Abstract
Background
Continuous peripheral nerve blocks can be administered as continuous infusion, patient-controlled boluses, automated boluses, or a combination of these modalities.
Material/Methods
Ten patients undergoing either ankle (5) or distal radius (5) open reduction and internal fixation received single-injection ropivacaine sciatic nerve block or infraclavicular brachial plexus block and catheter. Infusion pumps were set to begin administering additional ropivacaine 6 h following the initial block as automated boluses supplemented with patient-controlled boluses.
Results
Patients had similar pain scores when compared to previously published controls; however, local anesthetic consumption was lower in the patients, resulting in increased infusion and analgesia duration by 1 or more days in each group.
Conclusions
For infraclavicular and popliteal sciatic catheters, automated boluses may provide a longer duration of analgesia than continuous infusions following painful hand and ankle surgeries, respectively.
Keywords: Acute Pain, Nerve Block, Ultrasonography
Background
Continuous peripheral nerve blocks (cPNB) are frequently utilized to provide prolonged analgesia after surgery, leading to decreased opioid consumption and improved patient quality of life [1]. There are 3 general methods that are frequently combined for dosing of local anesthetic in a cPNB: continuous infusion, patient-administered boluses, and automated boluses.
Continuous infusion with patient-administered boluses is the most commonly utilized dosing strategy [1], providing the benefits of a constant relatively low-dose infusion of local anesthetic with boluses that can be initiated by the patient at times of increased pain.
Automated bolus dosing combined with patient-controlled boluses for popliteal sciatic nerve blocks has been shown to provide superior analgesia compared to a continuous infusion with patient-controlled boluses in some situations [2,3]. Bolus dosing has also been demonstrated to increase the duration of perineural ropivacaine infusions relative to continuous infusions [4].
The use of patient-controlled boluses alone, without automated boluses or continuous infusion, is a dosing strategy associated with intermittent, dramatic increases in breakthrough pain [4,5]. This is likely the result of patients falling asleep, failing to administer boluses, and then awaking in pain. While this effect might be mitigated by utilizing automated as opposed to patient-controlled boluses, most ambulatory infusion pumps have not been capable, until recently, of combining automated and patient-controlled boluses [6].
Because long-acting local anesthetics are generally used for cPNBs [1], the duration between automated boluses could potentially be increased beyond the hourly dosing that has most commonly utilized with automated boluses [2,3], with a resulting decrease in local anesthetic consumption and longer duration of the therapy. If combined with a delay in the infusion start time, a feature available on some electronic infusion pumps but which has never been investigated, patients could conceivably receive a significantly longer duration of analgesia using the same volume of local anesthetic after painful upper and lower extremity surgeries.
Consent for Publication
The University of California, San Diego Institutional Review Board (San Diego, CA) waives the review of case reports and small series. Written consent for HIPAA authorization and permission for publication of non-identifying medical information was obtained from each patient.
Material and Methods
Ten patients presenting for either ankle (n=5) or distal radius (n=5) open reduction and internal fixation underwent placement of either a popliteal sciatic or infraclavicular brachial plexus perineural catheter, respectively.
Popliteal Sciatic Catheter Technique
Patients were placed in the prone position. A 13- to 6-MHz 38-mm linear array ultrasound transducer (HFL, Edge II; SonoSite, Bothell, WA, USA) was used to visualize the sciatic nerve in short axis and identify its bifurcation point. The skin proximal to the bifurcation was cleansed with chlorhexidine and a sterile drape was applied. The skin was anesthetized with 1% lidocaine. Using an in-plane technique, a 17-gauge (G) Tuohy needle (FlexTip Plus; Teleflex Medical, Research Triangle Park, NC, USA) was inserted in a lateral-to-medial direction and advanced under ultrasound guidance until adjacent to the sciatic nerve. Ropivacaine 0.5% with 1: 400 000 epinephrine (15–20 mL) was injected in a circumferential pattern around the sciatic nerve. A 19G flexible catheter (FlexTip Plus) was inserted through the Tuohy needle. It was visualized coiling adjacent to the sciatic nerve.
Infraclavicular Catheter Technique
Patients were placed in the supine position with the arm abducted at 90 degrees. The infraclavicular area was cleansed with chlorhexidine and a sterile drape was applied. An 8- to 5-MHz 11-mm curvilinear transducer (C11, Edge II, SonoSite) in a sterile sleeve was used to visualize the brachial plexus in the short-axis view. The skin was anesthetized with 1% lidocaine. Using an in-plane technique, a 17G Tuohy needle was inserted into the skin and advanced until it was adjacent to the medial cord of the brachial plexus. Ropivacaine 0.5% with 1: 400 000 epinephrine (20 mL) was injected adjacent to the medial, posterior, and lateral cords. A 19G flexible catheter was inserted through the Tuohy needle. It was visualized coiling posterior to the axillary artery.
Local Anesthetic Administration
A programmed 6-h delay was set for the automated bolus doses to increase the total duration of the initial single-injection block combined with the postoperative local anesthetic administration. Patients received intermittent boluses of ropivacaine 0.2% by using an ambulatory electronic pump (Nimbus™ II PainPRO, InfuTronix, Natick, MA) with a 500-mL reservoir of ropivacaine. For sciatic catheters, patients received an 8-mL bolus administered every 2 h with a patient-controlled 4-mL bolus available every 30 min. For infraclavicular catheters, patients received an 11-mL bolus administered every 2 h with a patient-controlled 4-mL bolus available every 30 min.
Patient Data Collection
Patients were called daily by an anesthesia provider to assess pain control over the previous 24 h. Specifically, patients were asked to report the lowest and highest pain scores they had experienced over the previous 24 h measured on a numeric rating scale from 0 to 10, with “0” indicating “no pain” and “10” indicating “worst imaginable pain” reported verbally to the provider. Patients were additionally asked to provide a number on the same scale representing an average pain score over the previous 24 h. The reported pain scores for each group were compared to previously published controls, who received conventional dosing of either sciatic (6 mL/h continuous with 4 mL of patient-controlled bolus and 30-min lockout) or infraclavicular (8 mL/h continuous with 4 mL of patient-controlled bolus and 30-min lockout) cPNBs [7,8].
Results
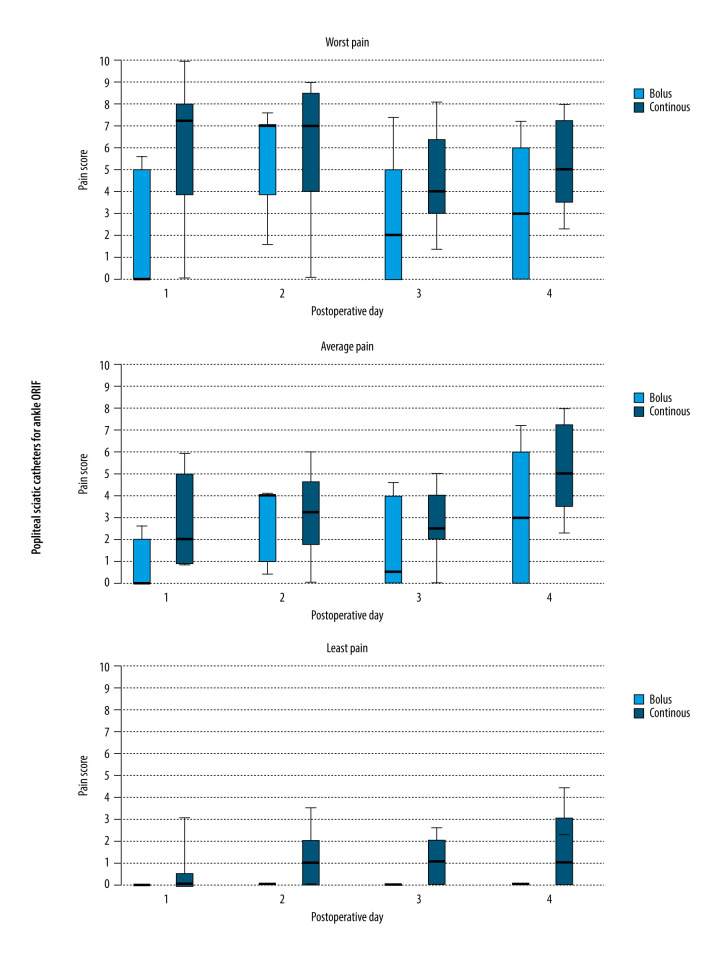
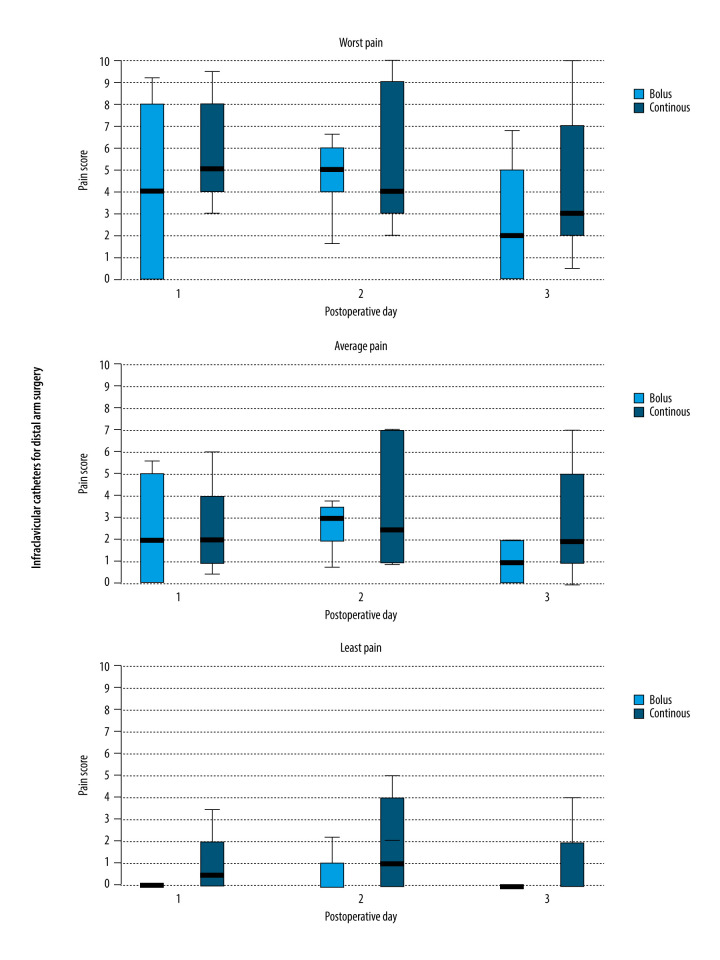
All patients in the present series had successful placement of either a popliteal sciatic or brachial plexus perineural catheter, and telephone follow-up was completed for all patients. The lowest, average, and highest pain scores were similar when comparing previously published controls who received continuous infusion with the patients who received automated boluses for both infraclavicular and popliteal sciatic blocks (Figures 1, 2).
Figure 1.
Worst, average, and lowest pain scores during the first 4 days following foot/ankle surgery with a popliteal sciatic catheter and ropivacaine administered by either automated boluses or continuous infusion. Data is expressed as median (horizontal bar), 25th to 75th percentile (box), and 10th to 90th percentile (whiskers), and statistics were not applied owing to the small sample size.
Figure 2.
Worst, average, and lowest pain scores during the first 3 days following wrist/hand surgery with an infraclavicular brachial plexus catheter and ropivacaine administered by either automated boluses or continuous infusion. Data is expressed as median (horizontal bar), 25th to 75th percentile (box), and 10th to 90th percentile (whiskers), and statistics were not applied owing to the small sample size.
For infraclavicular catheters, the local anesthetic reservoirs lasted until the third or fourth postoperative day in the patients who received automated intermittent boluses compared with only the second day in the previously published controls who received continuous infusions. For popliteal sciatic catheters, local anesthetic lasted until the fifth postoperative day in the patients who received automated intermittent boluses compared with only the second or third day in the previously published controls who received continuous infusions.
No infections or other complications were observed in any of the patients receiving automated boluses.
Discussion
The duration of analgesia provided by a continuous peripheral nerve block is dependent on the rate of local anesthetic consumption and reservoir volume. Because ambulatory patients are discharged with a set reservoir volume that rarely exceeds 500 mL owing to weight and bulk limitations, the only way to extend analgesia duration is by decreasing local anesthetic consumption [1]. The results of studies comparing automated boluses with continuous infusions have been mixed, with results varying from significant to no benefit in the analgesia provided [1–3,9–11]. However, previous studies comparing automated boluses with continuous infusions for continuous peripheral nerve blocks have largely used the same basal dosing in both groups (e.g., 5 mL/h of continuous infusion compared with 5 mL of bolus every hour) [2,3,9,11]. Because long-acting local anesthetics are generally used for cPNB [1], longer intervals between boluses might further lengthen the analgesic duration. The duration of ropivacaine without additive is 12 to 20 h for peripheral nerve blocks; therefore, we posited that a lower basal dose of local anesthetic could be used in patients receiving automated ropivacaine boluses by increasing the time between boluses [12].
Electronic infusion pump technology is continually improving. There are now pumps that have additional features, which could also decrease local anesthetic consumption. Start-delay timers, which are one such feature, allow for a programmed delay in the start of the infusion to increase the total duration of the block provided by the single injection of local anesthetic and the local anesthetic infusion. In the present case series, a programmed delay of 6 h was used to further increase the duration of the block. Importantly, patients should have a method to activate the pump prior to the programmed start time if the initial block begins to resolve faster than expected.
The results of this case series suggest that a pump start-delay timers and subsequent automated boluses may extend the duration of a cPNB by decreasing the amount of local anesthetic consumed, compared with traditional delivery methods. The 2-h interval between boluses was chosen as a conservative time period to prevent breakthrough pain. Given the duration of ropivacaine, the interval between boluses could potentially be further increased allowing for even more prolonged infusion, and, consequently, analgesia [12]. Many questions remain regarding automated boluses for prolonged analgesia following surgery, including (1) whether automated boluses provide superior analgesia in comparison to continuous infusions when sciatic catheters are placed with ultrasound guidance, as is done for catheters placed by nerve stimulation [2,3]; (2) the optimal duration between boluses to minimize breakthrough pain while maximizing the duration of the infusion; (3) the dose of local anesthetic delivered with each bolus to optimize analgesia; and (4) if the interval between boluses may be further increased by using an even longer-acting local anesthetic, like bupivacaine.
Conclusions
Continuous peripheral nerve blocks provide excellent pain relief following surgery, decreasing opioid consumption and improving patient quality of life [1]. This case-control series suggests that, for infraclavicular and popliteal sciatic catheters, intermittent boluses may provide similar analgesia with a decreased rate of local anesthetic consumption compared with continuous infusions following wrist and ankle surgeries, respectively. Thus, nerve blocks utilizing intermittent boluses may provide a longer duration of analgesia following these surgeries. Further investigation in the form of randomized, controlled trials is indicated.
Footnotes
Declaration of Figures’ Authenticity
All figures submitted have been created by the authors who confirm that the images are original with no duplication and have not been previously published in whole or in part.
Financial support: None declared
Conflict of interest: John J. Finneran’s institution has received funding and/or product for his research from Epimed, InfuTronix, and SPR Therapeutics. Paola Baskin reports no conflicts. William T. Kent reports no conflicts. Eric R. Hentzen reports no conflicts. Alexandra K. Schwartz is a paid speaker for DePuy Sythes (Raynham, MA) and consultant for Globus Medical (Audubon, PA). Brian M. Ilfeld’s institution has received funding and/or product for his research from Epimed, InfuTronix, and SPR Therapeutics
References
- 1.Ilfeld BM. Continuous peripheral nerve blocks. An update of the published evidence and comparison with novel, alternative analgesic modalities. Anesth Analg. 2017;124:308–35. doi: 10.1213/ANE.0000000000001581. [DOI] [PubMed] [Google Scholar]
- 2.Taboada M, Rodríguez J, Bermudez M, et al. A “new” automated bolus technique for continuous popliteal block: A prospective, randomized comparison with a continuous infusion technique. Anesth Analg. 2008;107(4):1433–37. doi: 10.1213/ane.0b013e3181824164. [DOI] [PubMed] [Google Scholar]
- 3.Taboada M, Rodríguez J, Bermudez M, et al. Comparison of continuous infusion versus automated bolus for postoperative patient-controlled analgesia with popliteal sciatic nerve catheters. Anesthesiology. 2009;110:150–54. doi: 10.1097/ALN.0b013e318191693a. [DOI] [PubMed] [Google Scholar]
- 4.Ilfeld BM, Morey TE, Enneking FK. Infraclavicular perineural local anesthetic infusion: A comparison of three dosing regimens for postoperative analgesia. Anesthesiology. 2004;100(2):395–402. doi: 10.1097/00000542-200402000-00032. [DOI] [PubMed] [Google Scholar]
- 5.Ilfeld BM, Thannikary LJ, Morey TE, et al. Popliteal sciatic perineural local anesthetic infusion: A comparison of three dosing regimens for postoperative analgesia. Anesthesiology. 2004;101(4):970–77. doi: 10.1097/00000542-200410000-00023. [DOI] [PubMed] [Google Scholar]
- 6.Ilfeld BM, Gabriel RA. Basal infusion versus intermittent boluses for perineural catheter: Should we take the “continuous” out of “continuous peripheral nerve blocks”? Reg Anesth Pain Med. 2019;44(3):285–86. doi: 10.1136/rapm-2018-100262. [DOI] [PubMed] [Google Scholar]
- 7.Mariano ER, Sandhu NS, Loland VJ, et al. A randomized comparison of infraclavicular and supraclavicular continuous peripheral nerve blocks for postoperative analgesia. Reg Anesth Pain Med. 2011;36(1):26–31. doi: 10.1097/AAP.0b013e318203069b. [DOI] [PubMed] [Google Scholar]
- 8.Finneran JJ, 4th, Swisher MW, Gabriel RA, et al. Suture-method versus through-the-needle catheters for continuous popliteal-sciatic nerve blocks: A randomized clinical trial. Anesthesiology. 2020;132(4):854–56. doi: 10.1097/ALN.0000000000003145. [DOI] [PubMed] [Google Scholar]
- 9.Hamdani M, Chassot O, Fournier R. Ultrasound-guided continuous interscalene block: The influence of local anesthetic background delivery method on postoperative analgesia after shoulder surgery, a randomized trial. Reg Anesth Pain Med. 2014;39(5):387–93. doi: 10.1097/AAP.0000000000000112. [DOI] [PubMed] [Google Scholar]
- 10.Oxlund J, Clausen AH, Venø S, et al. a randomized trial of automated intermittent ropivacaine administration vs. continuous infusion in an interscalene catheter. Acta Anaesthesiol Scand. 2018;62(1):85–93. doi: 10.1111/aas.13011. [DOI] [PubMed] [Google Scholar]
- 11.Kadam VR, Van Wijk RM, Moran JL, et al. Continuous transversus abdominis plane block vs. intermittent bolus for analgesia after abdominal surgery: a randomized trial. J Pain Res. 2017;10:1705–12. doi: 10.2147/JPR.S132891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rasmussen SB, Saied NN, Bowens C, Jr, et al. Duration of upper and lower extremity peripheral nerve blockade is prolonged with dexamethesone when added to ropivacaine: A retrospective database analysis. Pain Med. 2013;14:1239–47. doi: 10.1111/pme.12150. [DOI] [PubMed] [Google Scholar]