Progression after anti-CD19 chimeric antigen receptor T (CAR T) cells is a major limitation of this novel form of immunotherapy. Proposed mechanisms of resistance include downregulation of target antigen and loss of CAR T-cell persistence.1 As part of a phase I, single-center clinical trial, patients with relapsed, refractory (R/R) B-cell non-Hodgkin lymphoma were given bispecific lentiviral anti-CD20, anti-CD19 (LV20.19) CAR T cells to mitigate resistance due to CD19 antigen loss.2,3 Among treatment failures, loss of CD19 was not identified and several patients who experienced treatment failures maintained high levels of CAR T cells at relapse.2 We hypothesized that T-cell exhaustion or tumor checkpoint upregulation could be contributing to treatment failures. One proposed approach to mitigate these effects in CAR T cells has been through PD-1 inhibitors. For patients who progress after anti-CD19 CAR T, there are reports suggesting that PD-1 inhibitors at time of relapse could lead to response in some.4-6 However, currently published data is limited to anti-CD19 CAR T cells with no data on the use of PD-1 inhibitors after bispecific CAR T cells. We describe two cases, detailing the clinical and immunophenotypic impacts of giving pembrolizumab, a PD-1 inhibitor, after failure of LV20.19 CAR T therapy.
In order to evaluate the impact of pembrolizumab on CAR T cells, cryopreserved peripheral blood mononuclear cell (PBMC) samples from two patients treated on a phase I clinical trial with LV20.19 CAR T cells (clinicaltrials gov. Identifier: NCT03019055) were thawed and analyzed by flow cytometry to determine immunophenotypic profile and persistence. Markers of T-cell exhaustion (PD1 [CD279], LAG3 [CD223] and TIM-3 [CD366]) and T-cell activation (CD154 [CD40L], CD25 [IL2RA], and CD137 [4-1BB]) were assessed before and after administration of PD-1 inhibitor. Institutional Review Board submission was deferred per institutional policy for case series including less than three patients.
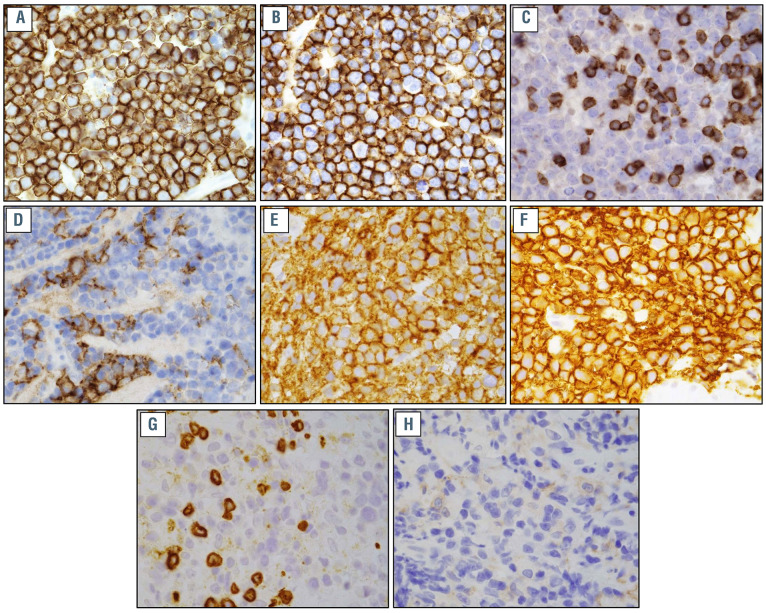
Patient 1 is a 56-year-old man diagnosed with stage IV c-MYC+ diffuse large B-cell lymphoma (DLBCL) who achieved a complete remission (CR) with R-CHOP. He progressed within 6 weeks and achieved a CR with R-ICE (rituximab, ifosfamide, carboplatin and etoposide). Autologous hematopoietic cell transplant (HCT) was planned but relapse occurred within 4 weeks. The patient’s disease was then refractory to R-GDP (rituximab, gemcitabine, cisplatin, and dexamethasone). LV20.19 CAR T cells were given at a dose of 2.5x105 cells/kg. His course was complicated by grade 1 cytokine release syndrome (CRS) and neurotoxicity which did not require treatment. Flow cytometry studies demonstrated exponential expansion of circulating CAR T cells (Figure 1A); however, day 28 positron emission tomography–computed tomography (PET/CT) demonstrated progressive disease (PD). Day 34 biopsy revealed no meaningful change in CD19 or CD20 expression (93% and 97% at relapse; 73% and 97% pre- CAR T) (Figure 2A and B). Infiltrating T cells in relapse biopsy were seen (Figure 2C). Flow cytometry revealed 21% of infiltrating T cells were CAR+ whereas 54% of circulating T cells were CAR+ at relapse. PD-L1 expression in the relapse specimen was 5-10% (Figure 2D). The patient’s pre-CAR T biopsy had absent PD-L1 expression. Pembrolizumab 200 mg was given intravenously (IV) on day 36. Day 51 CT revealed a decrease in the dominant retroperitoneal mass from 9.4x9 cm to 7.5x9.4 cm. On day 52, the patient was admitted with fever, consistent with grade 1 CRS. A second and third dose of pembrolizumab were given on days 60 and 81. Day 93 scans showed widespread disease progression.
Figure 1.
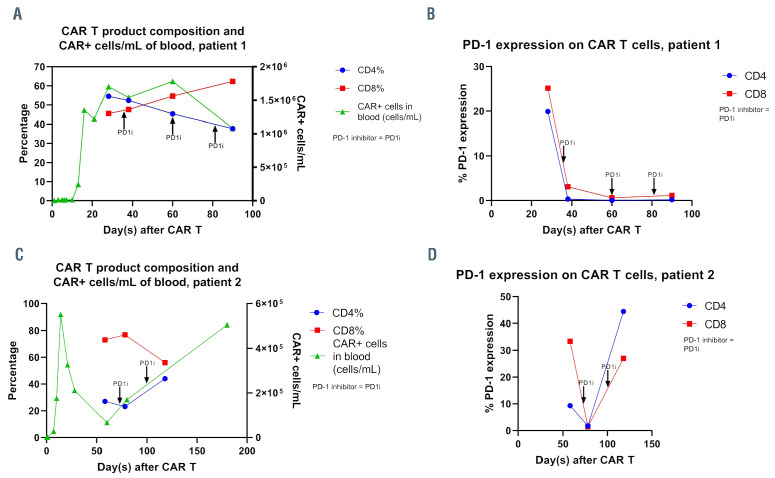
Chimeric antigen receptor (CAR) T-cell product composition, absolute numbers on of CAR+ cells in peripheral blood and PD-1 expression before and after PD-1 inhibitor. (A) Chimeric antigen receptor T (CAR T) product composition and CAR+ cells/mL of blood in patient 1; (B) PD-1 expression on CAR T cells in patient 1; (C) CAR T product composition and CAR+ cells/mL of blood in patient 2; (D) PD-1 expression on CAR T cells in patient 2.
Figure 2.
Immunohistochemical stains of post-chimeric antigen receptor (CAR) T-cell relapse tumor specimens. (A) CD19 stain, patient 1*; (B) CD20 stain, patient 1*; (C) CD3 stain, patient 1, showing T cells within the tumor specimen; (D) PDL1 stain, patient 1; (E) CD19 stain, patient 2*; (F) CD20 stain, patient 2*; (G) CD3 stain, patient 2, showing T cells within the tumor specimen; (H) PDL1 stain, patient 2. All images were taken at 1000x.*This work is licensed under a CC-BY Creative Commons attribution license, version 4.0. It was originally published in Nature Medicine and is attributed to Nirav N. Shah et al., and the original version can be found at the following URL: (https://www.nature.com/articles/s41591-020-1081-3). Minor formatting changes were made.
PBMC samples from patient 1 corresponding with days 28, 38, 60 and 90 were thawed and analyzed by flow cytometry. Composition of the circulating CAR T cells after pembrolizumab showed an increase in CD8:CD4 cell ratios and CAR T persistence remained high from days 28-90 (Figure 1A). PD-1 expression on CAR T cells decreased in a rapid and sustained fashion after pembrolizumab (Figure 1B). Other markers of T-cell exhaustion (LAG3 and TIM-3) and T-cell activation (CD40L, IL2RA, and 4-1BB) were minimally expressed and greatly unchanged after PD-1 inhibitor (not shown).
Patient 2 is a 69-year-old-man diagnosed with stage IV TP53-mutated DLBCL who achieved CR with R-CHOP but within 2 months had PD. He achieved CR with R-ICE and underwent autologous HCT. Three months after HCT the patient relapsed. LV20.19 cells were given at a dose of 2.5x106 cells/kg. Course was complicated by grade 1 CRS. Flow cytometry demonstrated exponential expansion of circulating LV20.19 CAR T cells (Figure 1C). Day 28 PET/CT showed a partial response (PR) but day 71 CT demonstrated PD. A biopsy confirmed relapse with no meaningful change in CD19 or CD20 expression (100% and 100% respectively at relapse; 100% and 99.5% pre- CAR T cells) (Figure 2E and F). Infiltrating T cells in the relapse biopsy were seen (Figure 2G). Flow cytometry revealed 18.5% of infiltrating T cells were CAR+ whereas 26% of circulating T cells were CAR+ at day 80.2 PD-L1 expression in the tumor sample was 1-5% (Figure 2H). Pembrolizumab 200 mg IV was given on day 73 and day 100 in combination with neck radiation. Day 120 scans demonstrated PD. The patient subsequently received rituximab, gemcitabine and oxaliplatin (R-GemOx) on day 127 and was transitioned to rituximab, bendamustine and polatuzumab (pola-BR) on day 141. Cycle 2 of pola-BR was given on day 162. The patient received six cycles of pola-BR achieving a CR and underwent allogeneic HCT. He remains in CR 1 year after transplant.
PBMC samples from patient 2 corresponding with days 58, 78 and 118 were thawed and analyzed by flow cytometry. Composition of the circulating CAR T cells after pembrolizumab showed minimal changes in CD8:CD4 cell ratios but a sustained rise in circulating CAR T cells (Figure 1C). PD-1 expression on CAR T cells initially decreased at day 78 but rose significantly by day 118 (Figure 1D). TIM- 3 expression decreased at day 78, then rose at day 118. LAG3 expression was low prior to pembrolizumab but rose after treatment (not shown). There were insufficient cells to analyze T-cell activation markers.
Mechanisms of resistance to bispecific CAR T are unclear to date. In both patients, target antigen loss was not identified, and there were high levels of CAR T persistence at relapse. While patient 1 had increased tumor bulk and an elevated pre-infusion lactate dehydrogenase (LDH) (501 mg/dL) which correlates with worse outcomes with CAR T therapy,7 patient 2 had no predictive markers of poor response. Off label pembrolizumab was administered to counter potential upregulation of tumoral checkpoint inhibitors and minimize T-cell exhaustion in the CAR T cells to augment CAR T efficacy. For patient 1, despite decreased PD-1 expression and an increase in cytotoxic T cells, there was minimal response with no meaningful durability. For patient 2, after pembrolizumab there was an initial decrease in PD-1 expression and increase in cytotoxic T cells, but this was quickly followed by increases in all markers of T-cell exhaustion and decrease in the cytotoxic T-cell ratio. For unclear reasons, CAR T levels in patient 2 continued to rise after PD-1 blockade despite the lack of efficacy. During this time the patient received RGemOx and pola-BR and ultimately achieved a CR with salvage therapy and remains in an ongoing remission postallogeneic HCT. Our experience appears similar to reports of PD-1 inhibitors after failure of anti-CD19 CAR T cells. While an initial report suggested pembrolizumab after progression with anti-CD19 CAR T enhanced CAR T activity in primary mediastinal B-cell lymphoma (PMBCL), this class of agents has meaningful single agent activity in PMBCL so it is unclear if the combinatorial regimen had added benefit.4,8 Another report described a patient with DLBCL with PET-CT confirmed progression on day 7 after axicabtagene ciloleucel.5 He was given nivolumab on days 11, 23 and 35 and day 35 CT showed PR, but day 64 scans demonstrated PD. In a prospective single-center study, only three of 11 patients who received pembrolizumab after failure of anti-CD19 CAR T had responses (one CR and two PR), which is only slightly higher than the single agent activity of PD-1 inhibitors in DLBCL.9 Although PDL1 expression was low in the post-CAR T relapse biopsies of these two patients at 1-10%, only approximately 30% of cases of DLBCL have ≥5% PD-L1 expression.10 Whereas PD-L1 expression seems to correlate with response rate in Hodgkin lymphoma and chronic lymphocytic leukemia, the predictive value of PD-L1 expression on response in DLBCL is less clear.11 Despite this, there remains high interest in combining PD-1 inhibitors with CAR T therapy, including an ongoing trial of pembrolizumab given with or immediately after dual targeted anti-CD19, anti-CD22 CAR T therapy.12 While this report and others have demonstrated low response rates with anti-PD1 at relapse after CAR T, the benefit of PD-1 inhibitors given concurrently with CAR T therapy remains unclear.7 Further research is needed to determine the etiology of treatment failures in these described cases. We have recently demonstrated that the baseline T-cell transcriptome along with cytokine signature and polyfunctionality of the CAR T product may predict clinical response in patients treated with LV20.19 CAR T cells.13,14 Whether this data can be used to create a more robust CAR T cell that overcomes treatment resistance remains to be seen; however, our experience suggests that after progression with bispecific CAR T cells, PD-1 inhibitors appear unlikely to provide clinically meaningful benefit.
References
- 1.Shah NN, Fry TJ. Mechanisms of resistance to CAR T cell therapy. Nat Rev Clin Oncol. 2019;16(6):372-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shah NN, Johnson BD, Schneider D, et al. Bispecific anti-CD20, anti-CD19 CAR T cells for relapsed B cell malignancies: a phase 1 dose escalation and expansion trial. Nat Med. 2020;26(10):1569-1575. [DOI] [PubMed] [Google Scholar]
- 3.Schneider D, Xiong Y, Wu D, et al. A tandem CD19/CD20 CAR lentiviral vector drives on-target and off-target antigen modulation in leukemia cell lines. J Immunother Cancer. 2017;5:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chong EA, Melenhorst JJ, Lacey SF, et al. PD-1 blockade modulates chimeric antigen receptor (CAR)-modified T cells: refueling the CAR. Blood. 2017;129(8):1039-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hill BT, Roberts ZJ, Xue A, Rossi JM, Smith MR. Rapid tumor regression from PD-1 inhibition after anti-CD19 chimeric antigen receptor T-cell therapy in refractory diffuse large B-cell lymphoma. Bone Marrow Transplant.2020;55(6):1184-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chong EA, Svoboda J, Dwivedy Nasta S, et al. Sequential anti-CD19 directed chimeric antigen receptor modified T-cell therapy (CART19) and PD-1 blockade with pembrolizumab in patients with relapsed or refractory B-cell non-hodgkin lymphomas. Blood. 2018; 132(Suppl 1):S4198. [Google Scholar]
- 7.Vercellino L, Di Blasi R, Kanoun S, et al. Predictive factors of early progression after CAR T-cell therapy in relapsed/refractory diffuse large B-cell lymphoma. Blood Adv. 2020;4(22):5607-5615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Armand P, Rodig S, Melnichenko V, et al. Pembrolizumab in relapsed or refractory primary mediastinal large B-cell lymphoma. J Clin Oncol. 2019;37(34):3291-3299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ansell SM, Minnema MC, Johnson P, et al. Nivolumab for relapsed/refractory diffuse large B-cell lymphoma in patients ineligible for or having failed autologous transplantation: a single-arm, phase II study. J Clin Oncol. 2019;37(6):481-489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Menter T, Bodmer-Haecki A, Dirnhofer S, Tzankov A.Evaluation of the diagnostic and prognostic value of PDL1 expression in Hodgkin and B-cell lymphomas. Hum Pathol. 2016;54:17-24. [DOI] [PubMed] [Google Scholar]
- 11.Xu-Monette ZY, Zhou J, Young KH. PD-1 expression and clinical PD-1 blockade in B-cell lymphomas. Blood. 2018;131(1):68-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ardeshna KM, Marzolini MAV, Norman J, et al. Phase 1/2 study of AUTO3 the first bicistronic chimeric antigen receptor (CAR) targeting CD19 and CD22 followed by an anti-PD1 in patients with relapsed/refractory (r/r) diffuse large B cell lymphoma (DLBCL): results of cohort 1 and 2 of the Alexander Study. Blood. 2019; 134(Suppl 1):S246. [Google Scholar]
- 13.Kearl T, Mei A, Brown R, et al. Single-cell RNA sequencing identifies expression patterns associated with clinical responses to dual-targeted CAR-T cell therapy. Blood. 2020;136(Suppl 1):S33-34. [Google Scholar]
- 14.Xu H, Chaney K, Johnson B, et al. Single-cell cytokine analysis of LV20.19 bispecific CAR T-cell products from a phase I clinical trial. Blood. 2020;136(Suppl 1):S22. [Google Scholar]