Despite an initial expectation of severe COVID-19 in patients with sickle cell disease (SCD) due to their wellknown susceptibility to infection-related acute respiratory distress syndrome, a low incidence of intensive care unit hospitalization and deaths were observed in SCD patients during the first outbreak of the pandemic.1 Regarding SCD children, it was unclear however, whether they were not prone to severe infection like their healthy peers or if they had been less exposed. A prospective outpatient survey of SARS-CoV-2 seroprevalence and related symptoms in SCD children was undertaken to address this issue and confirmed indeed very low morbidity despite substantial exposure (18.5 % immunoglobulin G [IgG] seropositivity). Subsequent monitoring of IgG titers showed a rapid and dramatic decrease after 3 months. In order to further address the absence of vulnerability of SCD children regarding SARSCoV- 2 complications, we analyzed the type I interferon (IFN-I) signature and found that 80% had an abnormally high IFN-I signature. Altogether, the basal activation of this pathway may explain partial protection against SARS-CoV-2 severe complications in SCD children.
The outbreak of the SARS-CoV-2 infection hit France at the beginning of 2020 resulting in a national shut down mid-March 2020. By mid-May 2020, the viral circulation was considered under control and the first wave of COVID-19 was over. COVID-19 was found to be particularly harmful in senior people and those presenting comorbidities and chronic illnesses. Regarding the general pediatric population, it was soon established that COVID-19 infection resulted in a very mild course.
The IFN-I response constitutes the major first line of defense against viruses. IFN-1 (including IFN-α and IFN-b) activate a powerful antiviral defense program of hundreds of interferon-stimulated genes (ISG), which have the capacity to interfere with every step of viral replication.2 Conversely, an exacerbated inflammatory response has been associated with a highly impaired IFNI response, characterized by low IFN-α production and activity in patients with severe COVID-19. A reduced type IFN-I signature in bronchoalveolar lavage macrophages was also demonstrated, altogether suggesting that IFN-I deficiency could be a hallmark of severe COVID-19.3
SCD was initially regarded as a disease potentially favoring poor outcome in case of SARS-CoV-2 infection because vaso-occlusive events are often triggered by infectious agents,4 particularly the acute chest syndrome (ACS), a severe acute respiratory distress syndrome. Previous viral epidemics like influenza H1N1 have been associated with increased morbidity and mortality in this population.5 Early reports of COVID-19 infection in SCD adult and pediatric patients were in line with expected severity.6 However, these reports focused on hospitalized patients with positive SARS-CoV-2 reverse transcription polymerase chain reaction (RT-PCR) and were hence potentially skewed towards severity. Moreover, comorbidities such as obesity or diabetes were generally not collected, nor were the local socio-economic conditions such as access to care, that may further influence outcomes. Last, but not least, these reports did not address the overall exposure of SCD patients to SARS-CoV-2.
In order to decipher the actual vulnerability of SCD children to SARS-CoV-2, we set up a prospective outpatient survey of SARS-CoV-2 seroprevalence and clinical symptoms during the first wave of COVID-19 outbreak. This survey took place in the largest pediatric SCD center of the Parisian area (Robert Debré Hospital) during the period ranging from 11/05/2020 to 31/08/2020. Symptoms compatible with COVID-19 were collected (fever, cough, loss of taste or smell, myalgia, fatigue) for all members living in the same household. COVID-19 serology was based on the detection of the IgG antinucleocapsid of SARS-CoV-2 in serum by chemiluminescent microparticle immuno assay (CMIA) (Architect- Abbott). A rate <0.49 was considered negative, a rate between 0.49 and 1.68 was considered intermediate, and a rate ≥1.68 was considered positive by the manufacturer, with a specificity of 100%.7
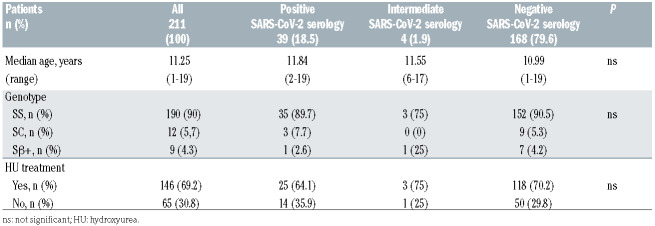
A total of 211 consecutive SCD children, all black Africans born in France, aged 1 to 19 years old (median age 11.25 years, interquartile range [IQR], 6.8-14.2) were analyzed, with 18.5 % (n=39) of them showing a positive SARS-CoV-2 serology. Clinical characteristics of patients are summarized in Table 1. Among seropositive patients, none had displayed symptoms except one who was hospitalized for mild vaso-occlusive symptoms with a favorable outcome. This cohort displayed a higher seroprevalence than that reported to date in children in Europe (0.8% among children aged 5-9 years-old, 9.6% among children aged 10-19 in Switzerland and 3.4% among children aged 5-19 in Spain8,9) albeit possibly comparable to the general pediatric population living in the same area characterized by a low socio-economic level (unavailable public data to date). Notwithstanding, this rather high rate of seropositivity shows that SCD children were actually exposed to SARS-CoV-2 and presented indeed a benign clinical course, comparable to the general population of the same age.
Table 1.
Patients’ characteristics.
This finding was somewhat puzzling given the deleterious inflammatory potential of SARS-CoV-2 infection and the involvement of inflammation in the pathobiology of SCD. An uncontrolled multifactorial inflammatory response (notably via activation of neutrophils and platelets) is responsible for both acute vaso-occlusive events (VOE) and longer-term organ damage. Importantly, children with SCD are, like their adult counterparts, susceptible to viral factors triggering the occurrence of ACS.4
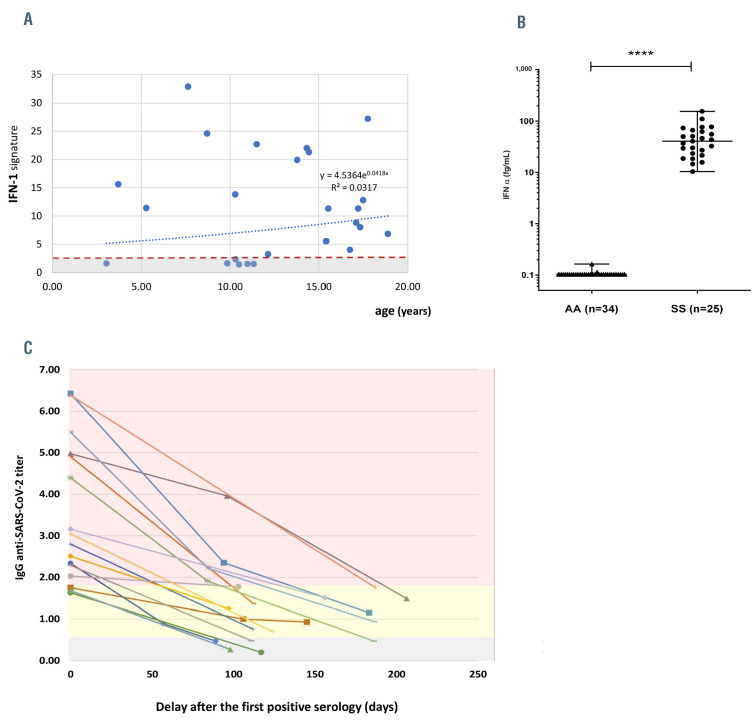
One explanation may pertain to a modified IFN basal status in SCD. Recently, it was shown that homozygous SCD patients had an increased basal high plasmatic IFN- α concentration associated with an abnormal activation of the IFN-I signaling pathway in neutrophils.10 Given that during SARS-CoV-2 infection, an increased IFN-I plasmatic level contributes to control viral load by regulating the expression of the cellular entry receptor for SARS-Cov-2 (ACE2), we sought to further explore the potential protective role of this pathway, by analyzing the IFN-I signature11 in a subgroup of children, regardless of their seroprevalence and at steady state (i.e., >2 months of a VOE or infectious episode). Of note, we did not test this pathway during an acute COVID-19 infection. Among 25 randomly selected homozygous patients, 80% of them (n=20) had an abnormally high IFN-I signature, with a median score of 11.3 (IQR, 3.2-32.9, normal range, <2.3) and a high plasmatic level of IFN-α (median 40.6 fg/mL; IQR, 22.4-64.4, normal range, <0.1), indicating basal activation of the IFN-I pathway in a large majority of patients, independently of age or chronic treatment with hydroxyurea (HU) (Figure 1A and B). We found no statistical correlation between the IFN-I signature and seroprevalence.
A number of factors specific to SCD may trigger IFN-I and explain its activation at steady state. Chronic hemolysis, for instance, generates circulating cell-free heme, which is a major erythrocyte damage-associated molecular pattern (DAMP) susceptible to trigger activation of the IFN-1 pathway. DAMP along with reactive oxygen species generation, Toll-like receptor 4 activation, neutrophil extracellular trap generation, DNA, and other unknown factors contribute to the baseline sterile inflammation in SCD by activating the inflammasome pathway in vascular and inflammatory cells. While this is a promising hypothesis, further studies will be needed to better decipher the underlying pathway of IFN-I activation in SCD as well as its specific contribution in the protection of SCD patients from serious COVID-19 complications.
Figure 1.
Interferon-I signature and kinetics of SARS-CoV-2 serology in sickle cell disease patients. (A) Interferon-I (IFN-I) signature in 25 sickle cell disease (SCD) patients according to age. IFN-I signature was considered increased above a score of 2.3. (B) Basal IFNα concentration in plasma from healthy donors (AA, n=34) and SS patients (SS, n=25) using a digital enzyme-linked immunsorbent assay (SIMOA). (C) Evolution of serological titer (immunoglobulin G [IgG]) in 17 SCD patients following the first wave of the SARSCoV- 2 epidemic. The different zones represent the level of IgG titers and interpretation: grey: negative IgG titers; yellow: intermediate IgG titers; red: positive IgG titers.
Finally, we monitored the serological titers in a subgroup of 17 seropositive patients to explore the persistence of humoral immunity. We observed a rapid and constant decrease in IgG levels of 63.4% after 3 months (Figure 1C), a decrease which may be greater than in the general population.12 In line, we observed one case of a 17-year-old homozygous patient considered as a having a severe phenotype of SCD (past history of ACS and frequent VOE). He had at the beginning of the study period a positive SARS-CoV-2 serology (titer of 2.3 UI/L) with no history of COVID-19 symptoms. He presented 90 days later with mild fever and a positive SARS-CoV-2 RT-PCR. His concomitant serology was negative and subsequent controls showed no secondary increase in IgG titers.
To our knowledge, there is no data on the duration of humoral immunity in SCD patients against SARS-CoV-2. These patients may also present an alteration of their immunity against other respiratory viruses such as influenza.13 Transient humoral immunity regarding the anti-nucleocapsid IgG response has been demonstrated in other populations, especially those with mild symptoms. 14 A recent study in a pediatric population showed an anti-nucleocapsid IgG decline 60 days after the first positive RT-PCR test but a positive titer still present at 90 days.15 More data on SCD patients are needed to confirm these findings.
In conclusion, SCD children were exposed to SARSCoV- 2 at least as much as their healthy peers during the first wave of the pandemic in France but despite theoretical susceptibility, they presented no severe complications. Nonetheless, humoral immunity appears to be transient in these patients and a second COVID-19 infection may occur. The basal activation of the IFN-I pathway in a majority of homozygous patients may represent a potential protective state to better control viral load. These findings raise important questions regarding the management of the ongoing pandemic in SCD patients. The negative outcome of COVID-19 in SCD patients in some reports may pertain to other factors including access to care and comorbidities, rather than SCD itself.6 Addressing these additional aspects may prove an effective strategy.
Acknowledgments
We are indebted to Labex GR-EX and URGEB Hôpital Universitaire Robert Debré.
References
- 1.Arlet J-B, de Luna G, Khimoud D, et al. Prognosis of patients with sickle cell disease and COVID-19: a French experience. Lancet Haematol. 2020;7(9):e632-e634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Park A, Iwasaki A.Type I and type III interferons – induction, signaling, evasion, and application to combat COVID-19. Cell Host Microbe. 2020;27(6):870-878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hadjadj J, Yatim N, Barnabei L, et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science. 2020;369(6504):718-724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vichinsky EP, Styles LA, Colangelo LH, Wright EC, Castro O, Nickerson B.Acute chest syndrome in sickle cell disease: clinical presentation and course. Cooperative Study of Sickle Cell Disease. Blood. 1997;89(5):1787-1792. [PubMed] [Google Scholar]
- 5.Inusa B, Zuckerman M, Gadong N, et al. Pandemic influenza A (H1N1) virus infections in children with sickle cell disease. Blood. 2010;115(11):2329-2330. [DOI] [PubMed] [Google Scholar]
- 6.Minniti CP, Zaidi AU, Nouraie M, et al. Clinical predictors of poor outcomes in patients with sickle cell disease and COVID-19 infection. Blood Adv. 2021;5(1):207-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meschi S, Colavita F, Bordi L, et al. Performance evaluation of Abbott ARCHITECT SARS-CoV-2 IgG immunoassay in comparison with indirect immunofluorescence and virus microneutralization test. J Clin Virol. 2020;129:104539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stringhini S, Wisniak A, Piumatti G, et al. Seroprevalence of anti- SARS-CoV-2 IgG antibodies in Geneva, Switzerland (SEROCoVPOP): a population-based study. Lancet. 2020;396(10247):313-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pollán M, Pérez-Gómez B, Pastor-Barriuso R, et al. Prevalence of SARS-CoV-2 in Spain (ENE-COVID): a nationwide, populationbased seroepidemiological study. Lancet. 2020;396(10250):535-544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hermand P, Azouzi S, Gautier EF, et al. The proteome of neutrophils in sickle cell disease reveals an unexpected activation of interferon alpha signaling pathway. Haematologica. 2020;105(12):2851-2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pescarmona R, Belot A, Villard M, et al. Comparison of RT-qPCR and Nanostring in the measurement of blood interferon response for the diagnosis of type I interferonopathies. Cytokine. 2019;113:446-452. [DOI] [PubMed] [Google Scholar]
- 12.Dan JM, Mateus J, Kato Y, et al. Immunological memory to SARSCoV- 2 assessed for up to 8 months after infection. Science. 2021;371(6529):eabf4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nagant C, Barbezange C, Dedeken L, et al. Alteration of humoral, cellular and cytokine immune response to inactivated influenza vaccine in patients with Sickle Cell Disease. PLoS One. 2019;14(10):e0223991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Long Q-X, Liu B-Z, Deng H-J, et al. Antibody responses to SARSCoV- 2 in patients with COVID-19. Nat Med. 2020;26(6):845-848. [DOI] [PubMed] [Google Scholar]
- 15.Interiano C, Muze S, Turner B, et al. Longitudinal evaluation of the Abbott ARCHITECT SARS-CoV-2 IgM and IgG assays in a pediatric population. Pract Lab Med. 2021;25:e00208. [DOI] [PMC free article] [PubMed] [Google Scholar]