Abstract
Cannabidiol (CBD), a non-psychotropic phytocannabinoid from the Cannabis plant, is increasingly being pursued as a treatment for differing ailments. The bioavailability and pharmacokinetics of CBD are not well understood, and proper dosing schemes have not been adequately developed for its clinical use. CBD is a lipophilic molecule and exhibits low water solubility, so its formulation expectedly impacts its gastrointestinal absorption and subsequent blood plasma concentrations. In this review article, the food effects on CBD pharmacokinetics were analyzed. Clinical trials focusing on the performance of Epidiolex, the FDA approved CBD formulation, were found in several databases and systematically analyzed in terms of administration method, dosing schedules, and patient characteristics. 44 data sets from clinical trials were found to be useful in the quantitative analysis. Following the normalization of all the pharmacokinetic data sets by dose and patient weight, CBD exhibited a much greater bioavailability in fed patients. For Epidiolex, administration in the fed state led to lower interindividual variability and more predictable pharmacokinetics. Considering all the different oral formulations of CBD, further analysis points to the main excipient of oral CBD formulations (refined sesame seed oil) as a major contributor to the dose-dependent variations in CBD pharmacokinetics, especially affecting the fasted state. We discuss the implications of these results on the downstream pharmacodynamics of endocannabinoid receptor modulation and its broad physiological implications.
Keywords: Epidiolex, Sativex, Pharmacokinetics, Cannabidiol, Food Effects, Drug Absorption
Introduction
Cannabidiol (CBD) is a non-psychotropic cannabinoid found in the Cannabis plant, as opposed to the psychoactive cannabinoid: Tetrahydrocannabinol (THC). 1, 2 CBD is found to have a very low oral bioavailability of approximately 9–13%, which has contributed to major difficulties in drug development.3 In terms of the timing and importance of this review article, CBD products containing no more than 0.3% THC by dry weight are no longer designated as scheduled products under the 2018 Farm Bill, which removed CBD products from a Schedule I substance designation under the Controlled Substances Act. Further, CBD is the active pharmaceutical ingredient of Epidiolex which is manufactured by GW Pharmaceuticals and is the first and only Food and Drug Administration (FDA) approved CBD drug product in the United States.2 Epidiolex is an oral solution with CBD that is formulated as a 100 mg/mL solution in sesame oil as the primary vehicle with dehydrated alcohol, strawberry flavoring, and sucralose excipients.2 Epidiolex is indicated for the treatment of seizures associated with Lennox-Gastaut syndrome, Dravet syndrome, or tuberous sclerosis complex.2 Epidiolex is recommended to be taken in 2.5 mg/kg doses or greater twice daily. Epidiolex, other oral solutions of CBD, and CBD-containing products such as Sativex (THC-CBD combination drug approved by the European Medicines Agency for use in Europe) have been increasingly used in clinical trials to test therapeutic efficacy, assess adverse events, and further advance understanding of the mechanisms of action of CBD.4 There is also an abundance of information available about how food-consumption affects the measured peak blood concentrations of the active pharmaceutical ingredients (Cmax) and the time to reach Cmax after oral administration (Tmax) of CBD.2
Overall, the factors that influence the pharmacokinetics of CBD have not been thoroughly evaluated, and what data are available show large interpatient variability. While this review is focused on oral drug product bioequivalence, the FDA considers understanding and controlling the systemic pharmacokinetics of a drug product as the critical parameter for drug product regulation.4, 6 Furthermore, there are no standard recommendations for dosing and administration of CBD. This is because current published clinical trials have not provided sufficient evidence to generate recommendations about such regimens that assess the food-effect on CBD bioavailability. To begin to address this deficit in knowledge, we performed a systematic review of clinical studies conducted to date on the impact of food on CBD pharmacokinetics. We considered drug formulations, dosing schemes, and patient demographics. To combine available raw data from the existing literature, we examined the reported Cmax, Tmax, and area under the concentration vs. time curve (AUC0−∞) in relation to the expected pharmacokinetic behavior of CBD as captured by a population pharmacokinetic model.4, 6, 7
By investigating CBD bioavailability under different fasted and fed state conditions using clinical trial data, we developed models that could be used to better guide evidence-based recommendations regarding CBD dosing and administration. Accordingly, the quantitative analysis on drug formulation, dosing schemes, and varying patient conditions conducted in this review article will help establish the importance of developing food-effect guidelines for CBD that will greatly benefit patients and clinicians in achieving their desired therapeutic aims. A possible mechanism of the effect of food on CBD is that fat and the caloric content of food can delay gastric emptying time, bile flow stimulation, and luminal metabolism.1 This review article also points to areas in which greater attention is needed to improve patient responses and better control key formulation and administration variables that exert the greatest effect on the biopharmaceutical performance of orally administered CBD.
Methods
A systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews (PRISMA) guidelines.8 Database searches using the a priori criteria were conducted in PubMed (from July 1980 to October 2020), clinicaltrials.gov (from September 18, 2014 to October 1, 2020), and the University of Michigan database (from July 1980 to December 2019). Additionally, a search was conducted with Google Scholar to identify other studies that were found to fit study criteria. A thorough search of all references was also conducted to find the greatest number of clinical trials that would fit within the scope of the review’s subject matter
The search study was conducted by the lead author (L.S.). Keywords and heading titles found within accepted search criteria included Bioavailability, CBD, Cannabidiol, Cannabinoids, duodenum, epilepsy, food-effect, gastric emptying, humans, lipid-based drug delivery, lipid excipient, liver function, lymphatic absorption, metabolism, oral drug delivery, and pharmacokinetics. For detailed descriptions of data compiled from each reference, please refer to Table 1. L.S. screened all titles, abstracts, and full text to determine viability for quantitative and qualitative use within the review article.
Table 1:
Administration Methods and Meal Contents
| Data Points | Drug | Fasted/Fed | Administration Time | FDA Guidance | Caloric Content (kcal) | Fat Content (g) | Published | Nation | Reference |
|---|---|---|---|---|---|---|---|---|---|
| A1-A3 | Epidiolex | Fasted | Dose given after an overnight fast | Yes | 0 | 0 | 3-Oct-18 | Canada | 21 |
| B1-B3 | Epidiolex | Fed | Dosed 30 mins after meal, evening dose given 12h after | Yes | 574 | 26.4 | 21-Feb-19 | Netherlands | 19 |
| C | Oral Capsule | Fed | Dosed 30 mins after light meal following an overnight fast | Yes | 450 | 12.5 | 10-Feb-16 | USA | 22 |
| D1 | Oral Capsule | Fed | Dosed 4h and 8h after meal | No | 574 | 26.4 | 28-Nov-17 | Israel | 3 |
| D2 | Sativex | Fed | Dosed 4h and 8h after meal | No | 574 | 26.4 | 28-Nov-17 | Israel | 3 |
| E1 - E2 | Oral Capsule | Fed | Dosed 2h after a light breakfast | No | 450 | 12.5 | 1-May-15 | USA | 23 |
| F1-F3 | Sativex | Fasted | 24h fast pre-dose with no food 15 mins before and after morning dose | No | 0 | 0 | 4-Nov-03 | UK | 16 |
| F4 | Oral Capsule | Fasted | 24h fast pre-dose with no food 15 mins before and after morning dose | No | 0 | 0 | 4-Nov-03 | UK | 16 |
| G | Sativex | Fasted | Fasted conditions | No | 0 | 0 | 16-Jun-10 | UK | 4 |
| H1-H5 | Epidiolex | Fasted | Overnight fast of at least 10h | Yes | 0 | 0 | 30-Oct-18 | Netherlands | 10 |
| H6 | Epidiolex | Fed | Overnight fast of at least 10h, high-fat breakfast given 30 mins before dose | Yes | 918 | 62 | 30-Oct-18 | Netherlands | 10 |
| H7-H8 | Epidiolex | Fasted | Overnight fast before first dose, 2h fast before evening dose (6 days), single dose on 7th day | Yes | 0 | 0 | 30-Oct-18 | Netherlands | 10 |
| I1-I4 | Oral Capsule | Fed | Light low-fat breakfast 1–2h before dose, snack 4h after dose | No | 450 | 12.5 | 12-Sep-19 | USA | 24 |
| J1 - J2 | Oral Capsule | Fed | Standard meal 30 mins before dose, after overnight fast | Yes | 574 | 26.4 | 10-Nov-17 | Israel | 20 |
| J3 | Sativex | Fed | Standard meal 30 mins before dose, after overnight fast | Yes | 574 | 26.4 | 10-Nov-17 | Israel | 20 |
| K1-K3 | Sativex | Fasted | Fasted 10h overnight and 4h post dose | Yes | 0 | 0 | 22-Nov-12 | UK | 17 |
| K4-K6 | Sativex | Fasted | Fasted 10h overnight and 4h post dose | Yes | 0 | 0 | 22-Nov-12 | UK | 17 |
| L1-L4 | Epidiolex | Fed | Low-protein breakfast 2h before dose after an 8h overnight fast | No | 450 | 12.5 | 28-Mar-19 | Hungary, Czech Republic, Slovakia | 14 |
| M1 | Epidiolex | Fasted | Fasted overnight for 10h, meal given 4h after dose | Yes | 0 | 0 | 27-Jun-19 | USA | 15 |
| M2 | Epidiolex | Fed | Fasted overnight 10h, high-fat breakfast burrito given 30 mins before dose | Yes | 850 | 60 | 27-Jun-19 | USA | 15 |
| N | Sativex | Fed | 36h fast before dose, standard low-fat breakfast 30 mins before dosing and standard meals 4h and 10h post-dose + digestive biscuits | Yes | 450 | 12.5 | 15-Apr-05 | UK | 18 |
Data point nomenclature is as follows: assigned letter corresponds to the publication and numbers (if any) correspond to individual studies within the publication
A total of 44 data sets from 14 publications that met search criteria were included in the review’s analysis. 3, 4, 10, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24 Clinical trials that were multi-crossover or parallel-group, open-label, randomized, double-blinded, and/or placebo-controlled that met the following search criteria were included (1) CBD oral administration in human subjects, (2) amount of dose or doses given with administration methods and information on drug product used, (3) pharmacokinetic data for CBD from blood plasma which included Cmax, Tmax, and AUC0−∞, and (4) a description of patient conditions in fed or fasted states.
Risk of Bias Assessment
Possible bias in the included clinical trials was analyzed with the Risk of Bias in Nonrandomized Studies – of Interventions (ROBINS-I) assessment tool (Table 2).13 Biases were defined as those due to (1) confounding, (2) selection of participants into the study, (3) misclassification of intervention bias, (4) deviations from interventions, (5) missing data, (6) bias in outcome measurement, and (7) selection of reported results.13 Each classification of bias was assessed by low, medium, high, and critical risk of bias per ROBINS-I.13 All studies scored low for risk of bias in selection, misclassification of intervention, missing data, and outcome measurement except for one study that did not include enough information.4 One study had a moderate risk of bias for confounding due to the stratification of individuals based on the severity of hepatic impairment.14 Another study had a moderate risk of bias for deviations from intervention because one subject needed to decrease their dosage by 100 mg based on subject characteristics.15 Two studies had a moderate risk of bias for selection of results due to knowledge of interventions that would be adequate for a non-randomized trial, but not comparable to a randomized control trial.14, 18
Table 2:
ROBINS-I Results
| Study | Confounding | Selection Bias | Misclassification of Intervention | Deviations from Intervention | Missing Data | Outcome Measurement | Selection of Results |
|---|---|---|---|---|---|---|---|
| 19 | Low | Low | Low | Low | Low | Low | Low |
| 3 | Low | Low | Low | Low | Low | Low | Low |
| 18 | Low | Low | Low | Low | Low | Low | Moderate |
| 4 | Low | NI | NI | NI | NI | NI | NI |
| 14 | Moderate | Low | Low | Low | Low | Low | Moderate |
| 15 | Low | Low | Low | Moderate | Low | Low | Low |
All risk of biases were scored as low, moderate, high, critical, or no information (NI)
Study Selection
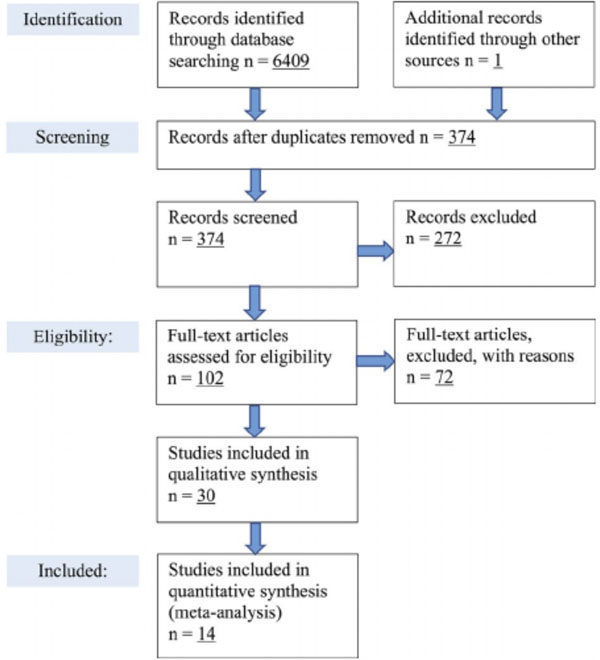
A total of 6,409 studies were found from searches in PubMed, the University of Michigan library database, and clinicaltrials.gov. One additional study was found in a search for the package insert of Sativex on Google,4 but no other studies were identified from the references that were not already found in the initial database search. After duplicate studies were removed, 374 studies were screened for eligibility of the search criteria, which were made up of 98 studies from PubMed, 275 studies from the University of Michigan library database, and one study from the Sativex package insert (Figure 1).4, 6
Figure 1.
PRISMA flow diagram showing database search results, exclusions, and inclusions for this reviews analysis
After screening, 272 studies were removed due to one or more of the following: absence of human subjects, review or pharmacology-based article, and not performed with CBD. Of the 102 articles that were deemed eligible, 30 were used for the preliminary analysis because the other trials lacked pharmacokinetic data and measurements for CBD, did not use oral administration methods, did not report dosing schemes and/or dose, and/or did not report patient demographic data. 14 publications that met all quantitative search criteria were reviewed based on abstract and title which were deemed eligible.3, 4, 10, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24 A full-text screening was done on each publication, and all 14 were included in the quantitative analysis. The screening flow diagram can be found in Figure 1.6
Of the 14 studies assessed, there were five publications from Phase I clinical trials,10, 14, 16, 17, 18 six publications that were open-label,14, 15, 16, 17, 19, 20 five publications that were crossover designs,3, 16, 18, 20 six publications that were double-blind,10, 16, 18, 21, 22, 24 and five publications that included a placebo.10, 18, 21, 23, 24 There were 527 subjects in total that were accounted for in the quantitative analysis of this review article. Five publications assessed GW Pharmaceuticals’ Drug Epidiolex,10, 14, 15, 19, 21 six publications assessed the CBD combination drug Sativex,3, 4, 16, 17, 18, 20 and six publications used oral capsule formulations containing CBD.3, 16, 20, 22, 23, 24 Additionally, one study assessed the drug-drug interactions with Epidiolex and three anti-epileptic drugs.19 One publication analyzed Epidiolex on subjects with varying levels of hepatic impairment,14 but all other included publications used healthy volunteers in their studies.
Assessing CBD Pharmacokinetics and the Impact of Food Based on a PopPK Modeling Approach
To assess whether the behavior of CBD in clinical trials conformed to predicted behavior, three Population Pharmacokinetic (PopPK) models were used to compare raw data blood CBD concentration data from clinical trials to the predicted pharmacokinetic values (Supplementary Figures 1 and 3).6 Dose- and patient weight-normalized averages for Epidiolex under fed and fasted conditions were calculated using Equation (1).
| (1) |
Graphical schematics of PopPK models in the fasted (Supplementary Figure 1) and fed state (Supplementary Figure 3) were created with GraphPad Prism 9 Version 9.0.0 by the lead author (L.S.).The dose- and patient weight-normalized fasted and fed averages for Epidiolex were plotted alongside the fasted and fed PopPK models using GraphPad Prism 9 (Supplementary Figure 4).
A root mean squared error (RMSE) equation was used to determine the degree of similarity between raw data from clinical trials and the values predicted with the PopPK models for the Cmax and of Epidiolex and is shown in Equation (2).
| (2) |
A modified root mean squared error (Normalized RMSE) equation was used to calculate the deviation, expressed as a fraction, of the clinical trial pharmacokinetic averages from the predicted PopPK models (Equation 3). The Normalized RMSE equation placed an even weight on the Cmax and averages, which accounted for the Cmax having a greater numerical value than the Tmax.
| (3) |
Construction of a Scientific Literature-derived PopPK Model
A three-compartment PopPK model was built based on a similar model used for analyzing THC concentrations in humans under fasted conditions (Supplementary Figures 1 and 3).17, 26, 27 The PopPK model consisted of a single oral (PO) dose under fasted conditions. Moreover, the CBD PopPK model assumed the same absorption (ka) and distribution parameters as the THC PopPK model due to CBD and THC early concentration profiles having a similar pattern when administered as a co-formulated product.27 THC and CBD possess very similar chemical structures and physicochemical properties (e.g. lipophilicity, molecular weight, charge, solubility, etc).27 Parameters including volume of distribution in the central compartment (V2), oral bioavailability (FPO), and elimination rate constant in the central compartment (k20) were modified to match the predicted behavior of CBD for a single dose of Epidiolex (2.5 mg/kg for a 75 kg adult). All modified parameter values are written in the code for the fasted (Supplementary Script 1) and fed (Supplementary Script 2) PopPK models. Raw NONMEM data for the fasted and fed PopPK models was transposed into a set number of time and blood plasma concentration values using RStudio Version 1.3.1073 (Supplementary Figures 1 and 3).
Assessing the Effect of Food on CBD Pharmacokinetics Using GW Pharmaceuticals’ PopPK Pharmacokinetic Model
A two-compartment PopPK model was used by GW Pharmaceuticals in the New Drug Application NDA submission for Epidiolex.6 GW Pharmaceuticals’ PopPK model was based on an Epidiolex clinical trial which included a single ascending dose phase (SAD), multiple ascending dose phase (MAD), and food-effect phase on healthy subjects.10 The food-effect phase consisted of a single dose of 1500 mg of Epidiolex which was given to 12 healthy subjects in a fasted state following an overnight fast, and 12 healthy subjects in a fed-state following an overnight fast and high-fat breakfast.10 GW Pharmaceuticals’ PopPK model was based on zeroth-order absorption, which increased CBD blood plasma concentration in proportion to the dose of Epidiolex.6
Result
General Considerations Regarding Epidiolex Pharmacokinetics
Pharmacokinetic parameters for all Epidiolex studies, as well as Sativex and oral capsule publications, were dose- and patient weight-normalized to make a proper comparison between values. A pharmacokinetic analysis was performed using quantitative data collected from the five publications that administered Epidiolex to subjects.10, 14, 15, 19, 21 Three pharmacokinetic measurements were utilized in the analysis: (1) peak concentration of CBD in the plasma (Cmax), (2) time to peak concentration of CBD in the plasma (Tmax), and (3) area under the curve from zero to infinity (AUC0−∞). Further information regarding the number of subjects, dose, number of doses, age, body mass index (BMI), primary race, patient conditions, and time of administration was collected and can be viewed in Table 3.
Table 3:
Raw Pharmacokinetic Parameters and Subject Specifics
| Drug | Subjects (n) | Primary Sex | Primary Race(s) | Median BMI (kg/m2), (SD) | Median Age (yrs), (SD) | Total Dose (mg) | # Doses | Time of Administration | Cmax (ng/mL), mean (CV%) | Tmax (hrs) | AUC(0-∞) (h*ng/mL), mean (CV%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| Epidiolex | |||||||||||
|
| |||||||||||
| A1 | 38 | M | Caucasian | 25.9 (2.7) | 37.7 (8.9) | 750 | 1 | Morning | 336.2 (46.7) | 5.116 | 1683.3 (46.7) |
| A2 | 39 | M | Caucasian | 25.9 (2.7) | 37.7 (8.9) | 1500 | 1 | Morning | 524.5 (64.9) | 6.133 | 2713 (64) |
| A3 | 40 | M | Caucasian | 25.9 (2.7) | 37.7 (8.9) | 4500 | 1 | Morning | 426.9 (112.8) | 4.067 | 2290.3 (104.1) |
| B1 | 15 | M | Caucasian/Multiple | 24.81 (3.52) | 27.7 (8.2) | 1500 | 2 | Morning & Evening | 840 (61.1) | 5 | - |
| B2 | 12 | M/F | Caucasian | 25.71 (3.52) | 35.1 (12.9) | 1500 | 2 | Morning & Evening | 852 (57.3) | 4.5 | - |
| B3 | 14 | M | Caucasian | 23.21 (2.22) | 29.9 (10.5) | 1500 | 2 | Morning & Evening | 838 (39.8) | 5 | - |
| H1 | 6 | M/F | Caucasian | 23.73 (2.45) | 26 (3.2) | 1500 | 1 | Morning | 292.4 (87.9) | 4 | 1618 (74.6) |
| H2 | 6 | M/F | Caucasian | 23.15 (2.33) | 25 (4.7) | 3000 | 1 | Morning | 533 (35.1) | 5 | 2802 (35.5) |
| H3 | 6 | M/F | Caucasian | 21.38 (1.71) | 25.8 (7.9) | 4500 | 1 | Morning | 722.1 (52.3) | 5 | 3426 (48.3) |
| H4 | 6 | M/F | Caucasian | 23.6 (2.53) | 22.8 (3.2) | 6000 | 1 | Morning | 782 (83) | 5 | 3900 (79.3) |
| H5 | 12 | M/F | Caucasian | 23.34 (1.9) | 25.1 (6.2) | 1500 | 1 | Morning | 335.4 (81.3) | 3.5 | 2198 (48.2) |
| H6 | 12 | M/F | Caucasian | 23.34 (1.9) | 25.1 (6.2) | 1500 | 1 | Morning | 1628 (51.4) | 3 | 8669 (33.9) |
| H7 | 9 | M/F | Caucasian | 22.28 (3.03) | 28.6 (8.5) | 750 | 13 | Morning and Evening (6 days), Morning dose on 7th day | Day 1 am: 290.8 (86.3), Day 1 pm: 732.4 (39.4), Day 7 am: 330.3 (40.8) | Day 1 am: 5, Day 1 pm: 2.5, Day 7 am: 3 | - |
| H8 | 9 | M/F | Caucasian/Multiple | 22.23 (2.33) | 25.1 (4.8) | 1500 | 13 | Morning and Evening (6 days), Morning dose on 7th day | Day 1 am: 361.8 (105.8), Day 1 pm: 1385 (52.4), Day 7 am: 541.2 (53.7) | Day 1 am: 5, Day 1 pm: 4, Day 7 am: 3 | - |
| L1 | 8 | M/F | Caucasian | 25.8 (5.7) | 57.5 (8.1) | 200 | 1 | Morning | 233 (70.5) | 2.8 | 699 (44.2) |
| L2 | 8 | M/F | Caucasian | 29.6 (3.8) | 55.6 (11.1) | 200 | 1 | Morning | 354 (42.3) | 2 | 1163 (39.9) |
| L3 | 6 | M/F | Caucasian | 30.0 (4.7) | 52.7 (6.9) | 200 | 1 | Morning | 381 (52.5) | 2.5 | 2439 (29.5) |
| L4 | 8 | M/F | Caucasian | 29.4 (3.2) | 55 (10) | 200 | 1 | Morning | 148 (65) | 2.3 | 474 (73.8) |
| M1 | 8 | M/F | Caucasian/Black | 90 kg | 49 | 300 | 1 | Morning | 9.0 (33.33) | 3.2 | 159 (49.06) |
| M2 | 8 | M/F | Caucasian/Black | 90 kg | 49 | 300 | 1 | Morning | 135 (66.67) | 2.4 | 771 (62.26) |
| Oral Capsule | |||||||||||
|
| |||||||||||
| C | 8 | M/F | Black/Caucasian/Mixed | - | 29.1 (9.1) | 800 | 1 | Morning | 77.9 | 3 | - |
| D1 | 9 | M | - | - | 25 | 10 | 1 | Dosed 4h and 8h after meal | 0.5 (20) | 3.1 (12.90) | |
| E1 | 6 | M/F | Black/Caucasian | - | 38.5 (2.2) | 400 | 1 | Morning | 181.2 (21.96) | 3 | 7040 (40.2) |
| E2 | 6 | M/F | Black/Caucasian | - | 38.5 (2.2) | 800 | 1 | Morning | 221.1 (16.10) | 3 | 8670 (35.06) |
| F4 | 12 | M/F | Caucasian | 24.33 (1.8) | 36.5 (8.38) | 10 | 1 | Morning | 2.47 | 1.267 | 362.04 |
| I1 | 12 | M | - | 25 (2) | 24 (4) | 45 | 1 | Morning | 16.8 (66.67) | 1.883 | 2252 (45.49) |
| I2 | 12 | M | - | 25 (2) | 24 (4) | 90 | 1 | Morning | 54.6 (43.04) | 2.05 | 7115 (41.86) |
| I3 | 12 | M | - | 25 (2) | 24 (4) | 45 | 1 | Morning | 21.2 (45.75) | 2.167 | 2860 (45.49) |
| I4 | 12 | M | 25 (2) | 24 (4) | 90 | 1 | Morning | 77.6 (52.32) | 1.833 | 10,865 (58.19) | |
| J1 | 13 | M | Caucasian | 24.8 | 30.7 | 10 | 1 | Morning | 3.22 (39.62) | 3 | 10.31 (40.14) |
| J2 | 13 | M | Caucasian | 24.8 | 30.7 | 100 | 1 | Morning | 47.44 (42.35) | 3.5 | 153.04 (22.67) |
| Sativex | |||||||||||
|
| |||||||||||
| D2 | 9 | M | - | - | 25 | 10 | 1 | Dosed 4h and 8h after meal | 2.1 (19.05) | 1 | 6.9 (18.84) |
| F1 | 12 | M/F | Caucasian | 24.33 (1.8) | 36.5 (8.38) | 10 | 1 | Moming | 2.5 | 1.633 | 427.33 |
| F2 | 12 | M/F | Caucasian | 24.33 (1.8) | 36.5 (8.38) | 10 | 1 | Morning | 3.02 | 2.8 | 407.79 |
| F3 | 12 | M/F | Caucasian | 24.33 (1.8) | 36.5 (8.38) | 10 | 1 | Morning | 2.61 | 2.05 | 496.98 |
| G | 12 | M/F | - | - | - | 10 | 1 | - | 5.4 (57.3) | 1 | - |
| J3 | 13 | M | Caucasian | 24.8 | 30.7 | 10 | 1 | Morning | 2.05 (53.83) | 3.5 | 7.81 (35.96) |
| K1 | 6 | M | Caucasian/Black/Asian | 23.5 | 32 | 5 | 1 | Morning | 0.39 (20.51) | 1 | 1.66 (30.72) |
| K2 | 12 | M | Caucasian/Black/Asian | 23.5 | 32 | 10 | 1 | Morning | 1.15 (64.35) | 1.39 | 5.64 (72.52) |
| K3 | 6 | M | Caucasian/Black/Asian | 23.5 | 32 | 20 | 1 | Morning | 2.17 (56.68) | 1 | 13.28 (96.84) |
| K4 | 6 | M | Caucasian/Black/Asian | 23.5 | 32 | 5 | 9 | Morning, 9 days | 0.49 (42.86) | 1.64 | 2.52 (28.97) |
| K5 | 12 | M | Caucasian/Black/Asian | 23.5 | 32 | 10 | 9 | Moming, 9 days | 1.14 (75.44) | 1.27 | 6.66 (46.55) |
| K6 | 6 | M | Caucasian/Black/Asian | 23.5 | 32 | 20 | 9 | Moming, 9 days | 3.22 (59.01) | 2 | 20.34 (35.84) |
| N | 24 | M | - | 24.5 | 34 | 10 | 1 | Moming | 3.33 | 4.22 | 43107.6 |
All data collected from clinical trials and categorized by Epidiolex, Sativex, or oral capsule
In the five publications that administered Epidiolex, a total of 20 studies were performed with varying dose strengths, subject conditions, number of doses, and timing of dose. Each study was dose- and patient weight-normalized with Equation (1) to correspond to a single starting dose of Epidiolex (2.5 mg/kg) recommended for a standard adult (75 kg).2 Thus, each pharmacokinetic measurement was normalized to a dose of 187.5 mg of Epidiolex for a 75 kg patient and is shown in Table 4.
Table 4:
Normalized Pharmacokinetic Parameters to 2.5 mg/kg for 75 kg
| Data Points | Raw Dose (mg) | Dose-normalized Dose (mg) | Dose-normalized Cmax (ng/mL) | Tmax (hrs) | Dose-normalized AUC(0-∞) (h*ng/mL) |
|---|---|---|---|---|---|
|
| |||||
| Predicted Fasted | 187.5 | 187.5 | 81.723 | 0.5 | - |
| Predicted Fed | 187.5 | 187.5 | 300.63 | 2.5 | - |
|
| |||||
| A1 | 750 | 187.5 | 84.05 | 5.12 | 420.83 |
| A2 | 1500 | 187.5 | 131.13 | 6.13 | 339.13 |
| A3 | 4500 | 187.5 | 106.73 | 4.07 | 95.43 |
| B1 | 1500 | 187.5 | 210 | 5 | - |
| B2 | 1500 | 187.5 | 213 | 4.5 | - |
| B3 | 1500 | 187.5 | 209.5 | 5 | - |
| C | 800 | 187.5 | 18.26 | 3 | - |
| D1 | 10 | 187.5 | 39.38 | 1 | 58 |
| D2 | 10 | 187.5 | 9.38 | 3 | 129 |
| E1 | 400 | 187.5 | 84.94 | 3 | 3300 |
| E2 | 800 | 187.5 | 51.82 | 3 | 2032 |
| F1 | 10 | 187.5 | 46.94 | 1.63 | 8012 |
| F2 | 10 | 187.5 | 56.63 | 2.8 | 7646 |
| F3 | 10 | 187.5 | 48.94 | 2.05 | 9318 |
| F4 | 10 | 187.5 | 46.31 | 1.27 | 6788 |
| G | 10 | 187.5 | 26.34 | 1.38 | - |
| H1 | 1500 | 187.5 | 36.55 | 4 | 202.25 |
| H2 | 3000 | 187.5 | 33.31 | 5 | 175.13 |
| H2 | 4500 | 187.5 | 30.09 | 5 | 142.75 |
| H4 | 6000 | 187.5 | 24.44 | 5 | 121.88 |
| H5 | 1500 | 187.5 | 41.93 | 3.5 | 274.75 |
| H6 | 1500 | 187.5 | 203.5 | 3 | 1083.63 |
| H7 | 750 | 187.5 | 82.58 | 3 | - |
| H8 | 1500 | 187.5 | 67.65 | 3 | - |
| I1 | 45 | 187.5 | 70 | 1.88 | - |
| I2 | 90 | 187.5 | 113.75 | 2.05 | - |
| I3 | 45 | 187.5 | 88.33 | 2.17 | - |
| I4 | 90 | 187.5 | 161.67 | 1.83 | - |
| J1 | 10 | 187.5 | 60.38 | 3 | 193.31 |
| J2 | 100 | 187.5 | 88.95 | 3.5 | 286.95 |
| J3 | 10 | 187.5 | 38.44 | 3.5 | 146.44 |
| K1 | 5 | 187.5 | 14.63 | 1 | 62.25 |
| K2 | 10 | 187.5 | 21.56 | 1.38 | 105.75 |
| K3 | 20 | 187.5 | 20.34 | 1 | 124.5 |
| K4 | 5 | 187.5 | 18.38 | 1.64 | - |
| K5 | 10 | 187.5 | 21.38 | 1.27 | - |
| K6 | 20 | 187.5 | 30.19 | 2 | - |
| L1 | 200 | 187.5 | 218.44 | 2.8 | 655.31 |
| L2 | 200 | 187.5 | 331.88 | 2 | 1090.31 |
| L3 | 200 | 187.5 | 357.19 | 2.5 | 2337.19 |
| L4 | 200 | 187.5 | 138.75 | 2.33 | 444.38 |
| M1 | 300 | 187.5 | 5.63 | 3.2 | 353.77 |
| M2 | 300 | 187.5 | 84.38 | 2.4 | 481.88 |
| N | 10 | 187.5 | 62.44 | 4.22 | 808267.5 |
Dose- and patient weight-normalized values correspond to the Population Pharmacokinetic (PopPK) plot generated by E.C. and transposed by A.W.
To ensure the inclusion of other methods of administrations and formulations for the comparison to Epidiolex, the remaining nine publications were analyzed under the same methods and separated by the administration of either oral capsule drug products or Sativex in each study.3, 4, 16, 17, 18, 20, 22, 23, 24 There were six publications that administered Sativex to subjects for a total of ten studies.3, 4, 16, 17, 18, 20 There were six publications that administered an oral capsule formulation of CBD for a total of 11 studies.3, 16, 20, 22, 23, 24
Assessing the Effects of Food on Epidiolex Pharmacokinetics
Upon comparison of fasted and fed pharmacokinetic values for Epidiolex, the fed state was observed to have a significantly larger maximum blood plasma concentration (Cmax) than the fasted state based on an unequal variances t-test (p = 0.0003). Dose-normalized and patient weight-normalized data from 20 studies collected for Epidiolex were further separated based on whether subjects were held under a fasted or fed condition upon administration and blood plasma collection. Nine total studies of Epidiolex were performed under fed conditions,10, 14, 15, 19 while the remaining 11 studies were performed under fasted conditions.10, 15, 21 Two of the studies that were performed under fasted conditions administered multiple doses of Epidiolex to subjects, but only the primary pharmacokinetic parameters from a single dose were included in the analysis to ensure consistency.17 In addition, three studies for Sativex were performed under fed conditions, 3, 20 and ten studies were performed under fasted conditions.4, 16, 17, 18 A total of ten studies of oral capsule formulations were performed under fed conditions,3, 20, 22, 23, 24 and one study was performed under a fasted condition.16
The mean Cmax, Tmax, AUC0−∞, and all corresponding standard deviations and coefficient of variation (CV%) measurements were calculated for the fasted and fed groups of Epidiolex using Microsoft Excel Version 16.42 and RStudio Version 1.3.1073 (Table 5). The same methods were performed among all studies of Sativex and oral capsule formulations (Table 5). Epidiolex had a dose- and patient weight-normalized Cmax of 218.51 ng/mL with a 36.4% coefficient of variation, a Tmax of 3.28 hours with a 34.6% coefficient of variation, and AUC0−∞ of 1015.5 h*ng/mL with a 63.54% coefficient of variation under fed patient conditions. The Cmax of Epidiolex under fed patient conditions (218.51 ng/mL) was nearly a four-fold increase from Epidiolex under fasted patient conditions (58.55 ng/mL). Thus, a fed patient state will lead to a significantly larger maximum blood plasma concentration of CBD. The Tmax of Epidiolex under fed patient conditions (3.28 hours) was shorter than fasted patient conditions (4.27 hours). Yet, the Tmax is less indicative of the predicted pharmacokinetic mechanisms of CBD based on the inability to dose- and patient weight-normalize the measurement. The unequal variances t-test was not statistically significant for Tmax (p = 0.069), showing that the Tmax was less important in the comparison of Epidiolex under fasted and fed conditions. The dose- and patient weight-normalized mean AUC0−∞ for Epidiolex under fed conditions (1015.5 h*ng/mL) was over four-fold greater than the mean AUC0−∞ under fasted conditions (236.21 h*ng/mL). The unequal variances t-test was statistically significant for AUC0−∞ (p = 0.04). However, there was less certainty in the comparison among fasted and fed groups for AUC0−∞ because the value was not reported in three of the fasted Epidiolex studies and three of the fed Epidiolex studies.
Table 5:
Normalized Pharmacokinetic Parameters to 2.5 mg/kg for 75 kg
| Drug | Cmax (ng/mL), (CV%) | Tmax (hrs), (CV%) | Dose-Normalized AUC(0-∞) (h*ng/mL), (CV%) | # Studies | Dose- and Weight-normalized RMSE | RMSE | Studies Included |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Epidiolex | |||||||
| Fed | 218.51 (36.37) | 3.28 (34.64) | 1015.45 (63.54) | 9 | 0.41 | 82.12 | B1-B3, H6, L1-L4, M2 |
| Fasted | 58.55 (60.52) | 4.27 (23.25) | 236.21 (46.05) | 11 | 7.55 | 23.48 | A1-A3, H1-H5, H7, H8, M1 |
| Sativex | |||||||
| Fed | 36.75 (59.04) | 3.57 (14.01) | 404206.97 (99.96) | 3 | 0.98 | 263.88 | D2, J3, N |
| Fasted | 30.53 (46.03) | 1.62 (32.35) | 4211.42 (98.43) | 10 | 2.33 | 51.21 | F1-F3, G, K1-K6 |
| Oral Capsule | |||||||
| Fed | 77.75 (49.27) | 2.44 (29.96) | 240.13 (19.5) | 10 | 0.74 | 222.88 | C, D1, E1, E2, I1-I4, J1, J2 |
| Fasted | 46.31 (0) | 1.27 (0) | 6788 (0) | 1 | 1.6 | 35.42 | F4 |
Mean dose- and patient weight-normalized values correspond to the Population Pharmacokinetic (PopPK) plot generated by E.C. and transposed by A.W.
The coefficient of variation (CV%) was used to assess the degree of interindividual variability among fasted and fed Epidiolex averages. All coefficient of variation values for the dose- and patient weight-normalized Cmax, Tmax, and AUC0−∞ measurements can be found in Table 5. The coefficient of variation for the Cmax of Epidiolex in a fed state (36.37%) was much lower than in the fasted state (60.52%), indicating that there was less interindividual variability among fed studies. Furthermore, the coefficient of variation for the Tmax of Epidiolex in the fed state (34.64%) was slightly larger than the fasted state (23.25%), and the coefficient of variation for AUC0−∞ in the fed state (63.54%) was larger than the fasted state (46.05%). As Cmax was the only statistically significantly different value between fasted and fed conditions, which included all 20 studies, the comparison between the coefficient of variation values for Cmax was the most significant in our analysis. A smaller interindividual variability among fed Epidiolex studies indicates that the fed state had a more predictable behavior for the concentration of CBD in the blood plasma.
Assessing How Meal Content Affects Epidiolex Pharmacokinetics
When assessing the studies that utilized a food effect in combination with Epidiolex administration, there was a considerable difference in the type of meal given, and a lack of information about meal contents. The FDA recommends several guidelines for food effect studies, with both high-fat/high-calorie and low-fat/low-calorie meals.4 The FDA states that a food effect study with a high-fat/high-calorie meal must contain between 800–1000 total kcal, 55–65 grams of fat which is equivalent to 500–600 kcal, 50% fat content within the meal, 150 kcals from protein, and 250 kcals from carbohydrates.4 An example of a high-fat/high-calorie meal consists of two fried eggs in butter, two strips of bacon, two slices of toast with butter, four ounces of hash browns, and eight ounces of whole milk.4 In contrast, the FDA notes that a food effect study with a low-fat/low-calorie meal must contain between 400–500 total kcal, 11–14 grams of fat which is equivalent to 100–125 kcal, and 25% fat content in the meal.4 An example of a low-fat/low-calorie meal would contain eight ounces of 1% milk, one boiled egg, and one packet of instant oatmeal.4 Furthermore, the FDA notes that a high-fat/high-calorie meal can likely delay gastric emptying, stimulate bile flow, change gastrointestinal pH, increase splanchnic bile flow, change luminal metabolism of a drug substance, and physically or chemically interact with a dosage form or a drug substance administered.4 Biopharmaceutics Class System (BCS) class II drugs, such as CBD, with high permeability and low solubility, will likely undergo an increased bioavailability upon administration with a high-fat/high-calorie meal.25 Thus, it is vital to understand how co-administration with food will impact the drug’s pharmacokinetics, which will determine the clinical success of Epidiolex.
Information on the meal contents in fed studies for Epidiolex was collected and summarized in Table 1. Fed studies for Sativex and oral capsule drug products were also included in Table 1 for comparison. Among the studies that listed the caloric content and fat content of the meal(s) given, data was entered and converted into standard units. For fed studies that did not provide information about specific caloric content or the percentage of fat, the average numbers for high- or low-fat/calorie FDA recommendations were implemented.4 For studies that did not give any indication of whether the meal was high- or low-fat/calorie, a mean imputation technique was performed for a standard meal, which contained 574 kcal with 26.4 g of fat. For all studies that co-administered Epidiolex with a meal, two included a high-fat/high-calorie meal,8, 15 three included the standard meal from mean imputation,19 and four included a low-fat/low-calorie meal.14 Table 6 includes all information regarding meal contents co-administered with Epidiolex, Sativex, and oral capsule drug products in fed studies. Conclusions cannot be made based on the small number of acceptable food-effect studies found in the database search.
Table 6:
Fed Study Meal Breakdown
| Drug | Meal Type | Studies Included | Mean Caloric Content (kcal) | Mean Fat Content (g) | Mean normalized Cmax (ng/mL) | Mean normalized Tmax (hours) | Mean normalized AUC (h*ng/mL) |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Epidiolex | High-fat | H6, M2 | 884 | 61 | 72.51 | 3.61 | 782.75 |
| Epidiolex | Standard | B1-B3 | 574 | 26.4 | 146.92 | 4.17 | - |
| Epidiolex | Low-fat | L1-L4 | 450 | 12.5 | 208.36 | 2.51 | 1131.80 |
| Sativex | Standard | D2, J3 | 574 | 26.4 | 49.78 | 2 | 137.91 |
| Sativex | Low-fat | N | 450 | 12.5 | 62.44 | 4.22 | 808267.5 |
| Oral Capsule | Standard | D1, J1, J2 | 574 | 26.4 | 45.59 | 3.33 | 179.46 |
| Oral Capsule | Low-fat | I1-I4 | 450 | 12.5 | 90.49 | 2.06 | - |
Assessing How Sesame Oil Vehicle Mimics the Fed State and Mainly Affects the Pharmacokinetics of CBD in the Fasted State
The food effect on CBD is largely correlated with the pharmacokinetics and pharmacodynamics of CBD. Following the breakdown of caloric and fat content within meals co-administered in fed patient studies, the oil content for the formulation of Epidiolex is hypothesized to mimic a fed state in the body at high doses.12 The main excipient (non-active pharmaceutical ingredient) in Epidiolex consists of a highly lipophilic substance, which has a high concentration of oil.2 736 mg of sesame seed oil and 100 mg of CBD is included per 1 mL of Epidiolex.2 The recommended starting dose of 2.5 mg/kg/dose in a human adult would include 0.02 mL/kg/dose of oil excipient.2 The largest oral dose of Epidiolex given to patients in clinical trials was 6 grams per day, which is equivalent to approximately 44.2 mL of sesame seed oil excipient.10 Based on the caloric content of sesame oil, the ~44.2 mL amount of sesame seed oil excipient was equivalent to approximately 353 calories.2, 10, 11 Research is lacking for the exact effect food has on the bioavailability of CBD, so a survey of published literature on the bioavailability of CBD under varying patient conditions, doses, and drug formulations was deemed to be crucial.
The total sesame seed oil concentration was calculated, in mL, for each study that administered Epidiolex to patients. In addition, the amount of oil derived from fat in the meals given in fed studies was added to the oil concentration of sesame oil in the corresponding dose. The sum of oil from the sesame seed oil excipient of Epidiolex and the meal(s) consumed was defined as the total amount of oil in a specific study. The total oil concentration consumed in Epidiolex studies, as well as Sativex and oral capsule formulation studies, can be found in Table 7.
Table 7:
Oil Concentration in Food and Excipients
| Drug | Excipients | Excipient oil Concentration (mL) | Oil from meal (mL) | Total amount of oil (mL) |
|---|---|---|---|---|
|
| ||||
| Epidiolex | ||||
|
| ||||
| A1 | Dehydrated alcohol, refined sesame oil, strawberry flavor, sucralose | 5.52 | 0 | 5.52 |
| A2 | Dehydrated alcohol, refined sesame oil, strawberry flavor, sucralose | 11.04 | 0 | 11.04 |
| A3 | Dehydrated alcohol, refined sesame oil, strawberry flavor, sucralose | 33.12 | 0 | 33.12 |
| B1 | Dehydrated alcohol, refined sesame oil, strawberry flavor, sucralose | 11.04 | 26.4 | 33.12 |
| B2 | Dehydrated alcohol, refined sesame oil, strawberry flavor, sucralose | 11.04 | 26.4 | 33.12 |
| B3 | Dehydrated alcohol, refined sesame oil, strawberry flavor, sucralose | 11.04 | 26.4 | 33.12 |
| H1 | Dehydrated alcohol, refined sesame oil, strawberry flavor, sucralose | 11.04 | 0 | 11.04 |
| H2 | Dehydrated alcohol, refined sesame oil, strawberry flavor, sucralose | 22.08 | 0 | 22.08 |
| H3 | Dehydrated alcohol, refined sesame oil, strawberry flavor, sucralose | 33.12 | 0 | 33.12 |
| H4 | Dehydrated alcohol, refined sesame oil, strawberry flavor, sucralose | 44.16 | 0 | 44.16 |
| H5 | Dehydrated alcohol, refined sesame oil, strawberry flavor, sucralose | 11.04 | 0 | 11.04 |
| H6 | Dehydrated alcohol, refined sesame oil, strawberry flavor, sucralose | 11.04 | 62 | 73.04 |
| H7 | Dehydrated alcohol, refined sesame oil, strawberry flavor, sucralose | 5.52 | 0 | 5.52 |
| H8 | Dehydrated alcohol, refined sesame oil, strawberry flavor, sucralose | 11.04 | 0 | 11.04 |
| L1 | Dehydrated alcohol, refined sesame oil, strawberry flavor, sucralose | 1.472 | 13.54 | 15.012 |
| L2 | Dehydrated alcohol, refined sesame oil, strawberry flavor, sucralose | 1.472 | 13.54 | 15.012 |
| L3 | Dehydrated alcohol, refined sesame oil, strawberry flavor, sucralose | 1.472 | 13.54 | 15.012 |
| L4 | Dehydrated alcohol, refined sesame oil, strawberry flavor, sucralose | 1.472 | 13.54 | 15.012 |
| M1 | Dehydrated alcohol, refined sesame oil, strawberry flavor, sucralose | 2.208 | 0 | 2.208 |
| M2 | Dehydrated alcohol, refined sesame oil, strawberry flavor, sucralose | 2.208 | 60 | 62.208 |
|
| ||||
| Oral Capsule | ||||
|
| ||||
| C | Dehydrated alcohol, refined sesame oil, strawberry flavor, sucralose | 8 | 12.5 | 20.5 |
| D1 | Piperine, tween, span, polyoxyl 40-hydroxy castor oil, lecithin, tricaprin, ethyl lactate | 0.22 | 26.4 | 26.62 |
| E1 | Corn Oil | - | 12.5 | - |
| E2 | Corn Oil | - | 12.5 | - |
| F4 | Granulated Lactose | - | 0 | - |
| I1 | Multi-spectrum organic hemp oil CO2 extract | 162.9 | 12.5 | 175.4 |
| I2 | American ginseng, Gingko Biloba, multi-spectrum organic hemp oil CO2 extract | 325.8 | 12.5 | 338.3 |
| I3 | Multi-spectrum organic hemp oil CO2 extract | 162.9 | 12.5 | 175.4 |
| I4 | American ginseng, Gingko Biloba, multi-spectrum organic hemp oil CO2 extract | 325.8 | 12.5 | 338.3 |
| J1 | Gelatin | - | 26.4 | - |
| J2 | Gelatin | - | 26.4 | - |
|
| ||||
| Sativex | ||||
|
| ||||
| D2 | Ethanol, propylene glycol, peppermint oil | 0 | 26.4 | 26.4 |
| F1 | Ethanol, propylene glycol, peppermint oil | 0 | 0 | 0 |
| F2 | Ethanol, propylene glycol, peppermint oil | 0 | 0 | 0 |
| F3 | Ethanol, propylene glycol, peppermint oil | 0 | 0 | 0 |
| G | Ethanol, propylene glycol, peppermint oil | 0 | 0 | 0 |
| J3 | Ethanol, propylene glycol, peppermint oil | - | 26.4 | - |
| K1 | Ethanol, propylene glycol, peppermint oil | 0 | 0 | 0 |
| K2 | Ethanol, propylene glycol, peppermint oil | 0 | 0 | 0 |
| K3 | Ethanol, propylene glycol, peppermint oil | 0 | 0 | 0 |
| K4 | Ethanol, propylene glycol, peppermint oil | 0 | 0 | 0 |
| K5 | Ethanol, propylene glycol, peppermint oil | 0 | 0 | 0 |
| K6 | Ethanol, propylene glycol, peppermint oil | 0 | 0 | 0 |
| N | Ethanol, propylene glycol, peppermint oil | 0 | 12.5 | 12.5 |
1 mL of Epidiolex contains 736 mg refined sesame oil and 100 mg CBD
The highest total concentration of oil consumed in a study for Epidiolex was 73 mL, with 62 mL of the total oil coming from 62 g of fat in the co-administered meal.15 The lowest total concentration of oil, 2.2 mL, was in a fasted study for Epidiolex. 15 Therefore, the total amount of the oil consumed was from the sesame seed oil excipient in a single 300 mg dose of Epidiolex.15 A human gastrointestinal and physiology study found that long chain fatty acids as little as 2 grams were found to stimulate gall bladder contraction and stimulate increased secretion of bile salts, phospholipids, and cholesterol in the intestine.12 The effect of increased bile salts and phospholipids are found to contribute to enhanced absorption of drugs with a low-solubility.12 Fatty acid excipients have also been linked to an improved uptake of CBD which may bypass first-pass liver metabolism and lead to increased absorption and bioavailability.24 Therefore, it is possible that a dose of Epidiolex of 272 mg or more will mimic a positive food-effect on CBD, even under fasted patient conditions.2 It is of great importance to understand the exact amount of sesame seed oil excipient in a certain dose of Epidiolex that will contribute to a positive food-effect with or without consumption of a meal.
Pharmacokinetic Considerations for Multi-dose Regimens of CBD
Only single-dose administrations of Epidiolex and other CBD-containing drug products were used in the analysis, as only two publications performed multi-dose regimens.10, 17 One study administered Epidiolex in two doses of either 750 mg or 1500 mg per day for six days, and a single dose on the seventh day.10 The Cmax and Tmax with respective coefficient of variation (CV%) values for the first dose of either 750 mg or 1500 mg was 290.8 ng/mL (86.3) at 5 hours and 361.8 ng/mL (105.8) at 5 hours, respectively.10 Subsequently, the Cmax and Tmax for the last single dose of either 750 mg or 1500 mg on the seventh day was 330.3 ng/mL (40.8) at 3 hours and 541.2 ng/mL (53.7) at 3 hours, respectively.10 Because the two studies that performed multiple-dose regimens were only conducted under fasted patient conditions, the results cannot be included in the review’s analysis. Further clinical trials and research on multiple doses of CBD under fasted and fed patient conditions are needed for the consideration of how multi-dose regimens may impact the bioavailability of CBD.
Assessing the Effects of Food on Circulating CBD Concentrations Using A Scientific Literature-derived 3-compartment PopPK Model
Using a three-compartment PopPK model based on published pharmacokinetic data, the predicted Cmax and Tmax under fasted conditions was 81.72 ng/mL at 30 minutes (Supplementary Figure 1).17, 26, 27 Additionally, the code written by Dr. Capparelli in NONMEM provided the modified absorption rate constant (ka) and oral bioavailability (FPO) for the predicted fed state (Supplementary Script 1). The fed-state absorption rate constant (ka) and oral bioavailability (FPO) were implemented and transposed into the corresponding time and plasma concentration values by co-author (A.R.W) (Supplementary Script 2).27 The predicted Cmax and Tmax under fed conditions was 300.63 ng/mL at 2.5 hours (Supplementary Figure 3). Average dose- and patient weight-normalized Epidiolex Cmax and values for clinical trial data under fasted and fed conditions were compared to the respective PopPK models and predicted Cmax and Tmax (Supplementary Figure 4). The RMSE and normalized RMSE calculations were used to determine whether data from clinical trials followed the predicted behavior of CBD in the respective PopPK model. All RMSE calculations pertaining to the fasted and fed PopPK models for a single dose of Epidiolex (2.5 mg/kg for a 75 kg adult) can be found in Table 5. The RMSE in the fed state was 82.12, and the normalized RMSE in the fed state was 0.41. For the fasted state, the RMSE was 23.48 and the normalized RMSE was 7.55. Because RMSE Equation (1) does not equally weigh the Cmax and Tmax values, the Cmax will mainly account for the result. The normalized RMSE calculations from Equation (2) were expressed as evenly weighted percentages of error from the predicted values, which were more indicative of how accurate the PopPK models predicted the behavior of CBD in clinical trials. The PopPK model for the fed state is found to be more accurate in its comparison to the pharmacokinetic data collected from clinical trials of Epidiolex. This shows that the fed state will be highly beneficial for future dosing regimens and predictions regarding the expected behavior of CBD in the patient population.
Assessing the Effects of Food on Circulating CBD Concentrations Using GW Pharmaceutical’s PopPK Model
Raw data was not accessible for the PopPK model provided in the Epidiolex NDA, which was obtained from the FDA filings and submitted by GW Pharmaceuticals.10 However, the clinical trial data used to generate the model can be analyzed to assess its accuracy.10 According to the clinical trial data used for the PopPK model of Epidiolex, the Cmax, Tmax, and AUC0−∞ for the fasted state was 335.4 ng/mL, 3.5 hours, and 2198 h*ng/mL, respectively. Consequently, the Cmax, Tmax, and AUC0−∞ for the fed state was 1628 ng/mL, 3 hours, and 8669 h*ng/mL, respectively.10 Dose- and patient weight-normalized values for clinical trial data can be found in Table 8. According to GW Pharmaceuticals’ PopPK model, the bioavailability of CBD was much greater in the fed state and is shown to increase by a 0.52 fractional change from non-fed conditions.6 RMSE and normalized RMSE calculations were performed for dose- and patient weight-normalized Epidiolex averages for a 1500 mg dose and a 75 kg adult under fasted and fed conditions (Table 9). The RMSE for Epidiolex under fed conditions was 1116.4, and the normalized RMSE was 3.89. The RMSE under fasted conditions was 250.9, and the normalized RMSE was 8.14. As previously mentioned, the normalized RMSE calculations were a better indicator of how accurate the PopPK model was in predicting the behavior of CBD in patients. The normalized RMSE for Epidiolex under fed patient conditions was much smaller than fasted conditions, meaning that the GW Pharmaceuticals’ PopPK model for Epidiolex yielded similar conclusions as those obtained with the three-compartment alternative model that was generated for the present review, based on previously published data from academic research labs.
Table 8:
Normalized PK Parameters to GW Pharmaceuticals’ PopPK model (1500 mg)
| Data points | Dose-normalized Cmax (ng/mL) | Tmax (hrs) | Dose-normalized AUC(0-∞) (h*ng/mL) |
|---|---|---|---|
|
| |||
| Predicted Fasted | 335.4 | 3.5 | 2198 |
| Predicted Fed | 1628 | 3 | 8669 |
|
| |||
| A1 | 672.4 | 5.12 | 3366.6 |
| A2 | 524.5 | 6.13 | 2713 |
| A3 | 142.3 | 4.07 | 763.43 |
| B1 | 840 | 5 | - |
| B2 | 852 | 4.5 | - |
| B3 | 838 | 5 | - |
| C | 146.063 | 3 | - |
| D1 | 75 | 1 | 465 |
| D2 | 315 | 3 | 1035 |
| E1 | 679.5 | 3 | 26400 |
| E2 | 414.56 | 3 | 16256.25 |
| F1 | 375 | 1.63 | 64099.5 |
| F2 | 453 | 2.8 | 61168.5 |
| F3 | 391.5 | 2.05 | 74547 |
| F4 | 370.5 | 1.27 | 54306 |
| G | 810 | 1.38 | - |
| H1 | 292.4 | 4 | 1618 |
| H2 | 266.5 | 5 | 1401 |
| H2 | 240.7 | 5 | 1154 |
| H4 | 195.5 | 5 | 975 |
| H5 | 335.4 | 3.5 | 2198 |
| H6 | 1628 | 3 | 8669 |
| H7 | 581.6 | 3 | - |
| H8 | 361.8 | 3 | - |
| I2 | 560 | 1.88 | 75066.67 |
| I2 | 910 | 2.05 | 118583.33 |
| I3 | 706.67 | 2.17 | 95333.33 |
| I4 | 1293.33 | 1.83 | 181083.33 |
| J1 | 483 | 3 | 1546.5 |
| J2 | 711.6 | 3.5 | 2295.6 |
| J3 | 307.5 | 3.5 | 1171.5 |
| K1 | 117 | 1 | 498 |
| K2 | 172.5 | 1.38 | 846 |
| K3 | 162.75 | 1 | 996 |
| K4 | 147 | 1.64 | - |
| K5 | 171 | 1.27 | - |
| K6 | 241.5 | 2 | - |
| L1 | 1747.5 | 2.8 | 5242.5 |
| L2 | 2655 | 2 | 8722.5 |
| L3 | 2857.5 | 2.5 | 18292.5 |
| L4 | 1110 | 2.33 | 3555 |
| M1 | 45 | 3.2 | 795 |
| M2 | 675 | 2.4 | 3855 |
| N | 499.5 | 4.22 | 6466140 |
Dose- and patient weight-normalized values correspond to the Population Pharmacokinetic (PopPK) plot generated by GW Pharmaceuticals
Table 9:
Interindividual Variability of Normalized PK Parameters in GW Pharmaceuticals’ PopPK Model
| Drug | Cmax (ng/mL), (CV%) | Tmax (hrs), (CV%) | Dose-Normalized AUC(0-∞) (h*ng/mL), (CV%) | # Studies | Dose- and Weight-normalized RMSE | RMSE | Studies Included |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Epidiolex | |||||||
| Fed | 1467 (52.66) | 3.28 (34.65) | 8056.08 (62.36) | 9 | 3.89 | 1166.37 | B1-B3, H6, L1-L4, M2 |
| Fasted | 332.55 (52.61) | 4.27 (23.25) | 1664.89 (51.65) | 11 | 8.14 | 250.86 | A1-A3, H1-H5, H7, H8, M1 |
| Sativex | |||||||
| Fed | 374 (23.74) | 3.57 (14.01) | 2156115.5 (141.35) | 3 | 0.49 | 73.38 | D2, J3, N |
| Fasted | 304.13 (66.53) | 1.62 (32.35) | 33692.5 (98.43) | 10 | 3.52 | 222.41 | F1-F3, G, K1-K6 |
| Oral Capsule | |||||||
| Fed | 597.97 (56.42) | 2.44 (29.96) | 57447.78 (105.16) | 10 | 0.99 | 297.34 | C, D1, E1, E2, I1-I4, J1, J2 |
| Fasted | 370.5 (0) | 1.27 (0) | 54306 (0) | 1 | 3.85 | 288.78 | F4 |
Mean dose- and patient weight-normalized values correspond to the PopPK plot created by GW Pharmaceuticals
Discussion
In accordance with the Epidiolex and Sativex drug package inserts, CBD pharmacokinetics were more accurate and efficacious in terms of lower interindividual variability and significantly greater bioavailability in the presence of food.2, 4 CBD has poor solubility and high permeability, meaning that the bioavailability in the fasted state was observed to be quite low, especially in orally administered products.2 Because CBD is categorized as a BCS II drug, and concomitant food administration has been reported to increase the bioavailability of CBD, 2 we hypothesized that the fed state will alter drug absorption in the gastrointestinal tract and possibly influence first-pass metabolism.1 Although the FDA recommends that food-effect bioavailability and bioequivalence studies are conducted under the administration of a high-calorie (800–1000 calories) and high-fat meal (50% of caloric content), only a fraction of food-effect studies on CBD have abided by the FDA’s guidelines.4 Knowing that a meal will significantly increase the maximum concentration of CBD, while reducing the interindividual variability of the CBD concentration in the plasma, is of great importance for future recommendations regarding dosing and administration schemes. An example of a dosing scheme would follow the assumption that there will be a larger amount of CBD absorbed in the body at the maximum concentration, and therefore greater bioavailability, and thus all patients should be advised to take Epidiolex with a meal. Any patient that may not need a high plasma concentration of CBD could take a reduced dose in the presence of food to achieve the desired therapeutic effect.
Based on the pharmacokinetic behavior of CBD in clinical trial data in relation to the PopPK models, a fed state was observed to achieve the current pharmacokinetic predictions more accurately (Supplementary Figure 4). 6 Both a low (187.5 mg) and high (1500 mg) dose of Epidiolex under fed conditions were found to have a more accurate behavior of CBD in comparison to the respective PopPK models. The low dose of Epidiolex had a much smaller normalized RMSE value (0.41) than that of the high dose (3.9).
Higher doses of Epidiolex, such as 1500 mg, contain a substantially larger amount of oil from excipients alone. A 1500 mg dose of Epidiolex includes approximately 11 mL of oil, whereas a 187.5 mg dose of Epidiolex would contain approximately 1.4 mL of oil.11 As previously mentioned, an oil concentration as low as 2 mg has been reported to stimulate increased bile salt secretion, elevate levels of phospholipid and cholesterol in the intestine, and trigger gall bladder contraction.12 Thus, CBD drug product excipients are likely contributing to the increased interindividual variability in fasted state studies –especially with higher doses as greater oil concentration may largely affect the gastrointestinal mechanisms in digestion as the high doses are administered in conjunction with large amounts of sesame oil vehicle.
It is expected that the effect of food on CBD pharmacokinetics is also poised to have an impact on endocannabinoid receptor signaling pharmacodynamics as exemplified by current research.28 The molecular signaling mechanisms that are perturbed by CBD, THC, and other phytocannabinoids are part of the biochemical pathways involved in inflammation, wound healing, and regeneration. Food effects are not only important in understanding the pharmacokinetics of Epidiolex, Sativex, Marinol, and other FDA-approved prescription drugs, but can also offer mechanistic insights into the possible health benefits of phytochemical cannabinoid receptor modulators present in hemp-derived nutritional supplements that are now sold in most health food stores across the United States. The interplay between endocannabinoid receptor modulation and greater amounts of amino acid and L-Carnitine metabolites in the circulation, together with food effect considerations, reveals an entirely new field centered on the interaction between phytocannabinoids, nutrition, organismic physiology, health, and disease.28
Conclusions
This review article outlines the necessity for factoring in patient food-effects in the therapeutic treatment and subsequent dosing and administration regimens of CBD. This review is of great importance due to the widespread availability of CBD products. Companies developing CBD drug products and supplements can use the findings in this review and the PopPK model analysis to help provide more accurate recommendations. Based on a thorough analysis of the data found in published clinical trials, Epidiolex is shown to have a statistically significant increase in maximum concentration in the plasma when taken with a meal. Furthermore, oil content in excipients and meal content should be considered when generating dosing regimens, as many drug products for CBD contain large amounts of oil excipients. Thus, dosing CBD in the presence of a meal leads to much improved, predictable pharmacokinetics, which should translate to improved pharmacodynamics in terms of any observed efficacy and side effects. To our knowledge, this is the only study addressing how high doses of a drug formulated in sesame oil, such as Epidiolex, can lead to a situation resembling a fed state based on the excipients alone.2 Moving forward, PopPK models for CBD should be modified to include the amount of vehicle and its impact on the food effect, especially in the case of multi-dose regimens, to achieve the most predictable pharmacotherapeutic outcomes in humans. Of noteworthy significance, the magnitude of the effect of food on the increase in Cmax and AUC0−∞ is well beyond the range that is typically observed with other FDA-approved drugs and is more akin to the effect of food on the absorption of vitamins.29, 30 This suggests that CBD absorption may be mediated by specific transport pathways involved in the uptake of nutrients into the body.
Supplementary Material
Acknowledgements
We greatly appreciate the support from the National Center for Complementary and Integrative Health for grant 1R01AT010381-01A1 and the National Institute of General Medical Sciences for grant 1R01GM127787-01A1. Thank you to Kathleen A. Stringer for helping determine proper statistical analyses and modeling parameters for the data collected in this review article. Thank you to Steven Harte, Daniel Clauw, Richard Harris, and Kevin Boehnke for your input and suggestions.
Funding
Research reported in this manuscript was supported by the National Center for Complementary and Integrative Health of the National Institutes of Health (NIH) under award number 1R01AT010381-01A1 (to Richard E. Harris and Steven Edward Harte) and the National Institute of General Medical Sciences of the National Institutes of Health (NIH) under award number 1R01GM127787-01A1 (to GRR). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Conflict of Interest
The authors declare no conflicts of interest.
Thank you to Merlin T. Steffensen for helping format all tables and generate visual representations of all data in this review article.
References
- 1.Perucca E, Bialer M. Critical Aspects Affecting Cannabidiol Oral Bioavailability and Metabolic Elimination, and Related Clinical Implications. CNS Drugs [Internet]. 2020. August 1 [cited 2020 Oct 15];34(8):795–800. Available from: https://doi.org/10.1007/s40263-020-00741-5 [DOI] [PubMed] [Google Scholar]
- 2.EPX-03633–1020_EPIDIOLEX_(cannabidiol)_USPI_v8.0.pdf [Internet]. [cited 2020 Nov 10]. Available from: https://www.epidiolex.com/sites/default/files/pdfs/1020/EPX-03633-1020_EPIDIOLEX_(cannabidiol)_USPI_v8.0.pdf
- 3.Cherniakov I, Izgelov D, Barasch D, Davidson E, Domb AJ, Hoffman A. Piperine-pro-nanolipospheres as a novel oral delivery system of cannabinoids: Pharmacokinetic evaluation in healthy volunteers in comparison to buccal spray administration. Journal of Controlled Release [Internet]. 2017. November 28 [cited 2020 Sep 30];266:1–7. Available from: http://www.sciencedirect.com/science/article/pii/S016836591730843X [DOI] [PubMed] [Google Scholar]
- 4.Sativex Oromucosal Spray - Summary of Product Characteristics (SmPC) - (emc) [Internet]. [cited 2020 Sep 30]. Available from: https://www.medicines.org.uk/emc/product/602
- 5.Assessing the Effects of Food on Drugs in Investigational New Drug Applications and New Drug Applications-Clinical Pharmacology Considerations; Draft Guidance for Industry; Availability [Internet]. Federal Register. 2019. [cited 2021 Feb 5]. Available from: https://www.federalregister.gov/documents/2019/02/26/2019-03247/assessing-the-effects-of-food-on-drugs-in-investigational-new-drug-applications-and-new-drug
- 6.210365Orig1s000ClinPharmR.pdf [Internet]. [cited 2020 Oct 15]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2018/210365Orig1s000ClinPharmR.pdf
- 7.Rowland M Clinical pharmacokinetics :concepts and applications / [Internet]. 3rd ed. Baltimore :; 1995. Available from: http://hdl.handle.net/2027/mdp.39015034842149 [Google Scholar]
- 8.Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement | The EQUATOR Network [Internet]. [cited 2020 Oct 15]. Available from: https://www.equator-network.org/reporting-guidelines/prisma/
- 9.GW Pharmaceuticals announces that EPIDYOLEX® (cannabidiol) has been reclassified by the UK Home Office as a Schedule 5 drug | GW Pharmaceuticals [Internet]. [cited 2021 Feb 5]. Available from: https://ir.gwpharm.com/news-releases/news-release-details/gw-pharmaceuticals-announces-epidyolexr-cannabidiol-has-been
- 10.Taylor L, Gidal B, Blakey G, Tayo B, Morrison G. A Phase I, Randomized, Double-Blind, Placebo-Controlled, Single Ascending Dose, Multiple Dose, and Food Effect Trial of the Safety, Tolerability and Pharmacokinetics of Highly Purified Cannabidiol in Healthy Subjects. CNS Drugs [Internet]. 2018. [cited 2020 Sep 30];32(11):1053–67. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6223703/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amazon.com : Roland Pure Sesame Oil, 56 oz. : Grocery & Gourmet Food [Internet]. [cited 2020 Oct 15]. Available from: https://www.amazon.com/Roland-Pure-Sesame-Oil-oz/dp/B005AK78LE
- 12.Kossena GA, Charman WN, Wilson CG, O’Mahony B, Lindsay B, Hempenstall JM, et al. Low dose lipid formulations: effects on gastric emptying and biliary secretion. Pharm Res. 2007November;24(11):2084–96. [DOI] [PubMed] [Google Scholar]
- 13.Risk of bias tools - ROBINS-I detailed guidance (2016) [Internet]. [cited 2020 Oct 29]. Available from: https://sites.google.com/site/riskofbiastool/welcome/home/current-version-of-robins-i/robins-i-detailed-guidance-2016?authuser=0
- 14.Taylor L, Crockett J, Tayo B, Morrison G. A Phase 1, Open-Label, Parallel-Group, Single-Dose Trial of the Pharmacokinetics and Safety of Cannabidiol (CBD) in Subjects With Mild to Severe Hepatic Impairment. The Journal of Clinical Pharmacology [Internet]. 2019. [cited 2020 Jun 8];59(8):1110–9. Available from: https://accp1.onlinelibrary.wiley.com/doi/abs/10.1002/jcph.1412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Birnbaum AK, Karanam A, Marino SE, Barkley CM, Remmel RP, Roslawski M, et al. Food effect on pharmacokinetics of cannabidiol oral capsules in adult patients with refractory epilepsy. Epilepsia [Internet]. 2019. [cited 2020 Oct 1];60(8):1586–92. Available from: http://onlinelibrary.wiley.com/doi/abs/10.1111/epi.16093 [DOI] [PubMed] [Google Scholar]
- 16.Guy GW, Robson PJ. A Phase I, Open Label, Four-Way Crossover Study to Compare the Pharmacokinetic Profiles of a Single Dose of 20 mg of a Cannabis Based Medicine Extract (CBME) Administered on 3 Different Areas of the Buccal Mucosa and to Investigate the Pharmacokinetics of CBME per Oral in Healthy Male and Female Volunteers (GWPK0112). Journal of Cannabis Therapeutics [Internet]. 2004. January 29 [cited 2020 Sep 30];3(4):79–120. Available from: http://www.tandfonline.com/doi/abs/10.1300/J175v03n04_01 [Google Scholar]
- 17.Stott CG, White L, Wright S, Wilbraham D, Guy GW. A phase I study to assess the single and multiple dose pharmacokinetics of THC/CBD oromucosal spray. Eur J Clin Pharmacol [Internet]. 2013. May 1 [cited 2020 Oct 1];69(5):1135–47. Available from: https://link-springer-com.proxy.lib.umich.edu/article/10.1007/s00228-012-1441-0 [DOI] [PubMed] [Google Scholar]
- 18.Guy GW, Robson PJ. A Phase I, Double Blind, Three-Way Crossover Study to Assess the Pharmacokinetic Profile of Cannabis Based Medicine Extract (CBME) Administered Sublingually in Variant Cannabinoid Ratios in Normal Healthy Male Volunteers (GWPK0215). Journal of Cannabis Therapeutics [Internet]. 2004. January 29 [cited 2020 Oct 1];3(4):121–52. Available from: http://www.tandfonline.com/doi/abs/10.1300/J175v03n04_02 [Google Scholar]
- 19.Morrison G, Crockett J, Blakey G, Sommerville K. A Phase 1, Open-Label, Pharmacokinetic Trial to Investigate Possible Drug-Drug Interactions Between Clobazam, Stiripentol, or Valproate and Cannabidiol in Healthy Subjects. Clinical Pharmacology in Drug Development [Internet]. 2019. [cited 2020 Sep 30];8(8):1009–31. Available from: https://accp1.onlinelibrary.wiley.com/doi/abs/10.1002/cpdd.665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Atsmon J, Heffetz D, Deutsch L, Deutsch F, Sacks H. Single-Dose Pharmacokinetics of Oral Cannabidiol Following Administration of PTL101: A New Formulation Based on Gelatin Matrix Pellets Technology. Clinical Pharmacology in Drug Development [Internet]. 2018. [cited 2020 May 5];7(7):751–8. Available from: http://accp1.onlinelibrary.wiley.com/doi/abs/10.1002/cpdd.408 [DOI] [PubMed] [Google Scholar]
- 21.Schoedel KA, Szeto I, Setnik B, Sellers EM, Levy-Cooperman N, Mills C, et al. Abuse potential assessment of cannabidiol (CBD) in recreational polydrug users: A randomized, double-blind, controlled trial. Epilepsy Behav [Internet]. 2018. November 1 [cited 2020 Oct 29];88:162–71. Available from: https://www.epilepsybehavior.com/article/S1525-5050(18)30483-9/abstract [DOI] [PubMed] [Google Scholar]
- 22.Haney M, Malcolm RJ, Babalonis S, Nuzzo PA, Cooper ZD, Bedi G, et al. Oral Cannabidiol does not Alter the Subjective, Reinforcing or Cardiovascular Effects of Smoked Cannabis. Neuropsychopharmacology [Internet]. 2016. July [cited 2020 May 5];41(8):1974–82. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4908634/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manini AF, Yiannoulos G, Bergamaschi MM, Hernandez S, Olmedo R, Barnes AJ, et al. Safety and pharmacokinetics of oral cannabidiol when administered concomitantly with intravenous fentanyl in humans. J Addict Med [Internet]. 2015. [cited 2020 Sep 30];9(3):204–10. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4449284/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patrician A, Versic-Bratincevic M, Mijacika T, Banic I, Marendic M, Sutlović D, et al. Examination of a New Delivery Approach for Oral Cannabidiol in Healthy Subjects: A Randomized, Double-Blinded, Placebo-Controlled Pharmacokinetics Study. Adv Ther [Internet]. 2019. November 1 [cited 2020 Sep 30];36(11):3196–210. Available from: https://doi.org/10.1007/s12325-019-01074-6 [DOI] [PubMed] [Google Scholar]
- 25.Omachi F, Kaneko M, Iijima R, Watanabe M, Itagaki F. Relationship between the effects of food on the pharmacokinetics of oral antineoplastic drugs and their physicochemical properties. J Pharm Health Care Sci [Internet]. 2019. December 3 [cited 2020 Nov 3];5(1):26. Available from: https://doi.org/10.1186/s40780-019-0155-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ohlsson A, Lindgren J-E, Andersson S, Agurell S, Gillespie H, Hollister LE. Single-dose kinetics of deuterium-labelled cannabidiol in man after smoking and intravenous administration. Biomedical & Environmental Mass Spectrometry [Internet]. 1986. [cited 2020 May 5];13(2):77–83. Available from: http://onlinelibrary.wiley.com/doi/abs/10.1002/bms.1200130206 [DOI] [PubMed] [Google Scholar]
- 27.Heuberger JAAC, Guan Z, Oyetayo O-O, Klumpers L, Morrison PD, Beumer TL, et al. Population Pharmacokinetic Model of THC Integrates Oral, Intravenous, and Pulmonary Dosing and Characterizes Short- and Long-term Pharmacokinetics. Clin Pharmacokinet [Internet]. 2015. February 1 [cited 2020 Nov 11];54(2):209–19. Available from: https://doi.org/10.1007/s40262-014-0195-5 [DOI] [PubMed] [Google Scholar]
- 28.Mohammed A,FK Alghetaa H, Miranda K, Wilson K,P Singh N, Cai G, et al. Δ9-Tetrahydrocannabinol Prevents Mortality from Acute Respiratory Distress Syndrome through the Induction of Apoptosis in Immune Cells, Leading to Cytokine Storm Suppression. International Journal of Molecular Sciences [Internet]. 2020. January [cited 2020 Dec 2];21(17):6244. Available from: https://www.mdpi.com/1422-0067/21/17/6244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Savla R, Browne J, Plassat V, Wasan KM, Wasan EK. Review and analysis of FDA approved drugs using lipid-based formulations. Drug Development and Industrial Pharmacy [Internet]. 2017. November 2 [cited 2020 Dec 2];43(11):1743–58. Available from: https://www.tandfonline.com/doi/full/10.1080/03639045.2017.1342654 [DOI] [PubMed] [Google Scholar]
- 30.Rose RC. Transport and metabolism of water-soluble vitamins in intestine. American Journal of Physiology-Gastrointestinal and Liver Physiology [Internet]. 1981. February 1 [cited 2020 Dec 2];240(2):G97–101. Available from: https://www.physiology.org/doi/10.1152/ajpgi.1981.240.2.G97 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.