Abstract
Memory T-cell responses are partitioned between the blood, secondary lymphoid organs, and nonlymphoid tissues. Tissue-resident memory T (Trm) cells are a population of immune cells that remain permanently in tissues without recirculating in blood. These nonrecirculating cells serve as a principal node in the anamnestic response to invading pathogens and developing malignancies. Here, we contemplate how T-cell tissue residency is defined and shapes protective immunity in the steady state and in the context of disease. We review the properties and heterogeneity of Trm cells, highlight the critical roles these cells play in maintaining tissue homeostasis and eliciting immune pathology, and explore how they might be exploited to treat disease.
The cardinal feature of an effective memory T-cell response is the ability to rapidly locate and contain the spread of disease. Early analyses of peripheral blood and lymph uncovered two major subsets of memory T cells: central memory T (Tcm) cells that typically traffic between secondary lymphoid organs (SLOs) and the blood, and effector memory T (Tem) cells that were found to circulate continuously through peripheral nonlymphoid tissues (NLTs) (Sallusto et al. 1999, 2004). However, these circulating memory T (Tcirc) cells can be restricted in their ability survey and quickly access barrier sites where the majority of pathogens gain entry to the body (Masopust et al. 2010; Wakim et al. 2010; Mackay et al. 2012a). We now know that a large proportion of memory T cells are actually anchored in peripheral NLTs, comprising a dedicated layer of tissue memory that is maintained independently of the Tcirc cell pool. These tissue-resident memory T (Trm) cells form a first line of defense against invading pathogens and developing malignancies and are critical to control tissue homeostasis. In this review, we will examine how T-cell residency is defined and shapes tissue memory in the context of infection, cancer, and autoimmunity. We will discuss how memory T-cell persistence is regulated within tissues, our evolving understanding of heterogeneity within the Trm cell pool, and the consequences of tissue residency for spatiotemporal immune protection.
DEFINING TISSUE RESIDENCY
In the context of T-cell memory, “tissue residency” refers to the permanent maintenance of cells in a given organ without recirculation through blood. Conclusive evidence for the existence of CD8+ Trm cells stemmed from experiments in mice where donor-derived CD8+ memory T cells were shown to be retained in grafted skin or gut tissue following transplantation (Gebhardt et al. 2009; Masopust et al. 2010). Several additional methods to demonstrate T-cell tissue residency have since been used, each with their own strengths and weaknesses (for review, see Masopust and Soerens 2019). Parabiosis—in which the circulatory systems of two animals are surgically fused—has been instrumental in revealing that the vast majority of CD4+ and CD8+ T cells situated in peripheral tissues are nonrecirculating, as they do not equilibrate between conjoined partners (Steinert et al. 2015; Beura et al. 2019). In addition, CD8+ Trm cells have been shown to persist in various tissues following antibody-mediated depletion of Tcirc cells (Gebhardt et al. 2009; Schenkel et al. 2013; Mackay et al. 2015b; Park et al. 2016) and are retained in peripheral tissues following treatment with the sphingosine-1-phosphate (S1P) receptor antagonist FTY720, which prevents T-cell egress from SLOs (Masopust et al. 2010). Using these methods, bona fide CD8+ and CD4+ Trm cells have now been identified in almost every mouse organ including the brain (Wakim et al. 2010), skin (Gebhardt et al. 2009; Jiang et al. 2012), intestines (Masopust et al. 2010; Beura et al. 2019), salivary glands (Hofmann and Pircher 2011), lung (Teijaro et al. 2011; Takamura et al. 2016), liver, kidney, pancreas (Steinert et al. 2015), female reproductive tract (FRT) (Mackay et al. 2012a; Schenkel et al. 2013; Iijima and Iwasaki 2014), adipose tissue (Han et al. 2017), thymus (Hofmann et al. 2013), spleen, and lymph nodes (LNs) (Schenkel et al. 2014b). Unlike blood-tropic T cells, CD8+ Trm cells in most tissues do not stain with intravascularly administered antibodies, indicating that they are situated within the tissue parenchyma (Anderson et al. 2012). Although intravascular labeling is useful to illustrate the spatial location of T cells within a single snapshot of time (Anderson et al. 2014), the use of this technique alone is insufficient to denote permanent residency.
The phenomenon of T-cell tissue residency has proven more difficult to examine in humans. Early work demonstrated that non-recirculating CD8+ and CD4+ T cells were retained in human skin following antibody-mediated depletion of blood Tcirc cells, where they improved resistance to infections (Clark et al. 2012; Watanabe et al. 2015). More recently, analyses of donated skin (Lian et al. 2014), gut (Zuber et al. 2016; Bartolomé-Casado et al. 2019), lung (Snyder et al. 2019), and kidneys (de Leur et al. 2019) after transplantation have revealed that donor-derived CD8+ and CD4+ Trm cells are maintained long term in grafted tissues without being detectable in blood. Collectively, these findings strongly support that non-recirculating Trm cells akin to those found in mice are also abundant in humans.
CD8+ Trm cells were first formally identified in the skin epithelium and intestinal intraepithelial layer of mice, where they were shown to differ from Tcirc cells by elevated expression of the surface markers CD69 and CD103 (αE integrin) (Gebhardt et al. 2009; Masopust et al. 2010). However, several studies have revealed that bona fide CD69+ Trm cells in nonepithelial tissue compartments such as the gut lamina propria (Bergsbaken and Bevan 2015) and visceral tissues including the liver (Fernandez-Ruiz et al. 2016), kidney, FRT (Steinert et al. 2015), and adipose tissue (Han et al. 2017) frequently lack expression of CD103. Populations of CD69+CD103+ and CD69+CD103– T cells are also ample across human NLTs and SLOs (Sathaliyawala et al. 2013) where they accumulate over the life span (Thome et al. 2014). Importantly, donor-derived CD8+ T cells retained in transplanted human tissues similarly express CD69, and most also express CD103 (Bartolomé-Casado et al. 2019; Snyder et al. 2019). Given that CD69 is stably expressed by the majority of Trm cells in both NLTs and SLOs (Schenkel et al. 2014a; Steinert et al. 2015), CD69 positivity is now frequently used to infer tissue residency in both mice and humans irrespective of CD103 expression. However, CD69 can be rapidly and transiently induced on recently activated T cells and Tcirc cells following antigen recognition or exposure to certain inflammatory cytokines (Beura et al. 2015; Cibrián and Sánchez-Madrid 2017; Duhen et al. 2018). As such, the veracity of CD69 as marker to identify Trm cells may be limited in some tissues or circumstances, especially in those where sustained local antigen presentation or ongoing inflammation occur (Fig. 1). For example, whereas CD8+CD69+ T cells in SLOs are resident and relatively rare in specific pathogen-free (SPF) mice (Schenkel et al. 2014b), their frequency increases following colonization with “wild” microbiota and they partially equilibrate during parabiosis (Beura et al. 2018b). CD69+CD103– CD8+ T cells have also been shown to recirculate in the skin (Mackay et al. 2015b), thymus (Park et al. 2016), kidney, and liver (Steinert et al. 2015) of SPF mice in some settings. These studies stress that expression of CD69 alone can be insufficient to demarcate T-cell residency with certitude. Profiling additional molecules that are typically up-regulated by CD8+ Trm cells including the chemokine receptor CXCR6 (Fernandez-Ruiz et al. 2016; Wong et al. 2016) or collagen-binding integrin CD49a (Gebhardt et al. 2009; Mackay et al. 2013) may increase confidence in attempts to distinguish Trm cells from their circulating counterparts. However, a considerable fraction of Trm cells in the pancreas, FRT, and salivary glands may evade detection altogether via phenotyping, as they express neither CD69 nor CD103 (Steinert et al. 2015).
Figure 1.
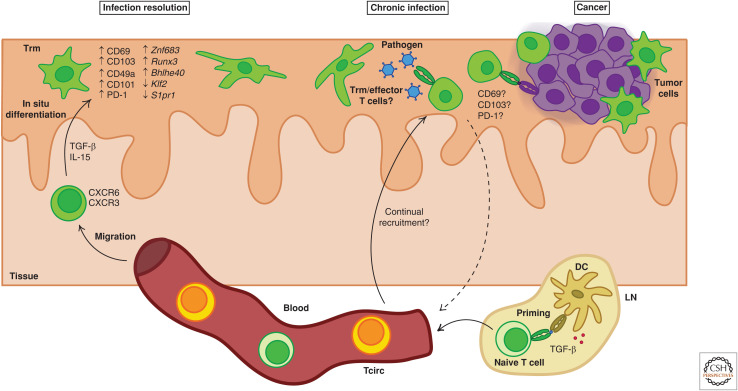
Regulation of T-cell residency formation and persistence in peripheral tissues. Trm cell formation is controlled at multiple stages of T-cell homeostasis, activation, and differentiation. Naive CD8+ T cells can be preconditioned for T-cell residency potential by interacting with dendritic cells (DCs) that activate transforming growth factor β (TGF-β) in lymph nodes (LNs) prior to T-cell priming. Activated T cells migrate to the periphery where Trm differentiation occurs in situ following exposure to tissue-derived cytokines that can include TGF-β and interleukin 15 (IL-15). Full commitment to the T-cell residency fate is often associated with the up-regulation of molecules including CD69 and CD103 and down-regulation of factors that promote T-cell egress from tissues such as Klf2 and S1pr1. In addition, Trm cells acquire a unique transcriptional profile that may include up-regulation of residency-promoting factors such as Znf683, Runx3, and Bhlhe40. In the context of chronic infections or cancer where persistent antigen may be present, residency-associated molecules might also be dynamically regulated in recently activated or effector T cells that could eventually return to the circulation.
CD8+ Trm cells across diverse tissues share a unifying transcriptional signature that distinguishes them from Tcirc cells following the resolution of infection and that is also enriched in resident innate immune cells (Wakim et al. 2012; Mackay et al. 2013, 2016). This transcriptional profile includes induction of genes that enhance T-cell retention including Cd69, Itgae (encoding CD103), and Cdh1 (encoding E-cadherin) and down-regulation of genes that promote tissue egress (e.g., Klf2, S1pr1) or acquisition of alternate memory T-cell fates (e.g., Tcf7, Eomes) (Mackay et al. 2013, 2015b, 2016; Skon et al. 2013). Comparing gene expression by putative tissue-resident cells to this canonical Trm cell genetic profile may reveal transcriptional similarities that can be used to imply tissue residency. However, many of the genes comprising this Trm-associated gene signature including Cd69, Itgae, S1pr1, and Klf2 can also be dynamically altered in Tcirc cells following antigen stimulation, exposure to cytokines, or in response to stress (Cyster and Schwab 2012; Duhen et al. 2018). Use of transcriptional profiling to identify Trm cells might therefore be confounded in the context of chronic infection or cancer, where gene expression by recently activated Tcirc cells or dysfunctional “exhausted” T cells could overlap with bona fide Trm cells. Moreover, although gene expression by human Trm cells generally mirrors that seen in mouse Trm cells (Kumar et al. 2017; Snyder et al. 2019), there may be differences between species. For example, whereas the critical residency-promoting gene Znf683 (encoding Hobit, Homologue of Blimp-1 in T cells) is elevated in Trm cells across multiple mouse tissues compared to Tcirc cells (Mackay et al. 2016), expression of Hobit by human Trm and Tcirc cells appears to be more variable (Kumar et al. 2017; Pallett et al. 2017; Stelma et al. 2017; Zhang et al. 2018; Snyder et al. 2019).
In sum, we still lack definitive markers that dependably and comprehensively denote tissue-resident cells in many settings. Coexpression of CD69 and CD103 can reliably identify bona fide CD8+ Trm cells under homeostatic conditions (Gebhardt et al. 2009; Masopust et al. 2010; Wakim et al. 2010), but these markers fail to capture all resident T-cell populations across tissues. Phenotypic hallmarks of Trm cells may be particularly intangible during chronic disease when ongoing exposure to antigen or inflammation is likely. Delineating combinations of markers that can reliably distinguish Trm cells from Tcirc cells across various organs and disease settings is a long-standing goal of the field and would greatly advance the study of these cells in humans, which typically rely on phenotypic and transcriptional analyses for Trm cell identification.
IMPRINTING AND REGULATION OF TISSUE-RESIDENT MEMORY T CELLS
The generation of Trm cells in peripheral tissues is a multistep process involving naive T-cell activation in SLOs, migration of effector cells to the site of challenge, in situ differentiation, and long-term retention (Fig. 1). Although complete commitment to the Trm cell fate occurs after effector T cells enter peripheral tissues (Mackay et al. 2013), initial priming or preconditioning events might also impact the potential for Trm cell development. Recent work suggests that migratory dendritic cells (DCs) facilitate transforming growth factor β (TGF-β) imprinting of naive CD8+ T cells in LNs, which predisposes these cells toward effective Trm cell differentiation following activation (Mani et al. 2019). Early signals integrated during T-cell activation, including inflammatory cues, direct differentiation into KLRG1loCD127hi memory precursor (MP) and KLRG1hiCD127lo terminal effector (TE) cells (Chang et al. 2014). Studies in mice indicate that Trm cells primarily develop from KLRG1lo MP-like effector cells (Mackay et al. 2013; Sheridan et al. 2014) or from MP cells that temporarily express KLRG1 but later down-regulate its expression (Herndler-Brandstetter et al. 2018). T-cell receptor (TCR) lineage tracking of recipient-derived T cells following organ transplantation suggests this may also be the case in humans (Bartolomé-Casado et al. 2019). Furthermore, CD8+ T cells activated by lower affinity antigens might be more likely to give rise to lung Trm cells than high-affinity clones in mice (Fiege et al. 2019), although the reverse may be true during persistent viral infection of the brain and kidney (Frost et al. 2015). Recent reports indicate that CD8+ Trm cells might depend more stringently on cross-presenting Batf3-dependent DCs for their activation than Tcirc cells (Iborra et al. 2016). However, Trm and Tcm cells can both be generated from naive T-cell progenitors bearing the same TCR (Gaide et al. 2015) and the requirement for Batf3+ DC priming in Trm cell formation is not absolute (Iborra et al. 2016).
T-cell migration to peripheral tissues is guided by homing and chemokine receptors that can be imprinted during activation and confer tissue-specific tropism (Agace 2006). For example, expression of the a4β7 integrin and chemokine receptor CCR9 can direct T-cell migration to the gut (Mora et al. 2003), whereas E- and P-selectin ligands, CCR4, CCR8, and CCR10, can promote migration toward the skin (Schaerli et al. 2004; Malik et al. 2017; Zaid et al. 2017). However, inflammatory cytokines induced in tissues following infection or injury might dampen the impact of tissue-directed imprinting and promote more promiscuous T-cell migration patterns. The inflammatory cytokines CXCL9 and CXCL10 are rapidly induced in diverse tissues during infection, drawing CXCR3-expressing T cells toward afflicted sites, promoting their epithelial positioning, and subsequent Trm cell differentiation (Shin and Iwasaki 2012; Mackay et al. 2013; Laidlaw et al. 2014; Bergsbaken and Bevan 2015; Fernandez-Ruiz et al. 2016; Gilchuk et al. 2016). E- and P-selectins can also be widely expressed on the vascular endothelium following infection (Hobbs and Nolz 2017) and CXCR6 can enhance Trm cell formation in diverse anatomical sites including the skin and liver (Tse et al. 2014; Zaid et al. 2017) and may promote Trm cell positioning in the lung airways (Takamura et al. 2019; Wein et al. 2019). Furthermore, strong systemic infections (Masopust et al. 2004; Casey et al. 2012), repeated reactivation of Tcirc cells via boosting (Jiang et al. 2012; Davies et al. 2017; Park et al. 2018b), and lymphopenia-driven homeostatic proliferation (Casey et al. 2012) can promote widespread dissemination of Trm cells throughout the body. Migratory receptors are often quickly down-regulated following T-cell activation, restricting peripheral migration, and thus Trm cell formation to a relatively short window of time after antigen encounter (Masopust et al. 2004, 2010; Wakim et al. 2010; Mackay et al. 2012a; Zhang and Bevan 2013). However, during chronic or persistent infection, the expression of homing receptors on Tcirc cells might be sustained for prolonged periods, promoting ongoing peripheral recruitment (Zhang and Bevan 2013; Beura et al. 2015) and potentially continuous Trm cell generation (Smith et al. 2015).
Following tissue entry, Trm cell differentiation can occur in an antigen-independent fashion (Gebhardt et al. 2009; Casey et al. 2012; Mackay et al. 2012a; Holz et al. 2018). Exceptions to this include in the nervous system (Wakim et al. 2010; Casey et al. 2012; Mackay et al. 2012a) and lung (Lee et al. 2011; Takamura et al. 2016), where in situ antigen recognition appears critical for Trm cell formation. Although noncompulsory, the presence of local antigen within the skin, liver, or intestines can boost the number of Trm cells formed after infection or vaccination (Gebhardt et al. 2009; Fernandez-Ruiz et al. 2016; Khan et al. 2016; Muschaweckh et al. 2016; Davies et al. 2017; Holz et al. 2018). Recent experiments in which major histocompatibility complex I (MHCI) expression was ablated following establishment of the Trm cell pool have revealed that ongoing MHCI:TCR interactions are dispensable for the long-term maintenance of CD8+ Trm cells in the skin (Lauron et al. 2019) and lung (Wang et al. 2019). In fact, prolonged antigen presentation may actually oppose Trm cell differentiation under certain circumstances by inhibiting CD103 up-regulation (Zhang and Bevan 2013; Tomov et al. 2017; Wang et al. 2019) or Znf683 expression (Kragten et al. 2018; Behr et al. 2019), which may have consequences for Trm cell generation in chronic infection or cancer.
Full commitment to the Trm cell fate requires induction or suppression of key molecules and transcription factors (TFs) that regulate T-cell retention and survival. CD69 can be induced upon antigen recognition or exposure to tissue-derived cytokines including type I interferon (IFN), TGF-β, and interleukin 33 (IL-33) (Casey et al. 2012; Skon et al. 2013; Mackay et al. 2015b) and can promote early local retention of Trm cell precursors (Mackay et al. 2015a; Takamura et al. 2016) through competitive inhibition of S1PR1, which facilitates emigration (Mackay et al. 2015a). Whereas CD69 expression is critical for Trm cell formation in the skin (Mackay et al. 2015a), lung (Lee et al. 2011), thymus (Park et al. 2016), and kidney (Walsh et al. 2019), it may not be essential in the intestines, salivary gland, or liver (Walsh et al. 2019). Nevertheless, suppression of tissue-egress mediators such as KLF2, S1PR1, and CCR7 represents an initial key step in Trm cell differentiation (Mackay et al. 2013; Skon et al. 2013), promoting accumulation of effector T cells in tissues where they can be further imprinted for long-term residency by local signals. TGF-β drives up-regulation of CD103 on Trm cells (El-Asady et al. 2005; Mackay et al. 2013; Zhang and Bevan 2013; Wakim et al. 2015), which can bind E-cadherin on surrounding cells (Cepek et al. 1994; Cresswell et al. 2001) and promote either accumulation (Sheridan et al. 2014) or long-term retention (Wakim et al. 2010; Lee et al. 2011; Casey et al. 2012; Mackay et al. 2013). Trm cells themselves also express E-cadherin, which has been shown to support their maintenance in the salivary glands (Hofmann and Pircher 2011). In addition, very late antigen-1 (VLA-1; CD49a) and LFA-1 have been shown to enhance Trm cell retention in the lungs (Ray et al. 2004), skin (Bromley et al. 2020), and liver (McNamara et al. 2017), respectively.
Multiple TFs cooperate to orchestrate Trm cell formation and function. Down-regulation of the T-box TFs T-bet and Eomes is necessary for Trm cell differentiation in the skin and lungs of mice (Laidlaw et al. 2014; Mackay et al. 2015b), but low levels of T-bet are maintained to ensure sustained IL-15Rα and CXCR3 expression (Mackay et al. 2015b). In addition, T-bet induces the TF Hobit in differentiating Trm cells, which collaborates with Blimp-1 to inhibit expression of tissue-egress genes including Klf2, S1pr1, and Ccr7 as well as the Tcm-associated TF Tcf7 (encoding T-cell factor 1 [TCF-1]) (Mackay et al. 2016). Loss of both Hobit and Blimp-1 leads to a reduction of both Trm cells and resident innate cells in multiple organs including the liver, skin, lung, and gut (Mackay et al. 2016; Behr et al. 2019). Similarly, the TF Runx3 is up-regulated during Trm cell development and promotes Trm cell survival and cytotoxicity in both tissues and tumors (Milner et al. 2017). Similarly, the TF Bhlhe40 has been shown to support Trm cell persistence and functionality in the lungs and melanoma (Li et al. 2019). Additional TFs that might promote Trm cell survival and metabolic homeostasis include the aryl hydrocarbon receptor (Ahr) (Zaid et al. 2014), Nr4a1 (Boddupalli et al. 2016b), and Notch (Hombrink et al. 2016).
Differentiation and maintenance of Trm cells is regulated by tissue-derived cytokines and requires metabolic adaptations to life in barrier tissues. Tissue-derived TGF-β is a key signal for Trm cell commitment. TGF-β can induce CD103 expression (Pauls et al. 2001) and KLRG1 down-regulation (Schwartzkopff et al. 2015) in vitro, and deficiencies in TGF-β signaling have been shown to result in defects in Trm cell generation in the skin (Mackay et al. 2013), intestines (Casey et al. 2012; Zhang and Bevan 2013), kidney (Ma et al. 2017), lungs (Lee et al. 2011), and salivary glands (Thom et al. 2015), which correlate with reduced CD103 expression (Casey et al. 2012; Mackay et al. 2013; Zhang and Bevan 2013; Bergsbaken and Bevan 2015). However, the role of TGF-β in Trm cell development extends beyond CD103 up-regulation, as TGF-βRII-deficient cells can display reduced Trm cell formation even when CD103 is dispensable (Sheridan et al. 2014). In line with this, TGF-β also regulates expression of CD69, CD49a (Zhang and Bevan 2013), Klf2, and S1pr1 (Skon et al. 2013) and drives the down-regulation of Eomes and T-bet following tissue entry (Mackay et al. 2015b). TGF-β is produced in a latent form and must be activated by αvβ6-expressing epithelial cells to license Trm cell differentiation (Mohammed et al. 2016). Blocking its activation after establishment of the memory T-cell pool has demonstrated that TGF-β is also required for long-term maintenance of gut and skin Trm cells (Mohammed et al. 2016). In addition, the cytokine IL-15 is required for Trm cell development and survival in several tissues (Mackay et al. 2013; Schenkel et al. 2016), where it can promote expression of residency-associated TFs including T-bet (Mackay et al. 2015b), Hobit (Mackay et al. 2016), and Runx3 (Milner et al. 2017), and modulate expression of Klf2 (Skon et al. 2013) and CD69 (Pallett et al. 2017). Other cytokines including IL-12, tumor necrosis factor (TNF), and IL-7 can also influence Trm cell development and function in some settings (Adachi et al. 2015; Bergsbaken et al. 2017). Trm cell formation and maintenance may also require metabolic adjustments; differentiating Trm cells gradually up-regulate certain isoforms of fatty acid–binding proteins determined by their tissue location to facilitate exogenous lipid uptake, whereas Tcirc cells do not (Pan et al. 2017; Frizzell et al. 2020). In addition, Trm cells in many tissues express the purinergic receptor P2RX7, which may serve as a means to sense microenvironmental stressors and disruptions to tissue homeostasis (Borges da Silva et al. 2018, 2020; Stark et al. 2018).
HETEROGENEITY OF TISSUE-RESIDENT MEMORY T CELLS
Although Trm cells across tissues share many unifying features, they can also exhibit key developmental and phenotypic differences. Whereas CD8+ Trm cells in the skin (Mackay et al. 2013, 2015b), liver (Herndler-Brandstetter et al. 2018; Holz et al. 2018), salivary gland, and kidney (Schenkel et al. 2016) require IL-15 signaling for differentiation and survival, CD8+ Trm cells in the gut, pancreas, FRT (Schenkel et al. 2016), and SLOs (Schenkel et al. 2014b) are IL-15 independent. These requirements may also be malleable within a single tissue in the context of chronic infection; salivary gland CD8+ Trm cells require IL-15 for formation following acute lymphocytic choriomeningitis virus (LCMV) but not during chronic murine cytomegalovirus (MCMV) infection (Smith et al. 2015; Schenkel et al. 2016). Moreover, whereas Trm cells across NLTs and SLOs share a degree of transcriptional overlap, they can also display tissue-specific gene-expression patterns (Mackay et al. 2013, 2016; Beura et al. 2018b). For instance, LN Trm cells share some transcriptional similarities with NLT Trm cells but others with Tcm cells (Beura et al. 2018b). Lung Trm cells may be particularly unique; unlike Trm cells in most other NLTs, they require antigen for their formation (Wakim et al. 2015; Takamura et al. 2016; McMaster et al. 2018), develop independently of Hobit (Behr et al. 2019), and experience a high rate of cell turnover (Wu et al. 2014; Slütter et al. 2017). Some studies indicate that lung Trm cell populations may be sustained by continual recruitment of Tcirc cells (Ely et al. 2006; Slütter et al. 2017), whereas Trm cells in other tissues are maintained independently of Tcirc cell input (Gebhardt et al. 2009; Masopust et al. 2010). These observations emphasize that Trm cells residing in different tissues can be functionally distinct and display tissue-specific requirements for local antigen, TFs, and cytokines.
Heterogeneity between Trm cells also exists at the CD4+ and CD8+ subset level. Parabiosis indicates that the majority of CD4+ T cells are resident in peripheral tissues such as the lung, gut, FRT, and liver (Teijaro et al. 2011; Kumar et al. 2017; Beura et al. 2019). Although mouse and human CD4+ and CD8+ Trm cells display similarities in their transcriptional signatures (Kumar et al. 2017; Strutt et al. 2018; Beura et al. 2019), they may also differ with respect to their expression of certain genes and molecules. For example, CD4+ Trm cells are less likely to localize to epithelial tissue compartments and tend to express lower levels of CD103 and Ly6C than CD8+ Trm cells (Gebhardt et al. 2011; Watanabe et al. 2015; Collins et al. 2016; Strutt et al. 2018; Beura et al. 2019). CD4+ T cells may be less prone to long-term residency than CD8+ T cells in epithelial barrier tissues including the skin and salivary glands following infection (Collins et al. 2016; Beura et al. 2019), and recent work suggests that human CD4+CD103+ Trm-like cells might continuously migrate between skin and blood despite expressing the core Trm cell–associated gene signature (Klicznik et al. 2019). CD4+ Trm cells may depend more stringently on IL-7 than CD8+ Trm cells with possible tissue- or context-specific requirements for IL-15 and IL-2 and a lower dependency on TGF-β (Adachi et al. 2015; Hondowicz et al. 2016; Romagnoli et al. 2017; Strutt et al. 2018). CD4+ Trm cells can also display diverse effector profiles depending on tissue location and stimulation history; in addition to resident Th1 cells (Beura et al. 2019), resident Treg (Sanchez Rodriguez et al. 2014), Th17 (Park et al. 2018a; Amezcua Vesely et al. 2019), and Th2 (Hondowicz et al. 2016; Turner et al. 2018; Bošnjak et al. 2019) subsets have been identified.
Trm cells and tissue-resident innate cells residing in the same organ share transcriptional similarities, implying a tissue-specific overlay of resident lymphocyte imprinting independent of cell type (Mackay et al. 2016). Despite this, even CD8+ Trm cells residing in the same tissue can be heterogeneous with respect to development and functionality. CD69+CD103+ and CD69+ CD103– Trm cells found in either the lamina propria or brain can exhibit distinct proliferative and cytotoxic potential and may display differential dependencies on cytokines including TGF-β, type I IFN, and IL-12 (Wakim et al. 2012; Bergsbaken et al. 2017; Bartolomé-Casado et al. 2019). In addition, CD69+CD8+ T cells in the lung parenchyma display enhanced cytokine and GzmB production and an altered surface phenotype compared to those residing in the airways (Wein et al. 2019). Furthermore, within the CD69+CD103+ subset, skin CD8+ Trm cells can display diverse cytokine profiles, whereby CD49a+ Trm cells producing IFN-γ display enhanced cytotoxicity and those lacking CD49a expression secrete IL-17 (Cheuk et al. 2017). In mice, both IL-17- and IFN-γ-producing skin Trm cells can develop from naive CD8+ T-cell precursors with identical TCRs and antigen specificity in response to the same commensal microbes (Naik et al. 2015; Linehan et al. 2018; Harrison et al. 2019). Together, these findings highlight disparities between Trm cells both within and among tissues and imply that infection- and tissue-independent factors can also influence Trm cell identity. Understanding interorgan and interpopulation variation between CD4+ and CD8+ Trm cells may allow for site-specific modulation of these cells for immunotherapy.
TISSUE MEMORY IN IMMUNE PROTECTION AND PATHOLOGY
Trm cells are poised for immediate detection and control of microbes that breach barrier surfaces (Mackay et al. 2012a; Park et al. 2018b), contrasting with Tcirc cells that may take days to be recruited to the site of infection. CD8+ Trm cells have been shown to enhance protection against a broad range of viral, bacterial, and parasitic pathogens in mice including herpes simplex virus (HSV) or vaccinia virus in the skin (Gebhardt et al. 2009; Jiang et al. 2012; Mackay et al. 2012b), LCMV infection of the salivary glands or brain (Hofmann and Pircher 2011; Steinbach et al. 2016), listeria infection of the gut (Sheridan et al. 2014), influenza infection of the lung (Ray et al. 2004; Wu et al. 2014; McMaster et al. 2015; Wakim et al. 2015; Pizzolla et al. 2017), and malaria infection of the liver (Fernandez-Ruiz et al. 2016). In some of these settings, Trm cells can efficiently protect against peripheral infections in the absence of Tcirc cells (Hofmann and Pircher 2011; Mackay et al. 2015b; Zens et al. 2016; Park et al. 2018b). CD4+ Trm cells have also been found to confer antimicrobial protection during influenza or streptococcal infections in the lung (Teijaro et al. 2011; Smith et al. 2018), Leishmania infection of the skin (Glennie et al. 2015), and HSV infection of the FRT (Iijima and Iwasaki 2014). In humans, CD8+ Trm cells cluster around nerve endings of neurons latently infected with HSV-2 where they are associated with reduced bouts of viral reactivation (Zhu et al. 2007, 2013; Schiffer et al. 2010; Peng et al. 2012) and accumulate at sites of viral persistence during Epstein–Barr virus (EBV) (Woodberry et al. 2005; Woon et al. 2016) and hepatitis (Pallett et al. 2017; Stelma et al. 2017) infection, suggesting that Trm cells in humans serve a similar protective function to those found in mice.
More recently, a critical role for Trm cells in cancer protection has emerged. CD8+ Trm-like tumor-infiltrating lymphocyte (TIL) expressing CD103 are abundant in human melanoma (Salerno et al. 2014; Boddupalli et al. 2016a; Edwards et al. 2018), breast (Wang et al. 2016; Savas et al. 2018), ovarian (Webb et al. 2010, 2014; Bösmüller et al. 2016), colorectal (Quinn et al. 2003), brain (Masson et al. 2007), lung (Djenidi et al. 2015; Ganesan et al. 2017), bladder (Wang et al. 2015; Hartana et al. 2018), endometrial (Workel et al. 2016), oropharyngeal (Solomon et al. 2019), and cervical (Komdeur et al. 2017) cancers where they often correlate with improved patient prognoses. Studies in mice have affirmed an anticancer role for Trm cells by revealing that they can protect against challenge with melanoma or head and neck cancer cells bearing their cognate antigen (Enamorado et al. 2017; Malik et al. 2017; Nizard et al. 2017; Gálvez-Cancino et al. 2018; Park et al. 2019). Trm-like TIL often exhibit additional transcriptional features of tissue residency including S1pr1 and Klf2 down-regulation and elevated Znf638 expression (Djenidi et al. 2015; Ganesan et al. 2017; Guo et al. 2018; Savas et al. 2018; Zhang et al. 2018; Clarke et al. 2019), although the extent to which these changes always reflect long-term residency is unclear. Heterogeneous populations of Trm-like TIL that differ with respect to proliferative capacity and effector function can coexist in human lung and breast tumors (Bengsch et al. 2018; Savas et al. 2018; Clarke et al. 2019). At least some of this diversity may result from the presence of bystander Trm cells that reside in transformed tissue but do not actually recognize cancer cells, and recent work indicates that coexpression of PD-1 and CD39 might distinguish tumor-reactive clones from Trm cells of unrelated specificities (Duhen et al. 2018; Simoni et al. 2018). Given that most cancers arise from epithelial tissue layers (Ferlay et al. 2015), Trm cells are likely vital during the early stages of carcinogenesis, as Tcirc cells might have difficulty entering these peripheral tissue compartments in the absence of overt inflammation. However, bona fide tissue-resident cells may become less critical in progressively growing tumors that can recruit their own vascular supply, spread beyond the epithelium, and provide a constant source of antigen. In line with this, Tcirc cells may be sufficient to protect against subcutaneously transplanted tumors in mice (Enamorado et al. 2017), although the extent to which this protection depends on their conversion into de novo Trm cells in the tumor microenvironment remains unclear.
In addition to providing protection against infection and cancer a role for Trm cells in orchestrating autoimmunity is becoming apparent. IFN-γ-producing CD8+CD103+ skin Trm cells have been shown to mediate or promote vitiligo in both mice (Malik et al. 2017; Richmond et al. 2018, 2019) and humans (Cheuk et al. 2017), whereas IL-17-producing Trm cells have been implicated in the pathogenesis of psoriasis (Boyman et al. 2004; Cheuk et al. 2017). However, commensal-specific IL-17+ Trm cells can also promote tissue homeostasis in other settings by accelerating wound healing after skin injury (Linehan et al. 2018), pointing to context-dependent functions of Trm cells that share functional profiles. Further evidence indicates that Trm cells might promote autoimmune pathology in multiple sclerosis (Sasaki et al. 2014; Steinbach et al. 2019), diabetes (Kuric et al. 2017), and inflammatory colitis (Zundler et al. 2019). Trm cells can also induce allergic reactions in the settings of asthma (Hondowicz et al. 2016; Bošnjak et al. 2019) and inflammatory skin hypersensitivity (Adachi et al. 2015; Gamradt et al. 2019). The permanent retention of these cells in afflicted tissues may explain relapsing and remitting bouts of disease at the same site over time.
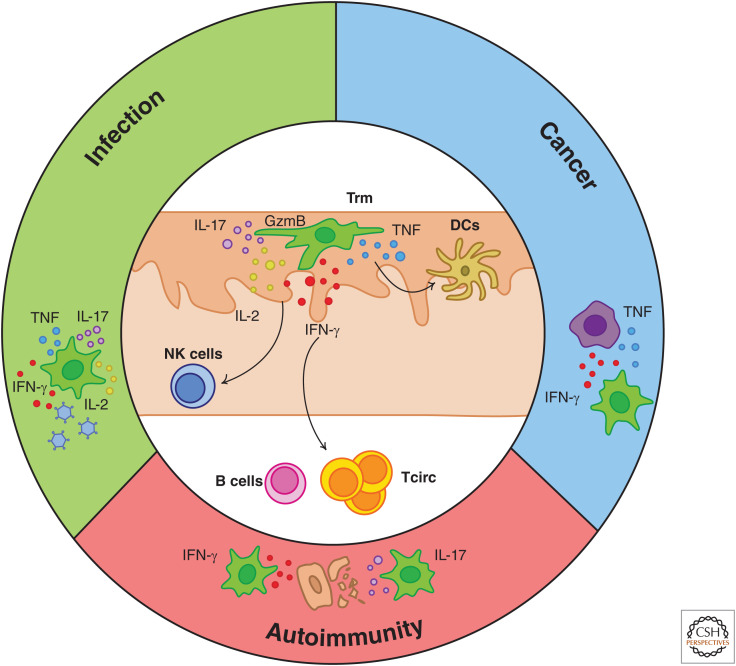
Trm cells constantly survey their surroundings for potential threats (Gebhardt et al. 2011; Ariotti et al. 2012; Zaid et al. 2014; Beura et al. 2018a) with the degree of immune protection they confer being linked to their density within the tissue (Schiffer et al. 2010; Park et al. 2018b). Whereas Trm cells in the skin and intestines display a slow and restricted mode of migration (Zaid et al. 2014; Thompson et al. 2019b), those in the FRT and liver exhibit more mobile behavior (Fernandez-Ruiz et al. 2016; Beura et al. 2018a; Holz et al. 2018). CD8+ Trm cells from both mice and humans can constitutively express cytotoxic molecules such as GzmB (Masopust et al. 2006; Casey et al. 2012; Mackay et al. 2012b; Ganesan et al. 2017) and transcripts for proinflammatory cytokines including IFN-γ (Wakim et al. 2012; Hombrink et al. 2016), although this is not always the case (McMaster et al. 2015; Pizzolla et al. 2017). Following antigen recognition, both CD4+ and CD8+ Trm cells rapidly up-regulate an arsenal of effector proteins including GzmB, IFN-γ, TNF, and IL-2 (Teijaro et al. 2011; Schenkel et al. 2014a; McMaster et al. 2015; Watanabe et al. 2015; Beura et al. 2019). Together, these molecules may enable Trm cells to limit the spread of infection or cancer by killing or inhibiting proliferation of infected or abnormal cells or may contribute to the induction of autoimmune pathology (Fig. 2). Direct Trm cell-mediated killing of target cells has been demonstrated in vitro (Djenidi et al. 2015), but whether this process also occurs in vivo remains unclear. CD8+ Trm cell-derived TNF and IFN-γ are critical for antiviral protection in the lung (McMaster et al. 2015), brain (Steinbach et al. 2016), and FRT (Schenkel et al. 2014a) and allow Trm cells to halt melanoma progression without completely eliminating cancer cells (Park et al. 2019). Similarly, Trm cells can suppress reactivation of latent HSV infections without mediating substantial tissue damage (Mackay et al. 2012b; Zhu et al. 2013). Trm cells in the skin (Collins et al. 2016; Lauron et al. 2019), brain (Wakim et al. 2010), FRT (Iijima and Iwasaki 2014), and gut (Bergsbaken and Bevan 2015; Bergsbaken et al. 2017) can be seen clustering with subsets of antigen-presenting cells including DCs that may cross-present antigen in situ to activate Trm cells in the absence of direct contact with infected cells (Wakim et al. 2010; Shin et al. 2016; Lauron et al. 2019). Importantly, cytokines such as IFN-γ produced by activated CD8+ and CD4+ Trm cells can recruit and activate downstream innate and adaptive effector cells including DCs, Tcirc cells, B cells, and natural killer (NK) cells to amplify local immunity and inducing a tissue-wide state of pathogen alert that helps to contain viral spread in an antigen-independent manner (Schenkel et al. 2013, 2014a; Ariotti et al. 2014; Beura et al. 2019). In addition, Trm cell activation can promote DC maturation and subsequent antigen spreading during tumor challenge (Menares et al. 2019). The “sense-and-alarm” function of Trm cells may explain why the protection they provide or pathology they induce is often amplified in the presence of Tcirc cells (Enamorado et al. 2017; Nizard et al. 2017; Park et al. 2019; Richmond et al. 2019).
Figure 2.
Functions of Trm cells in infection, cancer, and autoimmune disorders. Upon restimulation, Trm cells can rapidly produce a variety of effector molecules with diverse immune functions, including interferon γ (IFN-γ), GzmB, tumor necrosis factor (TNF), interleukin (IL)-2, and IL-17. Many of the cytokines produced by Trm cells can recruit and activate downstream immune cells found either locally in the surrounding tissue or in the circulation, including B cells, natural killer (NK) cells, and circulating T cells. This “sense-and-alarm” function of Trm cells may combine with their capacity for direct effector function (e.g., via cell killing or direct functions of cytokines on abnormal cells), allowing them to provide superior protection against infection and cancer by comparison with circulating T cells or endowing them with the capacity to orchestrate autoimmunity.
Following restimulation CD8+ Trm cells in the skin and FRT can proliferate in situ to maintain or expand the Trm cell pool (Beura et al. 2018a; Park et al. 2018b). Recently recruited Tcirc cells may also divide to give rise to de novo Trm cells at the site of rechallenge (Enamorado et al. 2017; Beura et al. 2018a; Park et al. 2018b; Osborn et al. 2019). The extent to which Trm cells and their progeny maintain functional and anatomical plasticity following reactivation is incompletely understood. Trm cells isolated from the gut and liver and transferred to the circulation of new hosts may retain the capacity to give rise to Tcirc cells and disseminate throughout the body when restimulated (Masopust et al. 2006; Behr et al. 2020; Fonseca et al. 2020). Skin or gut Trm cells reactivated in situ might also be capable of relocating to draining LNs (Beura et al. 2018b) and giving rise to secondary resident or effector memory T cells (Behr et al. 2020), respectively. Restimulated Trm cells that remain in their tissue of origin might also adapt their cytokine profile in response to environmental cues. Following tissue injury or exposure to the inflammatory cytokine IL-18 and antigen recognition, IL-17+ CD8+ skin Trm cells can acquire the ability to produce Th2-associated cytokines including IL-5 and IL-13 (Harrison et al. 2019). In addition, a proportion of CD4+ lung Trm cells that produce IL-17 during differentiation can instead acquire the ability to produce IFN-γ following restimulation (Menares et al. 2019). Whether Trm cell properties can also be altered in the absence of antigenic restimulation remains unclear, but these observations imply that Trm cells may maintain a degree of plasticity in the face of contextual fluidity. Understanding Trm cell fate following reactivation will be of particular importance in active or evolving immune environments including cancer or persistent infection.
EXPLOITING TISSUE-RESIDENCY FOR IMMUNOTHERAPY
The critical role of Trm cells in protecting against or inducing disease implies they could be exploited to improve immunotherapies. First, their regional localization allows them to rapidly curtail pathogen replication at its origin, making Trm cells a highly attractive target for vaccine design (Muruganandah et al. 2018). Immunization protocols designed to embed Trm cells at sites of infection can provide superior prophylactic protection against multiple pathogens when compared to strategies that only induce Tcirc cells, including HSV (Mackay et al. 2012a; Shin and Iwasaki 2012; Çuburu et al. 2015), influenza (Wakim et al. 2015; Zens et al. 2016; Pizzolla et al. 2017), and malaria (Fernandez-Ruiz et al. 2016; Holz et al. 2018) in mice, as well as HIV in primates (Petitdemange et al. 2019). Moreover, cancer vaccines that generate greater frequencies of Trm or Trm-like cells enhance tumor protection in both mice and humans (Murray et al. 2016; Nizard et al. 2017; Gálvez-Cancino et al. 2018). Immunizing at mucosal surfaces may enhance the generation of Trm cells and therefore the protective efficacy of T-cell-based vaccines against both infection (Liu et al. 2010; Jiang et al. 2012) and cancer (Sandoval et al. 2013), but intravenous vaccination can also generate large numbers of peripherally localized Trm cells in some settings (Thompson et al. 2019a). Repeated immunizations may lessen requirements for mucosal priming by encouraging widespread dissemination or boosted accumulation of protective Trm cells in tissues (Jiang et al. 2012; Davies et al. 2017; Park et al. 2018b; Van Braeckel-Budimir et al. 2018).
Antibody-mediated blockade of inhibitory checkpoint (IC) molecules has proven effective for the treatment of various solid cancers but not all patients respond favorably to therapy (Wei et al. 2018). The presence of high numbers of Trm-like TIL correlates with positive responses to immune checkpoint blockade (ICB) in melanoma (Edwards et al. 2018; Savas et al. 2018), and Trm cells can constitutively express various IC molecules including PD-1, CTLA-4, TIM-3, and LAG-3 (Mackay et al. 2013; Ganesan et al. 2017; Park et al. 2018b). Expression of IC molecules may be elevated on T cells following chronic antigen stimulation (Boldison et al. 2014; Gamradt et al. 2019; Wang et al. 2019) and in the tumor microenvironment (Djenidi et al. 2015; Boddupalli et al. 2016a; Workel et al. 2016; Ganesan et al. 2017; Clarke et al. 2019), but the impact these molecules have on Trm cell functionality is incompletely understood. Recent work suggests IC molecules can restrain Trm cell effector functions during persistent antigen recognition in the skin (Gamradt et al. 2019) or lung (Wang et al. 2019), possibly as a means to prevent unwanted autoimmune pathology. Blocking interactions between IC molecules and their ligands may therefore enhance Trm cell effector functions both in vitro (Djenidi et al. 2015; Shwetank et al. 2017) and in vivo (Gamradt et al. 2019; Wang et al. 2019). However, skin Trm cells retain the ability to efficiently protect against viral challenge despite expressing various IC molecules (Park et al. 2018b) and Trm-like TIL often express high levels of effector and IC molecules simultaneously (Djenidi et al. 2015; Savas et al. 2018; Clarke et al. 2019). Studies in mice indicate that only some CD8+ T-cell populations are efficiently targeted by ICB; in particular, proliferation of a “stem-like” TCF-1hi TIL subset appears critical for therapeutic success (Kurtulus et al. 2019; Siddiqui et al. 2019). Trm-like TIL may become more abundant following ICB (Edwards et al. 2018; Clarke et al. 2019), but whether this reflects direct proliferation of the Trm cell pool or expansion of less differentiated “stem-like” TIL that give rise to de novo Trm cells is unknown. Separate approaches through which Trm cell responses might be boosted for cancer treatment include adoptive cell therapy (ACT) and chimeric antigen receptor (CAR) T-cell therapies. In line with this, forced expression of the residency-promoting TFs Runx3 or Bhlhe40 in tumor-specific effector T cells can promote TIL accumulation and melanoma protection in mouse models of ACT (Milner et al. 2017; Li et al. 2019).
On the other hand, devising ways to dislodge Trm cells from tissues may prove beneficial for the treatment of localized autoimmune and allergic disorders. Trm cells in most tissues appear numerically and temporally stable and are not easily displaced by newly formed Trm cells (Holz et al. 2018; Park et al. 2018b). IL-15 blockade was recently shown to deplete skin Trm cells and alleviate disease in a mouse model of vitiligo (Richmond et al. 2018), suggesting that approaches that remove unwanted Trm cells might ameliorate autoimmunity. Ideally, identifying markers that are specifically expressed by Trm cells within afflicted tissues or expressed by subsets of pathological Trm cells may permit selective ablation of pathogenic Trm cells while preserving those that are essential for antipathogen and anticancer protection.
CONCLUDING REMARKS
The critical role for Trm cells in coordinating antipathogen and cancer immunity is now well established. Targeting these cells during vaccination or cancer immunotherapy is therefore an attractive prospect. However, many questions surrounding Trm cell responses remain unanswered and decoding these may unlock novel strategies to boost tissue memory to improve disease treatments. In particular, elucidating molecules and genes that can reliably identify Trm cells across diverse tissues and in active disease settings including cancer or chronic infection will be critical. Recent work has revealed that microbial exposure dramatically alters both the distribution and phenotype of memory T cells in mice (Beura et al. 2016), emphasizing the importance of studying Trm cell biology in humans in addition to laboratory animals. Uncovering more reliable markers for the identification of CD4+ and CD8+ Trm cells will be imperative to advance the study of these cells in patients where analyses are typically limited to phenotypic or transcriptional profiling.
Trm cells are frequently positioned in barrier tissues that can be coinhabited by commensal microbes and that are subjected to frequent tissue damage and low nutrient availability. Our understanding of how such microenvironmental factors influence the protective capacity and longevity of Trm cells remains incomplete. How are extrinsic cues such as nutrient availability and stress sensed and perceived by Trm cells and how might these regulate their functions, survival, and plasticity? How do Trm cells interact and communicate with surrounding commensals and immune, stromal, and neural cells that comprise the tissue networks in which they reside? Resolving how Trm cell responses are fine-tuned within tissues and tumors may provide insight into the genesis and regulation of both protective immune responses and detrimental autoimmune or inflammatory diseases.
The diversity of Trm cells in different organs and even within a single given tissue is becoming increasingly clear, but the extent of this heterogeneity is incompletely understood. Can we specifically target Trm cells in a particular given organ to better treat tissue-tropic infections, cancers, or autoimmune diseases? Are some Trm cells more functional than others, and if so can we identify those that are the most protective? Can Trm cells become “exhausted” or dysfunctional following exposure to persistent infection or cancer? Or do chronically stimulated Trm cells de-differentiate and give rise to alternate effector and memory T-cell subsets? Are the relative roles of Trm and Tcirc cell subsets different during acute infection compared to chronic disease? Deciphering differences in the requirements and functionality of Trm cells across different tissues and understanding how Trm cell generation and persistence might be differentially regulated during acute versus chronic infection or in the tumor microenvironment will be critical to manipulate these cells for therapeutic purposes.
ACKNOWLEDGMENTS
S.L.P. is supported by an NHMRC Emerging Leadership Investigator Grant. L.K.M. is supported by an NHMRC Leadership Investigator Grant and a Senior Medical Research Fellowship from the Sylvia & Charles Viertel Charitable Foundation.
Footnotes
Editors: David Masopust and Rafi Ahmed
Additional Perspectives on T-Cell Memory available at www.cshperspectives.org
REFERENCES
- Adachi T, Kobayashi T, Sugihara E, Yamada T, Ikuta K, Pittaluga S, Saya H, Amagai M, Nagao K. 2015. Hair follicle-derived IL-7 and IL-15 mediate skin-resident memory T cell homeostasis and lymphoma. Nat Med 21: 1272–1279. 10.1038/nm.3962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agace WW. 2006. Tissue-tropic effector T cells: generation and targeting opportunities. Nat Rev Immunol 6: 682–692. 10.1038/nri1869 [DOI] [PubMed] [Google Scholar]
- Amezcua Vesely MC, Pallis P, Bielecki P, Low JS, Zhao J, Harman CCD, Kroehling L, Jackson R, Bailis W, Licona-Limón P, et al. 2019. Effector TH17 cells give rise to long-lived TRM cells that are essential for an immediate response against bacterial infection. Cell 178: 1176–1188.e15. 10.1016/j.cell.2019.07.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson KG, Sung H, Skon CN, Lefrancois L, Deisinger A, Vezys V, Masopust D. 2012. Cutting edge: intravascular staining redefines lung CD8 T cell responses. J Immunol 189: 2702–2706. 10.4049/jimmunol.1201682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson KG, Mayer-Barber K, Sung H, Beura L, James BR, Taylor JJ, Qunaj L, Griffith TS, Vezys V, Barber DL, et al. 2014. Intravascular staining for discrimination of vascular and tissue leukocytes. Nat Protoc 9: 209–222. 10.1038/nprot.2014.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariotti S, Beltman JB, Chodaczek G, Hoekstra ME, van Beek AE, Gomez-Eerland R, Ritsma L, van Rheenen J, Marée AFM, Zal T, et al. 2012. Tissue-resident memory CD8+ T cells continuously patrol skin epithelia to quickly recognize local antigen. Proc Natl Acad Sci 109: 19739–19744. 10.1073/pnas.1208927109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariotti S, Hogenbirk MA, Dijkgraaf FE, Visser LL, Hoekstra ME, Song JY, Jacobs H, Haanen JB, Schumacher TN. 2014. Skin-resident memory CD8+ T cells trigger a state of tissue-wide pathogen alert. Science 346: 101–105. 10.1126/science.1254803 [DOI] [PubMed] [Google Scholar]
- Bartolomé-Casado R, Landsverk OJB, Chauhan SK, Richter L, Phung D, Greiff V, Risnes LF, Yao Y, Neumann RS, Yaqub S, et al. 2019. Resident memory CD8 T cells persist for years in human small intestine. J Exp Med 216: 2412–2426. 10.1084/jem.20190414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behr FM, Kragten NAM, Wesselink TH, Nota B, van Lier RAW, Amsen D, Stark R, Hombrink P, van Gisbergen KPJM. 2019. Blimp-1 rather than Hobit drives the formation of tissue-resident memory CD8+ T cells in the lungs. Front Immunol 10: 400. 10.3389/fimmu.2019.00400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behr FM, Parga-Vidal L, Kragten NAM, van Dam TJP, Wesselink TH, Sheridan BS, Arens R, van Lier RAW, Stark R, van Gisbergen KPJM. 2020. Tissue-resident memory CD8+ T cells shape local and systemic secondary T cell responses. Nat Immunol 21: 1070–1081. 10.1038/s41590-020-0723-4 [DOI] [PubMed] [Google Scholar]
- Bengsch B, Ohtani T, Khan O, Setty M, Manne S, O'Brien S, Gherardini PF, Herati RS, Huang AC, Chang KM, et al. 2018. Epigenomic-guided mass cytometry profiling reveals disease-specific features of exhausted CD8 T cells. Immunity 48: 1029–1045.e5. 10.1016/j.immuni.2018.04.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergsbaken T, Bevan MJ. 2015. Proinflammatory microenvironments within the intestine regulate the differentiation of tissue-resident CD8+ T cells responding to infection. Nat Immunol 16: 406–414. 10.1038/ni.3108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergsbaken T, Bevan MJ, Fink PJ. 2017. Local inflammatory cues regulate differentiation and persistence of CD8+ tissue-resident memory T cells. Cell Rep 19: 114–124. 10.1016/j.celrep.2017.03.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beura LK, Anderson KG, Schenkel JM, Locquiao JJ, Fraser KA, Vezys V, Pepper M, Masopust D. 2015. Lymphocytic choriomeningitis virus persistence promotes effector-like memory differentiation and enhances mucosal T cell distribution. J Leukoc Biol 97: 217–225. 10.1189/jlb.1HI0314-154R [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beura LK, Hamilton SE, Bi K, Schenkel JM, Odumade OA, Casey KA, Thompson EA, Fraser KA, Rosato PC, Filali-Mouhim A, et al. 2016. Normalizing the environment recapitulates adult human immune traits in laboratory mice. Nature 532: 512–516. 10.1038/nature17655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beura LK, Mitchell JS, Thompson EA, Schenkel JM, Mohammed J, Wijeyesinghe S, Fonseca R, Burbach BJ, Hickman HD, Vezys V, et al. 2018a. Intravital mucosal imaging of CD8+ resident memory T cells shows tissue-autonomous recall responses that amplify secondary memory. Nat Immunol 19: 173–182. 10.1038/s41590-017-0029-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beura LK, Wijeyesinghe S, Thompson EA, Macchietto MG, Rosato PC, Pierson MJ, Schenkel JM, Mitchell JS, Vezys V, Fife BT, et al. 2018b. T cells in nonlymphoid tissues give rise to lymph-node-resident memory T cells. Immunity 48: 327–338.e5. 10.1016/j.immuni.2018.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beura LK, Fares-Frederickson NJ, Steinert EM, Scott MC, Thompson EA, Fraser KA, Schenkel JM, Vezys V, Masopust D. 2019. CD4+ resident memory T cells dominate immunosurveillance and orchestrate local recall responses. J Exp Med 216: 1214–1229. 10.1084/jem.20181365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boddupalli CS, Bar N, Kadaveru K, Krauthammer M, Pornputtapong N, Mai Z, Ariyan S, Narayan D, Kluger H, Deng Y, et al. 2016a. Interlesional diversity of T cell receptors in melanoma with immune checkpoints enriched in tissue-resident memory T cells. JCI Insight 1: e88955. 10.1172/jci.insight.88955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boddupalli CS, Nair S, Gray SM, Nowyhed HN, Verma R, Gibson JA, Abraham C, Narayan D, Vasquez J, Hedrick CC, et al. 2016b. ABC transporters and NR4A1 identify a quiescent subset of tissue-resident memory T cells. J Clin Invest 126: 3905–3916. 10.1172/JCI85329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldison J, Chu CJ, Copland DA, Lait PJP, Khera TK, Dick AD, Nicholson LB. 2014. Tissue-resident exhausted effector memory CD8+ T cells accumulate in the retina during chronic experimental autoimmune uveoretinitis. J Immunol 192: 4541–4550. 10.4049/jimmunol.1301390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borges da Silva H, Beura LK, Wang H, Hanse EA, Gore R, Scott MC, Walsh DA, Block KE, Fonseca R, Yan Y, et al. 2018. The purinergic receptor P2RX7 directs metabolic fitness of long-lived memory CD8+ T cells. Nature 559: 264–268. 10.1038/s41586-018-0282-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borges da Silva H, Peng C, Wang H, Wanhainen KM, Ma C, Lopez S, Khoruts A, Zhang N, Jameson SC. 2020. Sensing of ATP via the purinergic receptor P2RX7 promotes CD8+ Trm cell generation by enhancing their sensitivity to the cytokine TGF–β. Immunity 53: 158–171.e6. 10.1016/j.immuni.2020.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bösmüller HC, Wagner P, Peper JK, Schuster H, Pham DL, Greif K, Beschorner C, Rammensee HG, Stevanović S, Fend F, et al. 2016. Combined immunoscore of CD103 and CD3 identifies long-term survivors in high-grade serous ovarian cancer. Int J Gynecol Cancer 26: 671–679. 10.1097/IGC.0000000000000672 [DOI] [PubMed] [Google Scholar]
- Bošnjak B, Kazemi S, Altenburger LM, Mokrović G, Epstein MM. 2019. Th2-TRMs maintain life-long allergic memory in experimental asthma in mice. Front Immunol 10: 840. 10.3389/fimmu.2019.00840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyman O, Hefti HP, Conrad C, Nickoloff BJ, Suter M, Nestle FO. 2004. Spontaneous development of psoriasis in a new animal model shows an essential role for resident T cells and tumor necrosis factor-α. J Exp Med 199: 731–736. 10.1084/jem.20031482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromley SK, Akbaba H, Mani V, Mora-Buch R, Chasse AY, Sama A, Luster AD. 2020. CD49a regulates cutaneous resident memory CD8+ T cell persistence and response. Cell Rep 32: 108085. 10.1016/j.celrep.2020.108085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey KA, Fraser KA, Schenkel JM, Moran A, Abt MC, Beura LK, Lucas PJ, Artis D, Wherry EJ, Hogquist K, et al. 2012. Antigen-independent differentiation and maintenance of effector-like resident memory T cells in tissues. J Immunol 188: 4866–4875. 10.4049/jimmunol.1200402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepek KL, Shaw SK, Parker CM, Russell GJ, Morrow JS, Rimm DL, Brenner MB. 1994. Adhesion between epithelial cells and T lymphocytes mediated by E-cadherin and the αEβ7 integrin. Nature 372: 190–193. 10.1038/372190a0 [DOI] [PubMed] [Google Scholar]
- Chang JT, Wherry EJ, Goldrath AW. 2014. Molecular regulation of effector and memory T cell differentiation. Nat Immunol 15: 1104–1115. 10.1038/ni.3031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheuk S, Schlums H, Sérézal IG, Martini E, Chiang SC, Marquardt N, Gibbs A, Detlofsson E, Introini A, Forkel M, et al. 2017. CD49a expression defines tissue-resident CD8+ T cells poised for cytotoxic function in human skin. Immunity 46: 287–300. 10.1016/j.immuni.2017.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cibrián D, Sánchez-Madrid F. 2017. CD69: from activation marker to metabolic gatekeeper. Eur J Immunol 47: 946–953. 10.1002/eji.201646837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RA, Watanabe R, Teague JE, Schlapbach C, Tawa MC, Adams N, Dorosario AA, Chaney KS, Cutler CS, LeBoeuf NR, et al. 2012. Skin effector memory T cells do not recirculate and provide immune protection in alemtuzumab-treated CTCL patients. Sci Transl Med 4: 117ra7. 10.1126/scitranslmed.3003008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke J, Panwar B, Madrigal A, Singh D, Gujar R, Wood O, Chee SJ, Eschweiler S, King EV, Awad AS, et al. 2019. Single-cell transcriptomic analysis of tissue-resident memory T cells in human lung cancer. J Exp Med 216: 2128–2149. 10.1084/jem.20190249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins N, Jiang X, Zaid A, Macleod BL, Li J, Park CO, Haque A, Bedoui S, Heath WR, Mueller SN, et al. 2016. Skin CD4+ memory T cells exhibit combined cluster-mediated retention and equilibration with the circulation. Nat Commun 7: 11514. 10.1038/ncomms11514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cresswell J, Robertson H, Neal DE, Griffiths TR, Kirby JA. 2001. Distribution of lymphocytes of the αEβ7 phenotype and E-cadherin in normal human urothelium and bladder carcinomas. Clin Exp Immunol 126: 397–402. 10.1046/j.1365-2249.2001.01652.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Çuburu N, Wang K, Goodman KN, Pang YY, Thompson CD, Lowy DR, Cohen JI, Schiller JT. 2015. Topical herpes simplex virus 2 (HSV-2) vaccination with human papillomavirus vectors expressing gB/gD ectodomains induces genital-tissue-resident memory CD8+ T cells and reduces genital disease and viral shedding after HSV-2 challenge. J Virol 89: 83–96. 10.1128/JVI.02380-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyster JG, Schwab SR. 2012. Sphingosine-1-phosphate and lymphocyte egress from lymphoid organs. Annu Rev Immunol 30: 69–94. 10.1146/annurev-immunol-020711-075011 [DOI] [PubMed] [Google Scholar]
- Davies B, Prier JE, Jones CM, Gebhardt T, Carbone FR, Mackay LK. 2017. Cutting edge: tissue-resident memory T cells generated by multiple immunizations or localized deposition provide enhanced immunity. J Immunol 198: 2233–2237. 10.4049/jimmunol.1601367 [DOI] [PubMed] [Google Scholar]
- de Leur K, Dieterich M, Hesselink DA, Corneth OBJ, Dor FJMF, de Graav GN, Peeters AMA, Mulder A, Kimenai HJAN, Claas FHJ, et al. 2019. Characterization of donor and recipient CD8+ tissue-resident memory T cells in transplant nephrectomies. Sci Rep 9: 5984. 10.1038/s41598-019-42401-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djenidi F, Adam J, Goubar A, Durgeau A, Meurice G, de Montpréville V, Validire P, Besse B, Mami-Chouaib F. 2015. CD8+CD103+ tumor-infiltrating lymphocytes are tumor-specific tissue-resident memory T cells and a prognostic factor for survival in lung cancer patients. J Immunol 194: 3475–3486. 10.4049/jimmunol.1402711 [DOI] [PubMed] [Google Scholar]
- Duhen T, Duhen R, Montler R, Moses J, Moudgil T, de Miranda NF, Goodall CP, Blair TC, Fox BA, McDermott JE, et al. 2018. Co-expression of CD39 and CD103 identifies tumor-reactive CD8 T cells in human solid tumors. Nat Commun 9: 2724. 10.1038/s41467-018-05072-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards J, Wilmott JS, Madore J, Gide TN, Quek C, Tasker A, Ferguson A, Chen J, Hewavisenti R, Hersey P, et al. 2018. CD103+ tumor-resident CD8+ T cells are associated with improved survival in immunotherapy-naïve melanoma patients and expand significantly during anti-PD-1 treatment. Clinical Cancer Res 24: 3036–3045. 10.1158/1078-0432.CCR-17-2257 [DOI] [PubMed] [Google Scholar]
- El-Asady R, Yuan R, Liu K, Wang D, Gress RE, Lucas PJ, Drachenberg CB, Hadley GA. 2005. TGF-β-dependent CD103 expression by CD8+ T cells promotes selective destruction of the host intestinal epithelium during graft-versus-host disease. J Exp Med 201: 1647–1657. 10.1084/jem.20041044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ely KH, Cookenham T, Roberts AD, Woodland DL. 2006. Memory T cell populations in the lung airways are maintained by continual recruitment. J Immunol 176: 537–543. 10.4049/jimmunol.176.1.537 [DOI] [PubMed] [Google Scholar]
- Enamorado M, Iborra S, Priego E, Cueto FJ, Quintana JA, Martínez-Cano S, Mejías-Pérez E, Esteban M, Melero I, Hidalgo A, et al. 2017. Enhanced anti-tumour immunity requires the interplay between resident and circulating memory CD8+ T cells. Nat Commun 8: 16073. 10.1038/ncomms16073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. 2015. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136: E359–E386. 10.1002/ijc.29210 [DOI] [PubMed] [Google Scholar]
- Fernandez-Ruiz D, Ng WY, Holz LE, Ma JZ, Zaid A, Wong YC, Lau LS, Mollard V, Cozijnsen A, Collins N, et al. 2016. Liver-resident memory CD8+ T cells form a front-line defense against malaria liver-stage infection. Immunity 45: 889–902. 10.1016/j.immuni.2016.08.011 [DOI] [PubMed] [Google Scholar]
- Fiege JK, Stone IA, Fay EJ, Markman MW, Wijeyesinghe S, Macchietto MG, Shen S, Masopust D, Langlois RA. 2019. The impact of TCR signal strength on resident memory T cell formation during influenza virus infection. J Immunol 203: 936–945. 10.4049/jimmunol.1900093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca R, Beura LK, Quarnstrom CF, Ghoneim HE, Fan Y, Zebley CC, Scott MC, Fares-Frederickson NJ, Wijeyesinghe S, Thompson EA, et al. 2020. Developmental plasticity allows outside-in immune responses by resident memory T cells. Nat Immunol 21: 412–421. 10.1038/s41590-020-0607-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frizzell H, Fonseca R, Christo SN, Evrard M, Cruz-Gomez S, Zanluqui NG, von Scheidt B, Freestone D, Park SL, McWilliam HEG, et al. 2020. Organ-specific isoform selection of fatty acid-binding proteins in tissue-resident lymphocytes. Sci Immunol 5: eaay9283. 10.1126/sciimmunol.aay9283 [DOI] [PubMed] [Google Scholar]
- Frost EL, Kersh AE, Evavold BD, Lukacher AE. 2015. Cutting edge: resident memory CD8 T cells express high-affinity TCRs. J Immunol 195: 3520–3524. 10.4049/jimmunol.1501521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaide O, Emerson RO, Jiang X, Gulati N, Nizza S, Desmarais C, Robins H, Krueger JG, Clark RA, Kupper TS. 2015. Common clonal origin of central and resident memory T cells following skin immunization. Nat Med 21: 647–653. 10.1038/nm.3860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gálvez-Cancino F, López E, Menares E, Díaz X, Flores C, Cáceres P, Hidalgo S, Chovar O, Alcántara-Hernández M, Borgna V, et al. 2018. Vaccination-induced skin-resident memory CD8+ T cells mediate strong protection against cutaneous melanoma. Oncoimmunology 7: e1442163. 10.1080/2162402X.2018.1442163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamradt P, Laoubi L, Nosbaum A, Mutez V, Lenief V, Grande S, Redoulès D, Schmitt AM, Nicolas JF, Vocanson M. 2019. Inhibitory checkpoint receptors control CD8+ resident memory T cells to prevent skin allergy. J Allergy Clin Immunol 143: 2147–2157.e9. 10.1016/j.jaci.2018.11.048 [DOI] [PubMed] [Google Scholar]
- Ganesan A-P, Clarke J, Wood O, Garrido-Martin EM, Chee SJ, Mellows T, Samaniego-Castruita D, Singh D, Seumois G, Alzetani A, et al. 2017. Tissue-resident memory features are linked to the magnitude of cytotoxic T cell responses in human lung cancer. Nat Immunol 18: 940–950. 10.1038/ni.3775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebhardt T, Wakim LM, Eidsmo L, Reading PC, Heath WR, Carbone FR. 2009. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat Immunol 10: 524–530. 10.1038/ni.1718 [DOI] [PubMed] [Google Scholar]
- Gebhardt T, Whitney PG, Zaid A, Mackay LK, Brooks AG, Heath WR, Carbone FR, Mueller SN. 2011. Different patterns of peripheral migration by memory CD4+ and CD8+ T cells. Nature 477: 216–219. 10.1038/nature10339 [DOI] [PubMed] [Google Scholar]
- Gilchuk P, Hill TM, Guy C, McMaster SR, Boyd KL, Rabacal WA, Lu P, Shyr Y, Kohlmeier JE, Sebzda E, et al. 2016. A distinct lung-interstitium-resident memory CD8+ T cell subset confers enhanced protection to lower respiratory tract infection. Cell Rep 16: 1800–1809. 10.1016/j.celrep.2016.07.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glennie ND, Yeramilli VA, Beiting DP, Volk SW, Weaver CT, Scott P. 2015. Skin-resident memory CD4+ T cells enhance protection against Leishmania major infection. J Exp Med 212: 1405–1414. 10.1084/jem.20142101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X, Zhang Y, Zheng L, Zheng C, Song J, Zhang Q, Kang B, Liu Z, Jin L, Xing R, et al. 2018. Global characterization of T cells in non-small-cell lung cancer by single-cell sequencing. Nat Med 24: 978–985. 10.1038/s41591-018-0045-3 [DOI] [PubMed] [Google Scholar]
- Han SJ, Glatman Zaretsky A, Andrade-Oliveira V, Collins N, Dzutsev A, Shaik J, Morais da Fonseca D, Harrison OJ, Tamoutounour S, Byrd AL, et al. 2017. White adipose tissue is a reservoir for memory T cells and promotes protective memory responses to infection. Immunity 47: 1154–1168.e6. 10.1016/j.immuni.2017.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison OJ, Linehan JL, Shih HY, Bouladoux N, Han S-J, Smelkinson M, Sen SK, Byrd AL, Enamorado M, Yao C, et al. 2019. Commensal-specific T cell plasticity promotes rapid tissue adaptation to injury. Science 363: eaat6280. 10.1126/science.aat6280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartana CA, Ahlén Bergman E, Broomé A, Berglund S, Johansson M, Alamdari F, Jakubczyk T, Huge Y, Aljabery F, Palmqvist K, et al. 2018. Tissue-resident memory T cells are epigenetically cytotoxic with signs of exhaustion in human urinary bladder cancer. Clin Exp Immunol 194: 39–53. 10.1111/cei.13183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herndler-Brandstetter D, Ishigame H, Shinnakasu R, Plajer V, Stecher C, Zhao J, Lietzenmayer M, Kroehling L, Takumi A, Kometani K, et al. 2018. KLRG1+ effector CD8+ T cells lose KLRG1, differentiate into all memory T cell lineages, and convey enhanced protective immunity. Immunity 48: 716–729.e8. 10.1016/j.immuni.2018.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbs SJ, Nolz JC. 2017. Regulation of T cell trafficking by enzymatic synthesis of O-glycans. Front Immunol 8: 600–600. 10.3389/fimmu.2017.00600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann M, Pircher H. 2011. E-cadherin promotes accumulation of a unique memory CD8 T-cell population in murine salivary glands. Proc Natl Acad Sci 108: 16741–16746. 10.1073/pnas.1107200108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann M, Oschowitzer A, Kurzhals SR, Krüger CC, Pircher H. 2013. Thymus-resident memory CD8+ T cells mediate local immunity. Eur J Immunol 43: 2295–2304. 10.1002/eji.201343519 [DOI] [PubMed] [Google Scholar]
- Holz LE, Prier JE, Freestone D, Steiner TM, English K, Johnson DN, Mollard V, Cozijnsen A, Davey GM, Godfrey DI, et al. 2018. CD8+ T cell activation leads to constitutive formation of liver tissue-resident memory T cells that seed a large and flexible niche in the liver. Cell Rep 25: 68–79.e4. 10.1016/j.celrep.2018.08.094 [DOI] [PubMed] [Google Scholar]
- Hombrink P, Helbig C, Backer RA, Piet B, Oja AE, Stark R, Brasser G, Jongejan A, Jonkers RE, Nota B, et al. 2016. Programs for the persistence, vigilance and control of human CD8+ lung-resident memory T cells. Nat Immunol 17: 1467–1478. 10.1038/ni.3589 [DOI] [PubMed] [Google Scholar]
- Hondowicz BD, An D, Schenkel JM, Kim KS, Steach HR, Krishnamurty AT, Keitany GJ, Garza EN, Fraser KA, Moon JJ, et al. 2016. Interleukin-2-dependent allergen-specific tissue-resident memory cells drive asthma. Immunity 44: 155–166. 10.1016/j.immuni.2015.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iborra S, Martínez-López M, Khouili SC, Enamorado M, Cueto FJ, Conde-Garrosa R, del Fresno C, Sancho D. 2016. Optimal generation of tissue-resident but not circulating memory T cells during viral infection requires crosspriming by DNGR-1+ dendritic cells. Immunity 45: 847–860. 10.1016/j.immuni.2016.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iijima N, Iwasaki A. 2014. A local macrophage chemokine network sustains protective tissue-resident memory CD4 T cells. Science 346: 93–98. 10.1126/science.1257530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Clark RA, Liu L, Wagers AJ, Fuhlbrigge RC, Kupper TS. 2012. Skin infection generates non-migratory memory CD8+ TRM cells providing global skin immunity. Nature 483: 227–231. 10.1038/nature10851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan TN, Mooster JL, Kilgore AM, Osborn JF, Nolz JC. 2016. Local antigen in nonlymphoid tissue promotes resident memory CD8+ T cell formation during viral infection. J Exp Med 213: 951–966. 10.1084/jem.20151855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klicznik MM, Morawski PA, Höllbacher B, Varkhande SR, Motley SJ, Kuri-Cervantes L, Goodwin E, Rosenblum MD, Long SA, Brachtl G, et al. 2019. Human CD4+CD103+ cutaneous resident memory T cells are found in the circulation of healthy individuals. Sci Immunol 4: eaav8995. 10.1126/sciimmunol.aav8995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komdeur FL, Prins TM, van de Wall S, Plat A, Wisman GBA, Hollema H, Daemen T, Church DN, de Bruyn M, Nijman HW. 2017. CD103+ tumor-infiltrating lymphocytes are tumor-reactive intraepithelial CD8+ T cells associated with prognostic benefit and therapy response in cervical cancer. Oncoimmunology 6: e1338230. 10.1080/2162402X.2017.1338230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kragten NA, Behr FM, Vieira Braga FA, Remmerswaal EBM, Wesselink TH, Oja AE, Hombrink P, Kallies A, van Lier RA, Stark R, van Gisbergen KP. 2018. Blimp-1 induces and Hobit maintains the cytotoxic mediator granzyme B in CD8 T cells. Eur J Immunol 48: 1644–1662. 10.1002/eji.201847771 [DOI] [PubMed] [Google Scholar]
- Kumar BV, Ma W, Miron M, Granot T, Guyer RS, Carpenter DJ, Senda T, Sun X, Ho S-H, Lerner H, et al. 2017. Human tissue-resident memory T cells are defined by core transcriptional and functional signatures in lymphoid and mucosal sites. Cell Rep 20: 2921–2934. 10.1016/j.celrep.2017.08.078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuric E, Seiron P, Krogvold L, Edwin B, Buanes T, Hanssen KF, Skog O, Dahl-Jørgensen K, Korsgren O. 2017. Demonstration of tissue resident memory CD8 T cells in insulitic lesions in adult patients with recent-onset type 1 diabetes. Am J Pathol 187: 581–588. 10.1016/j.ajpath.2016.11.002 [DOI] [PubMed] [Google Scholar]
- Kurtulus S, Madi A, Escobar G, Klapholz M, Nyman J, Christian E, Pawlak M, Dionne D, Xia J, Rozenblatt-Rosen O, et al. 2019. Checkpoint blockade immunotherapy induces dynamic changes in PD-1− CD8+ tumor-infiltrating T cells. Immunity 50: 181–194.e6. 10.1016/j.immuni.2018.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laidlaw BJ, Zhang N, Marshall HD, Staron MM, Guan T, Hu Y, Cauley LS, Craft J, Kaech SM. 2014. CD4+ T cell help guides formation of CD103+ lung-resident memory CD8+ T cells during influenza viral infection. Immunity 41: 633–645. 10.1016/j.immuni.2014.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauron EJ, Yang L, Harvey IB, Sojka DK, Williams GD, Paley MA, Bern MD, Park E, Victorino F, Boon ACM, et al. 2019. Viral MHCI inhibition evades tissue-resident memory T cell formation and responses. J Exp Med 216: 117–132. 10.1084/jem.20181077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YT, Suarez-Ramirez JE, Wu T, Redman JM, Bouchard K, Hadley GA, Cauley LS. 2011. Environmental and antigen receptor-derived signals support sustained surveillance of the lungs by pathogen-specific cytotoxic T lymphocytes. J Virol 85: 4085–4094. 10.1128/JVI.02493-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Zhu B, Son YM, Wang Z, Jiang L, Xiang M, Ye Z, Beckermann KE, Wu Y, Jenkins JW, et al. 2019. The transcription factor Bhlhe40 programs mitochondrial regulation of resident CD8+ T cell fitness and functionality. Immunity 51: 491–507.e7. 10.1016/j.immuni.2019.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian CG, Bueno EM, Granter SR, Laga AC, Saavedra AP, Lin WM, Susa JS, Zhan Q, Chandraker AK, Tullius SG, et al. 2014. Biomarker evaluation of face transplant rejection: association of donor T cells with target cell injury. Mod Pathol 27: 788–799. 10.1038/modpathol.2013.249 [DOI] [PubMed] [Google Scholar]
- Linehan JL, Harrison OJ, Han SJ, Byrd AL, Vujkovic-Cvijin I, Villarino AV, Sen SK, Shaik J, Smelkinson M, Tamoutounour S, et al. 2018. Non-classical immunity controls microbiota impact on skin immunity and tissue repair. Cell 172: 784–796.e18. 10.1016/j.cell.2017.12.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Zhong Q, Tian T, Dubin K, Athale SK, Kupper TS. 2010. Epidermal injury and infection during poxvirus immunization is crucial for the generation of highly protective T cell-mediated immunity. Nat Med 16: 224–227. 10.1038/nm.2078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C, Mishra S, Demel EL, Liu Y, Zhang N. 2017. TGF-β controls the formation of kidney-resident T cells via promoting effector T cell extravasation. J Immunol 198: 749–756. 10.4049/jimmunol.1601500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay LK, Stock AT, Ma JZ, Jones CM, Kent SJ, Mueller SN, Heath WR, Carbone FR, Gebhardt T. 2012a. Long-lived epithelial immunity by tissue-resident memory T (TRM) cells in the absence of persisting local antigen presentation. Proc Natl Acad Sci 109: 7037–7042. 10.1073/pnas.1202288109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay LK, Wakim L, van Vliet CJ, Jones CM, Mueller SN, Bannard O, Fearon DT, Heath WR, Carbone FR. 2012b. Maintenance of T cell function in the face of chronic antigen stimulation and repeated reactivation for a latent virus infection. J Immunol 188: 2173–2178. 10.4049/jimmunol.1102719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay LK, Rahimpour A, Ma JZ, Collins N, Stock AT, Hafon M-L, Vega-Ramos J, Lauzurica P, Mueller SN, Stefanovic T, et al. 2013. The developmental pathway for CD103+CD8+ tissue-resident memory T cells of skin. Nat Immunol 14: 1294–1301. 10.1038/ni.2744 [DOI] [PubMed] [Google Scholar]
- Mackay LK, Braun A, Macleod BL, Collins N, Tebartz C, Bedoui S, Carbone FR, Gebhardt T. 2015a. Cutting edge: CD69 interference with sphingosine-1-phosphate receptor function regulates peripheral T cell retention. J Immunol 194: 2059–2063. 10.4049/jimmunol.1402256 [DOI] [PubMed] [Google Scholar]
- Mackay LK, Wynne-Jones E, Freestone D, Pellicci DG, Mielke LA, Newman DM, Braun A, Masson F, Kallies A, Belz GT, et al. 2015b. T-box transcription factors combine with the cytokines TGF-β and IL-15 to control tissue-resident memory T cell fate. Immunity 43: 1101–1111. 10.1016/j.immuni.2015.11.008 [DOI] [PubMed] [Google Scholar]
- Mackay LK, Minnich M, Kragten NAM, Liao Y, Nota B, Seillet C, Zaid A, Man K, Preston S, Freestone D, et al. 2016. Hobit and Blimp1 instruct a universal transcriptional program of tissue residency in lymphocytes. Science 352: 459–463. 10.1126/science.aad2035 [DOI] [PubMed] [Google Scholar]
- Malik BT, Byrne KT, Vella JL, Zhang P, Shabaneh TB, Steinberg SM, Molodtsov AK, Bowers JS, Angeles CV, Paulos CM, et al. 2017. Resident memory T cells in the skin mediate durable immunity to melanoma. Sci Immunol 2: eaam6346. 10.1126/sciimmunol.aam6346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani V, Bromley SK, Äijö T, Mora-Buch R, Carrizosa E, Warner RD, Hamze M, Sen DR, Chasse AY, Lorant A, et al. 2019. Migratory DCs activate TGF-β to precondition naïve CD8+ T cells for tissue-resident memory fate. Science 366: eaav5728. 10.1126/science.aav5728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masopust D, Soerens AG. 2019. Tissue-resident T cells and other resident leukocytes. Ann Rev Immunol 37: 521–546. 10.1146/annurev-immunol-042617-053214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masopust D, Vezys V, Usherwood EJ, Cauley LS, Olson S, Marzo AL, Ward RL, Woodland DL, Lefrançois L. 2004. Activated primary and memory CD8 T cells migrate to nonlymphoid tissues regardless of site of activation or tissue of origin. J Immunol 172: 4875–4882. 10.4049/jimmunol.172.8.4875 [DOI] [PubMed] [Google Scholar]
- Masopust D, Vezys V, Wherry EJ, Barber DL, Ahmed R. 2006. Cutting edge: gut microenvironment promotes differentiation of a unique memory CD8 T cell population. J Immunol 176: 2079–2083. 10.4049/jimmunol.176.4.2079 [DOI] [PubMed] [Google Scholar]
- Masopust D, Choo D, Vezys V, Wherry EJ, Duraiswamy J, Akondy R, Wang J, Casey KA, Barber DL, Kawamura KS, et al. 2010. Dynamic T cell migration program provides resident memory within intestinal epithelium. J Exp Med 207: 553–564. 10.1084/jem.20090858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masson F, Calzascia T, Di Berardino-Besson W, de Tribolet N, Dietrich PY, Walker PR. 2007. Brain microenvironment promotes the final functional maturation of tumor-specific effector CD8+ T cells. J Immunol 179: 845–853. 10.4049/jimmunol.179.2.845 [DOI] [PubMed] [Google Scholar]
- McMaster SR, Wilson JJ, Wang H, Kohlmeier JE. 2015. Airway-resident memory CD8 T cells provide antigen-specific protection against respiratory virus challenge through rapid IFN-γ production. J Immunol 195: 203–209. 10.4049/jimmunol.1402975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMaster SR, Wein AN, Dunbar PR, Hayward SL, Cartwright EK, Denning TL, Kohlmeier JE. 2018. Pulmonary antigen encounter regulates the establishment of tissue-resident CD8 memory T cells in the lung airways and parenchyma. Mucosal Immunol 11: 1071–1078. 10.1038/s41385-018-0003-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara HA, Cai Y, Wagle MV, Sontani Y, Roots CM, Miosge LA, O'Connor JH, Sutton HJ, Ganusov VV, Heath WR, et al. 2017. Up-regulation of LFA-1 allows liver-resident memory T cells to patrol and remain in the hepatic sinusoids. Sci Immunol 2: eaaj1996. 10.1126/sciimmunol.aaj1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menares E, Gálvez-Cancino F, Cáceres-Morgado P, Ghorani E, López E, Díaz X, Saavedra-Almarza J, Figueroa DA, Roa E, Quezada SA, et al. 2019. Tissue-resident memory CD8+ T cells amplify anti-tumor immunity by triggering antigen spreading through dendritic cells. Nat Commun 10: 4401. 10.1038/s41467-019-12319-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner JJ, Toma C, Yu B, Zhang K, Omilusik K, Phan AT, Wang D, Getzler AJ, Nguyen T, Crotty S, et al. 2017. Runx3 programs CD8+ T cell residency in non-lymphoid tissues and tumours. Nature 552: 253–257. 10.1038/nature24993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammed J, Beura LK, Bobr A, Astry B, Chicoine B, Kashem SW, Welty NE, Igyártó BZ, Wijeyesinghe S, Thompson EA, et al. 2016. Stromal cells control the epithelial residence of DCs and memory T cells by regulated activation of TGF-β. Nat Immunol 17: 414–421. 10.1038/ni.3396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora JR, Bono MR, Manjunath N, Weninger W, Cavanagh LL, Rosemblatt M, von Andrian UH. 2003. Selective imprinting of gut-homing T cells by Peyer's patch dendritic cells. Nature 424: 88–93. 10.1038/nature01726 [DOI] [PubMed] [Google Scholar]
- Murray T, Fuertes Marraco SA, Baumgaertner P, Bordry N, Cagnon L, Donda A, Romero P, Verdeil G, Speiser DE. 2016. Very late antigen-1 marks functional tumor-resident CD8 T cells and correlates with survival of melanoma patients. Front Immunol 7: 573–573. 10.3389/fimmu.2016.00573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muruganandah V, Sathkumara HD, Navarro S, Kupz A. 2018. A systematic review: the role of resident memory T cells in infectious diseases and their relevance for vaccine development. Front Immunol 9: 1574. 10.3389/fimmu.2018.01574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muschaweckh A, Buchholz VR, Fellenzer A, Hessel C, König P-A, Tao S, Tao R, Heikenwälder M, Busch DH, Korn T, et al. 2016. Antigen-dependent competition shapes the local repertoire of tissue-resident memory CD8+ T cells. J Exp Med 213: 3075–3086. 10.1084/jem.20160888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik S, Bouladoux N, Linehan JL, Han S-J, Harrison OJ, Wilhelm C, Conlan S, Himmelfarb S, Byrd AL, Deming C, et al. 2015. Commensal–dendritic cell interaction specifies a unique protective skin immune signature. Nature 520: 104–108. 10.1038/nature14052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nizard M, Roussel H, Diniz MO, Karaki S, Tran T, Voron T, Dransart E, Sandoval F, Riquet M, Rance B, et al. 2017. Induction of resident memory T cells enhances the efficacy of cancer vaccine. Nat Commun 8: 15221–15221. 10.1038/ncomms15221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn JF, Hobbs SJ, Mooster JL, Khan TN, Kilgore AM, Harbour JC, Nolz JC. 2019. Central memory CD8+ T cells become CD69+ tissue-residents during viral skin infection independent of CD62L-mediated lymph node surveillance. PLoS Pathog 15: e1007633. 10.1371/journal.ppat.1007633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallett LJ, Davies J, Colbeck EJ, Robertson F, Hansi N, Easom NJ, Burton AR, Stegmann KA, Schurich A, Swadling L, et al. 2017. IL-2high tissue-resident T cells in the human liver: sentinels for hepatotropic infection. J Exp Med 214: 1567–1580. 10.1084/jem.20162115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y, Tian T, Park CO, Lofftus SY, Mei S, Liu X, Luo C, O'Malley JT, Gehad A, Teague JE, et al. 2017. Survival of tissue-resident memory T cells requires exogenous lipid uptake and metabolism. Nature 543: 252–256. 10.1038/nature21379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Mackay L, Gebhardt T. 2016. Distinct recirculation potential of CD69+CD103− and CD103+ thymic memory CD8+ T cells. Immunol Cell Biol 94: 975–980. 10.1038/icb.2016.60 [DOI] [PubMed] [Google Scholar]
- Park CO, Fu X, Jiang X, Pan Y, Teague JE, Collins N, Tian T, O'Malley JT, Emerson RO, Kim JH, et al. 2018a. Staged development of long-lived T-cell receptor αβ TH17 resident memory T-cell population to Candida albicans after skin infection. J Allergy Clin Immunol 142: 647–662. 10.1016/j.jaci.2017.09.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SL, Zaid A, Hor JL, Christo SN, Prier JE, Davies B, Alexandre YO, Gregory JL, Russell TA, Gebhardt T, et al. 2018b. Local proliferation maintains a stable pool of tissue-resident memory T cells after antiviral recall responses. Nat Immunol 19: 183–191. 10.1038/s41590-017-0027-5 [DOI] [PubMed] [Google Scholar]
- Park SL, Buzzai A, Rautela J, Hor JL, Hochheiser K, Effern M, McBain N, Wagner T, Edwards J, McConville R, et al. 2019. Tissue-resident memory CD8+ T cells promote melanoma–immune equilibrium in skin. Nature 565: 366–371. 10.1038/s41586-018-0812-9 [DOI] [PubMed] [Google Scholar]
- Pauls K, Schön M, Kubitza RC, Homey B, Wiesenborn A, Lehmann P, Ruzicka T, Parker CM, Schön MP. 2001. Role of integrin αE(CD103)β7 for tissue-specific epidermal localization of CD8+ T lymphocytes. J Invest Dermatol 117: 569–575. 10.1046/j.0022-202x.2001.01481.x [DOI] [PubMed] [Google Scholar]
- Peng T, Zhu J, Phasouk K, Koelle DM, Wald A, Corey L. 2012. An effector phenotype of CD8+ T cells at the junction epithelium during clinical quiescence of herpes simplex virus 2 infection. J Virol 86: 10587–10596. 10.1128/JVI.01237-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petitdemange C, Kasturi SP, Kozlowski PA, Nabi R, Quarnstrom CF, Reddy PBJ, Derdeyn CA, Spicer LM, Patel P, Legere T, et al. 2019. Vaccine induction of antibodies and tissue-resident CD8+ T cells enhances protection against mucosal SHIV-infection in young macaques. JCI Insight 4: e126047. 10.1172/jci.insight.126047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzolla A, Nguyen THO, Smith JM, Brooks AG, Kedzierska K, Heath WR, Reading PC, Wakim LM. 2017. Resident memory CD8+ T cells in the upper respiratory tract prevent pulmonary influenza virus infection. Sci Immunol 2: eaam6970. 10.1126/sciimmunol.aam6970 [DOI] [PubMed] [Google Scholar]
- Quinn E, Hawkins N, Yip Y, Suter C, Ward R. 2003. CD103+ intraepithelial lymphocytes—a unique population in microsatellite unstable sporadic colorectal cancer. Eur J Cancer 39: 469–475. 10.1016/S0959-8049(02)00633-0 [DOI] [PubMed] [Google Scholar]
- Ray SJ, Franki SN, Pierce RH, Dimitrova S, Koteliansky V, Sprague AG, Doherty PC, de Fougerolles AR, Topham DJ. 2004. The collagen binding α1β1 integrin VLA-1 regulates CD8 T cell-mediated immune protection against heterologous influenza infection. Immunity 20: 167–179. 10.1016/S1074-7613(04)00021-4 [DOI] [PubMed] [Google Scholar]
- Richmond JM, Strassner JP, Zapata L, Garg M, Riding RL, Refat MA, Fan X, Azzolino V, Tovar-Garza A, Tsurushita N, et al. 2018. Antibody blockade of IL-15 signaling has the potential to durably reverse vitiligo. Sci Transl Med 10: eaam7710. 10.1126/scitranslmed.aam7710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond JM, Strassner JP, Rashighi M, Agarwal P, Garg M, Essien KI, Pell LS, Harris JE. 2019. Resident memory and recirculating memory T cells cooperate to maintain disease in a mouse model of vitiligo. J Invest Dermatol 139: 769–778. 10.1016/j.jid.2018.10.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romagnoli PA, Fu HH, Qiu Z, Khairallah C, Pham QM, Puddington L, Khanna KM, Lefrançois L, Sheridan BS. 2017. Differentiation of distinct long-lived memory CD4 T cells in intestinal tissues after oral Listeria monocytogenes infection. Mucosal Immunol 10: 520–530. 10.1038/mi.2016.66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salerno EP, Olson WC, McSkimming C, Shea S, Slingluff CL Jr. 2014. T cells in the human metastatic melanoma microenvironment express site-specific homing receptors and retention integrins. Int J Cancer 134: 563–574. 10.1002/ijc.28391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallusto F, Lenig D, Förster R, Lipp M, Lanzavecchia A. 1999. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401: 708–712. 10.1038/44385 [DOI] [PubMed] [Google Scholar]
- Sallusto F, Geginat J, Lanzavecchia A. 2004. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol 22: 745–763. 10.1146/annurev.immunol.22.012703.104702 [DOI] [PubMed] [Google Scholar]
- Sanchez Rodriguez R, Pauli ML, Neuhaus IM, Yu SS, Arron ST, Harris HW, Yang SH-Y, Anthony BA, Sverdrup FM, Krow-Lucal E, et al. 2014. Memory regulatory T cells reside in human skin. J Clin Invest 124: 1027–1036. 10.1172/JCI72932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoval F, Terme M, Nizard M, Badoual C, Bureau M-F, Freyburger L, Clement O, Marcheteau E, Gey A, Fraisse G, et al. 2013. Mucosal imprinting of vaccine-induced CD8+ T cells is crucial to inhibit the growth of mucosal tumors. Sci Transl Med 5: 172ra20. 10.1126/scitranslmed.3004888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki K, Bean A, Shah S, Schutten E, Huseby PG, Peters B, Shen ZT, Vanguri V, Liggitt D, Huseby ES. 2014. Relapsing–remitting central nervous system autoimmunity mediated by GFAP-specific CD8 T cells. J Immunol 192: 3029–3042. 10.4049/jimmunol.1302911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathaliyawala T, Kubota M, Yudanin N, Turner D, Camp P, Thome JJC, Bickham Kara L, Lerner H, Goldstein M, Sykes M, et al. 2013. Distribution and compartmentalization of human circulating and tissue-resident memory T cell subsets. Immunity 38: 187–197. 10.1016/j.immuni.2012.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savas P, Virassamy B, Ye C, Salim A, Mintoff CP, Caramia F, Salgado R, Byrne DJ, Teo ZL, Dushyanthen S, et al. 2018. Single-cell profiling of breast cancer T cells reveals a tissue-resident memory subset associated with improved prognosis. Nat Med 24: 986–993. 10.1038/s41591-018-0078-7 [DOI] [PubMed] [Google Scholar]
- Schaerli P, Ebert L, Willimann K, Blaser A, Roos RS, Loetscher P, Moser B. 2004. A skin-selective homing mechanism for human immune surveillance T cells. J Exp Med 199: 1265–1275. 10.1084/jem.20032177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenkel JM, Fraser KA, Vezys V, Masopust D. 2013. Sensing and alarm function of resident memory CD8+ T cells. Nat Immunol 14: 509–513. 10.1038/ni.2568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenkel J, Fraser K, Beura L, Pauken K, Vezys V, Masopust D. 2014a. T cell memory. Resident memory CD8 T cells trigger protective innate and adaptive immune responses. Science 346: 98–101. 10.1126/science.1254536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenkel JM, Fraser KA, Masopust D. 2014b. Cutting edge: resident memory CD8 T cells occupy frontline niches in secondary lymphoid organs. J Immunol 192: 2961–2964. 10.4049/jimmunol.1400003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenkel JM, Fraser KA, Casey KA, Beura LK, Pauken KE, Vezys V, Masopust D. 2016. IL-15–independent maintenance of tissue-resident and boosted effector memory CD8 T cells. J Immunol 196: 3920–3926. 10.4049/jimmunol.1502337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffer JT, Abu-Raddad L, Mark KE, Zhu J, Selke S, Koelle DM, Wald A, Corey L. 2010. Mucosal host immune response predicts the severity and duration of herpes simplex virus-2 genital tract shedding episodes. Proc Natl Acad Sci 107: 18973–18978. 10.1073/pnas.1006614107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzkopff S, Woyciechowski S, Aichele U, Flecken T, Zhang N, Thimme R, Pircher H. 2015. TGF-β downregulates KLRG1 expression in mouse and human CD8+ T cells. Eur J Immunol 45: 2212–2217. 10.1002/eji.201545634 [DOI] [PubMed] [Google Scholar]
- Sheridan BS, Pham QM, Lee YT, Cauley LS, Puddington L, Lefrançois L. 2014. Oral infection drives a distinct population of intestinal resident memory CD8+ T cells with enhanced protective function. Immunity 40: 747–757. 10.1016/j.immuni.2014.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin H, Iwasaki A. 2012. A vaccine strategy that protects against genital herpes by establishing local memory T cells. Nature 491: 463–467. 10.1038/nature11522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin H, Kumamoto Y, Gopinath S, Iwasaki A. 2016. CD301b+ dendritic cells stimulate tissue-resident memory CD8+ T cells to protect against genital HSV-2. Nat Commun 7: 13346. 10.1038/ncomms13346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shwetank, Abdelsamed HA, Frost EL, Schmitz HM, Mockus TE, Youngblood BA, Lukacher AE. 2017. Maintenance of PD-1 on brain-resident memory CD8 T cells is antigen independent. Immunol Cell Biol 95: 953-959. 10.1038/icb.2017.62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui I, Schaeuble K, Chennupati V, Marraco SAF, Calderon-Copete S, Ferreira DP, Carmona SJ, Scarpellino L, Gfeller D, Pradervand S, et al. 2019. Intratumoral Tcf1+PD-1+CD8+ T cells with stem-like properties promote tumor control in response to vaccination and checkpoint blockade immunotherapy. Immunity 50: 195–211.e10. 10.1016/j.immuni.2018.12.021 [DOI] [PubMed] [Google Scholar]
- Simoni Y, Becht E, Fehlings M, Loh CY, Koo S-L, Teng KWW, Yeong JPS, Nahar R, Zhang T, Kared H, et al. 2018. Bystander CD8+ T cells are abundant and phenotypically distinct in human tumour infiltrates. Nature 557: 575–579. 10.1038/s41586-018-0130-2 [DOI] [PubMed] [Google Scholar]
- Skon CN, Lee JY, Anderson KG, Masopust D, Hogquist KA, Jameson SC. 2013. Transcriptional downregulation of S1pr1 is required for the establishment of resident memory CD8+ T cells. Nat Immunol 14: 1285–1293. 10.1038/ni.2745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slütter B, Van Braeckel-Budimir N, Abboud G, Varga SM, Salek-Ardakani S, Harty JT. 2017. Dynamics of influenza-induced lung-resident memory T cells underlie waning heterosubtypic immunity. Sci Immunol 2: eaag2031. 10.1126/sciimmunol.aag2031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CJ, Caldeira-Dantas S, Turula H, Snyder CM. 2015. Murine CMV infection induces the continuous production of mucosal resident T cells. Cell Rep 13: 1137–1148. 10.1016/j.celrep.2015.09.076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith NM, Wasserman GA, Coleman FT, Hilliard KL, Yamamoto K, Lipsitz E, Malley R, Dooms H, Jones MR, Quinton LJ, et al. 2018. Regionally compartmentalized resident memory T cells mediate naturally acquired protection against pneumococcal pneumonia. Mucosal Immunol 11: 220–235. 10.1038/mi.2017.43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder ME, Finlayson MO, Connors TJ, Dogra P, Senda T, Bush E, Carpenter D, Marboe C, Benvenuto L, Shah L, et al. 2019. Generation and persistence of human tissue-resident memory T cells in lung transplantation. Sci Immunol 4: eaav5581. 10.1126/sciimmunol.aav5581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon B, Young RJ, Bressel M, Cernelc J, Savas P, Liu H, Urban D, Thai A, Cooper C, Fua T, et al. 2019. Identification of an excellent prognosis subset of human papillomavirus-associated oropharyngeal cancer patients by quantification of intratumoral CD103+ immune cell abundance. Ann Oncol 30: 1638–1646. 10.1093/annonc/mdz271 [DOI] [PubMed] [Google Scholar]
- Stark R, Wesselink TH, Behr FM, Kragten NAM, Arens R, Koch-Nolte F, van Gisbergen KPJM, van Lier RAW. 2018. TRM maintenance is regulated by tissue damage via P2RX7. Sci Immunol 3: eaau1022. 10.1126/sciimmunol.aau1022 [DOI] [PubMed] [Google Scholar]
- Steinbach K, Vincenti I, Kreutzfeldt M, Page N, Muschaweckh A, Wagner I, Drexler I, Pinschewer D, Korn T, Merkler D. 2016. Brain-resident memory T cells represent an autonomous cytotoxic barrier to viral infection. J Exp Med 213: 1571–1587. 10.1084/jem.20151916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbach K, Vincenti I, Egervari K, Kreutzfeldt M, van der Meer F, Page N, Klimek B, Rossitto-Borlat I, Di Liberto G, Muschaweckh A, et al. 2019. Brain-resident memory T cells generated early in life predispose to autoimmune disease in mice. Sci Transl Med 11: eaav5519. 10.1126/scitranslmed.aav5519 [DOI] [PubMed] [Google Scholar]
- Steinert E, Schenkel J, Fraser K, Beura L, Manlove L, Igyártó B, Southern P, Masopust D. 2015. Quantifying memory CD8+ T cells reveals regionalization of immunosurveillance. Cell 161: 737–749. 10.1016/j.cell.2015.03.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelma F, de Niet A, Sinnige MJ, van Dort KA, van Gisbergen KP, Verheij J, van Leeuwen EM, Kootstra NA, Reesink HW. 2017. Human intrahepatic CD69+CD8+ T cells have a tissue resident memory T cell phenotype with reduced cytolytic capacity. Sci Rep 7: 6172. 10.1038/s41598-017-06352-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strutt TM, Dhume K, Finn CM, Hwang JH, Castonguay C, Swain SL, McKinstry KK. 2018. IL-15 supports the generation of protective lung-resident memory CD4 T cells. Mucosal Immunol 11: 668–680. 10.1038/mi.2017.101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamura S, Yagi H, Hakata Y, Motozono C, McMaster SR, Masumoto T, Fujisawa M, Chikaishi T, Komeda J, Itoh J, et al. 2016. Specific niches for lung-resident memory CD8+ T cells at the site of tissue regeneration enable CD69-independent maintenance. J Exp Med 213: 3057–3073. 10.1084/jem.20160938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamura S, Kato S, Motozono C, Shimaoka T, Ueha S, Matsuo K, Miyauchi K, Masumoto T, Katsushima A, Nakayama T, et al. 2019. Interstitial-resident memory CD8+ T cells sustain frontline epithelial memory in the lung. J Exp Med 216: 2736–2747. 10.1084/jem.20190557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teijaro JR, Turner D, Pham Q, Wherry EJ, Lefrançois L, Farber DL. 2011. Cutting edge: tissue-retentive lung memory CD4 T cells mediate optimal protection to respiratory virus infection. J Immunol 187: 5510–5514. 10.4049/jimmunol.1102243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thom JT, Weber TC, Walton SM, Torti N, Oxenius A. 2015. The salivary gland acts as a sink for tissue-resident memory CD8+ T cells, facilitating protection from local cytomegalovirus infection. Cell Rep 13: 1125–1136. 10.1016/j.celrep.2015.09.082 [DOI] [PubMed] [Google Scholar]
- Thome JJC, Yudanin N, Ohmura Y, Kubota M, Grinshpun B, Sathaliyawala T, Kato T, Lerner H, Shen Y, Farber DL. 2014. Spatial map of human T cell compartmentalization and maintenance over decades of life. Cell 159: 814–828. 10.1016/j.cell.2014.10.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson EA, Darrah PA, Foulds KE, Hoffer E, Caffrey-Carr A, Norenstedt S, Perbeck L, Seder RA, Kedl RM, Loré K. 2019a. Monocytes acquire the ability to prime tissue-resident T cells via IL-10-mediated TGF-β release. Cell Rep 28: 1127–1135.e4. 10.1016/j.celrep.2019.06.087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson EA, Mitchell JS, Beura LK, Torres DJ, Mrass P, Pierson MJ, Cannon JL, Masopust D, Fife BT, Vezys V. 2019b. Interstitial migration of CD8αβ T cells in the small intestine is dynamic and is dictated by environmental cues. Cell Rep 26: 2859–2867.e4. 10.1016/j.celrep.2019.02.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomov VT, Palko O, Lau CW, Pattekar A, Sun Y, Tacheva R, Bengsch B, Manne S, Cosma GL, Eisenlohr LC, et al. 2017. Differentiation and protective capacity of virus-specific CD8+ T cells suggest murine norovirus persistence in an immune-privileged enteric niche. Immunity 47: 723–738.e5. 10.1016/j.immuni.2017.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse SW, Radtke AJ, Espinosa DA, Cockburn IA, Zavala F. 2014. The chemokine receptor CXCR6 is required for the maintenance of liver memory CD8+ T cells specific for infectious pathogens. J Infect Dis 210: 1508–1516. 10.1093/infdis/jiu281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner DL, Goldklang M, Cvetkovski F, Paik D, Trischler J, Barahona J, Cao M, Dave R, Tanna N, D'Armiento JM, et al. 2018. Biased generation and in situ activation of lung tissue-resident memory CD4 T cells in the pathogenesis of allergic asthma. J Immunol 200: 1561–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Braeckel-Budimir N, Varga SM, Badovinac VP, Harty JT. 2018. Repeated antigen exposure extends the durability of influenza-specific lung-resident memory CD8+ T cells and heterosubtypic immunity. Cell Rep 24: 3374–3382.e3. 10.1016/j.celrep.2018.08.073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakim LM, Woodward-Davis A, Bevan MJ. 2010. Memory T cells persisting within the brain after local infection show functional adaptations to their tissue of residence. Proc Natl Acad Sci 107: 17872–17879. 10.1073/pnas.1010201107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakim LM, Woodward-Davis A, Liu R, Hu Y, Villadangos J, Smyth G, Bevan MJ. 2012. The molecular signature of tissue resident memory CD8 T cells isolated from the brain. J Immunol 189: 3462–3471. 10.4049/jimmunol.1201305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakim LM, Smith J, Caminschi I, Lahoud MH, Villadangos JA. 2015. Antibody-targeted vaccination to lung dendritic cells generates tissue-resident memory CD8 T cells that are highly protective against influenza virus infection. Mucosal Immunol 8: 1060–1071. 10.1038/mi.2014.133 [DOI] [PubMed] [Google Scholar]
- Walsh DA, Borges da Silva H, Beura LK, Peng C, Hamilton SE, Masopust D, Jameson SC. 2019. The functional requirement for CD69 in establishment of resident memory CD8+ T cells varies with tissue location. J Immunol 203: 946–955. 10.4049/jimmunol.1900052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Wu S, Zeng H, Liu Z, Dong W, He W, Chen X, Dong X, Zheng L, Lin T, et al. 2015. CD103+ tumor infiltrating lymphocytes predict a favorable prognosis in urothelial cell carcinoma of the bladder. J Urol 194: 556–562. 10.1016/j.juro.2015.02.2941 [DOI] [PubMed] [Google Scholar]
- Wang ZQ, Milne K, Derocher H, Webb JR, Nelson BH, Watson PH. 2016. CD103 and intratumoral immune response in breast cancer. Clin Cancer Res 22: 6290–6297. 10.1158/1078-0432.CCR-16-0732 [DOI] [PubMed] [Google Scholar]
- Wang Z, Wang S, Goplen NP, Li C, Cheon IS, Dai Q, Huang S, Shan J, Ma C, Ye Z, et al. 2019. PD-1hi CD8+ resident memory T cells balance immunity and fibrotic sequelae. Sci Immunol 4: eaaw1217. 10.1126/sciimmunol.aaw1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe R, Gehad A, Yang C, Scott LL, Teague JE, Schlapbach C, Elco CP, Huang V, Matos TR, Kupper TS, et al. 2015. Human skin is protected by four functionally and phenotypically discrete populations of resident and recirculating memory T cells. Sci Transl Med 7: 279ra39. 10.1126/scitranslmed.3010302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb JR, Wick DA, Nielsen JS, Tran E, Milne K, McMurtrie E, Nelson BH. 2010. Profound elevation of CD8+ T cells expressing the intraepithelial lymphocyte marker CD103 (αE/β7 integrin) in high-grade serous ovarian cancer. Gynecol Oncol 118: 228–236. 10.1016/j.ygyno.2010.05.016 [DOI] [PubMed] [Google Scholar]
- Webb JR, Milne K, Watson P, deLeeuw RJ, Nelson BH. 2014. Tumor-infiltrating lymphocytes expressing the tissue resident memory marker CD103 are associated with increased survival in high-grade serous ovarian cancer. Clin Cancer Res 20: 434–444. 10.1158/1078-0432.CCR-13-1877 [DOI] [PubMed] [Google Scholar]
- Wei SC, Duffy CR, Allison JP. 2018. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov 8: 1069–1086. 10.1158/2159-8290.CD-18-0367 [DOI] [PubMed] [Google Scholar]
- Wein AN, McMaster SR, Takamura S, Dunbar PR, Cartwright EK, Hayward SL, McManus DT, Shimaoka T, Ueha S, Tsukui T, et al. 2019. CXCR6 regulates localization of tissue-resident memory CD8 T cells to the airways. J Exp Med 216: 2748–2762. 10.1084/jem.20181308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong MT, Ong DEH, Lim FSH, Teng KWW, McGovern N, Narayanan S, Ho WQ, Cerny D, Tan HKK, Anicete R, et al. 2016. A high-dimensional atlas of human T cell diversity reveals tissue-specific trafficking and cytokine signatures. Immunity 45: 442–456. 10.1016/j.immuni.2016.07.007 [DOI] [PubMed] [Google Scholar]
- Woodberry T, Suscovich TJ, Henry LM, Davis JK, Frahm N, Walker BD, Scadden DT, Wang F, Brander C. 2005. Differential targeting and shifts in the immunodominance of Epstein–Barr virus-specific CD8 and CD4 T cell responses during acute and persistent infection. J Infect Dis 192: 1513–1524. 10.1086/491741 [DOI] [PubMed] [Google Scholar]
- Woon HG, Braun A, Li J, Smith C, Edwards J, Sierro F, Feng CG, Khanna R, Elliot M, Bell A, et al. 2016. Compartmentalization of total and virus-specific tissue-resident memory CD8+ T cells in human lymphoid organs. PLoS Pathog 12: e1005799. 10.1371/journal.ppat.1005799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Workel HH, Komdeur FL, Wouters MC, Plat A, Klip HG, Eggink FA, Wisman GBA, Arts HJ, Oonk MH, Mourits MJ, et al. 2016. CD103 defines intraepithelial CD8+PD1+ tumour-infiltrating lymphocytes of prognostic significance in endometrial adenocarcinoma. Eur J Cancer 60: 1–11. 10.1016/j.ejca.2016.02.026 [DOI] [PubMed] [Google Scholar]
- Wu T, Hu Y, Lee YT, Bouchard KR, Benechet A, Khanna K, Cauley LS. 2014. Lung-resident memory CD8 T cells (TRM) are indispensable for optimal cross-protection against pulmonary virus infection. J Leukoc Biol 95: 215–224. 10.1189/jlb.0313180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaid A, Mackay LK, Rahimpour A, Braun A, Veldhoen M, Carbone FR, Manton JH, Heath WR, Mueller SN. 2014. Persistence of skin-resident memory T cells within an epidermal niche. Proc Natl Acad Sci 111: 5307–5312. 10.1073/pnas.1322292111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaid A, Hor JL, Christo SN, Groom JR, Heath WR, Mackay LK, Mueller SN. 2017. Chemokine receptor–dependent control of skin tissue–resident memory T cell formation. J Immunol 199: 2451–2459. 10.4049/jimmunol.1700571 [DOI] [PubMed] [Google Scholar]
- Zens KD, Chen JK, Farber DL. 2016. Vaccine-generated lung tissue-resident memory T cells provide heterosubtypic protection to influenza infection. JCI Insight 1: e85832. 10.1172/jci.insight.85832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Bevan MJ. 2013. Transforming growth factor-β signaling controls the formation and maintenance of gut-resident memory T cells by regulating migration and retention. Immunity 39: 687–696. 10.1016/j.immuni.2013.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Yu X, Zheng L, Zhang Y, Li Y, Fang Q, Gao R, Kang B, Zhang Q, Huang JY. 2018. Lineage tracking reveals dynamic relationships of T cells in colorectal cancer. Nature 564: 268–272. 10.1038/s41586-018-0694-x [DOI] [PubMed] [Google Scholar]
- Zhu J, Koelle DM, Cao J, Vazquez J, Huang ML, Hladik F, Wald A, Corey L. 2007. Virus-specific CD8+ T cells accumulate near sensory nerve endings in genital skin during subclinical HSV-2 reactivation. J Exp Med 204: 595–603. 10.1084/jem.20061792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Peng T, Johnston C, Phasouk K, Kask AS, Klock A, Jin L, Diem K, Koelle DM, Wald A, et al. 2013. Immune surveillance by CD8αα+ skin-resident T cells in human herpes virus infection. Nature 497: 494–497. 10.1038/nature12110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuber J, Shonts B, Lau SP, Obradovic A, Fu J, Yang S, Lambert M, Coley S, Weiner J, Thome J, et al. 2016. Bidirectional intragraft alloreactivity drives the repopulation of human intestinal allografts and correlates with clinical outcome. Sci Immunol 1: eaah3732. 10.1126/sciimmunol.aah3732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zundler S, Becker E, Spocinska M, Slawik M, Parga-Vidal L, Stark R, Wiendl M, Atreya R, Rath T, Leppkes M, et al. 2019. Hobit- and Blimp-1-driven CD4+ tissue-resident memory T cells control chronic intestinal inflammation. Nat Immunol 20: 288–300. 10.1038/s41590-018-0298-5 [DOI] [PubMed] [Google Scholar]