Abstract
Background
The high prevalence of atrial fibrillation (AF) in the very elderly population (aged >80 years) might be underestimated. The elderly are at increased risk of both fatal stroke and bleeding. The Optimal Thromboprophylaxis in Elderly Chinese Patients with Atrial Fibrillation (ChiOTEAF) registry provides contemporary management strategies among the elderly Chinese patients in the new era of non‐vitamin K antagonists.
Objective
To present the 1‐year follow‐up data from the ChiOTEAF registry, focusing on the use of antithrombotic therapy, rate vs. rhythm control strategies, and determinants of mortality and stroke.
Methods
The ChiOTEAF registry analyzed consecutive AF patients presenting in 44 centers from 20 Chinese provinces from October 2014 to December 2018. Endpoints of interest were mortality, thromboembolism, major bleedings, cardiovascular comorbidities, and hospital re‐admissions.
Results
Of the 7077 patients enrolled at baseline, 657 patients (9.3%) were lost to the follow‐up and 435 deaths (6.8%) occurred. The overall use of anticoagulants remains low, approximately 38% of the entire cohort at follow‐up, with similar proportions of vitamin K antagonists (VKA) and non‐vitamin K antagonists (NOACs). Antiplatelet therapy was used in 38% of the entire cohort at follow‐up, and more commonly among high‐risk patients (41%). Among those on a NOAC at baseline, 22.4% switched to antiplatelet therapy alone after one year.
Independent predictors of stroke/transient ischemic attack/peripheral embolism and/or mortality were age, heart failure, chronic kidney disease, prior ischemic stroke, dementia, and chronic obstructive pulmonary disease.
Conclusions
The ChiOTEAF registry provides contemporary data on AF management, including stroke prevention. The poor adherence of NOACs and common use of antiplatelet in these high‐risk elderly population calls for multiple comorbidities management.
Keywords: atrial fibrillation, mortality, prognosis, registry, stroke prevention, thromboprophylaxis
General outcomes of the ChiOTEAF registry. AF, atrial fibrillation.
Abbreviations
- AF
atrial fibrillation
- AFFIRM
The Atrial Fibrillation Follow‐up Investigation of Rhythm Management
- CHA2DS2‐VASc
Congestive heart failure or left ventricular dysfunction Hypertension, Age ≥75 (doubled), Diabetes, Stroke (doubled)‐Vascular disease, Age 65‐74, Sex category
- CI
confidence interval
- ECG
electrocardiography
- EORP‐AF
EURObservational Research Programme Atrial Fibrillation
- ESC
European Society of Cardiology
- HAS‐BLED
hypertension, abnormal renal/ liver function, stroke, bleeding history or predisposition, labile international normalized ratio, elderly, drugs/ alcohol concomitantly
- HR
hazard ratio
- IQR
inter‐quartile range
- mAFA
mobile AF Application
- NOAC
non‐vitamin K antagonist
- OAC
oral anticoagulant
- RCT
randomized control trial
- SD
standard deviation
- TIA
transient ischemic attack
- VKA
vitamin K antagonist: ChiOTEAF: Optimal Thromboprophylaxis in Elderly Chinese Patients with Atrial Fibrillation
1. INTRODUCTION
Atrial fibrillation (AF) incidence is increasing over the past decade1; patients with AF are older and overburdened with multimorbidity.2, 3 The prevalence of AF in the elderly (aged >80 years) ranges from 10% to 17%4; however, the exact numbers might be underestimated due to asymptomatic AF. Likewise, age is an independent risk factor for adverse outcomes in patients with AF,5 and the elderly are at a high risk of fatal ischemic stroke and major bleeding.6
Recent data demonstrate the beneficial effect of oral anticoagulants (OACs) for stroke prevention in elderly patients with AF.7, 8, 9, 10, 11, 12 One meta‐analysis showed a significant reduction in the risk of stroke and systemic embolism without increasing major bleeding events among the elderly treated with non‐vitamin K antagonist OACs (NOACs) compared with vitamin K antagonists (VKAs).13 A Taiwanese cohort study of extreme elderly (aged >90) AF patients showed superior effectiveness and safety of NOACs compared with VKAs.11 Furthermore, the use of NOACs was associated with a reduction in adverse events, especially the risk of intracranial hemorrhage.11 Despite the clear benefit of OAC therapy is maintained in elderly patients with AF, “real‐world” data showed that OACs are substantially underused14, 15, 16, 17, 18 due to a fear of bleeding, especially among those with frailty or dementia.19 Of note, the NOACs showed better efficacy and safety among Asian patients compared with non‐Asians.20
The introduction of the NOACs has led to a major change in the landscape of stroke prevention in AF, but limited contemporary nationwide data are evident from China. Thus, the prospective, nationwide Optimal Thromboprophylaxis in Elderly Chinese Patients with Atrial Fibrillation (ChiOTEAF) registry aimed to explore contemporary regional management strategies, including antithrombotic therapy among the high‐risk AF population of the elderly Chinese patients, in the new era of the NOACs. In this analysis, we present the 1‐year follow‐up data from the ChiOTEAF registry, focusing on the use of antithrombotic therapy, rate vs. rhythm control strategies, and determinants of mortality and stroke.
2. METHODS
The protocol of the ChiOTEAF registry has previously been published.21 The study was approved by the Central Medical Ethics Committee of Chinese PLA General Hospital, Beijing, China (approval no S2014‐065‐01) and local institutional review boards.
The registry was conducted between October 2014 and December 2018. Briefly, the registry population comprises consecutive in‐ and outpatients presenting with AF to cardiologists (mainly), neurologist, and surgeons, enrolled in 44 sites from 20 Chinese provinces. The main inclusions criteria were age ≥65 years (for the extended analysis, AF patients aged >50 years were included) and the qualifying AF event in the 12 months prior to enrolment (recorded by a 12‐lead ECG or 24 hours ECG Holter).
Data were collected at the moment of enrolment and during the follow‐up visits (including patient visit and/or chart review and/or telephone follow‐up) by any investigator and reported into an electronic case report form. Follow‐up was performed by the local investigators, initially at 6 and 12 months in the first year and annually for the next 2 years. Endpoints of interest were mortality, thromboembolism, major bleedings, cardiovascular comorbidities, and hospital re‐admissions. For this analysis, we focused on 1‐year outcomes.
The ChiOTEAF registry has common definitions and protocol for the EURObservational Research Programme Atrial Fibrillation (EORP‐AF) General Registry.22 Based on the ESC guidelines,23 thromboembolic risk was categorized using the CHA2DS2‐VASc score.5 “Low‐risk” patients were defined as males with a CHA2DS2‐VASc 0 or females with a CHA2DS2‐VASc 1; “moderate risk” was defined as male patients with a CHA2DS2‐VASc score 1 or females with a CHA2DS2‐VASc 2; and “high risk” was defined as CHA2DS2‐VASc score ≥2. Bleeding risk was assessed based on the HAS‐BLED bleeding score.23
2.1. Statistical analyses
Univariate analysis was applied to continuous and categorical variables. Continuous variables were reported as mean+SD and/or as median and inter‐quartile range (IQR). Among‐group comparisons were made using a non‐parametric test (Kruskal–Wallis test). Categorical variables were reported as percentages, and the χ2 test or Fisher's exact test (if required) was used for among‐group comparisons.
All the statistically significant variables at univariate analysis and variables considered of relevant clinical interests were included in the multivariable model to distinguish the independent predictors of all‐cause death and/or stroke/transient ischemic attack (TIA)/peripheral embolism during the 1‐year follow‐up period. A Cox proportional hazard model was performed by adjusting for the following covariates: sex, hypertension, coronary artery disease, liver dysfunction, and prior major bleeding. All Cox regression analyses were reported as hazard ratio (HR) and 95% confidence interval [CI]. A two‐sided P‐value of <.05 was considered statistically significant.
3. RESULTS
Available data on patient demography and baseline characteristics in relation to clinical AF subtype are summarized in Table 1, and the patient disposition is shown in Figure 1. Of the 7077 patients enrolled at baseline, 657 patients (9.3%) were lost to follow‐up and 435 deaths (6.8%) occurred (Figure 1).
TABLE 1.
Patient demography in relation to clinical subtype of atrial fibrillation
| Total (n = 5474) | First detected (n = 886) | Paroxysmal (n = 2403) | Persistent (n = 980) | Long‐standing persistent AF (n = 181) | Permanent (n = 838) | Unknown (n = 185) | P‐value | |
|---|---|---|---|---|---|---|---|---|
| Age (years) [mean ± SD] | 73.4 ± 10.6 | 74.8 ± 10.5 | 71.7 ± 10.6 | 73.6 ± 10.6 | 74.9 ± 11.1 | 76.8 ± 9.4 | 70.7 ± 9.9 | <0.001 |
| Age (years) [Median (IQR)] | 75.0 (65.0‐82.0) | 77.0 67.7‐83.0) | 72.0 64.0‐80.0) | 75.0 (65.0‐81.0) | 76.0 (66.0‐83.5) | 79.0 (70.0‐84.0) | 70.0 (63.0‐78.5) | <0.001 |
| Age (years) | ||||||||
| ≤65, n (%) | 1396 (25.5%) | 186 (21.0%) | 738 (30.7%) | 245 (25.0%) | 43 (23.8%) | 122 (14.6%) | 62 (33.5%) | <0.001 |
| >65, n (%) | 4076 (74.5%) | 700 (79.0%) | 1665 (69.3%) | 734 (75.0%) | 138(76.2%) | 716 (85.4%) | 123 (66.5%) | <0.001 |
| Gender | ||||||||
| Male, n (%) | 3300 (60.3%) | 517 (58.4%) | 1437 (59.8%) | 610 (62.2%) | 111 (61.3%) | 513 (61.2%) | 112 (60.2%) | 0.612 |
| Female, n (%) | 2174 (39.7%) | 369 (41.6%) | 966 (40.2%) | 370 (37.8%) | 70 (38.7%) | 325 (38.8%) | 74 (39.8%) | |
| Stroke risk based on CHA2DS2‐VASc score | <0.001 | |||||||
| Low risk, n (%) | 312 (5.7%) | 39 (4.4%) | 186 (7.7%) | 38 (3.9%) | 5 (2.8%) | 24 (2.9%) | 20 9(10.8%) | |
| Moderate risk, n (%) | 628 (11.5%) | 96 (10.8%) | 328 (13.6%) | 111 (11.3%) | 15 (8.3%) | 51 (6.1%) | 27 (14.5%) | |
| High risk, n (%) | 4534 (82.8%) | 751 (84.8%) | 1889 (78.6%) | 831 (84.8%) | 161 (89.0%) | 763 (91.1%) | 139 (74.7%) | |
| HAS‐BLED score class | ||||||||
| 0‐2, n (%) | 4326 (79.0%) | 697 (78.7%) | 1974 (82.1%) | 760 (77.6%) | 133 (73.5%) | 593 (70.8%) | 169 (90.9%) | <0.001 |
| ≥ 3, n (%) | 1148 (21.0%) | 189 (21.3%) | 429 (17.9%) | 220 (22.4%) | 48 (26.5%) | 245 (29.2%) | 17 (9.1%) | |
| Comorbidities | ||||||||
| Hypertension, n (%) | 3426 (62.6%) | 574 (64.8%) | 1445 (60.1%) | 635 (64.8%) | 114 (63%) | 574 (68.4%) | 84 (45.4%) | <0.001 |
| Coronary artery disease, n (%) | 2453 (44.8%) | 416 (46.9%) | 1082 (45%) | 405 (41.3%) | 104 (57.5%) | 377 (45%) | 69 (37.3%) | <0.001 |
| Heart failure, n (%) | 1758 (32.1%) | 310 (3.5%) | 523 (21.8%) | 366 (37.3%) | 108 (59.7%) | 391 (46.7%) | 60 (32.4%) | <0.001 |
| Diabetes mellitus, n (%) | 1415 (25.8%) | 215 (24.3%) | 615 (25.6%) | 248 (25.3%) | 57 (31.5%) | 250 (29.8%) | 30 (16.2%) | 0.008 |
| Prior ischemic stroke, n (%) | 1100 (20.1%) | 152 (17.2%) | 443 (18.4%) | 200 (20.4%) | 38 (21.0%) | 247 (29.5%) | 20 (10.8%) | <0.001 |
| Chronic kidney disease, n (%) | 587 (10.7%) | 105 (11.9%) | 206 (8.6%) | 114 (11.6%) | 23 (12.7%) | 129 (15.4%) | 10 (5.4%) | <0.001 |
| Liver disease, n (%) | 199 (3.6%) | 35 (3.9%) | 84 (3.5%) | 10 (1%) | 11 (6%) | 20 (2.4%) | 9 (4.9%) | 0.098 |
| Chronic obstructive pulmonary disease, n (%) | 373 (6.8%) | 70 (7.9%) | 118 (4.9%) | 66 (6.7%) | 11 (6%) | 99 (11.8%) | 9 (4.9%) | <0.001 |
| Dementia, n (%) | 115 (2.1%) | 26 (2.9%) | 42 (1.7%) | 16 (1.6%) | 7 (3.9%) | 24 (2.9%) | 0 (0%) | 0.007 |
| Moderate/severe mitral stenosis | 153 (2.8%) | 19 (2.1%) | 47 (1.9%) | 27 (2.7%) | 13 (7.2%) | 46 (5.5%) | 1 (0.5%) | <0.001 |
| Current symptoms at 1‐year follow‐up, n (%) | 1098 (20.4%) | 160 (18.4%) | 456 (19.1%) | 242 (25.1%) | 50 (27.6%) | 154 (19%) | 37 (19.8%) | <0.001 |
| Palpitations, n (%) | 859 (15.9%) | 141 (16.3%) | 366 (15.4%) | 186 (19.3%) | 33 (18.6%) | 104 (12.9%) | 29 (15.4%) | <0.001 |
| Dizziness, n (%) | 19 (0.4%) | 3 (0.3%) | 4 (0.2%) | 4 (0.4%) | 0 (0) | 5 (0.6%) | 3 (1.6%) | 0.018 |
| General non‐wellbeing, n (%) | 98 (1.8%) | 35 (4.0%) | 19 (0.8%) | 21 (2.2%) | 7 (4.0%) | 14 (1.7%) | 2 (1.1%) | <0.001 |
| Fatigue, n (%) | 25 (0.5%) | 5 (0.6%) | 5 (0.2%) | 2 (0.2%) | 2 (1.1%) | 11 (1.4%) | 0 (0) | <0.001 |
| Shortness of breath, n (%) | 129 (2.4%) | 17 (2.0%) | 32 (1.3%) | 30 (3.1%) | 9 (5.1%) | 35 (4.3%) | 6 (3.2%) | <0.001 |
| Chest pain, n (%) | 45 (0.8%) | 8 (0.9%) | 12 (0.5%) | 13 (1.3%) | 0 (0) | 8 (1.0%) | 4 (2.1%) | <0.001 |
| Fear/anxiety, n (%) | 105 (1.9%) | 38 (4.4%) | 16 (0.7%) | 19 (2.0%) | 8 (4.5%) | 15 (1.9%) | 9 (4.8%) | <0.001 |
| Other, n (%) | 18 (0.3%) | 3 (0.3%) | 2 (0.1%) | 5 (0.5%) | 3 (1.7%) | 4 (0.5%) | 1 (0.5%) | 0.006 |
Abbreviations: CHA2DS2‐VASc, Congestive heart failure or left ventricular dysfunction Hypertension, Age ≥75 (doubled), Diabetes, Stroke (doubled)‐Vascular disease, Age 65‐74, Sex category; HAS‐BLED, hypertension, abnormal renal/ liver function, stroke, bleeding history or predisposition, labile international normalized ratio, elderly, drugs/ alcohol concomitantly; IQR, inter‐quartile range;SD, standard deviation.
FIGURE 1.
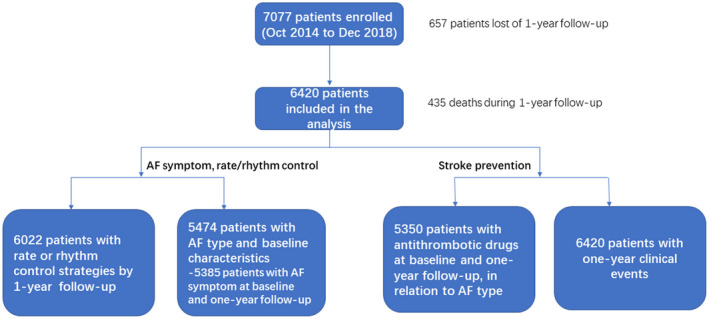
Patient flow as part of the ChiOTEAF registry. AF, atrial fibrillation
The median age of AF patients (n = 5474, 39.7% female) in relation to clinical subtype was 75.0 (65.0‐82.0) years, with a vast majority of patients at high risk of stroke (CHA2DS2‐VASc score ≥2) (Table 1). Analysis of AF subtypes showed that those patients with permanent AF were older, but no statistically significant difference was found in a gender ratio between groups. Differences in the risk of stroke and bleeding (HAS‐BLED score ≥3) strata were evident, with more high‐risk patients in the subgroups of permanent and long‐standing persistent AF (Table 1). Patients were overburdened with multi‐morbidity (particularly patients with long‐standing persistent and permanent AF), including hypertension (62.6%), coronary artery disease (44.8%), heart failure (32.1%), diabetes mellitus (25.8%), prior ischemic stroke (20.1%) chronic kidney disease (10.7%), chronic obstructive pulmonary disease (6.8%), and dementia (2.1%).
3.1. Symptoms at follow‐up
Of those patients with reported data, 1098 (20.4%) were symptomatic at 1‐year follow‐up (Table 1), most frequently among persistent and long‐standing persistent AF patients (25.1% and 27.6%, respectively). The most common symptoms at follow‐up were palpitations (15.9%), shortness of breath (2.4%), and fear/anxiety (1.9%).
3.2. Antithrombotic therapy
Overall, 5350 patients had available data on antithrombotic drugs at baseline and 1‐year follow‐up, in relation to AF type. The use of antithrombotic therapy at a 1‐year follow‐up visit, concerning antithrombotic therapy used at the baseline visit is shown in Figure 2. Of those on a vitamin K antagonist (VKA), 75.1% remained on a VKA, and 9.9% had switched to a NOAC during the follow‐up. Among those on a NOAC at baseline, 2.2% had changed to a VKA and 22.4% to antiplatelet therapy alone. Of those on antiplatelet therapy at the baseline, 14.8% had switched to OAC, and 4.5% had dual therapy (OAC and antiplatelet).
FIGURE 2.
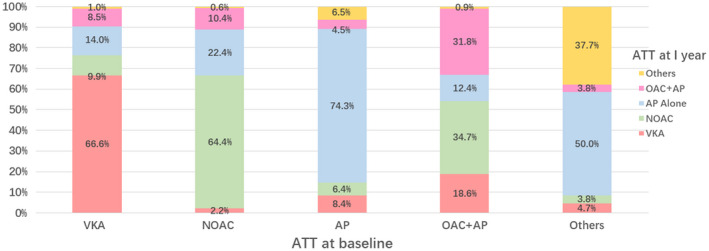
Antithrombotic therapy use at 1 year based on initial/baseline antithrombotic regimen. ATT, antithrombotic therapy; VKA, vitamin K antagonist; NOAC, non‐vitamin K anatagonist; AP, antiplatelet therapy (most commonly aspirin); OAC, oral anticoagulant therapy
Drug therapies prescribed at follow‐up are shown in Table 2, summarizing drugs used before (and after) the follow‐up consultation. The overall use of OACs remained low among 5350 AF patients, approximately 37.8%–38.8% of the entire cohort at follow‐up, with similar proportions of a VKA (18.0%–17.8%) and NOACs (20.1%–21.1%) pre and post the follow‐up consultation visit (Table 2, Figure 3). The use of OACs was the highest among persistent and long‐standing persistent AF patients (51.3%, 51.1%, respectively), with significantly lower intake in the subgroup of first detected and paroxysmal AF (33.4%, 34.6%, respectively). The NOACs were more common among long‐persistent AF (30.5%), while they were used only in 14.7% of those with persistent AF. Antiplatelet therapy was used in 37.9%–38.1% of the entire cohort at follow‐up and more commonly among first detected AF (44.0%–44.2%).
TABLE 2.
Drug therapies prescribed at follow‐up
| Total (n = 5350) | First detected (n = 859) | Paroxysmal (n = 2370) | Persistent (n = 958) | Long‐standing persistent AF (n = 174) | Permanent (n = 808) | Unknown (n = 181) | P‐value | |
|---|---|---|---|---|---|---|---|---|
| (a) Antithrombotic drugs by AF subgroup | ||||||||
| Oral anticoagulation drug | ||||||||
| Pre‐follow‐up consultation, n (%) | 2021 (37.8%) | 272 (31.7%) | 813 (34.3%) | 486 (51.3%) | 79 (45.4%) | 331 (40.9%) | 40 (22.1%) | <0.001 |
| After follow‐up consultation, n (%) | 2074 (38.8%) | 287 (33.4%) | 821 (34.6%) | 486 (51.3%) | 89 (51.1%) | 340 (42.1%) | 51 (28.2%) | <0.001 |
| VKA | ||||||||
| Pre‐follow‐up consultation, n (%) | 964 (18.0%) | 121 (14.1%) | 321 (13.5%) | 236 (24.6%) | 37 (21.3%) | 222 (27.5%) | 27 (14.9%) | <0.001 |
| After follow‐up consultation, n (%) | 950 (17.8%) | 122 (14.2%) | 308 (13.0%) | 230 (24.0%) | 37 (21.3%) | 221 (27.4%) | 32 (17.7%) | <0.001 |
| NOAC | ||||||||
| Pre‐follow‐up consultation, n (%) | 1074 (20.1%) | 152 (17.7%) | 500 (21.1%) | 253 (26.4%) | 42 (24.1%) | 113 (14.0%) | 14 (7.7%) | <0.001 |
| After follow‐up consultation, n (%) | 1129 (21.1%) | 165 (19.2%) | 515 (21.7%) | 258 (26.9%) | 53 (30.5%) | 119 (14.7%) | 19 (10.5%) | <0.001 |
| Antiplatelet drug | ||||||||
| Pre‐follow‐up consultation, n (%) | 2037 (38.1%) | 378 (44.0%) | 918 (38.7%) | 310 (32.4%) | 61 (35.1%) | 302 (37.4%) | 68 (37.6%) | <0.001 |
| After follow‐up consultation, n (%) | 2029 (37.9%) | 380 (44.2%) | 907 (38.3%) | 310 (32.4%) | 61 (35.1%) | 305 (37.7%) | 66 (36.5%) | <0.001 |
| Total (n = 5350) | Low (n = 376) | Moderate (n = 714) | High (n = 4260) | P‐value | |
|---|---|---|---|---|---|
| (b) Antithrombotic therapy by stroke risk strata | |||||
| Oral anticoagulation drug | |||||
| Pre‐follow‐up consultation, n (%) | 2021 (37.8%) | 105 (27.9%) | 244 (34.2%) | 1672 (39.2%) | <0.001 |
| After follow‐up consultation, n (%) | 2074 (38.8%) | 109 (29.0%) | 256 (35.8%) | 1709 (40.1%) | <0.001 |
| VKA | |||||
| Pre‐follow‐up consultation, n (%) | 964 (18.0%) | 50 (13.3%) | 116 (16.2%) | 798 (18.7%) | 0.013 |
| After follow‐up consultation, n (%) | 950 (17.8%) | 48 (12.8%) | 117 (16.4%) | 785 (18.4%) | 0.013 |
| NOAC | |||||
| Pre‐follow‐up consultation, n (%) | 1074 (20.1%) | 56 (14.9%) | 129 (18.1%) | 889 (20.9%) | 0.008 |
| After follow‐up consultation, n (%) | 1129 (21.1%) | 61 (16.2%) | 139 (19.5%) | 929 (21.8%) | 0.02 |
| Antiplatelet drug | |||||
| Pre‐follow‐up consultation, n (%) | 2037 (38.1%) | 89 (23.7%) | 189 (26.4%) | 1759 (41.3%) | <0.001 |
| After follow‐up consultation, n (%) | 2029 (37.9%) | 89 (23.7%) | 186 (26.1%) | 1754 (41.2%) | <0.001 |
|
Total (n = 5350) |
First detected (n = 859) |
Paroxysmal (n = 2370) |
Persistent (n = 958) |
Long‐standing persistent AF (n = 174) |
Permanent (n = 808) |
Unknown (n = 181) |
P‐value | |
|---|---|---|---|---|---|---|---|---|
| (c) Rhythm/rate control drugs (at follow‐up after consultation) | ||||||||
| Class Ic (propafenone), n (%) | 206 (3.9%) | 19 (2.2%) | 139 (5.9%) | 37 (3.9%) | 2 (1.1%) | 5 (0.6%) | 4 (2.2%) | <0.001 |
| Beta‐blockers, n (%) | 2918 (54.5%) | 466 (54.2%) | 1241 (52.4%) | 588 (61.4%) | 120 (69.0%) | 413 (51.1%) | 90 (49.7%) | <0.001 |
| Class III (amiodarone), n (%) | 465 (8.7%) | 62 (7.2%) | 301 (12.7%) | 70 (7.3%) | 15 (8.6%) | 10 (1.2%) | 7 (3.9%) | <0.001 |
| Digitalis (mainly digoxin), n (%) | 547 (10.2%) | 79 (9.2%) | 137 (5.8%) | 120 (12.5%) | 29 (16.7%) | 155 (19.2%) | 27 (14.9%) | <0.001 |
Abbreviations: AF, atrial fibrillation; NOAC, non‐vitamin K antagonist; VKA, vitamin K antagonist.
FIGURE 3.
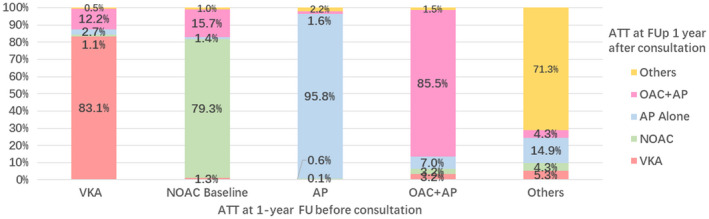
Antithrombotic therapy at 1 year comparing before vs. after visit/consultation. ATT, antithrombotic therapy; FU: follow up. VKA, vitamin K antagonist; NOAC, non‐vitamin K antagonist; AP, antiplatelet therapy (most commonly aspirin); OAC, oral anticoagulant therapy
Table 2B shows the use of antithrombotic therapy by stroke risk strata (based on the CHA2DS2‐VASc score). OACs were used in 27.9%–29.0% of low‐risk patients, in 34.2%–35.8% of moderate‐risk, and in 39.2%–40.1% of high‐risk patients, while the NOACs were used in 14.9%–16.2% of low, 18.1%–19.5% of moderate and 20.9%–21.8% of high‐risk patients, respectively. Antiplatelet therapy was used in 41.2%–41.3% of patients at high risk of stroke.
3.3. Rate and rhythm control strategy
For the analysis of rate and rhythm control strategies, 6022 patients with available data by 1‐year follow‐up were included. Drugs used for rhythm and rate control therapy at follow‐up are summarized in Table 2C. Beta‐blockers (54.5%) and digitalis (10.2%) remained the most common drugs used, especially in persistent and long‐standing persistent AF; while Class Ic and III drugs were more often used in paroxysmal AF (5.9% and 12.7%, respectively).
Among patients managed with rate control at baseline, only 4.2% continued a rate control strategy, while rhythm control was considered in 43.1% (Figure S1). Of those considered for a rhythm control at baseline, 23.9% continued the strategy, and 16.4% were eventually considered for a rate control therapy.
Table 3 shows the interventions performed by the 1‐year follow‐up. Any rhythm control intervention was performed in 9% of the overall cohort—especially among persistent and long‐standing persistent AF patients (12.8 and 17.6%, respectively). Catheter ablation was performed in 5.5% of the population, commonly among paroxysmal AF patients (8%); whereas pacemaker implantation was required in 6.9% of permanent AF patients.
TABLE 3.
Interventions performed by 1‐year follow‐up
|
Total (n = 6022) |
First detected (n = 125) | Paroxysmal (n = 3778) | Persistent (n = 802) | Long‐standing persistent AF (n = 210) | Permanent (n = 925) | Unknown (n = 182) | P‐value | |
|---|---|---|---|---|---|---|---|---|
| Rhythm control intervention, n (%) | 545 (9.05%) | 12 (9.60%) | 303 (8.02%) | 103 (12.84%) | 37 (17.62%) | 81 (8.76%) | 9 (4.95%) | <0.001 |
| Pharmacological cardioversion, n (%) | 140 (2.32%) | 3 (2.40%) | 107 (2.83%) | 16 (2.00%) | 9 (4.29%) | 4 (0.43%) | 1 (0.55%) | <0.001 |
| Electrical cardioversion, n (%) | 21 (0.35%) | 2 (1.60%) | 8 (0.21%) | 7 (0.87%) | 0 (0) | 2 (0.22%) | 2 (1.10%) | 0.003 |
| Catheter ablation, n (%) | 332 (5.51%) | 4 (3.20%) | 303 (8.02%) | 13 (1.62%) | 1 (0.48%) | 3 (0.32%) | 8 (4.40%) | <0.001 |
| Pacemaker implantation, n (%) | 329 (5.46%) | 4 (3.20%) | 206 (5.45%) | 40 (4.99%) | 14 (6.67%) | 64 (6.92%) | 1 (0.55%) | 0.013 |
| Implantable defibrillator, n (%) | 26 (0.43%) | 1 (0.80%) | 16 (0.42%) | 3 (0.37%) | 1 (0.48%) | 5 (0.54%) | 0 (0) | 0.911 |
| AF surgery, n (%) | 54 (0.90%) | 1 (0.81%) | 34 (0.91%) | 6 (0.75%) | 1 (0.48%) | 2 (0.22%) | 10 (5.81%) | <0.001 |
Abbreviation: AF, atrial fibrillation.
3.4. Mortality and morbidity
After one year, 6.8% (435/6420) of the patients enrolled in the study died between the enrolment and the 1‐year follow‐up visit (Table 4). Causes were categorized as cardiovascular (28.5%; 124/435) and non‐cardiovascular (56%; 244/435).
TABLE 4.
Mortality and morbidity during the 1‐year follow‐up
| Total (n = 6420) | First detected (n = 948) | Paroxysmal (n = 2461) | Persistent (n = 1017) | Long‐standing persistent AF (n = 188) | Permanent (n = 887) |
Unknown/missing data (n = 919) |
|
|---|---|---|---|---|---|---|---|
| (a) Mortality | |||||||
| Death, n (%) | 435 (6.8%) | 84 (8.9%) | 83 (3.4%) | 55 (5.4%) | 16 (8.5%) | 88 (9.9%) | 109 (11.9%) |
| Causes of death: | |||||||
| Cardiovascular, n (%) | 124 (28.5%) | 36 (42.9%) | 18 (21.7%) | 15 (27.3%) | 3 (18.8%) | 25 (28.4%) | 28 (25.7%) |
| Non‐cardiovascular, n (%) | 244 (56.1%) | 40 (47.6%) | 55 (66.3%) | 31 (56.3%) | 8 (50%) | 52 (59.1%) | 58 (53.2%) |
| Unknown | 67 (15.4%) | 8 (9.5%) | 10 (12%) | 9 (16.4) | 5 (31.2) | 11 (12.5%) | 23 (21.1%) |
| (b) Morbidities | |||||||
| ACS, n (%) | 67 (1%) | 15 (1.6%) | 9 (0.4%) | 10 (1%) | 4 (2.1%) | 12 (1.4%) | 17 (1.8%) |
| Heart failure, n (%) | 108 (1.7%) | 22 (2.3%) | 19 (0.8%) | 11 (1%) | 5 (2.7%) | 16 (1.8%) | 35 (3.80) |
| Any thromboembolic event | 102 (1.6%) | 13 (1.4%) | 17 (0.7%) | 16 (1.6%) | 5 (2.7%) | 24 (2.7%) | 27 (2.9%) |
| Ischemic stroke, n (%) | 62 (1%) | 8 (0.8%) | 13 (0.5%) | 5 (0.5%) | 4 (2.1%) | 17 (1.9%) | 15 (1.6%) |
| TIA, n (%) | 9 (0.1%) | 3 (0.3%) | 0 (0%) | 0 (0%) | 1 (0.5%) | 4 (0.5%) | 1 (0.1%) |
| Peripheral/pulmonary embolism, n (%) | 18 (0.2%) | 3 (0.3%) | 0 (0%) | 2 (0.2%) | 0 (0%) | 4 (0.5%) | 9 (1%) |
| Intracranial hemorrhage, n (%) | 18 (0.2%) | 0 (0%) | 3 (0.1%) | 5 (0.5%) | 0 (0%) | 8 (1%) | 2 (0.2%) |
| Extracranial bleeding, n (%) | 84 (1.3%) | 16 (1.7%) | 18 (0.7%) | 8 (0.8%) | 6 (3.2%) | 15 (1.7%) | 21 (2.3%) |
| Readmissions for arrhythmias, n (%) | 146 (2.3%) | 26 (2.7%) | 37 (1.5%) | 23 (2.3%) | 12 (6.4%) | 20 (2.3%) | 28 (3%) |
| Recurrent AF/atrial flutter, n (%) | 47 (0.7%) | 11 (1.2%) | 13 (0.5%) | 2 (0.2%) | 0 (0%) | 5 (0.6%) | 16 (1.7%) |
Abbreviations: ACS, acute coronary syndrome; AF, atrial fibrillation; TIA, transient ischemic attack.
During the 1‐year follow‐up, there were 321 cardiac re‐admissions reported; 146 for arrhythmias (47 for AF/atrial flutter recurrence), 67 for acute coronary syndrome, and 108 for heart failure. In this high‐risk cohort, 102 thromboembolism complications (including 62 ischemic strokes, 9 TIAs, and 18 systemic embolisms) and 102 major bleedings (including 18 intracranial hemorrhages) occurred. Patients with long‐standing persistent and permanent AF were at the highest risk of both ischemic stroke/TIA and bleeding.
3.5. Multivariate analysis
A Cox proportional hazard model was compiled to establish clinical factors associated with the composite outcome of stroke/TIA/peripheral embolism and/or death (Table 5). For stroke/TIA/peripheral embolism and/or mortality, independent predictors were age (HR: 3.75; 95% CI: 2.85‐4.94; P <.001), heart failure (HR: 1.93; 95% CI: 1.58‐2.34; P <.001), chronic kidney disease (HR: 1.82; 95% CI: 1.48‐2.25; P <.001), prior ischemic stroke (HR: 1.28; 95% CI: 1.05‐1.56; P =.015), dementia (HR: 2.40; 95% CI: 1.84‐3.14; P <.001) and chronic obstructive pulmonary disease (HR: 1.72; 95% CI: 1.38‐2.14; P <.001).
TABLE 5.
Multivariate analysis
| Clinical variable | Hazard ratio | 95% CI | P‐value | |
|---|---|---|---|---|
| Low | High | |||
| (a) Stroke/TIA/peripheral embolism and/or mortality | ||||
| Age >75 years | 3.75 | 2.85 | 4.94 | <0.001 |
| Heart failure | 1.93 | 1.58 | 2.34 | <0.001 |
| Chronic kidney disease | 1.82 | 1.48 | 2.25 | <0.001 |
| Prior ischemic stroke | 1.28 | 1.05 | 1.56 | 0.015 |
| Dementia | 2.40 | 1.84 | 3.14 | <0.001 |
| COPD | 1.72 | 1.38 | 2.14 | <0.001 |
| (b) Mortality | ||||
| Age >75 years | 4.02 | 2.95 | 5.49 | <0.001 |
| Heart failure | 2.24 | 1.78 | 2.81 | <0.001 |
| Chronic kidney disease | 1.98 | 1.59 | 2.48 | <0.001 |
| Prior ischemic stroke | 1.21 | 0.97 | 1.49 | 0.09 |
| Dementia | 2.41 | 1.80 | 3.21 | <0.001 |
| COPD | 1.59 | 1.26 | 2.03 | <0.001 |
| (c) Stroke/TIA/peripheral embolism | ||||
| Age >75 years | 2.66 | 1.42 | 5.00 | 0.002 |
| Heart failure | 1.11 | 0.66 | 1.87 | 0.696 |
| Chronic kidney disease | 1.00 | 0.52 | 1.91 | 0.997 |
| Prior ischemic stroke | 1.90 | 1.15 | 3.15 | 0.012 |
| Dementia | 1.90 | 0.86 | 4.20 | 0.11 |
| COPD | 2.85 | 1.60 | 5.07 | <0.001 |
Abbreviations: CI, confidence interval; COPD, chronic obstructive pulmonary disease; TIA—transient ischemic attack. Adjusted for sex, diabetes mellitus, hypertension, coronary artery disease, liver dysfunction, prior major bleeding.
4. DISCUSSION
In this 1‐year follow‐up analysis of high‐risk elderly patients with AF, our principal findings are as follows: (a) patients are frequently asymptomatic, but in a fifth of AF patients, symptoms are present (mostly palpitations, shortness of breath, and fear/anxiety); (b) the use of OAC remained low, less than 40% of patients, with similar proportions of VKA and NOACs; (c) rhythm control was infrequent, with any rhythm control intervention being performed in 9% of patients (and catheter ablation in only 5.5%); (d) 1‐year mortality was high (6.8%, with the majority being non‐cardiovascular deaths) and independent predictors of mortality were age, heart failure, chronic kidney disease, chronic obstructive pulmonary disease and dementia; and (e) hospital re‐admissions were common, especially for arrhythmic causes.
The ChiOTEAF registry is the first contemporary nationwide prospective survey focused on management practices among Chinese cardiologists, with associated follow‐up data, since the introduction of NOACs. It was designed to have aligned definitions of clinical outcomes and a common protocol with the EORP‐AF registry to compare AF management between European and Chinese populations.
While patients are frequently asymptomatic, symptoms at 1‐year follow‐up are common among persistent and long‐term persistent AF patients (but not paroxysmal AF), particularly palpitations and shortness of breath. Of note, over 50% of patients were treated with beta‐blockers; and rhythm control drugs were used in 12.6% of patients (particularly amiodarone in the paroxysmal AF subgroup). Consistent with symptom‐based management, any rhythm control intervention was limited and performed only in 9% of the overall cohort (most commonly in persistent and long‐term persistent AF).
Given that many elderly AF patients are asymptomatic, opportunistic screening is recommended for early AF detection in those aged ≥65 years.24 Likewise, the rhythm control strategy should be recommended for symptomatic patients to mitigate their symptoms and improve the quality of life.24 In the elderly, rate control is often the management of choice25; while rhythm control may be a preferable strategy among younger AF patients (aged <65 years), resulting in a higher rate of sinus rhythm restoration and a lower risk of all‐cause mortality than rate control strategy.26 An increasingly common approach is to use catheter ablation as first‐line treatment to reduce AF‐related adverse clinical outcomes among patients with recently diagnosed AF, with superior results compared to anti‐arrhythmic drugs.27, 28, 29
The ChiOTEAF registry showed that the overall use of OACs was relatively low (38% of patients at follow‐up), with similar uptake of a VKA (18%) and NOACs (20%–21%) among Chinese elderly. In comparison, data from European registries shows over 80% of AF patients being anticoagulated, and NOACs account for 40% of OACs.22, 30, 31, 32 However, an improvement in the use of OACs among Chinese patients can be observed as compared to the data from the Clinical Epidemiology of Atrial Fibrillation in Asia and previous Chinese registries.33, 34 The use of OACs is increasing steadily, and most recently, the Chinese Atrial Fibrillation Registry Study showed that 36.5% of patients with AF and CHA2DS2‐VASc scores ≥2 were anticoagulated.34 Indeed, prior papers33, 34 have highlighted the poor uptake of OACs in China, and the reasons may be multifactorial. These include patient's perceptions, physician/prescriber concerns about bleeding and costs (in the case of NOACs).
Despite guideline recommendations, we found that antiplatelet therapy (commonly aspirin) was still used in 23.7% of low‐risk and 41% of high‐risk patients. When a NOAC was discontinued, over a fifth of patients was started on antiplatelet therapy. However, the reasons for this antiplatelet “overuse” in Chinese patients are not evidence‐based; indeed, OACs were found to have superior efficacy with similar safety than aspirin among the elderly with AF.8, 35 In the EORP‐AF Long‐Term Registry, antiplatelet therapy was prescribed in 20% of patients, while 6.4% had no antithrombotic treatment.32 The poor adherence of OACs at 1‐year follow‐up and common use of antiplatelet in ChiOTEAF registry reflected the real‐world clinical practice, partly contributed by patient's risk profile, with complex comorbidities in these elderly population, thus highlighting cardiovascular risk and comorbidities management. Given that antiplatelet therapy is still commonly used in China,36 planned analyses of the ChiOTEAF registry might help addressing this “gap” and determine the reasons for OACs withholding in Chinese patients.
Given that stroke prevention is central to AF management, better education and awareness are needed to improve outcomes in this AF population.37 Indeed, guideline‐driven anticoagulation is related to significantly better outcomes in the elderly (including lower risk of all‐cause and cardiovascular deaths).38 In contrast, both under‐treatment and over‐treatment increases the risk of death and thromboembolism among AF patients (HR: 1.679; 95% CI: 1.202‐2.347 and HR: 1.622; 95% CI: 1.173‐2.23; respectively).39 Another study showed that multimorbidity was an independent factor of withholding OAC, while frequent falls and frailty were the most common reasons for non‐prescription of OACs in the elderly.40
Furthermore, our data show high morbidity and mortality rates. Indeed, 1‐year mortality was 6.8% in all cohort, particularly from non‐cardiovascular causes. During the follow‐up, 89 thromboembolism complications occurred. Independent predictors for stroke/TIA/peripheral embolism and/or mortality included age, heart failure, chronic kidney disease, prior ischemic stroke, dementia, and chronic obstructive pulmonary disease. Similarly, the European data of AF patients showed that overall mortality rates remained high (5%) during the 2‐year follow‐up, but mostly due to cardiovascular causes (61.8%).41 Accordingly in the AFFIRM trial, the diagnosis of heart failure, chronic obstructive pulmonary disease, and osteoporosis were associated with an increased risk of all‐cause mortality among elderly AF patients.3
Consistent with other registries, hospital re‐admissions were common in our cohort, especially for cardiac causes (atrial arrhythmias and heart failure). The increasing number of AF‐related hospitalizations is acknowledged as a major healthcare costs.42 A recent RCT43 assessed the impact of integrated care supported by the mobile AF Application (mAFA) on clinical outcomes among Chinese patients with AF and ≥2 stroke risk factors during a 12‐month follow‐up. The composite endpoint of ischemic stroke/systemic thromboembolism, death, and re‐hospitalization was lower in the mAFA patients than usual care.43, 44 Among the mAFA group, lower rates of OAC‐related bleeding (due to the mitigation of modifiable bleeding risk factors) and an increase in the use of OAC (from 63.4% to 70.2%) was observed as compared to standard care.45 Indeed, implementing digital healthcare models into holistic care pathways of patients with AF may improve patients awareness and treatment acceptance, resulting in better outcomes and OACs compliance.46, 47, 48
4.1. Limitations
The primary limitation of the study is its observational nature, and given its modest size, it was not powered to detect differences in some endpoints. Patients were enrolled in 44 centers, which implies a potential variability in the therapeutic strategies for AF. Moreover, the enrolment period was relatively long, which may affect the generalizability of the results. There was a moderate proportion of patients lost to follow‐up (9.3%) consistent with large European registries.49 Also, the causes of 67 deaths (15.4%) are unknown, and 919 patients have unknown (182 patients)/missing data (737 patients) of the AF type. Finally, data on anticoagulation control are not currently available for this cohort and cannot be considered in this analysis.
5. CONCLUSIONS
The Optimal Thromboprophylaxis in Elderly Chinese Patients with Atrial Fibrillation registry provides contemporary data on AF management, including stroke prevention. The rate of OAC use was <40%, and antiplatelet therapy is still commonly prescribed among high‐risk patients. Given that Chinese patients with AF are increasingly elderly and overburdened with multimorbidity, our large cohort data may help establish best practices to reduce morbidity and mortality.
5.1. Clinical perspectives
Stroke prevention is central to AF management; better education and awareness are needed to improve outcomes in high‐risk AF populations. Given the substantial clinical impact and healthcare burden associated with AF, the collection of prospective data from local AF cohorts may help establish best practice to reduce AF‐related morbidity and mortality.
DISCLOSURES
GYHL: Consultant and speaker for BMS/Pfizer, Boehringer Ingelheim, and Daiichi‐Sankyo. No fees are received personally. Other authors: None declared.
Supporting information
Supplementary Material
ACKNOWLEDGMENTS
A list of ChiOTEAF investigators in the appendix.
Guo Y, Wang H, Kotalczyk A, Wang Y, Lip GYH; the ChiOTEAF Registry Investigators . One‐year Follow‐up Results of the Optimal Thromboprophylaxis in Elderly Chinese Patients with Atrial Fibrillation (ChiOTEAF) registry. J Arrhythmia. 2021;37:1227–1239. 10.1002/joa3.12608
Drs Yutao Guo, Agnieszka Kotalczyk and Hao Wang Wang contributed equally to this manuscript.
Drs Yutang Wang, Yutao Guo and Gregory YH Lip are joint senior authors.
Funding information
The study was supported by Beijing Natural Science Foundation, China (Z141100002114050), and Chinese Military Health Care (17BJZ08).
REFERENCES
- 1.Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, et al. Worldwide epidemiology of atrial fibrillation: a global burden of disease 2010 study. Circulation. 2014;129(8):837–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Proietti M, Laroche C, Nieuwlaat R, Crijns HJGM, Maggioni AP, Lane DA, et al. Increased burden of comorbidities and risk of cardiovascular death in atrial fibrillation patients in Europe over ten years: a comparison between EORP‐AF pilot and EHS‐AF registries. Eur J Intern Med. 2018;55:28–34. [DOI] [PubMed] [Google Scholar]
- 3.Proietti M, Romiti GF, Olshansky B, Lane DA, Lip GYH. Comprehensive management with the ABC (Atrial Fibrillation Better Care) pathway in clinically complex patients with atrial fibrillation: a post hoc ancillary analysis from the AFFIRM Trial. J Am Heart Assoc. 2020;9(10):e014932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zoni‐Berisso M, Lercari F, Carazza T, Domenicucci S. Epidemiology of atrial fbrillation: European perspective. Clin Epidemiol. 2014;6(1):213–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lip GYH, Nieuwlaat R, Pisters R, Lane DA, Crijns HJGM, et al. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor‐based approach: the Euro Heart Survey on atrial fibrillation. Chest. 2010;137(2):263–72. [DOI] [PubMed] [Google Scholar]
- 6.Lip GYH, Clementy N, Pericart L, Banerjee A, Fauchier L. Stroke and major bleeding risk in elderly patients aged ≥75 years with atrial fibrillation: the Loire Valley atrial fibrillation project. Stroke. 2015;46(1):143–50. [DOI] [PubMed] [Google Scholar]
- 7.Chao T‐F, Liu C‐J, Lin Y‐J, Chang S‐L, Lo L‐W, Hu Y‐F, et al. Oral anticoagulation in very elderly patients with atrial fibrillation: a nationwide cohort study. Circulation. 2018;138(1):37–47. [DOI] [PubMed] [Google Scholar]
- 8.Mant J, Hobbs FDR, Fletcher K, Roalfe A, Fitzmaurice D, Lip GYH, et al. Warfarin versus aspirin for stroke prevention in an elderly community population with atrial fibrillation (the Birmingham Atrial Fibrillation Treatment of the Aged Study, BAFTA): a randomised controlled trial. Lancet. 2007;370(9586):493–503. [DOI] [PubMed] [Google Scholar]
- 9.Fauchier L, Blin P, Sacher F, et al. Reduced dose of rivaroxaban and dabigatran vs. vitamin K antagonists in very elderly patients with atrial fibrillation in a nationwide cohort study. Europace. 2020;22(2):205–15. [DOI] [PubMed] [Google Scholar]
- 10.Subic A, Cermakova P, Religa D, Han S, von Euler M, Kåreholt I, et al. Treatment of atrial fibrillation in patients with dementia: a cohort study from the Swedish Dementia Registry. J Alzheimer’s Dis. 2018;61(3):1119–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chao TF, Chiang CE, Liao JN, Chen TJ, Lip GYH, Chen SA. Comparing the effectiveness and safety of Nonvitamin K antagonist oral anticoagulants and warfarin in elderly asian patients with atrial fibrillation: a Nationwide Cohort Study. Chest. 2020;157(5):1266–77. [DOI] [PubMed] [Google Scholar]
- 12.Kotalczyk A, Mazurek M, Kalarus Z, Potpara TS, Lip GYH. Stroke prevention strategies in high‐risk patients with atrial fibrillation. Nat Rev Cardiol. 2021;18(4):276–90. [DOI] [PubMed] [Google Scholar]
- 13.Caldeira D, Nunes‐Ferreira A, Rodrigues R, Vicente E, Pinto FJ, Ferreira JJ. Non‐vitamin K antagonist oral anticoagulants in elderly patients with atrial fibrillation: a systematic review with meta‐analysis and trial sequential analysis. Arch Gerontol Geriatr. 2019;81:209–14. [DOI] [PubMed] [Google Scholar]
- 14.Bahri O, Roca F, Lechani T, Druesne L, Jouanny P, Serot J‐M, et al. Underuse of oral anticoagulation for individuals with atrial fibrillation in a nursing home setting in france: comparisons of resident characteristics and physician attitude. J Am Geriatr Soc. 2015;63(1):71–6. [DOI] [PubMed] [Google Scholar]
- 15.Wolff A, Shantsila E, Lip GYH, Lane DA. Impact of advanced age on management and prognosis in atrial fibrillation: insights from a population‐based study in general practice. Age Ageing. 2015;44(5):874–8. [DOI] [PubMed] [Google Scholar]
- 16.Yamashita Y, Hamatani Y, Esato M, et al. Clinical characteristics and outcomes in extreme elderly (age ≥85 years) Japanese patients with atrial fibrillation: the Fushimi AF registry. Chest. 2016;149(2):401–12. [DOI] [PubMed] [Google Scholar]
- 17.Saczynski JS, Sanghai SR, Kiefe CI, Lessard D, Marino F, Waring ME, et al. Geriatric elements and oral anticoagulant prescribing in older atrial fibrillation patients: SAGE‐AF. J Am Geriatr Soc. 2020;68(1):147–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mazurek M, Halperin JL, Huisman MV, et al. Antithrombotic treatment for newly diagnosed atrial fibrillation in relation to patient age: the GLORIA‐AF registry programme. Europace. 2020;22(1):47–57. [DOI] [PubMed] [Google Scholar]
- 19.Chan EW, Lau WCY, Siu CW, Lip GYH, Leung WK, Anand S, et al. Effect of suboptimal anticoagulation treatment with antiplatelet therapy and warfarin on clinical outcomes in patients with nonvalvular atrial fibrillation: a population‐wide cohort study. Heart Rhythm. 2016;13(8):1581–8. [DOI] [PubMed] [Google Scholar]
- 20.Wang K‐L, Lip GYH, Lin S‐J, Chiang C‐E. Non‐Vitamin K antagonist oral anticoagulants for stroke prevention in Asian patients with nonvalvular atrial fibrillation. Stroke. 2015;46(9):2555–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo Y, Wang Y, Li X, Shan Z, Shi X, Xi G, et al. Optimal thromboprophylaxis in elderly chinese patients with atrial fibrillation (ChiOTEAF) registry: protocol for a prospective, observational nationwide cohort study. BMJ Open. 2018;8(5):e020191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lip GYH, Laroche C, Dan G‐A, Santini M, Kalarus Z, Rasmussen LH, et al. A prospective survey in European Society of Cardiology member countries of atrial fibrillation management: baseline results of EURObservational Research Programme Atrial Fibrillation (EORP‐AF) Pilot General Registry. EP Eur. 2014;16(3):308–19. [DOI] [PubMed] [Google Scholar]
- 23.Camm AJ, Lip GYH, De Caterina R, Savelieva I, Atar D, Hohnloser SH, et al. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation. Eur Heart J. 2012;33(21):2719–47. [DOI] [PubMed] [Google Scholar]
- 24.Hindricks G, Potpara T, Dagres N, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio‐Thoracic Surgery (EACTS): the Task Force for the diagnosis and management of atrial fibrillation of the European. Eur Heart J. 2021;42:373–498. [DOI] [PubMed] [Google Scholar]
- 25.Fumagalli S, Said SAM, Laroche C, Gabbai D, Marchionni N, Boriani G, et al. Age‐related differences in presentation, treatment, and outcome of patients with atrial fibrillation in Europe: The EORP‐AF General Pilot Registry (EURObservational Research Programme‐Atrial Fibrillation). JACC Clin Electrophysiol. 2015;1(4):326–34. [DOI] [PubMed] [Google Scholar]
- 26.Chen S, Dong Y, Fan J, Yin Y. Rate vs. rhythm control in patients with atrial fibrillation – an updated meta‐analysis of 10 randomized controlled trials. Int J Cardiol. 2011;153(1):96–8. [DOI] [PubMed] [Google Scholar]
- 27.Andrade JG, Wells GA, Deyell MW, et al. Cryoablation or drug therapy for initial treatment of atrial fibrillation. N Engl J Med. 2021;384:305–15. [DOI] [PubMed] [Google Scholar]
- 28.Wazni OM, Dandamudi G, Sood N, et al. Cryoballoon ablation as initial therapy for atrial fibrillation. N Engl J Med. 2021;384:316–24. [DOI] [PubMed] [Google Scholar]
- 29.Kirchhof P, Camm AJ, Goette A, Brandes A, Eckardt L, Elvan A, et al. Early rhythm‐control therapy in patients with atrial fibrillation. N Engl J Med. 2020;383(14):1305–16. [DOI] [PubMed] [Google Scholar]
- 30.Boriani G, Proietti M, Laroche C, et al. Changes to oral anticoagulant therapy and risk of death over a 3‐year follow‐up of a contemporary cohort of European patients with atrial fibrillation final report of the EURObservational Research Programme on Atrial Fibrillation (EORP‐AF) pilot general. Int J Cardiol. 2018;271:68–74. [DOI] [PubMed] [Google Scholar]
- 31.Kotalczyk A, Gue YX, Potpara TS, Lip GYH. Current trends in the use of anticoagulant pharmacotherapy in the United Kingdom are changes on the horizon? Expert Opin Pharmacother. 2021;22:1061‐70. [DOI] [PubMed] [Google Scholar]
- 32.Boriani G, Proietti M, Laroche C, Fauchier L, Marin F, Nabauer M, et al. Contemporary stroke prevention strategies in 11 096 European patients with atrial fibrillation: a report from the EURObservational Research Programme on Atrial Fibrillation (EORP‐AF) Long‐Term General Registry. EP Eur. 2018;20(5):747–57. [DOI] [PubMed] [Google Scholar]
- 33.Bai Y, Wang Y‐L, Shantsila A, Lip GYH. The global burden of atrial fibrillation and stroke: a systematic review of the clinical epidemiology of atrial fibrillation in Asia. Chest. 2017;152(4):810–20. [DOI] [PubMed] [Google Scholar]
- 34.Chang S‐S, Dong J‐Z, Ma C‐S, Du X, Wu J‐H, Tang R‐B, et al. Current status and time trends of oral anticoagulation use among chinese patients with nonvalvular atrial fibrillation: the Chinese Atrial Fibrillation Registry Study. Stroke. 2016;47(7):1803–10. [DOI] [PubMed] [Google Scholar]
- 35.Rash A, Downes T, Portner R, Yeo WW, Morgan N, Channer KS. A randomised controlled trial of warfarin versus aspirin for stroke prevention in octogenarians with atrial fibrillation (WASPO). Age Ageing. 2007;36(2):151–6. [DOI] [PubMed] [Google Scholar]
- 36.Ma C, Riou França L, Lu S, Diener H‐C, Dubner SJ, Halperin JL, et al. Stroke prevention in atrial fibrillation changes after dabigatran availability in China: the GLORIA‐AF registry. J arrhythmia. 2020;36(3):408–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kotalczyk A, Potpara TS, Lip GYH. How effective is pharmacotherapy for stroke and what more is needed? A focus on atrial fibrillation. Expert Opin Pharmacother. 2021:1–4. 10.1080/14656566.2021.1921738 [DOI] [PubMed] [Google Scholar]
- 38.Proietti M, Nobili A, Raparelli V, Napoleone L, Mannucci PM, Lip GYH. Adherence to antithrombotic therapy guidelines improves mortality among elderly patients with atrial fibrillation: insights from the REPOSI study. Clin Res Cardiol. 2016;105(11):912–20. [DOI] [PubMed] [Google Scholar]
- 39.Lip GYH, Laroche C, Popescu MI, Rasmussen LH, Vitali‐Serdoz L, Dan G‐A, et al. Improved outcomes with European Society of Cardiology guideline‐adherent antithrombotic treatment in high‐risk patients with atrial fibrillation: a report from the EORP‐AF General Pilot Registry. Europace. 2015;17(12):1777–86. [DOI] [PubMed] [Google Scholar]
- 40.Dalgaard F, Xu H, Matsouaka RA, Russo AM, Curtis AB, Rasmussen PV, et al. Management of atrial fibrillation in older patients by morbidity burden: insights from get with the guidelines‐atrial fibrillation. J Am Heart Assoc. 2020;9(23):e017024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Proietti M, Laroche C, Opolski G, Maggioni AP, Boriani G, Lip GYH, et al. ‘Real‐world’ atrial fibrillation management in Europe: observations from the 2‐year follow‐up of the EURObservational Research Programme‐Atrial Fibrillation General Registry Pilot Phase. EP Eur. 2017;19(5):722–33. [DOI] [PubMed] [Google Scholar]
- 42.Burdett P, Lip GYH. Atrial Fibrillation in the United Kingdom: predicting costs of an emerging epidemic recognising and forecasting the cost drivers of atrial fibrillation‐related costs. Eur Hear J ‐ Qual Care Clin Outcomes. 2020:qcaa093. [DOI] [PubMed] [Google Scholar]
- 43.Guo Y, Lane DA, Wang L, et al. Mobile Health (mHealth) technology for improved screening, patient involvement and optimising integrated care in atrial fibrillation: the mAFA (mAF‐App) II randomised trial. Int J Clin Pract. 2019;73(7):e13352. [DOI] [PubMed] [Google Scholar]
- 44.Guo Y, Lane DA, Wang L, Zhang H, Wang H, Zhang W, et al. Mobile health technology to improve care for patients with atrial fibrillation. J Am Coll Cardiol. 2020;75(13):1523–34. [DOI] [PubMed] [Google Scholar]
- 45.Guo Y, Lane DA, Chen Y, Lip GYH. Regular bleeding risk assessment associated with reduction in bleeding outcomes: the mAFA‐II randomized trial. Am J Med. 2020;133(10):1195.e2–1202.e2. [DOI] [PubMed] [Google Scholar]
- 46.Kotalczyk A, Kalarus Z, Wright DJ, Boriani G, Lip GYH. Cardiac electronic devices: future directions and challenges. Med Devices Evid Res. 2020;13:325–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lane DA, McMahon N, Gibson J, Weldon JC, Farkowski MM, Lenarczyk R, et al. Mobile health applications for managing atrial fibrillation for healthcare professionals and patients: a systematic review. EP Eur. 2020;22(10):1567–78. [DOI] [PubMed] [Google Scholar]
- 48.Raparelli V, Proietti M, Cangemi R, Lip GYH, Lane DA, Basili S. Adherence to oral anticoagulant therapy in patients with atrial fibrillation focus on non‐vitamin k antagonist oral anticoagulants. Thromb Haemost. 2017;117(2):209–18. [DOI] [PubMed] [Google Scholar]
- 49.Boriani G, Proietti M, Laroche C, Fauchier L, Marin F, Nabauer M, et al. Association between antithrombotic treatment and outcomes at 1‐year follow‐up in patients with atrial fibrillation: the EORP‐AF General Long‐Term Registry. EP Eur. 2019;21(7):1013–22. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


