Abstract
Objective
Imbalance and fatigue are among the most common and disabling symptoms of multiple sclerosis (MS). Vestibular rehabilitation studies demonstrate not only improvements in balance but fatigue also, suggesting a relationship between central vestibular integration and fatigue. The objective of this study was to determine whether the relationship between balance and fatigue in people with MS is seen between other measures of central vestibular integration and fatigue and to understand how central vestibular integration measures interrelate.
Methods
This cross-sectional study consisted of 40 people with MS (age = 27–55 years, Expanded Disability Severity Scale score = 1.0–6.5) who completed vestibular ocular reflex testing, subjective visual vertical testing, static posturography, dynamic gait, 2 self-report fatigue surveys, and a 6-Minute Walk Test to assess walking capacity/physical fatigue was completed. Spearman correlations were calculated between variables.
Results
Measures of central vestibular integration were significantly correlated with measures of fatigue and walking capacity and with each other. The correlations between physical fatigue and central vestibular functions were larger than self-reported fatigue correlations with central vestibular functions.
Conclusion
The relationship between balance and fatigue extends to other measures requiring central vestibular integration, suggesting a deficit in central vestibular processing in people with MS. These measures may compliment balance assessment as outcome measures for vestibular rehabilitation in people with MS. Fatigue measures should be included in vestibular rehabilitation as secondary outcomes.
Impact
Correlations between central vestibular integration and fatigue in people with MS suggest that future studies of vestibular rehabilitation should include fatigue, as a secondary outcome measure as vestibular function and fatigue may share similar a similar etiology in people with MS.
Keywords: Balance, Fatigue, Multiple Sclerosis, Rehabilitation, Vestibular Functions
Introduction
Central integration of vestibular information is critical to multisensory processes such as balance and gait.1–3 Improvements in the ability to properly integrate and weigh sensory information represent a mechanism involved in improving balance and gait following vestibular rehabilitation.4 Research on central integration of vestibular information in people with multiple sclerosis (MS) suggests that this process is disrupted by disease pathology and represents a major cause of the balance deficits in upwards of 75% of people with MS.5–7 Central vestibular integration, as measured by postural sway, has been linked with self-reported fatigue in people with MS; improvements in postural sway coincide with significant improvements in self-reported fatigue following rehabilitation.8–11 Despite the fact that up to 80% of people with MS experience fatigue and that most report fatigue as their most debilitating symptom, interventions that significantly improve fatigue long term are lacking.12–14 Understanding the relationship between central vestibular integration and fatigue could inform treatment strategies that target both balance impairments and fatigue simultaneously.
Balance function is not the only function that requires central integration of vestibular information; if balance deficits in people with MS are due to impairment of central vestibular integration, other measures requiring central vestibular integration should also be impaired and correlate with balance deficits and fatigue. Two measures requiring central integration of vestibular information, but arising from integration of unique vestibular inputs, are vestibular ocular reflex (VOR) cancellation and subjective visual vertical (SVV). The VOR cancellation task requires integration of vestibular signals and the visual system to produce equal, but opposite, smooth pursuit eye movements to the VOR to keep vision stable on a visual target moving at the same speed as the participant’s head.15,16 The SVV task requires the integration of otolith graviceptive inputs and the visual system to orient a line to true (perceived) vertical.17 Both measures have been applied in people with MS and demonstrate impairments compared with controls.18–21 Tilt of SVV has been correlated with gait and balance but, to our knowledge, VOR cancellation has not been investigated alongside gait and balance, and the relationships between those 2 functions and fatigue have not been explored.20,21
This study examined whether measures of central vestibular integration, VOR cancellation and SVV, are related to balance, self-reported fatigue, and physical fatigue (walking capacity) in people with MS. We hypothesized that worse VOR cancellation and SVV would be correlated with worse balance performance due to shared central vestibular integration pathways and that impairment in performance on these measures would demonstrate correlations with higher self-reported fatigue and lower walking capacity.
Methods
Recruitment
We recruited 40 people with MS. This sample size was determined with the goal of expanding on a previous study that investigated fatigue and balance in people with MS and that used a sample size of 17 people with MS. To expand on their findings, the sample size was doubled for people with MS.6 The criteria for people with MS were as follows: age 21 to 55 years, diagnosis of relapsing–remitting MS, ability to walk with or without aid (Expanded Disability Severity Scale [EDSS] score ≤6.5), no additional neurological diagnosis unrelated to MS (ie, a diagnosis of optic neuritis was not disqualifying), and no diagnosis of vestibular disease. Our upper age cutoff of 55 years was set to lower the chance that vestibular deficits we measured were due to age-related vestibular degeneration while still ensuring we could include people with MS with more progressed disease.22 Sample demographics are provided in Table 1.
Table 1.
Sample Demographic and Clinical Characteristics for 40 People With MSa
| Characteristic | Value |
|---|---|
| Age, y, mean (SD) | 42.4 (7.7) |
| Sex, no. (%) women/no. (%) men | 35 (88)/5 (12) |
| EDSS score, median (IQR) | 2.5 (2.3) |
| Years since diagnosis, mean (SD) | 9.9 (7.2) |
a EDSS = Expanded Disability Status Scale; IQR = interquartile range; MS = multiple sclerosis.
People with MS were recruited in person from an outpatient clinic (n = 28) and from study flyers mailed to participants of previous studies (n = 12). Participants recruited from the clinic were screened by physicians for interest; the number not interested was not recorded. Of the potential participants who had MS and who indicated interest, 28 of 45 completed the study. Forty flyers were mailed to previous research participants; 12 responded and completed the study. A flowchart for recruitment is provided in the Figure.
Figure.
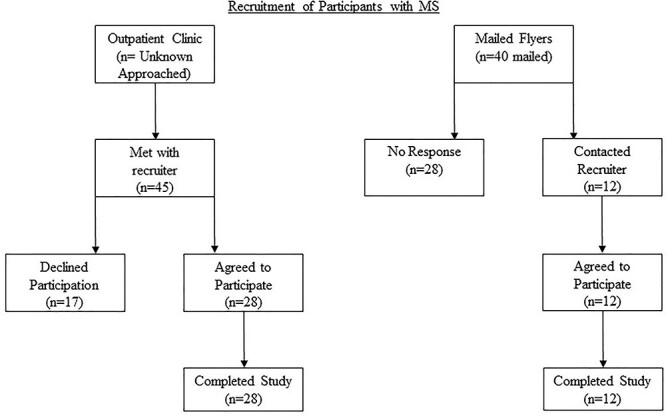
Study recruitment diagram. An unknown number of potential participants was approached by multiple sclerosis clinic physicians for interest in participation. All potential participants from the clinic who met with the recruiter met the inclusion/exclusion criteria; reasons for declining to participate were thus only from the potential participant’s point of view (ie, concerns about COVID-19 transmission, scheduling, long drive to laboratory, and rotary chair testing because of claustrophobia).
Participants completed a 3-hour battery of clinical vestibular, oculomotor, gait, and balance tests. The entire protocol is not described here; a previous analysis of the vestibular function tests of this battery suggested that VOR cancellation and SVV variance were the only vestibular measures this sample with MS performed worse on than controls and that predicted MS disease severity.23 Therefore, other vestibular measures that were also collected (such as dynamic visual acuity, video head-impulse testing, and vestibular-evoked myogenic potentials) are not included in this analysis. Reflexive VOR and SVV average deviation are included as control measures for VOR cancellation and SVV variance, respectively, despite not predicting disease severity in our previous analysis.
The testing battery was split into two 1.5-hour sessions that were completed within 2 weeks of each other. Day 1 of testing consisted of study explanation, obtaining informed consent, neurological examination, surveys, and tests of VOR and SVV. Day 2 consisted of balance and gait testing. All participants completed the same order of tests, which are ordered in that manner here.
Neurological Examination
The data collector was certified as a Neurostatus e-Test level C examiner and performed a neurological examination among people with MS for EDSS scoring. The examiner was a medical student who had additionally been formally evaluated on his neurological exam skills and interpretation of findings by clinical neurologists and other medical school faculty.
Surveys
All participants completed 2 fatigue surveys: the Modified Fatigue Impact Scale (MFIS)13 and the Fatigue Severity Scale (FSS).24 Both the MFIS and the FSS provide valid measures of fatigue in people with MS, but the MFIS allows for the assessment of different domains of fatigue through its subscales (ie, physical, cognitive, and psychosocial).25 Therefore, including both self-report measures allowed us to extrapolate our results to groups who may prefer one tool over another as well as to evaluate different domains of fatigue. Participants also completed the Activities-Specific Balance Confidence Scale (ABC),26 which has been shown to identify lower balance confidence in people with MS compared with controls.27 The Dizziness Handicap Inventory (DHI) was offered to all participants, but only those experiencing dizziness or imbalance were able to complete its items.28 Of our sample of 40 people, 19 with MS were able to complete the DHI.
MFIS
The MFIS is a self-reported measure of fatigue consisting of 21 items scored on a scale from 0 to 4, with 0 indicating “never” and 4 indicating “almost always.” Participants are asked to respond to how often fatigue has affected them as described by each item using the scale from 0 to 4. The variables of interest were the combined composite score (0–84, with higher scores representing higher fatigue) and the combined scores on the 3 MFIS subscales (physical [0–36], cognitive [0–40], and psychosocial [0–8]).
FSS
The FSS is a self-reported measure of fatigue consisting of 9 items scored on a scale from 1 to 7, with 1 indicating “strong disagreement” with the item and 7 indicating “strong agreement” with the item. Each item is a statement of how fatigue affects the individual. The variable of interest was the average score of the 9 items (0–7, with higher scores representing higher fatigue).
ABC
The ABC is a self-reported measure of balance confidence consisting of 16 scenarios, such as getting off an escalator or bending to pick up items. Participants score each scenario on a scale from 0 to 100, with 0 indicating that they are sure they would lose their balance in the scenario and 100 indicating that they are sure they would not lose their balance. The variable of interest was the average balance confidence score (0–100, with higher scores indicating greater confidence in balance).
DHI
The DHI is a self-reported measure of the impact of dizziness on the participant; it consists of 25 items scored as 0 (“never”), 2 (“sometimes”), or 4 (“always”). The variable of interest was the composite combined score (0–100, with higher scores indicating a greater impact of dizziness).
Clinical Vestibular Tests
Participants completed a 15-minute vestibular and oculomotor battery in a Neuroalign Technologies (Toronto, ON, Canada) (formerly NeuroKinetics, Inc) rotary chair system. Participants were immobilized while wearing 100-Hz infrared eye-tracking goggles and a headset for communication. The measures of interest for this study were the VOR and SVV subtests.
Rotary Chair VOR
Our VOR testing consisted of sinusoidal oscillations at 0.64 Hz in complete darkness for approximately 10 seconds under 2 conditions: complete darkness (reflexive VOR) and with a visual target that oscillated with the participant (VOR cancellation). For reflexive VOR testing, participants were asked distracting questions and told to keep their eyes open and relaxed. For VOR cancellation, participants were instructed to focus on a visual target that oscillated with them. The variable of interest for reflexive VOR was the velocity gain of the slow-phase VOR eye movement, calculated as the average ratio between eye velocity and chair (head) velocity; for VOR cancellation, the variable of interest was the percentage decrease of the velocity gain compared with the 0.64-Hz gain in darkness.
Rotary Chair SVV
The SVV test examined the participant’s perception of vertical. A line stimulus appeared tilted up to 30 degrees clockwise or counterclockwise. The participant pressed buttons on the rotary chair handles to tilt the line to perceived true vertical for 6 trials. The variables of interest were the absolute average degrees off true alignment (ie, a participant deviation average of −2° was treated as a deviation of +2°) and the variance across trials.
Balance and Gait Testing
Static Balance
Static balance was tested with the EquiTest (Natus, Pleasanton, CA, USA) Sensory Organization Test (SOT), which has been shown to be a reliable and valid measure of balance in people with MS.29 The SOT consists of 6 balance conditions in which the participant is asked to stand as still as possible. The conditions are as follows: condition 1—eyes open, stable surface and surround; condition 2—eyes closed, stable surface; condition 3—eyes open, stable surface, sway-referenced surround; condition 4—eyes open, sway-referenced surface, stable surround; condition 5—eyes closed, sway-referenced surface; and condition 6—eyes open, sway-referenced surface and surround. Three 20-second trials were completed for each condition. The equilibrium score calculated by the SOT software for each condition was averaged. The equilibrium score is calculated using the following equation: equilibrium score = [12.5 – (maximum anterior sway angle – maximum posterior sway angle)]/12.5; in this equation, 12.5 is the limit of sway in degrees in the sagittal plane. An equilibrium score of 100 indicates no sway, and 0 indicates a fall. The variables of interest were average equilibrium scores for each condition and the composite score (an average of the 6 conditions).
Functional Gait Assessment (FGA)
The FGA, a 10-item modification of the Dynamic Gait Index believed to be more specific to vestibular disorders than the Dynamic Gait Index and valid in people with MS, was completed over an approximately 0.3-m (1-ft)-wide, 6-m (20-ft)-long track.30,31 Participants were read standardized instructions for 9 different gait conditions and used a cane or walker if needed. Item 10 (steps) was not completed because a staircase was not available near the testing location; instead, participants were asked if they required a rail to ascend/descend stairs and if they alternate feet while ascending/descending stairs or need to put 2 feet onto each step for scoring that component. Each item was scored on the basis of its specific scale from 0 to 3. The variable of interest was the composite score of the items.
Six-Minute Walk Test (6MWT)
The 6MWT is a commonly used and valid measure of walking capacity in people with MS.32 Here, it was completed by instructing participants to walk “as fast and as far as you can” around an approximately 6-m (20-ft) oval track designated by cones using a cane or walker if needed. The tester followed 2 steps behind the participant with a rolling measuring wheel and recorded the distance traveled, reminding the participant every minute how much time remained. Participants were able to rest if needed; all participants who required a break were using walkers with built-in seats and would rest on those seats. The variable of interest was the distance the participant was able to walk in 6 minutes, measured in feet.
Data Analysis
All statistics were completed in SPSS v27.0 (IBM Corp., IBM SPSS Statistics for Windows, Version 27.0. Armonk, NY, USA). Variable histograms were inspected for normality. Positively skewed measures were ABC responses, VOR cancellation (percentage), SOT scores, and FGA composite scores, whereas SVV absolute deviation and variance were negatively skewed. All other measures were normally distributed.
Clinical Task Performance
Summary statistics (mean and SD or median and interquartile range) were calculated for all collected variables.
Correlations Among Vestibular Function, Self-reported Fatigue, Balance, and Walking Capacity
Spearman correlations, due to their lower bias in non-normal datasets, were calculated between EDSS scores, survey scores, including MFIS subscale scores, VOR function, SVV, SOT, FGA, and 6 MW distance. To reduce the number of total correlational tests run, individual SOT conditions were not included. To control for multiple comparisons, P values were corrected using the Benjamini-Hochberg method, and a false discovery rate of 0.10 was set as the threshold to determine statistical significance.33 Both uncorrected and corrected P values are presented; uncorrected P values of less than .05 were not considered statistically significant even if the Benjamini-Hochberg–corrected P value was less than .10.
Role of the Funding Source
The funders played no role in the design, conduct, or reporting of this study.
Results
Clinical Task Performance
Summary statistics for all collected variables are presented in Table 2.
Table 2.
Summary Statistics of Clinical Variables for 40 People With MSa
| Clinical Variable | Value |
|---|---|
| MFIS composite, mean (SD) | 36.7 (19.5) |
| FSS average, mean (SD) | 37.2 (14.9) |
| ABC composite, mean (SD) | 74.9 (27.2) |
| Reflexive VOR gain, mean (SD) | 0.59 (0.21) |
| VOR cancellation, %, mean (SD) | 63.0 (19.0) |
| SVV absolute deviation, median (IQR) | 2.09 (2.82) |
| SVV variance, median (IQR) | 1.5 (2.25) |
| SOT condition, median (IQR) | |
| 1 | 94.7 (3.4) |
| 2 | 89.7 (8.0) |
| 3 | 89.3 (9.4) |
| 4 | 81.7 (17.7) |
| 5 | 61.0 (34.4) |
| 6 | 63.0 (46.7) |
| SOT composite, mean (SD) | 72 (13.6) |
| FGA composite, median (IQR) | 27 (8) |
| 6MWT distance, mean (SD) | 1505 (499) |
a Data for normally distributed variables are presented as mean (SD), and those for nonnormal variables are presented as median (interquartile range [IQR]). For consistency, data for all Sensory Organization Test (SOT) conditions are presented as median (IQR) because of bimodal distributions (ie, falls) in the more difficult conditions. 6MWT = 6-Minute Walk Test; ABC = Activities-Specific Balance Confidence Scale; FGA = Functional Gait Assessment; FSS = Fatigue Severity Scale; MFIS = Modified Fatigue Impact Scale; MS = multiple sclerosis; SVV = subjective visual vertical; VOR = vestibular ocular reflex.
Correlations Among Vestibular Function, Self-reported Fatigue, Balance, and Walking Capacity
Spearman correlation results are presented in Table 3. To reduce table clutter, MFIS subscales are not included in the table but were included in the analysis (including for Benjamini-Hochberg corrections). SVV variance was significantly correlated with FSS and ABC scores, SOT and FGA, 6MWT distance, and EDSS scores. Only SVV variance and SOT were significantly correlated with the MFIS composite score. The MFIS physical subscale was significantly correlated with SVV variance, SOT, 6MWT distance, and FGA. The MFIS cognitive and psychosocial subscales were not correlated with any additional variable besides other MFIS, FSS, and ABC scores. Scores on VOR cancellation, SVV variance, SOT, and FGA had significant absolute correlations between 0.30 and 0.60 with each other, and each was significantly correlated with 6MWT distance. Reflexive VOR gain and SVV absolute deviation were not significantly correlated with any measure.
Table 3.
Spearman Correlations Between Variables in People With MSa
| Variable | EDSS | Surveys | VOR | SVV | Balance and Gait | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MFIS Composite | FSS Composite | ABC Composite | DHI Composite | Reflexive VOR Gain | VOR Cancellation Gain | SVV Absolute Deviation | SVV Variance | SOT Composite | FGA Composite | 6 MW Distance | ||
| EDSS | ||||||||||||
| MFIS composite | 0.33 | |||||||||||
| FSS composite | 0.43 | 0.80 | ||||||||||
| ABC composite | −0.72 | −0.54 | −0.59 | |||||||||
| DHI composite | 0.31 | 0.34 | 0.39 | −0.61 | ||||||||
| Reflexive VOR gain | −0.01 | 0.00 | −0.04 | −0.01 | 0.05 | |||||||
| VOR cancellation gain | −0.47 | −0.15 | −0.20 | 0.46 | −0.38 | 0.20 | ||||||
| SVV absolute deviation | 0.16 | 0.20 | 0.14 | −0.28 | 0.25 | −0.05 | −0.28 | |||||
| SVV variance | 0.45 | 0.33 | 0.35 | −0.56 | 0.15 | −0.05 | −0.35 | 0.16 | ||||
| SOT composite | −0.61 | −0.31 | −0.37 | 0.47 | 0.20 | −0.09 | 0.34 | −0.02 | −0.52 | |||
| FGA composite | −0.75 | −0.24 | −0.38 | 0.72 | −0.06 | −0.10 | 0.42 | −0.19 | −0.60 | 0.52 | ||
| 6MWT distance | −0.73 | −0.31 | −0.36 | 0.72 | −0.19 | −0.08 | 0.53 | 0.01 | −0.58 | 0.64 | 0.80 | |
a Correlations with significant P values after Benjamini-Hochberg correction are shown in bold type. 6MWT = 6-Minute Walk Test; ABC = Activities-Specific Balance Confidence Scale; DHI = Dizziness Handicap Inventory; EDSS = Expanded Disability Status Scale; FGA = Functional Gait Assessment; FSS = Fatigue Severity Scale; MFIS = Modified Fatigue Impact Scale; MS = multiple sclerosis; SOT = Sensory Organization Test; SVV = subjective visual vertical; VOR = vestibular ocular reflex.
Discussion
This study investigated whether vestibular functions requiring central sensory integration of vestibular information were associated with self-reported fatigue, balance, and walking capacity in people with MS. Overall, the results suggest that vestibular functions requiring central integration have stronger correlations with fatigue, balance, and walking capacity than reflexive vestibular functions and intercorrelate with each other.
We investigated correlations between vestibular functions, fatigue, balance, and walking capacity and hypothesized that functions requiring central integration of vestibular information (VOR cancellation, SVV, SOT, and FGA) would significantly correlate with each other and self-reported fatigue, walking capacity, and disease severity. Scores on the FSS and ABC and 6MWT distance were each significantly correlated with VOR cancellation, SVV variance, SOT, and FGA. The MFIS composite score was only correlated with SVV variance and SOT, but the MFIS physical additionally correlated with 6MWT distance and FGA, suggesting that central vestibular variables may best correlate with physical fatigue and not other fatigue domains. These central vestibular measures significantly correlated with each other, suggesting the presence of shared central processing pathways that are impaired by MS pathology. Although SVV absolute deviation should theoretically require central vestibular processing, it seems that SVV variance better relates to this phenomenon because SVV absolute deviation was not correlated with any other central vestibular measure. Reflexive VOR gain did not show correlations as VOR cancellation did, potentially because it did not require central processing pathways.
Together, our data suggest that central integration of vestibular information can be impaired in people with MS even in the presence of normal reflexive vestibular functions and that this ability to integrate is related to self-reported fatigue and walking capacity. Our data build upon the relationship between central vestibular integration and fatigue hypothesized by Hebert et al.6,8 To hypothesize what may lead to alterations of central vestibular integration in people with MS, we studied the limited amount of previous research on SVV variance. Variance on SVV in healthy controls appears to be governed by the signal-to-noise ratio of the primary vestibular driver of graviceptive information, the utricle otolith organ, and this signal-to-noise ratio changes as participants are rotated in the roll plane.34,35 As the SVV task in this study required the participant’s head be stabilized in a 0-degree roll position, a different mechanism must be responsible for altering this utricle signal-to-noise ratio. We therefore hypothesize that MS lesions lead to deficits in vestibular sensory gating, that is, the nervous system’s ability to suppress vestibular noise and preferentially process true signal. Sensory gating of proprioceptive and tactile information has been shown to be impaired in people with MS and correlated with motor control, walking impairment, and fatigue.36–38 Because SVV variance was correlated with fatigue surveys and walking capacity here, it may serve as a measure of vestibular sensory gating.
It is possible that this hypothesized relationship between central vestibular integration and fatigue is more specifically explained as impairment in vestibular sensory gating. Processing of unfiltered noise as true signal likely results in deficiencies in the cerebellum’s ability to cancel VOR eye movements, shifting perceptions of verticality, and improper shifts of center-of-gravity, resulting in higher postural sway. Judging by our correlations between central vestibular integration measures here and 6MWT distance, these changes are likely related to lower gait speeds and/or increased physical fatigue and possibly contribute to the fatigue consciously experienced by people with MS, as evidenced by correlations with MFIS and FSS scores.36,37 Experiences of physical fatigue may be more associated with central vestibular functions compared with cognitive and psychosocial fatigue because 6MWT distance showed the strongest correlations with central integration functions (Spearman r = 0.53–0.80 compared with MFIS/FSS scores at r = 0.32–0.38), and only the MFIS physical subscale was signficantly correlated with central integration measures. Vestibular rehabilitation may rehabilitate balance and fatigue simultaneously through rehabilitating sensory gating and weighting processes, which may also lead to improvements in walking capacity.4 Future studies of vestibular rehabilitation in people with MS should include measurements of fatigue and explore the relationships between changes in vestibular function and changes in fatigue and walking capacity following intervention.
Clinically, the data presented here suggest that VOR cancellation and SVV may serve as additional outcome measures for disease progression and vestibular rehabilitation in people with MS. Although balance function and DHI scores have typically been used as outcome measures for vestibular rehabilitation, our data support previous findings that DHI scores may not correlate well with objective vestibular functions.39 Although only 50% of our sample reported dizziness or imbalance and were able to complete the DHI, we believe this further strengthens our claims because, despite low prevalence of dizziness, our sample with MS had poor balance as measured by the SOT and FGA. Therefore, vestibular rehabilitation may be beneficial even to those who do not report dizziness, and central vestibular integration measures investigated here may serve as better outcome measures for those individuals. Although VOR cancellation requires specialized eye-tracking equipment, SVV can be tested affordably in any outpatient clinic by using the bucket test.40 Although the bucket test may not be as sensitive as the SVV tool used here, we have shown that variance on the bucket test is also impaired in people with MS.23
Limitations
There are several limitations of our study. In our sample with MS, only 10 people (25%) with MS had an EDSS score of 4.0 to 6.5, indicating that most participants had mild to moderate MS. This ensured participants with MS could complete balance and walking subtests; however, we do not know how these relationships translate to people with MS with more severe disease. Our participants with MS were recruited from a single clinic and by contacting participants of past research; our results may not be generalizable to larger populations of people with MS, and our sample may have different treatment and socioeconomic statuses than the average person with MS due to their active involvement in research. Finally, it is possible that correlations between central vestibular functions and fatigue exist because of a shared relationship to MS disease severity and not with each other. Future studies should attempt to reproduce our study in other samples to better understand if these deficits in central integration and relationships of central integration with fatigue and walking capacity are ubiquitous in people with MS.
Clinical measures that require central sensory integration of vestibular information are impaired in people with MS with mild to moderate disease. These central functions significantly correlate with self-reported fatigue, walking capacity, and disease severity. Impairments in central vestibular integration may reflect impairments in sensory gating of vestibular noise, which may lead to fatigue and gait impairment. Future studies should investigate these measures as potential outcome measures for vestibular rehabilitation studies and further investigate their relationship with fatigue and walking capacity.
Contributor Information
Graham D Cochrane, Department of Physical Therapy, School of Health Professions, University of Alabama at Birmingham, Birmingham, Alabama, USA; Medical Scientist Training Program, School of Medicine, University of Alabama at Birmingham, Birmingham, Alabama, USA.
Jennifer B Christy, Department of Physical Therapy, School of Health Professions, University of Alabama at Birmingham, Birmingham, Alabama, USA.
Robert W Motl, Department of Physical Therapy, School of Health Professions, University of Alabama at Birmingham, Birmingham, Alabama, USA.
Author Contributions
Concept/idea/research design: G.D. Cochrane, J.B. Christy, R.W. Motl
Writing: G.D. Cochrane, J.B. Christy, R.W. Motl
Data collection: G.D. Cochrane
Data analysis: G.D. Cochrane
Project management: G.D. Cochrane
Fund procurement: G.D. Cochrane, R.W. Motl
Providing facilities/equipment: R.W. Motl
Consultation (including review of manuscript before submitting): J. B. Christy, R. W. Motl
Funding
Graham Cochrane was awarded an MS Workforce of the Future program grant from the Foundation of the Consortium of Multiple Sclerosis Centers, which made this study possible. Graham Cochrane is also supported by award T33GM008361 from the National Institute of General Medical Sciences. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Ethics Approval
This study was approved by the University of Alabama at Birmingham Institutional Review Board, and all participants provided written informed consent before participating.
Disclosures
The authors completed the ICMJE Form for Disclosure of Potential Conflicts of Interest and reported no conflicts of interest.
References
- 1. Peterka RJ. Sensorimotor integration in human postural control. J Neurophysiol. 2002;88:1097–1118. [DOI] [PubMed] [Google Scholar]
- 2. Amblard B. Visuo-vestibular integration in the development of posture and gait. Arch Ital Biol. 1996;134:249–277. [PubMed] [Google Scholar]
- 3. Schniepp R, Mohwald K, Wuehr M. Gait ataxia in humans: vestibular and cerebellar control of dynamic stability. J Neurol. 2017;264:87–92. [DOI] [PubMed] [Google Scholar]
- 4. Appiah-Kubi KO, Wright WG. Vestibular training promotes adaptation of multisensory integration in postural control. Gait Posture. 2019;73:215–220. [DOI] [PubMed] [Google Scholar]
- 5. Cattaneo D, Jonsdottir J. Sensory impairments in quiet standing in subjects with multiple sclerosis. Mult Scler. 2009;15:59–67. [DOI] [PubMed] [Google Scholar]
- 6. Hebert JR, Corboy JR. The association between multiple sclerosis-related fatigue and balance as a function of central sensory integration. Gait Posture. 2013;38:37–42. [DOI] [PubMed] [Google Scholar]
- 7. Fling BW, Dutta GG, Schlueter H, Cameron MH, Horak FB. Associations between proprioceptive neural pathway structural connectivity and balance in people with multiple sclerosis. Front Hum Neurosci. 2014;8:814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hebert JR, Corboy JR, Manago MM, Schenkman M. Effects of vestibular rehabilitation on multiple sclerosis-related fatigue and upright postural control: a randomized controlled trial. Phys Ther. 2011;91:1166–1183. [DOI] [PubMed] [Google Scholar]
- 9. Hebert JR, Corboy JR, Vollmer T, Forster JE, Schenkman M. Efficacy of balance and eye-movement exercises for persons with multiple sclerosis (BEEMS). Neurology. 2018;90:e797–e807. [DOI] [PubMed] [Google Scholar]
- 10. Soysal Tomruk M, Uz MZ, Kara B, Idiman E. Effects of Pilates exercises on sensory interaction, postural control and fatigue in patients with multiple sclerosis. Mult Scler Relat Disord. 2016;7:70–73. [DOI] [PubMed] [Google Scholar]
- 11. van Emmerik REA, Remelius JG, Johnson MB, Chung LH, Kent-Braun JA. Postural control in women with multiple sclerosis: effects of task, vision and symptomatic fatigue. Gait Posture. 2010;32:608–614. [DOI] [PubMed] [Google Scholar]
- 12. Freal JE, Kraft GH, Coryell JK. Symptomatic fatigue in multiple sclerosis. Arch Phys Med Rehabil. 1984;65:135–138. [PubMed] [Google Scholar]
- 13. Fisk JD, Pontefract A, Ritvo PG, Archibald CJ, Murray TJ. The impact of fatigue on patients with multiple sclerosis. Can J Neurol Sci. 1994;21:9–14. [PubMed] [Google Scholar]
- 14. Khan F, Amatya B, Galea M. Management of fatigue in persons with multiple sclerosis. Front Neurol. 2014;5:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cullen KE. Physiology of central pathways. Handb Clin Neurol. 2016;137:17–40. [DOI] [PubMed] [Google Scholar]
- 16. Cullen KE. The vestibular system: multimodal integration and encoding of self-motion for motor control. Trends Neurosci. 2012;35:185–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bohmer A, Rickenmann J. The subjective visual vertical as a clinical parameter of vestibular function in peripheral vestibular diseases. J Vestib Res. 1995;5:35–45. [PubMed] [Google Scholar]
- 18. Sharpe JA, Goldberg HJ, Lo AW, Herishanu YO. Visual-vestibular interaction in multiple sclerosis. Neurology. 1981;31:427–433. [DOI] [PubMed] [Google Scholar]
- 19. Crevits L, Venhovens J, Vanoutrive J, Debruyne J. False perception of visual verticality in multiple sclerosis. Eur J Neurol. 2007;14:228–232. [DOI] [PubMed] [Google Scholar]
- 20. da Fonseca BA, Pereira CB, Jorge F, Simm R, Apostolos-Pereira S, Callegaro D. A disturbed processing of graviceptive pathways may be involved in the pathophysiology of balance disorders in patients with multiple sclerosis. Arq Neuropsiquiatr. 2016;74:106–111. [DOI] [PubMed] [Google Scholar]
- 21. Klatt BN, Sparto PJ, Terhorst L, Winser S, Heyman R, Whitney SL. Relationship between subjective visual vertical and balance in individuals with multiple sclerosis. Physiother Res Int. 2019;24:e1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zalewski CK. Aging of the human vestibular system. Semin Hear. 2015;36:175–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cochrane GD, Christy JB, Motl RW. Comprehensive clinical assessment of vestibular function in multiple sclerosis. J Neurol Phys Ther. 2021;45:228–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The Fatigue Severity Scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol. 1989;46:1121–1123. [DOI] [PubMed] [Google Scholar]
- 25. Tellez N, Rio J, Tintore M, Nos C, Galan I, Montalban X. Does the Modified Fatigue Impact Scale offer a more comprehensive assessment of fatigue in MS? Mult Scler. 2005;11:198–202. [DOI] [PubMed] [Google Scholar]
- 26. Powell LE, Myers AM. The Activities-Specific Balance Confidence (ABC) scale. J Gerontol A Biol Sci Med Sci. 1995;50A:M28–M34. [DOI] [PubMed] [Google Scholar]
- 27. Nilsagard Y, Carling A, Forsberg A. Activities-specific balance confidence in people with multiple sclerosis. Mult Scler Int. 2012;2012:613925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jacobson GP, Newman CW. The development of the Dizziness Handicap Inventory. Arch Otolaryngol Head Neck Surg. 1990;116:424–427. [DOI] [PubMed] [Google Scholar]
- 29. Hebert JR, Manago MM. Reliability and validity of the computerized dynamic posturography sensory organization test in people with multiple sclerosis. Int J MS Care. 2017;19:151–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wrisley DM, Marchetti GF, Kuharsky DK, Whitney SL. Reliability, internal consistency, and validity of data obtained with the Functional Gait Assessment. Phys Ther. 2004;84:906–918. [PubMed] [Google Scholar]
- 31. Forsberg A, Andreasson M, Nilsagard Y. The Functional Gait Assessment in people with multiple sclerosis. Int J MS Care. 2017;19:66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Goldman MD, Marrie RA, Cohen JA. Evaluation of the six-minute walk in multiple sclerosis subjects and healthy controls. Mult Scler J. 2008;14:383–390. [DOI] [PubMed] [Google Scholar]
- 33. Benjamini YHY. Controlling the false discovery rate: a practical and powerful approach to multiple hypothesis testing. J R Stat Soc B. 1995;57:289–300. [Google Scholar]
- 34. Tarnutzer AA, Bockisch C, Straumann D, Olasagasti I. Gravity dependence of subjective visual vertical variability. J Neurophysiol. 2009;102:1657–1671. [DOI] [PubMed] [Google Scholar]
- 35. Ward BK, Bockisch CJ, Caramia N, Bertolini G, Tarnutzer AA. Gravity dependence of the effect of optokinetic stimulation on the subjective visual vertical. J Neurophysiol. 2017;117:1948–1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Arpin DJ, Gehringer JE, Wilson TW, Kurz MJ. A reduced somatosensory gating response in individuals with multiple sclerosis is related to walking impairment. J Neurophysiol. 2017;118:2052–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Arpin DJ, Gehringer JE, Wilson TW, Kurz MJ. Movement-related somatosensory activity is altered in patients with multiple sclerosis. Brain Topogr. 2018;31:700–707. [DOI] [PubMed] [Google Scholar]
- 38. Conte A, Giannì C, Belvisi D, et al. Deep grey matter involvement and altered sensory gating in multiple sclerosis. Mult Scler. 2020;26:786–794. [DOI] [PubMed] [Google Scholar]
- 39. Yip CW, Strupp M. The Dizziness Handicap Inventory does not correlate with vestibular function tests: a prospective study. J Neurol. 2018;265:1210–1218. [DOI] [PubMed] [Google Scholar]
- 40. Zwergal A, Rettinger N, Frenzel C, Dieterich M, Brandt T, Strupp M. A bucket of static vestibular function. Neurology. 2009;72:1689–1692. [DOI] [PubMed] [Google Scholar]


