Abstract
Purpose
Women undergoing diagnosis and treatment for breast cancer may face challenges in employment. We investigated the impact of demographic, clinical, workplace, and psychosocial characteristics on loss of employment after a breast cancer diagnosis and treatment. We further describe changes in work status and work environment for cancer survivors who sustain employment.
Methods
We analyzed responses from a survey of breast cancer survivors from the Sister Study and the Two Sister Study cohorts who reported being employed at the time of their breast cancer diagnosis and who reported employment status (lost vs. sustained employment) at the time of survey administration. Multivariate logistic regression was used to identify the effects of lymphedema, neuropathy, problems with memory or attention, social support, health insurance, and sick leave on lost employment, adjusting for demographic characteristics, cancer stage, treatment, and general health.
Results
Of the 1675 respondents who reported being employed at the time of diagnosis, 83.5% reported being ‘currently’ employed at the time of the survey. Older age, peripheral neuropathy, lack of sick leave, late stage at diagnosis, a recurrence or a new cancer, problems with memory or attention, and poor general health were significantly associated with lost employment.
Conclusions
The long-term effects of breast cancer treatment and workplace provisions for leave and accommodation may have a substantial effect on women’s ability to sustain employment. The findings from this study highlight challenges reported by cancer survivors that may inform clinical and occupational interventions to support survivors’ return to work.
Keywords: Breast cancer, Employment, Disability, Cancer survivors, Return to work
Introduction
Each year, about a quarter million women in the United States are told they have breast cancer, and half are age 62 years and younger [1]. This means that for many women, breast cancer strikes during their peak earning years. Most breast cancers are diagnosed at early stages, and 5-year survival is nearly 90% [1]. A growing population at risk, [2] together with improvements in breast cancer treatment and survival, have resulted in an estimated 3.5 million women living with a breast cancer diagnosis (survivors) in the United States in 2016 [1]. That number is projected to climb to over 4.5 million by 2026 [3]. Some of these women will continue to work during diagnosis and treatment for breast cancer, and many may return to work once they complete treatment.
Employment benefits accrue to both society and to the individual. Society benefits economically from cancer survivors returning to work [4, 5]. For the cancer survivor, returning to work can mean a return to normalcy, an improved quality of life, financial security, and a sense of purpose or identity [6–9]. Despite the positive effects of work on women’s well-being, cancer survivors face many challenges that may result in reduced or lost employment. Over the past 15 years, reviews of U.S. and European studies describe higher unemployment rates among cancer survivors than comparison populations with an average employment rate of 62–64% (range 43–90%) [9–13].
Return to work is a complex process involving the health care system, the work environment, the course of disease, and the long-term effects of treatment [14]. Because of breast cancer and its treatment (that may include surgery, chemotherapy, targeted therapy, radiotherapy, hormonal therapy, or a combination), women may experience short- and long-term disabilities that influence employment [15, 16]. Over the past decades, studies in the United States and Europe have identified cancer treatments (such as chemotherapy and surgery), or the sequela of treatments (including cognitive limitations, neuropathy, lymphedema, fatigue, and psychological distress), as barriers to continued employment and work productivity [12, 17–24].
Factors that play a role in a cancer survivor’s continued employment and work performance include the work environment, and job demands [25]. A non-supportive workplace, lack of health insurance, sick leave policies, and physical demands at work can act as barriers to continued employment [9, 12, 22, 23, 26–29]. Finally, personal characteristics such as age, income, race/ethnicity, and education have been associated with sustained employment after diagnosis and treatment for breast cancer [12, 17, 19, 23, 25, 30].
The purpose of this paper is to identify determinants of sustained employment after a cancer diagnosis and treatment, and to describe the challenges faced by survivors who continue to work. We also aim to highlight important clinical and psychosocial factors operating within a cohort of breast cancer survivors with relatively high socioeconomic status. We based our analysis on the Cancer and Work model from Feuerstein and colleagues that provides a framework to examine return to work, work ability, and work performance [25]. We operationalize this framework using data from a survey of breast cancer survivors participating in the Sister Study and its companion study, the Two Sister Study [31] (www.sisterstudy.niehs.nih.gov). We examine demographic characteristics, cancer-related and treatment-related factors, workplace characteristics, economic factors, and availability of social support as predictors of sustained employment after diagnosis and treatment. Among women who continued working, we further describe their ongoing concerns about productivity, changes in job status or hours, workplace experiences, and perceived discrimination due to survivor status.
Methods
Study Population
In 2012, the Centers for Disease Control and Prevention (CDC) and the National Institute of Environmental Health Sciences conducted the Sister Study Survivorship Survey to examine breast cancer treatments, employment patterns, and health status among women diagnosed with breast cancer. The Survivorship Survey included participants from two related studies: the Sister Study, a cohort of women initially free of breast cancer who had a sister diagnosed with breast cancer, and who developed an incident breast cancer (invasive or ductal carcinoma in situ) during follow-up; and the Two Sister Study, a companion study of the breast cancer-affected sisters of the women in the original Sister Study who had been diagnosed with breast cancer at < 50 years and within 4 years of enrollment. Study design and methodology for the Sister Study, the Two Sister Study, and the Survivorship Survey are described in previous publications [31–35]. Sister Study participants were eligible for the survey if they had been diagnosed with incident breast cancer before October 2012 (self-reported and then confirmed by medical records review). All Two Sister Study participants were eligible. A total of 2540 women completed the survey; 3 women who were found not to have breast cancer were excluded, leaving 2537 eligible participants (response rate: 90.4% for Sister Study and 90.1% for Two Sister Study). For this analysis we included only women who were employed full- or part-time at the time of their breast cancer diagnosis (N = 1675). The study was approved by the Institutional Review Boards of the NIEHS/NIH and the Copernicus Group.
Outcome
Our outcome of interest was employment status at the time of the Survivorship Survey. Participants were asked if they were employed for pay at a job or business at the time of their breast cancer diagnosis (t1) and ‘currently’ i.e., at the time of the Survivorship Survey (t2) (Fig. 1). They were considered employed if they responded, ‘yes full time’, ‘yes part time’ or ‘other paid employment’. We grouped participants who were employed both at the time of diagnosis and at the time of the survey as ‘sustained employment’ (N = 1398). The participants who were employed at the time of diagnosis but were not working at the time of the survey were defined as ‘lost employment’ (n = 270). The primary outcome for this analysis was ‘lost employment’ after breast cancer diagnosis. We also present changes in work status and the effects of treatment on the working conditions of women who sustained employment.
Fig. 1.
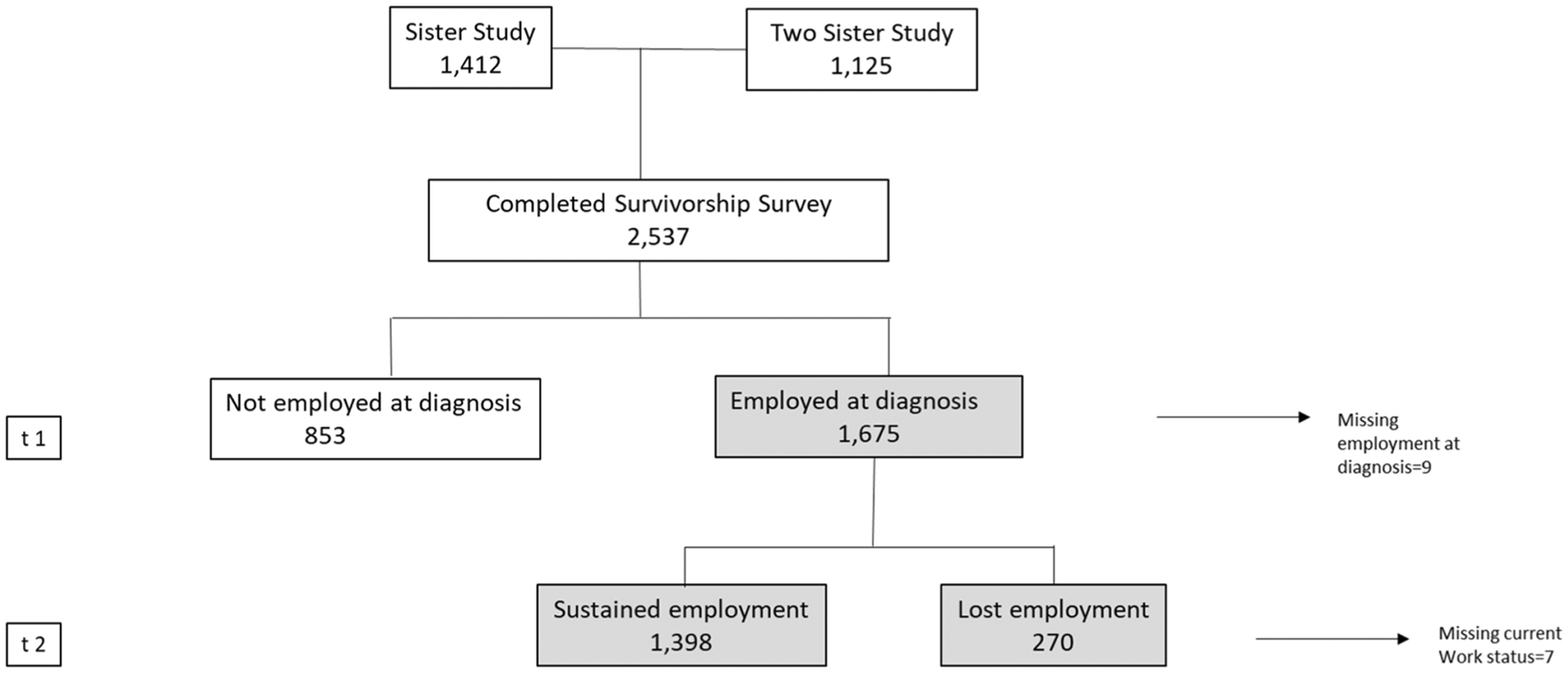
Work history of breast cancer survivors, sister study and two sister study survivorship study
Measures
We examined demographic factors (age at diagnosis, race/ethnicity, and marital status), and additional work-related characteristics at time of diagnosis (health insurance status, sick leave, income) (Table 1). All women, irrespective of age, were included in analyses because some women aged > 65 continued employment. Clinical characteristics (stage at breast cancer diagnosis, treatment status, recurrence of cancer or new cancer diagnosis, lymphedema, neuropathy, and problems with thinking, memory or attention), general health, social support were also examined (Table 2).
Table 1.
Demographic characteristics by current employment status among women who were employed at the time of breast cancer diagnosis, Sister Study Survivorship Survey, 2014
| Characteristics | Sustained employment (N = 1398) | Lost employment (N = 270) | Test^^ (P value) |
|---|---|---|---|
| N (%) | N (%) | ||
| Age at diagnosis in years: mean (SD) Race/ethnicity | 49.0 (7.4) | 53.9 (9.7) | <.0001 a |
| White, Non-Hispanic | 1246 (89.1) | 239 (88.5) | 0.9424 |
| Black, Non-Hispanic | 66 (4.7) | 14 (5.2) | |
| Other | 86 (6.2) | 17 (6.3) | |
| Marital status | |||
| Single/never married | 103 (7.4) | 16 (5.9) | 0.2504 |
| Married/Committed relationship | 1092 (78.1) | 205 (75.9) | |
| Divorced/Widowed/Separated | 203 (14.5) | 49 (18.1) | |
| Insurance type | |||
| Private | 1331 (95.2) | 239 (88.5) | <.0001 |
| Public | 55 (3.9) | 29 (10.7) | |
| Sick leave | |||
| Paid/Some paid | 786 (56.2) | 142 (52.6) | <.0001 |
| No sick leave provided | 36 (2.6) | 21 (7.8) | |
| unpaid only | 321 (23.0) | 72 (26.7) | |
| Did not take leave | 249 (17.8) | 32 (11.9) | |
| Household income | |||
| <$50,000 | 197 (14.1) | 42 (15.6) | 0.7328 |
| $50,000–99,999 | 566 (40.5) | 110 (40.7) | |
| >$100,000 | 602 (43.1) | 110 (40.7) | |
Numbers and percentages of columns may not add up to column totals as missing were not shown
P-values are from chi-square tests of categorical variables or t-test
for mean values (missing were excluded)
Table 2.
Clinical and psychosocial characteristics by current employment status among women who were employed at the time of breast cancer diagnosis, Sister Study Survivorship Survey, 2014
| Characteristics | Sustained employment (N = 1398) | Lost employment (N = 270) | Test^^ (P value) |
|---|---|---|---|
| N (%) | N (%) | ||
| Stage at Dx | |||
| 0/I | 838 (60.0) | 165 (61.1) | 0.0011 |
| II | 357 (25.5) | 57 (21.1) | |
| III | 171 (12.2) | 30 (11.1) | |
| IV | 27 (1.9) | 16 (5.9) | |
| Treatment status | |||
| Surg + Radio + Chemo | 543 (38.8) | 102 (37.8) | 0.6324 |
| Surg + Radio | 387 (27.7) | 81 (30.0) | |
| Surg + Chemo | 237 (17.0) | 42 (15.6) | |
| Surgery only | 103 (7.4) | 25 (9.3) | |
| Other | 128 (9.2) | 20 (7.4) | |
| Recurrence of cancer | |||
| Yes | 43 (3.1) | 31 (11.5) | <.0001 |
| No | 1347 (96.4) | 237 (87.8) | |
| Lymphedema and ability to work… | |||
| No Lymphedema | 1175 (84.0) | 210 (77.8) | 0.0011 |
| Yes, does not/slightly affect work | 196 (14.0) | 44 (16.3) | |
| Yes, affects work quite a bit/very much | 19 (1.4) | 12 (4.4) | |
| Neuropathy and ability to work… | |||
| No Neuropathy | 1116 (79.8) | 189 (70.0) | <.0001 |
| Yes, does not/slightly affect work | 237 (17.0) | 55 (20.4) | |
| Yes, affects work quite a bit/very much | 18 (1.3) | 18 (6.7) | |
| General health at survey | |||
| Excellent/very good | 846 (60.5) | 125 (46.3) | <.0001 |
| Good | 457 (32.7) | 84 (31.1) | |
| Fair/poor | 94 (6.7) | 61 (22.6) | |
| Problems with thinking, memory or attentions since diagnosis | |||
| No memory problems | 501 (35.8) | 85 (31.5) | <.0001 |
| Yes, does not/slightly affect work | 805 (57.6) | 85 (31.5) | |
| Yes, affects work quite a bit/very much | 82 (5.9) | 47 (17.4) | |
| Scores of relied on support mechanisms: instrumental support | |||
| Mean score (SD) | 5.5 (2.8) | 5.5 (2.9) | 0.7804 a |
| Scores of relied on support mechanisms: emotional support | |||
| Mean score (SD) | 2.7 (0.8) | 2.6 (0.8) | 0.3868 a |
Numbers and percentages of columns may not add up to column totals as missing were not shown
P-values are from chi-square tests of categorical variables or t-test
for mean values (missing were excluded)
Women were asked whether they took at least one week leave from work for cancer treatment and recovery (yes/no), and what kind of leave was provided. Sick leave was categorized as ‘paid/some paid’ if they received at least some paid leave; ‘unpaid only’ if they took only unpaid leave, Family Medical Leave Act leave, or other leave; ‘did not take leave’ if they did not take leave or took leave of less than one week; and ‘no sick leave provided.’ Women were asked the type of health insurance they had at the time of treatment for breast cancer. Insurance was classified as ‘private’ if they had a plan through their employer, a plan through someone else’s employer, a plan they bought themselves, or a plan bought by someone else. Insurance was classified as ‘public’ if they had Medicare, Medicaid, Military, Tri-Care, CHAMPUS, or other government insurance program.
Survey and medical records were used to characterize breast cancer stage of diagnosis. The survey elicited information on receipt of surgery, radiation, chemotherapy, and/or hormonal therapies (tamoxifen, anastrozole, exemestane or letrozole). Women responded ‘yes’ or ‘no’ to a cancer recurrence or new cancer diagnosis. Participants were asked whether a doctor or health professional told them that they had lymphedema and/or neuropathy since their breast cancer diagnosis and to what extent it affected their work, either at home or at their place of employment. They were also asked whether they had experienced any problems in thinking, memory or attention since being diagnosed with breast cancer. Response categories for these three symptoms were ‘not at all,’ ‘a little,’ ‘quite a bit,’ and ‘very much.’ We classified responses as ‘no (lymphedema, neuropathy, memory problems)’, ‘yes, but does not or slightly affects work,’ or ‘yes, affects work quite a bit or very much.’ We also examined instrumental and emotional social support. To assess instrumental support at the time of diagnosis and treatment, we asked whether participants had someone they relied on to remind them to take medications, help them cook meals, help them complete household chores, provide transportation, take care of children, help with caregiving responsibilities, attend doctors’ appointments, complete work responsibilities, and take care of other duties or responsibilities. Having emotional support was assessed by having someone with whom to confide in about feelings, having someone to provide comfort or support, and having someone with whom to share worries or fears. We assigned scores of ‘yes = 1’ and ‘no = 0’ for the responses for each of these items and calculated mean scores for the instrument and emotional support categories
Respondents who sustained employment were asked whether they remained in the same job, had the same hours, or had the same duties as they had at the time of diagnosis. They also were asked whether they felt that their cancer, its treatment, or the lasting effects of that treatment, interfered with their ability to perform physical or mental tasks required by the job. Finally, participants were asked whether they feared being forced to retire before they wanted to, whether they stayed at a job to retain health insurance, and whether they experienced job discrimination in the workplace.
Statistical Analysis
To examine the distribution of sociodemographic, clinical and psychosocial characteristics of study participants by employment status at the time of survey (sustained employment vs. lost employment), we used chi-square tests for each categorical characteristic, and mean differences and t-tests for continuous variables. Statistically significant variables at p < 0.05 in the descriptive analysis were included in multivariate logistic regression models to predict ‘lost employment’, our primary outcome of interest. We calculated adjusted odds ratios (ORs) and 95% confidence intervals for ‘lost employment’ including the following mutually adjusted variables: age at diagnosis; health insurance type; sick leave; stage at diagnosis; cancer recurrence/new cancer; general health; lymphedema or neuropathy and ability to work; and problems with thinking, memory and attention. Overall associations and differences between categories within each adjusted variable were assessed with the Wald F chi-square statistic, and associated p-values. We considered p-values < 0.05 as statistically significant in the final model. Additional descriptive analyses were conducted on workplace characteristics of women who sustained employment. All statistical analyses were performed using SAS v9.4 statistical software (SAS Institute Inc, Cary, NC).
Results
Participants ranged in age from 34 to 81 years at breast cancer diagnosis with a mean age of 55 years. Over half had a bachelor’s degree or higher (60.4%), and a large majority were married or partnered (77.7%), were non-Hispanic white (89.0%), and reported a relatively high annual household income (83.1% with income of at least $50,000). The average time between breast cancer diagnosis and the survey was 5.1 years (range 0.7 to 12.4). Of the 1675 respondents who reported being employed at the time of diagnosis, 83.5% reported being ‘currently’ employed at the time of the survey. Of those currently employed, 1062 reported working full time (76.0%) and 336 reported working part time (24.0%); this distribution did not substantially change from time of diagnosis (76.7% working full time, 23.3% working part time).
Table 1 displays the demographic and work-related characteristics at the time of diagnosis by employment status. Compared with cancer survivors who sustained employment, those who lost employment were, on average, older (mean age 53.9 vs. 49.0 years), and were more likely to have public insurance (10.7% vs. 3.9%). Type of sick leave provided also varied significantly by employment. Marital status, race/ethnicity, and income were not associated with employment status.
Table 2 presents clinical and psychosocial characteristics by employment status. Women who lost or sustained employment were equally likely to have been diagnosed at Stage 0/I (61.1% and 60.0%, respectively), however 5.9% of those who lost work were diagnosed at stage IV as compared with only 1.9% of those who sustained work. Treatments were generally similar for those who sustained and lost employment. Compared with cancer survivors who sustained employment, a higher percentage of survivors who lost employment experienced a recurrence or a new cancer (11.5% vs. 3.1%), reported lymphedema, neuropathy, or memory problems that very much affected their ability to work (4.4% vs. 1.4%; 6.7% vs. 1.3%; and 17.4% vs. 5.9%, respectively), and reported fair or poor health (22.6% vs. 6.7%). The mean scores for instrumental and for emotional social support did not differ by employment status.
Table 3 presents adjusted ORs and 95% confidence intervals from the multivariable logistic regression model for lost employment. Age was a strong predictor of lost employment, and overall, fewer women at older ages remained employed. However, despite lower numbers, we found 53.8% of women aged ≥ 65 years sustained employment after treatment and diagnosis—a similar percentage of employment held for those aged ≥ 70 years and older, as well as for those aged ≥ 75 years and older (data not shown).
Table 3.
Multivariate regression analysis for lost employment among women who were employed at the time of breast cancer diagnosis, Sister Study Survivorship Survey, 2014
| OR (95% CI)^^ | *P-value | |
|---|---|---|
| Age dx (continuous, 1 year) Insurance | 1.08 (1.06, 1.10) | < .0001 |
| Private | Ref | |
| Public | 1.61 (0.85, 3.08) | 0.1471 |
| Sick leave | ||
| Paid/Some paid | Ref | |
| No sick leave provided | 3.35 (1.61, 6.97) | 0.0013 |
| Unpaid only | 1.14 (0.77, 1.68) | 0.5146 |
| Did not take leave | 0.57 (0.34, 0.97) | 0.0374 |
| Stage at diagnosis | ||
| Stage 0/I | Ref | |
| Stage II | 0.74 (0.48, 1.13) | 0.1648 |
| Stage III | 0.76 (0.43, 1.35) | 0.3541 |
| Stage IV | 2.90 (1.24, 6.79) | 0.0144 |
| Recurrence or new cancer | ||
| Yes | 2.49 (1.32, 4.71) | 0.0049 |
| No | Ref | |
| General health | ||
| Excellent/very good | Ref | |
| Good | 1.07 (0.74, 1.56) | 0.7199 |
| Fair/poor | 2.73 (1.64, 4.55) | 0.0001 |
| Lymphedema and ability to work… | ||
| No Lymphedema | Ref | |
| Yes, does not/slightly affect work | 1.49 (0.94, 2.37) | 0.0929 |
| Yes, affects work quite a bit/very much | 1.29 (0.43, 3.85) | 0.6443 |
| Neuropathy and ability to work… | ||
| No neuropathy | Ref | |
| Yes, does not/slightly affect work | 1.07 (0.69, 1.65) | 0.7670 |
| Yes, affects work quite a bit/very much | 4.37 (1.78, 10.69) | 0.0013 |
| Problems with thinking, memory or attentions since diagnosis | ||
| No memory problems | Ref | |
| Yes, does not/slightly affect work | 0.74 (0.51, 1.07) | 0.1109 |
| Yes, affects work quite a bit/very much | 2.82 (1.62, 4.93) | 0.0003 |
Lost employment as outcome of interest
Adjusted for all the variables in the model
Lack of sick leave, late stage at diagnosis, a recurrence or a new cancer, poor general health, and having neuropathy or memory problems that very much affect the ability to work were also positively associated with losing employment. Although cancer survivors who took no leave at diagnosis and during treatment were less likely to have lost employment, having no sick leave was associated with an over three-fold increased odds of lost employment compared with survivors with paid sick leave. Other predictors of lost employment were a recurrence or new cancer (OR 2.49, 95% CI 1.32–4.71), and fair or poor health at survey compared with excellent or very good health (OR 2.73, 95% CI 1.64–4.55). One of the strongest predictors of lost employment was neuropathy that affects work ability quite a bit or very much vs. no neuropathy (OR 4.37, 95% CI 1.78–10.69). In the model that adjusted for all variables, neither health insurance type nor lymphedema were associated with lost employment.
We also found that almost 95% of women who sustained employment continued to work in the same job after treatment and recovery; 15.7% reported working fewer hours and 4.3% reported working more hours since diagnosis and treatment. Furthermore, less than 10% of survivors experienced a change in job duties or status. Table 4 presents additional job experiences of cancer survivors who continued to work after diagnosis and treatment. Among these women, nearly one in five reported that the effects of cancer or treatment interfered with their performance of physical tasks at work, and nearly one in four reported that effects of cancer or treatment affected their performance of mental tasks at work. Over one-third felt that cancer or cancer-related treatment effects made them less productive at work. About one-quarter feared being forced to retire or quit before they wanted to, and a slightly larger proportion stayed at work because they feared losing health insurance. A small proportion (5%) experienced discrimination in the workplace due to their cancer, treatment, or lasting effects of treatment.
Table 4.
The effects of cancer, its treatment, or the lasting effects of treatment on job performance for those who sustained employment, Sister Study Survivorship Survey, 2014
| Characteristics | Yes | No |
|---|---|---|
| N (%) | N (%) | |
| Interfered with performance of any physical tasks at work | 238 (18.0) | 1084 (82.0) |
| Interfered with performance any mental tasks required by your job | 305 (23.1) | 1018 (77.0) |
| Felt you were less productive at work | 495 (36.8) | 849 (63.2) |
| Feared being forced to retire or quit before you wanted to | 307 (22.8) | 1040 (77.2) |
| Stayed at a job because of concern with losing health insurance | 386 (28.6) | 965 (71.4) |
| Experienced discrimination in the workplace | 66 (5.0) | 1268 (95.1) |
Sustained employment (n = 1398): women who were employed at the time of diagnosis and currently employed at the time of survey
Row totals may not add up to subpopulation total as missing were not shown
Discussion
Over 80% of the breast cancer survivors in this study sustained employment after diagnosis and treatment for their cancer—a higher return-to-work percentage than the average calculated across several studies [4, 9, 13]. Perhaps due to the generally high socioeconomic status of this cohort, we did not find differences in sustained employment by race/ethnicity or income—characteristics previously reported to influence return to work [12, 22, 27].
We found that younger age predicted continued employment. However, more than half of the breast cancer survivors aged ≥ 65 years at diagnosis sustained employment after diagnosis and treatment. In the United States, older adults are staying in the workforce longer. The proportion adults aged ≥ 65 years, who are employed, increased from 12% in 1980 to 19% in 2015 [36]. Thus, the ‘traditional’ retirement age of 65 years is becoming less of a clear cut-off, especially in a population with higher education levels. Women with a higher socioeconomic status may choose either to stay employed in their careers through their 60s and 70s, or they may have the economic resources to retire earlier [37]. Thus, the proportion of women facing these challenges in returning to work is likely to increase with the aging of the population, the shifts in employment patterns, and the increasing risk for cancer associated with age [38].
Later stage at diagnosis and recurrence of cancer were strongly associated with lost employment—conditions which may require continued treatment that could limit employment or lead to disability. Treatment type alone did not appear to affect lost employment. Most women in this cohort underwent surgery and over half received chemotherapy, limiting the variability seen across treatment types. However, cognitive effects and neuropathy had significant effects on employment status. A strong body of evidence from imaging studies, animal studies, and neuropsychological studies demonstrate cognitive changes associated with chemotherapy and/or hormonal therapies manifest as problems in attention, memory, learning, and executive function [39]. Furthermore, these effects can last more than 20 years post-treatment [40]. Another sequela of chemotherapy is peripheral neuropathy affecting 11 to 80% of survivors with long-term effects on breast cancer survivor’s physical function and quality of life [41–43]. Chemotherapy and its effects have been well documented in both cross-sectional and cohort studies as barriers to returning to work in the US and in Europe [12, 17–20, 22–24, 44]. Finally, cancer survivors who reported fair or poor general health were almost three times more likely to report lost employment compared with survivors reporting excellent/very good or good general health.
In our final model lymphedema was not associated with loss of employment after adjustment for other cancer-related risk factors. Breast cancer surgery, especially involving lymph node dissection can lead to functional impairment that limits work ability and lowers quality of life [44–48]. Although most women underwent surgery, only 6% reported that lymphedema affected work quite a bit or very much. Because these women had relatively high income and education levels, they were likely to be white-collar workers with fewer physical demands. In this setting, lymphedema may not pose as serious an obstacle to continued employment as having physically demanding work [9, 12, 23].
Among the employment-related characteristics, lack of any time off strongly predicted loss of employment, despite the relatively small proportion of survivors in this category. Conversely, women who reported not taking more than one week of sick leave for diagnosis and treatment of their cancer were less likely to report lost employment. This group was largely made up of the earliest stage breast cancer survivors whose treatment may have been managed within a short time frame or managed through non-formal leave accommodation. After adjustment for all variables in the model, health insurance status did not predict loss of employment in this cohort, possibly because most survivors in the cohort had some private insurance.
Although most of the women in this cohort continued to work in the same job after diagnosis and treatment, one in six worked fewer hours, and one in ten reported a change in status, position or duties because of cancer diagnosis, treatment or side effects. Almost 30% of women continued to work to ensure maintenance of health insurance, a situation described in the literature as ‘job lock’[49]. Voluntary unemployment is unlikely unless patients have other resources for income [50]. As reported previously, a significant proportion of cancer survivors return to work with some impaired abilities [18].
Strengths and Limitations
The Sister Study and Two Sister Study provided the opportunity to examine the employment trajectory of breast cancer survivors by identifying characteristics associated with job loss. Thus, we were able to examine demographic, workplace, and clinical factors in a relatively large study population that included many women aged ≤ 50 years, a peak time of employment. A limitation of this study is that our data on variables other than breast cancer stage and treatment history are self-reported. Recall error may have affected reporting of symptoms such as memory loss or neuropathy. Women also self-reported employment status at breast cancer diagnosis and ‘currently’ at the time of the survey. Given the seriousness and emotional impact of a cancer diagnosis and treatment, women may be likely to recall their employment status and situations such as taking leave at the time of diagnosis and treatment.[51] In addition, we prefaced the questions about conditions and sequelae with the statement ‘since your breast cancer diagnosis.’ Finally, this sample was taken from a volunteer cohort of women which may limit the generalizability of study results, as might the higher income and education levels of these survivors.
Conclusions
Breast cancer, even when treated successfully, is a life-altering disease that primarily impacts women in midlife. Longer survival means that most women will return to employment after diagnosis and treatment. The extent to which a woman’s quality of life is limited by the long-term effects of treatments, financial stress, and her ability to work can be substantial. The findings from this study highlight important factors underlying unmet needs of cancer survivors. These may inform the ongoing efforts to reduce or mitigate treatment effects, integrate rehabilitative and supportive clinical services into optimal cancer care, and develop workplace accommodations that enable survivors to return to work [52, 53].
Funding
This research was funded, in part, by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences (Z01 ES-044005). The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. Web site addresses of nonfederal organizations are provided solely as a service to readers. Provision of an address does not constitute an endorsement of this organization by CDC or the federal government, and none should be inferred. CDC is not responsible for the content of other organizations’ Web pages.
Footnotes
Conflict of interest The authors declare that they have no conflict of interest.
Ethical Approval This project received IRB approval.
Informed Consent Informed consent was obtained from all individual participants included in the study.
References
- 1.National Cancer Institute, Surveillance E, and End Results (SEER). Cancer stat facts: female breast cancer. https://seer.cancer.gov/statfacts/html/breast.html.Accessed10 Mar 2020
- 2.Weir HK, Thompson TD, Soman A, Møller B, Leadbetter S. The past, present, and future of cancer incidence in the United States: 1975 through 2020. Cancer. 2015;121(11):1827–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics. CA Cancer J Clin. 2016;66(4):271–89. [DOI] [PubMed] [Google Scholar]
- 4.de Boer AM, Taskila T, Ojajärvi A, van Dijk FH, Verbeek JM. Cancer survivors and unemployment: a meta-analysis and meta-regression. JAMA. 2009;301(7):753–62. [DOI] [PubMed] [Google Scholar]
- 5.Fu AZ, Chen L, Sullivan S, Christiansen NP. Absenteeism and short-term disability associated with breast cancer. Breast Cancer Res Treat. 2011;130:235–42. [DOI] [PubMed] [Google Scholar]
- 6.Banning M. Employment and breast cancer: a meta-ethnography. Eur J Cancer Care. 2011;20:708–19. [DOI] [PubMed] [Google Scholar]
- 7.Blinder VS, Murphy MM, Vahdat LT, et al. Employment after a breast cancer diagnosis: a qualitative study of ethnically diverse urban women. J Commun Health. 2012;37(4):763–72. [DOI] [PubMed] [Google Scholar]
- 8.de Boer AGEM, Taskila TK, Tamminga SJ, Feuerstein M, Frings-Dresen MHW, Verbeek JH. Interventions to enhance return-to-work for cancer patients. Cochrane Database Syst Rev. 2015;2015(9):007569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spelten ER, Sprangers MAG, Verbeek JHAM. Factors reported to influence the return to work of cancer survivors: a literature review. Physcooncology. 2002;11(2):124–31. [DOI] [PubMed] [Google Scholar]
- 10.de Boer AGEM, Frings-Dresen MHW. Employment and the common cancers: return to work of cancer survivors. Occup Med. 2009;59(6):378–80. [DOI] [PubMed] [Google Scholar]
- 11.Livestrong Foundation. Survivors’ experiences with employment. A Livestrong brief 2013. www.LIVESTRONG.ore/we-can-help/managing-your-life-during-treatment/employment-issues/. Accessed 16 Nov 2019. [Google Scholar]
- 12.Islam T, Dahlui M, Majid HA, Nahar AM, Mohd Taib NA, Su TT. Factors associated with return to work of breast cancer survivors: a systematic review. BMC Public Health. 2014;14(3):S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun Y, Shigaki CL, Armer JM. Return to work among breast cancer survivors: a literature review. Support Care Cancer. 2017;25(3):709–18. [DOI] [PubMed] [Google Scholar]
- 14.Tamminga SJ, Braspenning AM, Haste A, Sharp L, Frings-Dresen MHW, de Boer AGEM. Barriers to and facilitators of implementing programs for return to work (RTW) of cancer survivors in four European countries: a qualitative study. J Occup Rehabil. 2019;29(3):550–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silver JK, Baima J, Mayer RS. Impariment-driven cancer rehabilitation: an essential component of quality care and survivorship. CA Cancer J Clin. 2013;63:295–317. [DOI] [PubMed] [Google Scholar]
- 16.Yabroff KR, McNeel TS, Waldron WR. Helath limitations and quality of life associated with cancer and other chronic diseases by phase of care. Med Care. 2007;45:629–37. [DOI] [PubMed] [Google Scholar]
- 17.Balak F, Roelen CAM, Koopmans PC, ten Berge EE, Groothoff JW. Return to work after early-stage breast cancer: a cohort study into the effects of treatment and cancer-related symptoms. J Occup Rehabil. 2008;18(3):267–72. [DOI] [PubMed] [Google Scholar]
- 18.Duijts SFA, van Egmond MP, Spelten E, van Muijen P, Anema JR, van der Beek AJ. Physical and psychosocial problems in cancer survivors beyond return to work: a systematic review. Psycho-Oncology. 2014;23(5):481–92. [DOI] [PubMed] [Google Scholar]
- 19.Eaker S, Wigertz A, Lambert PC, et al. Breast cancer, sickness absence, income and marital status. A study on life situation 1 year prior diagnosis compared to 3 and 5 years after diagnosis. PLoS ONE. 2011;6(3):e18040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fantoni SQ, Peugniez C, Duhamel A, Skrzypczak J, Frimat P, Leroyer A. Factors related to return to work by women with breast cancer in Northern France. J Occupat Rehabil. 2010;20(1):49–58. [DOI] [PubMed] [Google Scholar]
- 21.Hassett MJ, O’Malley AJ, Keating NL. Factors influencing changes in employment among women with newly diagnosed breast cancer. Cancer. 2009;115(12):2775–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Timperi AW, Ergas IJ, Rehkopf DH, Roh JM, Kwan ML, Kushi LH. Employment status and quality of life in recently diagnosed breast cancer survivors. Psycho-Oncology. 2013;22(6):1411–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Muijen P, Duijts SFA, van der Beek AJ, Anema JR. Prognostic factors of work disability in sick-listed cancer survivors. J Cancer Surviv. 2013;7(4):582–91. [DOI] [PubMed] [Google Scholar]
- 24.Jagsi R, Hawley ST, Abrahamse P, et al. Impact of adjuvant chemotherapy on long-term employment of early stage breast cancer survivors. Cancer. 2014. 10.1002/cncr.28607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fuerstein M, Todd BL, Moskowitz MC, et al. Work in cancer survivors: a model for practice and research. J Cancer Surv. 2010;4:414–37. [DOI] [PubMed] [Google Scholar]
- 26.Bouknight RR, Bradley CJ, Luo Z. Correlates of return to work for breast cancer survivors. J Clin Oncol. 2006;24(3):345–53. [DOI] [PubMed] [Google Scholar]
- 27.Ekenga CC, Perez M, Margenthaler JA, Jeffe DB. Early-stage breast cancer and employment participation after 2 years of follow-up: a comparison with age-matched controls. Cancer. 2018;124:2026–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carlsen K, Jensen AJ, Rugulies R, et al. Self-reported work ability in long-term breast cancer survivors. A population-based questionnaire study in Denmark. Acta Oncol. 2013;52(2):423–9. [DOI] [PubMed] [Google Scholar]
- 29.Caron M, Durand M-J, Tremblay D. Perceptions of breast cancer survivors on the supporting practices of their supervisors in the return-to-work process: a qualitative descriptive study. J Occup Rehabil. 2018;28(1):89–96. [DOI] [PubMed] [Google Scholar]
- 30.Blinder V, Patil S, Eberle C, Griggs J, Maly RC. Early predictors of not returning to work in low-income breast cancer survivors: a 5-year longitudinal study. Breast Cancer Res Treat. 2013;140(2):407–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fei C, Deroo LA, Sandler DP, Weinberg CR. Fertility drugs and young-onset breast cancer: results from the two sister study. J Natl Cancer Inst. 2012;104(13):1021–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sandler DP, Hodgson ME, Deming-Halverson SL, et al. The sister study cohort: baseline methods and participant characteristics. Environ Health Perspect. 2017;125(12):127003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anderson C, Islam JY, Elizabeth Hodgson M, et al. Long-term satisfaction and body image after contralateral prophylactic mastectomy. Ann Surg Oncol. 2017;24(6):1499–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buchanan ND, Dasari S, Rodriguez JL, et al. Post-treatment neurocognition and psychosocial care among breast cancer survivors. Am J Prev Med. 2015;49(6 Suppl 5):S498–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bressler L, Mersereau JE, Anderson C, et al. Fertility-related experiences after breast cancer diagnosis in the sister and two sister studies. Cancer. 2019;125(15):2675–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pew Research Center. The State of American jobs: how the shifting economic landscape is reshaping work and society and affecting the way people think about the skills and training they need to get ahead. https://www.pewsocialtrends.org/wp-content/uploads/sites/3/2016/10/ST_2016.10.06_Future-of-Work_FINAL4.pdf. Accessed 16 Oct 2019
- 37.Yoe J Why are older people working longer? Monthly Labor Review Bureau of Labor Statistics. June 2019. [Google Scholar]
- 38.Bradley CJ, Brown KL, Haan M, et al. Cancer survivorship and employment: intersection of oral agents, changing workforce dynamics, and employers’ perspectives. J Natl Cancer Inst. 2018;110:1292–9. [DOI] [PubMed] [Google Scholar]
- 39.Ahles TA, Root JC, Ryan EL. Cancer- and cancer treatment-associated cognitive change: an update on the state of the science. J Clin Oncol. 2012;30(30):3675–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koppelmans V, Breteler MMB, Boogerd W, Seynaeve C, Gundy C, Schagen SB. Neuropsychological performance in survivors of breast cancer more than 20 years after adjuvant chemotherapy. J Clin Oncol. 2012;30(10):1080–6. [DOI] [PubMed] [Google Scholar]
- 41.Hershman DL, Lacchetti C, Loprinzi CL. Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline summary. J Clin Oncol. 2014;10(6):e421–4. [DOI] [PubMed] [Google Scholar]
- 42.Rivera DR, Ganz PA, Weyrich MS, Bandos H, Melnikow J. Chemotherapy-associated peripheral neuropathy in patients with early-stage breast cancer: a systematic review. J Natl Cancer Inst. 2018;110(2):djx140. 10.1093/jnci/djx140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Winters-Stone KM, Horak F, Jacobs PG, et al. Falls, functioning, and disability among women with persistent symptoms of chemotherapy-induced peripheral neuropathy. J Clin Oncol. 2017;35(23):2604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Johnsson A, Fornander T, Rutqvist L-E, Vaez M, Alexanderson K, Olsson M. Predictors of return to work ten months after primary breast cancer surgery. Acta Oncol. 2009;48(1):93–8. [DOI] [PubMed] [Google Scholar]
- 45.DiSipio T, Rye S, Newman B, Hayes S. Incidence of unilateral arm lymphodema after breast cancer: a systematic review and meta-analysis. Lancet Oncol. 2013;14(6):500–15. [DOI] [PubMed] [Google Scholar]
- 46.Gillespie TC, Sayegh HE, Brunelle CL, Daniell KM, Taghian AG. Breast cancer-related lymphedema: risk factors, precautionary measures, and treatments. Gland Surgery. 2018;7(4):379–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Magno S, Filippone A, Forcina L, Maggi L, Ronconi G, Amabile E, Ferrera PE. Physical rehabilitation after breast cancer. Transl Cancer Res. 2018;7:S351–5. [Google Scholar]
- 48.Norman SA, Localio AR, Potashnik SL, et al. Lymphedema in breast cancer survivors: incidence, degree, time course, treatment, and symptoms. J Clin Oncol. 2009;27(3):390–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kent EE, de Moor JS, Zhao J, Ekwueme DU, Han X, Yabroff KR. Staying at one’s job to maintain employer-based health insurance among cancer survivors and their spouses/partners. JAMA Oncol. 2020;6(6):929–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sawyer M, Spencer D. On the definition of involuntary unemployment. J Soc Econom. 2008;37(2):718–35. [Google Scholar]
- 51.Buchanan TW. Retrieval of emotional memories. Psychol Bull. 2007;133(5):761–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.de Boer AGEM, Verbeek JHAM, Spelten ER, et al. Work ability and return-to-work in cancer patients. Br J Cancer. 2008;98(8):1342–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stout NL, Alfano CM, Belter CW, et al. A bibliometric analysis of the landscape of cancer rehabilitation research (1992–2016). J Natl Cancer Inst. 2018;110(8):815–24. [DOI] [PMC free article] [PubMed] [Google Scholar]


