Abstract
Background
Acute respiratory distress syndrome (ARDS) is a life-threatening clinical syndrome whose potential to become one of the most grievous challenges of the healthcare system evidenced by the COVID-19 pandemic. Considering the lack of target-specific treatment for ARDS, it is absolutely exigent to have an effective therapeutic modality to reduce hospitalization and mortality rate as well as to improve quality of life and outcomes for ARDS patients. ARDS is a systemic inflammatory disease starting with the pulmonary system and involves all other organs in a morbid bidirectional fashion. Mounting evidence including our findings supporting the notion that cannabinoids have potential to be targeted as regulatory therapeutic modalities in the treatment of inflammatory diseases. Therefore, it is plausible to test their capabilities as alternative therapies in the treatment of ARDS. In this study, we investigated the potential protective effects of cannabichromene (CBC) in an experimental model of ARDS.
Methods
We used, for the first time, an inhalant CBC treatment as a potential therapeutic target in a murine model of ARDS-like symptoms. ARDS was induced by intranasal administration of Poly(I:C), a synthetic mismatched double-stranded RNA, into the C57BL/6 mice (6–10 male mice/group, including sham, placebo, and CBC treated), three once-daily doses followed by a daily dose of inhalant CBC or placebo for the period of 8 days starting the first dose 2 h after the second Poly(I:C) treatment. We employed histologic, immunohistochemistry, and flow cytometry methods to assess the findings. Statistical analysis was performed by using one way analysis of variance (ANOVA) followed by Newman–Keuls post hoc test to determine the differences among the means of all experimental groups and to establish significance (p < 0.05) among all groups.
Results
Our data showed that CBC was able to reverse the hypoxia (increasing blood O2 saturation by 8%), ameliorate the symptoms of ARDS (reducing the pro-inflammatory cytokines by 50% in lung and blood), and protect the lung tissues from further destruction. Further analysis showed that CBC may wield its protective effects through transient receptor potential (TRP) cation channels, TRPA1 and TRPV1, increasing their expression by 5-folds in lung tissues compared to sham and untreated mice, re-establishing the homeostasis and immune balance.
Conclusion
Our findings suggest that inhalant CBC may be an effective alternative therapeutic target in the treatment of ARDS. In addition, Increased expression of TRPs cation channels after CBC treatment proposes a novel role for TRPs (TRPA1 and TRPV2) as new potential mechanism to interpret the beneficial effects of CBC as well as other cannabinoids in the treatment of ARDS as well as other inflammatory diseases. Importantly, delivering CBC through an inhaler device is a translational model supporting the feasibility of trial with human subjects, authorizing further research.
Keywords: Cannabichromene, CBC, ARDS, TRPA1, TRPV1, COVID-19
Introduction
Acute respiratory distress syndrome (ARDS) is a serious complication of sepsis, initiated by cytokine storm due to imbalance of host immunity with exaggerated inflammatory responses (Hu et al., 2020; Matthay et al., 2019). Despite controversy in the definition of ARDS, there is a consensus over the crucial role of Immune response dysregulation in the pathophysiology of ARDS (Matthay et al., 2019; Matthay et al., 2021). As a systemic inflammatory disease, ARDS is initiated by activation of alveolar macrophages, recruitment of neutrophils, and circulating macrophages to the stressed lung tissues. As a consequent of such cellular activations, ARDS advances with elevated level of pro-inflammatory cytokines such as IL-6, IL-17, and IFNγ, damaging lung tissues and eliciting accumulation of macromolecules manifested by edema and hypoxemia (Han & Mallampalli, 2015). Contrary to all advancements in understanding the mechanisms and pathophysiology of ARDS, however, no pharmacologic modality for the treatment of ARDS has been yet identified. As evidenced by the COVID-19 pandemic, supportive therapies along with combinatorial therapies such as corticosteroid and IL-6 inhibitor have shown beneficial effects in the management of ARDS (Pan et al., 2018; Harahwa et al., 2020; Catherine, 2014). Therefore, the need to develop alternative novel therapeutic targets for the prevention and treatment of ARDS persists.
Cannabinoids are naturally occurring compounds in Cannabis plants (Atakan, 2012). Numerous studies suggest beneficial effects of cannabinoids in clinical settings (Kogan, 2007; The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research, 2017; Maione et al., 2011). Of over 100 known cannabinoids, four, including tetrahydrocannabinol (Δ9-THC), cannabidiol (CBD), cannabinol (CBN), and cannabichromene (CBC), have attracted the most attention due to supporting evidence of their potential as therapeutic targets (Maione et al., 2011; Izzo et al., 2012; Patil et al., 2020; Martin et al., 2021.; Anderson et al., 2021). Despite their structural differences, both CBD and CBC are non-psychoactive compounds with potential immunomodulatory effects and several other health benefits (Maione et al., 2011; Izzo et al., 2012; Pollastro et al., 2018). More recently, in a set of preclinical studies, we showed for the first time that CBD could ameliorate the symptoms of ARDS (Salles et al., 2020; Khodadadi et al., 2020). Importantly, unlike THC and CBD, CBC is not categorized as a scheduled compound by US Drug Enforcement Agency (DEA), which may facilitate the use and assessment of CBC in clinical settings (BayMedica White paper on Cannabichromene, n.d.). Further, several reports suggest that CBC may act through the transient receptor potential (TRP) cation channel subfamily A member 1 (TRPA1) as well as transient receptor potential cation channel subfamily V member 1 (TRPV1) with less affinity. TRPs are involved in vast variety of vital cellular processes, influencing the dynamic of physiologic and immunologic interactions within the multicellular organism (Muller et al., 2019; Froghi et al., 2021; Kun et al., 2014; Tsumura et al., 2012; Romano et al., 2013). CBC binding to TRPs would likely affect tissue homeostasis and immune balance, modulating the inflammatory responses as well as endocannabinoid cellular reuptake (Pollastro et al., 2018; De Petrocellis et al., 2011). CBC has also been reported to be an agonist to the CB2 receptor, activation of which may further modulate inflammatory responses (Izzo et al., 2012; Udoh et al., 2019).
In this study, we investigated the potential protective effects of CBC, possible mechanisms of action, and whether CBC can improve symptoms in an experimental model of ARDS.
Materials and methods
Animals
The studies utilized male, 10–12 weeks of age, C57BL/6 mice which were obtained from Jackson Laboratory USA and housed in the laboratory animal facilities of the Augusta University with free access to food and water. These studies conformed to guidelines of Institutional Animal Care and Use Committee.
Experimental design
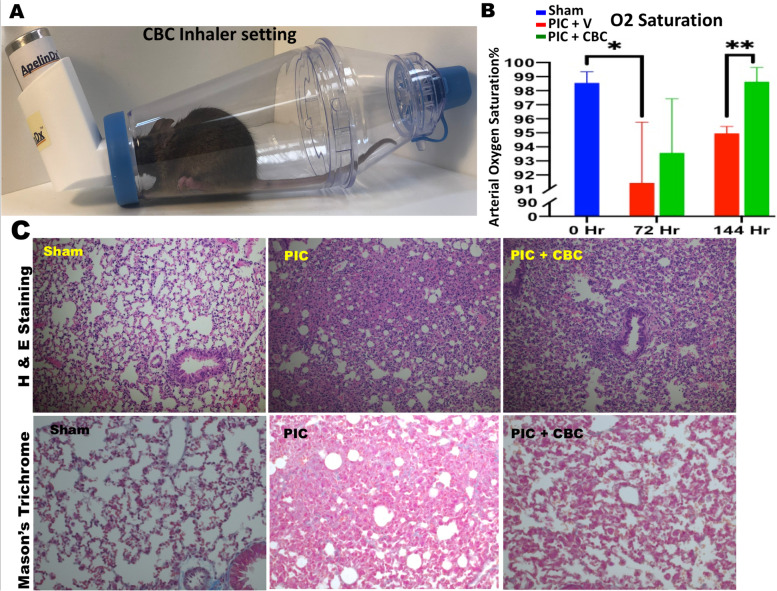
ARDS was induced as described previously (Salles et al., 2020; Khodadadi et al., 2020). Briefly, mice were divided into three groups: sham, control, and treatment (n = 6–10) in an open-labeled fashion to explore the efficacy of the treatment. All experiments were performed in accordance with the rules and regulations of the Augusta University Institutional Animal Care and Use Committee (IACUC). All mice were anesthetized with isoflurane. Sham group received phosphate-buffered saline (PBS) while control and treatment groups were administered Poly(I:C) (Sigma Aldrich, St. Louis, MO, USA) (100 mg in 50 mL sterile PBS) intranasally (IN) three once-daily doses. CBC was generously provided by BayMedica USA (ProdiolTM CBC) and was administered through an inhaler (ApelinDxtm, TM Global Bioscience) (5 mg/kg, Fig. 1A), first dose 2 h after the second Poly(I:C) treatment, and every other day for a total of three doses to the treatment group. The dose and administration route of the drug were determined based on previous independent as well as our own studies (Khodadadi et al., 2020; Millar et al., 2019; Borghardt et al., 2018; Rau, 2005). Inhalant delivery provides advantages including local drug delivery with expected minimal side effects, rapid onset and high efficiency impact on the target site and simulating common route of administration seen in clinical scenarios. Sham and control groups received placebo through inhaler carrying the vehicle only. All mice were sacrificed at 8 days after the first Poly(I:C) application. Blood and lung tissues were harvested and subjected to further analysis. Blood oxygen saturation was measured using portable pulse oximeter through carotid arteries, before and after any treatment.
Fig. 1.
Inhalant cannabichromene (CBC) improved pulmonary function. A Treating mouse model of acute respiratory distress syndrome (ARDS) with inhalant CBC, specifically designed cone-shaped spout of the inhaling device (ApelinDxtm) connected to spacer completely sealed for a soft, comfortable, and insuring delivery of inhalant CBC to the mice. B Blood oxygen saturation was increased by ~8% towards a normal level (Blue bar) in mice treated with Poly (I:C) (PIC) followed by the treatment of inhalant CBC (PIC + CBC) (Green bar) compared to the untreated group (PIC) (Red bar) (**p<0.05, bars show means +/− standard deviation, SD). C Histological analysis, hematoxylin and eosin (H&E upper panels), and Masson’s trichrome (MT lower panels) demonstrated that Poly (I:C) caused the destruction of morphology and architecture of lung in mice with ARDS compared to the normal control group (sham). Inhalant CBC was able to improve the lung structure towards a normal status (PIC + CBC)
Histology and immunohistochemistry
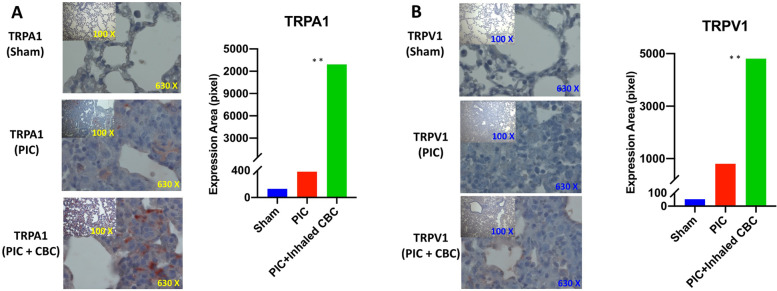
Multiple 5 μm midcoronal paraffin-embedded sections of lung tissues were cut and stained with hematoxylin and eosin, or trichrome. As for inflammatory indices, immunohistochemistry was performed as described previously (Salles et al., 2020; Khodadadi et al., 2020). Briefly, paraffin sections of lung tissues were deparaffinized in xylene and rehydrated by passing the slides through graded alcohol solutions. Endogenous peroxidase was quenched with 3% H2O2 in PBS. The sections were then washed in distilled water and heated at 95 °C in antigen retrieval buffer (Dako, Glostrup, Denmark). Nonspecific staining was blocked with 5% normal goat serum in PBS for 1 h. Endogenous biotin was inhibited with an avidin/biotin blocking kit (SP2001, Vector Laboratories, Burlingame, CA). The sections were then incubated with anti-murine TRPA1, and TRPV1. Preparations were counterstained with hematoxylin and mounted in Faramount and imaged by bright field microscopy. The expression of TRPA1 and TRPV1 were quantified by using particle count and color intensity measurement in the ImageJ software version 1.53.
Flow cytometry analyses
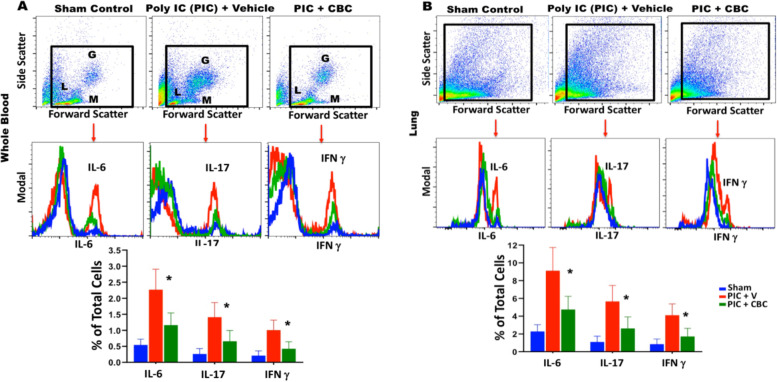
For analytical flow cytometry, single-cell suspension was prepared from lung and blood tissues as described previously (Salles et al., 2020; Khodadadi et al., 2020). In brief, tissue samples were sieved through a 100 μM cell strainer (BD Biosciences, San Diego, CA, USA), followed by centrifugation (252 g, 10 min) to prepare single-cell suspensions. Then cells were fixed and permeabilized and stained intracellularly for cytokines including IL-6, IL-17, and interferon gamma (IFNγ) (proinflammatory cytokines). All samples were run through a 4-Laser LSR II flow cytometer. Cells were gated based on forward and side scatter properties and on marker combinations to select cells of interest. All acquired flow cytometry data were analyzed using FlowJo V10. Graphs and summary statistics were also used to assess the results.
Statistics
In order to determine the statistical differences among the means of all experimental groups, data were analyzed using one way analysis of variance (ANOVA) followed by Newman–Keuls post hoc test for multiple comparison and to establish significance (p < 0.05) among all groups.
Results
Inhalant CBC reversed hypoxia, increasing blood oxygen saturation positively towards the normal level by 8% from 90% to 98% (+/− 0.5%) (Fig. 1A, B). Histological examination of lung tissues, hematoxylin and eosin staining (Fig. 1C upper panels), and Masson’s trichrome staining (Fig. 1C lower panels) demonstrated that CBC treatment reduced the ARDS-like lung destruction, improved the structural damages to the lung. Further histological examination, immunohistochemistry showed that CBC treatment increased the level of expression for transient receptor potential (TRP) channels of ankyrin type-1 (TRPA1) and vanilloid type-1 (TRPV1), suggesting an active interaction between CBC and TRPs (Fig. 2). Flow cytometry analysis of blood and lung demonstrated that CBC treatment curtailed the expression levels of pro-inflammatory cytokines including IL-6, IL-17, and IFNγ in both tissues compared to the untreated counterparts (p < 0.01) (Fig. 3A, B). The histograms show the quantified level of expression of IL-6, IL-17, and IFNγ in both tissues treated with CBC compared to the control sham (untreated with CBC) group.
Fig. 2.
Inhalant cannabichromene (CBC) may interact with the endocannabinoid system through enhancement of transient receptor potential (TRP) cation channels. Immunohistochemical staining of lung tissues with A transient receptor potential cation channel subfamily A member 1 (TRPA1) and B the transient receptor potential cation channel subfamily V member 1 (TRPV1) with less affinity showed an increase in the expression level of TRPA1 and TRPV1 in lung tissues of CBC treated group (PIC + CBC) compared to normal sham or Poly (I:C) treated one (sham or PIC) (images are all ×630 and inner images are ×100 magnification) (**p<0.01, means +/-SD). The bar graphs demonstrate the quantified analysis of the staining using particle count and color intensity measurement in the ImageJ software version 1.53
Fig. 3.
Inhalant cannabichromene (CBC) reduced ARDS-like induced inflammatory indices. Flow cytometry analysis of A whole blood and B lung tissues showed that Intranasal administration of Poly(I:C) effectuated a significant increase of pro-inflammatory cytokines including IL-6, IL-17, and IFNγ (PIC group) compared to the control group (sham). These effects were reversed with inhalant CBC (PIC + CBC), resulted in marked downregulation of all three pro-inflammatory cytokines (*p<0.03, means +/− SD). Further, the flow cytometry analysis of whole blood in the PIC group showed increased granulocytes (G) and monocytes (M) while lymphocytes were decreased compared to mice treated with inhalant CBC (PIC + CBC) supporting the notion that inhalant CBC can reverse the adverse effects of ARDS and re-establish the immune balance, resolving lymphopenia and restoring homeostasis
Discussion
Our findings demonstrated, for the first time, that inhalant CBC protected lung structure, contained excessive cytokine production, and curtailed inflammatory responses both in lung and blood tissues in an experimental ARDS model. These results were in consistent with findings by other independent studies demonstrating counter-inflammatory effects of CBC in LPS-induced paw edema (DeLong et al., 2010). In addition, CBC is a non-psychoactive cannabinoid which is not categorized as a scheduled compound by US Drug Enforcement Agency (DEA). This may facilitate the use and assessment of CBC in clinical settings (BayMedica White paper on Cannabichromene, n.d.). More importantly, using inhalers for the first time in a therapeutic setting would provide an effective and safer way to deliver CBC to small airways, lowering required doses and reducing any potential systemic adversarial symptom in a more cost-effective way. Additionally, delivery of CBC through an inhaler increases the translational values of this study and potential for trials in a clinical setting with implications for the treatment of respiratory complications such as development of ARDS in severe cases of COVID-19 and several respiratory diseases. It is noteworthy that we exclusively employed male animals in our studies to prevent additional confounding factors based on gender differences (e.g., different stages of estrous cycle, hormonal effects), staying focused on the proof of concept for CBC sole effects in ARDS. However, increasing evidence suggests a crucial role for sex dimorphism in occurrence and outcomes of ARDS, warranting further investigations at the pre-clinical level as well as for the potential human clinical trials in the future (Scully et al., 2020).
Further, our data showed that CBC can increase the expression level of transient receptor potential ankyrin 1 (TRPA1) and vanilloid receptor 1 (TRPV1), supporting the notion of potential interactions between CBC and TRPA1 as well as TRPV1 (TRPs). The TRP channels superfamily play vital roles in a wide range of physiological and immunologic processes such as cellular polarization, cytokine production, phagocytosis, and cytotoxicity (Muller et al., 2019; Froghi et al., 2021; Kun et al., 2014; Tsumura et al., 2012). Therefore, it is very plausible to hypothesize that binding CBC to TRPs may downregulate the inflammatory responses by shifting the so-called “cytokine storm” to a “cytokine breeze,” re-establishing the homeostasis and preventing additional structural damages to the vital organs. Although the interaction between CBC and TRPs has been previously reported (Maione et al., 2011; De Petrocellis et al., 2011), to the best of our knowledge, this is the first report to show the beneficial effects of cross-talk between CBC and TRPs in ARDS. In addition, since several studies have indicated that CBC may bind to and activate CB2 receptor of the endocannabinoid system, suggesting a potential synergy between TRPs and CB2 activation as a potential mechanism contributing to the beneficial effects of CBC in ARDS. In fact, such potential engagement of the endocannabinoid system by CBC may establish a synergistic relationship between exogenous phytocannabinoids (CBC) and endogenous endocannabinoids (e.g., anandamide and 2-AG). Such bi-directional dynamic would have the potential as a therapeutic modality in the treatment of ARDS as well as a wide variety of inflammatory diseases, neurodegenerative diseases, pain control, cancer, chronic wounds, and sleeping disorders.
Conclusion
These novel findings render a new therapeutic role for CBC in the treatment of ARDS as well as a wide range of respiratory diseases including a potential for COVID-19 and other inflammatory conditions. The use of inhalant CBC and direct delivery of CBC to the lungs offers a more rapid onset of action, allows smaller doses to be used, and has a better efficacy to safety ratio compared to systemic therapy. Further, as TRPs are emerging as novel therapeutic targets through their potential roles in cellular interactions and tissue homeostasis (Zhao et al., 2021), therefore, it is plausible to envision more prominent roles for CBC in tissue homeostasis, modulation of inflammatory responses, and orchestrating a set of cellular interactions as an immunotherapeutic target, warranting further research.
Acknowledgements
Authors would like to thank BayMedica (BayMedica.com) for the generous supply of CBC for this study.
Abbreviations
- ARDS
Acute respiratory distress syndrome
- CBC
Cannabichromene
- CBD
Cannabidiol
- CBN
Cannabinol
- DEA
Drug Enforcement Agency
- IL-6
Interleukin-6
- IL-17
Interleukin-17
- IFNγ
Interferon gamma
- LPS
Lipopolysaccharides
- PIC
Poly I:C
- PBS
Phosphate-buffered saline
- TRP
Transient receptor potential
- TRPA1
Transient receptor potential ankyrin 1
- TRPV1
Transient receptor potential vanilloid receptor 1
- 2-AG
2-Arachidonoylglycerol
- Δ9-THC
Tetrahydrocannabinol
Authors’ contributions
HK, ELS, ES, AJ, KV, and BB performed experiments and analyzed the data. HK and BB performed statistical analysis. BB, KMD, and HK wrote the manuscript. VC, PK, JCY, JCM, DCH, KMD, and BB contributed to the conceptualization and editing of the text. All authors read and approved the final manuscript.
Funding
This work was partially supported by funding provided by TM Global Bioscience, LLC, a medically-focused division of ThriftMaster Global Holdings., as well as by awards from the NIH (NS110378 to KMD/BB, and NS114560 to KV).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The experiments, materials, and methods were performed in accordance with the rules and regulations of the Augusta University Institutional Animal Care and Use Committee (IACUC).
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests. Thriftmaster Holding Group (THG) is the provider of inhalers and BayMedica as the provider of CBC which have a contract with Augusta University. THG and BayMedica had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Anderson LL, Ametovski A, Lin Luo J, Everett-Morgan D, McGregor IS, Banister SD, Arnold JC. Cannabichromene, Related Phytocannabinoids, and 5-Fluoro-cannabichromene Have Anticonvulsant Properties in a Mouse Model of Dravet Syndrome. ACS Chem Neurosci. 2021;12(2):330–339. doi: 10.1021/acschemneuro.0c00677. [DOI] [PubMed] [Google Scholar]
- Atakan Z. Cannabis, a complex plant: different compounds and different effects on individuals. Ther Adv Psychopharmacol. 2012;2(6):241–254. doi: 10.1177/2045125312457586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chris Meiering., Prodiol CBC: Cannabichromene Whitepaper, Baymedica Inc, USA., 2021. p. 2. (baymedica.com).
- Borghardt JM, Kloft C, Sharma A. Inhaled Therapy in Respiratory Disease: The Complex Interplay of Pulmonary Kinetic Processes. Can Respir J. 2018;2018:2732017. doi: 10.1155/2018/2732017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catherine L. Hough, Should we ever give steroids to ARDS patients? Clin Chest Med. 2014;35(4):781–795. doi: 10.1016/j.ccm.2014.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Petrocellis L, Ligresti A, Moriello AS, Allarà M, Bisogno T, Petrosino S, Stott CG, Di Marzo V. Effects of cannabinoids and cannabinoid-enriched Cannabis extracts on TRP channels and endocannabinoid metabolic enzymes. Br J Pharmacol. 2011;163(7):1479–1494. doi: 10.1111/j.1476-5381.2010.01166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLong GT, Wolf CE, Poklis A, Lichtman AH. Pharmacological evaluation of the natural constituent of Cannabis sativa, cannabichromene and its modulation by Δ(9)-tetrahydrocannabinol. Drug Alcohol Depend. 2010;112(1-2):126–133. doi: 10.1016/j.drugalcdep.2010.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froghi S, Grant CR, Tandon R, Quaglia A, Davidson B, Fuller B. New Insights on the Role of TRP Channels in Calcium Signalling and Immunomodulation: Review of Pathways and Implications for Clinical Practice. Clin Rev Allergy Immunol. 2021;60:271–292. doi: 10.1007/s12016-020-08824-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S, Mallampalli RK. The acute respiratory distress syndrome: from mechanism to translation. J Immunol. 2015;194(3):855–860. doi: 10.4049/jimmunol.1402513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harahwa TA, Khan IH, Harky A. Ventilatory support for COVID-19 patients. Acta Biomed. 2020;91(4):e2020122. doi: 10.23750/abm.v91i4.9895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Q, Hao C, Tang S. From sepsis to acute respiratory distress syndrome (ARDS): emerging preventive strategies based on molecular and genetic researches. Biosci Rep. 2020;40(5):BSR20200830. doi: 10.1042/BSR20200830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izzo AA, Capasso R, Aviello G, Borrelli F, Romano B, Piscitelli F, Gallo L, Capasso F, Orlando P, Di Marzo V. Inhibitory effect of cannabichromene, a major non-psychotropic cannabinoid extracted from Cannabis sativa, on inflammation-induced hypermotility in mice. Br J Pharmacol. 2012;166(4):1444–1460. doi: 10.1111/j.1476-5381.2012.01879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodadadi H, Salles ÉL, Jarrahi A, Chibane F, Costigliola V, Yu JC, Vaibhav K, Hess DC, Dhandapani KM, Baban B. Cannabidiol Modulates Cytokine Storm in Acute Respiratory Distress Syndrome Induced by Simulated Viral Infection Using Synthetic RNA. Cannabis Cannabinoid Res. 2020;5(3):197–201. doi: 10.1089/can.2020.0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogan NM. Cannabinoids in health and disease. Dialogues Clin Neurosci. 2007;9(4):413–430. doi: 10.31887/DCNS.2007.9.4/nkogan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kun J, Szitter I, Kemény A, Perkecz A, Kereskai L, Pohóczky K, Vincze A, Gódi S, Szabó I, Szolcsányi J, Pintér E, Helyes Z. Upregulation of the transient receptor potential ankyrin 1 ion channel in the inflamed human and mouse colon and its protective roles. PLoS One. 2014;9(9):e108164. doi: 10.1371/journal.pone.0108164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maione S, Piscitelli F, Gatta L, Vita D, De Petrocellis L, Palazzo E, de Novellis V, Di Marzo V. Non-psychoactive cannabinoids modulate the descending pathway of antinociception in anaesthetized rats through several mechanisms of action. Br J Pharmacol. 2011;162(3):584–596. doi: 10.1111/j.1476-5381.2010.01063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LJ, Cairns EA, Heblinski M, Fletcher C, Krycer JR, Arnold JC, McGregor IS, Bowen MT, Anderson LL. Cannabichromene and Δ9-Tetrahydrocannabinolic Acid Identified as Lactate Dehydrogenase-A Inhibitors by in Silico and in Vitro Screening. J Nat Products. 2021;84(5):1469–1477. doi: 10.1021/acs.jnatprod.0c01281. [DOI] [PubMed] [Google Scholar]
- Matthay MA, Thompson BT, Ware LB. The Berlin definition of acute respiratory distress syndrome: should patients receiving high-flow nasal oxygen be included? Lancet Respir Med. 2021;26 S2213-2600(21)00105-3. [DOI] [PMC free article] [PubMed]
- Matthay MA, Zemans RL, Zimmerman GA, Arabi YM, Beitler JR, Mercat A, Herridge M, Randolph AG, Calfee CS. Acute respiratory distress syndrome. Nat Rev Dis Primers. 2019;5(1):18. doi: 10.1038/s41572-019-0069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar SA, Stone NL, Bellman ZD, Yates AS, England TJ, O'Sullivan SE. A systematic review of cannabidiol dosing in clinical populations. Br J Clin Pharmacol. 2019;85(9):1888–1900. doi: 10.1111/bcp.14038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller C, Morales P, Reggio PH. Cannabinoid Ligands Targeting TRP Channels. Front Mol Neurosci. 2019;11:487. doi: 10.3389/fnmol.2018.00487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan C, Liu L, Xie J-F, Qiu H-B. Acute Respiratory Distress Syndrome: Challenge for Diagnosis and Therapy. Chin Med J (Engl). 2018;131(10):1220–1224. doi: 10.4103/0366-6999.228765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil AS, Mahajan UB, Agrawal YO, Patil KR, Patil CR, Ojha S, Sharma C, Goyal SN. Plant-derived natural therapeutics targeting cannabinoid receptors in metabolic syndrome and its complications: A review. Biomed Pharmacother. 2020;132:110889. doi: 10.1016/j.biopha.2020.110889. [DOI] [PubMed] [Google Scholar]
- Pollastro F, Caprioglio D, Del Prete D, et al. Cannabichromene. Nat Product Commun. 2018;13(9):1189–1194. [Google Scholar]
- Rau JL. The inhalation of drugs: advantages and problems. Respir Care. 2005;50(3):367–382. [PubMed] [Google Scholar]
- Romano B, Borrelli F, Fasolino I, Capasso R, Piscitelli F, Cascio M, Pertwee R, Coppola D, Vassallo L, Orlando P, Di Marzo V, Izzo A. The cannabinoid TRPA1 agonist cannabichromene inhibits nitric oxide production in macrophages and ameliorates murine colitis. Br J Pharmacol. 2013;169(1):213–229. doi: 10.1111/bph.12120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salles ÉL, Khodadadi H, Jarrahi A, Ahluwalia M, Paffaro VA, Jr, Costigliola V, Yu JC, Hess DC, Dhandapani KM, Baban B. Cannabidiol (CBD) modulation of apelin in acute respiratory distress syndrome. J Cell Mol Med. 2020;24(21):12869–12872. doi: 10.1111/jcmm.15883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scully EP, Haverfield J, Ursin RL, Tannenbaum C, Klein SL. Considering how biological sex impacts immune responses and COVID-19 outcomes. Nat Rev Immunol. 2020;20(7):442–447. doi: 10.1038/s41577-020-0348-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research., Committee on the Health Effects of Marijuana: An Evidence Review and Research Agenda. Washington (DC): National Academies Press (US); 2017. [PubMed]
- Tsumura M, Sobhan U, Muramatsu T, Sato M, Ichikawa H, Sahara Y, Tazaki M, Shibukawa Y. TRPV1-mediated calcium signal couples with cannabinoid receptors and sodium-calcium exchangers in rat odontoblasts. Cell Calcium. 2012;52(2):124–136. doi: 10.1016/j.ceca.2012.05.002. [DOI] [PubMed] [Google Scholar]
- Udoh M, Santiago M, Devenish S, McGregor IS, Connor M. Cannabichromene is a cannabinoid CB2 receptor agonist. Br J Pharmacol. 2019;176(23):4537–4547. doi: 10.1111/bph.14815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, McVeigh BM, Moiseenkova-Bell VY. Structural pharmacology of TRP channels. Rev J Mol Biol. 2021;166914. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.