopkins Verbal Learning Test Revised; JoLO, Benton Judgment of Line Orientation Test; GDS, Geriatric Depression Scale 15‐item; STAI, Spielberger's State–Trait Anxiety Inventory.
TABLE 2.
Follow‐up clinical characteristics
| Characteristic | PD with aggressive dreams, n = 58 | PD without aggressive dreams, n = 166 | Statistic | P |
|---|---|---|---|---|
| Hoehn and Yahr stage | χ2 = 13.77 | 0.003 | ||
| 1 | 2 (3.4) | 18 (10.8) | ||
| 2 | 47 (81.0) | 143 (86.1) | ||
| 3 | 8 (13.8) | 4 (2.4) | ||
| 4 | 0 (0) | 0 (0) | ||
| 5 | 1 (1.7) | 1 (0.6) | ||
| MDS‐UPDRS III total score | 36.0 (12.2) | 29.6 (12.1) | U = 3217 | <0.001 |
| DEBs, n (%) | 47 (81.0) | 96 (57.8) | χ2 = 10.02 | 0.002 |
| Vivid dreams, n (%) | 49 (84.5) | 87 (52.4) | χ2 = 18.54 | <0.001 |
| ESS score | 9.5 (5.3) | 6.8 (4.0) | U = 3278 | <0.001 |
| SCOPA‐AUT score | 17.3 (9.5) | 12.3 (7.0) | U = 3171 | <0.001 |
| MCI or PDD, n (%) | 17 (29.3) | 26 (15.7) | χ2 = 5.16 | 0.02 |
| MoCA score | 25.5 (3.9) | 27.0 (2.9) | U = 3706 | 0.008 |
| SFT score | 46.7 (13.5) | 49.3 (13.3) | t = 1.26 | 0.21 |
| SDMT score | 34.5 (12.9) | 42.6 (10.8) | U = 3111 | <0.001 |
| LNS score | 10.2 (2.8) | 10.6 (2.6) | U = 3227 | <0.001 |
| HVLT‐R, total recall | 23.1 (6.2) | 24.9 (6.3) | U = 4120 | 0.10 |
|
HVLT‐R, recognition |
11.0 (1.8) |
11.2 (1.8) |
U = 4794 |
0.96 |
|
JoLO score |
11.8 (3.0) | 12.6 (2.0) | U= 4242 | 0.17 |
|
GDS‐15 score |
3.4 (2.8) | 2.4 (2.6) | U = 3591 | 0.003 |
| STAI total score | 69.6 (17.2) | 62.2 (17.9) | U = 3438 | 0.001 |
Bold indicates characteristics that significantly differed between PD groups at follow‐up after correcting for multiple comparisons (Bonferroni). Values are presented as mean (standard deviation) except for Hoehn and Yahr stage, DEBs, vivid dreams, and MCI or PDD.
For MDS‐UPDRS III, ESS, SCOPA‐AUT, GDS‐15, and STAI, higher scores represent more severe symptoms. For MoCA, SFT, SDMT, LNS, HVLT‐R, and JoLO, lower scores represent worse cognitive function.
Abbreviations: PD, Parkinson's disease; MDS‐UPDRS III, Movement Disorder Society–Unified Parkinson's Disease Rating Scale Part III; DEBs, dream enactment behaviors; ESS, Epworth Sleepiness Scale; SCOPA‐AUT, Scales for Outcomes in Parkinson's Disease–Autonomic; MCI, mild cognitive impairment; PDD, Parkinson's disease dementia; MoCA, Montreal Cognitive Assessment; SFT, Semantic Fluency Test; SDMT, Symbol Digit Modalities Test; LNS, Letter‐Number Sequencing Test; HVLT‐R, Hopkins Verbal Learning Test Revised; JoLO, Benton Judgment of Line Orientation Test; GDS‐15, Geriatric Depression Scale 15‐item; STAI, Spielberger's State–Trait Anxiety Inventory.
Cognitive Decline
At follow‐up, the mean MoCA score for the overall group had decreased by 0.39 points from baseline (26.64 vs. 27.03). Patients with aggressive dreams had a mean MoCA score decrease 33 times greater than patients without aggressive dreams (−1.40 vs. −0.04; P = 0.014). Furthermore, the decline in MoCA scores for patients with aggressive dreams was significantly reduced (25.5 vs. 26.9; P = 0.010), whereas the decline for patients without aggressive dreams was not (27.04 vs. 27.08; P = 0.65).
A total of 43 patients (19.2%) at follow‐up had cognitive impairment compared with 32 (14.3%) with MCI at baseline. Patients with aggressive dreams were nearly twice as likely to have cognitive impairment at follow‐up compared with patients without aggressive dreams (29.3% vs. 15.7%; P = 0.023). With regard to affected cognitive domains, patients with frequent aggressive dreams at baseline performed significantly worse on tests of attention and working memory at follow‐up when compared with patients without aggressive dreams (see Table 2).
In univariate linear analysis, there was a significant association between dream content and MoCA change (β = −1.35; P = 0.003) and between DEBs and MoCA change (β = −1.04; P = 0.009) (Table 3). However, there was no association between dream vividness and MoCA change (P = 0.18). In the adjusted model for dream content, the association with MoCA change remained significant (β = −1.49; 95% confidence interval [CI], −0.61 to −2.38; P = 0.001). In the adjusted model for DEBs, the association with MoCA change also remained significant (β = −1.03; 95% CI, −0.25 to −1.82; P = 0.010), although the effect size was smaller and of less significance than for dream content.
TABLE 3.
Predictors of MoCA change
| Variable | Univariate model | Multivariate model | ||||||
|---|---|---|---|---|---|---|---|---|
| t | β | SE | P | t | β | SE | P | |
| Age | −3.14 | −0.06 | 0.02 | 0.002 | −3.19 | −0.07 | 0.02 | 0.002 |
| Male sex | 1.77 | 0.75 | 0.42 | 0.079 | 1.32 | 0.54 | 0.41 | 0.19 |
| Education | 0.87 | 0.06 | 0.07 | 0.39 | 1.25 | 0.09 | 0.07 | 0.21 |
| Disease duration | 0.65 | 0.09 | 0.14 | 0.52 | 0.51 | 0.07 | 0.13 | 0.61 |
| MDS‐UPDRS III | −0.06 | −0.001 | 0.03 | 0.95 | 1.01 | 0.03 | 0.03 | 0.31 |
| Aggressive dreams | −3.02 | −1.35 | 0.45 | 0.003 | −3.34 | −1.49 | 0.45 | 0.001 |
| Vivid dreams | −1.35 | −0.55 | 0.41 | 0.18 | – | – | – | – |
| DEBs | −2.62 | −1.04 | 0.40 | 0.009 | – | – | – | – |
| GDS‐15 | −1.88 | −0.17 | 0.09 | 0.06 | – | – | – | – |
| STAI | −2.13 | −0.03 | 0.01 | 0.03 | ‐ | ‐ | ‐ | ‐ |
| ESS | −1.03 | −0.07 | 0.06 | 0.30 | – | – | – | – |
| SCOPA‐AUT | −4.40 | −0.13 | 0.03 | <0.001 | – | – | – | – |
Values in bold denote significant predictors of MoCA change in univariate and adjusted multivariate models in the primary analysis.
Abbreviations: MoCA, Montreal Cognitive Assessment; SE, standard error; MDS‐UPDRS III, Movement Disorder Society–Unified Parkinson's Disease Rating Scale Part III; DEBs, dream enactment behaviors; GDS‐15, Geriatric Depression Scale 15‐item; STAI, Spielberger's State–Trait Anxiety Inventory; ESS, Epworth Sleepiness Scale; SCOPA‐AUT, Scales for Outcomes in Parkinson's Disease–Autonomic.
In univariate logistic analysis, there was a significant positive association between cognitive impairment and both dream content (odds ratio [OR], 2.23; P = 0.025) and DEBs (OR, 2.04; P = 0.050). However, there was no association between dream vividness (P = 0.97) and cognitive impairment. In the adjusted model for dream content, the association with cognitive impairment remained significant (OR, 2.35; 95% CI, 1.12–4.91; P = 0.023) (Table 4). However, DEBs no longer predicted cognitive impairment in the respective adjusted model (P = 0.069).
TABLE 4.
Predictors of cognitive impairment
| Variable | Univariate model | Multivariate model | ||||
|---|---|---|---|---|---|---|
| OR | SE | P | OR | SE | P | |
| Age | 1.02 | 0.02 | 0.42 | 1.00 | 0.02 | 0.64 |
| Male sex | 0.47 | 0.41 | 0.064 | 0.48 | 0.42 | 0.079 |
| Education | 0.95 | 0.06 | 0.39 | 0.94 | 0.06 | 0.30 |
| Disease duration | 0.84 | 0.14 | 0.22 | 0.83 | 0.14 | 0.18 |
| MDS‐UPDRS III | 1.02 | 0.02 | 0.38 | 1.02 | 0.02 | 0.50 |
| Aggressive dreams | 2.23 | 0.36 | 0.025 | 2.35 | 0.38 | 0.023 |
| Vivid dreams | 0.99 | 0.35 | 0.97 | – | – | – |
| DEBs | 2.04 | 0.36 | 0.050 | – | – | – |
| GDS‐15 | 1.16 | 0.07 | 0.03 | – | – | – |
| STAI | 1.03 | 0.10 | 0.002 | – | – | – |
| ESS | 1.13 | 0.05 | 0.03 | – | – | – |
| SCOPA‐AUT | 1.05 | 0.03 | 0.052 | – | – | – |
Values in bold denote significant predictors of cognitive impairment in univariate and adjusted multivariate models in the primary analysis.
Abbreviations: OR, odds ratio; SE, standard error; MDS‐UPDRS III, Movement Disorder Society–Unified Parkinson's Disease Rating Scale Part III; DEBs, dream enactment behaviors; GDS‐15, Geriatric Depression Scale 15‐item; STAI, Spielberger's State–Trait Anxiety Inventory; ESS, Epworth Sleepiness Scale; SCOPA‐AUT, Scales for Outcomes in Parkinson's Disease–Autonomic.
Motor Decline
At follow‐up, the mean MDS‐UPDRS III score for the overall group had increased by 12.1 points from baseline (31.2 vs. 19.1). The MDS‐UPDRS III scores of patients with PD with (36.0 vs. 21.6; P < 0.001) and without aggressive dreams (29.6 vs. 18.3; P < 0.001) had significantly increased from baseline. However, patients with aggressive dreams had a mean MDS‐UPDRS III change on average 27% greater than patients without aggressive dreams, although this did not reach significance (14.4 vs. 11.3; P = 0.13).
At follow‐up, 14 patients (6.3%) had progressed to H&Y ≥ 3. Patients with PD with aggressive dreams were more than 5 times more likely than patients without aggressive dreams to have progressed to H&Y ≥ 3 (15.5% vs. 3.0%; P = 0.001).
In univariate linear analyses, no association was found between MDS‐UPDRS III change and dream content (P = 0.075), DEBs (P = 0.88), or dream vividness (P = 0.63). However, in the respective adjusted linear models, the association between dream content and MDS‐UPDRS III change became significant (β = 4.64; 95% CI, 1.27–8.02; P = 0.007) (Table 5), whereas the associations for DEBs (P = 0.74) and dream vividness (P = 0.42) remained nonsignificant.
TABLE 5.
Predictors of MDS‐UPDRS III change
| Variable | Univariate model | Multivariate model | ||||||
|---|---|---|---|---|---|---|---|---|
| t | β | SE | P | t | β | SE | P | |
| Age | 1.44 | 0.11 | 0.08 | 0.15 | 2.26 | 0.18 | 0.08 | 0.03 |
| Male sex | −1.84 | −3.02 | 1.64 | 0.067 | −1.59 | −2.52 | 1.58 | 0.11 |
| Education | −0.97 | −0.26 | 0.27 | 0.33 | −1.03 | −0.27 | 0.26 | 0.30 |
| Disease duration | −0.50 | −0.03 | 0.53 | 0.96 | 0.67 | −0.34 | 0.51 | 0.50 |
| MDS‐UPDRS III | −3.61 | −0.34 | 0.10 | 0.001 | −4.45 | −0.44 | 0.10 | <0.001 |
| Aggressive dreams | 1.78 | 3.15 | 1.76 | 0.075 | 2.71 | 4.64 | 1.71 | 0.007 |
| Vivid dreams | 0.49 | 0.77 | 1.59 | 0.63 | – | – | – | – |
| DEBs | −0.16 | −0.25 | 1.57 | 0.88 | – | – | – | – |
| GDS‐15 | 1.18 | 0.40 | 0.34 | 0.24 | – | – | – | – |
| STAI | −0.08 | −0.004 | 0.05 | 0.94 | – | – | – | – |
| ESS | −0.65 | −0.16 | 0.25 | 0.52 | – | – | – | – |
| SCOPA‐AUT | 0.68 | 0.08 | 0.12 | 0.50 | – | – | – | – |
Values in bold denote significant predictors of MDS‐UPDRS III change in univariate and adjusted multivariate models in the primary analysis.
Abbreviations: MDS‐UPDRS III, Movement Disorder Society–Unified Parkinson's Disease Rating Scale Part III; SE, standard error; DEBs, dream enactment behaviors; GDS‐15, Geriatric Depression Scale 15‐item; STAI, Spielberger's State–Trait Anxiety Inventory; ESS, Epworth Sleepiness Scale; SCOPA‐AUT, Scales for Outcomes in Parkinson's Disease–Autonomic.
In univariate logistic analysis, there was a significant positive association between dream content and progression to H&Y ≥ 3 (OR, 5.91; P = 0.002). However, there was no association found between DEBs (P = 0.25) or dream vividness (P = 0.40) and progression to H&Y ≥ 3. In the adjusted model for dream content, the association with progression to H&Y ≥ 3 remained significant (OR, 5.82; 95% CI, 1.70–19.94; P = 0.005) (Table 6). However, DEBs (P = 0.53) and dream vividness (P = 0.38) remained nonsignificant in their respective adjusted models.
TABLE 6.
Predictors of progression to Hoehn and Yahr stage ≥ 3
| Variable | Univariate model | Multivariate model | ||||
|---|---|---|---|---|---|---|
| OR | SE | P | OR | SE | P | |
| Age | 1.06 | 0.03 | 0.077 | 1.07 | 0.04 | 0.07 |
| Male sex | 1.57 | 0.56 | 0.43 | 2.56 | 0.64 | 0.14 |
| Education | 1.06 | 0.10 | 0.56 | 0.98 | 0.11 | 0.83 |
| Disease duration | 1.08 | 0.17 | 0.66 | 0.98 | 0.20 | 0.93 |
| MDS‐UPDRS III | 1.10 | 0.03 | 0.003 | 1.08 | 0.03 | 0.02 |
| Aggressive dreams | 5.91 | 0.58 | 0.002 | 5.82 | 0.63 | 0.005 |
| Vivid dreams | 1.67 | 0.61 | 0.40 | – | – | – |
| DEBs | 2.03 | 0.61 | 0.25 | – | – | – |
| GDS‐15 | 1.21 | 0.10 | 0.04 | – | – | – |
| STAI | 1.02 | 0.02 | 0.17 | – | – | – |
| ESS | 1.12 | 0.08 | 0.18 | – | – | – |
| SCOPA‐AUT | 1.08 | 0.04 | 0.04 | – | – | – |
Values in bold denote significant predictors of motor deterioration in univariate and adjusted multivariate models in the primary analysis.
Abbreviations: OR, odds ratio; SE, standard error; MDS‐UPDRS III, Movement Disorder Society–Unified Parkinson's Disease Rating Scale Part III; DEBs, dream enactment behaviors; GDS‐15, Geriatric Depression Scale 15‐item; STAI, Spielberger's State–Trait Anxiety Inventory; ESS, Epworth Sleepiness Scale; SCOPA‐AUT, Scales for Outcomes in Parkinson's Disease–Autonomic.
Sensitivity Analyses
When further adjusting for MCI at baseline, the association between aggressive dream content and all motor and cognitive outcomes remained significant (P's < 0.05). When further adjusting for anxiety, depression, daytime sleepiness, and autonomic symptoms at baseline, the association between aggressive dream content and change in MoCA scores, change in MDS‐UPDRS III scores, and progression to H&Y ≥ 3 remained significant (P's < 0.05). However, the association between aggressive dream content and the development of cognitive impairment was reduced to a trend toward significance (P = 0.069). Finally, when including both aggressive dream content and DEBs as covariates, the association between aggressive dream content and MoCA change, MDS‐UPDRS III change, and progression to H&Y ≥ 3 again remained significant (P's < 0.05). However, the association with cognitive impairment was once more reduced to a trend toward significance (P = 0.070).
Discussion
In this prospective study using the PPMI cohort, it is demonstrated for the first time that subjectively reported dream content alterations in newly diagnosed PD may independently predict motor decline. It was found that patients with frequent aggressive dreams at baseline were more than five times more likely (adjusted OR, 5.82) to have progressed to H&Y ≥ 3 within 5 years. This widens the findings of previous studies that identified a cross‐sectional association between aggressive dream content and higher H&Y stages in PD.14 In addition, this study has shown for the first time that altered dream content in PD is associated with a faster increase in MDS‐UPDRS III scores.
The results also confirm findings from smaller studies that showed dream content alterations in PD could represent independent predictors of cognitive decline.12, 13 In the present study, the decline in MoCA scores was 33 times greater in patients with PD who reported frequent aggressive dreams at baseline (P = 0.001). Notably, the minor decline in MoCA scores in the group without aggressive dreams was not statistically significant.
In an earlier study, in only 23 patients,13 negative emotions in patients' dreams predicted a faster rate of MoCA decline, whereas aggression in dreams did not. Although aggression instigated by the dreamer—as opposed to aggression directed toward the dreamer—had the largest effect size in the aforementioned study, the p‐value nevertheless failed to reach statistical significance (P = 0.084). However, in the present study, it was found that aggressive dream content even predicted the development of cognitive impairment using standardized neuropsychological tests (adjusted OR, 2.35).
It was also found that patients with PD with frequent aggressive dreams had significantly worse performance on tests of attention and working memory at follow‐up (Table 2) despite the 2 groups being similar at baseline (Table 1). However, there were no between group differences at either baseline or follow‐up with respect to other cognitive domains. As such, these results are consistent with previous cross‐sectional findings, linking aggressive dream content in early PD to frontal dysfunction.6
Although the majority of previous studies have found that RBD (both probable and polysomnography‐confirmed) in PD predicts cognitive decline,26, 27, 28 there have, however, been recent conflicting findings.13, 29, 30 Nonetheless, given that it is well established that RBD is associated with worse cognition in PD cross‐sectionally and that idiopathic RBD is associated with the subsequent development of dementia and parkinsonism, we would therefore expect an association between RBD and cognitive and/or motor decline in PD to be genuine. Nevertheless, in the present study, no association was found between DEBs and rate of change in MDS‐UPDRS III scores or with progression to H&Y ≥ 3. However, an association was found between DEBs and MoCA decline and between DEBs and progression to cognitive impairment, as expected, although these effects were smaller and less significant than for aggressive dream content. However, after controlling for potential confounders in the multivariate analysis, DEBs no longer predicted cognitive impairment. Furthermore, when the analyses were repeated after including both aggressive dream content and DEBs as covariates, DEBs were no longer significant predictors of MoCA decline (data not shown). In contrast, when aggressive dream content and DEBs were included as covariates, the associations between dream content and both motor and cognitive outcomes remained robust.
As such, this study suggests that RBD, a condition for which recurrent DEBs are the defining clinical feature,31 may not independently increase the risk for cognitive or motor decline in PD. Interestingly, although not all patients with RBD report exclusively aggressive dreams,32 aggression in dreams is nevertheless more common in PD with RBD than in PD without RBD3, 4, 7—as this study further confirmed (Fig. 1). Therefore, these results may indicate that the relationship between RBD and cognitive decline in PD is mediated by dream content alterations. Consistent with this hypothesis, a recent study in PD found negative dream content, but not polysomnography‐confirmed RBD, to predict a faster decline in MoCA scores in a multivariable analysis.13
FIG. 1.
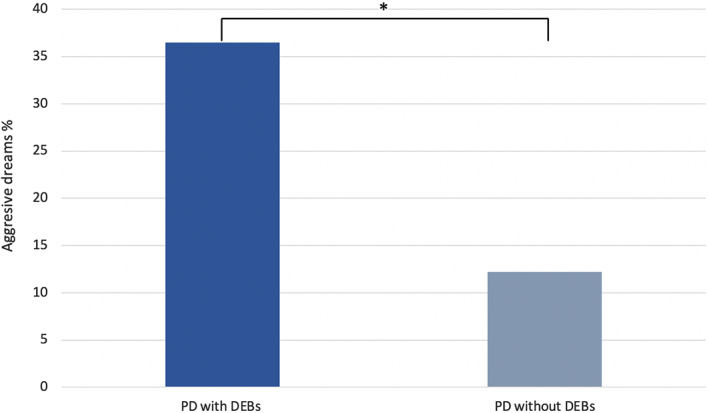
The dark blue (36.5%) and light blue (12.2%) bars represent the percentage of patients with PD with and without a positive history for dream enactment behaviors (DEBs) at baseline reporting frequent aggressive dreams. *P < 0.001, χ2 test.
Less than 3% of older adults report weekly nightmares.33 However, in PD and PDD, this increases to 18% and 78%, respectively.7, 8 In dementia with Lewy bodies, this is even higher at 83%.8 In the current study, 25.9% of this exclusively nondemented PD cohort reported frequent aggressive dreams at baseline. Previous studies have confirmed that the elevated rate of aggression in dreams of patients with PD cannot be explained by increased daytime aggressiveness or heightened testosterone levels.4, 34 Although intriguingly, a recent neuroimaging study identified structural alterations in the frontal lobe (right superior frontal gyrus and right anterior cingulate) to be correlated with the frequency of distressing dreams in PD.35
The severity of nightmares in PD has also previously been shown to correlate with the severity of mood disturbances.23 In the present study, it was found that patients with PD with frequent aggressive dreams at baseline were both more depressed and more anxious, with this difference becoming even more pronounced at follow‐up (Table 2). Given the brain regions implicated in neurobiological studies of distressing dreams in PD,35, 36 overlap with the brain regions known to be involved in the downregulation of negative emotions,37 it is possible that severe frontal dysfunction in a subgroup of patients with PD leads to a reduced ability to downregulate negative emotions across both conscious states. This would explain the dysphoric, negatively toned, and aggressive dream content as well as daytime mood disturbances and would be consistent with the neurocognitive model of nightmares.38
The rate of PD progression in the initial years after diagnosis is highly variable.39 In recent years, it has been proposed that there are at least 3 subtypes of PD39 characterized by differing rates of neuropathologic progression.40 This would explain why some patients have a more rapid and diffuse progression than others. In line with this, several studies have aimed to predict motor and cognitive progression in PD using complex machine‐learning algorithms41 or clinical and nonclinical tests that can cumulatively take hours to administer42 or may be invasive. Despite these efforts, few variables have been shown to be strong predictors of motor or cognitive decline in PD, and even fewer have been shown to predict both.41, 42, 43
The current study suggests that in patients with early PD, a simple screening question from the diagnosing clinician at the initial visit, asking about their nightly dream experiences, could identify patients at substantially higher risk for motor and cognitive deterioration in the near future. Furthermore, dream content alterations are easily recognized by the patient, unlike RBD, in which half of the affected patients are unaware of their DEBs.44 As such, inquiring about dream content changes would be particularly advantageous for the purposes of clinical practice and research.
The main limitation of this study is the absence of polysomnography to confirm RBD. As such, the possibility that some of the patients with frequent aggressive dreams, but without a history of DEBs, did still have a mild form of RBD— detectable only via video‐polysomnography, cannot be definitively ruled out. However, given that a clinical history for DEBs is usually required to diagnose RBD (alongside REM Sleep Without Atonia [RSWA]) according to the International Classification of Sleep Disorders45 and that RSWA without coexisting DEBs predicts neither cognitive nor motor decline in PD,30, 46 it therefore seems unlikely that the inclusion of polysomnography would have meaningfully changed the results. In any case, the RBDSQ subscore corresponding to DEBs used in this study has previously been shown to have a high sensitivity (0.93) for detecting polysomnography‐confirmed RSWA.18 Another limitation of this study is that many patients from the baseline cohort did not have complete data at follow‐up. As such, there remains a possibility that loss to follow‐up was nonrandom, with patients having frequent aggressive dreams but milder disease progression being less likely to be followed up. However, there are no a priori reasons to believe that this would be likely.
The strengths of this study include its prospective nature, large sample size, longer follow‐up relative to previous studies, the availability of neuropsychological tests to determine cognitive impairment, and the fact that all patients were early stage and unmedicated at baseline. Furthermore, this study is the first multicenter investigation on the predictive value of dream content in PD. The inclusion of several sensitivity analyses made it possible to confirm that the difference in trajectories between patients with and without aggressive dreams could not be explained by confounding baseline variables and were independent of a history of DEBs. Future studies will need to confirm the neuroanatomical basis of altered dream content in PD, which may also help to inform pharmacological treatment strategies. In addition, subsequent studies should use more detailed questionnaires that have been designed specifically for assessing dream content47 to determine whether other changes besides increased aggression might also predict progression in PD.
In conclusion, this study has shown for the first time that frequent aggressive dreams in newly diagnosed PD may independently predict motor decline. Furthermore, this study has confirmed, in the largest cohort to date, that abnormal dream content in PD independently predicts cognitive decline. If replicated in independent cohorts, these findings could have significant value for the routine clinical evaluation of patients with PD. In addition, these findings could have important value for researchers by identifying a succinct questionnaire item that could be used in screening large PD cohorts. Finally, given that distressing dreams usually precede the onset of PD motor symptoms by more than a decade,48 this study suggests that a simple questionnaire item on dream content could even have potential for identifying prodromal PD in the general population.
Disclosures
Ethical Compliance Statement
Each Parkinson's Progression Markers Initiative site received approval from a local ethical committee on human experimentation before commencement of the study. Written informed consent was obtained from all individuals who participated in the study. The present study received approval from the University of Birmingham (reference no. ERN_21–0427). I confirm that I have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Funding Sources and Conflicts of Interest
The Parkinson's Progression Markers Initiative—a public–private partnership—is funded by The Michael J. Fox Foundation for Parkinson's Research and funding partners, including Abbvie, Allergan, Amathus Therapeutics, Avid Radiopharmaceuticals, BIAL Biotech, Biogen, BioLegend, Bristol‐Myers Squibb, Calico, Celgene, Denali, 4D Pharma plc, GE Healthcare, Genentech, GlaxoSmithKline, Golub Capital, Handl Therapeutics, Insitro, Janssen Neuroscience, Lilly, Lundbeck, Merck, Meso Scale Discovery, Neurocrine Biosciences, Pfizer, Piramal, Prevail, Roche, Sanofi Genzyme, Servier, Takeda, Teva, UCB, Verily, and Voyager Therapeutics. The author declares that there are no conflicts of interest relevant to this work.
Financial Disclosures for the Previous 12 Months
The author is funded by a National Institute for Health Research academic clinical fellowship.
Acknowledgments
I thank Professor Carl E. Clarke for helpful comments on this article. Data used in the preparation of this article were obtained from the Parkinson's Progression Markers Initiative (PPMI) database (www.ppmi-info.org/data). For up‐to‐date information on the study, visit www.ppmi-info.org. Data were downloaded from the PPMI database on December 22, 2020.
Relevant disclosures and conflicts of interest are listed at the end of this article.
References
- 1.Siclari F, Valli K, Arnulf I. Dreams and nightmares in healthy adults and in patients with sleep and neurological disorders. Lancet Neurol 2020;19:849–859. [DOI] [PubMed] [Google Scholar]
- 2.Chaudhuri KR, Prieto‐Jurcynska C, Naidu Y, et al. The nondeclaration of nonmotor symptoms of Parkinson's disease to health care professionals: an international study using the nonmotor symptoms questionnaire. Mov Disord 2010;25:704–709. [DOI] [PubMed] [Google Scholar]
- 3.Borek LL, Kohn R, Friedman JH. Phenomenology of dreams in Parkinson's disease. Mov Disord 2007;22:198–202. [DOI] [PubMed] [Google Scholar]
- 4.Fantini ML, Corona A, Clerici S, Ferini‐Strambi L. Aggressive dream content without daytime aggressiveness in REM sleep behavior disorder. Neurology 2005;65:1010–1015. [DOI] [PubMed] [Google Scholar]
- 5.De Gennaro L, Lanteri O, Piras F, et al. Dopaminergic system and dream recall: an MRI study in Parkinson's disease patients. Hum Brain Mapp 2016;37:1136–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bugalho P, Paiva T. Dream features in the early stages of Parkinson's disease. J Neural Transm 2011;118:1613–1619. [DOI] [PubMed] [Google Scholar]
- 7.Ylikoski A, Martikainen K, Partinen M. Parasomnias and isolated sleep symptoms in Parkinson's disease: a questionnaire study on 661 patients. J Neurol Sci 2014;346:204–208. [DOI] [PubMed] [Google Scholar]
- 8.Pistacchi M, Gioulis M, Contin F, Sanson F, Marsala SZ. Sleep disturbance and cognitive disorder: epidemiological analysis in a cohort of 263 patients. Neurol Sci 2014;35:1955–1962. [DOI] [PubMed] [Google Scholar]
- 9.Goetz CG, Ouyang B, Negron A, Stebbins GT. Hallucinations and sleep disorders in PD: ten‐year prospective longitudinal study. Neurology 2010;75:1773–1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moskovitz C, Moses H 3rd, Klawans HL. Levodopa‐induced psychosis: a kindling phenomenon. Am J Psychiatry 1978;135:669–675. [DOI] [PubMed] [Google Scholar]
- 11.Kim EJ, Baek JH, Shin DJ, et al. Correlation of sleep disturbance and cognitive impairment in patients with Parkinson's disease. J Mov Disord 2014;7:13–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kwon KY, Kang SH, Kim M, et al. Nonmotor symptoms and cognitive decline in de novo Parkinson's disease. Can J Neurol Sci 2014;41:597–602. [DOI] [PubMed] [Google Scholar]
- 13.Bugalho P, Ladeira F, Barbosa R, et al. Do dreams tell the future? Dream content as a predictor of cognitive deterioration in Parkinson's disease. J Sleep Res 2020;30:e13163. [DOI] [PubMed] [Google Scholar]
- 14.Paiva T, Bugalho P, Bentes C. Dreaming and cognition in patients with frontotemporal dysfunction. Conscious Cogn 2011;20:1027–1035. [DOI] [PubMed] [Google Scholar]
- 15.Kumar S, Bhatia M, Behari M. Sleep disorders in Parkinson's disease. Mov Disord 2002;17:775–781. [DOI] [PubMed] [Google Scholar]
- 16.Marek K, Chowdhury S, Siderowf A, et al. Parkinson's progression markers initiative. The Parkinson's Progression Markers Initiative (PPMI)—establishing a PD biomarker cohort. Ann Clin Transl Neurol 2018;31:1460–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stiasny‐Kolster K, Mayer G, Schäfer S, Möller JC, Heinzel‐Gutenbrunner M, Oertel WH. The REM Sleep Behavior Disorder Screening Questionnaire–a new diagnostic instrument. Mov Disord 2007;22:2386–2393. [DOI] [PubMed] [Google Scholar]
- 18.Bolitho SJ, Naismith SL, Terpening Z, et al. Investigating rapid eye movement sleep without atonia in Parkinson's disease using the rapid eye movement sleep behavior disorder screening questionnaire. Mov Disord 2014;29:736–742. [DOI] [PubMed] [Google Scholar]
- 19.Ehgoetz Martens KA, Lukasik EL, Georgiades MJ, et al. Predicting the onset of freezing of gait: a longitudinal study. Mov Disord 2018;33:128–135. [DOI] [PubMed] [Google Scholar]
- 20.Chahine LM, Urbe L, Caspell‐Garcia C, et al. Cognition among individuals along a spectrum of increased risk for Parkinson's disease. PLoS One 2018;13:e0201964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Litvan I, Aarsland D, Adler CH, et al. MDS task force on mild cognitive impairment in Parkinson's disease: critical review of PD‐MCI. Mov Disord 2011;26:1814–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Evans JR, Mason SL, Williams‐Gray CH, et al. The natural history of treated Parkinson's disease in an incident, community based cohort. J Neurol Neurosurg Psychiatry 2011;82:1112–1118. [DOI] [PubMed] [Google Scholar]
- 23.Borek LL, Kohn R, Friedman JH. Mood and sleep in Parkinson's disease. J Clin Psychiatry 2006;67:958–963. [DOI] [PubMed] [Google Scholar]
- 24.Paul F, Schredl M, Alpers GW. Nightmares affect the experience of sleep quality but not sleep architecture: an ambulatory polysomnographic study. Borderline Personal Disord Emot Dysregul 2015;2:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nielsen T, Paquette T, Solomonova E, et al. Changes in cardiac variability after REM sleep deprivation in recurrent nightmares. Sleep 2010;33:113–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bugalho P, Magriço M, Alves L, Borbinha C. Objective sleep data as predictors of cognitive decline in dementia with Lewy bodies and Parkinson's disease. Sleep Med 2021;80:273–278. [DOI] [PubMed] [Google Scholar]
- 27.Anang JB, Gagnon JF, Bertrand JA, et al. Predictors of dementia in Parkinson disease: a prospective cohort study. Neurology 2014;83:1253–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Duarte Folle A, Paul KC, Bronstein JM, Keener AM, Ritz B. Clinical progression in Parkinson's disease with features of REM sleep behavior disorder: a population‐based longitudinal study. Parkinsonism Relat Disord 2019;62:105–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mollenhauer B, Zimmermann J, Sixel‐Döring F, et al. Baseline predictors for progression 4 years after Parkinson's disease diagnosis in the De novo Parkinson cohort (DeNoPa). Mov Disord 2019;34:67–77. [DOI] [PubMed] [Google Scholar]
- 30.Bugalho P, Ladeira F, Barbosa R, et al. Polysomnographic predictors of sleep, motor and cognitive dysfunction progression in Parkinson's disease: a longitudinal study. Sleep Med 2021;77:205–208. [DOI] [PubMed] [Google Scholar]
- 31.Baltzan M, Yao C, Rizzo D, Postuma R. Dream enactment behavior: review for the clinician. J Clin Sleep Med 2020;16:1949–1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oudiette D, De Cock VC, Lavault S, et al. Nonviolent elaborate behaviors may also occur in REM sleep behavior disorder. Neurology 2009;10:551–557. [DOI] [PubMed] [Google Scholar]
- 33.Park D, Kim S, Shin C, Suh S. Prevalence of and factors associated with nightmares in the elderly in a population based cohort study. Sleep Med 2021;78:15–23. [DOI] [PubMed] [Google Scholar]
- 34.Chou KL, Moro‐De‐Casillas ML, Amick MM, et al. Testosterone not associated with violent dreams or REM sleep behavior disorder in men with Parkinson's. Mov Disord 2007;22:411–414. [DOI] [PubMed] [Google Scholar]
- 35.Radziunas A, Deltuva VP, Tamasauskas A, et al. Brain MRI morphometric analysis in Parkinson's disease patients with sleep disturbances. BMC Neurol 2018;18:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stavitsky K, McNamara P, Durso R, Harris E, Auerbach S, Cronin‐Golomb A. Hallucinations, dreaming, and frequent dozing in Parkinson disease: impact of right‐hemisphere neural networks. Cogn Behav Neurol 2008;21:143–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morawetz C, Bode S, Derntl B, et al. The effect of strategies, goals and stimulus material on the neural mechanisms of emotion regulation: a meta‐analysis of fMRI studies. Neurosci Biobehav Rev 2017;72:111–128. [DOI] [PubMed] [Google Scholar]
- 38.Nielsen T, Levin R. Nightmares: a new neurocognitive model. Sleep Med Rev 2007;11:295–310. [DOI] [PubMed] [Google Scholar]
- 39.Bloem BR, Okun MS, Klein C. Parkinson's disease. Lancet 2021;397:P2284–P2303. [DOI] [PubMed] [Google Scholar]
- 40.De Pablo‐Fernández E, Lees AJ, Holton JL, et al. Prognosis and neuropathologic correlation of clinical subtypes of Parkinson disease. JAMA Neurol 2019;76:470–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Latourelle JC, Beste MT, Hadzi TC, et al. Large‐scale identification of clinical and genetic predictors of motor progression in patients with newly diagnosed Parkinson's disease: a longitudinal cohort study and validation. Lancet Neurol 2017;16:908–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schrag A, Siddiqui UF, Anastasiou Z, Weintraub D, Schott JM. Clinical variables and biomarkers in prediction of cognitive impairment in patients with newly diagnosed Parkinson's disease: a cohort study. Lancet Neurol 2017;16:66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bugalho P, Ladeira F, Barbosa R, et al. Progression in parkinson's disease: variation in motor and non‐motor symptoms severity and predictors of decline in cognition, motor function, disability, and health‐related quality of life as assessed by two different methods. Mov Disord Clin Pract 2021. 10.1002/mdc3.13262 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fernández‐Arcos A, Iranzo A, Serradell M, et al. The clinical phenotype of idiopathic rapid eye movement sleep behavior disorder at presentation: a study in 203 consecutive patients. Sleep 2016;39:121–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.American Academy of Sleep Medicine . International Classification of Sleep Disorders. 3rd ed. Darien, IL: American Academy of Sleep Medicine; 2014. [Google Scholar]
- 46.Nomura T, Inoue Y, Kagimura T, Nakashima K. Clinical significance of REM sleep behavior disorder in Parkinson's disease. Sleep Med 2013;14:131–135. [DOI] [PubMed] [Google Scholar]
- 47.Godin I, Montplaisir J, Nielsen T. Dreaming and nightmares in REM sleep behavior disorder. Dreaming 2015;25:257–273. [Google Scholar]
- 48.Pont‐Sunyer C, Hotter A, Gaig C, et al. The onset of nonmotor symptoms in Parkinson's disease (the ONSET PD study). Mov Disord 2015;2:229–237. [DOI] [PubMed] [Google Scholar]


