Abstract
Matrilin 1, or cartilage matrix protein, is a member of a novel family of extracellular matrix proteins. To date, four members of the family have been identified, but their biological role is unknown. Matrilin 1 and matrilin 3 are expressed in cartilage, while matrilin 2 and matrilin 4 are present in many tissues. Here we describe the generation and analysis of mice carrying a null mutation in the Crtm gene encoding matrilin 1. Anatomical and histological studies demonstrated normal development of homozygous mutant mice. Northern blot and biochemical analyses show no compensatory up-regulation of matrilin 2 or 3 in the cartilage of knockout mice. Although matrilin 1 interacts with the collagen II and aggrecan networks of cartilage, suggesting that it may play a role in cartilage tissue organization, studies of collagen extractability indicated that collagen fibril maturation and covalent cross-linking were unaffected by the absence of matrilin 1. Ultrastructural analysis did not reveal any abnormalities of matrix organization. These data suggest that matrilin 1 is not critically required for cartilage structure and function and that matrilin 1 and matrilin 3 may have functionally redundant roles.
Matrilin 1, formerly called cartilage matrix protein, is the first and best characterized member of a newly discovered multidomain family. Matrilins are extracellular matrix (ECM) proteins consisting of a von Willebrand factor A (vWFA)-like domain(s), an epidermal growth factor (EGF)-like domain(s), and a coiled-coil α-helical motif (for review, see reference 10). To date, four members of the family have been identified. Matrilin 1 and matrilin 3 are expressed mainly in hyaline cartilage, while matrilin 2 and matrilin 4 are expressed in a wide variety of extracellular matrices (6, 9, 15–17).
Matrilin 1 was originally isolated from bovine tracheal cartilage as a protein cofractionating with cartilage proteoglycan (14). The monomer consists of two vWFA-like domains separated by one EGF-like module and a C-terminal coiled-coil trimerization domain (11, 13). In addition to the formation of homo-oligomeric assemblies, hetero-oligomeric forms of matrilin 1 and matrilin 3 were detected in bovine epiphyseal cartilage (19). The functional importance of the hetero-oligomers is unknown, and the abundance of hetero-oligomers in other types of cartilage, and cartilages from other species has not been established.
In the mouse, matrilin 1 expression is restricted to certain types of cartilage. Matrilin 1 is an abundant component of tracheal, nasal septal, auricular, and epiphyseal cartilages, but it is not present in articular and intervertebral disc cartilages (1, 2). In situ hybridization experiments revealed a zonal distribution of matrilin 1 mRNA in the growth plate of long bones. The matrilin 1 transcript was found in the proliferative and upper hypertrophic zones, whereas the lower hypertrophic, calcified regions were negative (2). In contrast, immunostaining revealed the presence of matrilin 1 throughout the growth plate (2).
The role of matrilin 1 in cartilage matrix assembly is unclear. The age-dependent cross-linking of matrilin 1 to the aggrecan core protein (12) as well as its association with type II collagen-containing fibrils (18) was demonstrated, suggesting a possible organizational role. Furthermore, it was reported that matrilin 1 can also form a collagen-independent filamentous network in chondrocyte culture (7).
The in vivo function of matrilin 1 during skeletal development is unknown. In this study, we report the targeted disruption of the Crtm gene encoding matrilin 1 in mice. Both heterozygous and homozygous mutant mice are viable and show no detectable abnormalities.
MATERIALS AND METHODS
Generation of matrilin 1-deficient mice.
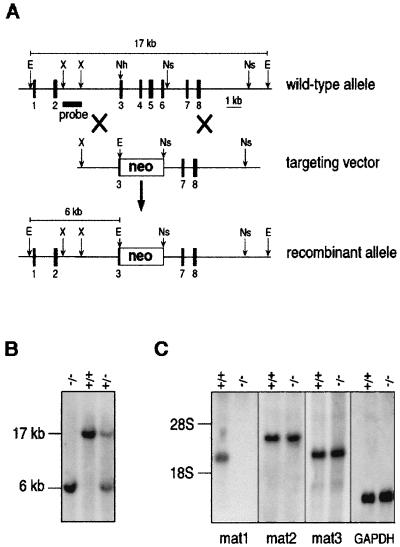
Genomic clones of Crtm encoding matrilin 1 were isolated from a 129/Sv genomic cosmid library as previously described (3). Crtm was disrupted by inserting the phosphoglycerate kinase-neomycin (PGKneo) cassette into the NheI-NsiI sites (see Fig. 1A), thereby deleting exons 4 to 6 and part of exon 3. After electroporation of R1 embryonic stem cells with the linearized targeting vector DNA (Fig. 1A), neomycin-resistant clones were isolated and analyzed by Southern blot assay. Two individually targeted embryonic stem cell clones were injected into blastocysts to generate germline chimeras. Chimeric males were mated with C57BL/6 females to test for germline transmission or with 129/Sv females to establish an inbred strain of Crtm-null mice.
FIG. 1.
Targeted disruption of mouse Crtm encoding matrilin 1. (A) Structure of wild-type allele, targeting construct, and recombinant locus. Dark boxes represent exons of Crtm. The expected fragment sizes after EcoRI digestion are 17 kb for the wild-type allele and 6 kb for the recombinant allele. E, EcoRI; Nh, NheI; Ns, NsiI; X, XhoI. (B) Southern blot analysis of mouse tail DNA isolated from the progeny of a mating between heterozygous parents. DNAs were digested with EcoRI and hybridized with the probe indicated in panel A. +/+, wild-type mouse; +/−, heterozygous mouse; −/−, homozygous mutant mouse. (C) Northern blot analysis of total RNA from limb cartilage, derived from 3-day-old wild-type (+/+) and homozygous mutant mice. The same filter was hybridized with cDNA probes specific for matrilin 1 (mat1), matrilin 2 (mat2), matrilin 3 (mat3), and glyceraldehyde phosphodehydrogenase (GAPDH).
RNA isolation and Northern blot analysis.
Total RNA from newborn mouse limb cartilage was isolated as previously described (2). For Northern analysis, 20 μg of total RNA was size fractionated on a 1% agarose–2.2 M formaldehyde gel, transferred to Hybond N+ membrane (Amersham), and consecutively hybridized with 32P-labelled cDNA probes specific for mouse matrilin 1 (nucleotides [nt] 856 to 1455) (2), matrilin 2 (nt 2762 to 3091 [provided by Ibolya Kiss and Ferenc Deák, Szeged, Hungary]) matrilin 3 (nt 308 to 823 [provided by Raimund Wagener, Cologne, Germany]), and glyceraldehyde phosphodehydrogenase.
Gross morphological analysis of skeleton.
Skeletons of 17.5-day-old embryos and newborn mice were prepared and stained with alcian blue and alizarin red as described previously (4). For X-ray analysis, 8- or 16-week-old matrilin 1-null and control mice were anesthetized with Avertin, and X-ray images were taken with a Siemens Polymat 70 at 48 kV, 0.2 mA (Siemens, Germany).
Histology, immunohistochemistry, and electron microscopy.
For histological analysis, knees or trunks dissected from 17.5-day-old embryos and newborn, 5-day-old, 2-week-old, and 1-, 6-, and 12-month-old animals were fixed overnight in fresh 4% paraformaldehyde in phosphate-buffered saline (PBS [pH 7.2]). Samples from mice analyzed after birth were decalcified in 10% EDTA–PBS for 1 week. After being embedded in paraffin, sections were cut at 6 to 8 μm and stained with hematoxylin-eosin, with safranin orange-van Kossa stain or with 0.1% toluidine blue at pH 2 (1).
For immunohistochemistry, knees dissected from 17.5-day-old embryos or newborn or 1-week-old animals were fixed overnight at 4°C in 95% ethanol–5% acetic acid, dehydrated in absolute ethanol, and embedded in paraffin. Immunostaining was performed by the avidin-biotin-complex (ABC) procedure as described earlier (4). Primary antibodies against matrilin 1, collagens II, IX, X, and XI, aggrecan, and cartilage oligomeric protein (COMP) are described in reference 4. Polyclonal antibodies against matrilin 2 (15) and matrilin 3 (gifts of Mats Paulsson and Raimund Wagener, Cologne, Germany) were diluted 1:400 and 1:500, respectively. Electron microscopic analysis of newborn cartilage tissue was performed as described earlier (4).
Biochemical analysis of cartilage.
Cartilage samples were dissected from limbs or trachea of wild-type and homozygous mutant mice at different time points postpartum. To analyze the expression of matrilins 1, 2, and 3 and aggrecan, cartilage tissues were extracted in 10 mM Tris-HCl buffer (pH 7.4) containing 4 M guanidine hydrochloride (GuHCl), 10 mM EDTA, 2 mM phenylmethylsulfonyl fluoride (Sigma), and 2 mM N-ethylmaleimide (Sigma). Extraction proceeded for 48 h at 4°C under gentle agitation. Unextracted material was removed by centrifugation at 8,000 × g for 30 min at 4°C. In some experiments, cartilage was extracted sequentially with 0.25 M NaCl in 50 mM Tris-HCl buffer (pH 7.4) and then with 0.25 M NaCl (Tris-HCl buffer [pH 7.4]) containing 10 mM EDTA prior to GuHCl extraction (11). Proteins were separated on sodium dodecyl sulfate (SDS)-polyacrylamide gels (5% or 4 to 15% gradient) and blotted onto ProBlott membranes (Applied Biosystem). The blots were subsequently hybridized with polyclonal antibodies specific for matrilins 1, 2, and 3 and the G1 subunit of the aggrecan core protein. Bound antibodies were hybridized to horseradish peroxidase-conjugated swine anti-rabbit immunoglobulin G (Sigma) and detected by using the ECL (enhanced chemiluminescence) kit (Amersham).
For collagen analysis, pooled freeze-milled cartilages were extracted with 0.15 M NaCl, 50 mM Tris-HCl buffer, containing 10 mM EDTA, 0.1 mM phenylmethylsulfonyl fluoride, and 2 mM N-ethylmaleimide for 24 h at 4°C, and then with 4 M GuHCl (Tris-HCl buffer [pH 7.4]) and two digestions with pepsin at 100 μg/ml in 0.5 M acetic acid to sequentially extract the successively more cross-linked and thus mature collagen matrix II (4). Collagen chains in each extract were analyzed on SDS–5% polyacrylamide gels, visualized by Coomassie staining, and quantified as described previously (5).
RESULTS
Generation of Crtm-null mice.
The genomic organization and detailed restriction map of the Crtm gene encoding matrilin 1 were published earlier (3). Crtm was inactivated with a targeting vector in which a part of exon 3 and exons 4 to 6 were replaced by a neomycin resistance cassette (Fig. 1A). Out of 240 ES cell clones surviving G418 selection, two targeted clones were identified by Southern blot analysis of EcoRI-digested genomic DNA (data not shown). Both clones were used to produce germline chimeric males, which were crossed with C57/B6 and 129/Sv females to generate outbred and inbred strains, respectively. Southern blot genotyping (Fig. 1B) of 272 offspring from heterozygous intercrosses showed that 23.9% were wild type, 51.2% were heterozygous, and 24.9% were homozygous for the Crtm mutation.
To test whether the mutation abolishes the expression of matrilin 1, Northern blot analyses of total RNA derived from limb and tracheal cartilages were performed. Figure 1C shows the complete absence of matrilin 1 mRNA in homozygous mice. Immunohistology of newborn knee joints (see Fig. 3F) and immunoblot analyses of proteins extracted from cartilages (see Fig. 4A) confirmed that the matrilin 1 protein was absent in the null mice.
FIG. 3.
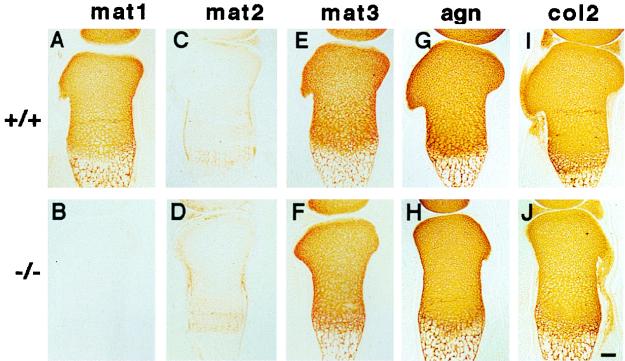
Immunostaining of cartilage. Consecutive sections of the tibia from wild-type (+/+ [A, C, E, G, and I]) and matrilin 1-deficient (−/− [B, D, F, H, and J]) newborn littermates were stained with specific antibodies against matrilin 1 (mat1), matrilin 2 (mat2), matrilin 3 (mat3), aggrecan (agn), and type II collagen (col2). Bar, 100 μm.
FIG. 4.
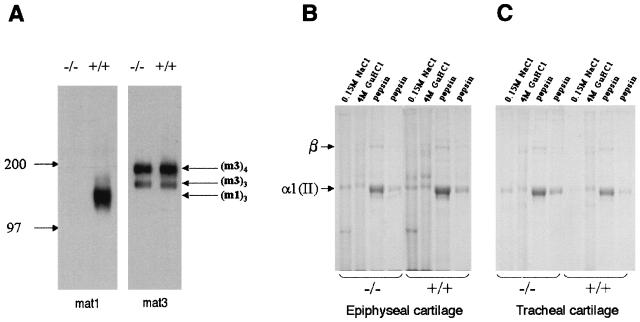
Biochemical analysis of wild type (+/+) and mutant (−/−) epiphyseal and tracheal cartilages. (A) Western blot analysis of matrilin 1 (mat1) and matrilin 3 (mat3) expression in pooled 4 M GuHCl extracts from 3-day-old mouse epiphyseal cartilage. The identities of the matrilin 1 trimers (m1)3 and matrilin 3 trimers (m3)3 and tetramers (m3)4 were determined by comparison with the migration position of protein molecular mass markers (kilodaltons). (B and C) Coomassie-stained SDS–5% polyacrylamide gel of collagens sequentially extracted with neutral salt (0.15 M NaCl), 4 M GuHCl, and pepsin from pooled 7-day-old mouse epiphyseal (B) and tracheal (C) cartilages. The identities of the cartilage collagen α1(II) band and the covalently cross-linked collagen β-components are indicated.
Analysis of skeletal development in matrilin 1-deficient mice.
Adult mice lacking matrilin 1 display no obvious abnormalities, are fertile, and have a normal lifespan. Since matrilin 1 is an abundant component of cartilage ECM, we performed a detailed macroscopical and histological analysis of the endoskeleton at different time points of embryonic and adult development.
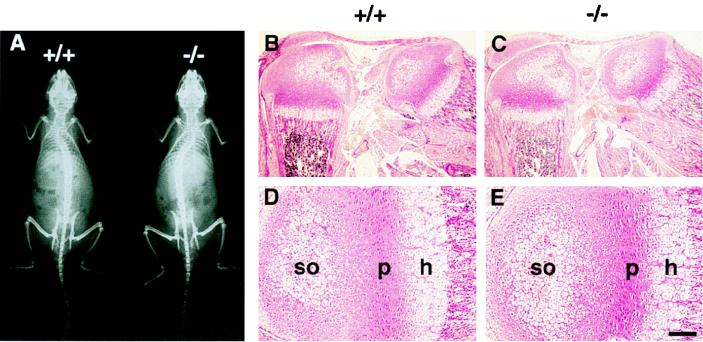
Intact skeletons of normal and matrilin 1-deficient mice were compared by two different methods. Neither skeletal staining of 17.5-day-old embryos and newborn mice nor X-ray analyses of 4- and 8-week-old animals revealed skeletal malformation in mutants (Fig. 2A and data not shown). Histological examination of limb and trunk development showed no differences between control and homozygous mutant mice up to 1 year of age (Fig. 2B to E and data not shown). The structure of growth plates and the appearance of the primary and secondary ossification centers of long bones were identical in control (Fig. 2B and D) and null mutant (Fig. 2C and E) mice. Results of safranin orange-van Kossa staining and toluidine blue staining of the cartilage matrix for proteoglycans and minerals were also indistinguishable between wild-type and matrilin 1-deficient mice (data not shown). Finally, ultrastructural analysis of limb cartilage of Crtm-null mice by electron microscopy showed normal chondrocyte morphology and the presence of a normal collagen fibrillar network in the extracellular matrix (data not shown).
FIG. 2.
Analysis of the skeleton in wild-type (+/+) and matrilin 1-deficient (−/−) mice. (A) X-ray of an 8-week-old mutant mouse shows no gross skeletal abnormalities compared to a normal littermate. (B to E) Hematoxylin-eosin staining of knee region (B and C) and tibia (D and E) from 3-day-old wild-type (B and D) and homozygous mutant (C and E) mice. h, hypertophic zone; p, proliferative zone; so, center of secondary ossification. Bars, 200 μm for panels B and C and 50 μm for panels D and E.
Immunohistochemical distribution of matrix proteins in cartilage.
To compare the expression patterns of matrix molecules in wild-type and Crtm-null cartilage, a detailed immunohistochemical analysis of limb sections at various stages of development was performed. At E17.5, matrilin-1 was deposited into the epiphyseal and growth plate cartilage, but was absent at the superficial zone of the epiphyses in normal animals (Fig. 3A). No matrilin 1 was detected in Crtm-null cartilage (Fig. 3B). Matrilin 2 was expressed in the perichondrium-periosteum, in the superficial zone, and, very weakly, in the hypertrophic zone of growth plate (Fig. 3C), while matrilin 3 showed the same staining pattern as matrilin 1 (Fig. 3E). Immunostaining for matrilin 2 and matrilin 3 in mutant tissue revealed no alterations in either distribution or staining intensity compared to those of the wild type (Fig. 3D and F).
The distributions of aggrecan, collagens II, IX, X, and XI, and COMP were similar in limb cartilage of normal and Crtm-null mice (Fig. 3G, I, H, and J, respectively, and data not shown). In adult tracheal cartilage, codistribution of matrilins 1 and 3 was detected in control mice, while the mutant tissue completely lacked matrilin 1 expression (data not shown).
Biochemical analysis of cartilage.
For biochemical analyses of matrilins and aggrecan, limb epiphyseal cartilage tissue was isolated from normal and Crtm-null mice of different ages (3, 7, and 14 days old) by extraction with 4 M GuHCl. The expression of matrilins 1, 2, and 3 and aggrecan was examined by immunoblotting after nonreducing SDS-polyacrylamide gel electrophoresis. Wild-type cartilage contained matrilin 1 trimers, matrilin 2 oligomers, and matrilin 3 trimers and tetramers (Fig. 4A and data not shown), but there was no evidence of matrilin 1 and 3 hetero-oligomeric assemblies in GuHCl extracts of epiphyseal cartilage. The expression levels of matrilins 2 and 3 in mutant cartilage were similar to those of the control (Fig. 4A and data not shown). Immunoblot analysis of aggrecan extracted with GuHCl revealed no apparent differences between control and mutant cartilage (data not shown).
Electrophoretic analysis of collagen chains isolated from day 7 epiphyseal cartilage (Fig. 4B) and tracheal cartilage (Fig. 4C) revealed a similar solubility profile of collagen II in the different extracts (0.15 M NaCl, GuHCl, or pepsin) between control and mutant tissues (Fig. 4B and C). Analysis of day 11 cartilages also showed no detectable changes in collagen solubility (data not shown). Detailed quantitative analysis of tracheal cartilage demonstrated a very slight, but consistent, increase in the amount of the total collagen II extracted by neutral salt buffer from the mutant (3.2%) compared to wild-type tracheal cartilage (1.3%). However, this small increase was not statistically significant.
DISCUSSION
The cartilage-specific expression pattern of matrilin 1 during development and the results obtained by biochemical and in vitro cell culture experiments suggested an important function of matrilin 1 in skeletogenesis. Based on the age-dependent covalent cross-linking of matrilin 1 with the aggrecan core protein (12), as well as its association with the surface of type II collagen-containing fibrils (18), it has been proposed that matrilin 1 might play an integrative role as a bridging molecule between the two major components of cartilage matrix. The recently described collagen-independent filamentous network of matrilin 1 in the pericellular compartments of cultured chondrocytes (7, 8) further supports the hypothesis that matrilin 1 has a role in the assembly of cartilage ECM.
Surprisingly, the presence of normal cartilage in Crtm-null mice clearly demonstrated that matrilin 1 is not essential for cartilage development and endochondral bone formation in vivo. The deposition of cartilage matrix proteins such as collagens II, IX, X, and XI, aggrecan, and COMP is normal in matrilin 1-deficient mice, suggesting that the lack of matrilin 1 has no influence on the expression or deposition of these molecules into the cartilage matrix. Our biochemical analyses showed apparently normal expression levels and extractability profiles of both collagen II and aggrecan in mutant epiphyseal cartilage. This suggests that the lack of matrilin 1 does not significantly alter the biochemical properties of these macromolecules, as measured by extractability, a crude assay of the extent of interaction and subsequent cross-linking. The very small increase in readily soluble collagen II in tracheal cartilage is most likely of no functional significance in terms of collagen maturation or matrix structure. Finally, the normal ultrastructure of collagen fibers in the cartilage matrix of null mice further suggests that matrilin 1 expression does not play a critically important role in collagen fibrillogenesis and cartilage function. However, it remains possible that during aging and/or in mechanical stress or disease and repair situations, matrilin 1 and its interactions with the collagen and proteoglycan networks may be of functional importance.
A possible explanation for the lack of a cartilage phenotype in matrilin 1-deficient mice is that its function in normal development and structure is redundant or compensated for by other, structurally related molecule(s). The recently described matrilin 2 and matrilin 3 are among the possible candidates, since they are expressed in developing bones and share a similar modular structure to matrilin 1. The matrilin 2 monomer consists of two vWFA domains connected by 10 EGF-like modules, an oligomerization domain, and a unique sequence (9). It is deposited in many organs and all forms of connective tissues (9, 15). In long bones, it is localized in perichondrium-periosteum and weakly in the hypertophic chondrocytes (15). In this study, we demonstrated that matrilin 2 is also present at the forming articular surface. Matrilin 3, the closest relative of matrilin 1, is composed of one vWFA-like domain followed by four EGF-like motifs and a putative oligomerization domain (6, 16). By Northern hybridization, the mouse matrilin 3 was detected only in cartilaginous tissues (16). Our immunohistochemical data showed an identical tissue distribution of matrilin 1 and matrilin 3 in cartilage of developing long bones and trachea, which suggests that these two matrilins might perform similar functions during skeletogenesis.
Our biochemical and immunohistochemical analyses of matrilin 1-deficient and wild-type cartilage revealed some interesting findings. First, we detected comparable amounts of matrilin 1 and matrilin 3 in both epiphyseal and tracheal cartilage of normal mice. This observation is in contrast with a recent publication in which the lack of matrilin 3 in adult bovine tracheal cartilage has been reported (19). Second, we did not observe matrilin 1-matrilin 3 hetero-oligomers in the epiphyseal cartilage of control animals, as described for bovine cartilage (19). These findings might indicate species-specific differences and suggest that matrilin 1 and matrilin 3 function independently in mouse cartilage tissue. Finally, in agreement with the Northern blot analysis, we observed no compensatory up-regulation of either matrilin 2 or matrilin 3 in Crtm-null mice compared to controls.
In conclusion, our data suggest redundant function of matrilins in cartilage ECM and might explain the lack of a detectable phenotype in matrilin 1-deficient mice. In order to test the in vivo relevance of such a redundancy, the generation of matrilin 2 and matrilin 3 knockout strains and their intercrossing with the Crtm-null mice are required.
ACKNOWLEDGMENTS
We thank Ray Boot-Handford and Mats Paulsson for discussion and critical reading of the manuscript. Sue Golub is thanked for technical assistance.
This study was supported by the Swedish Medical Research Council (no. K98-12X-12531-01A), the Volkswagen-Stiftung (no. I/70621), OTKA (no. T023838), and the University of Melbourne Collaborative Research Grants Scheme.
REFERENCES
- 1.Aszódi A, Módis L, Páldi A, Rencendorj A, Kiss I, Bösze Z. The zonal expression of chicken cartilage matrix protein in the developing skeleton of transgenic mice. Matrix Biol. 1994;14:181–190. doi: 10.1016/0945-053x(94)90007-8. [DOI] [PubMed] [Google Scholar]
- 2.Aszódi A, Hauser N, Studer D, Paulsson M, Hiripi L, Bösze Z. Cloning, sequencing and expression analysis of mouse cartilage matrix protein cDNA. Eur J Biochem. 1996;236:970–977. doi: 10.1111/j.1432-1033.1996.00970.x. [DOI] [PubMed] [Google Scholar]
- 3.Aszódi A, Beier D, Hiripi L, Bösze Z, Fässler R. Sequence, structure and chromosomal localization of Crtm gene encoding mouse cartilage matrix protein and its exclusion as a candidate for murine achondroplasia. Matrix Biol. 1998;16:563–573. doi: 10.1016/s0945-053x(98)90067-1. [DOI] [PubMed] [Google Scholar]
- 4.Aszódi A, Chan D, Hunziker E, Bateman J F, Fässler R. Collagen II is essential for the removal of the notochord and the formation of intervertebral discs. J Cell Biol. 1998;143:1399–1412. doi: 10.1083/jcb.143.5.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bateman J F, Golub S. Deposition and selective degradation of structurally-abnormal type I collagen in an in vitro collagen matrix produced by osteogenesis imperfecta fibroblasts. Matrix Biol. 1994;14:251–262. doi: 10.1016/0945-053x(94)90189-9. [DOI] [PubMed] [Google Scholar]
- 6.Belluoccio D, Trueb B. Matrilin-3 from chicken cartilage. FEBS Lett. 1997;415:212–216. doi: 10.1016/s0014-5793(97)01126-5. [DOI] [PubMed] [Google Scholar]
- 7.Chen Q, Johnson D M, Haudenschild D R, Tondravi M M, Goetinck P F. Cartilage matrix protein forms a type collagen-independent filamentous network: analysis in primary cell cultures with a retrovirus expression system. Mol Biol Cell. 1995;76:238–252. doi: 10.1091/mbc.6.12.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen Q, Zhang Y, Johnson D M, Goetinck P F. Assembly of a novel cartilage matrix protein filamentous network: molecular basis of differential requirement of von Willebrand factor A domain. Mol Biol Cell. 1999;10:2149–2162. doi: 10.1091/mbc.10.7.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deák F, Pichea D, Bachrati C, Paulsson M, Kiss I. Primary structure and expression of matrilin-2, the closest relative of cartilage matrix protein within the von Willebrand factor type A-like module superfamily. J Biol Chem. 1997;272:9268–9274. doi: 10.1074/jbc.272.14.9268. [DOI] [PubMed] [Google Scholar]
- 10.Deák F, Wagener R, Kiss I, Paulsson M. The matrilins: a novel family of oligomeric extracellular matrix proteins. Matrix Biol. 1999;18:55–64. doi: 10.1016/s0945-053x(98)00006-7. [DOI] [PubMed] [Google Scholar]
- 11.Hauser N, Paulsson M. Native cartilage matrix protein. J Biol Chem. 1994;269:25747–25753. [PubMed] [Google Scholar]
- 12.Hauser N, Paulsson M, Heinegård D, Mörgelin M. Interaction of cartilage matrix protein with aggrecan. J Biol Chem. 1996;271:32247–32252. doi: 10.1074/jbc.271.50.32247. [DOI] [PubMed] [Google Scholar]
- 13.Kiss I, Deák F, Holloway R G, Delius H, Mebust K A, Frimberger E, Argraves W S, Tsonis P A, Winterbottom N, Goetinck P F. Structure of the gene for cartilage matrix protein, a modular protein of the extracellular matrix. J Biol Chem. 1989;264:8126–8134. [PubMed] [Google Scholar]
- 14.Paulsson M, Heinegård D. Matrix proteins bound to associatively prepared proteoglycans from bovine cartilage. Biochem J. 1979;183:539–545. doi: 10.1042/bj1830539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Piecha D, Muratoglu S, Mörgelin M, Hauser N, Studer D, Kiss I, Paulsson M, Deák F. Matrilin-2, a large, oligomeric matrix protein, is expressed by a great variety of cells and forms fibrillar networks. J Biol Chem. 1994;274:13353–13361. doi: 10.1074/jbc.274.19.13353. [DOI] [PubMed] [Google Scholar]
- 16.Wagener R, Kobbe B, Paulsson M. Primary structure of matrilin-3, a new member of a family of extracellular matrix proteins related to cartilage matrix protein (matrilin 1) and von Willebrand factor. FEBS Lett. 1997;413:129–134. doi: 10.1016/s0014-5793(97)00895-8. [DOI] [PubMed] [Google Scholar]
- 17.Wagener R, Kobbe B, Paulsson M. Matrilin-4, a new member of the family of extracellular matrix proteins. FEBS Lett. 1998;436:123–127. doi: 10.1016/s0014-5793(98)01111-9. [DOI] [PubMed] [Google Scholar]
- 18.Winterbottom N, Tondravi M M, Harrington T L, Klier F G, Vertel B M, Goetinck P F. Cartilage matrix protein is a component of collagen fibril of cartilage. Dev Dyn. 1992;193:266–276. doi: 10.1002/aja.1001930307. [DOI] [PubMed] [Google Scholar]
- 19.Wu J J, Eyre D R. Matrilin-3 forms disulfide-linked oligomers with matrilin-1 in bovine epiphyseal cartilage. J Biol Chem. 1998;273:17433–17438. doi: 10.1074/jbc.273.28.17433. [DOI] [PubMed] [Google Scholar]