Abstract
Background
To compare the risk of stroke/systemic embolism (S/SE) and major bleeding (MB) between non‐vitamin K antagonist oral anticoagulants (NOACs) in patients with atrial fibrillation (AF), this retrospective study was conducted using the Korean Health Insurance Review & Assessment Service (HIRA) claims database.
Methods
Patients with AF who initiated NOACs (apixaban, dabigatran, and rivaroxaban) from July 1, 2015 to November 30, 2016 were included. We applied inverse probability of treatment weighting (IPTW) method using propensity score to make weighted populations having similar characteristics between groups. Hazard ratio (HR) of S/SE and MB were estimated by Cox proportional hazard model.
Results
Of the 39 783 patients with AF, 10 564; 11 418; and 17 801 used apixaban, dabigatran, and rivaroxaban, respectively. The mean CHA2DS2‐VASc and HAS‐BLED scores were 4.59 ~ 4.69 and 3.58 ~ 3.62, respectively, among all patients after applying IPTW. For S/SE, there were no significant differences between NOACs (HR [95% confidence interval (CI)]): apixaban vs dabigatran (0.99 [0.87‐1.13]), apixaban vs rivaroxaban (0.95 [0.84‐1.07]), and dabigatran vs rivaroxaban (0.96 [0.85‐1.08]). For MB (HR [95% CI]), both apixaban (0.77 [0.68‐0.86]) and dabigatran (0.88 [0.79‐0.98]) had a significantly lower risk compared with rivaroxaban. Apixaban also had a significantly lower risk of MB compared with dabigatran (0.87 [0.76‐0.99]).
Conclusions
In real‐world practice among Korean AF patients with relatively high risk of stroke and bleeding, there were no significant differences in the risk of S/SE between all NOAC comparisons. Apixaban was associated with lower risk of MB than dabigatran and rivaroxaban.
Keywords: anticoagulants, atrial fibrillation, intracranial hemorrhage, stroke, thromboembolism
In real‐world practice among Koreans with AF, there were no significant differences in the risk of S/SE between all NOAC comparisons. Apixaban was associated with lower risk of MB than dabigatran and rivaroxaban.
![]()
1. INTRODUCTION
Atrial fibrillation (AF), the most common arrhythmia, has been estimated to affect 1%‐2% of the general population, with a progressive increase in thromboembolic events and death.1 Due to the high risk of stroke in addition to morbidity and mortality, proactively preventing AF‐related ischemic stroke has been one of the most important treatment objectives, and effective stroke prevention essentially requires oral anticoagulant (OAC) therapy.2, 3 Recently, there has been a dramatic increase in the use of non‐vitamin K antagonist oral anticoagulants (NOACs) globally, largely driven by strong evidences of large randomized clinical trials that demonstrated non‐inferior or superior reduction in stroke/systemic embolism (S/SE) as well as critical bleeding events by NOACs compared with that by warfarin in patients with AF.4, 5, 6, 7
NOAC was first approved in Korea in 2009 starting with rivaroxaban, which obtained insurance coverage for non‐valvular AF patients in reducing their risk of S/SE in 2013. At that time, the reimbursement policy of NOAC from national health insurance was strict and patients showing 2 or more of the CHADS2 score and intolerability with warfarin were able to be reimbursed. Finally, in July of 2015, the insurance coverage of NOAC has expanded and NOACs could be prescribed as first‐line therapy with reimbursement in AF patients.
Although physicians now have a choice between the available NOACs, they have insufficient evidence to guide their decision‐making because of no head‐to‐head trials for these drugs. As Asian patients with AF are known to have different traits compared with non‐Asian patients as regards higher bleeding tendency and poorer anticoagulation quality with warfarin,8 more evidence reflecting real‐world practice would be needed for optimal treatment of choice in various clinical situations. Recently, some observational studies comparing NOACs have been reported9, 10, 11, 12; however, fewer studies have focused on Asian population, with limited information on bleeding events, such as gastrointestinal (GI) bleeding.13, 14
Therefore, the present study aimed to compare the risk of S/SE and major bleeding (MB) between NOACs in Korean patients with AF, based on the claims data in Korea's nationwide insurance database.
2. METHODS
2.1. Data source
We used the Korean Health Insurance Review and Assessment (HIRA) Service database to obtain data between January 2007 and November 2016, which includes data that cover the entire population due to the universal health insurance system in South Korea.15 The data include patients’ baseline demographics as well as medical and pharmacy claims and were provided after de‐identification.15 Diagnoses in this database were coded according to the Korean Standard Classification of Diseases, which is based on the International Classification of Diseases 10th Revision (ICD‐10) code.15 The Pusan National University Institutional Review Board exempted this study from ethical review (PNU IRB/2016_137_HR).
2.2. Study population
We included OAC‐naïve patients with diagnosis of AF who had ≥1 prescription for apixaban, dabigatran, or rivaroxaban during the intake period (from July 1, 2015 to November 30, 2016). The first prescription date of OAC was considered as the index date. All patients were required to be aged ≥18 years on the index date and have at least 2 outpatient visits or 1 hospitalization with AF diagnosis defined using ICD‐10 code I48 before or on the index date. The criteria that 2 outpatient visits or 1 hospitalization with AF diagnosis (ICD‐10 code I48) is required gave good results in a previous validation study, with a positive predictive value of 94.1%.16
Patients were excluded if they had evidence of hip/knee replacement surgery within 6 weeks before the index date; valvular AF, venous thromboembolism, thyrotoxicosis, hypertrophic cardiomyopathy, elective defibrillation, radiofrequency ablation, left atrial appendage occlusion, end‐stage chronic kidney disease, kidney transplant, dialysis, or pericarditis during the 12 months baseline period; or >1 OAC prescription on the index date.
Patients were followed‐up from the index date until the switch date from index OAC treatment to another OAC, discontinuation date, death, or November 30, 2016, whichever came first. Discontinuation was defined as having no prescription of any OAC treatment within 30 days after the last day of supply of the last filled prescription. Switching was defined as having a prescription for non‐index OAC treatment within 30 days after the last day of supply of the last filled prescription. For example, if the last day of supply of OAC (A) was "day X" and OAC (B) was started on "day X + 25", it would be defined as “switched” instead of “discontinued”, because there should be no prescription of any OAC within 30 days after the last day of supply of the last filled prescription to be defined as “discontinued”.
2.3. Study endpoints
The effectiveness outcome was S/SE, which includes ischemic stroke, hemorrhagic stroke, and systemic embolism. The safety outcome was MB, which includes intracranial hemorrhage (ICH), GI bleeding, and other bleeding. Other bleeding indicates bleeding from sites other than intracranial or GI (eg, eye, genitourinary system, or respiratory passages). To identify stroke, diagnosis codes with hospitalization and brain computed tomography/magnetic resonance imaging (CT/MRI) records were used.13, 17 For SE events, diagnosis codes with hospitalization and any CT/MRI records were used. For safety outcomes, ICH events were defined using diagnosis codes with hospitalization and brain CT/MRI records. The rest of safety outcomes (GI bleeding and other bleeding) were defined using diagnosis codes with hospitalization. To define each outcome, we used primary and all secondary diagnosis codes. Further details regarding study endpoints with ICD‐10 codes are summarized in Table S1.
2.4. Statistical analysis
We performed the inverse probability of treatment weighting (IPTW) method using the propensity score to balance baseline demographics and clinical characteristics. We calculated stabilized weights,18 because the weights would be extremely large for patients who received treatment contrary to prediction.19 Propensity scores were estimated using logistic regression, which included information on baseline demographics and clinical characteristics, including age; sex; insurance type; baseline medication use; baseline comorbidities; Charlson Comorbidity Index (CCI); CHA2DS2‐VASc and HAS‐BLED scores, and their components (congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, stroke, vascular disease, age 65‐74 years, female, abnormal renal and liver function, bleeding history or predisposition, age ≥65 years, antiplatelets and NSAIDs use, and alcoholism were included; labile international normalized ratio was not included; instead of abnormal renal function, renal disease (CKD3/4) was used). Details of CHA2DS2‐VASc and HAS‐BLED scores, baseline medication use, and CCI are presented in Tables S2‐S5. We evaluated the balance between treatment groups using standardized differences in the weighted sample.19 A standardized difference of <10% was considered acceptable.19 When we found any imbalance, this variable was included in the Cox proportional hazards model.
We used the Cox proportional hazards model to compare outcomes between treatment groups. We assessed the proportional hazard (PH) assumption using several methods (eg, log‐log plots, Schoenfeld's residuals, and time‐dependent covariates).20 If the PH assumption was not met, we reported hazard ratio (HR) at year 1 estimated using the extended Cox model, which contained the product term of variable with a log of time.20
Subgroup analyses were performed based on baseline characteristics of patients, including CHA2DS2‐VASc score, HAS‐BLED score, age, and sex. Two‐sided P values <.05 were considered statistically significant. We used SAS 9.4 (SAS Institute Inc) for all analyses.
2.5. Sensitivity analysis
To assess the influence of stroke definition, we conducted sensitivity analyses as follows. First, we assessed the crude event rates by restricting the definition of ischemic stroke events that met all the following criteria (sensitivity analysis 1): (i) inpatient claims with ischemic stroke (ICD‐10 codes I63, I693, and G459) as the primary diagnosis, (ii) claims at the Department of Neurology and Neurosurgery, and (iii) claims with brain CT/MRI records. Second, we assessed the crude event rates by restricting the ischemic and hemorrhagic stroke events that met all the following criteria (sensitivity analysis 2): (i) inpatient claims with ischemic stroke (ICD‐10 codes I63 and G459) and hemorrhagic stroke (ICD‐10 codes I61and I62) as the primary diagnosis, (ii) claims at the Department of Neurology and Neurosurgery, and (iii) claims with brain CT/MRI records.
3. RESULTS
3.1. Baseline characteristics
A total of 39 783 NOAC‐naïve patients were identified. Of these, 10 564; 11 418; and 17 801 used apixaban, dabigatran, and rivaroxaban, respectively (Figure 1). Before weighting, patients who initiated dabigatran were younger and had a lower risk score (CHA2DS2‐VASc score) and CCI than those who initiated apixaban and rivaroxaban. After weighting using the IPTW method, number of patients were 10 567.04 and 11 415.02 for apixaban and dabigatran, respectively for apixaban‐dabigatran comparison; 10 545.47 and 17 812.73 for apixaban and rivaroxaban, respectively for apixaban‐rivaroxaban comparison; 11 416.37 and 17 801.82 for dabigatran and rivaroxaban, respectively for dabigatran‐rivaroxaban comparison; and all differences in baseline characteristics were balanced (P value >.05; absolute standardized difference <0.1; Table 1). The mean CHA2DS2‐VASc and HAS‐BLED scores were 4.59 ~ 4.69 and 3.58 ~ 3.62, respectively, among all NOAC users after applying IPTW. Before applying IPTW, the percentage of standard dose, reduced dose, and unapproved dose in each NOAC were 35.6%, 49.8%, and 14.6% in apixaban group, 26.2%, 52.9%, and 20.9% in dabigatran group, and 45.7%, 42.8%, and 11.6% in rivaroxaban group, respectively. After applying IPTW, the percentage of standard dose, reduced dose, and unapproved dose were: 37.6%, 48.7%, and 13.7% in apixaban group and 24.8%, 53.9%, and 21.4% in dabigatran group for apixaban‐dabigatran comparison; 36.6%, 49.5%, and 13.9% in apixaban group and 45.3%, 43.0%, and 11.8% in rivaroxaban group for apixaban‐rivaroxaban comparison; and 25.1%, 54.4%, and 20.5% in dabigatran group and 46.5%, 42.2%, and 11.3% in rivaroxaban group for dabigatran‐rivaroxaban comparison. The median follow‐up periods of apixaban, dabigatran, and rivaroxaban groups were 148, 171, and 175 days, respectively.
FIGURE 1.
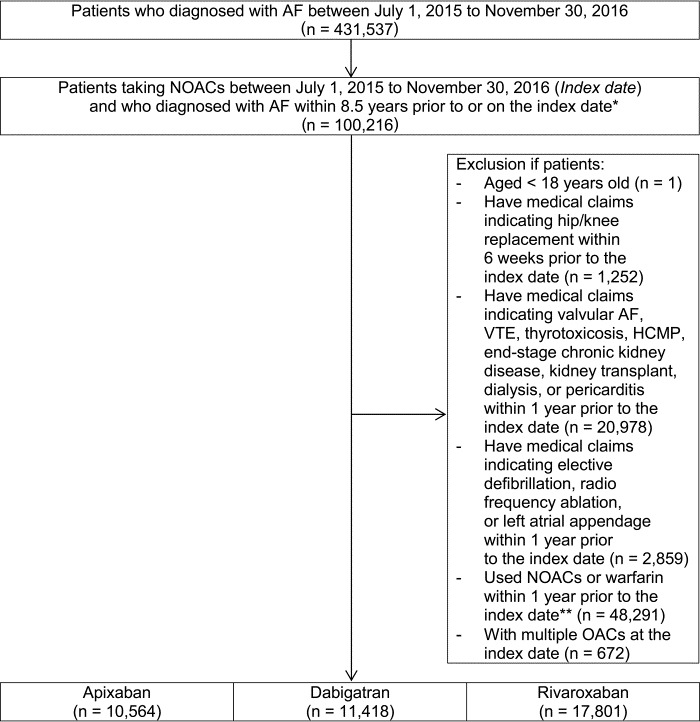
Cohort creation flow of non‐vitamin K antagonist oral anticoagulants users. AF, atrial fibrillation; HCMP, hypertrophic cardiomyopathy; ICD‐10, International Classification of Diseases 10 th Revision; NOAC, non‐vitamin K antagonist oral anticoagulants; OAC, oral anticoagulants; VTE, venous thromboembolism. *Patients should have at least one inpatient or two outpatient claims with diagnosis of ICD‐10 code I48 (atrial fibrillation and flutter) within 8.5 years prior to or on the index date. **Any OACs (apixaban, dabigatran, rivaroxaban, or warfarin) should not be prescribed one year prior to the index date.
TABLE 1.
Baseline characteristics of patients receiving oral anticoagulants
| Inverse probability of treatment weighting | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before | After a , b | |||||||||||
| A (n = 10 564) | D (n = 11 418) | A (n = 10 564) | R (n = 17 801) | D (n = 11 418) | R (n = 17 801) | A | D | A | R | D | R | |
| Age (years), mean | 73.89 | 72.24 | 73.89 | 73.27 | 72.24 | 73.27 | 73.01 | 73.01 | 73.55 | 73.52 | 72.87 | 72.87 |
| Female, % | 48.81 | 43.17 | 48.81 | 45.92 | 43.17 | 45.92 | 45.86 | 45.88 | 47.22 | 47.07 | 44.79 | 44.84 |
| Insurance, % | ||||||||||||
| National Health Insurance | 93.31 | 92.10 | 93.31 | 92.33 | 92.10 | 92.33 | 92.58 | 92.60 | 92.65 | 92.69 | 92.20 | 92.22 |
| Medical aid | 6.69 | 7.90 | 6.69 | 7.67 | 7.90 | 7.67 | 7.42 | 7.40 | 7.35 | 7.31 | 7.80 | 7.78 |
| CHA2DS2‐VASc, mean | 4.78 | 4.58 | 4.78 | 4.6 | 4.58 | 4.6 | 4.68 | 4.68 | 4.69 | 4.68 | 4.59 | 4.59 |
| HAS‐BLED, mean | 3.65 | 3.58 | 3.65 | 3.58 | 3.58 | 3.58 | 3.62 | 3.62 | 3.61 | 3.61 | 3.58 | 3.58 |
| CCI, mean | 4.33 | 4.05 | 4.33 | 4.11 | 4.05 | 4.11 | 4.19 | 4.19 | 4.21 | 4.20 | 4.09 | 4.09 |
| Medical history, % | ||||||||||||
| Heart failure | 43.81 | 40.95 | 43.81 | 43.91 | 40.95 | 43.91 | 42.31 | 42.33 | 44.06 | 43.95 | 42.86 | 42.81 |
| Hypertension | 88.46 | 89.17 | 88.46 | 89.80 | 89.17 | 89.80 | 88.83 | 88.83 | 89.31 | 89.30 | 89.63 | 89.59 |
| Diabetes | 56.76 | 54.89 | 56.76 | 54.60 | 54.89 | 54.60 | 55.77 | 55.77 | 55.58 | 55.47 | 54.81 | 54.74 |
| Ischemic stroke | 36.77 | 37.69 | 36.77 | 31.37 | 37.69 | 31.37 | 37.43 | 37.37 | 33.79 | 33.56 | 33.79 | 33.81 |
| Vascular disease | 29.70 | 29.16 | 29.70 | 30.04 | 29.16 | 30.04 | 29.42 | 29.43 | 29.98 | 29.93 | 29.70 | 29.70 |
| Renal disease (CKD3/4) | 2.17 | 0.91 | 2.17 | 1.44 | 0.91 | 1.44 | 1.50 | 1.50 | 1.72 | 1.72 | 1.23 | 1.23 |
| Bleeding | 12.80 | 8.73 | 12.80 | 10.27 | 8.73 | 10.27 | 10.68 | 10.68 | 11.30 | 11.25 | 9.68 | 9.68 |
| Medication history, % | ||||||||||||
| NSAIDs | 80.92 | 81.21 | 80.92 | 82.20 | 81.21 | 82.20 | 81.03 | 81.04 | 81.69 | 81.68 | 81.83 | 81.81 |
| Antiplatelets | 75.44 | 75.65 | 75.44 | 76.78 | 75.65 | 76.78 | 75.58 | 75.58 | 76.16 | 76.24 | 76.39 | 76.36 |
| Antiarrhythmics | 52.22 | 47.30 | 52.22 | 42.60 | 47.30 | 42.60 | 49.76 | 49.79 | 46.53 | 46.31 | 44.66 | 44.55 |
| Statins | 59.78 | 61.46 | 59.78 | 55.73 | 61.46 | 55.73 | 60.69 | 60.65 | 57.28 | 57.24 | 57.89 | 57.93 |
| Proton pump inhibitors | 45.08 | 42.89 | 45.08 | 42.51 | 42.89 | 42.51 | 43.96 | 43.98 | 43.55 | 43.49 | 42.79 | 42.72 |
| H2‐receptor antagonists | 68.89 | 68.68 | 68.89 | 67.43 | 68.68 | 67.43 | 68.76 | 68.79 | 67.99 | 67.98 | 67.95 | 67.94 |
| Digoxin | 23.80 | 25.04 | 23.80 | 26.00 | 25.04 | 26.00 | 24.37 | 24.38 | 25.04 | 25.13 | 25.81 | 25.69 |
| Dose, %c | ||||||||||||
| Standard dose | 35.60 | 26.20 | 35.60 | 45.70 | 26.20 | 45.70 | 37.64 | 24.84 | 36.63 | 45.31 | 25.09 | 46.54 |
| Low dose | 49.80 | 52.90 | 49.80 | 42.77 | 52.90 | 42.77 | 48.66 | 53.86 | 49.48 | 42.96 | 54.43 | 42.20 |
| Unapproved dose | 14.63 | 20.95 | 14.63 | 11.58 | 20.95 | 11.58 | 13.73 | 21.35 | 13.92 | 11.78 | 20.53 | 11.31 |
Abbreviations: A, Apixaban; CCI, Charlson Comorbidity Index; CHA2DS2‐VASc, congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, stroke, vascular disease, age 65‐74 years, and sex; CHADS2, congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, prior stroke, or transient ischemic attack; CKD, chronic kidney disease; D, Dabigatran; HAS‐BLED, hypertension, abnormal renal and liver function, stroke, bleeding, labile international normalized ratio, elderly, drugs, or alcohol; R: Rivaroxaban.
P‐values were not significant for all comparisons (apixaban vs dabigatran; apixaban vs rivaroxaban; dabigatran vs rivaroxaban) except for dose variable.
Absolute standardized differences were not above 10% for all comparisons (apixaban vs dabigatran; apixaban vs rivaroxaban; dabigatran vs rivaroxaban) except for dose variable.
Standard dose was defined as the general recommended dose for atrial fibrillation patients as specified in the package insert. Low dose was defined as the recommended dose for patients with renal dysfunction and/or low body weight or old age as specified in the package insert. Unapproved dose was defined as a dose lower than the low dose on label or higher than the standard dose.
3.2. Effectiveness outcomes
The Kaplan‐Meier curves for S/SE showed no significant differences among the 3 NOAC groups (Figure 2A). The crude incidence rates for S/SE showed no significant differences among the 3 NOAC groups (P = .4131). The weighted event rate for S/SE ranged from 7.01 to 7.75 per 100 person‐years among NOACs (Table 2).
FIGURE 2.
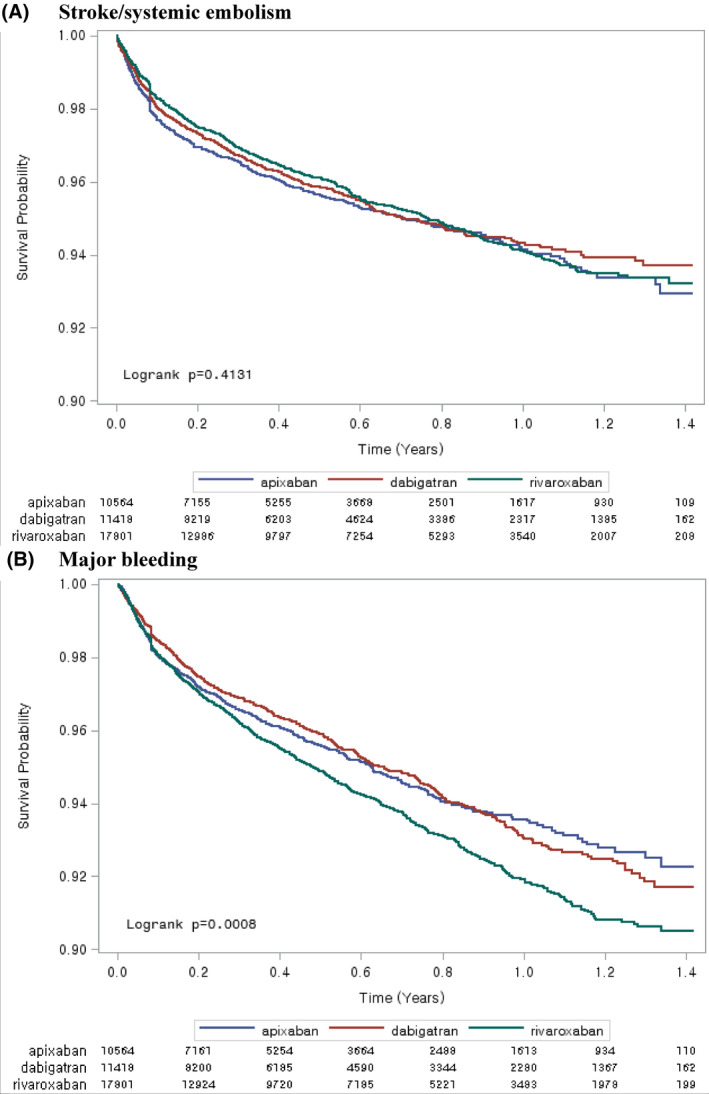
Crude Kaplan‐Meier curves of stroke/systemic embolism and major bleeding
TABLE 2.
Number of events and event rates for treatment groups
| Inverse probability of treatment weighting | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before | After | |||||||||||
| Apixaban (N = 10 564) | Dabigatran (N = 11 418) | Rivaroxaban (N = 17 801) | Apixaban | Dabigatran | Apixaban | Rivaroxaban | Dabigatran | Rivaroxaban | ||||
| Events | Crude IRa | Events | Crude IRa | Events | Crude IRa | Adjusted IR a , b | ||||||
| S/SE | 419 | 8.00 | 451 | 7.14 | 700 | 7.07 | 7.75 | 7.47 | 7.35 | 7.48 | 7.01 | 7.31 |
| MB | 432 | 8.25 | 491 | 7.81 | 927 | 9.44 | 7.70 | 8.65 | 7.77 | 9.86 | 8.18 | 9.36 |
| ICH | 76 | 1.43 | 68 | 1.06 | 140 | 1.39 | 1.39 | 1.12 | 1.35 | 1.43 | 1.08 | 1.41 |
| GIb | 188 | 3.55 | 234 | 3.68 | 432 | 4.33 | 3.23 | 4.18 | 3.28 | 4.59 | 3.97 | 4.25 |
Abbreviations: GIb, gastrointestinal bleeding; ICH, intracranial hemorrhage; IR, incidence rate; MB, major bleeding; S/SE, stroke/systemic embolism.
“Incidence rate” means number of patients with event divided by 100 person‐years.
Obtained using inverse probability of treatment weights.
Apixaban vs dabigatran (HR 0.99, 95% Confidence Interval [CI] 0.87‐1.13), apixaban vs rivaroxaban (HR 0.95, 95% CI 0.84‐1.07), and dabigatran vs rivaroxaban (HR 0.96, 95% CI 0.85‐1.08) did not show significant differences between the risk of S/SE (Figure 3).
FIGURE 3.

Hazard ratios for stroke/systemic embolism, major bleeding, intracranial hemorrhage, and gastrointestinal bleeding in comparison with each non‐vitamin K antagonist oral anticoagulants
3.3. Safety outcomes
The Kaplan‐Meier curves for major bleeding showed significant differences among the 3 NOAC groups (Figure 2B). The crude incidence rate for MB in patients who initiated rivaroxaban was higher than the corresponding event rates for those who initiated apixaban and dabigatran (P =.0008). The weighted event rate for MB ranged from 7.70 to 9.86 per 100 person‐years among NOACs (Table 2).
For MB, both apixaban (HR 0.77, 95% CI 0.68‐0.86, P value <.0001) and dabigatran (HR 0.88, 95% CI 0.79‐0.98, P value .0158) had a significantly lower risk of MB compared to rivaroxaban. Apixaban (HR 0.87, 95% CI 0.76‐0.99, P value .0283) had a significantly lower risk of MB than dabigatran (Figure 3). For ICH, there were no significant differences among all comparisons (Figure 3). For GI bleeding, apixaban had a significantly lower risk than dabigatran and rivaroxaban (HR 0.75, 95% CI 0.62‐0.91, P value .0035; and HR 0.70, 95% CI 0.59‐0.83, P value <.0001, respectively); however, dabigatran vs rivaroxaban (HR 0.94, 95% CI 0.80‐1.09) did not show significant differences in the risk of GI bleeding (Figure 3).
3.4. Subgroup analyses
In all 3 NOAC comparisons, the treatment effect did not interact with subgroups of CHA2DS2‐VASc score, HAS‐BLED score, age, and sex (Figure S1).
3.5. Sensitivity analyses
In sensitivity analyses restricted to stroke events that achieved stricter definition (sensitivity analyses 1 and 2), the crude event rates were overall lower than those of the main analysis (Table S6). There were statistical differences in event rates of hemorrhagic stroke between NOAC groups (P < .05), whereas there was no statistically significant difference in event rates of ischemic stroke (Table S6). The result showed that dabigatran had a significantly lower event rate of hemorrhagic stroke than apixaban and rivaroxaban.
4. DISCUSSION
In the present study, we found that MB events were significantly different among NOACs, although the risk of S/SE was similar. Apixaban and dabigatran showed a significantly lower risk of MB compared with rivaroxaban, and the risk of MB with apixaban was lower than that with dabigatran as well. Apixaban also showed a lower risk of GI bleeding compared with the other 2 NOACs.
This study has strengths as direct comparison results of NOACs are available with regards to ischemic stroke risks as well as bleeding‐related outcomes in Asians. The data source, Korea's HIRA claims database that covers 98% of the total population, including patients’ diagnoses, prescriptions, and procedures can, therefore, provide general representative data under routine practice, with broader clinical situations than clinical trial settings.15 Furthermore, we have carefully adjusted the confounders using IPTW to minimize potential confounding. IPTW has the advantage of being able to retain all data and reduce more bias compared with the methods of stratification and covariate adjustment using propensity scores.21
Reliable data are available as regards direct comparison between NOACs in real clinical practice, and overall results indicated similar trends with this study.10, 22, 23, 24, 25 Graham et al reported that rivaroxaban was associated with increased risks of major extracranial bleeding compared to dabigatran (HR 1.32, 95% CI 1.21‐1.45) or apixaban (HR 2.70, 95% CI 2.38‐3.05), whereas similar thromboembolic stroke risk has been reported for the 3 NOACs based on the United States (US) Medicare data.22 In this study, most of the major extracranial bleeding was GI bleeding. There was no significant difference in GI bleeding when dabigatran was compared with rivaroxaban; however, apixaban had a significantly lower risk of GI bleeding compared with both dabigatran and rivaroxaban. The lower bleeding tendency in apixaban users was also observed in a study recently conducted in Korea.23 Prior to this, Hernandez et al also mentioned about lower bleeding risk of apixaban (HR 0.69, 95% CI 0.60‐0.79) and dabigatran (HR 0.79, 95% CI 0.69‐0.92) in comparison to rivaroxaban, through claims from the Medicare beneficiaries samples.10 Another US claims database cohorts based research by Noseworthy et al also found a lower risk of MB with apixaban compared to rivaroxaban (HR 0.39, 95% CI 0.28‐0.54) or dabigatran (HR 0.50, 95% CI 0.36‐0.70), without significant difference in the risk of S/SE.24 A recent Scotland study by Mueller et al showed that the risk of MB was higher in the rivaroxaban group compared with the apixaban (HR 1.50, 95% CI 1.10‐2.03) and dabigatran (HR 1.58, 95% CI 1.01‐2.48) group. Especially, the risks of GI bleeding and overall bleeding in the rivaroxaban group were higher than those in apixaban group.25
Meanwhile, little evidence is available comparing NOACs in Asians. Therefore, Asian‐specific, real‐world evidence is crucial in the context of known ethnic differences in clinical factors and relevant outcomes in patients with AF, for example, higher bleeding tendency, lower time in therapeutic range, and higher rate of S/SE during warfarin management.8 Chan et al reported the result of comparison analyses between 4 NOACs and warfarin using Taiwan's National Health Insurance Research Database and the results were similar to that in the current study. Although the primary comparison was between NOAC and warfarin, according to the direct comparison between NOACs, rivaroxaban (HR 1.61, 95% CI 1.07‐2.43), and dabigatran (HR 1.64, 95% CI 1.09‐2.48) had a higher risk of MB than apixaban.26 Although Chan et al published the comparison results between rivaroxaban and dabigatran through a Taiwan cohort study earlier, which obtained similar outcomes between the 2 agents except a higher risk for hospitalization for GI bleeding in the rivaroxaban group,14 it did not include the apixaban group. Although several real‐world data via Korean national claims database have been reported,13, 27, 28, 29, 30 almost all included comparisons between NOACs and warfarin; therefore, only indirect interpretations were possible when it came to drawing a comparison between each of the NOACs. Lee et al very recently conducted direct comparison between 4 NOACs, and the result was generally comparable with that of our study in terms of major and GI bleeding; however, different results were observed for the comparison between stroke and ICH. Apixaban showed lower ischemic stroke than others, whereas dabigatran was associated with lower ICH events than other NOACs.23 The differences in the study period and ICH codes for the operational definitions may be considerable factors when it comes to interpreting these discrepancies23; however, further research with longer follow‐up durations will be helpful.
Our study cohort from the national representative data showed a high risk of stroke and bleeding in relation to CHA2DS2‐VASc score and HAS‐BLED score. The time‐period of HIRA data used for this study was relatively short since NOAC was launched in Korea. As such, the reimbursement policy of NOAC from national health insurance was strict and only patients showing 2 or more of CHADS2 score and intolerability with warfarin were able to be reimbursed at that time. So, AF patients with a relatively high risk of stroke were captured in the HIRA database and this may have led to higher incidence rate of S/SE and MB in NOAC users of this study compared to a previous Japanese study.31 Moreover, rates of patients with medication history of nonsteroidal anti‐inflammatory drugs and antiplatelets were high ranging from 81.03% to 81.83% and 75.58 to 76.39%, respectively, among NOAC users after applying IPTW, which may have also increased the occurrence of major bleeding (Table 1). However, a study by Lee et al23 which analyzed data from the period after the Korean insurance coverage was expanded to cover AF patients with lower risk of stroke, documented similar clinical characteristics and incidence rates of stroke and major bleeding with Japanese study results.31 In a network meta‐analysis of 16 Asian real‐world studies (including patients from 3 studies with CHA2DS2‐VASc score ≥4), there were no significant differences in the risk of stroke and systemic embolism between each NOAC while rivaroxaban showed a higher risk of major bleeding than apixaban.32 These results were similar to what we have found from our research. Therefore, our study results suggest that NOACs can be administered safely and effectively to AF patients with high risk of thromboembolism in Asia.
4.1. Limitations
This study has several inherent limitations of retrospective analysis using claims data. First, although we carefully adjusted confounders using IPTW and achieved a close balance of confounding factors, unmeasured confounding factors might have existed. Second, because claims data were collected for reimbursement purposes, coding errors for comorbidities and outcomes may exist in the database. Third, we assumed that the patients took all the medications as prescribed, but we could not accurately measure whether patients were fully adherent or the precise time point at which patients discontinued treatment. Fourth, the HIRA database does not contain laboratory data (eg, body weight and renal and liver function data) and over‐the‐counter medication use data. Fifth, the follow‐up period of this study was relatively short because reimbursement of NOAC as the first‐line therapy began since July 1, 2015. Thus, further studies may be required to explore the long‐term effectiveness and safety of NOACs.
5. CONCLUSIONS
Both apixaban and dabigatran were associated with a significantly lower risk of MB compared to rivaroxaban in real‐world practice among Asians with AF. Apixaban was also associated with lower risk of GI bleeding compared to dabigatran and rivaroxaban. The results may be especially relevant to patients with relatively high risk of thromboembolism. Further research including various data sources may be helpful for validating the findings of the current study.
CONFLICT OF INTEREST
Authors Y. Park, J. Lee, and S. Kang are full‐time employees and stockholders of Pfizer Inc. M. Won is previous employee of Pfizer Inc in connection with the development of this manuscript. Authors O. Bang, Y. On, M. Lee, S. Jang, S. Han, S. Han, HS Suh, and Y. Kim are paid consultants for Pfizer Korea Ltd.
Supporting information
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to thank Enago (www.enago.co.kr) for the English language review, which was funded by Pfizer and Bristol Myers Squibb.
Han S, Han S, Suh HS, Bang OY, On YK, Lee M‐Y, et al. Effectiveness and safety of non‐vitamin K antagonist oral anticoagulants in patients with non‐valvular atrial fibrillation: A nationwide, population‐based study in Korea. J Arrhythmia. 2021;37:1240–1249. 10.1002/joa3.12607
Seongwook Han, Sola Han, and Hae Sun Suh have contributed equally to this work (co‐first authors).
Funding information
This work was supported by Pfizer and Bristol‐Myers Squibb.
DATA AVAILABILITY STATEMENT
The study data were extracted and analyzed from the Korea Health Insurance Review & Assessment Service (HIRA) claims database, and additional data may be obtained from third party (with appropriate authorization approval) but are not publicly available.
REFERENCES
- 1.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 2015;131(4):e29–322. 10.1161/CIR.0000000000000152 [DOI] [PubMed] [Google Scholar]
- 2.Savelieva I, Bajpai A, Camm AJ. Stroke in atrial fibrillation: update on pathophysiology, new antithrombotic therapies, and evolution of procedures and devices. Ann Med. 2007;39(5):371–91. 10.1080/07853890701320662 [DOI] [PubMed] [Google Scholar]
- 3.January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association task force on practice guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64(21):e1–e76. 10.1016/j.jacc.2014.03.022 [DOI] [PubMed] [Google Scholar]
- 4.Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361(12):1139–51. 10.1056/NEJMoa0905561 [DOI] [PubMed] [Google Scholar]
- 5.Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369(22):2093–104. 10.1056/NEJMoa1310907 [DOI] [PubMed] [Google Scholar]
- 6.Granger CB, Alexander JH, McMurray JJV, Lopes RD, Hylek EM, Hanna M, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365(11):981–92. 10.1056/NEJMoa1107039 [DOI] [PubMed] [Google Scholar]
- 7.Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365(10):883–91. 10.1056/NEJMoa1009638 [DOI] [PubMed] [Google Scholar]
- 8.Chiang CE, Wang KL, Lip GY. Stroke prevention in atrial fibrillation: an Asian perspective. Thromb Haemost. 2014;111(05):789–97. 10.1160/TH13-11-0948 [DOI] [PubMed] [Google Scholar]
- 9.Deitelzweig S, Luo X, Gupta K, Trocio J, Mardekian J, Curtice T, et al. Comparison of effectiveness and safety of treatment with apixaban vs. other oral anticoagulants among elderly nonvalvular atrial fibrillation patients. Curr Med Res Opin. 2017;33(10):1745–54. 10.1080/03007995.2017.1334638 [DOI] [PubMed] [Google Scholar]
- 10.Hernandez I, Zhang Y, Saba S. Comparison of the effectiveness and safety of apixaban, dabigatran, rivaroxaban, and warfarin in newly diagnosed atrial fibrillation. Am J Cardiol. 2017;120(10):1813–9. 10.1016/j.amjcard.2017.07.092 [DOI] [PubMed] [Google Scholar]
- 11.Lip GYH, Keshishian A, Kamble S, Pan X, Mardekian J, Horblyuk R, et al. Real‐world comparison of major bleeding risk among non‐valvular atrial fibrillation patients initiated on apixaban, dabigatran, rivaroxaban, or warfarin: a propensity score matched analysis. Thromb Haemost. 2016;116(5):975–86. 10.1160/TH16-05-0403 [DOI] [PubMed] [Google Scholar]
- 12.Lip GYH, Pan X, Kamble S, Kawabata H, Mardekian J, Masseria C, et al. Major bleeding risk among non‐valvular atrial fibrillation patients initiated on apixaban, dabigatran, rivaroxaban or warfarin: a "real‐world" observational study in the United States. Int J Clin Pract. 2016;70(9):752–63. 10.1111/ijcp.12863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cha M‐J, Choi E‐K, Han K‐D, Lee S‐R, Lim W‐H, Oh S, et al. Effectiveness and safety of non‐vitamin k antagonist oral anticoagulants in Asian patients with atrial fibrillation. Stroke. 2017;48(11):3040–8. 10.1161/STROKEAHA.117.018773 [DOI] [PubMed] [Google Scholar]
- 14.Chan YH, Kuo CT, Yeh YH, Chang S‐H, Lung‐Sheng W, Lee H‐F, et al. Thromboembolic, bleeding, and mortality risks of rivaroxaban and dabigatran in Asians with nonvalvular atrial fibrillation. J Am Coll Cardiol. 2016;68(13):1389–401. 10.1016/j.jacc.2016.06.062 [DOI] [PubMed] [Google Scholar]
- 15.Kim JA, Yoon S, Kim LY, Kim DS. Towards actualizing the value potential of Korea health insurance review and assessment (HIRA) data as a resource for health research: strengths, limitations, applications, and strategies for optimal use of HIRA data. J Korean Med Sci. 2017;32(5):718–28. 10.3346/jkms.2017.32.5.718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim TH, Yang PS, Uhm JS, Kim JY, Pak HN, Lee MH, et al. Cha2ds2‐vasc score (congestive heart failure, hypertension, age ≥75 [doubled], diabetes mellitus, prior stroke or transient ischemic attack [doubled], vascular disease, age 65–74, female) for stroke in Asian patients with atrial fibrillation: a Korean nationwide sample cohort study. Stroke. 2017;48(6):1524–30. 10.1161/STROKEAHA.117.016926 [DOI] [PubMed] [Google Scholar]
- 17.Park TH, Choi JC. Validation of stroke and thrombolytic therapy in Korean national health insurance claim data. J Clin Neurol. 2016;12(1):42–8. 10.3988/jcn.2016.12.1.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stürmer T, Wyss R, Glynn RJ, Brookhart MA. Propensity scores for confounder adjustment when assessing the effects of medical interventions using nonexperimental study designs. J Intern Med. 2014;275(6):570–80. 10.1111/joim.12197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Statis Med. 2015;34(28):3661–79. 10.1002/sim.6607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kleinbaum DG, Klein M. Survival analysis: a self‐learning text, 2nd ed. New York: Springer Science+Business Media Inc; 2005. [Google Scholar]
- 21.Deb S, Austin PC, Tu JV, Ko DT, Mazer CD, Kiss A, et al. A review of propensity‐score methods and their use in cardiovascular research. Can J Cardiol. 2016;32(2):259–65. 10.1016/j.cjca.2015.05.015 [DOI] [PubMed] [Google Scholar]
- 22.Graham DJ, Baro E, Zhang R, Liao J, Wernecke M, Reichman ME, et al. Comparative stroke, bleeding, and mortality risks in older medicare patients treated with oral anticoagulants for nonvalvular atrial fibrillation. Am J Med. 2019;132(5):596–604.e11. 10.1016/j.amjmed.2018.12.023 [DOI] [PubMed] [Google Scholar]
- 23.Lee S‐R, Choi E‐K, Kwon S, Han K‐D, Jung J‐H, Cha M‐J, et al. Effectiveness and safety of contemporary oral anticoagulants among Asians with nonvalvular atrial fibrillation. Stroke. 2019;50(8):2245–9. 10.1161/STROKEAHA.119.025536 [DOI] [PubMed] [Google Scholar]
- 24.Noseworthy PA, Yao X, Abraham NS, Sangaralingham LR, McBane RD, Shah ND. Direct comparison of dabigatran, rivaroxaban, and apixaban for effectiveness and safety in nonvalvular atrial fibrillation. Chest. 2016;150(6):1302–12. 10.1016/j.chest.2016.07.013 [DOI] [PubMed] [Google Scholar]
- 25.Mueller T, Alvarez‐Madrazo S, Robertson C, Wu O, Bennie M. Comparative safety and effectiveness of direct oral anticoagulants in patients with atrial fibrillation in clinical practice in Scotland. Br J Clin Pharmacol. 2019;85(2):422–31. 10.1111/bcp.13814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chan YH, Lee HF, See LC, Tu HT, Chao TF, Yeh YH, et al. Effectiveness and safety of four direct oral anticoagulants in Asian patients with nonvalvular atrial fibrillation. Chest. 2019;156(3):529–43. 10.1016/j.chest.2019.04.108 [DOI] [PubMed] [Google Scholar]
- 27.Cho MS, Yun JE, Park JJ, Kim YJ, Lee J, Kim H, et al. Outcomes after use of standard‐ and low‐dose non‐vitamin K oral anticoagulants in Asian patients with atrial fibrillation. Stroke. 2019;50(1):110–8. 10.1161/STROKEAHA.118.023093 [DOI] [PubMed] [Google Scholar]
- 28.Kim HM, Choi E‐K, Park CS, Cha M‐J, Lee S‐Y, Kwon J‐M, et al. Effectiveness and safety of non‐vitamin k antagonist oral anticoagulants in octogenarian patients with non‐valvular atrial fibrillation. PLoS One. 2019;14(3):e0211766. 10.1371/journal.pone.0211766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee S‐R, Choi E‐K, Han K‐D, Jung J‐H, Cha M‐J, Oh S, et al. Non‐vitamin K antagonist oral anticoagulants in Asian patients with supranormal renal function. Stroke. 2019;50(6):1480–9. 10.1161/STROKEAHA.118.024264 [DOI] [PubMed] [Google Scholar]
- 30.Lee SR, Choi EK, Park CS, Han KD, Jung JH, Oh S, et al. Direct oral anticoagulants in patients with nonvalvular atrial fibrillation and low body weight. J Am Coll Cardiol. 2019;5(73):919–31. 10.1016/j.jacc.2018.11.051 [DOI] [PubMed] [Google Scholar]
- 31.Kohsaka S, Katada J, Saito K, Jenkins A, Li B, Mardekian J, et al. Safety and effectiveness of non‐vitamin K oral anticoagulants versus warfarin in real‐world patients with non‐valvular atrial fibrillation: a retrospective analysis of contemporary Japanese administrative claims data. Open Heart. 2020;1(7):e001232. 10.1136/openhrt-2019-001232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang J, Tang J, Cui X, Wang BO, Bu M, Bai Y, et al. Indirect comparison of novel oral anticoagulants among Asians with non‐valvular atrial fibrillation in the real world setting: a network meta‐analysis. BMC Cardiovasc Disord. 2019;19(1):182. 10.1186/s12872-019-1165-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
The study data were extracted and analyzed from the Korea Health Insurance Review & Assessment Service (HIRA) claims database, and additional data may be obtained from third party (with appropriate authorization approval) but are not publicly available.


