Abstract
Our sense of touch emerges from an array of mechanosensory structures residing within the fabric of our skin. These tactile end organ structures convert innocuous forces acting on the skin into electrical signals that propagate to the CNS via the axons of low-threshold mechanoreceptors (LTMRs). Our rich capacity for tactile discrimination arises from the dissimilar intrinsic properties of the LTMR subtypes present in different regions of the skin and the structurally distinct end organ complexes with which they associate. These end organ structures include a range of non-neuronal cell types, which may themselves actively contribute to the transformation of tactile forces into neural impulses within the LTMR afferents. Though the mechanism and site of transduction across end organs remains unclear, Piezo2 has emerged as the prominent mechanosensitive channel involved in light touch. Here we review the physiological properties of LTMRs and how disparate and conserved features of their cutaneous end organ complexes shape tuning across the diverse LTMR subtypes.
Introduction
Touch lies at the heart of our interaction with, and experience of the world around us. It enables us to perceive a passing breeze, the rumbling of an approaching train, physical contact of a loved one, and the fine textural details and shape of an object held in hand, as well as providing the rapid sensory feedback essential for grip control and object manipulation. These capacities all arise from a preponderance of mechanically sensitive neurons that reside within the integumentary system. In mammals and many other animals, the skin serves as the resident site of innervation for these tactile sensors. The cell bodies of mammalian cutaneous mechanosensory neurons reside within dorsal root ganglia (DRG) and trigeminal ganglia and each gives rise to a single axon that branches to form a distal, cutaneous axonal projection and a centrally-projecting axon. It is within the distal, cutaneous endings of mechanosensory neurons that the transformation of mechanical forces into electrical signals occurs.
The sensory neurons of touch can be categorized in terms of the preferred mechanical stimulus and force threshold for spike initiation, the rate of adaption to static indentation, and their action potential conduction velocity. Mechanosensory neurons that respond to light, innocuous forces are termed low-threshold mechanoreceptors (LTMRs), and most are activated by indentation forces as low as 0.5–2 mN1–7. High-threshold mechanoreceptors (HTMRs) are activated by higher intensity mechanical forces and, though not engaged exclusively under painful conditions, are thought to be the main neural conduit of painful, or noxious, mechanical stimuli8–21.
LTMRs associate with end organ structures, which are diverse in size, shape, morphology, and location within the skin (Fig. 1, Fig. 2). Central to understanding our sense of touch, therefore, is knowing how the biomechanical properties of each end organ and surrounding tissue together with the mechanotransduction machinery within LTMR endings lead to patterns of spikes in LTMRs and their engagement of central circuits. The past decade has witnessed an explosion in our understanding of the properties and functions of LTMR subtypes and their cutaneous end organ structures. Indeed, advances in genetic tools, as well as optical and electrophysiological methods and preparations for imaging LTMR activity, are enabling an ever-finer dissection of the neural players involved in light touch. In this Review, we summarize recent advances in defining the properties of mammalian LTMRs, their principal mechanosensitive proteins, and the ultrastructural features of LTMR end organs that underlie mechanosensation.
Figure 1. The Cutaneous Mechanoreceptors of Glabrous Skin.

(A) Within glabrous skin there are at least four end organ structures involved in innocuous touch: the Merkel cell complex, Meissner corpuscle, Pacinian corpuscle, and Ruffini ending.
(B) Each end organ is innervated by distinct LTMR subtypes with unique tuning properties. Two molecularly distinct Aβ LTMR subtypes innervate the Meissner corpuscle located at the apex of dermal papillae. The TrkB+ afferent exhibits the classic Aβ RAI-LTMR response property, with rapidly adapting ON/OFF response to indentation, while the Ret+ afferent is more slowly adapting in its ON response, rarely has an OFF response, and has a higher threshold for activation. Both subtypes are equally sensitive to vibration. In deep dermal tissue, Pacinian corpuscles are innervated by Aβ RAII-LTMRs, which are unique in their capacity to detect high-frequency vibrations. At the rete ridge along the stratum basale are the Aβ SAI-LTMRs that form an intimate, synapse-like association with the mechanically sensitive epidermal Merkel cells (shown in red). Lastly, The Aβ SAII-LTMR is proposed to associate with Ruffini endings, although this relationship remains controversial. Responses shown are schematic illustrations of typical responses, see: Loewenstein and Mendelson (1965), Mountcastle et al. (1967), Knibestol and Vallbo (1970), Chambers et al. (1972), Gottschaldt and Vahle-Hinz (1981), and Neubarth, Emanuel, Liu et al. (2020).
(C) Unlike the Aβ SAI-LTMR, the Aβ RAI- and RAII-LTMR sensory afferents associated with the Meissner and Pacinian corpuscle, respectively, have a frequency dependence in their force sensitivity. The Meissner corpuscle is optimally sensitive to light forces in the range of 40–60 Hz. The Pacinian corpuscle is sensitive to force in the range of 100–300 Hz and insensitive to comparable forces delivered at lower frequencies. Adapted with permission from REF. 45.
Figure 2. The Cutaneous Mechanoreceptors of Hairy Skin.
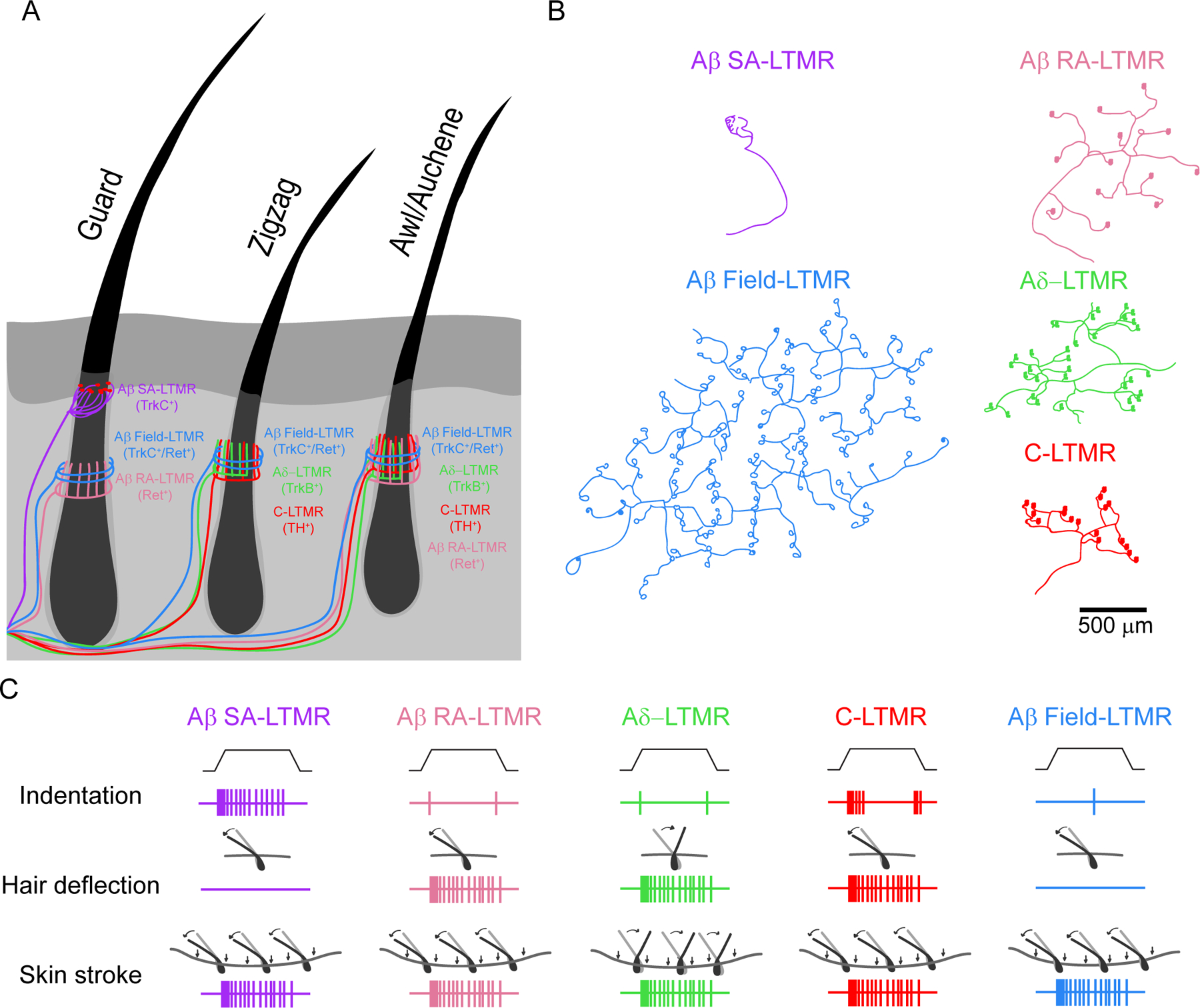
(A) Blanketing the majority of the body of mice and most mammals are three hairs (guard, zigzag, and awl/auchene hairs) that each receive a unique innervation ensemble of distinct LTMR subsets. While the Aβ RA-LTMR, Aδ-LTMR, and C-LTMRs all form lanceolate endings, the Aβ Field-LTMRs form circumferential endings and the Aβ SAI-LTMRs form touch domes with Merkel cells (shown in red). While in mice, the Aβ SAI-LTMRs form touch domes restricted to the mouth of the guard hair, the longest but least prevalent body hair, in humans, the Aβ SAI-LTMR forms touch domes in between hair follicles.
(B) Unlike the Aβ SAI-LTMRs that have a restricted terminal field area within the skin, innervating only a single guard hair in the mouse, the lanceolate-forming LTMRs innervate numerous hair follicles over a large terminal area. The lanceolate endings formed by Aδ-LTMRs are unique in their polarized innervation pattern restricted to the caudal (downward) side of the follicle. Adapted with permission from REF. 1.
(C) Each LTMR subtype innervating hairy skin has a unique set of responses to common tactile stimuli. Though Aβ SAI-LTMR, Aβ RA-LTMR, Aδ-LTMR, C-LTMR, and Aβ field-LTMRs all respond with stroke across the surface of the skin, the Aβ SAI- and Aβ field-LTMRs are insensitive to deflection of individual hairs, and the Aδ-LTMRs are sensitive to deflection of awl/auchene or zigzag hairs in trunk skin specifically along the caudal-to-rostral axis and to deflection of the hairs in paw skin specifically along the rostral-to-caudal axis. The Aβ field-LTMRs are the only hairy LTMR subtype insensitive to indentation. Though the Aβ SAI-LTMR, Aβ RA-LTMR, Aδ-LTMR, and C-LTMRs all respond to indentation, their rate of adaptation with the Aβ RA-LTMR and Aδ-LTMRs adapting the fastest and the Aβ SAI-LTMRs the slowest. Responses shown are schematic illustrations of typical responses, see: Iggo and Muir (1969), Li, Rutlin et al. (2011), Rutlin et al. (2014), Bai, Lehnert et al. (2015), and Walcher at al. (2018).
Mechanosensory end organs and LTMRs
Within an individual animal and across different animal species exist morphologically, functionally and neurophysiologically distinct regions of skin. This includes the hairy skin that blankets the majority of the body of most mammals, the beak of birds, the snout of the star-nosed mole, whisker pads of rodents and most other mammals, the non-hairy, or glabrous, skin of finger tips and genitals, and the mucocutaneous skin regions of the tongue, cheek, and lips22–31. These regions of skin differ in their tactile sensitivity, spatial acuity and the sensory-motor circuitry that they engage, as well as their unique ensembles and distribution patterns of mechanosensory end organs and associated LTMR types. Despite this wide range of neurophysiologically distinct skin regions, much of what we know about the properties of LTMR subtypes and their associated end organ structures comes from studies of glabrous digit skin and trunk hairy skin of mammals.
The LTMRs of glabrous and trunk hairy skin are diverse, with notably distinct physiological properties (Table 1, Fig. 1, Fig. 2). At one level, LTMRs are broadly classified based on conduction velocity. The heavily myelinated Aβ-LTMR nerve fibers propagate action potentials at roughly 15–100 m/s, depending on the species, while the lightly myelinated Aδ- and unmyelinated C-LTMRs conduct at intermediate (5–30 m/s) and slow (0.2–2 m/s) velocities, respectively14,28,32–34. LTMRs also differ in their rate of adaption to sustained skin indentation. The slowly adapting Aβ-LTMRs (Aβ SA-LTMRs) exhibit spiking throughout the duration of a sustained step indentation, displaying graded responses with increasing force35–38. In contrast, the rapidly adapting Aβ-LTMRs (Aβ RA-LTMRs), on the other hand, fire action potentials (often a single action potential) restricted to the onset, and in some cases the offset, of a step indentation32,39. A third distinction arises from the examination of receptive field properties. While both cutaneous Aβ SA-LTMRs and Aβ RA-LTMRs typically have small receptive field sizes, only the receptive fields of the RA-LTMRs grow with increasing force40. And yet, even under high forces, the receptive fields of glabrous skin Meissner-associated RA-LTMRs are well-defined and restricted in size, unlike the receptive fields of Pacinian corpuscle RA-LTMRs, which can span nearly the entire hand33,41. Finally, while all LTMRs can entrain to dynamic, periodic stimuli, the force threshold for activation of both cutaneous and dermal RA-LTMR subtypes is uniquely frequency dependent (Figure 1C)42–45. These and other differences in physiological properties render each LTMR subtype suited to convey distinct features of a tactile stimulus to the CNS.
Table 1.
Cutaneous Mechanoreceptor Afferents and Their End Organ Structures
| Subtype and conduction velocity1 | Skin type2 | End organ/ending type2 | Location2 | Optimal stimulus3 | Response properties3 | Labeling strategy4 |
|---|---|---|---|---|---|---|
| Aβ SAI-LTMR 16–96 m/s |
Glabrous | Merkel cell | Basal epidermis | Indentation |

|
TrkCcreER embryonic TAM |
| Hairy | Merkel cell (touch dome) | Around Guard hair follicles | ||||
| Aβ SAII-LTMR5 20–100 m/s |
Glabrous and Hairy | Ruffini? | Dermis? | Stretch |

|
unknown |
| Aβ RAI-LTMR 26–91 m/s |
Glabrous | Meissner corpuscle | Dermal papillae | Skin movement |

|
Npy2r-GFP RetcreER /TrkBcreER embryonic TAM |
| Hairy | Longitudinal lanceolate ending | Guard/Awl-Auchene hair follicles | Skin movement Hair deflection | |||
| Aβ Field-LTMR 15–25 m/s |
Hairy | Circumferential ending | Guard/Awl-Auchene/Zigzag hair follicles | Stroke |

|
TrkCcreER; RetLSL-CFP postnatal TAM |
| Aβ RAII-LTMR 26–91 m/s |
Glabrous and Hairy | Pacinian corpuscle | Deep dermis | Vibration |

|
RetcreER embyonic TAM |
| Aδ-LTMR 5–30 m/s |
Hairy | Longitudinal lanceolate ending | Awl-Auchene/Zigzag hair follicles | Skin movement Hair deflection rostral-caudal axis |

|
TrkBcreER embryonic or postnatal TAM |
| C-LTMR 0.2–2 m/s |
Hairy | Longitudinal lanceolate ending | Awl-Auchene/Zigzag hair follicles | Skin movement Hair deflection |

|
THcreER postnatal TAM |
| Aβ/Aδ/C HTMR 0.5–100 m/s |
Glabrous | Free nerve ending | Dermis/epidermis | Noxious indentation Hair pull |

|
MrgDEGFP CGRPαEGFP others |
| Hairy | Free nerve ending Circumferential ending | Dermis/epidermis Guard/Awl-Auchene/Zigzag hair follicles |
The conduction velocities of LTMR and HTMR subtypes vary across species. Please see the following references: Cain et al. (2001), Schmidt et al. (1995), Leem et al. (1993), Brown and Iggo (1967), Burgess et al. (1968), Perl (1968), and Knibestol (1973).
For reference on skin and end organ type for each mechanoreceptor subtype please see: Iggo and Muir (1969), Moll, Moll and Franke (1984), Pare, Smith and Rice (2002), Pare, Behets and Cornu (2003), Cuana and Ross (1960), Lishi and Ginty (2014), Bai, Lehnert et al. (2015), Pease and Quilliam (1957), Li et al. (2011), Halata (1993), and Ghitani et al. (2017).
In addition to indentation and hair deflection, in the case of LTMRs innervating hairy skin, many LTMR subtypes are sensitive to vibratory stimuli in a frequency-dependent (Aβ RAI- and RAII-LTMR) and frequency-independent (Ab SAI-LTMR) manner. The frequency-dependence in force sensitivity of lanceolate endings is unknown. Please see the following references for further description of response properties: Zotterman (1939), Loewenstein and Mendelson (1965), Mountcastle et al. (1967), Bessou and Perl (1969), Iggo and Muir (1969), Chambers et al. (1972), Iggo and Ogawa (1976), Gottschaldt and Vahle-Hinz (1981), Li, Rutlin et al. (2011), Rutlin et al. (2014), Bai, Lehnert et al. (2015), and Ghitani et al. (2017).
Early (P3) postnatal tamoxifen (TAM) will label Ret+ and TrkB+ LTMR afferents but will also label small diameter fibers, in the case of RetcreER and epidermal cells and satellite glia cells, in the case of TrkBcreER. Please see the following references for further description of labeling strategies: Bai, Lehnert et al. (2015), Neubarth, Emanuel, Liu et al (2020), Luo et al (2009), Rutlin et al. (2014), and Li, Rutlin et al. (2011).
Though neurons with electrophysiological properties matching the Aβ SAII-LTMR have been reported in the mouse, Ruffini endings in the mouse have not been identified. It has also been suggested the Aβ SAII-LTMR associates with unique cutaneous structures such as claws and teeth, see: Byers et al. (1985), Rice et al. (2000), and Paré et al. (2002).
The end organ structures that house LTMR terminals in glabrous and hairy skin provide an additional means of classifying the touch neuron types (Table 1). Within glabrous skin, the Aβ SAI-LTMRs form the Merkel cell-neurite complex, Aβ RAI-LTMRs associate with Meissner corpuscles, and Aβ RAII-LTMRs terminate within Pacinian corpuscles (Figure 1A-C)39,46,47. In contrast to their glabrous skin counterparts, the hairy skin innervating Aβ RA-LTMRs form the longitudinal lanceolate endings that envelope certain hair follicle types7,48–50 and the Aβ SAI-LTMRs associate with large groups of Merkel cells that comprise the crescent shaped touch dome (Figure 2A-B)51. In addition to these slowly and rapidly adapting Aβ-LTMRs, the Aβ field-LTMR exhibits an expansive, highly branched axon that forms circumferential endings that wrap around hair follicles like a corkscrew (Figure 2A-B)1. Though typically activated by static indentation at high forces and responding in a sustained manner, the Aβ field-LTMR is highly sensitive to gentle stroke across the skin (Figure 2C). Two additional LTMR subtypes, the rapidly adapting Aδ-LTMRs and slowly to intermediately adapting C-LTMRs, exclusively innervate hairy skin where, like hairy skin Aβ RA-LTMRs, they both form lanceolate endings associated with hair follicles (Figure 2A-B)7,52–56. Finally, the Aβ SAII-LTMR subtype innervates both hairy and glabrous skin and may form the Ruffini corpuscle; however, the existence, prevalence, and physiological properties of this end organ across skin regions remain debated in the field57.
It is noteworthy that, while the simplistic distinction of slowly, intermediately, and rapidly adapting LTMRs is useful to discern subtypes under conditions of static indentation of the skin, most LTMR types respond to dynamic stimuli, such as vibration and skin stroke, in a manner distinct from their static response profile. Indeed, Aβ SAI-LTMRs entrain to high frequency vibrations, firing a single action potential per stimulus cycle, while Aβ RA-LTMRs, Aδ-LTMRs and C-LTMRs fire repetitively in response to skin stroke (Figure 2C)1,7,58–60. Moreover, while each end organ and its resident LTMR subtype represents a distinct sensory unit, unique in architecture, tuning preferences, and response kinetics, the individual LTMR endings of a particular subtype often exhibit a spatially tiled and largely non-overlapping morphological arrangement across the skin (Figure 2B)1,2,61. On the other hand, between the distinct LTMR subtypes there is extensive territorial overlap in the skin. These features point to a population coding model for touch, in which our appreciation of the vast richness of touch arises from the central integration of myriad ensembles of impulses originating from LTMR subtypes, physiologically distinct in their intrinsic properties, the cutaneous end organ structures with which they associate, and their spatial arrangements across the skin.
Transduction of forces into impulses
Central to our understanding of any sensory system is an appreciation for how a stimulus is detected and converted into electrical signals, the main currency of the nervous system. It is therefore essential to understand the biomechanics of skin and end organ receptor cells, how the engulfing tissue propagates and filters mechanical forces, the proteins that form the mechanoelectrical transduction channel, the mechanism by which the channel is gated, the cell type(s) in which the channel functions, how the distribution of ion channels within receptor cells shape the receptor potential, and the site of action potential initiation within the LTMR terminal. While our appreciation of most of these is limited, recent breakthrough findings have revealed the mechanically sensitive cation channel Piezo2 as a main component of the transduction apparatus in both slowly and rapidly adapting LTMRs53,62–67. Indeed, mice and humans lacking Piezo2 exhibit profound deficits in light touch sensation, including tactile sensitivity, texture discrimination and vibration sensitivity53,62,65,66,68.
Despite the landmark discovery of Piezo2, we still understand little regarding how cutaneous stress is filtered through the ~0.1 mm epidermis and 1–2 mm dermis and how the remaining strain acts on cutaneous receptors to activate Piezo2 and trigger spiking. Indeed, the diverse microenvironments surrounding each end organ structure suggest that the same force likely yields distinct strain within the different end organs, rendering it difficult to extrapolate biomechanical properties across diverse skin regions. Furthermore, additional proteins likely play a role in scaffolding Piezo2 or in modulating tactile responses within the LTMR afferents. One such protein is stomatin-like protein 3 (STOML3), the mammalian MEC2 homologue, and mice harboring a mutation in Stoml3 have impaired tactile responsivity with 35% of skin mechanoreceptors insensitive to mechanical stimuli69. Along these lines, cell culture assays have shown that displacement thresholds are elevated in the STOML3 deficient DRG neurons and that overexpression of STOML3 lowers the threshold for mechanical currents in Piezo2-expressing neuroblastoma cells70. Thus, an appreciation of the ultrastructural and biomechanical properties of each LTMR end organ, with a focus on the subcellular localization of Piezo2, STOML3, and the other components of the mechanotransduction machinery, will further our understanding of how the various LTMR subtypes acquire their distinct response properties and kinetics.
The Merkel Cell/Aβ SAI-LTMR Complex
At the dermal-epidermal boundary of hairy and glabrous skin resides the Merkel cell-neurite complex71. The Merkel cell-neurite complex consists of Aβ SAI-LTMR terminal endings closely associated with the Merkel cells that reside in the inner most region of the epidermis, the stratum basale (Figure 1B, 3A-B). In response to sustained skin indentation within its 2–3 mm receptive field, the Aβ SAI-LTMR exhibits two physiological response phases. The initial phase has a discharge frequency that changes rapidly with the dynamic (onset) component of indentation. This early phase is dependent on force amplitude and velocity and precedes a second, force amplitude dependent steady-state phase, in which responses can be maintained for 30 minutes or longer (Figure 3C)35,51.
Figure 3. The Merkel Cell and Aβ SAI-LTMR Complex.

(A) In both hairy and glabrous skin the Aβ SAI-LTMRs (shown in purple) associate with the epidermal Merkel cells (shown in red) that lie within the basal layer of the epidermis. In hairy skin, the Merkel cells cluster to form a crescent shaped structure that either surrounds the mouth of the longest body hair, known as the guard hair, or lies between hair follicles, as is the case in humans. A single Aβ SAI-LTMR gives rise to branches that form intimate associations with the Merkel cells apical to the lanceolate and circumferential endings that surround the bulge region of the follicle. The asymmetric distribution of heminodes (green semicircles) across the branches of the Aβ SAI-LTMR is suspected of enhancing the spatial resolution of the afferent to be finer than the morphological receptive field area of the neuron. Within glabrous skin, the Aβ SAI-LTMR branches and associated with clustered Merkel cells lying within the rete ridge of the epidermis. The morphological receptive field area of a single Aβ SAI-LTMR in glabrous skin is unknown.
(B) The Aβ SAI-LTMR forms a synapse-like contact with the Merkel cells, which anchor to neighboring keratinocytes through desmosomes. On the basal side of the complex are lamellar cells than anchor the structure to the epidermis. The mechanosensitive ion channel, Piezo2, is expressed in both the Aβ SAI-LTMR axon and the Merkel cells. Adapted with permission from REF. 51.
(C) The Aβ SAI-LTMR’s response to indentation is subdivided into two stages: the initial dynamic phase (dark gray bar) when the skin is being indented and responses scale with both the amplitude and velocity of displacement, and the static phase (light gray bar) when indentation is maintained and responses are force amplitude dependent. Deletion of Piezo2 within the Merkel cells predominantly impairs the static phase of the Aβ SAI-LTMR’s response to indentation, suggesting that Piezo2 within the Aβ SAI-LTMR is involved in mediating the majority of the dynamic phase and likely part of the static phase as well. Responses shown are schematic illustrations of typical responses and are adapted with permission from REF. 63.
Aβ SAI-LTMRs also entrain to high-frequency tactile stimuli (Figure 1C) and are capable of firing a single action potential per sine wave up to 300 Hz with phase jitter of less than 10 µsec and a receptor delay of 0.2–0.3 msec42,58,72. This capacity for high frequency firing may arise from the rapid activation and inactivation kinetics of the constellation of voltage-gated channels expressed by the principal Aβ-LTMR subtypes, including Nav1.6 and Nav1.1, and the expression of the tension-activated K2P channels, TRAAK and TREK-1 within the heminode and subsequent nodes of Ranvier along Aβ-LTMR axons21,73–75. The Aβ SAI-LTMR/Merkel cell complex also responds to discontinuities in surface texture and their response is suppressed by additional neighboring indentations40,76. This sensitivity to spatial variation suggests that, in addition to the strain energy density related to direct vertical compression of the skin, shear stress across the skin surface influences receptor elements to enhance detection of fine surface details across its relatively small, invariant receptive field40,51. These three salient features of the Merkel cell-Aβ SAI-LTMR complex render it well-suited for reporting points and edges of objects.
Genetic access to the cellular components of the Aβ SAI-LTMR/Merkel complex, including Aβ SAI-LTMRs using tools that exploit the cell-type specific TrkC gene1 and Merkel cells using the transcription factor Atoh177, has enabled its morphological and functional characterization. In the dermis, a single Aβ SAI-LTMR axon branches and associates with multiple, clustered Atoh1+ Merkel cells (Figure 3A)40,51,61,78. Within glabrous skin, Merkel cell-neurite complexes lie at the trough of the dermal papillae groove with a density of roughly 500 Merkel cells/mm2 in humans79. The hairy skin Aβ SAI-LTMR counterpart associates with a touch dome located at the apical region of the longest body hair follicle type, the guard hair, in mice1,80 and adjacent to hairs in humans81,82. The touch dome is a polar, crescent-like complex ranging ~50–500 µm in diameter and contains columnar keratinocytes that lie apical to a dense collection of dozens (in some cases as many as 265) of Merkel cells83,84. In the mouse, one Aβ SAI-LTMR typically branches extensively near to the touch dome complex61. While each Merkel cell is typically associated with a single terminal branch, the complexes are often asymmetrically distributed across the heminodes of a single Aβ SAI fiber (Figure 3A)73. Modeling suggests that this arrangement heightens the reactivity of the Aβ SAI-LTMR in comparison to evenly distributed transduction units73. Extrapolating from this model, it is possible that this asymmetric convergence underlies the non-uniform receptive fields of Aβ SAI-LTMRs in humans and monkeys that enables spatial resolution finer than that predicted solely by their anatomical receptive field size85,86.
Morphological and ultrastructural features of the skin may provide insight into the cutaneous forces that activate the Merkel cell-Aβ SAI-LTMR complex. On the apical surface of the complex, desmosomes anchor Merkel cells to neighboring keratinocytes (Figure 3B)51. Along this boundary, Merkel cells extend cytoplasmic protrusions deep into the surrounding epidermis, enabling adhesion to surrounding structures51. At the base of the complex, S100+ non-myelinating Schwann cells surround the Aβ SAI-LTMR axon, securing the axon terminal to the apical side of the basement membrane51,87. Cushioning and supporting the entire complex is a dense network of collagen fibrils that extend from the dermis88. Together, the connections between the dermis and epidermis at sites close to the Merkel cell-neurite complex may underlie the biophysical coupling of local tactile forces to membrane strain within the Merkel cell and its associated Aβ SAI-LTMR ending and account for the invariant receptive field properties under conditions of increasing force.
Recent work has investigated whether Merkel cells themselves play an active role in mechanotransduction and the shaping of Aβ SAI-LTMR firing properties63,65,87,89. Classic electron micrograph studies revealed similarities between the axon-Merkel cell junction and synapses51. In addition, the presence of numerus spherical granules and synaptic proteins in Merkel cells have suggested a glutamatergic mode of communication from Merkel cells to Aβ SAI-LTMRs90,91. Recordings from Atoh1 mutant mice using an ex vivo skin nerve preparation revealed a requirement of Merkel cells for the Aβ SAI-LTMR’s response to skin indentation89. Strikingly, optogenetically-evoked depolarization of Merkel cells can elicit action potentials in Aβ SAI-LTMRs87. Moreover, electrophysiological recordings of Merkel cells demonstrated their inherent mechanosensitivity63,87 with calcium-dependent action potentials in Merkel cells required for normal Aβ SAI-LTMR responses and touch-evoked behaviors65. Though deletion of Piezo2 in Merkel cells attenuated the dynamic, initial phase of Aβ SAI-LTMRs firing, it disproportionately impaired the sustained response during the static phase of indentation (Figure 3C)62,65. These findings suggest an excitatory connection between Merkel cells and the Aβ SAI-LTMR afferent, although the nature of this communication remains debated. Indeed, recent studies of the whisker follicle suggest that the signal may be serotonergic92,93, whereas single unit recordings in skin-nerve preparations of β2 adrenergic receptor mutant animals and live-cell imaging experiments have pointed to a role of adrenergic signaling94. Follow-up experiments in the whisker system using pharmacological approaches failed to demonstrate a clear role for adrenergic signaling in the whisker Merkel cell-neurite complex95, emphasizing the need for future experiments to resolve the controversy of signaling mechanisms between Merkel cells and Aβ SAI-LTMRs and to address the possibility of co-transmitter release or differential reliance on distinct biogenic amine neurotransmitters depending on skin type.
These findings have given rise to a two-site model for mechanotransduction in the Merkel cell/Aβ SAI-LTMR complex. According to this model, the kinetics of the rapid receptor delay of Aβ SAI-LTMRs and the Aβ SAI-LTMR response that remains following Piezo2 deletion in Merkel cells, implicate the LTMR afferent as the initial site of transduction, mediating the response during the initial dynamic phase of indentation. The second site lies within the Merkel cells themselves where Piezo2-dependent activation and signal propagation to the Aβ SAI-LTMR ending underlies or enhances the sustained response of the Aβ SAI-LTMR, recorded during the static phase of indentation63,65,96. Though functional imaging experiments have revealed a cell-autonomous, neuronal dependence on Piezo2 across nearly all molecularly distinct classes of Aβ-LTMRs for air-puff, vibration, and brush responses, experiments in which step indentation, an optimal stimulus for Aβ SAI-LTMR activation, is applied to Piezo2 depleted Aβ SAI-LTMRs, with Piezo2 levels normal in Merkel cells, will be informative in addressing the cell autonomous role of Piezo2 in the Aβ SAI-LTMR for both phases of responses to step indentation and will inform our understanding of mechanotransduction in this complex.
The Pacinian Corpuscle and the Aβ RAII-LTMR
The Pacinian corpuscle (Figure 4A)97 has long served as a model for studying mechanotransduction because of its large size and prevalence across skin types. The Pacinian corpuscle is ~1 mm long in humans and is found in the deep dermis of both glabrous and hairy skin98. It is noteworthy that not all small mammals have Pacinian corpuscles/Aβ RAII-LTMRs in the skin; in mice, for example, though Pacinians are not observed in the skin, they are enriched in the periosteum of digit and limb bones and exhibit physiological properties similar to those observed in the skin of larger mammals99,100. Proper development and function of Pacinian corpuscles in both rodents and humans depend on c-Maf, a transcription factor important for the development of all end organs innervated by Aβ-RA LTMRs and useful for studying the function of these LTMRs101. The Pacinian corpuscle is structurally related to the Herbst corpuscle found in the bill of tactile foraging ducks31, revealing a conserved dependence on this structure across species and skin types that rely on vibrotactile feedback.
Figure 4. The Structure and Function of the Pacinian Corpuscle.

(A) The Pacinian corpuscle is comprised of a single neurofilament+ (NFH) Aβ RAII-LTMR (pink) axon that travels in an unbranching manner throughout the entirety of the corpuscle before terminating in the bulbous ultraterminal region. Proximal to the ultraterminal region is the terminal region of the corpuscle. Though the Aβ RAII-LTMR is initially myelinated upon entry of the main, terminal region, the axon is unmyelinated throughout the majority of the terminal and ultraterminal region of the corpuscle where it instead forms extensive associations with S100+ lamellar cells (blue). The S100+ lamellar cells form the tightly packed inner core of the Pacinian corpuscle and organize into two sets of semi-annular, cytoplasmic lamellae that extend processes to surround the central axon. Adapted with permission from REF. 109.
(B) The Aβ RAII-LTMR is unique among LTMR afferents in its insensitivity to innocuous forces delivered at low frequencies. The Aβ RAII-LTMR’s threshold for spiking decreases as frequency increases with the afferent able to entrain to light forces delivered at 500–600 Hz. Responses shown are schematic illustrations of typical responses, see: Mountcastle et al. (1967) and Talbot et al. (1968).
(C) The receptor potential of an intact Pacinian corpuscle in response to a mechanical stimulus is rapidly adapting. The potential rises during the dynamic phase of indentation and rapidly declines during the static phase of indentation, allowing only one or two impulses during the onset and offset of indentation (left). Loewenstein and colleagues showed that removal of the outer core region lead to the prolongation of the receptor potential (right), suggesting that adaptation rate is partly determined by mechanical properties of the corpuscle. Responses shown are schematic illustrations of typical responses, see: Loewenstein, W. and Mendelson, M. (1965).
The Pacinian’s Aβ RAII-LTMR responds to the dynamic phase of static indentation, exhibiting rapid adaptation at the onset and offset of a stimulus (Figure 1C)102. However, unlike other LTMR subtypes, Aβ RAII-LTMR afferents have enormous receptive fields—some even spanning nearly half the human hand33,41. Moreover, while the Aβ RAII-LTMR responds to skin deformation as little as 10 nm when applied at an optimal frequency of 200 Hz, it exhibits impressive filtering of low-frequency stimuli (Figure 4B)103. This remarkable frequency dependent tuning of the Aβ RAII-LTMR enables high-fidelity responses selectively to high-frequency, dynamic light forces104,105.
In mice, Aβ RAII-LTMRs can be labeled using genetic tools relying on the Ret gene106. A cross section through a Pacinian corpuscle reveals a single Ret+ Aβ RAII-LTMR axon that is centrally located and spans the entire length of the corpuscle (Figure 4A). Within the corpuscle, the axon is surrounded by two sets of semi-annular, cytoplasmic lamellae107. The first set of lamellae originates from S100+ non-myelinating Schwann cells that send centripetally and bilaterally arranged lamellar projections around the axon108, creating a longitudinal cleft that contains cytoplasmic branches originating from peripherally located Schwann cells and small cytoplasmic protrusions emerging from the central axon. Although the extent of these axonal protrusions is not yet appreciated, they appear to meander within an extensive collagen network that fills the interlamellar spaces and often form contacts with the centripetally arranged lamellar processes109. Vesicles of approximately 50 nm in diameter aggregate within the axon in the vicinity of these clefts, suggesting that strain along these axonal protrusions may initiate chemical communication between the neurite and surrounding glial cells107,109. Beyond this inner core region exists a more spacious peripheral zone made up of concentrically arranged lamellae of perineurial origin110,111. Piercing of the outer core lamellae reveals this structure is under turgor pressure, an attribute that may be central to the physiological responsivity of the Pacinian complex107. Interestingly, at the apex of the corpuscle, the axon branches and gives rise to a number of small protrusions that collectively form a complex, bulbous ultraterminal region (Figure 4A)109. Though the morphology of the axon in this region is distinct in its size and shape, its full extent and role in the Aβ RAII-LTMR’s response to corpuscle compression and vibration are unknown.
The large size of the Pacinian corpuscle has permitted its dissection and isolation. In classic experiments, Loewenstein and colleagues isolated single corpuscles, dissected away their lamellar cell wrappings, and recorded mechanically-evoked responses to define physical attributes that shape the receptor potential of the Aβ RAII-LTMR112–114. These studies localized the mechanoreceptor to the unmyelinated portion of axon within the central canal of the corpuscle and suggested that local summation occurs across the area of excited membrane following mechanical strain. However, whether receptor potentials are uniquely generated along the entire length of the unmyelinated axon or specifically within the axonal protrusions or within the ultraterminal region and how high frequency forces optimally activate the mechanotransduction apparatus remain unknown.
Recordings in the reduced preparation suggest that the axonal membrane serves as a main site of mechanotransduction, however it remains to be determined whether the Pacinian’s lamellar cells are themselves mechanically sensitivity and the identity of the mechanosensitive channel or channels within this structure remains unknown. Recent work in the Pacinian-like Herbst corpuscle of the duck bill has demonstrated the inherent mechanosensitvity of resident lamellar-like cells115; yet whether the lamellar cells of the mammalian Pacinian corpuscle are sensors of mechanical forces remains unexplored. Furthermore, though Piezo2 immunoreactivity has so far been undetectable in Pacinian corpuscles116, studies examining Piezo2 loss of function mutations in humans and neuron specific deletion of Piezo2 in the mouse revealed a deficit in behavioral and neural responses, respectively, to 150 Hz vibration53,66,67. These experiments suggest Piezo2 is the relevant mechanotransducer in Aβ RAII-LTMRs, though cell-type specific recordings and localization of Piezo2 within the end organ would provide a more complete model for transduction within the Pacinian corpuscle.
While the naked axon in the reduced Pacinian preparation retains mechanoreceptive properties, the characteristic rapid adaptation and off response is lost, suggesting the numerous lamellar processes and fluid-filled spaces between them creates a biomechanical filter (Figure 4C). Indeed, modeling suggests that the mechanics of outer and inner lamellar processes may amplify oscillatory strain by 8–12 fold within the corpuscle’s optimal frequency response range117. Furthermore, the turgor pressure of the fluid-filled space between lamellar wrappings and the corpuscle’s close association with resonant tissue, such as bone and vasculature, are both likely essential for maintaining the electrical resonance of the Aβ RAII-LTMR to high-frequency vibrations. Beyond this biomechanical role, the Pacinian’s lamellar cells are also hypothesized to release GABA in response to axonal excitation, leading to inhibition of neural responses during the static phase of an indentation118. Thus, the Pacinian’s lamellar cells may have complex structural, biophysical, and chemical-transmission roles in shaping the sensitivity and kinetics of Aβ RAII-LTMR responses. Nevertheless, the precise steps involved in mechanotransduction and the cellular and biophysical basis of the enigmatic Aβ RAII-LTMR’s frequency-dependent responsivity remain largely a mystery. A greater appreciation of the 3-dimensional structure of the Pacinian’s axonal and lamellar processes, biophysical measurements using intact preparations paired with selective genetic perturbations, and identification and subcellular localization of the relevant mechanosensitive ion channel complex are needed to explain the Aβ RAII-LTMR’s unique physiological properties.
The Meissner corpuscle
Because of its invariant location at the apex of dermal papillae of non-hairy skin, the ovoid shaped Meissner corpuscle119 may be the end organ of greatest proximity to the surface of glabrous skin. The corpuscle’s superficial location renders its resident Aβ RAI-LTMRs exquisitely sensitive to skin deformation by contact points that are as small as 2 µm high (Figure 5A)120. Though roughly one fifth the size of the Pacinian corpuscle, the Meissner corpuscle’s Aβ RAI-LTMRs share some of the properties of the Pacinian’s Aβ RAII-LTMRs. Indeed, the Aβ RAI-LTMR receptive field size, albeit much smaller than that of the Aβ RAII-LTMR, scales with force, and Aβ RAI-LTMRs exhibit frequency dependence in force sensitivity40,43,121. However, unlike Aβ RAII-LTMRs of the Pacinian, Aβ RAI-LTMRs of the Meissner are optimally tuned to light forces in a frequency range more often associated with slight movements across the surface of the skin (40–60 Hz) (Figure 1A), leading some to suggest they play an important role in sensing microslips to regulate grip control and object manipulation2,120,122,123. The Meissner Aβ RAI-LTMRs lie closely situated to the Merkel cell associated Aβ SAI-LTMRs, sharing similar morphological receptive fields. Despite innervation similarities between the Aβ RAI-LTMRs and Aβ SAI-LTMRs, distinctions in their physiological receptive field, frequency dependence, and adaptation kinetics have led to the suggestion that they play complementary roles in somatosensation, loosely analogous to rods and cones of the visual system124.
Figure 5. The Structure of the Meissner Corpuscle and the Unique Tuning Properties of the Sensory Afferents.

(A) Meissner corpuscles are located at the apex of the dermal papillae that extend through the layers of the epidermis (gray), nearly reaching the surface of the epidermis, in glabrous skin. The corpuscle is composed of neurofilament+ axons (red) that are surrounded by S100+ lamellar cells (blue). Within the corpuscle, which is roughly 10–15 μm in diameter in the mouse, the axons take a tortuous path while maintaining a close association with the lamellar cell processes.
(B) The majority of Meissner corpuscles are innervated by two molecularly distinct Aβ LTMRs, including the Ret+ and TrkB+ neurons. Both the Ret+ and TrkB+ neurons innervate 6–8 spatially contiguous corpuscles. Though each genetic subtype homotypically tiles glabrous skin, the receptive field area of the Ret+ and TrkB+ neurons are heterotypically offset from one another, see: Neubarth, Emanuel, Liu et al. (2020).
(C) In response to force steps, TrkB+ neurons act in a manner matching the well-described rapidly adapting Meissner afferent, exhibiting a low-force threshold and responding with only one or two spikes restricted to the onset and offset of the indentation. In contrast, the Ret+ neuron has a higher threshold for activation and rarely responds at the offset of indentation. A handful of the Ret+ neurons also have a more slowly adapting response, raising the possibility that not all Meissner afferents are rapidly adapting. Responses shown are schematic illustrations of typical responses, see: Neubarth, Emanuel, Liu et al. (2020).
(D) A cross section through a Meissner corpuscle reveals numerous ellipsoid shaped axon profiles (pink) each cushioned by numerous lamellar cell processes (blue). Nearly all axon profiles have regions of bare axon (arrow heads) where the axon extends a filopodial process. Higher magnification images reveal that each lamellar cell process is studded with a specialized membrane microdomain called caveolae (double arrowheads). Adapted with permission from REF. 2.
Recently, genetic labeling analyses found that within most Meissner corpuscles exists the endings of two genetically and physiologically distinct Aβ LTMR subtypes, a TrkB+ population and a Ret+ population (Figure 5B)2. Single unit in vivo DRG recordings revealed the TrkB+ Meissner afferent population to be the stereotypical, highly sensitive Aβ RAI-LTMR, responding exclusively at the onset and offset of skin indentations with force as low as 1–2 mN. In contrast, the Ret+ Aβ subtype was, on average, less sensitive to skin indentation and exhibited greater variability in its degree of adaptation to static indentation, indicating that not all Meissner Aβ afferents are rapidly adapting to static indentation (Figure 5C). While the role of these distinct Aβ LTMRs in Meissner function remains unclear, their unique physiological response properties suggest that they may enhance the coding capacity of the cutaneous light touch afferent system.
Within the dermis of mice, the Aβ LTMR afferents of the Meissner corpuscle branch and extend into multiple, spatially contiguous papillae in a single digit or palmar pad2. In the mouse, individual Ret+ and TrkB+ afferents branch within glabrous skin and innervate roughly 6–8 Meissner corpuscles with each afferent laying claim to a terminal zone of roughly 0.01 mm2. Across the surface of the skin, the two Meissner LTMR subtypes are homotypically tiled with their receptive fields offset from one another (Figure 5B). As both Meissner afferents extend into dermal papillae they shed their myelination and form close associations with the corpuscle’s resident non-myelinating Schwann cells, called lamellar cells. Within the corpuscle, the two, interdigitated Meissner LTMR axons take a tortuous path, sometimes running parallel and at other times perpendicular to the skin surface, perhaps ensuring reliable detection of forces originating from all angles tangential to the surface of the skin. Despite their convoluted path, lamellar cell processes, studded with caveolae-like membranous pits, maintain close association with these LTMR axons. (Figure 5D)2,125–128. Similar to the afferents of Pacinian corpuscles, clear and dense-cored vesicles are scattered throughout the axons128,129. Electron micrographs of the ellipsoid afferent axon profiles have revealed regions lacking the intimate lamellar association2,129. At these regions, the sensory afferent protrudes a filopodial extension into the surrounding extracellular matrix (Figure 5D)127. As with the Pacinian complex, the relevance of these axonal extensions for Meissner corpuscle LTMR responses is unknown.
Analogous to models of the Pacinian corpuscle, the lamellar processes of the Meissner corpuscle may serve as elastic shells amplifying strain from the outermost lamellar process to the central axon, where the mechanically gated Piezo2 cation channel likely resides62,114,116,117. Interestingly, comparative analysis of the two Meissner afferent subtypes indicated that more lamellar cell wrappings are associated with the highly sensitive TrkB+ afferents as compared to the less sensitive Ret+ afferents, suggesting a role for lamellar cell processes in setting the sensory afferent’s tactile responsivity 2. Alternatively, it is possible that the sensitivity and adaptation differences emerge from other anatomical or intrinsic properties, such as area of excitable membrane within the corpuscle130 or varying levels in expression of ion channels, such as KCNQ4131. Another striking feature of the Meissner corpuscle is the high prevalence of caveolae-like membrane invaginations within its lamellar cell processes. It can be speculated that these membrane pits enable elasticity and pliability of the axon/lamellar complex, possibly influencing the force threshold or enabling frequency-dependent activation of Meissner Aβ LTMRs. Gaining insight into the molecular constituents of these structures will be essential in defining whether they are indeed caveolae and what role they may play in LTMR physiology.
While direct evidence of Piezo2’s role in transducing mechanical forces within the corpuscle is lacking, histological and functional experiments in the mouse and human point to a neuron-specific role of Piezo2 in mediating responses in the Meissner sensory axons62,68,116. Moreover, recent evidence suggests that Meissner corpuscle lamellar cells play a critical role in touch transduction. Indeed, a recent finding revealed the transmembrane, tether-like protein USH2A, expressed within the Meissner lamellar cells, plays a critical role in shaping low-frequency vibration tuning in the Meissner Aβ RAI-LTMRs100. Indeed, extracellular protein tethers have been implicated in mechanotransduction of cultured sensory neurons132,133. Together these results suggest a model where USH2A physically links lamellar cells to the mechanically sensitive Aβ RAI-LTMR100, allowing for efficient coupling of strain across the corpuscle structure. Additionally, evidence in an analogous, but not identical, corpuscle structure within the duck bill indicates that lamellar cells themselves are mechanically sensitive, revealing the possibility that both the sensory axon and local lamellar cells may play an active role in transduction in the mammalian Meissner. As a recurring theme of this review, the relevant location of Piezo2 and other structural proteins composing the transduction apparatus within Meissner corpuscle is not known, and how Piezo2 converts mechanical strain imposed by lamellar wrappings onto both the central axon and its finger-like protrusions into electrical signals is still unknown. It is also unclear how the unique ultrastructural features of the corpuscle underlie the distinct adaptation properties and frequency dependence of the Meissner afferents. Nevertheless, with genetic access to both Meissner afferent subtypes as well as corpuscle lamellar cells, the precise steps leading from skin compression to action potential initiation at the Meissner Aβ LTMR spike initiation site in a vibration frequency-dependent manner may soon be within our grasp.
Hair follicle-associated LTMRs
Recent progress has furthered our understanding of the properties and functional organization of hairy skin LTMRs, which form associations with hair follicles to extend the sense of touch beyond the surface of the skin. The complexity of LTMR-hair follicle associations is impressive. Within murine hairy skin exist several unique hair follicle types: these include the whisker follicles and the guard, awl/auchene, and zigzag hairs, which are distributed throughout the body, exhibit distinct distribution and anatomical properties, and are innervated by unique combinations of LTMR subtype endings 1,2,7,15,53,61,106,130,134,135.
Hairy skin LTMRs forming lanceolate endings:
Guard, awl/auchene, and zigzag hairs of mice receive innervation by distinct, invariant combinations of Aβ RA-LTMRs, Aδ-LTMRs, and C-LTMRs, each of which form fence-like lanceolate endings that run parallel to the long axis of the hair follicle shaft beneath the skin’s surface7. Though the lanceolate endings of the guard hair are exclusively formed by Aβ RA-LTMRs, zigzag hairs receive interdigitated innervation by C-LTMR and Aδ-LTMR lanceolate endings, and awl/auchene hairs are innervated by lanceolate endings formed by all three subtypes, in an interdigitated manner (Figure 2A). While hairy skin Aβ RA-LTMRs and Aδ-LTMRs are rapidly adapting to static indentation of the skin and the C-LTMRs intermediately to slowly adapting, all three lanceolate ending types are highly sensitive to hair deflections and skin stroke (Figure 2C)1,7,32,136. It is reasonable to assume, therefore, that the lanceolate ending structure endows these three neurons with their robust sensitivity to hair deflection, and we speculate that their high sensitivity to skin indentation reflects subtle movements of the follicle. Nevertheless, each ending type exhibits distinct tuning properties.
The asymmetric organization of Aδ-LTMR lanceolate endings around hair follicles renders the Aδ-LTMRs uniquely direction sensitive, with the strongest responses evoked by deflection of awl/auchene or zigzag hairs of trunk skin along the caudal-to-rostral axis59 and to deflection of the hairs in paw skin along the rostral-to-caudal axis130. Despite its distinct morphology, the Aδ-LTMR, labeled using TrkB genetic tools, responds to skin indentation in a manner identical to the TrkB+ sensory afferent that innervates the Meissner corpuscle, raising the question of whether the rapidly adapting response shared by both arises from a shared ensemble of ion channels or from shared ultrastructural features. Unique among LTMR subtypes are the C-LTMRs, which were initially hypothesized to be associated with the sensation of “tickling”137 and more recently shown to respond to intermediate stroke velocities deemed “pleasurable” and linked to social and emotional aspects of touch60,138. C-LTMRs are efficiently labeled using THCreER genetic tools7,139 and express Kv4.3 channels, which in vitro experiments indicated play a role in their characteristic delayed firing in response to current injection21. Furthermore, expression of the low-voltage activated T-type calcium channel, Cav3.2, selectively in Aδ- and C-LTMRs may lower the excitability threshold of these LTMR subtypes and increase firing frequency, potentially rendering them ultrasensitive to movement across the skin140,141. Each lanceolate-forming LTMR subtype is unique in both the terminal area and total number of hair follicles they innervate within the skin, permitting distinct patterns of signal integration across their respective receptive field areas. As such, hairy skin Aβ RA-LTMRs, Aδ-LTMRs, and C-LTMRs have distinct physiological properties arising both from morphological and intrinsic biophysical differences.
The relationship between ultrastructure and function is easy to appreciate in the stereotyped and well-organized architecture of the lanceolate endings48,49,142,143. As the LTMR axon approaches the base of the follicle’s lanceolate complex domain, it branches to form many individual lanceolate processes that extend along the outer root sheath of the hair shaft1,143. Viewed in cross section, each individual lanceolate complex is comprised of a triplet or quadruplet unit containing a central lanceolate axon flanked by two or three processes originating from terminal Schwann cells (TSCs) whose cell bodies reside at the base of the complex (Figure 6A-C)143. The lanceolate complex is embedded within a matrix of longitudinally oriented collagen fibrils and is sandwiched between the epithelial cells forming the outer root sheath of the hair follicle on one side and a distal matrix of circumferentially oriented collagen fibrils on the other.
Figure 6. Ultrastructural Features of the Lanceolate and Circumferential Endings of the Guard Hair.

(A) Transverse electron micrographs through the guard hair reveal the highly organized ultrastructural features of the lanceolate and circumferential endings surrounding the hair shaft. Epidermal cells surround the hair shaft, creating the outer root sheath that is separated from the lanceolate endings by the thin basement membrane. The lanceolate endings are created by individual axon profiles, in this case the Aβ RA-LTMRs in pink, that are sandwiched by terminal Schwann cell (TSC) processes. The lanceolate endings are embedded within a longitudinal collagen matrix. The Aβ field-LTMRs lie more distal to the outer root sheath and are embedded within a circumferentially oriented collagen matrix that runs at a perpendicular angle relative to the lanceolate endings and longitudinal collagen matrix.
(B-C) Higher magnification electron micrographs reveal the intimate proximity of the Aβ RA-LTMRs and TSC processes to the basement membrane and outer root sheath (B). This close proximity has been proposed to underlie the sensitivity of the Aβ RA-LTMR to innocuous forces that indent or deflect the hair shaft. The membrane of the TSC associated with the Aβ RA-LTMRs (B) and the Aβ field-LTMRs (C) are studded with caveolae. (A-C) Adapted with permission from REF. 1.
Remarkably similar to lamellar cells of the Meissner, the membrane of the lanceolate complex TSCs is studded with caveolae-like invaginations143,144 although the molecular properties and role of these invaginations in LTMR physiology and light touch has yet to be addressed. Also noteworthy, the TSC processes form intermittent associations with one another while also often leaving a small portion of lanceolate axon membrane closest to the outer root sheath epithelial cells and the distal circumferential collagen matrix exposed (Figure 6B)143,144. It has been suggested that these intermittent regions of naked axonal membrane serve as a site of mechanotransduction143. Indeed, fine filaments of ~100 nm length, observed in both cell culture133 and in situ experiments143, may serve to anchor the sensory axon to neighboring structures. In addition to these potential “tethers”, N-cadherin adherens junctions are enriched along the boundary between the lanceolate axon and TSC membrane145. Though no in vivo functional experiments have addressed the role of these putative tether structures and adherens junctions in physiology and behavior, they may serve to stabilize the lanceolate complex and could play a central role in converting mechanical strain from hair deflection or follicle displacement into receptor potentials in the axon. Scanning electron microscopy of a lanceolate ending associated with the rat whisker follicle reveals another intriguing structural feature146. On the side distal to the hair shaft, numerous axonal processes emanate from the central lanceolate axon and extend beyond the longitudinally-oriented TSC processes, though the role of this structural feature in transduction is unexplored. Thus, while skin-nerve recording preparations and light microscopy analyses suggest Piezo2 plays a central role in the lanceolate complex62, we propose that future experiments that reveal the three-dimensional shape of the end organs, the precise localization of the mechanosensitive channel and its proximity to protein partners, adherens junctions, and putative tethers will help resolve the key question of how movement of the hair shaft triggers receptor potentials and action potentials in the three LTMR subtypes that form lanceolate endings.
Hairy skin LTMRs forming circumferential endings:
Described first in the 1960s, the Aβ field-LTMR is unique among hairy skin LTMRs due to its large, non-punctate receptive field, relatively high threshold for skin indentation responses, and insensitivity to hair deflection or air puff (Figure 2A-C)1,28. These properties are consistent with the Aβ field-LTMR being an Aβ HTMR or mechanonociceptor. However, single unit recordings in the intact animal reveal the exquisite sensitivity of Aβ field-LTMRs to gentle stroke across a large area of skin, and so we favor an LTMR designation of this neuron type. At indentation forces near threshold, Aβ field-LTMR responses are rapidly adapting while responses to higher forces are more intermediately adapting, suggesting again that this neuron does not easily fit into the simple categories outlined above. Recent genetic access to these neurons has related the physiological properties of the Aβ field-LTMR to its axon terminal structure and arborization pattern1. Perhaps surprisingly, its cutaneous endings associate with hair follicles (Figure 6A, 6C). However, the Aβ field-LTMR forms circumferential endings, embedded within the circumferentially-oriented collagen matrix just distal to the lanceolate complexes, where it winds like a corkscrew around the hair follicle lanceolate complex while maintaining close association with circumferentially oriented TSC processes. While there also exists a CGRP+ circumferential ending around hair follicles, intertwined with Aβ field-LTMR circumferential endings1,15,147, the Aβ field-LTMR may be unique in its sensitivity to innocuous forces.
These findings have suggested that the insensitivity of the Aβ field-LTMR to hair deflection arises from the lack of an intimate association with the hair follicle’s outer root sheath epithelial cells that likely serve as the fulcrum for the cantilever bending of the hair shaft. This stands in contrast to the more proximal lanceolate endings that are sensitive to even the slightest movement of the hair shaft (Figure 6B-C). The insensitivity of the Aβ field-LTMR to hair deflection and skin indentation may also correspond to dampening arising from the additional circumferentially organized collagen matrix within which the Aβ field-LTMR’s circumferential endings are embedded. However, recent anatomical and in vivo functional imaging experiments have revealed a Piezo2-dependent circumferential ending formed by Calb2-expressing Aβ-LTMRs that is sensitive to air-puff and vibration but insensitive to brush53, raising the possibility that anatomy and ultrastructure alone is not sufficient to account for tuning properties; further characterization of the ultrastructural properties of the Calb2 Aβ-LTMRs is needed to properly compare structure-function relationships across circumferential endings.
Unlike the punctate receptive fields of lanceolate ending LTMR subtypes, Aβ field-LTMRs have very large receptive fields, spanning 3–4 mm2 in size, where stimulation hotspots are separated by insensitive regions of skin1. These physiological properties align nicely with the spatial innervation pattern of the Aβ field-LTMR, where an individual neuron innervates between 20–180 hair follicles over a 3–4 mm2 area of skin (Figure 2B)1. In addition, the Aβ field-LTMR’s spike initiation sites are localized at a distance from the terminal circumferential endings, a feature that suggests subthreshold integration of receptor potentials across multiple circumferential ending complexes leading to spike initiation1. As such, the Aβ field-LTMR is unique in its high number of associations with hair follicles across an unusually large receptive field and distal spike initiation sites that may allow integration of signals from individual branches to drive the neuron’s distinctive sensitivity to gentle stroke across large regions of hairy skin. Despite the well-defined anatomical and physiological properties of the Aβ field-LTMR, ultrastructural features of this circumferential endings are not yet well understood. As with lanceolate endings of the hairy skin Aβ RA-LTMR, Aδ-LTMR and C-LTMR, the Aβ field-LTMR circumferential ending associates with TSCs; however, the 3-dimensional structure of this association is not yet appreciated. Moreover, whether Piezo2 is the relevant mechanotransduction channel in Aβ field-LTMRs is not yet known.
The elusive Aβ SAII-LTMR
The Aβ SAII-LTMRs, which some groups have hypothesized to terminate in Ruffini corpuscles148, are slowly adapting neurons with an early dynamic phase and sustained static phase linked by a period of adaptation (Figure 1A-B). Aβ SAII-LTMRs exhibit a more regular inter-spike interval during the sustained phase of firing compared to Aβ SAI-LTMRs, and unlike all other LTMRs they are reported to have baseline firing in the absence of a mechanical stimulus148,149. Their receptive field is roughly five times larger than Merkel cell-associated Aβ SAI-LTMRs and though they are ~six times less sensitive to indentation in comparison to the Aβ SAI-LTMRs, they are more sensitive to skin stretch. The Aβ SAII-LTMRs may be directionally sensitive, excited by stretch along one axis within its receptive field area and silenced by stretching the skin at a 90º offset, leading some to suggest the Aβ SAII-LTMRs play a role in the perception of hand and finger orientation105,150.
While recordings from mice to humans have revealed neurons with Aβ SAII-LTMR properties, corresponding morphological analyses often fail to identify Ruffini endings within the expected skin region29,47,151. For example, while neurons with Aβ SAII-LTMR properties that share no overlap with Merkel cells have been identified by physiological recordings in the mouse, the terminal anatomy and potential end organ structure with which these neurons associate in the mouse remains unclear149. In the hairy skin of cats, Ruffini corpuscles were described as defined structures encasing a single axon that branches, forming multiple terminals associated with longitudinally oriented collagen bundles. Surrounding the afferent is a perineurial capsule of 4–5 layers that serves to retain fluid within the corpuscle and separate the terminal endings from surrounding tissue148. Unencapsulated Ruffini-like endings have also been described at the base of fingernails or claws in monkeys and racoons and surrounding teeth in rodents29,152,153. The identification of a molecular marker for neurons with Aβ SAII-LTMR properties, if indeed they exist in genetically tractable animals, and characterization of the end organ structure innervated by these neurons will aid in clarifying their role in somatosensation and the mechanism by which this poorly characterized LTMR responds to skin stretch.
Perspectives and Future Prospects
Across the surface of our body exist anatomically and physiologically distinct skin regions specialized for detection and discrimination of an array of tactile forces. Though decades of histological examination have revealed a handful of anatomically distinct end organ types uniquely positioned and structured to detect mechanical forces acting on the skin, only recently has genetic access to LTMR subtypes enabled more detailed analysis of the tuning and adaptation properties that vary across the afferents embedded within these cutaneous tactile receptors. Indeed, with the seminal discovery of Piezo2 as a major mechanically sensitive cation channel in LTMRs, understanding how cutaneous forces are transformed into electrical signals in LTMR afferents is now within reach. Indeed, appreciating the precise subcellular localization of Piezo2 and additional components of the transduction machinery within the different LTMR subtypes will aid in our understanding of how skin indentation, hair deflection, skin stretch, or vibration gate Piezo2 across the different cutaneous end organ structures. Furthermore, transcriptomic analysis of LTMR subtypes reveals genes differentially expressed across subtypes19–21,53,154,155, and sophisticated experiments coupling the use of genetically encoded calcium sensors expressed across all LTMR subtypes with multiplexed in situ hybridization have defined a new LTMR subtype and allowed elegant comparison of transcriptomic classes and their distinct functional responses to touch, enabling a wholistic perspective on how genetically distinct LTMR subtypes represent and process touch53. Moreover, while transcriptomic datasets are enabling targeted generation of new LTMR subtype specific driver lines, relatively few molecular distinctions have been identified that fully account for the diversity of physiological properties across LTMR types. For example, the Aβ RA- and SAI-LTMR subtypes are distinct in their activation threshold, frequency tuning, receptive field stability, and adaptation kinetics yet are surprisingly similar in the ensembles of voltage-gated ion channels they express21. This underscores the prominent role of end organ structure, biomechanics of surrounding tissue, and sites of receptor potential initiation, signal integration and spike initiation, in shaping LTMR response properties. We speculate that complementing the analysis of LTMR physiology with 3-dimensional reconstructions of each of the major end organ structures and precise localization within these structures of the relevant mechanotransduction channel machinery is likely to reveal how the different tactile receptors generate patterns of action potentials that ultimately underlie our sense of touch.
Historically, LTMR afferents have been classified as the primary receptors for forces acting on the skin, with the surrounding Schwann cells serving to filter, modulate, or couple forces acting within the structure. This model is supported by the presence of Piezo2 in the LTMR membrane within the Meissner corpuscle, but not within surrounding lamellar cells116, and by the classic work suggesting the site of receptor potential generation on the central axon of the Pacinian corpuscle113,114. However, recent work points to the epithelial Merkel cell, keratinocytes, and epidermal and dermal Schwann cells as key players in light and painful touch63,87,89,115,156,157. Indeed, recent experiments have shown the inherent mechanosensitivity of non-neuronal cells, including the Merkel cell63,87, lamellar cells of Grandry and Herbst corpuscles of the duck bill115, and epidermal Schwann cells that associate with nociceptive C-fibers156, and additional work has demonstrated a role of non-neuronal cells, such as keratinocytes and lamellar cells, in modulating touch-evoked responses in the associated neurite118,157. Future experiments will be needed to determine whether the cutaneous lamellar cells and terminal Schwann cells within mammalian end organs are capable of initiating mechanical responses, whether mechanotransduction within these cells is required for LTMR physiological responses, and to clarify the nature of communication between lamellar or terminal Schwann cell processes and the neurite.
Genetic access to well-characterized LTMR subtypes has advanced the field of somatosensation research into an era in which the cellular, physical and molecular determinants that shape the sense of touch are now being addressed. Moving forward, insight into the touch sensors present in more specialized skin regions158–163 should become a focus. As an example, though it is well established that physical stimulation of the nipple by suckling elicits milk let down164, little is known about end organ structures within the nipple and the mechanosensory neurons and neural circuits involved in this essential response. Some species have evolved specialized skin regions that contain end organ structures, such as the Grandry and Herbst corpuscles of the duck bill30,31 and Eimer’s organ of the star-nosed mole24, that at a basic level resemble those found in humans, revealing a conservation in receptor structures involved in somatosensation22,115. The distinct organization of end organ structures of these tactile foraging specialists suggests that variation in tactile receptor structure, location, and density across species serve as an evolutionary solution to meet the somatosensory demands of species within their niche. Expanding comparative analyses of neurophysiology, end organ structure, and underlying molecular features will provide new clues to mechanisms of tactile transduction.
Finally, our percipience for the heterogenous properties of LTMRs and their roles in somatosensation is rooted in single unit recordings during the delivery of well-controlled, static or periodic stimuli to the skin. An appreciation for how the diverse end organs and associated LTMRs are engaged during dynamic, naturalistic touch is needed. As the German psychologist, David Katz, has said “movement [is] as indispensable for touch as light is for color sensations,”165. Indeed, enhanced coding efficiency in vision and hearing has been demonstrated using stimuli that reflect those encountered in the natural world 166,167. As with color vision and visual perception, any particular innocuous tactile stimulus can likely be represented by some unique combination of our cutaneous end organs with their resident LTMR types, each one uniquely tuned and firing with distinct patterns. Understanding the basis of touch, therefore, requires in depth appreciation for how each LTMR subtype and end organ structure gains its distinct firing pattern and how the different LTMR subtypes respond collectively during naturalistic touch. Indeed, experiments in rodents, primates and humans using more complex, naturalistic tactile stimuli have revealed a logic of compound neural activity patterns evoked across the various LTMR subtypes122,168–172. On top of this, HTMR populations likely contribute to our detection and perception of innocuous touch forces, yet relatively little is appreciated about how they are activated and how they contribute to everyday touch. The use of in vivo calcium or voltage imaging across a variety of LTMR and HTMR subtypes using naturalistic stimuli will aid our understanding for how tactile input is differentially represented across molecularly defined populations, and will reveal how both populations are engaged in innocuous and painful touch17,53. Together, this more global picture of how mechanically sensitive neurons respond to innocuous touch will move us one step closer to understanding how the disparate, yet concerted activity of mechanically sensitive neurons integrate to engage central circuits and give rise to the sense of touch and our appreciation of the richness of the physical world.
Acknowledgements
We would like to thank A. Emanuel, C. Santiago, C. Koutsioumpa, Q. Zhang, A. Tasnim, and B. Lehnert for helpful comments on the manuscript. This work was supported by NIH grant NS97344 (D.D.G.), the Edward R. and Anne G. Lefler Center for Neurodegenerative Disorders (D.D.G.), and an HHMI/Jane Coffin Childs Fellowship (A.H.). D.D.G. is an investigator of the Howard Hughes Medical Institute.
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Bai L. et al. Genetic Identification of an Expansive Mechanoreceptor Sensitive to Skin Stroking. Cell (2015) [DOI] [PMC free article] [PubMed]
- 2.Neubarth NL et al. Meissner corpuscles and their spatially intermingled afferents underlie gentle touch perception. Science (80-. ). 368, 2751 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lewin GR & McMahon SB Physiological properties of primary sensory neurons appropriately and inappropriately innervating skin in the adult rat. J. Neurophysiol. (1991) [DOI] [PubMed]
- 4.Koltzenburg M, Stucky CL & Lewin GR Receptive properties of mouse sensory neurons innervating hairy skin. J. Neurophysiol. (1997) [DOI] [PubMed]
- 5.Johansson RS, Vallbo ÅB & Westling G. Thresholds of mechanosensitive afferents in the human hand as measured with von Frey hairs. Brain Res. (1980) [DOI] [PubMed]
- 6.Cain DM, Khasabov SG & Simone DA Response properties of mechanoreceptors and nociceptors in mouse glabrous skin: An in vivo study. J. Neurophysiol. 85, 1561–1574 (2001). [DOI] [PubMed] [Google Scholar]
- 7.Li L. et al. The Functional Organization of Cutaneous Low-Threshold Mechanosensory Neurons. Cell 147, 1615–1627 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmidt R. et al. Novel classes of responsive and unresponsive C nociceptors in human skin. J. Neurosci. (1995) [DOI] [PMC free article] [PubMed]
- 9.Bessou P & Perl ER Response of cutaneous sensory units with unmyelinated fibers to noxious stimuli. J. Neurophysiol. (1969) [DOI] [PubMed]
- 10.Cavanaugh DJ et al. Distinct subsets of unmyelinated primary sensory fibers mediate behavioral responses to noxious thermal and mechanical stimuli. Proc. Natl. Acad. Sci. U. S. A. 106, 9075–9080 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Braz JM, Nassar MA, Wood JN & Basbaum AI Parallel ‘pain’ pathways arise from subpopulations of primary afferent nociceptor. Neuron 47, 787–793 (2005). [DOI] [PubMed] [Google Scholar]
- 12.Woodbury CJ, Kullmann FA, McIlwrath SL & Koerber HR Identity of myelinated cutaneous sensory neurons projecting to nocireceptive laminae following nerve injury in adult mice. J. Comp. Neurol. (2008) [DOI] [PMC free article] [PubMed]
- 13.Woodbury CJ & Koerber HR Widespread projections from myelinated nociceptors throughout the substantia gelatinosa provide novel insights into neonatal hypersensitivity. J. Neurosci. (2003) [DOI] [PMC free article] [PubMed]
- 14.Perl ER Myelinated afferent fibres innervating the primate skin and their response to noxious stimuli. J. Physiol. (1968) [DOI] [PMC free article] [PubMed]
- 15.Ghitani N. et al. Specialized Mechanosensory Nociceptors Mediating Rapid Responses to Hair Pull. Neuron 95, 944–954.e4 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hill RZ & Bautista DM Getting in Touch with Mechanical Pain Mechanisms. Trends Neurosci. 43, 311–325 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arcourt A. et al. Touch Receptor-Derived Sensory Information Alleviates Acute Pain Signaling and Fine-Tunes Nociceptive Reflex Coordination. Neuron 93, 179–193 (2017). [DOI] [PubMed] [Google Scholar]
- 18.Basbaum AI, Bautista DM, Scherrer G & Julius D. Cellular and Molecular Mechanisms of Pain. Cell 139, 267–284 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Usoskin D. et al. Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing. Nat. Neurosci. (2015) [DOI] [PubMed]
- 20.Sharma N. et al. The emergence of transcriptional identity in somatosensory neurons. Nature (2020). [DOI] [PMC free article] [PubMed]
- 21.Zheng Y. et al. Deep Sequencing of Somatosensory Neurons Reveals Molecular Determinants of Intrinsic NeuroResource Deep Sequencing of Somatosensory Neurons Reveals Molecular Determinants. Neuron 103, 598–616.e7 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schneider ER, Gracheva EO & Bagriantsev SN Evolutionary specialization of tactile perception in vertebrates. Physiology (2016) [DOI] [PMC free article] [PubMed]
- 23.Gregory JE An electrophysiological investigation of the receptor apparatus of the duck’s bill. J. Physiol. (1973) [DOI] [PMC free article] [PubMed]
- 24.Catania KC Structure and innervation of the sensory organs on the snout of the star-nosed mole. J. Comp. Neurol. (1995) [DOI] [PubMed]
- 25.Moayedi Y, Duenas-Bianchi LF & Lumpkin EA Somatosensory innervation of the oral mucosa of adult and aging mice. Sci. Rep. (2018) [DOI] [PMC free article] [PubMed]
- 26.Cottrell DF, Iggo A & Kitchell RL Electrophysiology of the afferent innervation of the penis of the domestic ram. J. Physiol. (1978) [DOI] [PMC free article] [PubMed]
- 27.Seto H Studies on the sensory innervation (human sensibility). (1963).
- 28.Burgess PR, Petit D & Warren RM Receptor types in cat hairy skin supplied by myelinated fibers. J. Neurophysiol. (1968) [DOI] [PubMed]
- 29.Paré M, Smith AM & Rice FL Distribution and terminal arborizations of cutaneous mechanoreceptors in the glabrous finger pads of the monkey. J. Comp. Neurol. 445, 347–359 (2002). [DOI] [PubMed] [Google Scholar]
- 30.Grandry M. Recherches sur les corpuscules de Pacini. J. anat. physiol. 6, 390–395 (1869). [Google Scholar]
- 31.Herbst G. Die Pacinischen Körper und ihre Bedeutung. Ein Beitrag zur Kenntnis der Nervenprimitivfasern. Vandenhoeck & Ruprecht, Göttingen. (1848).
- 32.Brown AG & Iggo A. A quantitative study of cutaneous receptors and afferent fibres in the cat and rabbit. J. Physiol. 193, 707–733 (1967). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Knibestöl M. Stimulus—response functions of rapidly adapting mechanoreceptors in the human glabrous skin area. J. Physiol. (1973) [DOI] [PMC free article] [PubMed]
- 34.Leem W, Chung J & Willis J. Cutaneous sensory receptors in the rat foot. J. Neurophysiol. (1993). [DOI] [PubMed]
- 35.Werner G & Mountcastle VB Neural activity in mechanoreceptive cutaneous afferents: Stimulus-response relations, Weber functions, and information transmission. J. Neurophysiol. (1965) [DOI] [PubMed]
- 36.Knibestöl M & Vallbo B. Intensity of sensation related to activity of slowly adapting mechanoreceptive units in the human hand. J. Physiol. (1980) [DOI] [PMC free article] [PubMed]
- 37.Coleman GT, Bahramali H, Zhang HQ & Rowe MJ Characterization of tactile afferent fibers in the hand of the marmoset monkey. J. Neurophysiol. (2001) [DOI] [PubMed]
- 38.Harrington T & Merzenich MM Neural coding in the sense of touch: Human sensations of skin indentation compared with the responses of slowly adapting mechanoreceptive afferents innervating the hairy skin of monkeys. Exp. Brain Res. (1970) [DOI] [PubMed]
- 39.Iggo BA & Ogawa H Correlative physiological and morphological studies of rapidly adapting mechanoreceptors in cat’s glabrous skin. 275–296 (1977). [DOI] [PMC free article] [PubMed]
- 40.Vega-Bermudez F & Johnson KO SA1 and RA receptive fields, response variability, and population responses mapped with a probe array. J. Neurophysiol. 81, 2701–2710 (1999). [DOI] [PubMed] [Google Scholar]
- 41.Vallbo a B. & Johansson RS The tactile sensory innervation of the glabrous skin of the human hand. Act. Touch, Mech. Recognit. Objects by Manip. (1978).
- 42.Talbot WH, Darian-Smith I, Kornhuber HH & Mountcastle VB The sense of flutter-vibration: comparison of the human capacity with response patterns of mechanoreceptive afferents from the monkey hand. J. Neurophysiol. 31, 301–334 (1968). [DOI] [PubMed] [Google Scholar]
- 43.Mountcastle VB, Talbot WH, Dar-Smith I & Kornhuber HH Neural basis of the sense of flutter-vibration. Science (80-. ). 155, 597–600 (1967). [DOI] [PubMed] [Google Scholar]
- 44.Vallbo AB & Johansson RS Properties of cutaneous mechanoreceptors in the human hand related to touch sensation. Hum. Neurobiol. (1984). [PubMed]
- 45.Roe A, Friedman R & Chen L Handbook of neurochemistry and molecular neurobiology: Sensory neurochemistry. in Handbook of Neurochemistry and Molecular Neurobiology: Sensory Neurochemistry (eds. Johnson DA & LLajtha A) 1–16 (Springer Science+Business Media, LLC., 2007). [Google Scholar]
- 46.Knibestöl M & Vallbo B. Single Unit Analysis of Mechanoreceptor Activity from the Human Glabrous Skin. Acta Physiol. Scand. (1970) [DOI] [PubMed]
- 47.Johansson RS & Vallbo AB Tactile sensibility in the human hand: relative and absolute densities of four types of mechanoreceptive units in glabrous skin. J. Physiol. (1979) [DOI] [PMC free article] [PubMed]
- 48.Yamamoto T. The fine structure of the palisade-type sensory endings in relation to hair follicles. J. Electron Microsc. (Tokyo). (1966) [PubMed]
- 49.Halata Z. Sensory innervation of the hairy skin (light-and electronmicroscopic study). J. Invest. Dermatol. (1993) [DOI] [PubMed]
- 50.Halata Z & Munger BL Sensory nerve endings in rhesus monkey sinus hairs. J. Comp. Neurol. (1980) [DOI] [PubMed]
- 51.Iggo A & Muir A The structure and function of a slowly adapting touch corpuscle in hairy skin. 1882, 763–796 (1969). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Seal RP et al. Injury-induced mechanical hypersensitivity requires C-low threshold mechanoreceptors. Nature 462, 651–655 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.von Buchholtz LJ et al. Decoding Cellular Mechanisms for Mechanosensory Discrimination. Neuron 109, 285–298.e5 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nordin BYM With Unmyelinated ( C ) Fibres in the Human. Physiology 229–240 (1990). [DOI] [PMC free article] [PubMed]
- 55.Iggo A & Kornhuber HH A quantitative study of C-mechanoreceptors in hairy skin of the cat. J. Physiol. (1977) [DOI] [PMC free article] [PubMed]
- 56.Lynn B & Carpenter SE Primary afferent units from the hairy skin of the rat hind limb. Brain Res. (1982) [DOI] [PubMed]
- 57.Paré M, Behets C & Cornu O. Paucity of presumptive Ruffini corpuscles in the index finger pad of humans. J. Comp. Neurol. (2003) [DOI] [PubMed]
- 58.Gottschaldt KM & Vahle-Hinz C. Merkel cell receptors: Structure and transducer function. Science (80-. ). 214, 183–186 (1981). [DOI] [PubMed] [Google Scholar]
- 59.Rutlin M. et al. The cellular and molecular basis of direction selectivity of Aδ-LTMRs. Cell 159, 1640–1651 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Löken LS, Wessberg J, Morrison I, McGlone F & Olausson H. Coding of pleasant touch by unmyelinated afferents in humans. Nat. Neurosci. (2009) [DOI] [PubMed]
- 61.Kuehn ED, Meltzer S, Abraira VE, Ho CY & Ginty DD Tiling and somatotopic alignment of mammalian low-threshold mechanoreceptors. Proc. Natl. Acad. Sci. U. S. A. 116, 9168–9177 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ranade SS et al. Piezo2 is the major transducer of mechanical forces for touch sensation in mice. Nature 516, 121–125 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Woo S. et al. Piezo2 is required for Merkel-cell mechanotransduction. Nature 509, 622–626 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Coste B. et al. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science (80-. ). (2010) [DOI] [PMC free article] [PubMed]
- 65.Ikeda R. et al. Merkel cells transduce and encode tactile stimuli to drive aβ-Afferent impulses. Cell 157, 664–675 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Szczot M. et al. PIEZO2 mediates injury-induced tactile pain in mice and humans. Sci. Transl. Med. 10, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chesler A. et al. The role of PIEZO2 in human mechanosensation. N. Engl. J. Med. 375, 1355–1364 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chesler A. et al. The Role of PIEZO2 in Human Mechanosensation Alexander. N Engl. J. Med. (2016). [DOI] [PMC free article] [PubMed]
- 69.Wetzel C. et al. A stomatin-domain protein essential for touch sensation in the mouse. Nature 445, 206–209 (2007). [DOI] [PubMed] [Google Scholar]
- 70.Poole K, Herget R, Lapatsina L, Ngo HD & Lewin GR Tuning Piezo ion channels to detect molecular-scale movements relevant for fine touch. Nat. Commun. 5, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Merkel F. Tastzellen und Tastkörperchen bei den Hausthieren und beim Menschen. Arch. für Mikroskopische Anat. (1875)
- 72.Ribot-Ciscar E, Vedel JP & Roll JP Vibration sensitivity of slowly and rapidly adapting cutaneous mechanoreceptors in the human foot and leg. Neurosci. Lett. 104, 130–135 (1989). [DOI] [PubMed] [Google Scholar]
- 73.Lesniak DR et al. Computation identifies structural features that govern neuronal firing properties in slowly adapting touch receptors. Elife 3, 1–20 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brohawn SG et al. The mechanosensitive ion channel TRAAK is localized to the mammalian node of Ranvier. Elife (2019) [DOI] [PMC free article] [PubMed]
- 75.Kanda H. et al. TREK-1 and TRAAK Are Principal K+ Channels at the Nodes of Ranvier for Rapid Action Potential Conduction on Mammalian Myelinated Afferent Nerves. Neuron 104, 960–971.e7 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vega-Bermudez F & Johnson KO Surround suppression in the responses of primate SA1 and RA mechanoreceptive afferents mapped with a probe array. J. Neurophysiol. 81, 2711–2719 (1999). [DOI] [PubMed] [Google Scholar]
- 77.Ben-Arie N. et al. Functional conservation of atonal and Math1 in the CNS and PNS. Development (2000). [DOI] [PubMed]
- 78.Lindblom U & Tapper DN Integration of impulse activity in a peripheral sensory unit. Exp. Neurol. (1966) [DOI] [PubMed]
- 79.Moll R, Moll I & Franke WW Identification of Merkel cells in human skin by specific cytokeratin antibodies: Changes of cell density and distribution in fetal and adult plantar epidermis. Differentiation 28, 136–154 (1984). [DOI] [PubMed] [Google Scholar]
- 80.Woodbury CJ & Koerber HR Central and peripheral anatomy of slowly adapting type I low-threshold mechanoreceptors innervating trunk skin of neonatal mice. J. Comp. Neurol. (2007) [DOI] [PubMed]
- 81.Kabata Y, Orime M, Abe R & Ushiki T. The morphology, size and density of the touch dome in human hairy skin by scanning electron microscopy. Microscopy (2019) [DOI] [PubMed]
- 82.Zelena J Nerves and Mechanoreceptors. (1994).
- 83.Orime M, Ushiki T, Koga D & Ito M. Three-dimensional morphology of touch domes in human hairy skin by correlative light and scanning electron microscopy. Journal of Investigative Dermatology (2013) [DOI] [PubMed]
- 84.Owens DM & Lumpkin EA Diversification and specialization of touch receptors in skin. Cold Spring Harb. Perspect. Med. (2014) [DOI] [PMC free article] [PubMed]
- 85.Vallbo AB, Olausson H, Wessberg J & Kakuda N. Receptive field characteristics of tactile units with myelinated afferents in hairy skin of human subjects. J. Physiol. (1995) [DOI] [PMC free article] [PubMed]
- 86.Pruszynski JA & Johansson RS Edge-orientation processing in first-order tactile neurons. Nat. Neurosci. 17, 1404–1409 (2014). [DOI] [PubMed] [Google Scholar]
- 87.Maksimovic S. et al. Epidermal Merkel cells are mechanosensory cells that tune mammalian touch receptors. Nature 509, 617–621 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ebara S, Kumamoto K, Baumann KI & Halata Z. Three-dimensional analyses of touch domes in the hairy skin of the cat paw reveal morphological substrates for complex sensory processing. Neurosci. Res. (2008) [DOI] [PubMed]
- 89.Maricich SM et al. Merkel cells are essential for light-touch responses. Science (80-. ). 324, 1580–1582 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hitchcock IS, Genever PG & Cahusac PMB Essential components for a glutamatergic synapse between Merkel cell and nerve terminal in rats. Neurosci. Lett. 362, 196–199 (2004). [DOI] [PubMed] [Google Scholar]
- 91.Fagan BM & Cahusac PMB Evidence for glutamate receptor mediated transmission at mechanoreceptors in the skin. Neuroreport (2001) [DOI] [PubMed]
- 92.Chang W. et al. Merkel disc is a serotonergic synapse in the epidermis for transmitting tactile signals in mammals. Proc. Natl. Acad. Sci. U. S. A. (2016) [DOI] [PMC free article] [PubMed]
- 93.Chang W & Gu JG Effects on tactile transmission by serotonin transporter inhibitors at Merkel discs of mouse whisker hair follicles. Mol. Pain (2020) [DOI] [PMC free article] [PubMed]
- 94.Hoffman BU et al. Merkel Cells Activate Sensory Neural Pathways through Adrenergic Synapses. Neuron (2018) [DOI] [PMC free article] [PubMed]
- 95.Sonekatsu M, Gu SL, Kanda H & Gu JG Effects of norepinephrine and β2 receptor antagonist ICI 118,551 on whisker hair follicle mechanoreceptors dissatisfy Merkel discs being adrenergic synapses. Mol. Brain 12, 10–13 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Woo S-H, Lumpkin EA & Patapoutian A. Merkel cells and neurons keep in touch. 100, 130–134 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pacini F. Sopra un particolare genere di piccoli corpi globosi scoperti nel corpo umano da Filippo Pacini. Lett. to Accad. Medico-Fisica di Firenze (1835).
- 98.Bell J, Bolanowski S & Holmes MH The structure and function of pacinian corpuscles: A review. Progress in Neurobiology (1994) [DOI] [PubMed]
- 99.Zelená J. The development of Pacinian corpuscles. J. Neurocytol. (1978) [DOI] [PubMed]
- 100.Schwaller F. et al. USH2A is a Meissner’s corpuscle protein necessary for normal vibration sensing in mice and humans. Nat. Neurosci. (2020) [DOI] [PubMed]
- 101.Wende H. et al. The transcription factor c-Maf controls touch receptor development and function. Science (80-. ). 335, 1373–1376 (2012). [DOI] [PubMed] [Google Scholar]
- 102.Alvarez-Buylla R & de Arellano J. Local responses in Pacinian corpuscles. Am. J. Physiol. 172, 237–244 (1953). [DOI] [PubMed] [Google Scholar]
- 103.Brisben AJ, Hsiao SS & Johnson KO Detection of vibration transmitted through an object grasped in the hand. J. Neurophysiol. (1999) [DOI] [PubMed]
- 104.Mountcastle VB, LaMotte RH & Carli G. Detection thresholds for stimuli in humans and monkeys: comparison with threshold events in mechanoreceptive afferent nerve fibers innervating the monkey hand. J. Neurophysiol. (1972) [DOI] [PubMed]
- 105.Johnson KO The roles and functions of cutaneous mechanoreceptors. Curr. Opin. Neurobiol. 11, 455–461 (2001). [DOI] [PubMed] [Google Scholar]
- 106.Luo W, Enomoto H, Rice FL, Milbrandt J & Ginty DD Molecular Identification of Rapidly Adapting Mechanoreceptors and Their Developmental Dependence on Ret Signaling. Neuron (2009) [DOI] [PMC free article] [PubMed]
- 107.Pease DC & Quilliam TA Electron microscopy of the pacinian corpuscle. J. Biophys. Biochem. Cytol. 3, 331–342 (1957). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Vega JA et al. The inner-core, outer-core and capsule cells of the human pacinian corpuscles: An Immunohistochemical study. Eur. J. Morphol. (1994). [PubMed]
- 109.Spencer PS & Schaumburg HH An ultrastructural study of the inner core of the Pacinian corpuscle. J. Neurocytol. (1973) [DOI] [PubMed]
- 110.Vega JA, García-Suárez O, Montaño JA, Pardo B & Cobo JM The Meissner and Pacinian sensory corpuscles revisited new data from the last decade. Microsc. Res. Tech. (2009) [DOI] [PubMed]
- 111.García-Piqueras J. et al. Endoneurial-CD34 positive cells define an intermediate layer in human digital Pacinian corpuscles. Ann. Anat. (2017) [DOI] [PubMed]
- 112.Loewenstein W. On the ‘specificity’ of a sensory receptor. J. Neurophysiol. 24, 150–158 (1961). [DOI] [PubMed] [Google Scholar]
- 113.Loewenstein W. Excitation and Inactivation in a Receptor Membrane. Ann. N. Y. Acad. Sci. 94, 510–534 (1961). [DOI] [PubMed] [Google Scholar]
- 114.Loewenstein W & Mendelson M. Components of receptor adaptation in a Pacinian corpuscle. J. Physiol. 177, 377–397 (1965). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nikolaev YA et al. Lamellar cells in Pacinian and Meissner corpuscles are touch sensors. Sci. Adv. (2020). [DOI] [PMC free article] [PubMed]
- 116.García-Mesa Y. et al. Merkel cells and Meissner’s corpuscles in human digital skin display Piezo2 immunoreactivity. J. Anat. 231, 978–989 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Quindlen JC, Stolarski HK, Johnson MD & Barocas VH A multiphysics model of the Pacinian corpuscle. Integr. Biol. (United Kingdom) 8, 1111–1125 (2016). [DOI] [PubMed] [Google Scholar]
- 118.Pawson L. et al. GABAergic/glutamatergic-glial/neuronal interaction contributes to rapid adaptation in pacinian corpuscles. J. Neurosci. 29, 2695–2705 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wagner R & Meissner G. Uber das Vorhandensein bisher unbekannter eigenthumlicher Tastkorperchen (corpuscula tactus) in den Gefuhlswarzchen der menschlichen Haut und uber die Endausbreitung sensitiver Nerven. v. d. Georg. u. d. Konigl. Ges. d. Wiss. zu Gottingen 17–30 (1852).
- 120.LaMotte RH & Whitehouse J. Tactile detection of a dot on a smooth surface: Peripheral neural events. J. Neurophysiol. (1986) [DOI] [PubMed]
- 121.Lindblom U. Properties of touch receptors in distal glabrous skin of the monkey. J. Neurophysiol. (1965) [DOI] [PubMed]
- 122.Srinivasan MA, Whitehouse JM & LaMotte RH Tactile detection of slip: Surface microgeometry and peripheral neural codes. J. Neurophysiol. 63, 1323–1332 (1990). [DOI] [PubMed] [Google Scholar]
- 123.Freeman AW & Johnson KO A model accounting for effects of vibratory amplitude on responses of cutaneous mechanoreceptors in macaque monkey. J. Physiol. (1982) [DOI] [PMC free article] [PubMed]
- 124.Johnson KO, Yoshioka T & Vega Bermudez F. Tactile functions of mechanoreceptive afferents innervating the hand. J. Clin. Neurophysiol. 17, 539–558 (2000). [DOI] [PubMed] [Google Scholar]
- 125.Idé C. Development of meissner corpuscle of mouse toe pad. Anat. Rec. 188, 49–67 (1977). [DOI] [PubMed] [Google Scholar]
- 126.Hashimoto K. Fine structure of the Meissner corpuscle of human palmar skin. J. Invest. Dermatol. (1973) [DOI] [PubMed]
- 127.Takahashi-Iwanaga H. The three-dimensional microanatomy of Meissner corpuscles in monkey palmar skin. Journal of Neurocytology vol. 32 (2003). [DOI] [PubMed] [Google Scholar]
- 128.Cuana N & Ross LL The fine structure of Meissner’s touch corpuscles of human fingers. J. Biophys. Biochem. Cytol. 8, 467–482 (1960). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Idé C. The fine structure of the digital corpuscle of the mouse toe pad, with special reference to nerve fibers. Am. J. Anat. 147, 329–355 (1976). [DOI] [PubMed] [Google Scholar]
- 130.Walcher J. et al. Specialized mechanoreceptor systems in rodent glabrous skin. J. Physiol. 596, 4995–5016 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Heidenreich M. et al. KCNQ4 K + channels tune mechanoreceptors for normal touch sensation in mouse and man. Nat. Neurosci. (2012) [DOI] [PubMed]
- 132.Chiang LY et al. Laminin-332 coordinates mechanotransduction and growth cone bifurcation in sensory neurons. Nat. Neurosci. 14, 993–1000 (2011). [DOI] [PubMed] [Google Scholar]
- 133.Hu J, Chiang LY, Koch M & Lewin GR Evidence for a protein tether involved in somatic touch. EMBO J. (2010) [DOI] [PMC free article] [PubMed]
- 134.Wu H, Williams J & Nathans J. Morphologic diversity of cutaneous sensory afferents revealed by genetically directed sparse labeling. Elife (2012) [DOI] [PMC free article] [PubMed]
- 135.Bourane S. et al. Low-Threshold Mechanoreceptor Subtypes Selectively Express MafA and Are Specified by Ret Signaling. Neuron (2009) [DOI] [PubMed]
- 136.Iggo A. Cutaneous mechanoreceptors with afferent C fibres. J. Physiol. (1960) [DOI] [PMC free article] [PubMed]
- 137.Zotterman Y. Touch, pain and tickling: an electro-physiological investigation on cutaneous sensory nerves. J. Physiol. (1939) [DOI] [PMC free article] [PubMed]
- 138.Vallbo Å, Olausson H, Wessberg J & Norrsell U. A system of unmyelinated afferents for innocuous mechanoreception in the human skin. Brain Res. (1993) [DOI] [PubMed]
- 139.Abraira V. et al. The Cellular and Synaptic Architecture of the Mechanosensory Dorsal Horn. Cell 168, 295–310.e19 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.François A. et al. The Low-Threshold Calcium Channel Cav3.2 Determines Low-Threshold Mechanoreceptor Function. Cell Rep. (2015) [DOI] [PubMed]
- 141.Wang R & Lewin GR The Cav3.2 T-type calcium channel regulates temporal coding in mouse mechanoreceptors. J. Physiol. (2011) [DOI] [PMC free article] [PubMed]
- 142.Hashimoto K. Fine structure of perifollicular nerve endings in human hair. J. Invest. Dermatol. 59, 432–441 (1972). [DOI] [PubMed] [Google Scholar]
- 143.Li L & Ginty DD The structure and organization of lanceolate mechanosensory complexes at mouse hair follicles. Elife 3, 1–24 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kaidoh T & Inoué T. Intercellular junctions between palisade nerve endings and outer root sheath cells of rat vellus hairs. J. Comp. Neurol. 420, 419–427 (2000). [DOI] [PubMed] [Google Scholar]
- 145.Kaidoh T & Inoué T. N-Cadherin Expression in Palisade Nerve Endings of Rat Vellus Hairs. J. Comp. Neurol. 506, 525–534 (2008). [DOI] [PubMed] [Google Scholar]
- 146.Takahashi-Iwanaga H. Three-dimensional microanatomy of longitudinal lanceolate endings in rat vibrissae. J. Comp. Neurol. 426, 259–269 (2000). [DOI] [PubMed] [Google Scholar]
- 147.Fünfschilling U. et al. TrkC kinase expression in distinct subsets of cutaneous trigeminal innervation and nonneuronal cells. J. Comp. Neurol. (2004) [DOI] [PMC free article] [PubMed]
- 148.Chambers MR, Andres KH, Duering M & Iggo A The structure and function of the slowly adapting type II mechanoreceptor in hairy skin. 417–445 (1972). [DOI] [PubMed]
- 149.Wellnitz SA, Lesniak DR, Gerling GJ & Lumpkin EA The regularity of sustained firing reveals two populations of slowly adapting touch receptors in mouse hairy skin. J. Neurophysiol. (2010) [DOI] [PMC free article] [PubMed]
- 150.Fleming MS & Luo W. The anatomy, function, and development of mammalian Aβ low-threshold mechanoreceptors. Frontiers in Biology vol. 8 408–420 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Rasmusson DD & Turnbull BG Sensory innervation of the raccoon forepaw: 2. Response properties and classification of slowly adapting fibers. Somatosens. Mot. Res. (1986) [DOI] [PubMed]
- 152.Rice FL & Rasmusson DD Innervation of the digit on the forepaw of the raccoon. J. Comp. Neurol. (2000) [DOI] [PubMed]
- 153.Byers MR Sensory innervation of periodontal ligament of rat molars consists of unencapsulated Ruffini-like mechanoreceptors and free nerve endings. J. Comp. Neurol. (1985) [DOI] [PubMed]
- 154.Li CL et al. Somatosensory neuron types identified by high-coverage single-cell RNA-sequencing and functional heterogeneity. Cell Res. (2016) [DOI] [PMC free article] [PubMed]
- 155.Nguyen MQ, Wu Y, Bonilla LS, von Buchholtz LJ & Ryba NJP Diversity amongst trigeminal neurons revealed by high throughput single cell sequencing. PLoS One (2017) [DOI] [PMC free article] [PubMed]
- 156.Abdo H. et al. Specialized cutaneous schwann cells initiate pain sensation. Science (80-. ). 365, 695–699 (2019). [DOI] [PubMed] [Google Scholar]
- 157.Moehring F. et al. Keratinocytes mediate innocuous and noxious touch via ATP-P2X4 signaling. Elife 7, 1–35 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Winkelmann RK The Mucocutaneous End-Organ: The Primary Organized Sensory Ending in Human Skin. A. M. A. Arch. Dermatology (1957) [DOI] [PubMed]
- 159.Yamada K. On the sensory nerve terminations in clitoris in human adult. Tohoku J. Exp. Med. (1951) [DOI] [PubMed]
- 160.Gairns FW THE SENSORY NERVE ENDINGS OF THE HUMAN PALATE. Q. J. Exp. Physiol. Cogn. Med. Sci. (1955) [DOI] [PubMed]
- 161.Cathcart EP, Gairns FW & Garven HSD XXIV.”The Innervation of the Human Quiescent Nipple, with Notes on Pigmentation, Erection, and Hyperneury. Trans. R. Soc. Edinburgh (1949)
- 162.Ohmori D. Über die Entwicklung der Innervation der Genitalapparate als peripheren Aufnahmeapparat der genitalen Reflexe. Z. Anat. Entwicklungsgesch. (1924)
- 163.Krause W. Uber die Nervenendigung in den Geschlechtsorganen. Zeitschrift rate. Med. 28, 86–88 (1866). [Google Scholar]
- 164.McNeilly AS Physiology of lactation. J. Biosoc. Sci. (1977)
- 165.Katz D. The World of Touch. Z. Psychol. (1925).
- 166.Rieke F, Bodnar DA & Bialek W. Naturalistic stimuli increase the rate and efficiency of information transmission by primary auditory afferents. Proc. R. Soc. B Biol. Sci. (1995) [DOI] [PubMed]
- 167.Lewicki MS Efficient coding of natural sounds. Nat. Neurosci. (2002) [DOI] [PubMed]
- 168.Leiser SC & Moxon KA Responses of Trigeminal Ganglion Neurons during Natural Whisking Behaviors in the Awake Rat. Neuron (2007) [DOI] [PubMed]
- 169.Blake DT, Hsiao SS & Johnson KO Neural coding mechanisms in tactile pattern recognition: The relative contributions of slowly and rapidly adapting mechanoreceptors to perceived roughness. J. Neurosci. 17, 7480–7489 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Macefield VG, Häger-Ross C & Johansson RS Control of grip force during restraint of an object held between finger and thumb: Responses of cutaneous afferents from the digits. Exp. Brain Res. 108, 155–171 (1996). [DOI] [PubMed] [Google Scholar]
- 171.Severson KS et al. Active Touch and Self-Motion Encoding by Merkel Cell-Associated Afferents. Neuron (2017) [DOI] [PMC free article] [PubMed]
- 172.Hulliger M, Nordh E, Thelin AE & Vallbo AB The responses of afferent fibres from the glabrous skin of the hand during voluntary finger movements in man. J. Physiol. (1979) [DOI] [PMC free article] [PubMed]


