Abstract
Polyploidy is defined as a cell with three or more whole genome sets and enables cell growth across the kingdoms of life. Studies in model organisms have revealed that polyploid cell growth can be required for optimal tissue repair and regeneration. In mammals, polyploid cell growth contributes to repair of many tissues, including the liver, heart, kidney, bladder, and eye, and similar strategies have been identified in Drosophila and zebrafish tissues. This review discusses the heterogeneity and versatility of polyploidy in tissue repair and regeneration. Polyploidy has been shown to restore tissue mass and maintain organ size as well as protect against oncogenic insults and genotoxic stress. Polyploid cells can also serve as a reservoir for new diploid cells in regeneration. The numerous mechanisms to generate polyploid cells provide an unlimited resource for tissues to exploit to undergo repair or regeneration.
Throughout the course of an animal's life, its cells need to be replaced due to damage caused by injury, aging, and disease. Tissue repair and regeneration are the two main strategies to compensate for cell loss. Regeneration replicates the missing cells to restore the exact tissue, organ, or limb without scar formation (Goldman and Poss 2020). Whereas tissue repair heals the wound and compensates for cell loss albeit often with scar formation and changes in cellular organization (Gjelsvik et al. 2019; Lazzeri et al. 2019). Cells are then generated by either cell division or cell growth, where cell size increases with a corresponding increase in ploidy (DNA content, C-value). A cell with more than the diploid copy of its genome (>3C) is referred to as a polyploid cell.
Polyploid cell growth is an integral part of development and homeostasis across multiple organisms (Edgar et al. 2014; Orr-Weaver 2015). While polyploidy is commonplace among plants and insects, it is now recognized that vertebrates possess many somatic polyploid cell types in numerous tissues, including within the placenta, muscle, heart, liver, pancreas, bladder, kidney, and eye (Edgar et al. 2014; Gjelsvik et al. 2019). This is in part because of the many advances in cell imaging and genomics in the last decade. Developmentally programmed polyploidy is recognized to be an essential step in many organisms’ cellular differentiation programs and has been extensively reviewed (Edgar et al. 2014; Schoenfelder and Fox 2015; Fox et al. 2020). Here, we focus on the emerging and diverse roles of polyploidy in tissue repair and regeneration. It is now recognized that polyploidization in some cases is desired, or even necessary, to replace lost cells when cell proliferation is limited. This review will focus on the conserved signals that give rise to polyploid cells in tissue repair and regeneration as well as their long-term role in tissue homeostasis and disease progression.
THE STRATEGIES TO GENERATE A POLYPLOID CELL
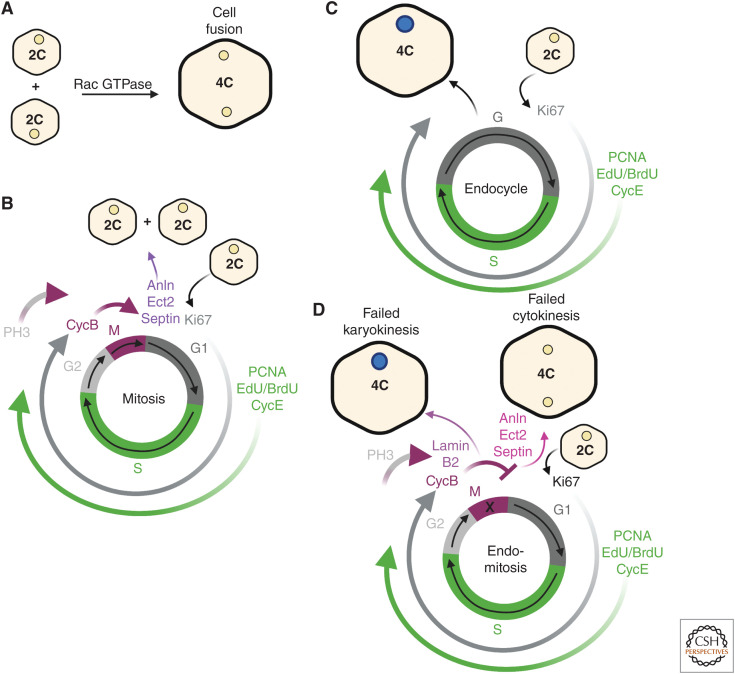
Polyploid cells can be generated by cell-cycle-dependent and independent mechanisms. Cell fusion occurs independent of cell cycle and involves two or more cells fusing together to form a multinucleated cell, known as a syncytium (Fig. 1A). The most well-studied examples of cell fusion occur in skeletal muscle development and regeneration in mice and fruit fly larvae (Lee and Chen 2019; Lehka and Rędowicz 2020). Muscle cell–cell fusion in Drosophila and mice requires conserved genes that are necessary for remodeling the cytoskeletal network and exerting mechanical forces to facilitate invasion and fusion of the founder cell (fruit fly) or stem cell (mice) with the developing or regenerating myotube. Other mammalian tissues require cell fusion for development including osteoclasts in the bone and syncytiotrophoblasts in the placenta, but the role of cell fusion in tissue repair or regeneration has yet to be determined.
Figure 1.
Polyploid cells are generated by cell fusion and endoreplication. (A) Cell fusion generates a polyploid syncytium when individual cells fuse together. The syncytium's cellular ploidy (C) equals the sum of its nuclear ploidy. This process is mediated by cytoskeletal remodeling proteins, including Rac GTPase in both epithelial and muscle cell types. (B) Mitosis produces two identical daughter cells with 2C ploidy after completing cytokinesis. (C) An endocycle produces a polyploid cell by bypassing M phase and moving between G and S phases only. This generates a daughter cell with double the DNA content as the parent cell. Diploid nuclei (beige) and ≥4C polyploid nuclei (dark blue). (D) Endomitosis results in polyploid cells by failed karyokinesis (nuclear division) generating a mononucleated, polyploid daughter cell with double the ploidy or failed cytokinesis (cell division) creating a binucleated 4C polyploid daughter cell with two diploid nuclei. Cells may use one or more of these processes to form polyploid cells in tissue repair and regeneration. (This figure was created with BioRender.com.)
In Drosophila, cell fusion is dispensable for epithelial development, but required, in part, for wound repair in all postembryonic stages of epithelial development (Galko and Krasnow 2004; Losick et al. 2013; Wang et al. 2015). Needle puncture, pinch, or laser wounds induce epithelial cell–cell fusion. In the adult fruit fly, epithelial cell fusion is dependent on Rac GTPase, which is also required for muscle cell fusion and speeds epithelial wound closure (Fig. 1A; Fernandes et al. 2005; Losick et al. 2013). In development, the fly rectal papilla was found to become multinucleated by a novel Rab5 and Rab11 GTPase-dependent remodeling of the cell apical membranes (Peterson et al. 2020). Overall, it is difficult to detect cell fusion events and there are still many unknowns to how multinucleated cells are generated and contribute to tissue repair.
More often, polyploid cells are generated by endoreplication, an incomplete cell cycle encompassing both the endocycle and endomitosis (Øvrebø and Edgar 2018; Gjelsvik et al. 2019). The endocycle increases nuclear ploidy by allowing cells to bypass M phase of the mitotic cell cycle through elevated expression of an E3 ubiquitin ligase activator, Fizzy-related (Fzr) (Fig. 1B,C; Cohen et al. 2018; Grendler et al. 2019). Fzr, known as Cdh1 in humans, activates the anaphase-promoting complex ubiquitin ligase to mediate the degradation of the M phase CDK components cyclin A and cyclin B (Sigrist and Lehner 1997). In flies, the down-regulation of cyclin A is critical for entry into endocycle, whereas mammals also require the atypical E2F7 and E2F8 repressors to reduce M phase gene expression (Chen et al. 2012; Pandit et al. 2012; Rotelli et al. 2019). The endocycle consists of only G-S phases, which are regulated by oscillations of CycE/Cdk2 (Zielke et al. 2011). Sequential endocycles will then double the cell's nuclear genome content leading to increasing nuclear ploidy (i.e., 4C, 8C, 16C, etc.).
Endomitosis occurs when cells fail to complete either karyokinesis (nuclear division) or cytokinesis (cellular division) (Fig. 1D). Failed cytokinesis occurs when there is a block in chromosome segregation or furrow formation resulting in a binucleated or multinucleated, polyploid cell. Failed cytokinesis has been shown to generate binucleated cells in the liver, heart, and bladder in response to injury and is dependent on the cytokinesis regulators, including Anln, Ect2, and Septin (González-Rosa et al. 2018; Wang et al. 2018; Jiang et al. 2019; Derks and Bergmann 2020; Donne et al. 2020). However, failed karyokinesis will result in a mononucleated polyploid cell, which enters M phase (labeled by P-Histone 3 or CycB), unlike the mononucleated polyploid cells generated by the endocycle, which do not express any mitotic markers (Fig. 1C,D). Lamin B2 was recently shown to be required for cardiomyocyte cell-cycle progression, as its genetic loss led to karyokinesis failure during heart development and regeneration (Han et al. 2020). As will be discussed, any one of these mechanisms can occur simultaneously or in sequence, adding to the complexity in how polyploid cells are generated in tissue repair and regeneration (Fig. 2).
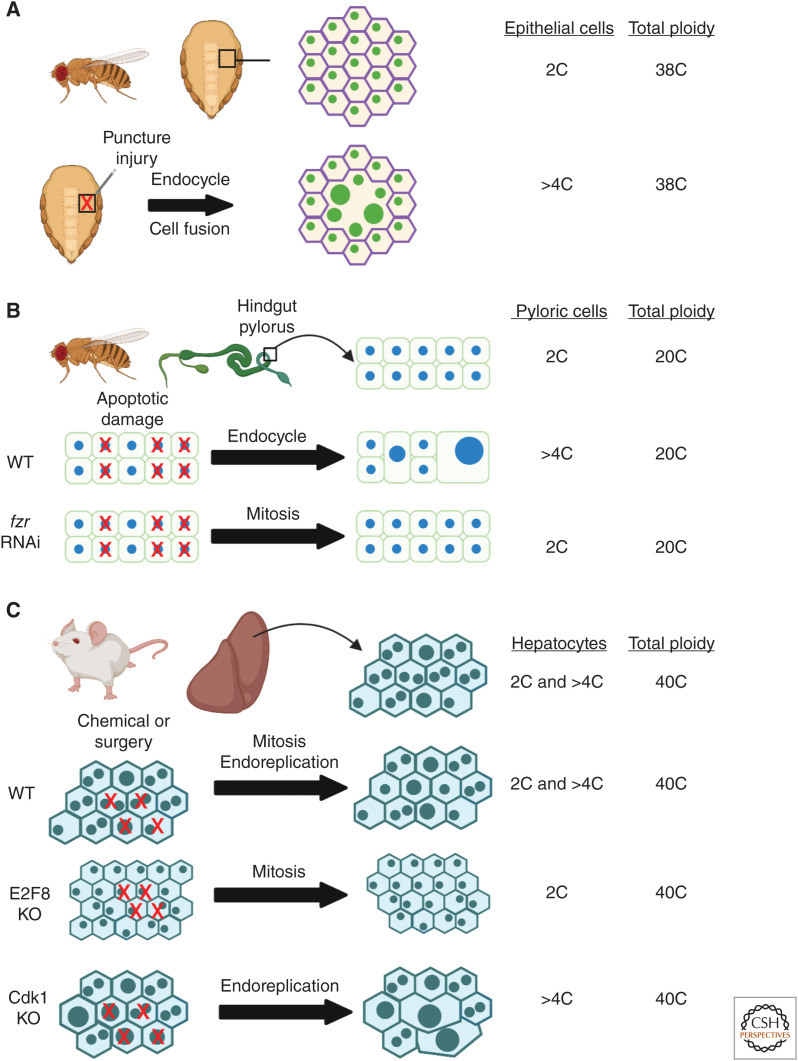
Figure 2.
Polyploid cell growth restores tissue mass and maintains organ size in fruit fly tissues and in mouse liver. Examples of tissues in the fruit fly and mouse where polyploidy has been demonstrated to precisely restore tissue mass. (A) Epithelial cells underlying the abdomen cuticle in the adult fruit fly heal a needle puncture wound by the endocycle and cell fusion. The endocycle boosts epithelial ploidy to >4C and cell fusion speed wound closure (Losick et al. 2013). (B) The hindgut pylorus region of the fruit fly intestine is made of diploid cells that endocycle to repair genetically induced apoptotic cell damage (Cohen et al. 2018). Pyloric cells can be switched to a mitotic cell cycle by knocking down frz to restore organ size. (C) Mammalian liver regeneration relies on a combination of diploid and polyploid division as well as endoreplication of its hepatocytes (Wilkinson et al. 2018; Zhang et al. 2018; Lin et al. 2020). The mouse liver is able to regrow and restore organ size by solely relying on diploid hepatocyte division or polyploid cell growth, as observed in Cdk1 and E2F8 mouse mutants (Diril et al. 2012; Pandit et al. 2012). Total tissue ploidy equals number of cells times each cell's ploidy. (This figure was created with BioRender.com.)
GROWING BIGGER CELLS TO RESTORE TISSUE MASS AND MAINTAIN ORGAN SIZE
The regulation of organ size (mass) is essential to generate a functional and correctly proportioned tissue for both tissue growth and regeneration. An organ's size is determined by the number of its cells and their sizes. Cell proliferation will increase cell number, whereas polyploidization will boost cell size (Fig. 1). Therefore, after injury, tissue mass can be restored by either cell proliferation or polyploidization or more often a combination of both.
In the fruit fly, polyploid cells arise in response to injury or genetically induced damage in many somatic tissues including ovarian follicle cells, hindgut pyloric cells, abdominal epithelial cells, and intestinal enterocytes (Losick et al. 2013; Tamori and Deng 2013; Xiang et al. 2017; Cohen et al. 2018). Likewise, mouse hepatocytes in the liver, tubule epithelial cells in the kidney, urothelial cells in the bladder, and epicardial cells in the zebrafish heart become polyploid or boost ploidy in response to injury (Cao et al. 2017; Lazzeri et al. 2018; Wang et al. 2018; Wilkinson et al. 2018; Zhang et al. 2018). In most of these examples, the polyploid cell growth acts to compensate for cell loss and restore tissue mass as polyploidization enables the cells to grow bigger to maintain total tissue ploidy. This is most evident in the fruit fly and mouse liver, where studies have been able to rigorously measure ploidy, cell number, and/or organ size (Fig. 2).
In the fly adult abdominal epithelium, a puncture wound destroys roughly 100 cells and in turn the surviving cells adjacent to the wound site endocycle and fuse (Fig. 2A; Losick et al. 2013). Remarkably, the epithelial cells were found to boost their ploidy to precisely compensate for the cell loss (Losick et al. 2016). The Hippo-Yorkie signal transduction pathway tunes the extent of endocycling in fly abdominal epithelium in response to injury. The Yorkie transcription factor regulates entry into the endocycle by transcriptional control of G-S phase regulators: Myc, E2F1, and cycE (Grendler et al. 2019). In fly hindgut pylorus, conditional expression of apoptotic genes caused cell loss and a corresponding increase in cell ploidy via the endocycle, which is essential to maintain an epithelial barrier in this organ (Fig. 2B; Fox and Spradling 2009; Sawyer et al. 2017; Cohen et al. 2018). The pyloric cells can be genetically forced to divide by knocking down fzr, which switches the endocycle into a mitotic cell cycle (Fig. 1B,C; Cohen et al. 2018). Remarkably, the pylorus can still regrow after injury to its normal size via cell proliferation instead of polyploid cell growth. However, this sensitizes the pylorus to oncogenic growth and epithelial barrier permeability when a constitutively active Ras GTPase is expressed. Similarly, the mammalian liver relies on the ability of its hepatocytes to polyploidize, as well as divide as either diploid or polyploid cells (Fig. 2C; Duncan et al. 2010; Wilkinson et al. 2018; Zhang et al. 2018). Hepatocyte cell growth was found to precede hepatocyte division following surgical removal of the liver mass (Miyaoka et al. 2012). However, polyploid cells are less efficient at cell division and acute injury or chronic liver injury is mostly repaired by the proliferation of the diploid hepatocytes (Wilkinson et al. 2018; Zhang et al. 2018; Chen et al. 2020).
The hepatocyte ploidy state has been investigated by genetically inhibiting the generation of polyploid or diploid cells in the liver. Genetic loss of cell-cycle regulators, including Cdk1, Skp2, and p53, will cause cells to bypass M phase and default into an endocycle (Figs. 1 and 2C; Minamishima et al. 2002; Diril et al. 2012; Kurinna et al. 2013; Sladky et al. 2020). Likewise, conditional knockout of atypical E2F7 and/or E2F8, which are required for endoreplication in mammals, will prevent generation of polyploid cells (Chen et al. 2012; Pandit et al. 2012). In so doing, it was strikingly discovered that hepatocyte polyploidization or diploid cell proliferation on its own is sufficient to grow and regenerate the liver (Fig. 2C). The decrease in the mouse liver's total cell number and corresponding increase in cell size still allowed the liver to develop or regrow to its normal organ size. Some studies have found that the more polyploid livers were able to function normally without significantly comprising its detoxifying capacity (Fig. 2C; Lin et al. 2020; Sladky et al. 2020). Whereas others observed that enhanced polyploidy in the liver led to a disease-like state characterized by inflammation, fibrosis, and dysregulation of lipid metabolism (Dewhurst et al. 2020; Ow et al. 2020). The difference may lie in the genetic perturbation used to enrich for the polyploid hepatocytes, so further studies are necessary to determine long-term impact of polyploidy on liver function.
Polyploidy is often associated with disease states; however, similar to the Drosophila pylorus, polyploid hepatocytes were found to protect against oncogenic growth. Livers genetically devoid of polyploid cells, by conditionally knocking out E2F7/8, more frequently developed tumors compared with wild-type or Anln mutant enriched for polyploid hepatocytes (Wilkinson et al. 2018; Zhang et al. 2018; Lin et al. 2020). In particular, polyploidy buffered against tumor suppressor mutations, but was equally susceptible to oncogenic activation. The extra gene copies in a polyploid cell acts as a backup to protect against loss of heterozygosity. Overall, polyploid cell growth offers a means to regrow tissue mass and restore organ size, while providing additional protection against tumorigenic insults.
POLYPLOID HETEROGENEITY AND VERSATILITY
Tissue repair and regeneration often rely on a combination of cell division and polyploid cell growth as observed in the mammalian liver. Depending on the animal and its tissue, polyploidy alone or in combination with diploid division can be necessary for optimal tissue repair. This is the case for the Drosophila midgut, which relies on both resident stem cells and polyploid enterocytes for homeostasis and regeneration. The physiological demands of the fly midgut have been found to dictate the cellular mode of repair. InR, PI3K, and Tor signaling stimulate stem cell division and enterocytes to endocycle during homeostasis, whereas tissue damage activates EGFR, Ras GTPase, and MAPK signaling to boost enterocytes ploidy (Xiang et al. 2017). Under conditions of severe stem cell loss caused by starvation, the polyploid enterocytes can then be a source for the generation of new intestinal stem cells. In these rare instances, the immediate stem cell daughter, a 4C polyploid pre-enterocyte, was found to undergo a reductive cell division known as amitosis. Amitosis occurred independently of the mitotic spindle to generate two diploid daughter cells that restored the intestinal stem cell pool (Fig. 3; Lucchetta and Ohlstein 2017). Reductive polyploid divisions, via multipolar spindle formation, have also been observed during mouse liver growth, regeneration, and cancer (Duncan et al. 2010; Wilkinson et al. 2018; Matsumoto et al. 2021). Therefore, amitosis and/or reductive cell division may offer a means to resupply the organ with a diploid cell pool that has more proliferative potential.
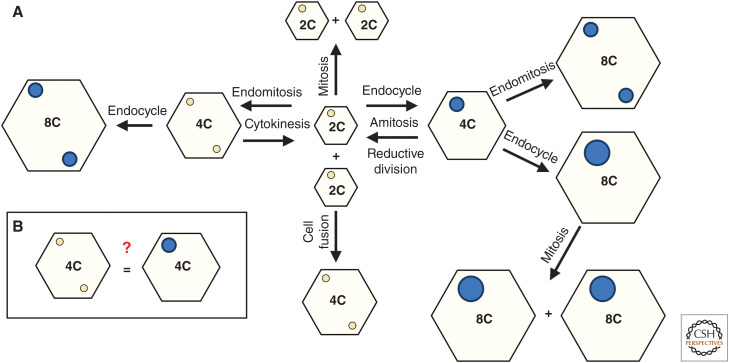
Figure 3.
Polyploidy heterogeneity: equal or not? (A) Illustration of many cellular mechanisms in which polyploid cells are generated and in turn generate diploid cells by amitosis, reductive division, or cytokinesis. Polyploid cells can also continue to grow increasing ploidy and cell size or mitotically divide as polyploid. (B) The enigma is whether cells with equivalent ploidy are equal in terms of their structure, gene expression, and physiological function or whether polyploidy and the mechanism used to generate polyploid cells dictates its output. Diploid nuclei (beige) and >4C polyploid nuclei (dark blue). (This figure was created with BioRender.com.)
The complexity of cell-cycle options available provides an unlimited resource for tissues to exploit to undergo repair or regeneration (Fig. 1B–D). But, this also complicates the interpretation of the cellular mechanisms used by a given cell type, as diploid cells can either mitotically divide or endoreplicate, either via the endocycle or endomitosis. Once polyploid, cells can then continue to grow in size by endoreplication, divide to generate new polyploid cells, or even undergo reductive division to be the source for new diploid cells (Fig. 3A). The complexity of possible cell-cycle outcomes has led to the realization that cell-cycle activity does not always equal diploid cell division (Fig. 1). For example, the S phase markers, PCNA and BrdU/EdU, will not distinguish between mitotic cell cycles, endocycles, or endomitosis, nor inform whether the starting cell was diploid or polyploid. To do so, studies have to rely on a combination of cell-cycle markers with ploidy analysis (both number of nuclei per cell and ploidy of each cell's nucleus). In addition, live imaging or lineage analysis is necessary to confirm whether or not a cell can successfully divide and generate daughter cells.
Using these methods, polyploidy is now recognized to be as ubiquitous in mammalian organs as it is in plants and insects. What still remains unanswered is why a cell opts to become polyploid through one mechanism versus another (Figs. 1 and 3A). For example, a tetraploid (4C) cell could be generated by either cell fusion, endomitosis, or the endocycle resulting in mono- or binucleated polyploid cell. This raises the question of whether there are selective pressures that generate a bi- versus mononucleated polyploid cell or whether these polyploid cell types are equivalent (Fig. 3B).
Studies on developmentally programmed polyploidy have begun to provide insights into these questions that have yet to be explored in the context of tissue repair and regeneration. In mice, megakaryocyte maturation in the blood is dependent on endomitosis, but genetic knockdown of Cdk1 (M phase regulator) can reprogram megakaryocytes into an endocycle without compromising platelet production (Trakala et al. 2015). Therefore, a mononucleated polyploid megakaryocyte is functionally equivalent to multinucleated polyploid megakaryocyte in development. Another example, but with the opposite outcome, occurs in development of the blood–brain barrier in Drosophila larvae. The blood–brain barrier is comprised of both mono- and multinucleated polyploid subperineurial glia (SPG) (Unhavaithaya and Orr-Weaver 2012). In development, SPGs first endocycle and then enter either another endocycle or endomitosis generating both mono- and multinucleated polyploid SPGs (Von Stetina et al. 2018). Genetically inhibiting the generation of either mono- or multinucleated SPGs disrupted barrier formation indicating that both polyploid cell states are necessary and the multi- and mononucleated polyploid SPGs cannot compensate for one another in development of the blood–brain barrier.
The recent advances in genomic sequencing could help to elucidate the distinction between mono-, bi-, and multinucleated cells. Indeed, single-cell transcriptomics have been used to compare gene expression in diploid and polyploid hepatocytes, revealing a distinct gene expression program in polyploid rat hepatocytes that was not previously detected in bulk transcriptome analysis (Katsuda et al. 2020). Even single-nuclei RNA sequencing is now being used to identify the gene expression program of individual myonuclei from syncytial skeletal muscle fibers. Several studies have shown both coordinated and differential gene-expression programs in myonuclei depending on muscle fiber type and its physiological state (Dos Santos et al. 2020; Kim et al. 2020; Petrany et al. 2020). Therefore, researchers are on the precipice of identifying the genomic distinction between cellular and nuclear ploidy states.
POLYPLOIDY IN HEART REPAIR: A BARRIER OR POTENTIAL REGENERATIVE RESOURCE?
A major focus in heart regenerative medicine is to elucidate the role and regulation of polyploidy in repair and regeneration. Unlike hepatocytes in the liver, polyploid cardiomyocytes in mammalian heart do not adequately divide or grow to compensate for cell loss. Injury caused by a myocardial infarction leads to scar formation and eventual heart failure. Whereas the zebrafish heart retains diploid cardiomyocytes that rapidly divide to regenerate the heart with minimal scar formation (Poss et al. 2002). Cardiomyocyte ploidy differences were found to be linked to the animal's energy metabolism and thermogenesis, in particular thyroid hormone presence (Hirose et al. 2019). In the mouse, blockade of thyroid hormone receptors reduced the percentage of polyploid cardiomyocytes that form during development, and improved cardiac regenerative capacity. Likewise, incubation of zebrafish with T3 hormone increased the incidence of binucleated cardiomyocytes and disrupted heart regeneration.
Mammalian cardiomyocytes become polyploid during their normal differentiation program and exhibit differences in nuclear number and ploidy depending on the animal species (Hirose et al. 2019; Derks and Bergmann 2020). For example, humans have ∼57% mononucleated and ∼25% multinucleated polyploid cardiomyocytes, whereas mice have predominantly ∼78% multinucleated polyploid cardiomyocytes. However, both species retain a subset (2%–10%) of mononucleated, diploid cardiomyocytes, which are thought to be privileged, like in zebrafish, and retain the capacity, if only latent, to divide (Patterson and Swift 2019). In support of this, mouse strains have natural variation in the number of their mononucleated diploid cardiomyocytes and strains with more diploid cells were found to be more regenerative (Patterson et al. 2017). The percentage of diploid mononuclear cardiomyocytes was found to be dependent on the cardiomyocyte-specific kinase, Tnni3k. Genetic loss of Tnni3k in C57BL/6J mouse strain doubled the diploid cardiomyocyte population, whereas overexpression of Tnni3k in zebrafish cardiomyocytes increased ploidy and impaired heart regeneration similar to expression of dominant-negative Ect2, which blocks cytokinesis in zebrafish (González-Rosa et al. 2018; Gan et al. 2019).
The biological significance of cardiomyocyte ploidy for heart growth and repair still remains to be elucidated. New genetic and cell biological tools have allowed the outcome of cycling cardiomyocytes to be tracked in vivo, and researchers have found that nine out of 10 cardiomyocytes that reenter the cell-cycle endoreplicate, not completing cytokinesis postinjury (Hesse et al. 2012; Bradley et al. 2021). In addition, genetic ablation of these cycling cells compromised myocardial function postinjury demonstrating that endoreplication is beneficial, to some extent, for heart repair (Bradley et al. 2021). Additionally, polyploidy may be a potential resource for new cardiomyocytes, as binucleated cardiomyocytes can be genetically forced to complete cytokinesis via β-catenin-induced expression of Ect2 (Figs. 1 and 3; Jiang et al. 2019). Therefore, like hepatocytes in the liver, stimulating polyploid cardiomyocytes to undergo cytokinesis or endoreplication may offer an alternative means worth exploiting to compensate for lost heart mass.
Still, a major challenge is the ability to stimulate a sufficient number of cardiomyocytes to reenter the cell cycle. Many studies have focused on strategies to boost cardiomyocyte cell-cycle activity (Derks and Bergmann 2020). In the pig heart, a microRNA therapy was shown to stimulate cardiomyocyte proliferation after a myocardial infarction, but this protocol also led to electric uncoupling of the myocardium (Gabisonia et al. 2019). Therefore, functional recovery of the heart tissue mass requires a balance in proliferation and differentiation. Hence, polyploid cell growth may be a necessary means to retain electric coupling and restore heart mass. It is therefore paramount to determine the physiological function of diploid versus polyploid (both mono- versus multinucleated) cardiomyocytes and whether there are benefits to repairing the heart through one cellular mechanism versus another (Fig. 3B).
MECHANICAL FORCES: A DRIVER OF POLYPLOIDY TOO!
Biophysical forces, including shear stress and tension, instruct tissue morphogenesis by controlling cell proliferation, movement, and shape (Coravos et al. 2017). Mechanical forces have been mostly studied under conditions of diploid mitotic cell division, but these forces also regulate polyploid cell growth. Regeneration of the zebrafish epicardium, the outer layer of heart, induces transient endoreplication (both the endocycle and endomitosis) at the wound leading edge, where tension is increased concomitant with accumulation and activation of motor protein myosin (Cao et al. 2017). Endomitosis, via failed cytokinesis, was found to be integrin-dependent, as higher tractional forces delayed closure of cytokinetic ring in zebrafish epicardial cells (Uroz et al. 2019). Similarly, in vitro, a rigid extracellular matrix resulted in binucleation of neonatal cardiomyocytes (Yahalom-Ronen et al. 2015). Other biophysical forces, including shear stress, are also required to induce polyploidization of megakaryocytes during maturation (Jiang et al. 2014).
In Drosophila, integrin signaling was shown to regulate epithelial wound-induced polyploidization (Besen-McNally et al. 2021). Components of focal adhesion complex, including β-integrin, focal adhesion kinase (Fak), and Talin are required for the adult Drosophila abdominal epithelial cells to endoreplicate postinjury. In this model, integrin activates the endocycle via Yorkie and is also necessary for optimal cell fusion and wound closure. Mechanical forces are critical in Drosophila muscle development as well. Myosin increases tension necessary for resistance in the receiving cell and formation of the fusion pore for cell–cell fusion (Kim et al. 2015). Likewise, mechanical forces within the myotube instruct myonuclear position and nuclear ploidy (Windner et al. 2019). There are still many unknowns in how mechanics instruct polyploid cell growth via endoreplication and cell fusion and in how polyploid cells, in turn, affect tissue mechanics as polyploid cells can persist after injury and accumulate with age.
THE INTERPLAY BETWEEN GENOTOXIC STRESS AND POLYPLOIDY
Cells are consistently challenged with genotoxic stress, both exogenously (i.e., radiation, chemical) and endogenously (reactive oxygen species). These stressors can result in DNA damage, including double-stranded breaks (DSBs) that activate the p53-dependent DNA damage response (DDR) resulting in cell-cycle arrest or cell death. However, endoreplication is able to promote cell growth in the presence of DNA damage (Mehrotra et al. 2008). It was discovered that endocycling cells in the Drosophila ovary are resistant to irradiation, whereas mitotically dividing cells undergo apoptosis (Hassel et al. 2014; Zhang et al. 2014). The endocycling follicle cells reduce p53 signaling by epigenetic silencing of its gene targets and via proteolytical degradation p53 itself to prevent apoptosis. This is integral to the endocycle as DNA damage is normally generated due to underreplication of the cell's genome during these cycles (Nordman et al. 2011; Yarosh and Spradling 2014).
In context of tissue repair, endoreplication offers a means to regrow tissues in the presence of genotoxic stress when diploid cell proliferation would otherwise be inhibited. Switching the adult Drosophila abdominal epithelium to the mitotic cycle prevented wound repair, whereas the fly epithelium could still heal via polyploid cell growth even after exposure to UV irradiation (Grendler et al. 2019). DNA damage is known to accumulate with age in many tissues, including the brain, where neurons in Drosophila were found to become polyploid with age via the endocycle (Nandakumar et al. 2020). As in tissue repair, endoreplication offers a means to compensate for cell loss due to normal aging. This also occurs in the human corneal disease Fuchs endothelial dystrophy, where terminally differentiated endothelial cells turn over at a faster rate yet the remaining endothelial cells boost their ploidy to compensate for cell loss, maintaining total tissue ploidy (Losick et al. 2016). Oxidative stress is thought to be one of the drivers of Fuchs dystrophy, causing both mitochondrial and nuclear DNA damage (Jurkunas 2018). Oxidative stress was also found to be a driver of nonalcoholic fatty liver disease, where highly polyploid hepatocytes accumulate (Gentric et al. 2015). Interestingly, oxidative stress-induced DNA damage appears to be a widespread signal to initiate endoreplication. Both the polyploidization of cardiomyocytes in development and generation of giant granuloma macrophages during inflammation are caused by DNA damage (Puente et al. 2014; Herrtwich et al. 2016). However, it is still unknown how specifically polyploidy (via cell fusion, endomitosis, or endocycle) in these different tissue contexts subverts the DDR and whether enhanced polyploidy itself is the cause or consequence of disease progression (Fig. 3).
CONCLUDING REMARKS
From fruit flies to humans, it is now recognized that polyploidy serves as an essential strategy for cells to grow and compensate for cell loss in tissue repair and regeneration. Polyploidy provides versatility as more than one cellular mechanism can be used to restore tissue mass after injury. Polyploid cell growth also enables repair in the presence of genotoxic stress and while protecting against oncogenic stimuli. The remaining enigma lies in why one ploidy state is used over another (mono- vs. multinucleated) and why some polyploid cell types, like liver hepatocytes, are competent to undergo division, reductive division, and growth, whereas others, like mammalian cardiomyocytes, are limited. In the future, it will be critical to understand how the cell's ploidy state is regulated and how it affects cellular and tissue function, as inducing polyploid cell growth may offer a more natural means to restore organ size by maintaining cell contacts and physiological function.
ACKNOWLEDGMENTS
We thank Ari Dehn and Levi Duhaime from the Losick laboratory for review of this manuscript. All figures in this article were created with BioRender.com using a BioRender premium license generously supported by the Boston College Biology Department. This publication was also supported by the National Institute of General Medical Sciences under Award No. R35GM124691 to V.P.L.
Footnotes
Editors: Kenneth D. Poss and Donald T. Fox
Additional Perspectives on Regeneration available at www.cshperspectives.org
References
- Besen-McNally R, Gjelsvik KJ, Losick VP. 2021. Wound-induced polyploidization is dependent on Integrin-Yki signaling. Biol Open 10: bio055996. 10.1242/bio.055996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley LA, Young A, Li H, Billcheck HO, Wolf MJ. 2021. Loss of endogenously cycling adult cardiomyocytes worsens myocardial function. Circ Res 128: 155–168. 10.1161/CIRCRESAHA.120.318277 [DOI] [PubMed] [Google Scholar]
- Cao J, Wang J, Jackman CP, Cox AH, Trembley MA, Balowski JJ, Cox BD, De Simone A, Dickson AL, Di Talia S, et al. 2017. Tension creates an endoreplication wavefront that leads regeneration of epicardial tissue. Dev Cell 42: 600–615.e4. 10.1016/j.devcel.2017.08.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HZ, Ouseph MM, Li J, Pécot T, Chokshi V, Kent L, Bae S, Byrne M, Duran C, Comstock G, et al. 2012. Canonical and atypical E2Fs regulate the mammalian endocycle. Nat Cell Biol 14: 1192–1202. 10.1038/ncb2595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Jimenez RJ, Sharma K, Luu HY, Hsu BY, Ravindranathan A, Stohr BA, Willenbring H. 2020. Broad distribution of hepatocyte proliferation in liver homeostasis and regeneration. Cell Stem Cell 26: 27–33.e4. 10.1016/j.stem.2019.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen E, Allen SR, Sawyer JK, Fox DT. 2018. Fizzy-Related dictates a cell cycle switch during organ repair and tissue growth responses in the Drosophila hindgut. eLife 7: e38327. 10.7554/eLife.38327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coravos JS, Mason FM, Martin AC. 2017. Actomyosin pulsing in tissue integrity maintenance during morphogenesis. Trends Cell Biol 27: 276–283. 10.1016/j.tcb.2016.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derks W, Bergmann O. 2020. Polyploidy in cardiomyocytes: roadblock to heart regeneration? Circ Res 126: 552–565. 10.1161/CIRCRESAHA.119.315408 [DOI] [PubMed] [Google Scholar]
- Dewhurst MR, Ow JR, Zafer G, van Hul NKM, Wollmann H, Bisteau X, Brough D, Choi H, Kaldis P. 2020. Loss of hepatocyte cell division leads to liver inflammation and fibrosis. PLoS Genet 16: e1009084. 10.1371/journal.pgen.1009084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diril MK, Ratnacaram CK, Padmakumar VC, Du T, Wasser M, Coppola V, Tessarollo L, Kaldis P. 2012. Cyclin-dependent kinase 1 (Cdk1) is essential for cell division and suppression of DNA re-replication but not for liver regeneration. Proc Natl Acad Sci 109: 3826–3831. 10.1073/pnas.1115201109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donne R, Saroul-Aïnama M, Cordier P, Celton-Morizur S, Desdouets C. 2020. Polyploidy in liver development, homeostasis and disease. Nat Rev Gastroenterol Hepatol 17: 391–405. 10.1038/s41575-020-0284-x [DOI] [PubMed] [Google Scholar]
- Dos Santos M, Backer S, Saintpierre B, Izac B, Andrieu M, Letourneur F, Relaix F, Sotiropoulos A, Maire P. 2020. Single-nucleus RNA-seq and FISH identify coordinated transcriptional activity in mammalian myofibers. Nat Commun 11: 5102. 10.1038/s41467-020-18789-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan AW, Taylor MH, Hickey RD, Hanlon Newell AE, Lenzi ML, Olson SB, Finegold MJ, Grompe M. 2010. The ploidy conveyor of mature hepatocytes as a source of genetic variation. Nature 467: 707–710. 10.1038/nature09414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar BA, Zielke N, Gutierrez C. 2014. Endocycles: a recurrent evolutionary innovation for post-mitotic cell growth. Nat Rev Mol Cell Biol 15: 197–210. 10.1038/nrm3756 [DOI] [PubMed] [Google Scholar]
- Fernandes JJ, Atreya KB, Desai KM, Hall RE, Patel MD, Desai AA, Benham AE, Mable JL, Straessle JL. 2005. A dominant negative form of Rac1 affects myogenesis of adult thoracic muscles in Drosophila. Dev Biol 285: 11–27. 10.1016/j.ydbio.2005.05.040 [DOI] [PubMed] [Google Scholar]
- Fox DT, Spradling AC. 2009. The Drosophila hindgut lacks constitutively active adult stem cells but proliferates in response to tissue damage. Cell Stem Cell 5: 290–297. 10.1016/j.stem.2009.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox DT, Soltis DE, Soltis PS, Ashman TL, Van de Peer Y. 2020. Polyploidy: a biological force from cells to ecosystems. Trends Cell Biol 30: 688–694. 10.1016/j.tcb.2020.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabisonia K, Prosdocimo G, Aquaro GD, Carlucci L, Zentilin L, Secco I, Ali H, Braga L, Gorgodze N, Bernini F, et al. 2019. MicroRNA therapy stimulates uncontrolled cardiac repair after myocardial infarction in pigs. Nature 569: 418–422. 10.1038/s41586-019-1191-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galko MJ, Krasnow MA. 2004. Cellular and genetic analysis of wound healing in Drosophila larvae. PLoS Biol 2: E239. 10.1371/journal.pbio.0020239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan P, Patterson M, Velasquez A, Wang K, Tian D, Windle JJ, Tao G, Judge DP, Makita T, Park TJ, et al. 2019. Tnni3k alleles influence ventricular mononuclear diploid cardiomyocyte frequency. PLoS Genet 15: e1008354. 10.1371/journal.pgen.1008354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentric G, Maillet V, Paradis V, Couton D, L'Hermitte A, Panasyuk G, Fromenty B, Celton-Morizur S, Desdouets C. 2015. Oxidative stress promotes pathologic polyploidization in nonalcoholic fatty liver disease. J Clin Invest 125: 981–992. 10.1172/JCI73957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gjelsvik KJ, Besen-McNally R, Losick VP. 2019. Solving the polyploid mystery in health and disease. Trends Genet 35: 6–14. 10.1016/j.tig.2018.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman JA, Poss KD. 2020. Gene regulatory programmes of tissue regeneration. Nat Rev Genet 21: 511–525. 10.1038/s41576-020-0239-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Rosa JM, Sharpe M, Field D, Soonpaa MH, Field LJ, Burns CE, Burns CG. 2018. Myocardial polyploidization creates a barrier to heart regeneration in Zebrafish. Dev Cell 44: 433–446.e7. 10.1016/j.devcel.2018.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grendler J, Lowgren S, Mills M, Losick VP. 2019. Wound-induced polyploidization is driven by Myc and supports tissue repair in the presence of DNA damage. Development 146: dev1730005. 10.1242/dev.173005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han L, Choudhury S, Mich-Basso JD, Ammanamanchi N, Ganapathy B, Suresh S, Khaladkar M, Singh J, Maehr R, Zuppo DA, et al. 2020. Lamin B2 levels regulate polyploidization of cardiomyocyte nuclei and myocardial regeneration. Dev Cell 53: 42–59.e11. 10.1016/j.devcel.2020.01.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassel C, Zhang B, Dixon M, Calvi BR. 2014. Induction of endocycles represses apoptosis independently of differentiation and predisposes cells to genome instability. Development 141: 112–123. 10.1242/dev.098871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrtwich L, Nanda I, Evangelou K, Nikolova T, Horn V, Erny D, Stefanowski J, Rogell L, Klein C, et al. 2016. DNA damage signaling instructs polyploid macrophage fate in granulomas. Cell 167: 1264–1280.e18. 10.1016/j.cell.2016.09.054 [DOI] [PubMed] [Google Scholar]
- Hesse M, Raulf A, Pilz GA, Haberlandt C, Klein AM, Jabs R, Zaehres H, Fügemann CJ, Zimmermann K, Trebicka J, et al. 2012. Direct visualization of cell division using high-resolution imaging of M-phase of the cell cycle. Nat Commun 3: 1076. 10.1038/ncomms2089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose K, Payumo AY, Cutie S, Hoang A, Zhang H, Guyot R, Lunn D, Bigley RB, Yu H, Wang J, et al. 2019. Evidence for hormonal control of heart regenerative capacity during endothermy acquisition. Science 364: 184–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J, Woulfe DS, Papoutsakis ET. 2014. Shear enhances thrombopoiesis and formation of microparticles that induce megakaryocytic differentiation of stem cells. Blood 124: 2094–2103. 10.1182/blood-2014-01-547927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang YH, Zhu Y, Chen S, Wang HL, Zhou Y, Tang FQ, Jian Z, Xiao YB. 2019. Re-enforcing hypoxia-induced polyploid cardiomyocytes enter cytokinesis through activation of β-catenin. Sci Rep 9: 17865. 10.1038/s41598-019-54334-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurkunas UV. 2018. Fuchs endothelial corneal dystrophy through the prism of oxidative stress. Cornea 37: S50–S54. 10.1097/ICO.0000000000001775 [DOI] [PubMed] [Google Scholar]
- Katsuda T, Hosaka K, Matsuzaki J, Usuba W, Prieto-Vila M, Yamaguchi T, Tsuchiya A, Terai S, Ochiya T. 2020. Transcriptomic dissection of hepatocyte heterogeneity: linking ploidy, zonation, and stem/progenitor cell characteristics. Cell Mol Gastroenterol Hepatol 9: 161–183. 10.1016/j.jcmgh.2019.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Ren Y, Ng WP, Li S, Son S, Kee YS, Zhang S, Zhang G, Fletcher DA, Robinson DN, et al. 2015. Mechanical tension drives cell membrane fusion. Dev Cell 32: 561–573. 10.1016/j.devcel.2015.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, Franke V, Brandt B, Lowenstein ED, Schöwel V, Spuler S, Akalin A, Birchmeier C. 2020. Single-nucleus transcriptomics reveals functional compartmentalization in syncytial skeletal muscle cells. Nat Commun 11: 6375. 10.1038/s41467-020-20064-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurinna S, Stratton SA, Coban Z, Schumacher JM, Grompe M, Duncan AW, Barton MC. 2013. P53 regulates a mitotic transcription program and determines ploidy in normal mouse liver. Hepatology 57: 2004–2013. 10.1002/hep.26233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazzeri E, Angelotti ML, Peired A, Conte C, Marschner JA, Maggi L, Mazzinghi B, Lombardi D, Melica ME, Nardi S, et al. 2018. Endocycle-related tubular cell hypertrophy and progenitor proliferation recover renal function after acute kidney injury. Nat Commun 9: 1344. 10.1038/s41467-018-03753-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazzeri E, Angelotti ML, Conte C, Anders HJ, Romagnani P. 2019. Surviving acute organ failure: cell polyploidization and progenitor proliferation. Trends Mol Med 25: 366–381. 10.1016/j.molmed.2019.02.006 [DOI] [PubMed] [Google Scholar]
- Lee DM, Chen EH. 2019. Drosophila myoblast fusion: invasion and resistance for the ultimate union. Annu Rev Genet 53: 67–91. 10.1146/annurev-genet-120116-024603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehka L, Rędowicz MJ. 2020. Mechanisms regulating myoblast fusion: a multilevel interplay. Semin Cell Dev Biol 104: 81–92. 10.1016/j.semcdb.2020.02.004 [DOI] [PubMed] [Google Scholar]
- Lin YH, Zhang S, Zhu M, Lu T, Chen K, Wen Z, Wang S, Xiao G, Luo D, Jia Y, et al. 2020. Mice with increased numbers of polyploid hepatocytes maintain regenerative capacity but develop fewer hepatocellular carcinomas following chronic liver injury. Gastroenterology 158: 1698–1712.e14. 10.1053/j.gastro.2020.01.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losick VP, Fox DT, Spradling AC. 2013. Polyploidization and cell fusion contribute to wound healing in the adult Drosophila epithelium. Curr Biol 23: 2224–2232. 10.1016/j.cub.2013.09.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losick VP, Jun AS, Spradling AC. 2016. Wound-induced polyploidization: regulation by Hippo and JNK signaling and conservation in mammals. PLoS ONE 11: e0151251. 10.1371/journal.pone.0151251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucchetta EM, Ohlstein B. 2017. Amitosis of polyploid cells regenerates functional stem cells in the Drosophila intestine. Cell Stem Cell 20: 609–620.e6. 10.1016/j.stem.2017.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto T, Wakefield L, Peters A, Peto M, Spellman P, Grompe M. 2021. Proliferative polyploid cells give rise to tumors via ploidy reduction. Nat Commun 12: 646. 10.1038/s41467-021-20916-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehrotra S, Maqbool SB, Kolpakas A, Murnen K, Calvi BR. 2008. Endocycling cells do not apoptose in response to DNA rereplication genotoxic stress. Genes Dev 22: 3158–3171. 10.1101/gad.1710208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minamishima YA, Nakayama K, Nakayama K. 2002. Recovery of liver mass without proliferation of hepatocytes after partial hepatectomy in Skp2-deficient mice. Cancer Res 62: 995–999. [PubMed] [Google Scholar]
- Miyaoka Y, Ebato K, Kato H, Arakawa S, Shimizu S, Miyajima A. 2012. Hypertrophy and unconventional cell division of hepatocytes underlie liver regeneration. Curr Biol 22: 1166–1175. 10.1016/j.cub.2012.05.016 [DOI] [PubMed] [Google Scholar]
- Nandakumar S, Grushko O, Buttitta LA. 2020. Polyploidy in the adult Drosophila brain. eLife 9: e54385. 10.7554/eLife.54385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordman J, Li S, Eng T, Macalpine D, Orr-Weaver TL. 2011. Developmental control of the DNA replication and transcription programs. Genome Res 21: 175–181. 10.1101/gr.114611.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr-Weaver TL. 2015. When bigger is better: the role of polyploidy in organogenesis. Trends Genet 31: 307–315. 10.1016/j.tig.2015.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Øvrebø JI, Edgar BA. 2018. Polyploidy in tissue homeostasis and regeneration. Development 145: dev156034. 10.1242/dev.156034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ow JR, Cadez MJ, Zafer G, Foo JC, Li HY, Ghosh S, Wollmann H, Cazenave-Gassiot A, Ong CB, Wenk MR, et al. 2020. Remodeling of whole-body lipid metabolism and a diabetic-like phenotype caused by loss of CDK1 and hepatocyte division. eLife 9: e63835. 10.7554/eLife.63835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandit SK, Westendorp B, Nantasanti S, van Liere E, Tooten PC, Cornelissen PW, Toussaint MJ, Lamers WH, de Bruin A. 2012. E2f8 is essential for polyploidization in mammalian cells. Nat Cell Biol 14: 1181–1191. 10.1038/ncb2585 [DOI] [PubMed] [Google Scholar]
- Patterson M, Swift SK. 2019. Residual diploidy in polyploid tissues: a cellular state with enhanced proliferative capacity for tissue regeneration? Stem Cells Dev 28: 1527–1539. 10.1089/scd.2019.0193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson M, Barske L, Van Handel B, Rau CD, Gan P, Sharma A, Parikh S, Denholtz M, Huang Y, Yamaguchi Y, et al. 2017. Frequency of mononuclear diploid cardiomyocytes underlies natural variation in heart regeneration. Nat Genet 49: 1346–1353. 10.1038/ng.3929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson NG, Stormo BM, Schoenfelder KP, King JS, Lee RR, Fox DT. 2020. Cytoplasmic sharing through apical membrane remodeling. eLife 9: e58107. 10.7554/eLife.58107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrany MJ, Swoboda CO, Sun C, Chetal K, Chen X, Weirauch MT, Salomonis N, Millay DP. 2020. Single-nucleus RNA-seq identifies transcriptional heterogeneity in multinucleated skeletal myofibers. Nat Commun 11: 6374. 10.1038/s41467-020-20063-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poss KD, Wilson LG, Keating MT. 2002. Heart regeneration in zebrafish. Science 298: 2188–2190. 10.1126/science.1077857 [DOI] [PubMed] [Google Scholar]
- Puente BN, Kimura W, Muralidhar SA, Moon J, Amatruda JF, Phelps KL, Grinsfelder D, Rothermel BA, Chen R, Garcia JA, et al. 2014. The oxygen-rich postnatal environment induces cardiomyocyte cell-cycle arrest through DNA damage response. Cell 157: 565–579. 10.1016/j.cell.2014.03.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotelli MD, Policastro RA, Bolling AM, Killion AW, Weinberg AJ, Dixon MJ, Zentner GE, Walczak CE, Lilly MA, Calvi BR. 2019. A Cyclin A—Myb-MuvB—Aurora B network regulates the choice between mitotic cycles and polyploid endoreplication cycles. PLoS Genet 15: e1008253. 10.1371/journal.pgen.1008253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer JK, Cohen E, Fox DT. 2017. Interorgan regulation of Drosophila intestinal stem cell proliferation by a hybrid organ boundary zone. Development 144: 4091–4102. 10.1242/dev.153114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenfelder KP, Fox DT. 2015. The expanding implications of polyploidy. J Cell Biol 209: 485–491. 10.1083/jcb.201502016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigrist SJ, Lehner CF. 1997. Drosophila fizzy-related down-regulates mitotic cyclins and is required for cell proliferation arrest and entry into endocycles. Cell 90: 671–681. 10.1016/S0092-8674(00)80528-0 [DOI] [PubMed] [Google Scholar]
- Sladky VC, Knapp K, Soratroi C, Heppke J, Eichin F, Rocamora-Reverte L, Szabo TG, Bongiovanni L, Westendorp B, Moreno E, et al. 2020. E2F-family members engage the PIDDosome to limit hepatocyte ploidy in liver development and regeneration. Dev Cell 52: 335–349.e7. 10.1016/j.devcel.2019.12.016 [DOI] [PubMed] [Google Scholar]
- Tamori Y, Deng WM. 2013. Tissue repair through cell competition and compensatory cellular hypertrophy in postmitotic epithelia. Dev Cell 25: 350–363. 10.1016/j.devcel.2013.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trakala M, Rodríguez-Acebes S, Maroto M, Symonds CE, Santamaria D, Ortega S, Barbacid M, Méndez J, Malumbres M. 2015. Functional reprogramming of polyploidization in megakaryocytes. Dev Cell 32: 155–167. 10.1016/j.devcel.2014.12.015 [DOI] [PubMed] [Google Scholar]
- Unhavaithaya Y, Orr-Weaver TL. 2012. Polyploidization of glia in neural development links tissue growth to blood–brain barrier integrity. Genes Dev 26: 31–36. 10.1101/gad.177436.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uroz M, Garcia-Puig A, Tekeli I, Elosegui-Artola A, Abenza JF, Marin-Llaurado A, Pujals S, Conte V, Albertazzi L, Roca-Cusachs P, et al. 2019. Traction forces at the cytokinetic ring regulate cell division and polyploidy in the migrating zebrafish epicardium. Nat Mater 18: 1015–1023. 10.1038/s41563-019-0381-9 [DOI] [PubMed] [Google Scholar]
- Von Stetina JR, Frawley LE, Unhavaithaya Y, Orr-Weaver TL. 2018. Variant cell cycles regulated by Notch signaling control cell size and ensure a functional blood-brain barrier. Development 145: dev157115. 10.1242/dev.157115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Antunes M, Anderson AE, Kadrmas JL, Jacinto A, Galko MJ. 2015. Integrin adhesions suppress syncytium formation in the Drosophila larval epidermis. Curr Biol 25: 2215–2227. 10.1016/j.cub.2015.07.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Batourina E, Schneider K, Souza S, Swayne T, Liu C, George CD, Tate T, Dan H, Wiessner G, et al. 2018. Polyploid superficial cells that maintain the urothelial barrier are produced via incomplete cytokinesis and endoreplication. Cell Rep 25: 464–477.e4. 10.1016/j.celrep.2018.09.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson PD, Delgado ER, Alencastro F, Leek MP, Roy N, Weirich MP, Stahl EC, Otero PA, Chen MI, Brown WK, et al. 2018. The polyploid state restricts hepatocyte proliferation and liver regeneration in mice. Hepatology 69: 1242–1258. 10.1002/hep.30286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windner SE, Manhart A, Brown A, Mogilner A, Baylies MK. 2019. Nuclear scaling is coordinated among individual nuclei in multinucleated muscle fibers. Dev Cell 49: 48–62.e3. 10.1016/j.devcel.2019.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang J, Bandura J, Zhang P, Jin Y, Reuter H, Edgar BA. 2017. EGFR-dependent TOR-independent endocycles support Drosophila gut epithelial regeneration. Nat Commun 8: 15125. 10.1038/ncomms15125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahalom-Ronen Y, Rajchman D, Sarig R, Geiger B, Tzahor E. 2015. Reduced matrix rigidity promotes neonatal cardiomyocyte dedifferentiation, proliferation and clonal expansion. eLife 4: e07455. 10.7554/eLife.07455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarosh W, Spradling AC. 2014. Incomplete replication generates somatic DNA alterations within Drosophila polytene salivary gland cells. Genes Dev 28: 1840–1855. 10.1101/gad.245811.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Mehrotra S, Ng WL, Calvi BR. 2014. Low levels of p53 protein and chromatin silencing of p53 target genes repress apoptosis in Drosophila endocycling cells. PLoS Genet 10: e1004581. 10.1371/journal.pgen.1004581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Zhou K, Luo X, Li L, Tu HC, Sehgal A, Nguyen LH, Zhang Y, Gopal P, Tarlow BD, et al. 2018. The polyploid state plays a tumor-suppressive role in the liver. Dev Cell 44: 447–459.e5. 10.1016/j.devcel.2018.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielke N, Kim KJ, Tran V, Shibutani ST, Bravo MJ, Nagarajan S, van Straaten M, Woods B, von Dassow G, Rottig C, et al. 2011. Control of Drosophila endocycles by E2F and CRL4CDT2. Nature 480: 123–127. 10.1038/nature10579 [DOI] [PMC free article] [PubMed] [Google Scholar]