Abstract
This 27-color flow cytometry panel was developed in order to assess immunological changes over the course of an immunization and challenge regimen in two experimental malaria vaccine trials. The aim of the study was to find correlates of vaccine-induced protection. Several studies have indicated that protection against malaria appears to involve immune responses at various immunological sites, with liver-resident responses playing an essential role. As it is not feasible to monitor the immune responses within the liver in humans, this panel is developed with the aim to thoroughly characterize the immune responses over time in blood in addition to detecting changes that might reflect what happens in other immunological sites like the liver. The focus of this panel is to detect several innate lymphoid cell populations, including NK cells and their activation status. Moreover, unconventional T cells like mucosal associated invariant T cells and γδ T cells are assessed in the panel.
Keywords: flow cytometry, human PBMC, γδ T cells, NK cells, T cells, MAIT cells, innate lymphoid cells
BACKGROUND
Malaria is still a major health threat, with 200 million cases and approximately 400,000 deaths annually, mostly among children under 5 (1–3). The disease is caused by mosquito-transmittedPlasmodiumparasites that have a complicated lifecycle which occurs in multiple sites of the body, including the liver and the blood. Although efforts to design a potent anti-malaria vaccine have been ongoing for nearly a century, there is still no vaccine that confers adequate durable immunity to infection. Furthermore, naturally occurring sterilizing immunity to malaria is rare, despite repeated infections (4,5). Immunization with radiation-attenuated sporozoites (RAS), which are parasites that have retained their ability to infect the liver, has been shown to confer sterilizing protection. However, the mechanism behind this protection is incompletely understood (1,6–9). Remarkably, the occurrence of natural infection seems to inhibit the development of protective sterilizing immunity, as clinical trials in nonendemic regions consistently report higher vaccine efficacies than those in malaria-endemic regions (10). It has been suggested that tolerogenic responses, immune exhaustion, and senescence play a role (11). Identifying the underlying immune mechanisms of anti-malarial immunity or the lack thereof will aid the development of a protective vaccine that is suitable for mass distribution. High parameter multicolor flow cytometry enables thorough characterization of immune responses against pathogens. Here we describe a 27-color panel that aims to detect immune responses that correlate with protection in experimental malaria vaccine trials, in addition to comparing responses in individuals from malaria endemic regions with those from nonendemic regions. This panel was developed for use with three other panels, in order to extensively phenotype the immune responses triggered by RAS-immunizations. The other panels we developed focus on T and B cells and we used a dendritic/monocyte panel published as an optimized multicolor immunofluorescence panel (OMIP) (Table 1) (12).
Table 1.
Summary table for application of OMIP-064
| Purpose | Extensive phenotyping |
| Species | Human |
| Celltype | PBMC |
| Cross-references | OMIP-029, OMIP-039, OMIP-044, OMIP-055, OMIP-056, and OMIP-058 |
The panel described here is developed with a focus on innate lymphoid cells (ILCs), conventional NK cells and their activation status, γδ T cells, mucosal associated invariant T (MAIT) cells in addition to the major lineages including T cells, B cells and monocytes, both for exclusion of other lineages and to characterize potential expression of NK-relevant markers in these other cell types. All the reagents are listed in Table 2. The panel includes lineage markers CD14 and CD33 to gate out monocytes, dendritic cells, and granulocytes and CD19 for the exclusion of B-cells. For the gating of T cells, CD3, CD4, and CD8 are used to identify the conventional T cell subsets, although these markers can be expressed on several other cell types detected by this panel, such as NK cells. CD161 and TCRvα7.2 are included to gate on MAIT cells. These cells are detected in peripheral blood, although they are more abundant at mucosal sites and in the liver. Their role in the protection against malaria infection is unknown, although MAIT cells were observed to contract and then expand in a controlled human malaria-infection(CHMI) study (13).
Table 2.
Reagents used for OMIP-0
| MARKER | ANTIBODY CLONE | FLUOROCHROME | PURPOSE |
|---|---|---|---|
|
| |||
| CD16 | 3G8 | BUV395 | NK, monocyte, ILC gating |
| Viability | NA | Fixable blue | Dead cell dump |
| CD3 | UCHT1 | BUV496 | T cells |
| CD19 | SJ25C1 | BUV563 | B cells |
| HLA-DR | G46–6 | BUV661 | Activation |
| CD27 | L128 | BUV737 | NK phenotyping |
| CD8 | SK1 | BUV805 | CD8+ T cells |
| TCRγδ | 11F2 | BV421 | Pan-γδ T cells |
| NKG2D | 1D11 | BV480 | NK phenotyping |
| CD56 | HCD56 | BV570 | NK phenotyping |
| Vδ2 TCR | B6 | BV605 | γδ T cell subset vδ2 |
| CD38 | HB-7 | BV650 | Activation |
| CD57 | HNK-1 | BV711 | NK phenotyping |
| CD4 | SK3 | BV750 | CD4+ T cells |
| TCRvα7.2 | 3C10 | BV786 | MAIT cells |
| FCεRIγ | Poly | FITC | NK phenotyping |
| Ki-67 | Ki67 | BB660 | Proliferation |
| CD14 | M®P9 | PerCP-Cy5.5 | Monocytes |
| CD127 | HIL-7R-M21 | BB790 | ILC phenotyping |
| NKp46 | 9E2/NKp46 | PE | NK phenotyping |
| CRTh2 | BM16 | PE-CF594 | ILC phenotyping |
| CD33 | WM53 | PE-Cy5 | Granulocyte/monocyte exclusion |
| c-kit | 104D2 | PE-Cy5.5 | ILC phenotyping |
| NKG2A | Z199 | PE-Cy7 | NK phenotyping |
| CD337/Nkp30 | AF29–4D12 | APC | NK phenotyping |
| NKG2C | 134,591 | Ax700 | NK phenotyping |
| CD161 | HP-3G10 | APC-Fire750 | MAIT cells/ILC phenotyping |
Another unconventional T cell subset that has generated attention in the malaria immunology field is the subset of T cells expressing the γδ T cell receptor (TCR). These γδ T cells have been shown to be expanded in acute malaria infection, and a recent study showed that the Vδ2 subset was found to correlate with protection in a large cohort of healthy, malaria-exposed individuals that were immunized with a RAS-vaccine (14). In addition, their relevance to malaria has also been demonstrated in animal models, for example, γδ T cells are required for the induction of sterile immunity in a rodent model (14). We have therefore included antibodies detecting the γδ TCR and Vδ2 to identify γδ T cells and the Vδ2 subset.
Animal models have helped elucidate some of the mechanisms needed for protective immune responses in the liver, and NK cells have previously been shown to be important in the immune responses during the early phases of liver stage infection. NK cells likely contribute through the production of IFNγ and potentially through direct cytolysis of infected hepatocytes (15,16).
In humans, NK cells have been shown to play a role in malaria disease, both as having protective effects against the pathogen in addition to contributing to pathology in cerebral malaria (17). We have included several markers to extensively characterize NK phenotypes, in which maturation status, differentiation, and activation markers are included. To phenotype NK cells, we used CD56 in combination with CD16 to delineate different NK subsets. An extensive set of NK cells markers were included to monitor maturation and differentiation, which can also indirectly indicate the functionality of these NK subsets. Recently, so-called adaptive NK cells that lack FCεRI-γ were associated with protective effects in a large cohort of seasonal malaria transmission monitoring (18). This marker is combined with NKG2C, CD57 and a lack of NKG2A expression to identify these adaptive NK cells (19). CD27 has been implicated as another maturation or memory-like marker, and has therefore been included (20). CD27 on NK cells mark mature NK cells with low cytotoxic potential (21). CD27-expressing NK cells were also indicated as being memory-like NK cells in a murine tuberculosis model (22). NKp30, NKp46, and CD38 are included to monitor activation of NK cells. CD38 was recently described to be a key regulator in NK cells that are enhanced in their cytotoxic abilities and cytokine producing potential (23,24). The activating receptor NKG2D was shown to be highly expressed in liver-resident NK cells in a rodent model, and implicated in humans (20,25,26). NKG2D ligands are upregulated in response to type I interferons, which have been shown to be induced in plasmodium-infected hepatocytes in mice, and these cells could therefore be of interest to monitor (15,27). Several of the NK markers in this panel have been shown to be expressed on the conventional and unconventional T cells detected by this panel. For instance, NKG2A and CD27 can be detected on γδ T cells as indirect markers for IFNγ-producing γδ T cells (28), and CD57 expression on T cells can be used as an exhaustion marker, previously shown to be upregulated in Plasmodium falciparum infection (11).
The markers CD16, CD161, CD127 c-kit, and CRTH2 can together be used to differentiate the ILC subsets, which are found in low abundance in the blood, although they are more prevalent in tissues (29). The role of ILCs in malaria vaccine responses has not been identified yet, although sparse data indicate that ILCs may have a role in infection. For instance, blood stage infection led to a rapid loss of group 1 ILCs in the blood of subjects participating in a CHMI study (30). Another study suggested that group 2 ILCs were involved in protection against cerebral malaria (31). As an additional marker to phenotype cellular responses, we included Ki67 as a marker for proliferation and recent in vivo activation. Figure 1 shows an example of how these markers can be used to gate on different cell subsets. This panel can be used for preipheral blood mononuclear cells (PBMC), and possibly for other sample types.
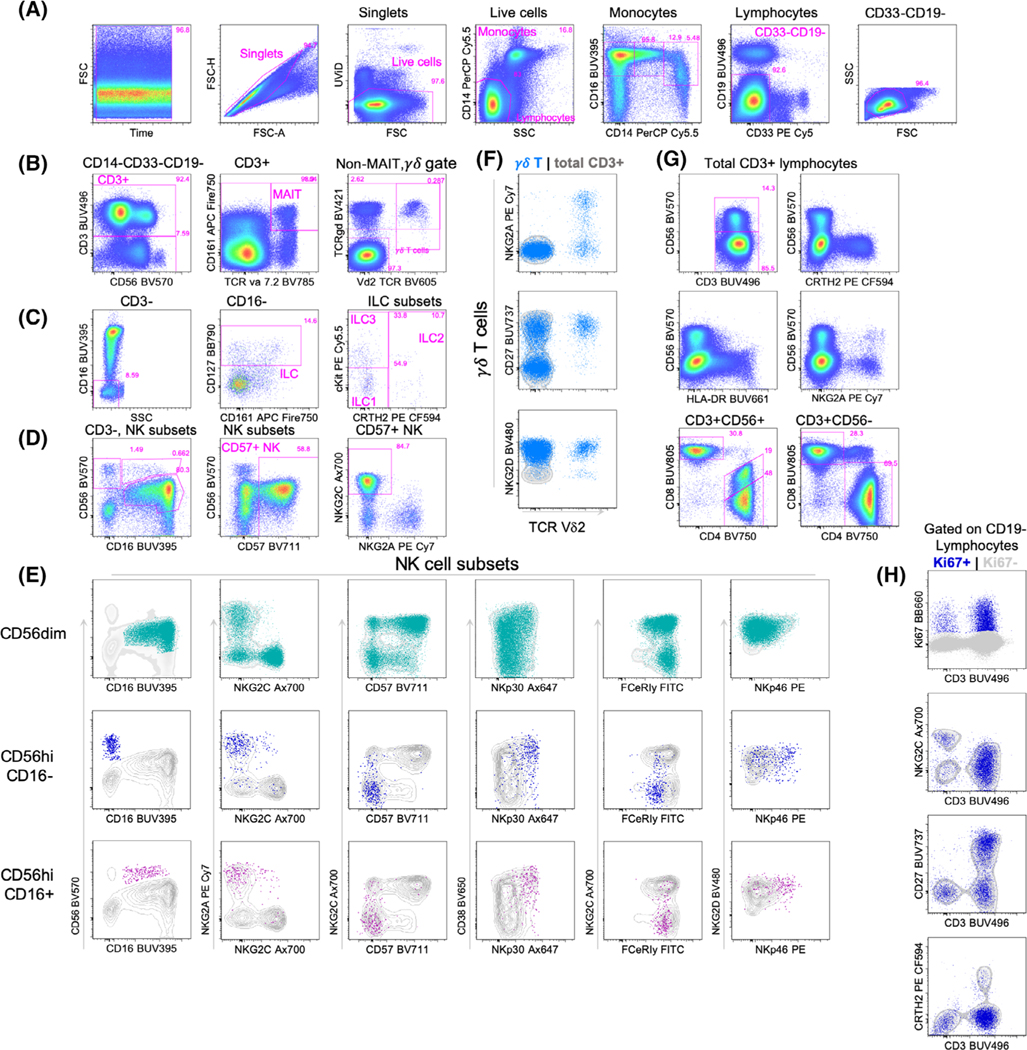
FIGURE 1.
Example of the gating strategy of the 27-color measurement of cryopreserved PBMCs. (A) The cells from a healthy donor were stained and the data were subsequently acquired on a BD FACSymphony instrument. The plot depicting forward scatter (FSC) vs. time is to verify that there are no pressure fluctuations that might affect fluorescent signals. The FSC-Area (A) vs. FSC-Height (H) is to gate on single cells and to exclude doublets. Next, the UViD-negative events are gated to exclude dead or damaged cells. CD14 vs. Side scatter (SSC)-A is used to gate monocytes (CD14+ and SSChi) vs. lymphocytes (CD14− and SSClo), and CD14 and CD16 are used to gate on the different monocyte subsets. In parallel, CD19 and CD33 are used as exclusion channels, to gate out B cells and granulocytes. Another clean-up gate for lymphocytes is included based on FSC and SSC. (B) The negative fraction is then plotted for CD3 to gate on T cell subsets. First, MAIT cells are gated as CD3+ TCRγδ− CD161+ and TCRva7.2+. The non-MAIT cells are then used to gate γδ T cells, as CD3+ and TCRγδ+. The vδ2 T cells are double positive for TCRγδ+ and the vδ2TCR. The negative fraction is then used to gate on conventional T cells that are either CD4+ or CD8+. (C) The CD3-gate is use for further delineation of ILCs. The ILC subsets are gated as CD3-CD16-CD127 + CD161+, although ILC1 and LTi have been shown to lack CD161 expression, so ILCs can be gated on without this marker as well. Further differentiation between ILC1, ILC2, and ILC3 can be done with CRTH2 and cKit, with CRTh2-CKit- cells gated as ILC1, CRTh2+ cells gated on as ILC2 and ILC3 are cKit+. (D) The CD3-fraction is alternatively gated as CD56 vs. CD16 for NK subsets. The second plot in this row shows that CD57 is mainly expressed on CD56dim NK cells. These cells are then plotted as NKG2A vs. NKG2C, the latter of which are adaptive NK cells. (E) The 3 NK subsets as shown in the left plot in each row are plotted separately for the remaining NK subset markers (CD57, NKG2A, NKG2C, NKG2D, NKp30, and NKp46), overlaid on the total CD3− population in gray. Also the expression of FCεRIγ is measured, “adaptive NK cells” have a low expression of this marker (36). (F) Depiction of the γδ T cells (blue overlay) and NKG2A, NKG2D and CD27 against vδ2.The background depicts total CD3+ cells, in gray. (G) A subset of CD3+ cells is positive for CD56 and these will include NK T cells. The expression of CRTH2, HLA-DR, and NKG2A is plotted vs. CD56 to demonstrate several phenotypic differences between the CD56+ and CD56− populations. The two plots in the third row show the CD4 versus CD8 pattern and how the CD56+ CD3+ cells have a CD4+ population that dimly expressed CD8. (H) Ki67+ is used as a proliferation marker and plotted against CD3+ on all CD14-CD33-CD19− lymphocytes, and shown as overlay combined with several markers that are different between the CD3+ and CD3− populations. [Color figure can be viewed at wileyonlinelibrary.com]
SIMILARITY TO PUBLISHED OMIPs
This panel is unique, although there is some overlap with OMIP-056 (32), which also looks at MAIT, γδ T cells, and NK cells (Table 1). However, the focus of that panel is more on functional responses in the context of HIV infection, and does not deeply phenotype subsets of NK cells and unconventional T cells, as in this panel. The 21-marker panel described in OMIP-055 (33) has nine markers in common with this panel, although four of those are lineage markers and all lineage markers are assigned to the same fluorochrome, which limits the depth of the analysis to ILC subsets. OMIP-039 (34) and OMIP-029 (35) describe panels that phenotype NK cells that partly overlap with this panel, although both panels are less elaborate, as they include 14 and 13 colors, respectively, and can therefore not combine both NK cell markers with MAIT and γδ T cells. OMIP-058 also has several markers that overlap with our panel, although the emphasis is on γδ T cells and iNKT cells and the depth of NK subset phenotyping is more limited than the panel we describe here. Overall, our panel is unique because it enables a deep assessment of NK subsets in addition to conventional and unconventional T cells, combined with an extensive set of activation and maturation markers.
Supplementary Material
ACKNOWLEDGMENTS
We would like to thank all the technologists in the HIV Vaccine Trial Network Laboratory for their help with the procedures in the lab and with the use of their instruments. We thank Dr. Catherine Blish for her advice on the NK markers, and Alicia Annamalay and Jason Carnes for ordering of the reagents.
Grant sponsor: Center for AIDS Research, University of Washington, Grant numberAI027757; Grant sponsor: Division of Intramural Research, National Institute of Allergy and Infectious Diseases, Grant number5U19AI128914-04, Grant numberUM1 AI068618
Footnotes
Additional Supporting Information may be found in the online version of this article.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
LITERATURECITED
- 1.Hoffman SL, Goh LML, Luke TC, Schneider I, le TP, Doolan DL, Sacci J, de la Vega P, Dowler M, Paul C, et al. Protection of humans against malaria by immunization with radiation-attenuatedPlasmodium falciparum sporozoites. J Infect Dis 2002;185:1155–1164. [DOI] [PubMed] [Google Scholar]
- 2.Fernandez-Ruiz D, Ng WY, Holz LE, Ma JZ, Zaid A, Wong YC, Lau LS, Mollard V, Cozijnsen A, Collins N, et al. Liver-resident memory CD8+ T cells form a front-line defense against malaria liver-stage infection. Immunity 2016;45:889–902. [DOI] [PubMed] [Google Scholar]
- 3.WHO. Malaria. WHO; 2019. Available at: https://www.who.int/malaria/en/.Accessed June 27, 2019. [Google Scholar]
- 4.Hoffman SL, Oster CN, Plowe CV, Woollett GR, Beier JC, Chulay JD, Wirtz RA, Hollingdale MR, Mugambi M. Naturally acquired antibodies to sporozoites do not prevent malaria: Vaccine development implications. Science 1987;237:639–642. [DOI] [PubMed] [Google Scholar]
- 5.Doolan DL, Dobaño C, Baird JK. Acquired immunity to malaria. Clin Microbiol Rev 2009;22:13–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goswami D, Minkah NK, Kappe SHI. Designer parasites: Genetically engineered plasmodium as vaccines to prevent malaria infection. J Immunol 2019;202:20–28. [DOI] [PubMed] [Google Scholar]
- 7.Nussensweig RS, van der Berg J, Most H, Orton C. Protective immunity produced by the injection of X-irradiated sporozoites of Plasmodium berghei. Nature 1967; 216:160–162. [DOI] [PubMed] [Google Scholar]
- 8.Clyde DF, Most H, McCarthy VC, Van der Berg JP. Immunization of man against sporozoite-induced falciparum malaria. Am J Med Sci 1973;266:169–177. [DOI] [PubMed] [Google Scholar]
- 9.Crompton PD, Moebius J, Portugal S, Waisberg M, Hart G, Garver LS, Miller LH, Barillas-Mury C, Pierce SK. Malaria immunity in man and mosquito: Insights into unsolved mysteries of a deadly infectious disease. Annu Rev Immunol 2014;32:157–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beeson JG, Kurtovic L, Dobaño C, Opi DH, Chan JA, Feng G, Good MF, Reiling L, Boyle MJ. Challenges and strategies for developing efficacious and long-lasting malaria vaccines. Sci Transl Med 2019;11:eaau1458. [DOI] [PubMed] [Google Scholar]
- 11.Frimpong A, Kusi KA, Adu-Gyasi D, Amponsah J, Ofori MF, Ndifon W. Phenotypic evidence of T cell exhaustion and senescence during symptomatic Plasmodium falciparum malaria. Front Immunol 2019;10:1345. 10.3389/fimmu.2019.01345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mair F, Prlic M. OMIP-044: 28-color immunophenotyping of the human dendritic cell compartment. Cytometry Part A 2018;93A:402–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mpina M, Maurice NJ, Yajima M, Slichter CK, Miller HW, Dutta M, McElrath MJ, Stuart KD, De Rosa SC, McNevin JP, et al. Controlled human malaria infection leads to long-lasting changes in innate and innate-like lymphocyte populations. J Immunol 2017;199:107–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zaidi I, Diallo H, Conteh S, Robbins Y, Kolasny J, Orr-Gonzalez S, Carter D, Butler B, Lambert L, Brickley E, et al. γδ T cells are required for the induction of sterile immunity during irradiated sporozoite vaccinations. J Immunol 2017;199:3781–3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller JL, Sack BK, Baldwin M, Vaughan AM, Kappe SHI. Interferon-mediated innate immune responses against malaria parasite liver stages. Cell Rep 2014;7:436–447. [DOI] [PubMed] [Google Scholar]
- 16.Burrack KS, Hart GT, Hamilton SE. Contributions of natural killer cells to the immune response against plasmodium. Malar J 2019;18:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wolf A- S, Sherratt S, Riley EM. NK cells: Uncertain allies against malaria. Front Immunol 2017;8:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hwang I, Zhang T, Scott JM, Kim AR, Lee T, Kakarla T, Kim A, Sunwoo JB, Kim S. Identification of human NK cells that are deficient for signaling adaptor FcRγ and specialized for antibody-dependent immune functions. Int Immunol 2012;24: 793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luetke-Eversloh M, Killig M, Romagnani C. Signatures of human NK cell development and terminal differentiation. Front Immunol 2013;4:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paust S, Blish CA, Reeves RK. Redefining memory: Building the case for adaptive NK cells. J Virol 2017;91:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vossen MTM, Matmati M, Hertoghs KML, Baars PA, Gent MR, Leclercq G, Hamann J, Kuijpers TW, van Lier RAW. CD27 defines phenotypically and functionally different human NK cell subsets. J Immunol 2008;180:3739–3745. [DOI] [PubMed] [Google Scholar]
- 22.Choreño Parra JA, Martínez Zúñiga N, Jiménez Zamudio LA, Jiménez Alvarez LA, Salinas Lara C, Zúñiga J. Memory of natural killer cells: A new chance against Mycobacterium tuberculosis? Front Immunol 2017;8:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Le Gars M, Seller C, Kay AW, Bayless NL, Sola E, Starosvetsky E, Moore L, Shen-Orr SS, Aziz N, Khatri P, et al. CD38 is a key regulator of enhanced NK cell immune responses during pregnancy through its role in immune synapse formation. bioRxiv 349084; 2018. doi: 10.1101/349084 [DOI] [Google Scholar]
- 24.Terhorst C, van Agthoven A, Leclair K, Snow P, Reinherz E, Schlossman S. Biochemical studies of the human thymocyte cell-surface antigens T6, T9 and T10. Cell 1981;23:771–780. [DOI] [PubMed] [Google Scholar]
- 25.Oliviero B, Varchetta S, Paudice E, Michelone G, Zaramella M, Mavilio D, De Filippi F, Bruno S, Mondelli MU. Natural killer cell functional dichotomy in chronic hepatitis B and chronic hepatitis C virus infections. Gastroenterology 2009;137: 1151–1160. [DOI] [PubMed] [Google Scholar]
- 26.Wang Y, Wang W, Shen C, Wang Y, Jiao M, Yu W, Yin H, Shang X, Liang Q, Zhao C. NKG2D modulates aggravation of liver inflammation by activating NK cells in HBV infection. Sci Rep 2017;7:1–11. 10.1038/s41598-017-00221-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Minkah NK, Wilder BK, Sheikh AA, Martinson T, Wegmair L, Vaughan AM, Kappe SHI. Innate immunity limits protective adaptive immune responses against pre-erythrocytic malaria parasites. Nat Commun 2019;10:3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.D’Ombrain MC, Hansen DS, Simpson KM, Schofield L. γδ-T cells expressing NK receptors predominate over NK cells and conventional T cells in the innate IFN-γ response to Plasmodium falciparum malaria. Eur J Immunol 2007;37:1864–1873. [DOI] [PubMed] [Google Scholar]
- 29.Hazenberg MD, Spits H. Human innate lymphoid cells. Blood 2014;124:700–709. [DOI] [PubMed] [Google Scholar]
- 30.Ng SS, Souza-Fonseca-Guimaraes F, Rivera FL, Amante FH, Kumar R, Gao Y, Sheel M, Beattie L, Montes de Oca M, Guillerey C, et al. Rapid loss of group 1 innate lymphoid cells during blood stage plasmodium infection. Clin Transl Immunol 2018;7:e1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Palomo J, Quesniaux VFJ, Togbe D, Reverchon F, Ryffel B. Unravelling the roles of innate lymphoid cells in cerebral malaria pathogenesis. Parasite Immunol 2018;40 (2):e12502. 10.1111/pim.12502. [DOI] [PubMed] [Google Scholar]
- 32.Dintwe O, Rohith S, Schwedhelm KV, McElrath MJ, Andersen-Nissen E, de Rosa SC. OMIP-056: Evaluation of human conventional T cells, donor-unrestricted T cells, and NK cells including memory phenotype by intracellular cytokine staining. Cytometry Part A 2019;95A:722–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bianca Bennstein S, Riccarda Manser A, Weinhold S, Scherenschlich N, Uhrberg M.OMIP-055: Characterization of human innate lymphoid cells from neonatal and peripheral blood. Cytometry Part A 2019;95A:427–430. [DOI] [PubMed] [Google Scholar]
- 34.Hammer Q, Romagnani C. OMIP-039: Detection and analysis of human adaptive NKG2C+ natural killer cells. Cytometry Part A 2017;91A:997–1000. [DOI] [PubMed] [Google Scholar]
- 35.Mahnke YD, Beddall MH, Roederer M. OMIP-029: Human NK-cell phenotypization. Cytometry Part A 2015;87A:986–988. [DOI] [PubMed] [Google Scholar]
- 36.Lee J, Zhang T, Hwang I, Kim A, Nitschke L, Kim MJ, Scott JM, Kamimura Y, Lanier LL, Kim S. Epigenetic modification and antibody-dependent expansion of memory-like NK cells in human cytomegalovirus-infected individuals. Immunity 2015;42:431–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.