Abstract
Metronidazole susceptibility of 100 Helicobacter pylori strains was assessed by determining the inhibition zone diameters by disk diffusion test and the MICs by agar dilution and PDM Epsilometer test (E test). Linear regression analysis was performed, allowing the definition of significant linear relations, and revealed correlations of disk diffusion results with both E-test and agar dilution results (r2 = 0.88 and 0.81, respectively). No significant differences (P = 0.84) were found between MICs defined by E test and those defined by agar dilution, taken as a standard. Reproducibility comparison between E-test and disk diffusion tests showed that they are equivalent and with good precision. Two interpretative susceptibility schemes (with or without an intermediate class) were compared by an interpretative error rate analysis method. The susceptibility classification scheme that included the intermediate category was retained, and breakpoints were assessed for diffusion assay with 5-μg metronidazole disks. Strains with inhibition zone diameters less than 16 mm were defined as resistant (MIC > 8 μg/ml), those with zone diameters equal to or greater than 16 mm but less than 21 mm were considered intermediate (4 μg/ml < MIC ≤ 8 μg/ml), and those with zone diameters of 21 mm or greater were regarded as susceptible (MIC ≤ 4 μg/ml). Error rate analysis applied to this classification scheme showed occurrence frequencies of 1% for major errors and 7% for minor errors, when the results were compared to those obtained by agar dilution. No very major errors were detected, suggesting that disk diffusion might be a good alternative for determining the metronidazole sensitivity of H. pylori strains.
Helicobacter pylori is strongly associated with some gastric pathologies, such as duodenal and gastric ulcers, and is an important risk factor in gastric cancer evolution (25). Treatment of infection caused by this organism has been shown to require a combination of antibiotics, and metronidazole is frequently used in the eradication therapies. However, the high prevalence of resistant strains to this antimicrobial agent can lead to therapeutic failure. Determination of strain susceptibility to antibiotics, particularly to metronidazole, is very important (7, 10), but there are several problems with antimicrobial susceptibility testing of H. pylori (9, 11, 15, 16). Standardization of simple and fast test procedures that can be applied in routine laboratories, to allow a faster identification of resistant strains and the selection of more effective therapies, is now a real need (6, 7, 9, 10, 13, 16, 29). Disk diffusion testing, since it is simple and economical, is very often used. However, the resistance breakpoints for H. pylori are not well defined. This is particularly evident from the different breakpoints, ranging from 4 to >32 μg/ml, already used by several authors (1, 7, 9, 15, 19, 22, 26, 30). The lack of standardization of the breakpoint MIC for metronidazole can lead to the misclassification of strains into the susceptibility interpretative categories, with important clinical implications. The aim of this study was the standardization of the disk diffusion method for metronidazole, by correlating the results with MICs determined both by the agar dilution method (which is accepted as a standard and has been used in several studies) (17, 20) and by a quantitative gradient diffusion test (E test) (4, 5, 14, 23, 27). Based on a breakpoint interpretative error rate analysis (21), we defined three susceptibility categories (susceptible, intermediate, and resistant) and the inhibition zone diameters corresponding to them.
Bacteria and inoculum preparation.
One hundred H. pylori strains from gastric biopsy specimens were isolated on Pylori selective medium (BioMérieux) and stored at −70°C in brucella broth (Gibco Europe) with 25% glycerol. Frozen clinical isolates were thawed and inoculated on Mueller-Hinton agar (MHA) plates (Oxoid) supplemented with 10% horse blood (12) and incubated under microaerophilic conditions produced by a gas-generating system (Campylobacter system; Oxoid). For inoculum preparation, strains were incubated in MHA plus 10% horse blood for 48 h at 37°C under microaerophilic conditions, produced as described above. Given the importance of inoculum homogeneity (3, 12), cellular viability was controlled microscopically by morphological observation with gram staining, in order to check the proportions of coccoid cells in cultures (18). Cultures were always used after 48 h of incubation, when they generally did not present coccoid forms. Suspensions were prepared in sterile distilled water to an opacity of 3 to 4 McFarland standard (ca. 109 CFU/ml).
Agar dilution susceptibility test.
Metronidazole (Sigma) was dissolved in dimethylformamide (DMF) (Merck) and diluted in sterile distilled water to produce serial log2 dilutions (ranging from 0.125 to 64 μg/ml) in 50°C MHA supplemented with 10% horse blood. Final concentrations of DMF were always lower than the MICs assessed for H. pylori strains. The plates were inoculated with 1 to 2 μl of suspensions at a 3 to 4 McFarland standard (ca. 106 CFU) by means of an automated multipoint inoculator (Denley) and incubated for 72 h at 37°C under microaerophilic conditions, produced as described above. An antibiotic-free control plate was also inoculated in each assay. The MIC was defined as the lowest concentration producing no visible colonies (a slight haze of apparent growth was ignored).
Disk diffusion and E test susceptibility methods.
Plates containing MHA plus 10% horse blood were inoculated with a swab from the suspension at a 3 to 4 McFarland standard. Then, a metronidazole disk (5-μg; Oxoid) and an E-test strip, which has a built-in gradient from high to low content of metronidazole in semi-log2 steps (E test; AB Biodisk, Solna, Sweden), were placed on the same plate for each strain. All plates were incubated for 72 h at 37°C in a microaerophilic atmosphere. Storage conditions and preuse conditions of metronidazole disks and E-test strips were strictly in accordance with the manufacturers’ instructions.
For the disk diffusion method, the inhibition zone diameter was measured, in millimeters, with a ruler. For the E test, MICs, corresponding to the intersection of the inhibition zone with the strip, were read directly from the strip.
Data and statistical analysis.
Possible significant differences between MICs defined by each method (α = 0.05) were assessed by means of a paired t test (2). For the average difference of MICs (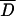 ), a 95% confidence interval based on the standard error of
), a 95% confidence interval based on the standard error of 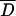 was also calculated (2). All correlations (for agar dilution MICs with E-test MICs and for inhibition zone diameters with both agar dilution MICs and E-test MICs) were determined by regression analysis. The last two functions obtained were linearized by logarithmic conversion of MICs. Determination coefficients (r2) were calculated for all regression lines. Validation of these linear models was carried out by an F test (28). The confidence intervals (α = 0.05) for the inhibition zone diameters, estimated from each linear function and corresponding to the breakpoints defined, were also calculated (2). In order to evaluate the reproducibility of the three methods, a random sample of 14 strains was tested in three independent assays. The average coefficient of variation (CV) (28) was calculated for each method by using the set of values obtained with the 14 strains (n = 42). For the disk diffusion test, the CV was calculated after translation of inhibition zone diameters into MICs, via the corresponding regression lines, to generate numbers of the same dimension. Confidence intervals (α = 0.05) for the average CV associated with each method were also defined, to evaluate the precision and reproducibility of each diffusion method (E-test strip and metronidazole disks) by comparison with agar dilution, as the latter is considered a reference method (28). An interpretative error rate analysis was performed in order to investigate the occurrence of strain misclassification when disk diffusion breakpoints were applied.
was also calculated (2). All correlations (for agar dilution MICs with E-test MICs and for inhibition zone diameters with both agar dilution MICs and E-test MICs) were determined by regression analysis. The last two functions obtained were linearized by logarithmic conversion of MICs. Determination coefficients (r2) were calculated for all regression lines. Validation of these linear models was carried out by an F test (28). The confidence intervals (α = 0.05) for the inhibition zone diameters, estimated from each linear function and corresponding to the breakpoints defined, were also calculated (2). In order to evaluate the reproducibility of the three methods, a random sample of 14 strains was tested in three independent assays. The average coefficient of variation (CV) (28) was calculated for each method by using the set of values obtained with the 14 strains (n = 42). For the disk diffusion test, the CV was calculated after translation of inhibition zone diameters into MICs, via the corresponding regression lines, to generate numbers of the same dimension. Confidence intervals (α = 0.05) for the average CV associated with each method were also defined, to evaluate the precision and reproducibility of each diffusion method (E-test strip and metronidazole disks) by comparison with agar dilution, as the latter is considered a reference method (28). An interpretative error rate analysis was performed in order to investigate the occurrence of strain misclassification when disk diffusion breakpoints were applied.
Equivalence between agar dilution and E test and breakpoint definition.
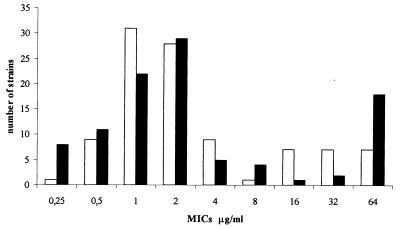
We obtained MICs ranging from 0.125 to >32 μg/ml by the E test and ranging from 0.25 to 64 μg/ml by the agar dilution method. The MICs for 11 of the 100 strains were discordant, with discrepancies of more than ±1 log2 concentration steps (agar dilution, standard error) but not more than ±2 log2. No significant differences were found between MICs determined by the agar dilution method and the E test (P = 0.84; 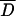 = 0.02 ± 0.260). A good correlation was found between these two methods (MICE test = 1.22 MICagar dilution + 2.89; r2 = 0.73), allowing us to use both quantitative methods as references and to correlate results obtained with them with inhibition zone diameters for the standardization of the disk diffusion test. Based on the distribution of strains among the MICs obtained (Fig. 1) and breakpoint values already described (22, 30), we defined two susceptibility classification schemes: one with a unique breakpoint (8 μg/ml), which separates the strains into two categories (MIC ≤ 8 μg/ml [susceptible strains] and MIC > 8 μg/ml [resistant strains]), and the other with two breakpoints (4 and 8 μg/ml) resulting in three interpretative categories (MIC ≤ 4 μg/ml [susceptible strains], 4 μg/ml < MIC ≤ 8 μg/ml [intermediate strains], and MIC > 8 μg/ml [resistant strains]).
= 0.02 ± 0.260). A good correlation was found between these two methods (MICE test = 1.22 MICagar dilution + 2.89; r2 = 0.73), allowing us to use both quantitative methods as references and to correlate results obtained with them with inhibition zone diameters for the standardization of the disk diffusion test. Based on the distribution of strains among the MICs obtained (Fig. 1) and breakpoint values already described (22, 30), we defined two susceptibility classification schemes: one with a unique breakpoint (8 μg/ml), which separates the strains into two categories (MIC ≤ 8 μg/ml [susceptible strains] and MIC > 8 μg/ml [resistant strains]), and the other with two breakpoints (4 and 8 μg/ml) resulting in three interpretative categories (MIC ≤ 4 μg/ml [susceptible strains], 4 μg/ml < MIC ≤ 8 μg/ml [intermediate strains], and MIC > 8 μg/ml [resistant strains]).
FIG. 1.
Distribution of the 100 H. pylori strains among the MICs obtained by agar dilution (□) and E test (■).
Reproducibility and precision of the methods.
In the evaluation of methods for susceptibility testing, precision and reproducibility are the most important criteria. Table 1 shows the average MICs obtained in three repeat tests with 14 strains and the corresponding standard errors for E-test and agar dilution methods. The table also shows the inhibition zone diameter ranges obtained for each MIC with disk diffusion method. CV determination showed that the two diffusion techniques are equivalent (CVdisk = 26.5% ± 11.8% and CVE test = 30% ± 11.8%). The agar dilution method, which is considered a reference method, has a similar variation (CVagar dilution = 30% ± 11.8%), confirming the good reproducibility and precision of the other methods tested. Furthermore, it should be noted that the variation associated with the agar dilution technique can be an artifact generated by the large concentration steps of the log2 scale, especially at the higher doses. Thus, the variation value associated with agar dilution is probably higher than those calculated for the two diffusion tests, reinforcing the reliability and good reproducibility of the latter. These data are in agreement with results for reproducibility and correlation found by others (7, 15, 30). The consistency observed for the three independent repeat tests, made with independent cultures, is evidence of inoculum homogeneity, which is an issue of major importance in antimicrobial susceptibility determination (4).
TABLE 1.
MICs defined by E-test and agar dilution methods and inhibition zone diameter ranges for a group of 14 strains obtained with three repeat tests
| Strain reference no. | MIC
|
||
|---|---|---|---|
| E test (avg ± SE) | Agar dilution (avg ± SE) | Disk inhibition zone diam range (mm) | |
| B43/91 | 0.67 ± 0.127 | 0.5a | 36–38 |
| B52/91 | 0.29 ± 0.091 | 0.50 ± 0.179 | 37–38 |
| B7/92 | 32.0a | 64.0a | 6 |
| I51/93 | 1.67 ± 0.179 | 2.0a | 24–30 |
| B6/95 | 8.0a | 8.0a | 14–16 |
| B7/95 | 0.67 ± 0.179 | 1.00 ± 0.253 | 30–38 |
| B24/96 | 1.67 ± 0.179 | 2.0a | 28–30 |
| B32/96 | 32.0a | 32.0a | 6 |
| B39/96 | 0.67 ± 0.236 | 1.0a | 30–38 |
| B18/97 | 1.42 ± 0.264 | 2.0a | 24–26 |
| B26/97 | 4.0a | 4.00 ± 0.358 | 20–24 |
| B30/97 | 0.83 ± 0.179 | 1.00 ± 0.179 | 32–36 |
| B38/97 | 32.0a | 32.0a | 6 |
| B67/97 | 10.67 ± 0.507 | 16.0a | 12–14 |
Identical values were obtained for the three repeat tests.
Regression analysis and correlation between methods.
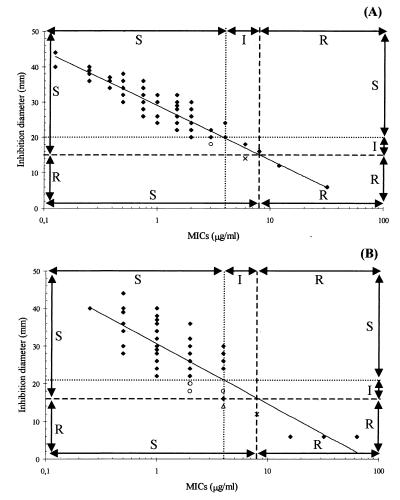
After logarithmic conversion of MICs, regression analysis was applied to our results in order to define linear functions correlating inhibition zone diameters with MICs obtained by the two quantitative methods and correlating the MICs with the inhibition zone diameters. The analytical expressions were log MIC = −0.06 ⊘ + 1.69 (r2 = 0.88), where ⊘ is the inhibition zone diameter, for calculation of E-test MICs from inhibition zone diameters and log MIC = −0.05 ⊘ + 1.64 (r2 = 0.81) for calculation of agar dilution MICs from inhibition zone diameters. The reverse equations, for estimation of inhibition zone diameters from E-test MICs (Fig. 2A) and agar dilution MICs (Fig. 2B), were ⊘ = −15.31 log MIC + 29.06 (r2 = 0.88) and ⊘ = − 15.98 log MIC + 30.63 (r2 = 0.81), respectively. It should be noted that the maximum concentration of metronidazole in the E-test strip is 32 μg/ml. Thus, only 81 strains were retained, as MICs higher than 32 μg/ml were not used in the E-test regression analysis. The linearity of all functions (P < 0.05) was confirmed by an F test. From each reverse regression line (Fig. 2A and B), it was possible to estimate 95% confidence intervals for the inhibition zone diameters corresponding to the breakpoints 4 and 8 μg/ml. From the correlation between disks and the E test we obtained the values 20 ± 0.9 and 15 ± 0.9 mm, whereas from the correlation between disks and agar dilution we obtained the values 21 ± 0.9 and 16 ± 0.9 mm, respectively.
FIG. 2.
Interpretative error rate analysis for each susceptibility classification scheme, performed by using reverse regressions of inhibition zone diameters with log MIC defined by E test (A) and with log MIC defined by agar dilution method (B). Top and right arrows indicate the ranges for the three-category scheme (susceptible [S], intermediate [I], and resistant [R]). Left and bottom arrows indicate the ranges for the two-category scheme (S and R). Interpretative errors are displayed (▵, major error; ○, minor error; and ×, major or minor error when the two-category or three category scheme is applied).
Interpretative error rate analysis.
Given our susceptibility classification model, when just one breakpoint is applied (8 μg/ml), allowing the classification of strains into two categories (susceptible and resistant strains), two types of error may occur. A very major error is assumed to be present when the strain is classified as resistant by the reference method and as susceptible by the method tested, and a major error is deemed present when the opposite happens. When the third susceptibility category is defined (classifying strains as intermediate, with breakpoints between 4 and 8 μg/ml), a minor error is considered to be present when strains are (i) classified as intermediate or resistant by the reference method and, respectively, as susceptible or intermediate by the method tested or (ii) classified as intermediate or susceptible by the reference method and, respectively, as resistant or intermediate by the method tested. The results obtained by performing this analysis are shown in Table 2 and Fig. 2. A higher frequency of interpretative errors for disk diffusion was observed with agar dilution as the reference method than with E test. However, no very major errors were found for disk diffusion when these quantitative methods were each used as reference, pointing to a high consistency of susceptibility classification (as well as MIC determinations) obtained by all methods.
TABLE 2.
Frequencies of interpretative errors, obtained by comparison with disk diffusion method, for the two susceptibility classification schemes applied to 100 H. pylori strains
| Reference method | Breakpointsa (μg/ml) | Frequency (%) of:
|
|||
|---|---|---|---|---|---|
| Very major error | Major error | Minor error | Total | ||
| E test, three-class scheme | 4 < I ≤ 8 | 0 | 0 | 2 | 2 |
| E test, two-class scheme | R > 8 | 0 | 1 | NAb | 1 |
| Agar dilution, three-class scheme | 4 < I ≤ 8 | 0 | 1 | 7 | 8 |
| Agar dilution, two-class scheme | R > 8 | 0 | 2 | NA | 2 |
R, resistant; I, intermediate.
NA, not applicable.
Validation of metronidazole disk diffusion test.
In a susceptibility classification scheme, the existence of an intermediate category is particularly important, since it acts as a buffer zone to prevent the misclassification of resistant strains as susceptible and susceptible strains as resistant (very major and major errors) (24). When the scheme with the intermediate class was used, the occurrence of major errors was reduced, emphasizing the advantage of the three-category scheme.
Agar dilution has been accepted as the reference method (17, 20). However, in our study, the E test showed a higher correlation with the disk diffusion test (r2 = 0.88) than did agar dilution (r2 = 0.81). This was also evident from the interpretative error rate analysis, as agar dilution showed a higher frequency of errors (major and minor) (Table 2). As previously stated, this could be due to the larger antibiotic concentration steps used in agar dilution (log2) than on the E-test strip (semi-log2) and also to the common property of diffusion of E-test and disk methods.
The inhibition zone diameters for metronidazole breakpoints (4 and 8 μg/ml), estimated from regression lines with both methods, are not significantly different, as the confidence intervals overlapped. This fact, associated with the overall agreement of susceptibility classification of strains by the other two methods, points to a level of accuracy of the disk agar diffusion method that makes it suitable as an alternative method. The range of MICs used and the statistical significance of the fittings are in good support of the linearity between methods and make the proposed equations important and useful tools to translate susceptibility data among these three methods. Although schemes based on a unique breakpoint classification (8 μg/ml) have been proposed (22, 19), the interpretative error rate analysis performed in our study is more compatible with a classification scheme including the intermediate category. Thus, strains for which the MIC is ≤4 μg/ml should be considered susceptible, those for which the MIC is >4 μg/ml and ≤8 μg/ml should be regarded as intermediate, and those for which the MIC is >8 μg/ml should be deemed resistant. Average inhibition zone diameters of 20.5 and 15.5 mm were calculated for the respective breakpoints 4 and 8 μg/ml, by combining the diameters obtained when each quantitative method was used as a reference. For practical reasons, we propose that the diameters 21 and 16 mm be adopted. Thus, when the disk diffusion test is applied with 5-μg metronidazole disks, a strain with an inhibition zone diameter less than 16 mm shall be considered resistant, when the diameter is equal to or higher than 21 mm it shall be considered susceptible, and when the value obtained is between 21 mm and 16 mm, the strain shall be considered intermediate. Xia et al. (30) proposed the same interpretative susceptibility classification scheme but with inhibition zone diameters of 26 and 20 mm, values that are not included in the confidence intervals for the disk breakpoints calculated in the present study. The discrepancies between disk breakpoint values may be explained by the lower determination coefficient (r2 = 0.59) of the regression analysis performed by those authors compared to those obtained in our study (r2 = 0.81 and r2 = 0.88).
The implications of H. pylori antimicrobial resistance to metronidazole are well known. Therapies in patients infected with resistant strains are likely to fail and subsequent treatment will be more difficult (7, 10). Routine testing of H. pylori sensitivities to this antimicrobial agent will allow the use of alternative treatments. Agar dilution is a laborious and time-consuming method, and the E test has the drawback of being costly. The disk diffusion test is a simple and economical method, suitable for testing single isolates and for differentiating resistant subpopulations in any routine laboratory (8). Although it has not been recommended for bacterial species requiring long incubation periods, because of the pattern of antibiotic release from the disk (4, 13), this method is reliable when the procedures are standardized (7, 15). Our study strongly suggests that the good reproducibility, precision, and accuracy of the disk diffusion method potentially make it a good alternative for determining antibiotic susceptibility of H. pylori, particularly to metronidazole. Furthermore, the routine implementation of this method may help to define more efficient eradication therapies.
REFERENCES
- 1.Alarcón T, Domingo D, López-Brea M. Discrepancies between E-test and agar dilution methods for testing metronidazole susceptibility of Helicobacter pylori. J Clin Microbiol. 1998;36:1165–1166. doi: 10.1128/jcm.36.4.1165-1166.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Armitage P, Berry G. Statistical methods in medical research. 2nd ed. Oxford, United Kingdom: Blackwell Scientific Publications; 1990. [Google Scholar]
- 3.Berger S A, Gorea A, Moskowitz M, Santo M, Gilat T. Effect of inoculum size on antimicrobial susceptibility of Helicobacter pylori. Eur J Clin Microbiol Infect Dis. 1993;12:782–783. doi: 10.1007/BF02098470. [DOI] [PubMed] [Google Scholar]
- 4.Brown D J, Brown L. Evaluation of the E-test, a novel method of quantifying antimicrobial activity. J Antimicrob Chemother. 1991;27:183–190. doi: 10.1093/jac/27.2.185. [DOI] [PubMed] [Google Scholar]
- 5.Cederbrant G, Kahlmeter G, Ljungh A. The E test for antimicrobial susceptibility testing of Helicobacter pylori. J Antimicrob Chemother. 1993;31:65–71. doi: 10.1093/jac/31.1.65. [DOI] [PubMed] [Google Scholar]
- 6.De Boer W A, Tytgat N J. How to treat Helicobacter pylori infection—should treatment strategies be based on testing bacterial susceptibility? A personal viewpoint. Eur J Gastroenterol Hepatol. 1996;8:709–716. [PubMed] [Google Scholar]
- 7.DeCross A J, Marshall B J, McCallum R W, Hoffman S R, Barrett L J, Guerrant R L. Metronidazole susceptibility testing for Helicobacter pylori: comparison of disk, broth, and agar dilution methods and their clinical relevance. J Clin Microbiol. 1993;31:1971–1974. doi: 10.1128/jcm.31.8.1971-1974.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glupczynski G Y. Contribution to the diagnosis, epidemiology, pathogenesis and treatment of Helicobacter pylori. Ph.D. thesis. Brussels, Belgium: University of Brussels; 1993. [Google Scholar]
- 9.Glupczynski Y, Labbé M, Hansen W, Crokaert F, Yourassowsky E. Evaluation of the E test for quantitative antimicrobial susceptibility testing of Helicobacter pylori. J Clin Microbiol. 1991;29:2072–2075. doi: 10.1128/jcm.29.9.2072-2075.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goddard A F, Logan R P. Antimicrobial resistance and Helicobacter pylori. J Antimicrob Chemother. 1996;37:639–643. doi: 10.1093/jac/37.4.639. [DOI] [PubMed] [Google Scholar]
- 11.Hachem C Y, Clarridge J E, Reddy R, Flamm R, Evans D G, Tanaka S K, Graham D Y. Antimicrobial susceptibility testing of Helicobacter pylori. Comparison of E-test, broth microdilution, and disk diffusion for ampicillin, clarithromycin, and metronidazole. Diagn Microbiol Infect Dis. 1996;24:37–41. doi: 10.1016/0732-8893(95)00252-9. [DOI] [PubMed] [Google Scholar]
- 12.Hartzen S H, Andersen L P, Bremmelgaard A, Colding H, Arpi M, Kristiansen J, Justesen T, Espersen F, Frimodt-Møller N, Bonnevie O. Antimicrobial susceptibility testing of 230 Helicobacter pylori strains: importance of medium, inoculum, and incubation time. Antimicrob Agents Chemother. 1997;41:2634–2639. doi: 10.1128/aac.41.12.2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henriksen T H, Brorson O, Schoyen R, Thoresen T. Risks of lack of standardization of tests to detect in vitro metronidazole resistance in Helicobacter pylori. Eur J Clin Microbiol Infect Dis. 1996;15:484–488. doi: 10.1007/BF01691316. [DOI] [PubMed] [Google Scholar]
- 14.Henriksen T H, Brorson O, Schoyen T, Lia A. A simple method for determining metronidazole resistance of Helicobacter pylori. J Clin Microbiol. 1997;35:1424–1426. doi: 10.1128/jcm.35.6.1424-1426.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirschi M A, Hirschi M M, Rotter M L. Comparison of three methods for determination of the sensitivity of Helicobacter pylori to metronidazole. J Antimicrob Chemother. 1993;32:45–49. doi: 10.1093/jac/32.1.45. [DOI] [PubMed] [Google Scholar]
- 16.Knapp C C, Ludwig M D, Washington J A. In vitro activity of metronidazole against Helicobacter pylori as determined by agar dilution and agar diffusion. Antimicrob Agents Chemother. 1991;35:1230–1231. doi: 10.1128/aac.35.6.1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lambert T, Mégraud F, Gerbaud G, Courvalin P. Susceptibility of Campylobacter pyloridis to 20 antimicrobial agents. Antimicrob Agents Chemother. 1986;30:510–511. doi: 10.1128/aac.30.3.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee A. Les hélicobacters: bactériologie, ecologie. In: Mégraud F, Lamouliatte H, editors. Helicobacter pylori. Paris, France: Elsevier; 1996. pp. 58–61. [Google Scholar]
- 19.Loo V G, Fallone C A, De Souza E, Lavallee J, Barkun A N. In vitro susceptibility of Helicobacter pylori to ampicillin, clarithromycin, metronidazole and omeprazole. J Antimicrob Chemother. 1997;40:881–883. doi: 10.1093/jac/40.6.881. [DOI] [PubMed] [Google Scholar]
- 20.McNulty C M A, Dent J, Wise R. Susceptibility of clinical isolates of Campylobacter pyloridis to 11 antimicrobial agents. Antimicrob Agents Chemother. 1985;28:837–838. doi: 10.1128/aac.28.6.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Metzler C M, DeHaan R M. Susceptibility tests of anaerobic bacteria: statistical and clinical considerations. J Infect Dis. 1974;130:588–594. doi: 10.1093/infdis/130.6.588. [DOI] [PubMed] [Google Scholar]
- 22.Midolo P D, Turnidge J, Lambert J R, Bell J M. Validation of a modified Kirby-Bauer disk diffusion method for metronidazole susceptibility testing of Helicobacter pylori. Diagn Microbiol Infect Dis. 1995;21:135–140. doi: 10.1016/0732-8893(95)00066-j. [DOI] [PubMed] [Google Scholar]
- 23.Midolo P D, Bell J M, Lambert J R, Turnidge J D, Grayson M L. Antimicrobial resistance testing of Helicobacter pylori: a comparison of E test and disk diffusion methods. Pathology. 1997;29:411–414. doi: 10.1080/00313029700169415. [DOI] [PubMed] [Google Scholar]
- 24.National Committee for Clinical Laboratory Standards. Approved standard M7-A3. Methods for dilution antimicrobial susceptibility test for bacteria that grow aerobically. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1995. [Google Scholar]
- 25.Nomura A, Stemmerman G N, Chyou R H, Kato I, Pérez-Pérez G L, Blaser M J. Helicobacter pylori infection and gastric carcinoma in a population of Japanese-Americans in Hawaii. N Engl J Med. 1991;325:1132–1136. doi: 10.1056/NEJM199110173251604. [DOI] [PubMed] [Google Scholar]
- 26.Piccolomini R, Bonaventura G D, Catamo G, Carbone F, Neri M. Comparative evaluation of the E test, agar dilution, and broth microdilution for testing susceptibilities of Helicobacter pylori strains to 20 antimicrobial agents. J Clin Microbiol. 1997;35:1842–1846. doi: 10.1128/jcm.35.7.1842-1846.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Recklinghausen G V, Ansorg R. Metronidazole susceptibility testing of Helicobacter pylori with the PDM Epsilometer test (E test) Int J Med Microbiol Virol Parasitol Infect Dis. 1995;282:83–85. doi: 10.1016/s0934-8840(11)80799-1. [DOI] [PubMed] [Google Scholar]
- 28.Sokal R R, Rohlf F J. Biometry. 3rd ed. New York, N.Y: W. H. Freeman and Company; 1995. [Google Scholar]
- 29.Weiss K, Laverdiere M, Restieri C. Comparison of activity of 10 antibiotics against clinical strains of Helicobacter pylori by three different techniques. Can J Gastroenterol. 1998;12:181–185. doi: 10.1155/1998/765795. [DOI] [PubMed] [Google Scholar]
- 30.Xia H, Keane C T, Beattie S, O’Morain C A. Standardization of disk diffusion test and its clinical significance for susceptibility testing of metronidazole against Helicobacter pylori. Antimicrob Agents Chemother. 1994;38:2357–2361. doi: 10.1128/aac.38.10.2357. [DOI] [PMC free article] [PubMed] [Google Scholar]