Abstract
Introduction:
Freezing of gait (FOG) is a debilitating feature of Parkinson’s disease (PD). Evidence suggests patients with FOG have increased cortical control of gait. The supplementary motor area (SMA) may be a key structure due to its connectivity with locomotor and cognitive networks. The objectives of this study were to determine (1) if SMA connectivity is disrupted in patients with FOG and (2) if “inhibitory” repetitive transcranial magnetic stimulation can decrease maladaptive SMA connectivity.
Methods:
Two experiments were performed. In experiment 1 resting-state (T2* BOLD imaging) was compared between 38 PD freezers and 17 PD controls. In experiment 2, twenty PD patients with FOG were randomized to either 10 sessions of real or sham rTMS to the SMA (1 Hz, 110% motor threshold, 1200 pulses/session) combined with daily gait training.
Results:
(Experiment 1) Freezers had increased connectivity between the left SMA and the vermis of the cerebellum and decreased connectivity between the SMA and the orbitofrontal cortex (pFDR-corr <0.05). (Experiment 2) 10 sessions of active TMS reduced SMA connectivity with the anterior cingulate, angular gyrus and the medial temporal cortex, whereas sham TMS did not reduce SMA connectivity. From a behavioral perspective, both groups showed nFOG-Q improvements (F(4, 25.7) = 3.87, p=0.014).
Conclusions:
The SMA in freezers is hyper-connected to the cerebellum, a key locomotor region which may represent maladaptive compensation. In this preliminary study, 1Hz rTMS reduced SMA connectivity however, this was not specific to the locomotor regions. Intervention outcomes may be improved with subject specific targeting of SMA.
Keywords: Freezing, Parkinson’s Disease, Connectivity, Gait, Transcranial Magnetic Stimulation
Introduction
Freezing of gait (FOG) is a severely disabling feature of Parkinson’s disease (PD) for which there are no effective therapies[1]. Defined as an episodic loss in forward stepping despite the intention to do so, patients with FOG have a higher prevalence of falling, and a loss of overall mobility[1, 2]. One conceptual model for FOG is that automaticity of gait, which typically does not require cognitive resources, begins to erode[3, 4]. The cost of executing a cognitive task while walking (i.e., dual tasking) is a well-established approach for quantifying gait automaticity[4]. Among participants with FOG, dual-task walking leads to a disproportionate reduction in velocity and increase in freezing [5]. This suggests automaticity of gait is reduced in FOG and higher-level cognitive resources are needed to compensate. Cortical control of gait, however, is not necessarily an effective compensatory strategy[6, 7].
In healthy individuals, the supplementary motor area (SMA) is a key node within the brain that links neural resources from cognitive and motor domains[8]. The SMA has connectivity to brain regions involved in attention and locomotor behavior[9, 10].
Recent studies have implicated the SMA in the development of FOG and dual tasking[1, 9]. Generally, studies have assumed enhanced activation and connectivity of the SMA is adaptive and reduces freezing behavior. A recent study testing this hypothesis, used a facilitatory form of high frequency transcranial magnetic stimulation (TMS) targeting the SMA and found stimulation improved some aspects of gait [11]. There is, however, increasing support SMA hyper-connectivity is associated with worsened freezing severity [9, 12] and may in fact interfere with smooth, automatic gait. To date, it remains unclear whether the SMA plays an adaptive or maladaptive role in FOG. Specifically, no studies have performed “inhibitory” stimulation to the SMA to directly evaluate the maladaptive hypothesis.
We sought to address two main objectives: (1) characterize maladaptive SMA connectivity in FOG and (2) determine whether maladaptive connectivity can be reversed. Objectives were accomplished using two experiments. In Experiment 1 we compared resting-state connectivity in 38 PD freezers to 17 PD non-freezers. In Experiment 2 we employed the use of 1 Hz repetitive transcranial magnetic stimulation (rTMS), to decrease cortical excitability and connectivity [13]. We tested the hypothesis that SMA hyper-connectivity, which represents an aberrant increase in volitional control of gait, can be reversed using 1Hz TMS when combined with gait training. To accomplish this, we performed a sham-controlled study in which brain connectivity (primary outcome) and freezing severity (secondary outcome) were assessed.
Materials and Methods
Participants. Experiment 1 Participants. Fifty-four PD participants were recruited for a cross-sectional, observational study comparing PD participants with (n=38) and without FOG (n=17). Experiment 2 Participants. Twenty participants with PD and FOG were recruited for a 10-day, double-blind, parallel assignment, randomized (12 active, 8 sham), sham controlled rTMS intervention study (Clinical-Trials.gov: NCT03273270) (Supplemental Figure 4). Recruitment began April of 2017 and ended in March of 2020. The sample size for Experiment 1 and 2 were determined by the number of subjects needed to detect a difference in SMA connectivity between groups at 80% power using the mean and standard deviation of connectivity estimates from a previous study[9].
Participants for Experiment 1 & 2 were recruited from the Movement Disorders clinics Medical University of South Carolina (MUSC) or the Ralph H Johnson VA hospital. Participants were informed of study procedures and provided consent approved by the MUSC Institutional Review Board (IRB). All participants had a PD diagnosis as defined by UK Brain Bank diagnostic criteria. “Freezer” status was based on Item 14 of the UPDRS, and Item 1 of the nFOG-Q. Presence of FOG was confirmed by direct observation by a movement disorders neurologist at screening. Exclusion criteria included MRI or TMS (Experiment 2 only) contraindications, cognitive dysfunction (<26 on the Mini-Mental State Examination), and inability to walk along a 30-foot walkway while in the OFF state.
Experiment 1 Methods.
MRI Assessment
MRI scans were collected at MUSC’s Center for Biomedical Imaging on a 3T Siemens Prisma scanner. T1 structural scans (32 channel head coil, TR = 2300 ms, TE = 2.26 ms, TI = 900 ms, slice thickness = 1 mm, field of view = 256 mm, flip angle = 8°) and resting-state functional scans were acquired in the ON-state while participants fixated on a cross (T2*; TR = 2200 ms, 36 transverse slices, thickness = 3.0 mm, interleaved or ascending slice order, 119 volumes, 4 minutes 30 seconds).
Behavioral Assessment
Behavioral assessments included the new Freezing of Gait Questionnaire (nFOG-Q), the Unified Parkinson’s Disease Rating Scale (UPDRS), and objective gait assessments performed in the ON and OFF states. OFF assessments were made at least 12 hours OFF all dopaminergic medication (24 hours OFF extended-release formulations), ON assessments were made at least 30 minutes after taking the first dose of medications on the same day. Since the PD control group did not experience motor fluctuations, tasks were only performed in the ON state. Objective gait metrics were collected using a GAITRite© digital walkway (Supplemental Methods 1.1). Demographic and behavioral group comparisons were performed using a 2-sample t-tests or χ2 tests (significant if p<0.05).
RS-fMRI Connectivity Analysis: PD Freezers vs. PD Controls
A seed-to-voxel approach was used to determine left and right SMA (AAL atlas) connectivity (Supplement Methods 1.2). Differences in SMA connectivity between groups was assessed using a general linear regression framework and included age, disease duration, and sex as covariates. This analysis was performed both with and without levodopa equivalent daily dose (LEDD) as a covariate to determine its effect on connectivity. Whole brain voxel-wise differences between groups were carried out using a 2-sample t-test (voxel threshold p<0.01, cluster threshold p<0.05 FDR). Relationship of SMA connectivity and behavior. The relationship between SMA connections (increased in freezers relative to PD-controls) and FOG severity (nFOG-Q, single and dual task time to turn OFF) were assessed using partial correlations (significant if p<0.05, Bonferroni corrected) correcting for age, and disease duration. Behavioral data were log transformed due to their non-normal distribution.
Experiment 2 Methods.
Transcranial Magnetic Stimulation (TMS)
TMS was performed in the ON state using a MagStim Rapid2 stimulator and 70mm air cooled figure-of-eight coil. Resting motor thresholds (rMT) were estimated on the first visit using an adaptive algorithm. Participants were randomized to receive either active or sham rTMS (Supplemental Figure 1). The visit-to-visit stability of the SMA target location (40% of the distance nasion to inion along the midline of the scalp) was aided with Brainsight neuro-navigation (Rogue Research Inc., Montreal, Canada) (Supplemental Methods 1.4). TMS was delivered at 1Hz (110% rMT, 1200 pulses per session, 20 minutes total). Sham setup is described in the supplement (Supplemental Methods 1.5).
Gait Training
A 20-minute gait training session was performed immediately following each active or sham treatment. Repetitive trials of walking were performed with a concurrent cognitive task at a consistent speed while accompanied by the therapist. Consistent with prior work [14, 15], the difficulty of the cognitive task (working memory, language and calculation) was increased at each session while maintaining a consistent walking velocity. Participants were instructed to focus on the cognitive task rather than their gait with the goal of improving gait automaticity.
Pre and Post MRI Assessments
MRI scans were collected in the ON-state at baseline and within 24 hours completing the intervention. Structural (T1) and functional (T2*) scans were equivalent to those described in Experiment 1.
Pre and Post Behavioral Assessments
Motor assessments (see Experiment 1) were performed before and again within 24 hours of the last treatment session (Supplemental Figure 1, Supplemental Methods 1.1). The nFOG-Q was additionally performed at 1, 2 and 3-months follow-ups.
RS-fMRI Connectivity Analysis: Pre vs. Post
Functional connectivity preprocessing (Supplemental Methods 1.2) was equivalent to those described in Experiment 1. A general linear regression framework was used to determine changes (Pre>Post, Post>Pre contrasts) in SMA whole-brain connectivity. To identify if SMA connectivity changed to a greater extent following active stimulation compared to sham stimulation the interaction between time (Pre, Post) and treatment group (Active, Sham) was analyzed (voxel threshold p<0.01, cluster threshold FDR corrected p<0.05).
Statistical Analysis of Baseline Assessments and Behavior
To assess group differences in demographics independent 2-sample t-tests were performed (significant if p<0.05). Interactions between time and treatment group for measures of freezing behavior were assessed using a linear mixed model with a first-order autoregression (AR(1)) model and Restricted Maximum Likelihood (REML) estimation. For each model fixed factors included time (pre vs post) and treatment group (active vs sham) and LEDD was included as a covariate. Statistics were performed using IBM SPSS Statistics (V25).
E-field Modeling
SimNIBS software (Version 3.1.2) was used to simulate the electric field produced by SMA TMS (Supplemental Methods 1.3). Individual Brainsight coordinates were used to determine coil location.
Results
Experiment 1.
Demographics, disease severity and gait measures.
See Supplemental Table 1 for group demographic, disease severity and gait measures. Years of education was greater among PD controls (t = 3.25, p = 0.029). Levodopa Equivalent Daily Dose (LEDD) (t = 4.13, p = 0.00015) was greater in freezers.
SMA Connectivity in PD FOG vs. PD Non-FOG.
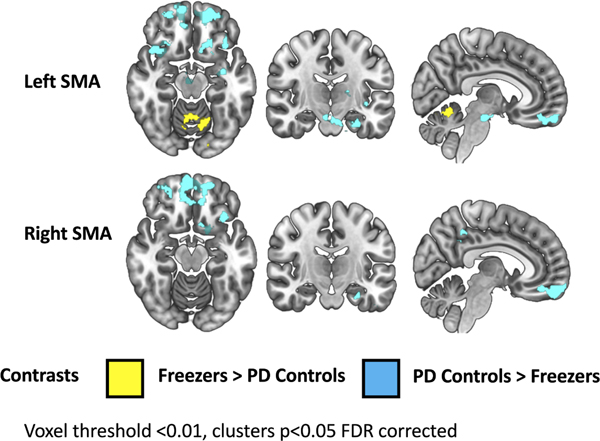
Left SMA Seed. Relative to PD controls, FOG participants had elevated connectivity to the vermis of the cerebellum (pFDR-corr = 0.047, Z = 3.85). PD controls had greater connectivity to the bilateral orbitofrontal cortex and a large cluster extending from entorhinal cortex and amygdala to the brainstem (Figure 1, Supplemental Table 2). Right SMA Seed. Relative to freezers, PD controls had greater connectivity to the left basal forebrain, orbitofrontal cortex and precuneus (Figure 1, Supplemental Table 2). The addition of LEDD as a covariate did not change which regions were detected to be significantly different between the freezer and non-freezer group (Supplemental Table 3). Relationship of increased SMA connectivity and FOG behavior. SMA connectivity with the cerebellum did not correlate with the nFOG-Q or spatiotemporal measures (ON or OFF state).
Figure 1. SMA resting-state functional connectivity in freezers versus PD controls.
Brain areas in which SMA connectivity is greater in freezers than PD controls (yellow) and greater in PD controls than freezers (blue) on an MNI template brain. Clusters shown are p<0.05, FDR corrected.
Experiment 2.
Demographics, disease severity and thresholds.
The active and sham groups did not differ significantly in mean age, level of education, LEDD, disease duration or PD motor impairment severity (Table 1).
Table 1.
Demographics, disease severity, and TMS parameters for Experiment II
| Active (n=12) | Sham (n=8) | |
|---|---|---|
|
| ||
| Age (mean years ± SD) | 66.6±7.5 years | 64.5±8.9 years |
| Sex (M,F) | 7,5 | 7,1 |
| Education (mean ± SD) | 15.25±4 years | 14.75±2.5 years |
| Disease Duration (mean ± SD) | 8.7±7.12 years | 8.0±5.63 years |
| Stimulation Intensity (mean ± SD) | 71.583±11.5 %MSO | N/A |
| MagStim rMT (mean ± SD) | 65±10.3 %MSO | 57.5±8.2 %MSO |
| MMSE (mean ± SD) | 29.08±1.16 a.u. | 28.38±1.69 a.u. |
| UPDRS (OFF) (mean ± SD) | 43.83±10.82 a.u. | 47.38±11.10 a.u. |
| UPDRS III (OFF) (mean ± SD) | 25.08±7.012 a.u. | 25.38±10.25 a.u. |
| H&Y Staging (OFF) (mean ± SD) | 2.32±0.405 a.u. | 2.29±0.267 a.u. |
| LEDD (mean ± SD) | 1074.4±493.9 mg | 1304.4±757.3mg |
M = male, F = female, a.u. = arbitrary units, %MSO = percentage of maximum machine output
Functional Connectivity. Left SMA Seed.
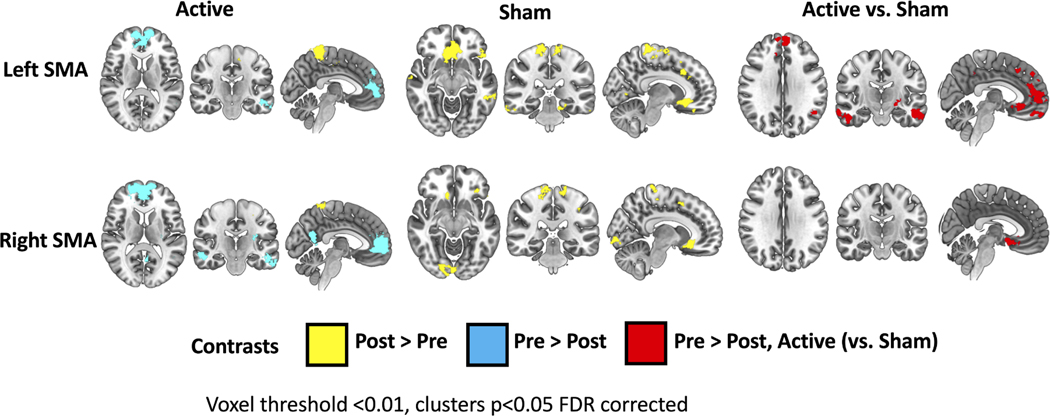
Following active rTMS, there was a decrease in connectivity to the anterior cingulate, medial prefrontal cortex, angular gyrus, and middle temporal gyrus and an increase in connectivity to postcentral gyrus. Following sham rTMS, there were no decreases in connectivity (Supplemental Table 4, Figure 2). Right SMA Seed. Following active rTMS there was a decrease in connectivity to the anterior cingulate, medial prefrontal cortex, parietal operculum, angular gyrus, precuneus and bilateral middle temporal gyrus and an increase to the postcentral gyrus and precuneus (Supplemental Table 4, Figure 2).
Figure 2. SMA resting-state functional connectivity changes following active vs sham rTMS and gait training.
Brain regions with either increased (yellow) or decreased (blue) SMA connectivity following the intervention are shown on an MNI template. Brain regions are shown where active stimulation (relative to sham) had a greater SMA connectivity decrease (red). Clusters p<0.05, FDR corrected.
E-field modeling.
E-fields exceeding 50 V/m are seen on gyri of both the left and right SMA in all participants (Supplemental Figure 3). The mean peak (99.9%) e-field was 81.4 ± 10.1 V/m (range: 62.5–99.1 V/m).
Change in Behavior. nFoG-Q.
There was a significant effect of time (F(4, 25.7) = 3.87, p=0.014), wherein scores decreased (improved) from pre intervention to post intervention timepoints. There was no interaction between time and group (F(4, 25.75) = 0.600, p=0.666) (Table 2, Supplemental Figure 2).
Table 2.
Effect of Active versus Sham Stimulation on Motor Behavior
| nFOG-Q | |||
|
| |||
| Timepoint | Active (mean ± SD) | Sham (mean ± SD) | |
| Baseline | 21.42±2.74 | 19.88±4.88 | |
| Post | 16.58±6.79 | 18.13±5.93 | |
| 1 Month F/U | 18.22±4.92 | 18.43±6.08 | |
| 2 Month F/U | 16.75±6.30 | 16.33±6.77 | |
| 3 Month F/U | 18.13±7.77 | 13.75±10.81 | |
| UPDRS-III score | |||
|
| |||
| Timepoint | Active | Sham | |
| UPDRS-III (ON) | Baseline | 16.82±4.12 | 15.75±5.97 |
| Post | 12.73±5.46 | 17.13±8.43 | |
| UPDRS-III (OFF) | Baseline | 25.08±7.01 | 25.38±10.25 |
| Post | 22.08±7.30 | 22.88±8.94 | |
| Time to Turn (seconds) | |||
|
| |||
| Timepoint | Active | Sham | |
| Single Task (ON) | Baseline | 7.40±4.35 | 7.42±4.58 |
| Post | 6.52±4.76 | 6.89±2.42 | |
| Single Task (OFF) | Baseline | 13.30±10.08 | 37.63±83.38 |
| Post | 8.68±6.52 | 8.92±5.07 | |
| Dual Task (ON) | Baseline | 10.37±8.31 | 30.14±54.24 |
| Post | 12.10±20.72 | 10.91±7.61 | |
| Dual Task (OFF) | Baseline | 25.58±36.09 | 17.12±13.65 |
| Post | 13.53±17.22 | 11.25±7.53 | |
| Spatiotemporal Measures | |||
|
| |||
| Measure | Timepoint | Active | Sham |
| Velocity (ON) | Baseline | 105.68±21.38 | 100.46±20.16 |
| Post | 105.81±21.03 | 93.99±19.17 | |
| Velocity (OFF) | Baseline | 89.62±37.96 | 74.81±37.93 |
| Post | 95.17±19.62 | 88.20±25.01 | |
| Cadence (ON) | Baseline | 117.35± 10.41 | 108.76±16.95 |
| Post | 119.51±9.89 | 108.46±14.53 | |
| Cadence (OFF) | Baseline | 114.11±11.95 | 91.70±26.01 |
| Post | 119.21±9.42 | 103.61± 10.12 | |
UPDRS-III.
There was a trend toward a main effect of time (F(1,18) = 4.202, p=0.055) for the UPDRS-III OFF wherein scores improved pre to post intervention, however, there was no interaction with group (time x group, F(1,18) = 0.035, p=0.854). UPDRS-III ON did not have main effects of time or interactions (Table 2).
Spatiotemporal measures.
Cadence during the OFF state did not have a significant main effect of time (F (1,11.9) = 3.820, p=0.075) but did have a significant interaction (time x group, F (2, 13.7) = 4.349, p=0.035). Velocity during the OFF state did not have a significant main effect of time (F (1, 12.3) = 2.510, 0.138, or interaction (time x group, F (2,14) = 0.804, p=0.467). Velocity and cadence during the ON state did not have main effects of time or interactions (Table 2).
Time to turn.
There were no significant effects of time (pre versus post) on turn duration for either the single or dual task conditions (Table 2).
Discussion
In this two-part study we set out to (1) characterize SMA connectivity in freezers relative to non-freezers and (2) determine whether “inhibitory” SMA stimulation could reduce hyper-connectivity and subsequently improve freezing behavior. While the majority of rTMS studies in FOG have attempted to enhance adaptive cortical control of gait using facilitatory forms of stimulation, there is evidence that increased SMA activity and connectivity is maladaptive and may in fact interfere with automatic gait[9, 12]. In support of this maladaptive hypothesis, we found the SMA to be hyper-connected to cerebellar locomotor regions in participants with FOG when compared to PD controls. Furthermore, we demonstrate that low frequency stimulation in participants with FOG reduces connectivity to regions involved in executive control (e.g., the medial PFC/ACC), and cognition/attention (e.g., the angular gyrus). Low frequency stimulation combined with dual-task gait training produced variable, but no significant changes in nFOG-Q scores.
The SMA in FOG: Adaptive vs Maladaptive Theory
Previous studies have argued that the use of excitatory (10 Hz) rTMS applied to the SMA can improve freezing because cortical control of gait is an adaptive, compensatory mechanism. Indeed, 10 Hz stimulation was previously shown to improve nFOG-Q scores, however, effect sizes were small, and the sham condition did not account for differences in scalp sensation between sham and active rTMS[11]. Alternatively, we suggested that SMA may actually interfere with subcortical automatic gait control by the brain’s locomotor network. The concept of maladaptive, compensatory plasticity for motor control can be seen in other neurological diseases including, stroke wherein the less affected cortex interferes with effective motor execution [16]. In FOG, maladaptive functional reorganization is evidenced by imaging studies showing the SMA is hyper-connected with brainstem locomotor structures (e.g., the mesencephalic locomotor region)[9, 12]. Our study extends these findings by demonstrating the SMA is hyper-connected to the vermis of the cerebellum, a region critical for adjustments in posture and locomotion with direct target of anatomical projections from cortex[17]. FOG participants also demonstrated decreased SMA connectivity to the orbitofrontal cortex and limbic regions which likely reflect its complexity [18] and relationship to the progressive degeneration of brain tissue[19].
Effects of SMA rTMS and Gait Training on Functional Connectivity
We observed a reduction in SMA connectivity to cortical regions including the ACC, angular gyrus, and middle temporal gyrus, demonstrating “inhibitory” rTMS can indeed reduce SMA connectivity to regions which influence executive and attentional control during gait. The ACC in particular has been shown to support coordination of movement, task switching, and adaptation of motor plans[20, 21], while the angular gyrus is associated with visuospatial attention [22]. Despite these changes, locomotor network connectivity appeared largely unchanged. This may reflect the heterogeneity of SMA connectivity. Distinct sub-regions of the SMA differ substantially in their efferent and afferent connections. For example, anterior regions of the SMA (pre-SMA) have dense connections to pre-frontal (cognitive) regions, while posterior portions of SMA (SMA-proper) are strongly connected to the motor network[23]. Variability in SMA targeting in this study is demonstrated by e-field models, wherein the particular gyri stimulated varied substantially between individuals. Thus, more targeted stimulation protocols may be needed to reduce variability in outcomes. Observed increases in SMA connectivity (with cerebellum) and decreases in SMA connectivity (with cognitive and limbic regions) demonstrated in Experiment 1 further emphasizes the need for individualized stimulation strategies for FOG. While “facilitatory” 10 Hz stimulation may benefit those with disrupted SMA-prefrontal connectivity, individuals with SMA-cerebellum hyper-connectivity may primarily benefit from “inhibitory” 1 Hz rTMS. Recent studies have supported the concept of multiple FOG subtypes which can be characterized by intrinsic connectivity [24]. Finally, it’s important to consider that rTMS effects on functional networks can be inconsistent or even paradoxical to our current understanding of frequency dependent neuromodulation[25, 26]. Observed connectivity changes may have differed from previous investigations due to several factors including pulse dose, and number of sessions.
Influence of Gait Training on Connectivity
Gait training by itself has been known to cause lasting changes in brain connectivity. Rehabilitation associated improvements in walking for example are associated with sensorimotor and pre-motor plasticity [27] [28]. While our study was not specifically designed to evaluate the effects of gait training on brain connectivity, both groups received it as part of the intervention. Interestingly both groups displayed increased connectivity between the SMA and the medial primary sensory cortex, suggesting the effect may be attributable to gait training. Within the sham group connectivity to visual areas known to be impaired in FOG[29] were increased, potentially reflecting visual-spatial adaptations as a result of gait training.
Effects of paired rTMS and Gait Training on Behavior
While we did not see a statistically significant difference between active and sham nFOG-Q scores, we did see a numerical reduction in nFOG-Q scores which was greater than the sham group. Consistent with our connectivity findings, this may represent the heterogeneity of targeting within each individual’s SMA as well as individual differences in FOG subtypes. Interestingly, we did not see a significant improvement in overall motor symptoms of PD (UPDRS-III) which suggesting improvements in freezing were not related to global improvements in PD severity. It is important to note that there were likely real improvements as the result of gait training in both the sham and active rTMS groups. This may in turn have minimized our ability to detect between group differences in behavioral outcome measures. Lastly, cadence in the OFF state demonstrated a significant group by time interaction, suggesting the sham group had a greater increase in cadence following the intervention. We believe this difference is primarily attributable to the substantially lower and highly variable cadence performance measures at baseline in the sham group. This difference in baseline cadence may have magnified the improvement in the sham group relative to the active group.
Limitations
It’s important to consider that connectivity in this study was evaluated at rest and thus may not directly reflect brain activity during walking behavior. Furthermore, interaction between dopaminergic medication and functional connectivity is incompletely understood. Connectivity within and between the motor, default mode and attentional networks are known to increase following L-DOPA administration[30]. This confound was minimized by including LEDD as a regressor in our analyses. The small sample size, and lack of individualized targeting may have limited our ability to detect a significant difference in freezing behavior. Responder analysis in a larger sample may help determine the relationship between FOG subtype and rTMS response. Finally, we had some participants which did not complete follow-up FOG-Q assements which limited our ability to determine the long-term effects of stimulation on freezing behavior.
Conclusions
The SMA in FOG is hyper-connected to cerebellum, but hypo-connected to prefrontal and limbic regions. In this preliminary 10-day study of “inhibitory” rTMS and gait training, connectivity was reduced to prefrontal regions. The lack of SMA-locomotor modulation and heterogeneity in behavioral response to rTMS emphasizes the need for individualized stimulation approaches and suggests the SMA likely does not play a purely adaptive or maladaptive role in FOG. Future studies may consider individualizing targets based on FOG subtypes to improve response.
Supplementary Material
A battery of gait and motor assessments were performed before and immediately following sham or active treatment. The nFOG-Q was repeated at 1,2 and 3 months.
a. The nFOG-Q scores at baseline and following 10 days of either active (blue lines) or sham (orange line) rTMS and gait training. Error bars represent standard error. b. Variability in nFOG-Q change within the active rTMS treatment group.
Mesh reconstructed head models are shown for individual participants within the active group (n=12). Cortical e-field distribution using the SMA stimulation parameters is shown by the heat map where blue represents 0 V/m and red represents 100+ V/m.
Supplemental Table 1. Demographics, disease severity, and gait measures for Experiment I
Supplemental Table 2. SMA connectivity in Freezers vs PD controls
Supplemental Table 3. SMA connectivity in Freezers vs PD controls LEDD corrected
Supplemental Table 4. Effect of Active versus Sham Stimulation on SMA connectivity
Highlights.
Unknown if supplementary motor area is adaptive or maladaptive for freezing of gait
The supplemental motor area is hyperconnected to the cerebellum in freezers
Low frequency stimulation of the supplemental motor area and gait training reduces connectivity
Low frequency stimulation and gait training has variable effects on freezing outcomes
Acknowledgements:
We would like to thank the Center for Biomedical Imaging at the Medical University of South Carolina for their help in scanning participants and for their technical knowledge.
Funding Agencies: This work was supported by the National Institutes of Health grant numbers 1K23NS091391-01A1, P2CHD086844, P2-GM109040 and by the Department of Veteran Affairs RR&D IK6RX003075.
Full Financial Disclosure (for the previous 12 months).
D.H.L., W.D., T.K., A.C., E.M., A.E., and J.D. have nothing to disclose. C.A.H. receives support from the NIH. S.A.K., and G.J.R. receive support from the NIH and the Department of Veteran Affairs.
Abbreviations
- SMA
Supplemental Motor Area
- ACC
Anterior Cingulate Cortex
- MTG
Middle Temporal Gyrus
- AG
Angular Gyrus
- fMRI
Functional Magnetic Resonance Imaging
- PD
Parkinson’s Disease
- FOG
Freezing of Gait
- L-DOPA
Levodopa
- rMT
Resting Motor Threshold
- (r)TMS
(Repetitive) Transcranial Magnetic Stimulation
- FDR
False Discovery Rate
- LEDD
Levodopa Equivalent Daily Dose
Footnotes
Relevant Financial Disclosure/Conflict of Interest: The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Nutt JG, Bloem BR, Giladi N, Hallett M, Horak FB, Nieuwboer A, Freezing of gait: moving forward on a mysterious clinical phenomenon, Lancet Neurol 10(8) (2011) 734–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Perez-Lloret S, Negre-Pages L, Damier P, Delval A, Derkinderen P, Destee A, Meissner WG, Schelosky L, Tison F, Rascol O, Prevalence, determinants, and effect on quality of life of freezing of gait in Parkinson disease, JAMA Neurol 71(7) (2014) 884–90. [DOI] [PubMed] [Google Scholar]
- [3].Vandenbossche J, Deroost N, Soetens E, Coomans D, Spildooren J, Vercruysse S, Nieuwboer A, Kerckhofs E, Freezing of gait in Parkinson’s disease: disturbances in automaticity and control, Front Hum Neurosci 6 (2012) 356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Clark DJ, Automaticity of walking: functional significance, mechanisms, measurement and rehabilitation strategies, Front Hum Neurosci 9 (2015) 246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bekkers EMJ, Dockx K, Devan S, Van Rossom S, Verschueren SMP, Bloem BR, Nieuwboer A, The Impact of Dual-Tasking on Postural Stability in People With Parkinson’s Disease With and Without Freezing of Gait, Neurorehabil Neural Repair 32(2) (2018) 166–174. [DOI] [PubMed] [Google Scholar]
- [6].Maidan I, Jacob Y, Giladi N, Hausdorff JM, Mirelman A, Altered organization of the dorsal attention network is associated with freezing of gait in Parkinson’s disease, Parkinsonism Relat Disord 63 (2019) 77–82. [DOI] [PubMed] [Google Scholar]
- [7].Shine JM, Naismith SL, Palavra NC, Lewis SJ, Moore ST, Dilda V, Morris TR, Attentional set-shifting deficits correlate with the severity of freezing of gait in Parkinson’s disease, Parkinsonism Relat Disord 19(3) (2013) 388–90. [DOI] [PubMed] [Google Scholar]
- [8].Nachev P, Kennard C, Husain M, Functional role of the supplementary and pre-supplementary motor areas, Nat Rev Neurosci 9(11) (2008) 856–69. [DOI] [PubMed] [Google Scholar]
- [9].Fling BW, Cohen RG, Mancini M, Carpenter SD, Fair DA, Nutt JG, Horak FB, Functional reorganization of the locomotor network in Parkinson patients with freezing of gait, PLoS One 9(6) (2014) e100291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Richard A, Van Hamme A, Drevelle X, Golmard JL, Meunier S, Welter ML, Contribution of the supplementary motor area and the cerebellum to the anticipatory postural adjustments and execution phases of human gait initiation, Neuroscience 358 (2017) 181–189. [DOI] [PubMed] [Google Scholar]
- [11].Mi TM, Garg S, Ba F, Liu AP, Wu T, Gao LL, Dan XJ, Chan P, McKeown MJ, High-frequency rTMS over the supplementary motor area improves freezing of gait in Parkinson’s disease: a randomized controlled trial, Parkinsonism Relat Disord 68 (2019) 85–90. [DOI] [PubMed] [Google Scholar]
- [12].Lench DH, Embry A, Hydar A, Hanlon CA, Revuelta G, Increased on-state cortico-mesencephalic functional connectivity in Parkinson disease with freezing of gait, Parkinsonism Relat Disord 72 (2020) 31–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Fitzgerald PB, Fountain S, Daskalakis ZJ, A comprehensive review of the effects of rTMS on motor cortical excitability and inhibition, Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology 117(12) (2006) 2584–96. [DOI] [PubMed] [Google Scholar]
- [14].Fritz NE, Cheek FM, Nichols-Larsen DS, Motor-Cognitive Dual-Task Training in Persons With Neurologic Disorders: A Systematic Review, J Neurol Phys Ther 39(3) (2015) 142–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Brauer SG, Morris ME, Can people with Parkinson’s disease improve dual tasking when walking?, Gait Posture 31(2) (2010) 229–33. [DOI] [PubMed] [Google Scholar]
- [16].Murase N, Duque J, Mazzocchio R, Cohen LG, Influence of interhemispheric interactions on motor function in chronic stroke, Ann Neurol 55(3) (2004) 400–9. [DOI] [PubMed] [Google Scholar]
- [17].Coffman KA, Dum RP, Strick PL, Cerebellar vermis is a target of projections from the motor areas in the cerebral cortex, Proc Natl Acad Sci U S A 108(38) (2011) 16068–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Gilat M, Ehgoetz Martens KA, Miranda-Dominguez O, Arpan I, Shine JM, Mancini M, Fair DA, Lewis SJG, Horak FB, Dysfunctional Limbic Circuitry Underlying Freezing of Gait in Parkinson’s Disease, Neuroscience 374 (2018) 119–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Pietracupa S, Suppa A, Upadhyay N, Gianni C, Grillea G, Leodori G, Modugno N, Di Biasio F, Zampogna A, Colonnese C, Berardelli A, Pantano P, Freezing of gait in Parkinson’s disease: gray and white matter abnormalities, J Neurol 265(1) (2018) 52–62. [DOI] [PubMed] [Google Scholar]
- [20].Diwadkar VA, Asemi A, Burgess A, Chowdury A, Bressler SL, Potentiation of motor subnetworks for motor control but not working memory: Interaction of dACC and SMA revealed by resting-state directed functional connectivity, PLoS One 12(3) (2017) e0172531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kerns JG, Cohen JD, MacDonald AW 3rd, Cho RY, Stenger VA, Carter CS, Anterior cingulate conflict monitoring and adjustments in control, Science 303(5660) (2004) 1023–6. [DOI] [PubMed] [Google Scholar]
- [22].Studer B, Cen D, Walsh V, The angular gyrus and visuospatial attention in decision-making under risk, Neuroimage 103 (2014) 75–80. [DOI] [PubMed] [Google Scholar]
- [23].Kim JH, Lee JM, Jo HJ, Kim SH, Lee JH, Kim ST, Seo SW, Cox RW, Na DL, Kim SI, Saad ZS, Defining functional SMA and pre-SMA subregions in human MFC using resting state fMRI: functional connectivity-based parcellation method, Neuroimage 49(3) (2010) 2375–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ehgoetz Martens KA, Shine JM, Walton CC, Georgiades MJ, Gilat M, Hall JM, Muller AJ, Szeto JYY, Lewis SJG, Evidence for subtypes of freezing of gait in Parkinson’s disease, Mov Disord 33(7) (2018) 1174–1178. [DOI] [PubMed] [Google Scholar]
- [25].Eldaief MC, Halko MA, Buckner RL, Pascual-Leone A, Transcranial magnetic stimulation modulates the brain’s intrinsic activity in a frequency-dependent manner, Proc Natl Acad Sci U S A 108(52) (2011) 21229–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ji GJ, Yu F, Liao W, Wang K, Dynamic aftereffects in supplementary motor network following inhibitory transcranial magnetic stimulation protocols, Neuroimage 149 (2017) 285–294. [DOI] [PubMed] [Google Scholar]
- [27].Enzinger C, Dawes H, Johansen-Berg H, Wade D, Bogdanovic M, Collett J, Guy C, Kischka U, Ropele S, Fazekas F, Matthews PM, Brain activity changes associated with treadmill training after stroke, Stroke 40(7) (2009) 2460–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Droby A, Maidan I, Jacob Y, Giladi N, Hausdorff JM, Mirelman A, Distinct Effects of Motor Training on Resting-State Functional Networks of the Brain in Parkinson’s Disease, Neurorehabil Neural Repair 34(9) (2020) 795–803. [DOI] [PubMed] [Google Scholar]
- [29].Velu PD, Mullen T, Noh E, Valdivia MC, Poizner H, Baram Y, de Sa VR, Effect of visual feedback on the occipital-parietal-motor network in Parkinson’s disease with freezing of gait, Front Neurol 4 (2014) 209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Evangelisti S, Pittau F, Testa C, Rizzo G, Gramegna LL, Ferri L, Coito A, Cortelli P, Calandra-Buonaura G, Bisquoli F, Bianchini C, Manners DN, Talozzi L, Tonon C, Lodi R, Tinuper P., L-Dopa Modulation of Brain Connectivity in Parkinson’s Disease Patients: A Pilot EEG-fMRI Study, Front Neurosci 13 (2019) 611. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A battery of gait and motor assessments were performed before and immediately following sham or active treatment. The nFOG-Q was repeated at 1,2 and 3 months.
a. The nFOG-Q scores at baseline and following 10 days of either active (blue lines) or sham (orange line) rTMS and gait training. Error bars represent standard error. b. Variability in nFOG-Q change within the active rTMS treatment group.
Mesh reconstructed head models are shown for individual participants within the active group (n=12). Cortical e-field distribution using the SMA stimulation parameters is shown by the heat map where blue represents 0 V/m and red represents 100+ V/m.
Supplemental Table 1. Demographics, disease severity, and gait measures for Experiment I
Supplemental Table 2. SMA connectivity in Freezers vs PD controls
Supplemental Table 3. SMA connectivity in Freezers vs PD controls LEDD corrected
Supplemental Table 4. Effect of Active versus Sham Stimulation on SMA connectivity