Abstract
Understanding the burden of NAFLD among adolescents and young adults has become increasingly relevant. Our aim was to estimate the prevalence of NAFLD among adolescents and young adults in the United States. Data were obtained from National Health and Nutrition Examination Survey from 2007‐2016. Adolescents and young adults aged 12 to 29 years were included. NAFLD was determined by the U.S. Fatty Liver Index in the absence of secondary causes of liver disease, and the differences in prevalence trends were analyzed based on age, gender, and race. Complete data were available for 4,654 adolescents and young adults (mean age 21 years; 50.9% male; 56.8% White, 20.9% Hispanic, and 13.3% Black). The overall prevalence of NAFLD among adolescents and young adults was 18.5%, ranging from 13.2% among early and middle adolescents (12‐17 years) to 18.7% among late adolescents and young adults (18‐24 years), to 24.0% among older young adults (25‐30 years) (trend P < 0.001). The prevalence of NAFLD was higher for boys than for girls (aged 12‐17: 15.1% vs. 11.3%; aged 18‐24: 21.1% vs. 16.2%; aged 25‐30: 28.7% vs. 19.2%, all P < 0.030). Among all age groups, Hispanics had a higher prevalence of NAFLD than Whites and Blacks (pairwise P < 0.001). Over the study time period, the prevalence of NAFLD among early and middle adolescents and young adults did not change (trend P > 0.80). In contrast, NAFLD prevalence among late adolescents increased (trend P = 0.018). In fact, White and Hispanic late adolescents were the drivers behind this increase in the prevalence of NAFLD. Conclusion: These data indicate an increasing trend in NAFLD prevalence among 18‐24‐year‐olds. These data have important public health and policy implications.
Over the past decade, the prevalence of NAFLD among the youth (12‐29 years old) is increasing primarily among the 18‐24‐year‐old age group. This increase is partly driven by increases among young Hispanic males. We also found the prevalence of complications of obesity such as insulin resistance and hyperlipidemia parallels that of NAFLD.

Abbreviations
- ALT
alanine aminotransferase
- BMI
body mass index
- CI
confidence interval
- CLD
chronic liver disease
- GGT
gamma‐glutamyltransferase
- HOMA‐IR
Homeostatic Model Assessment for Insulin Resistance
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- NCHA
National Center for Health Statistics
- NHANES
National Health and Nutrition Examination Survey
- OR
odds ratio
- PIR
poverty‐income ratio
- T2DM
type 2 diabetes mellitus
- US‐FLI
U.S. Fatty Liver Index
Nonalcoholic fatty liver disease (NAFLD) is defined as hepatic fat accumulation in the absence of other causes of hepatic fat or chronic liver disease (CLD).( 1 ) NAFLD is commonly observed in the obese and those with type 2 diabetes.( 1 )
Although NAFLD is commonly diagnosed in obese persons, it can also be present in nonobese patients who are metabolically unhealthy.( 2 ) In general, patients with NAFLD and components of metabolic syndrome (especially visceral obesity and type 2 diabetes) are not only at higher risk for NAFLD, but are also at increased risk for the progressive form of NAFLD, nonalcoholic steatohepatitis (NASH) and its' associated fibrosis.( 3 ) Additionally, patients with NAFLD and multiple components of metabolic syndrome are at high risk of mortality.( 4 ) In this context, not only is the presence of these clinical risk factors associated with adverse outcomes, but these risk factors drive the stage of liver fibrosis, which is also an independent predictor of mortality.( 5 ) In fact, hepatic fibrosis stage 2 or higher among patients with NAFLD is an independent risk factor for liver‐related mortality and overall mortality.( 6 )
The high burden of NAFLD in the United States has resulted in NAFLD becoming the second most common indication for liver transplantation.( 7 ) In addition, NAFLD carries a high economic and patient‐reported outcome burden due to the ever‐increasing rate of obesity around the world.( 8 ) In this context, the global burden of NAFLD has increased and contributes to higher mortality and disability‐adjusted life years.( 9, 10 ) However, despite this growing body of evidence about the burden of NAFLD, most data have been generated for the adult population. In fact, there is a paucity of data related to NAFLD among children and young adolescent population. This issue is of great importance, as the metabolic diseases that promote NASH (obesity and its complication) are increasing at an alarming rate in the young population. It is estimated that currently approximately 20% of children/adolescence (>6 years old) are considered to be overweight or obese, and 8% are considered morbidly obese.( 11 ) Furthermore, the prevalence of type 2 diabetes (T2DM) in this age group has also risen dramatically from 0.34 per 1,000 (95% confidence interval [CI], 0.31‐0.37) in 2001 to 0.46 per 1,000 (95% CI, 0.43‐0.49) in 2009, which translated into a 30.5% (95% CI, 17.3%‐45.1%) overall increase in the prevalence of T2DM in this age group.( 12 )
Additionally, the few studies in this field have reported a substantial increase in the prevalence of NAFLD, ranging from 7.6% (the general pediatric population) to 38% (in the obese pediatric population).( 13, 14, 15, 16 ) Unfortunately, these studies were not recent and may not reflect the current status of NAFLD among children, adolescences, and young adults.
Given the paucity of these data and the potential future impact of adolescent and young‐adult NAFLD on the burden of liver disease, assessment of the prevalence and risk factors of NAFLD among this age group is important. Therefore, our aim was to identify the prevalence and trend of NAFLD among adolescents and young adults over the past decade (2007‐2016).
Methods
Data Source and Population
We used the public data files for the 2007‐2008, 2009‐2010, 2011‐2012, 2013‐2014, and 2015‐2016 cycles of the National Health and Nutrition Examination Survey (NHANES). The NHANES is a population‐based program of studies conducted by the National Center for Health Statistics. To monitor the health and nutritional status of civilian, noninstitutionalized individuals in the U.S. population, cross‐sectional socio‐demographic, dietary, and medical data were collected through interviews, standardized physical examination, and laboratory testing with oversampling of certain subgroups of the U.S. population (people over the age of 60, Hispanic, African American). Full details of each survey have been described elsewhere.( 17 )
Definition of NAFLD
NAFLD was defined using the improved Fatty Liver Index for the multiethnic U.S. population (US‐FLI), a surrogate for the clinical diagnosis of NAFLD. The US‐FLI is a biochemical model that predicts the presence of fatty liver based on age, race/ethnicity, waist circumference, gamma‐glutamyltransferase (GGT) activity, fasting insulin and fasting glucose, defined as follows:
This model has been previously validated with an area under the receiver operating characteristic curve of 0.80 (95% confidence interval [CI] 0.77‐0.83) for the detection of NAFLD in subjects with values ≥30.( 18 ) In this study, subjects were presumed to have NAFLD, if they had a US‐FLI score of ≥30 in the absence of any other possible causes of CLD and excessive alcohol consumption. As a sensitivity analysis, NAFLD was also defined using a fatty liver index (FLI) of at least 60( 19 ) and elevated alanine aminotransferase (ALT; >30 U/L for males and >19 U/L for females) with body mass index (BMI) ≥ 25 kg/m2.( 20 )
Other Definitions
General demographic characteristics were collected from self‐reported information, including age (years), sex, race/ethnicity, income level (poverty‐income ratio [PIR] < 1.3 as low, PIR 1.3‐3.5 as middle, and PIR > 3.5 as high),( 21 ) college degree, and history of medical conditions (e.g., cardiovascular disease, any cancer, kidney).
Age was grouped as early and middle adolescents aged 12 to 17 years, late adolescents aged 18 to 24 years, and young adults aged 25 to 29 years. Race/ethnicity was categorized as non‐Hispanic White, non‐Hispanic Black, Hispanic, and other, because reliable estimates for non‐Hispanic Asian was not available across all the survey periods. Obesity pattern was categorized into lean (BMI: 18.5‐25 kg/m2), overweight (25‐29.9 kg/m2) and obese (≥30 kg/m2). For adolescents and young adults aged ≤20 years, obesity was defined as a BMI of ≥ the sex‐specific 95th percentile on the U.S. Centers for Disease Control and Prevention (CDC) BMI‐for‐age growth charts.( 22 ) T2DM was defined by a fasting glucose level greater than or equal to 126 mg/dL, self‐reported medical history of diabetes, oral hypoglycemic agents, insulin use, or hemoglobin A1c of ≥6.5%. Hypertension was defined by systolic blood pressure measure greater than or equal to 130 mm Hg or diastolic blood pressure measurements greater than or equal 80 mmHg from an average of three measurements, or history of high blood measurements.( 23 ) Hyperlipidemia was defined by either a serum cholesterol level greater than or equal to 200 mg/dL, low density lipoprotein level greater than or equal to 130 mg/dL, high‐density lipoprotein cholesterol level less than or equal to 40 mg/dL for men and 50 for women, or history of hyperlipidemia. Insulin resistance was defined as a homeostasis model assessment of insulin resistance (HOMA‐IR) > 3.( 24 )
Statistical Analysis
Estimates of age‐specific NAFLD prevalence and 95% CIs were examined by sex, and race/ethnicity using five NHANES (2007‐2008 to 2015‐2016). For combining all the survey periods, appropriate selection of sampling weights and adjustment coefficients was implemented in compliance with the NHANES Analytic and Reporting Guidelines.( 25 ) Differences across groups were tested using Rao‐Scott chi square for categorical variables or Wald test for continuous variables.
Analyses of trends in NAFLD prevalence across gender and race/ethnicity were performed using the 2‐year cycles of NHANES. Unadjusted trends as well as trends adjusted for sex and race/ethnicity were tested using logistic regression models by treating the midpoint of the period as the time point of a continuous variable.
Examination sample weights, accounting for nonresponse, noncoverage, and unequal selection probabilities for certain categories of the population, were incorporated to produce national estimates for all analyses. Sampling errors were estimated by the Taylor series linearization method.( 26 ) Because we used sample weight, weighted sample size for each group determined by multiplying the estimated corresponding percentage by the total number of individuals in the full sample was also reported. All analyses were performed with SAS software, version 9.4 (SAS Institute, Cary, NC) using “SURVEY” procedure, which incorporates the sample design. Statistical tests were considered significant at P < 0.05 (two tails).
Results
Of the 11,707 adolescents and young adults aged 12 to 29 years in five cycles of NHANES (2007‐2016), 7,053 were excluded based on study criteria (Supporting Fig. S1), so the final cohort included 4,654 participants. There was no difference among the demographic characteristics between subjects included and excluded (Supporting Table S1). Clinico‐demographic features of the study population for the five periods are presented in Table 1 and Supporting Table S2.
TABLE 1.
Demographic and Clinical Characteristics of Adolescents and Young Adults Aged 12‐29 Years by Age group: NHANES 2007‐2016
| Characteristics | Early and Middle Adolescents Aged 12‐17 | Late Adolescents Aged 18‐24 | Young Adults Aged 25‐29 | P Value for Trend* | Total Sample Aged 12‐29 |
|---|---|---|---|---|---|
| Age, mean (SEM) | 14.70 (0.05) | 21.20 (0.07) | 27.44 (0.07) | <0.0001 | 20.98 (0.13) |
| Male, % | 49.98 (1.63) | 52.42 (1.48) | 50.15 (1.40) | 0.904 | 50.93 (0.93) |
| Race, % | |||||
| Non‐Hispanic White | 57.93 (2.41) | 55.42 (2.32) | 57.33 (2.55) | 0.8017 | 56.83 (2.01) |
| Non‐Hispanic Black | 14.09 (1.26) | 13.61 (1.29) | 12.01 (1.17) | 0.1305 | 13.28 (1.02) |
| Hispanic | 20.40 (1.84) | 21.57 (1.88) | 20.49 (1.86) | 0.9503 | 20.86 (1.54) |
| Other race | 7.58 (0.94) | 9.40 (1.04) | 10.16 (1.19) | 0.0440 | 9.04 (0.75) |
| Low income, % | 30.04 (1.84) | 39.82 (2.04) | 28.03 (1.92) | 0.4654 | 32.98 (1.43) |
| Current smoker, % | 0 | 21.76 (1.72) | 26.16 (1.72) | 0.0431 | 23.95 (1.38) |
| Obese, % | 20.34 (1.23) | 24.50 (1.56) | 30.31 (1.90) | <0.0001 | 24.92 (0.99) |
| Hypertension, % | 3.83 (0.55) | 11.69 (0.92) | 17.02 (1.27) | <0.0001 | 10.78 (0.55) |
| Hyperlipidemia, % | 32.72 (1.48) | 40.22 (1.74) | 51.73 (2.12) | <0.0001 | 41.28 (1.05) |
| Insulin resistance, % | 40.50 (1.51) | 32.41 (1.72) | 31.53 (1.60) | 0.0001 | 34.79 (1.06) |
| Diabetes, % | 1.05 (0.32) | 2.35 (0.48) | 2.77 (0.59) | 0.0119 | 2.05 (0.28) |
| Other CLD | 0.07 (0.05) | 6.69 (0.82) | 8.91 (1.11) | <0.0001 | 5.52 (0.47) |
| Excess alcohol use, % | 0 | 7.93 (0.97) | 9.42 (1.18) | 0.3727 | 8.63 (0.74) |
| History of cancer, % | 0 | 1.07 (0.40) | 2.66 (0.64) | 0.0438 | 1.92 (0.39) |
| BMI, mean (SEM) | 23.39 (0.16) | 26.43 (0.27) | 27.88 (0.29) | <0.0001 | 25.88 (0.15) |
| Waist, cm, mean (SEM) | 80.58 (0.43) | 89.41 (0.70) | 94.27 (0.73) | <0.0001 | 88.00 (0.40) |
| HOMA‐IR, mean (SEM) | 3.44 (0.09) | 3.23 (0.16) | 3.10 (0.11) | <0.0001 | 3.26 (0.08) |
Data are displayed as weighted percentages (SEM) except where otherwise noted.
Based on logistic regression models by treating age group as a continuous variable.
NAFLD Prevalence Among Adolescents and Young Adults in 2007‐2016
Among the 4,654 adolescents and young adults, the mean age was 21 years; 50.9% were male; 56.8% were White, 20.9% were Hispanic, and 13.3% were Black. Of these, 32.7% were early and middle adolescents, 36.7% were late adolescents, and 30.6% were young adults. The distribution of sex and race/ethnicity were similar across age groups of adolescents and young adults. However, significant differences in all of the metabolic components were observed, with the exception that the HOMA‐IR score was significantly higher in adolescents than in young adults (Table 1). The unweighted as well as weighted sample sizes by sex, age group, and race/ethnicity are displayed in Supporting Table S3.
The weighted prevalence of NAFLD by age group, sex, and race/ethnicity are given in Table 2. The overall prevalence of NAFLD among adolescents and young adults was 18.5%, ranging from 13.2% among early and middle adolescents (12‐17 years) to 18.7% among late adolescents and young adults (18‐24 years), to 24.0% among older young adults (25‐29 years). The prevalence of NAFLD was higher among males than among females (aged 12‐17: 15.1% vs. 11.3%; aged 18‐24: 21.1% vs. 16.2%; aged 25‐30: 28.7% vs. 19.2%; all P < 0.030). This pattern remained the same across race/ethnicity group but not among young Black adults (9.6% among males vs. 17.8% among females; P = 0.022). Among early and middle adolescents, Hispanics (26.9%) had a higher prevalence of NAFLD than Whites (9.8%) and Blacks (7.9%) (pairwise P < 0.001). This was also true for late adolescents aged 18‐24 (32.2% vs. 17.7% and 7.5%; P < 0.001) and young adults aged 25‐29 (38.3% vs. 22.4% and 14.0%; P < 0.001).
TABLE 2.
Prevalence of NAFLD Among Adolescents and Young Adults Aged 12‐29 Years, by Age Group, Sex, and Race/Ethnicity: NHANES 2007‐2016
| Characteristics | Weighted Prevalence, % (95% CI) | |||
|---|---|---|---|---|
| Early and Middle Adolescents Aged 12‐17 | Late Adolescents Aged 18‐24 | Young Adults Aged 25‐29 | Total Sample Aged 12‐29 | |
| Both sexes | 13.16 (11.37‐14.96) | 18.74 (15.88‐21.60) | 23.95 (20.70‐27.20) | 18.51 (16.80‐20.22) |
| Females | 11.27 (8.85‐13.70) | 16.19 (12.80‐19.59) | 19.22 (15.72‐22.73) | 15.49 (13.74‐17.25) |
| Males | 15.05 (12.66‐17.45) | 21.06 (17.26‐24.86) | 28.65 (23.84‐33.46) | 21.42 (18.86‐23.97) |
| White | ||||
| Both sexes | 9.82 (7.35‐12.29) | 17.74 (13.55‐21.94) | 22.43 (17.98‐26.87) | 16.54 (14.28‐18.81) |
| Females | 7.72 (4.81‐10.63) | 16.21 (10.67‐21.74) | 15.58 (10.85‐20.32) | 12.99 (10.54‐15.44) |
| Males | 12.16 (8.33‐15.98) | 19.06 (13.89‐24.23) | 29.17 (22.11‐36.23) | 20.01 (16.43‐23.59) |
| Black | ||||
| Both sexes | 7.87 (5.66‐10.08) | 7.53 (4.95‐10.12) | 14.03 (10.33‐17.74) | 9.45 (7.82‐11.07) |
| Females | 6.56 (3.55‐9.57) | 7.16 (3.76‐10.56) | 17.76 (12.12‐23.40) | 10.05 (7.43‐12.67) |
| Males | 8.97 (6.10‐11.83) | 8.00 (3.73‐12.27) | 9.61 (4.46‐14.77) | 8.80 (6.52‐11.09) |
| Hispanic | ||||
| Both sexes | 26.90 (23.81‐29.99) | 32.23 (27.82‐36.63) | 38.34 (32.64‐44.04) | 32.36 (29.44‐35.27) |
| Females | 25.93 (20.41‐31.44) | 28.74 (22.30‐35.17) | 34.10 (25.12‐43.08) | 29.46 (24.89‐34.03) |
| Males | 27.83 (22.78‐32.87) | 34.89 (28.55‐41.22) | 42.24 (34.05‐50.42) | 34.86 (30.69‐39.04) |
| Other race | ||||
| Both sexes | 11.58 (8.07‐15.08) | 9.94 (5.81‐14.06) | 15.27 (10.06‐20.47) | 12.22 (9.80‐14.64) |
| Females | 9.21 (5.45‐12.97) | 6.64 (3.25‐10.04) | 12.71 (6.66‐18.76) | 9.34 (6.92‐11.75) |
| Males | 13.25 (8.45‐18.05) | 13.96 (5.00‐22.92) | 17.81 (9.85‐25.77) | 15.05 (10.76‐19.34) |
Data are displayed as weighted percentages (CI).
Logistic regression analyses were performed to assess the differences in NAFLD prevalence by age, sex, and race/ethnicity after adjusting for the calendar year (Table 3). In the entire study cohort, compared with early and middle adolescents, late adolescents and young adults were at increased risk of NAFLD (odds ratio [OR] 1.51 [95% CI: 1.18‐1.92] and OR 2.12 [1.68‐2.68]). The odds of NAFLD were higher among Hispanic males (OR 3.61 [2.72‐4.80]), followed by Hispanic females (OR 2.81 [1.99‐3.97]) and White males (OR 1.67 [1.23‐2.27]), whereas it was lower among Black males (OR 0.67 [0.46‐0.97]) compared with White females. This pattern remained the same across the age group. Notably, the disparities in the odds of NAFLD between White females with Hispanic males, Hispanic females, and White males were smaller among late adolescents than among both early and middle adolescents and young adults. Across all age groups, male gender, Hispanic race, lower income, less education, and having more metabolic components were associated with NAFLD (Table 4).
TABLE 3.
Comparisons in NAFLD Prevalence by Age Group, Sex, and Race/Ethnicity: NHANES 2007‐2016
| Characteristics | Total Sample Aged 12‐29 | Early and Middle Adolescents Aged 12‐17 | Late Adolescents Aged 18‐24 | Young Adults Aged 25‐29 | ||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |
| Aged 12‐17 | Reference | |||||||
| Aged 18‐24 | 1.51 (1.18‐1.92) | 0.0013 | ||||||
| Aged 25‐29 | 2.12 (1.68‐2.68) | <0.0001 | ||||||
| Sex and race | ||||||||
| White female | Reference | Reference | Reference | Reference | ||||
| White male | 1.67 (1.23‐2.27) | 0.0013 | 1.65 (0.97‐2.83) | 0.0663 | 1.26 (0.79‐2.01) | 0.3366 | 2.23 (1.38‐3.62) | 0.0087 |
| Black female | 0.74 (0.49‐1.11) | 0.1366 | 0.84 (0.40‐1.78) | 0.6421 | 0.40 (0.20‐0.80) | 0.0106 | 1.17 (0.72‐1.92) | 0.5260 |
| Black male | 0.67 (0.46‐0.97) | 0.0363 | 1.18 (0.63‐2.20) | 0.6058 | 0.47 (0.24‐0.92) | 0.029 | 0.58 (0.29‐1.16) | 0.1202 |
| Hispanic female | 2.81 (1.99‐3.97) | <0.0001 | 4.18 (2.47‐7.08) | <0.0001 | 2.17 (1.25‐3.78) | 0.0069 | 2.81 (1.60‐4.91) | 0.0004 |
| Hispanic male | 3.61 (2.72‐4.80) | <0.0001 | 4.61 (2.81‐7.55) | <0.0001 | 2.80 (1.74‐4.50) | <0.0001 | 3.96 (2.42‐6.50) | <0.0001 |
| Other female | 0.64 (0.33‐1.25) | 0.1848 | 1.21 (0.38‐3.84) | 0.7442 | 0.37 (0.11‐1.26) | 0.1107 | 0.79 (0.33‐1.87) | 0.5880 |
| Other male | 1.16 (0.72‐1.87) | 0.5484 | 1.83 (0.82‐4.07) | 0.1392 | 0.82 (0.32‐2.10) | 0.6797 | 1.17 (0.55‐2.51) | 0.6760 |
Logistic regression models were adjusted for calendar year as a continuous variable.
TABLE 4.
Demographic and Clinical Characteristics of Adolescents and Young Adults Aged 12‐29 Years by the Presence of NAFLD Across Age Groups: NHANES 2007‐2016
| Characteristics | Early and Middle Adolescents Aged 12‐17 | Late Adolescents Aged 18‐24 | Young Adults Aged 25‐29 | Total Sample Aged 12‐29 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NAFLD | No NAFLD* | P | NAFLD | No NAFLD* | P | NAFLD | No NAFLD* | P | NAFLD | No NAFLD* | P | |
| Age, mean (SEM) | 14.74 (0.11) | 14.70 (0.06) | 0.7542 | 21.48 (0.14) | 21.13 (0.07) | 0.0289 | 27.70 (0.09) | 27.36 (0.07) | 0.0051 | 22.37 (0.22) | 20.66 (0.14) | <0.0001 |
| Male, % | 57.16 (3.51) | 48.89 (1.70) | 0.0224 | 58.80 (2.97) | 50.93 (1.77) | 0.0319 | 60.00 (2.82) | 47.05 (1.76) | 0.0004 | 58.89 (1.94) | 49.11 (1.06) | <0.0001 |
| Race, % | ||||||||||||
| Non‐Hispanic White | 43.22 (4.64) | 60.16 (2.35) | <0.0001 | 52.60 (4.79) | 56.11 (2.29) | 0.4399 | 53.69 (3.96) | 58.48 (2.57) | 0.1716 | 50.84 (3.27) | 58.20 (1.95) | 0.0044 |
| Non‐Hispanic Black | 8.42 (1.87) | 14.95 (1.31) | 0.003 | 5.24 (0.92) | 15.48 (1.51) | <0.0001 | 7.04 (1.23) | 13.58 (1.33) | <0.0001 | 6.69 (0.83) | 14.75 (1.13) | <0.0001 |
| Hispanic | 41.70 (4.20) | 17.18 (1.57) | <0.0001 | 37.17 (4.46) | 17.99 (1.58) | <0.0001 | 32.80 (3.96) | 16.61 (1.57) | <0.0001 | 36.49 (3.12) | 17.31 (1.26) | <0.0001 |
| Other Race | 6.66 (1.88) | 7.71 (0.97) | 0.5887 | 5.00 (1.47) | 10.42 (1.15) | 0.0076 | 6.48 (1.97) | 11.33 (1.37) | 0.0654 | 5.97 (1.10) | 9.73 (0.80) | 0.0042 |
| Low income, % | 41.95 (3.63) | 28.24 (1.98) | 0.0003 | 44.94 (3.64) | 38.61 (2.30) | 0.1236 | 32.69 (3.62) | 26.56 (2.17) | 0.1258 | 39.35 (2.27) | 31.52 (1.56) | 0.0011 |
| Current smoker, % | 0 | 0 | 18.79 (3.10) | 22.49 (1.89) | 0.2875 | 19.84 (2.72) | 28.15 (2.04) | 0.0169 | ||||
| Obese, % | 77.21 (3.20) | 11.70 (1.17) | <0.0001 | 72.71 (3.07) | 13.46 (1.27) | <0.0001 | 77.54 (2.78) | 15.42 (1.51) | <0.0001 | 75.68 (1.86) | 13.41 (0.82) | <0.0001 |
| Hypertension, % | 10.46 (2.37) | 2.82 (0.50) | <0.0001 | 19.20 (2.63) | 9.97 (0.97) | 0.0002 | 34.69 (3.20) | 11.45 (1.31) | <0.0001 | 23.34 (1.80) | 7.93 (0.58) | <0.0001 |
| Hyperlipidemia, % | 62.00 (2.70) | 28.28 (1.50) | <0.0001 | 68.48 (2.89) | 33.74 (1.69) | <0.0001 | 79.38 (2.83) | 43.02 (2.61) | <0.0001 | 71.28 (1.75) | 34.48 (1.12) | <0.0001 |
| Insulin resistance, % | 97.34 (1.63) | 31.88 (1.50) | <0.0001 | 96.15 (1.16) | 17.74 (1.31) | <0.0001 | 87.88 (2.52) | 13.78 (1.37) | <0.0001 | 93.15 (1.14) | 21.55 (0.85) | <0.0001 |
| Diabetes, % | 3.83 (1.38) | 0.63 (0.29) | 0.0003 | 6.90 (1.87) | 1.31 (0.35) | <0.0001 | 7.01 (2.03) | 1.44 (0.42) | <0.0001 | 6.23 (0.95) | 1.11 (0.21) | <0.0001 |
| Other CLD | 0 | 0.07 (0.05) | 0 | 8.10 (1.01) | 0 | 11.47 (1.39) | 0 | 6.26 (0.52) | ||||
| Excess alcohol Use, % | 0 | 0 | 0 | 9.73 (1.21) | 0 | 12.09 (1.49) | 0 | 10.81 (0.94) | ||||
| Advanced fibrosis, % (FIB‐4 > 2.67) | 0 | 0 | 0 | 0 | 0.22 (0.22) | 0.09 (0.09) | ||||||
| History of cancer, % | 0 | 0 | 2.04 (1.34) | 0.84 (0.42) | 0.2914 | 1.79 (0.96) | 2.93 (0.75) | 0.3754 | 1.90 (0.69) | 1.93 (0.44) | 0.9692 | |
| BMI, mean (SEM) | 32.01 (0.48) | 22.09 (0.14) | <0.0001 | 34.76 (0.43) | 24.53 (0.18) | <0.0001 | 35.62 (0.42) | 25.45 (0.21) | <0.0001 | 34.46 (0.35) | 23.94 (0.11) | <0.0001 |
| Waist, cm, mean (SEM) | 103.86 (1.29) | 77.05 (0.34) | <0.0001 | 110.84 (0.99) | 84.47 (0.47) | <0.0001 | 114.10 (1.02) | 88.02 (0.53) | <0.0001 | 110.50 (0.82) | 82.89 (0.28) | <0.0001 |
| HOMA, mean (SEM) | 8.58 (0.33) | 2.66 (0.05) | <0.0001 | 7.92 (0.45) | 2.15 (0.06) | <0.0001 | 6.71 (0.29) | 1.96 (0.05) | <0.0001 | 7.60 (0.24) | 2.27 (0.04) | <0.0001 |
Data are displayed as weighted percentages (SEM) except where otherwise noted.
Subject without the presence of NAFLD.
Changes in NAFLD Prevalence Among Adolescents and Young Adults From 2007 to 2016
Trends in the prevalence of NAFLD are displayed in Fig. 1 by age group and sex, and by race, age group and sex in Fig. 2 and Supporting Tables S4 and S5. Over the study period, the prevalence of NAFLD among both early and middle adolescents and young adults (24‐29 years) did not change (trend P > 0.80). In contrast, NAFLD prevalence among late adolescents aged 18‐24 increased from 10.7% (2007‐2008) to 24.8% (2015‐2016) (trend P = 0.018). NAFLD with metabolic components was similar through the period of study across age groups, with the exception of obesity and diabetes among late adolescents aged 18‐24 with NAFLD, which increased over time (P < 0.02) (Table 5).
FIG. 1.
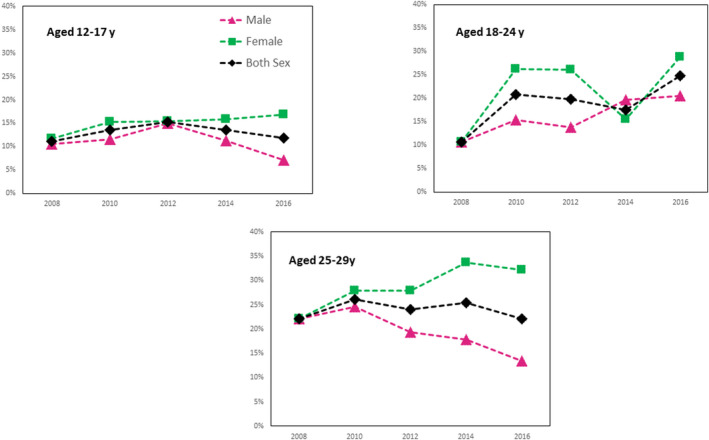
Prevalence of NAFLD among adolescents and young adults aged 12‐29 years in NHANES by age group and Sex.
FIG. 2.
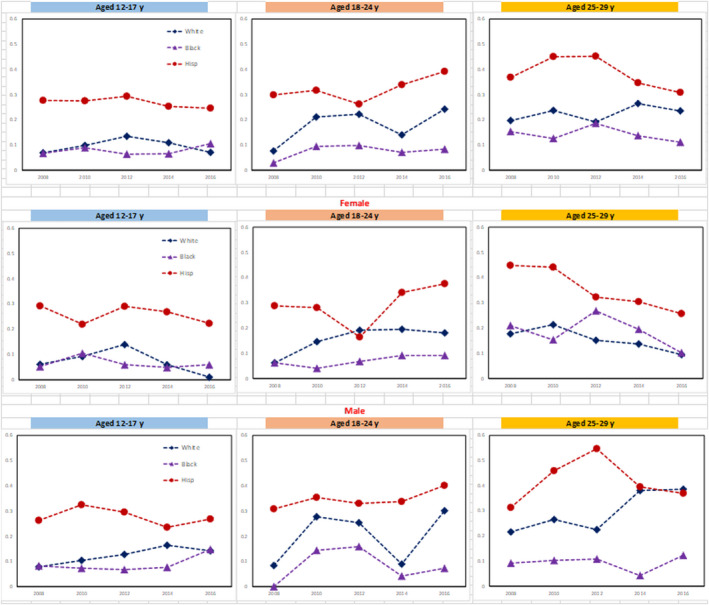
Prevalence of NAFLD among adolescents and young adults aged 12‐29 years in NHANES by age group, sex, and race/ethnicity.
TABLE 5.
Trends in Metabolic Components of Adolescents and Young Adults Aged 12‐29 Years With the presence of NAFLD: NHANES 2007‐2016
| Year | NAFLD | ||
|---|---|---|---|
| Early and Middle Adolescents Aged 12‐17 | Late Adolescents Aged 18‐24 | Young Adults Aged 25‐29 | |
| Obese | |||
| 2007‐2008 | 76.39 (6.72) | 63.40 (10.52) | 78.17 (7.65) |
| 2009‐2010 | 71.84 (8.39) | 63.38 (6.80) | 73.07 (3.89) |
| 2011‐2012 | 81.80 (5.06) | 66.00 (5.21) | 78.14 (3.43) |
| 2013‐2014 | 73.80 (6.37) | 85.14 (4.07) | 86.42 (4.71) |
| 2015‐2016 | 81.77 (6.47) | 80.54 (4.97) | 70.79 (7.19) |
| P value for trend | 0.5322 | 0.0191 | 0.9844 |
| Hypertension | |||
| 2007‐2008 | 5.42 (4.19) | 14.40 (4.24) | 30.07 (5.98) |
| 2009‐2010 | 11.75 (4.03) | 13.21 (5.14) | 48.64 (6.44) |
| 2011‐2012 | 10.76 (5.47) | 27.89 (6.13) | 31.43 (5.48) |
| 2013‐2014 | 6.84 (3.89) | 22.55 (3.57) | 36.81 (5.92) |
| 2015‐2016 | 16.79 (7.15) | 16.58 (3.85) | 25.16 (4.28) |
| P value for trend | 0.3585 | 0.523 | 0.2159 |
| Hyperlipidemia | |||
| 2007‐2008 | 54.08 (6.32) | 68.35 (7.56) | 83.89 (5.88) |
| 2009‐2010 | 54.72 (3.24) | 69.07 (6.01) | 76.06 (6.33) |
| 2011‐2012 | 75.21 (4.76) | 73.54 (4.84) | 83.55 (4.64) |
| 2013‐2014 | 63.37 (7.99) | 74.39 (4.49) | 86.56 (4.62) |
| 2015‐2016 | 57.59 (6.23) | 58.92 (6.13) | 65.78 (5.31) |
| P value for trend | 0.4335 | 0.3485 | 0.1938 |
| Insulin resistance | |||
| 2007‐2008 | 100.00 (0.00) | 86.79 (6.23) | 85.21 (5.55) |
| 2009‐2010 | 100.00 (0.00) | 96.90 (1.91) | 91.76 (4.44) |
| 2011‐2012 | 92.17 (5.61) | 100.00 (0.00) | 86.90 (3.24) |
| 2013‐2014 | 97.48 (1.89) | 96.88 (0.57) | 87.64 (4.36) |
| 2015‐2016 | 98.98 (1.12) | 95.59 (0.42) | 87.56 (2.64) |
| P value for trend | 0.2601 | 0.2413 | 0.9939 |
| Diabetes | |||
| 2007‐2008 | 2.89 (2.88) | 3.30 (3.38) | 9.55 (4.14) |
| 2009‐2010 | 1.57 (1.52) | 2.35 (1.84) | 9.15 (5.09) |
| 2011‐2012 | 2.02 (1.67) | 4.96 (1.46) | 4.85 (1.41) |
| 2013‐2014 | 4.62 (1.98) | 7.66 (4.07) | 2.41 (2.58) |
| 2015‐2016 | 9.16 (6.20) | 13.56 (2.77) | 9.86 (6.45) |
| P value for trend | 0.2110 | 0.0032 | 0.6993 |
Data are displayed as weighted percentages (SEM).
Logistic regression models by treating the midpoint of the period as the time point as a continuous variable after adjustments for sex and race/ethnicity.
Among late adolescent males aged 18‐24, there was a significant increasing trend in the prevalence of NAFLD from 10.7% to 28.7% (trend P = 0.037), whereas a significant decreasing trend from 22.1% to 13.4% (trend P = 0.031) was observed among female young adults aged 25‐29. Interestingly, the prevalence of other liver diseases including excessive alcohol and viral hepatitis decreased from 9.1% to 4.6% (trend P = 0.004) among adolescents and young adults (data not shown).
Discussion
As our understanding of the burden of NAFLD among adults expands, better appreciation of the burden of this important liver disease among adolescents and young adults has become increasingly important. In this study, we used national data from the United States to provide prevalence estimates of NAFLD among adolescents and young adults. Our data show that the overall prevalence of NAFLD among this cohort of patients was 18.5%, ranging from 13.2% among those aged 12‐17 years to 18.7% among those 18‐24 years, and 24.0% among those aged 25‐29 years. These data confirm that there is a high prevalence of NAFLD among adolescence as well as young adults, and suggests that the NAFLD prevalence increases with age. Additionally, our analysis showed that young Hispanic males were more likely to have NAFLD, followed Hispanic females and White males, whereas young Black Americans had the lowest prevalence rates. The higher prevalence of NAFLD among young Hispanics is similar to the rates reported for adults.( 27 )
Interestingly, in our overall study cohort, only approximately 25% were considered to be obese; however, among those with NAFLD, over 75% were considered obese. In contrast, 13% of obese subjects did not have NAFLD. This finding suggests that among this age group, obesity is a significant risk factor for NAFLD and should be considered in determining whether a further work‐up for NAFLD is warranted. In addition, when subjects with NAFLD were compared to those without NAFLD, the prevalence of other NAFLD risk factors (insulin resistance, hypertension, hyperlipidemia, and diabetes) were significantly higher among those with NAFLD. In fact, over 93% of those with NAFLD had insulin resistance and 71% had hyperlipidemia, compared with 21% and 34% (respectively) among the non‐NAFLD group.
In addition, while the mean HOMA‐IR was considered moderately high across all age groups, the youngest group (12‐17 years old) had the highest mean HOMA‐IR at 3.44, with over 40% in this group being diagnosed with insulin resistance, which decreased to 32% for those aged 18‐29. On the other hand, the prevalence of diabetes among our overall cohort increased dramatically, from 1% (12 to 17‐year‐old group) to 2.3% (18‐24 years old). This represents a 164% increase. On the other hand, there was only a moderate increase of 2.7% from the group of 18‐24‐year‐olds versus the 25‐29‐year‐olds.
In addition to obesity, we assessed the prevalence of T2DM in this cohort. Among those with NAFLD, the overall prevalence rate of T2DM was 6.23%, which was significantly higher than the group of subjects without NAFLD (1.11%). It is important to emphasize that the reported prevalence of T2DM among this NAFLD group is higher than what is currently reported by the CDC, which reports a prevalence rate of 4.2%.( 28, 29 ) Consistent with reports from the general population, we also found that the prevalence rates for both those with and without NAFLD increased significantly over time.( 28 )
Another notable trend we found was that while the prevalence of NAFLD among both early and middle adolescents (12‐17 years old) and young adults (25‐29 years) remained the same over the course of the study, the NAFLD prevalence among those aged 18‐24 increased from 10.7% (2007‐2008) to 24.8% (2015‐2016), a significant increase of +132%. The apparent drivers behind this significant increase were obesity and diabetes, which significantly increased +27% and +300%, respectively, over time, especially among White and Hispanic late adolescents. These changes occurred in the backdrop of significant decreases in CLD from either viral hepatitis or excessive alcohol use. Together these findings present a disturbing picture of what the future may hold for our adolescents and young adults, especially for Hispanic males and females if the present trajectory for obesity, diabetes, and NAFLD continues.( 30 )
In addition to obesity, the high prevalence of obesity‐related complications such as hyperlipidemia and insulin resistance among adolescents and young adults with NAFLD are also worrisome. Many of these findings may be attributed to the changing food environment, where the availability of healthy food choices is limited while foods high in saturated fat and fructose are readily available—a scenario that is especially prevalent among the low‐income group in which 40% of the 18‐24‐year‐olds comprise.( 31, 32, 33, 34, 35, 36 ) As such, efforts to change the environment to provide healthier food choices must start with healthy eating education by both caregivers and schools, while at the same time city planners develop ways to encourage food stores to offer healthier and affordable food choices as well as providing these healthy choices within easy walking distances to those most affected.( 37, 38, 39, 40, 41 )
In addition, schools must also work toward providing healthy school breakfasts and lunches to all children, but especially those from low‐income families, as we know that these children and adolescents get over half their daily caloric from the food provided by schools, in which 22% of their calories come from school breakfast and close to 31% of their calories come from school lunches.( 42 ) We also know that those who eat school‐provided food have a healthier dietary intake.( 43 ) Finally, providing a healthy environment that promotes physical activity both for schools and the general community is vital to reversing these trends.( 44 ) However, it must be noted that although the current coronavirus disease‐2019 pandemic has altered the landscape of obtaining food, especially for school aged children, schools and other public agencies have developed mechanisms to provide food for those most in need. Although this could be a viable long‐term strategy, these agencies must come up with alternative ways to providing healthy, nutritious food to those they serve. In this context, the U.S. government has established a program for healthy eating called “My Plate” (https://www.myplate.gov/), which can be used to guide the food choices provided as well as to educate the public about healthy eating.
There are a few limitations to this study. First, we have used US‐FLI as a noninvasive diagnostic method to establish NAFLD. Although a radiologic‐based or histologic‐based diagnosis of NAFLD may be more accurate, ultrasound data are only available for the early cycle of NHANES (1988‐1994). It is also important to note that US‐FLI has been established as a reliable method for noninvasive diagnosis of NAFLD in the U.S. population.( 45 ) In this study, we went further and validated US‐FLI in our study population using the subgroup that had both liver ultrasound and US‐FLI. In fact, of 11,532 NHANES III subjects (aged 20‐74 years), the prevalence of radiologic NAFLD and NAFLD based on US‐FLI were 19.4% (17.7%‐21.3%) and 23.6% (21.7%‐25.5%), respectively. Late adolescents and young adults (18‐24 years) and older young adults (25‐29 years) accounted for 5.25% and 9.47% of radiologic NAFLD, in comparison with 4.31% and 5.61% of NAFLD based on US‐FLI. Similar trend patterns in NAFLD prevalence by using other noninvasive markers (FLI and elevated ALT) were observed. In addition, among adolescents, there was significant agreement between US‐FLI with FLI (kappa statistic = 0.696 [95% CI: 0.617‐0.775) and elevated ALT (kappa = 0.607 [0.523‐0.691]), respectively.
Despite these study limitations, NHANES provides a nationally representative sample of the U.S. population, whose measures are standardized over time, reducing the potential for recording errors. However, true prevalence of NAFLD in this population is hindered by the lack of noninvasive testing, and most of the diagnoses have been made using liver enzyme elevations and or ultrasound—both of which have significant shortcomings, especially liver enzymes, which have been shown not to be elevated in many patients with NAFLD as well as the inaccuracy of ultrasound in those younger than 18 years old.( 45, 46 ) In addition, we were not able to identify advanced fibrosis among this cohort due to the lack of noninvasive tests that are validated for the younger population, to include the Fibrosis‐4 index, which is not accurate for those younger than 35 years of age. Further research is urgently needed to determine appropriate noninvasive tests for the presence of fibrosis in this age group, given the increasingly burden of NAFLD for this younger population.( 47, 48 )
In summary, our data show that the overall prevalence of NAFLD among children, adolescents, and young adults (12‐29 years old) is 18.5% with a range from 13% for 12‐17‐year‐olds to 24% for those 25‐29 years old. Over the past decade, the prevalence of NAFLD also is increasing, primarily among the 18‐24‐year‐old age group, and this increase is partly driven by increases among young Hispanic males. We also found that the prevalence of complications of obesity, such as insulin resistance and hyperlipidemia, parallels that of NAFLD. In this context, we believe environmental factors, especially food choices, are the major drivers of obesity and NAFLD. Therefore, we suggest that work must continue to improve the immediate food environment, both in schools as well as one’s living vicinity, so that healthy and affordable food choices and increased physical activity options are available—especially for those with a low income. In addition, education must also be provided to better inform all stakeholders about complications of obesity, including NAFLD. Finally, we have provided provisional validated NAFLD prevalence using the US‐FLI in this study population; however, we recommend further research to validate our findings.
Supporting information
Fig S1
Table S1‐S6
Supported by the Beatty Liver and Obesity Research Funds, Inova Medicine Service Line, Inova Health System (Falls Church, Virginia).
Potential conflict of interest: Dr. Younossi is a consultant to BMS, Gilead, Intercept, NovoNordisk, Novartis, Terns, Merck, Viking, Madrigal, Quest, and Siemens.
References
- 1.Younossi ZM, Henry L. The impact of obesity and type 2 diabetes on chronic liver disease. Am J Gastroenterol 2019;114:1714‐1715. [DOI] [PubMed] [Google Scholar]
- 2.Golabi P, Paik JM, Arshad T, Younossi Y, Mishra A, Younossi ZM. Mortality of NAFLD according to the body composition and presence of metabolic abnormalities. Hepatol Commun 2020;4:1136‐1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hossain N, Afendy A, Stepanova M, Nader F, Srishord M, Rafiq N, et al. Independent predictors of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2009;7:1224‐1229.e1‐e2. [DOI] [PubMed] [Google Scholar]
- 4.Golabi P, Otgonsuren M, de Avila L , Sayiner M, Rafiq N, Younossi ZM. Components of metabolic syndrome increase the risk of mortality in nonalcoholic fatty liver disease (NAFLD). Medicine (Baltimore) 2018;97:e0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Younossi ZM, Stepanova M, Rafiq N, Henry L, Loomba R, Makhlouf H, et al. Nonalcoholic steatofibrosis independently predicts mortality in nonalcoholic fatty liver disease. Hepatol Commun 2017;1:421‐428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dulai PS, Singh S, Patel J, Soni M, Prokop LJ, Younossi Z, et al. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: systematic review and meta‐analysis. Hepatology 2017;65:1557‐1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Younossi ZM, Stepanova M, Ong J, Trimble G, AlQahtani S, Younossi I, et al. Nonalcoholic steatohepatitis is the most rapidly increasing indication for liver transplantation in the United States. Clin Gastroenterol Hepatol 2020;19:580‐589. [DOI] [PubMed] [Google Scholar]
- 8.Younossi ZM, Henry L, Bush H, Mishra A. Clinical and economic burden of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Clin Liver Dis 2018;22:1‐10. [DOI] [PubMed] [Google Scholar]
- 9.Paik JM, Henry L, De Avila L, Younossi E, Racila A, Younossi ZM. Mortality related to nonalcoholic fatty liver disease is increasing in the United States. Hepatol Commun 2019;3:1459‐1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paik JM, Golabi P, Younossi Y, Mishra A, Younossi ZM. Changes in the global burden of chronic liver diseases from 2012 to 2017: the growing impact of NAFLD. Hepatology 2020;72:1605‐1616. [DOI] [PubMed] [Google Scholar]
- 11.Schwimmer JB, Deutsch R, Kahen T, Lavine JE, Stanley C, Behling C. Prevalence of fatty liver in children and adolescents. Pediatrics 2006;118:1388‐1393. [DOI] [PubMed] [Google Scholar]
- 12.Loomba R, Sirlin CB, Schwimmer JB, Lavine JE. Advances in pediatric nonalcoholic fatty liver disease. Hepatology 2009;50:1282‐1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ogden CL, Fryar CD, Martin CB, Freedman DS, Carroll MD, Gu Q, et al. Trends in obesity prevalence by race and Hispanic origin—1999‐2000 to 2017‐2018. JAMA 2020;324:1208‐1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dabelea D, Mayer‐Davis EJ, Saydah S, Imperatore G, Linder B, Divers J, et al.; SEARCH for Diabetes in Youth Study . Prevalence of type 1 and type 2 diabetes among children and adolescents from 2001 to 2009. JAMA 2014;311:1778‐1786.24794371 [Google Scholar]
- 15.Schwimmer JB, McGreal N, Deutsch R, Finegold MJ, Lavine JE. Influence of gender, race, and ethnicity on suspected fatty liver in obese adolescents. Pediatrics 2005;115:e561‐e565. [DOI] [PubMed] [Google Scholar]
- 16.Wanless IR, Lentz JS. Fatty liver hepatitis (steatohepatitis) and obesity: an autopsy study with analysis of risk factors. Hepatology 1990;12:1106‐1110. [DOI] [PubMed] [Google Scholar]
- 17.Johnson CL, Dohrmann SM, Burt VL, Mohadjer LK. National health and nutrition examination survey: sample design, 2011‐2014. Vital Health Stat 2014;2:1‐33. [PubMed] [Google Scholar]
- 18.Ruhl CE, Everhart JE. Fatty liver indices in the multiethnic United States National Health and Nutrition Examination Survey. Aliment Pharmacol Ther 2015;41:65‐76. [DOI] [PubMed] [Google Scholar]
- 19.Bedogni G, Bellentani S, Miglioli L, Masutti F, Passalacqua M, Castiglione A, et al. The Fatty Liver Index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol 2006;2:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mrad RA, Merjaneh N, Mubarak G, Lopez R, Zein NN, Alkhouri N. The increasing burden of nonalcoholic fatty liver disease among young adults in the United States: a growing epidemic. Hepatology 2016;64:1386‐1387. [DOI] [PubMed] [Google Scholar]
- 21.Center for Disease Control and Prevention . National Center for Health Statistics (NCHS). Analytic and Reporting Guidelines: The Third National Health and Nutrition Examination Survey, NHANES III (1988‐94). Center for Disease Control and Prevention. https://www.cdc.gov/nchs/nhanes/index.htm?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fnchs%2Fnhanes.htm. Accessed December 18, 2020.
- 22.Kuczmarski RJ, Ogden CL, Grummer‐Strawn LM, Flegal KM, Guo SS, Wei R, et al. CDC growth charts: United States. Advance data from vital and health statistics. Vol. 314. Hyattsville, MD: National Center for Health Statistics; 2000. [PubMed] [Google Scholar]
- 23.Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Dennison Himmelfarb C, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults. J Am Coll Cardiol 2018;71:e127‐e248. [DOI] [PubMed] [Google Scholar]
- 24.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care 2004;27:1487‐1495. [DOI] [PubMed] [Google Scholar]
- 25.Johnson CL, Paulose‐Ram R, Ogden CL, Carroll MD, Kruszon‐Moran D, Dohrmann SM, et al. National health and nutrition examination survey: analytic guidelines, 1999‐2010. Vital Health Stat 2013;2:1‐24. [PubMed] [Google Scholar]
- 26.Wolter KM. Introduction to variance estimation. In: Taylor Series Methods. 2nd Edition. New York, NY: Springer; 2007. [Google Scholar]
- 27.Saab S, Manne V, Nieto J, Schwimmer JB, Chalasani NP. Nonalcoholic fatty liver disease in Latinos. Clin Gastroenterol Hepatol 2016;14:5‐12; quiz e9‐e10. [DOI] [PubMed] [Google Scholar]
- 28.CDC . National Diabetes Report 2020. https://www.cdc.gov/diabetes/pdfs/data/statistics/national‐diabetes‐statistics‐report.pdf. Accessed April 1, 2021.
- 29.Divers J, Mayer‐Davis EJ, Lawrence JM, Isom S, Dabelea D, Dolan L, et al. Trends in Incidence of type 1 and type 2 diabetes among youths ‐ selected counties and Indian Reservations, United States, 2002‐2015. MMWR Morb Mortal Wkly Rep 2020;69:161‐165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol 2018;15:11‐20. [DOI] [PubMed] [Google Scholar]
- 31.Schwimmer JB, Ugalde‐Nicalo P, Welsh JA, Angeles JE, Cordero M, Harlow KE, et al. Effect of a low free sugar diet vs usual diet on nonalcoholic fatty liver disease in adolescent boys: a randomized clinical trial. JAMA 2019;321:256‐265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leslie T, Pawloski L, Kallman‐Price J, Escheik C, Hossain N, Fang Y, et al. Survey of health status, nutrition and geography of food selection of chronic liver disease patients. Ann Hepatol 2014;13:533‐540. [PubMed] [Google Scholar]
- 33.Taillie LS, Grummon AH, Miles DR. Nutritional profile of purchases by store type: disparities by income and food program participation. Am J Prev Med 2018;55:167‐177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taillie LS, Ng SW, Popkin BM. Global growth of “big box” stores and the potential impact on human health and nutrition. Nutr Rev 2016;74:83‐97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Larson NI, Story MT, Nelson MC. Neighborhood environments: disparities in access to healthy foods in the US. Am J Prev Med 2009;36:74‐81. [DOI] [PubMed] [Google Scholar]
- 36.Boone‐Heinonen J, Gordon‐Larsen P, Kiefe CI, Shikany JM, Lewis CE, Popkin BM. Fast food restaurants and food stores: longitudinal associations with diet in young to middle‐aged adults: the CARDIA study. Arch Intern Med 2011;171:1162‐1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scaglioni S, De Cosmi V, Ciappolino V, Parazzini F, Brambilla P, Agostoni C. Factors influencing children’s eating behaviours. Nutrients 2018;10:706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mackenbach JD, Nelissen KGM, Dijkstra SC, Poelman MP, Daams JG, Leijssen JB, et al. A systematic review on socioeconomic differences in the association between the food environment and dietary behaviors. Nutrients 2019;11:2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zarnowiecki DM, Dollman J, Parletta N. Associations between predictors of children’s dietary intake and socioeconomic position: a systematic review of the literature. Obes Rev 2014;15:375‐391. [DOI] [PubMed] [Google Scholar]
- 40.Zarnowiecki DM, Parletta N, Dollman J. Socio‐economic position as a moderator of 9–13‐year‐old children’s non‐core food intake. Public Health Nutr 2016;19:55‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Micha R, Karageorgou D, Bakogianni I, Trichia E, Whitsel LP, Story M, et al. Effectiveness of school food environment policies on children's dietary behaviors: a systematic review and meta‐analysis. PLoS One 2018;13:e0194555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gordon AR, Cohen R, Crepinsek MK, Fox MK, Hall J, Zeidman E. The third School Nutrition Dietary Assessment Study: background and study design. J Am Diet Assoc 2009;109(Suppl 2):S20‐S30. [DOI] [PubMed] [Google Scholar]
- 43.Au LE, Gurzo K, Gosliner W, Webb KL, Crawford PB, Ritchie LD. Eating school meals daily is associated with healthier dietary intakes: the Healthy Communities Study. J Acad Nutr Diet 2018;118:1474‐1481.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Healthy People 2030 . Reduce the proportion of children and adolescents with obesity—NWS‐04. https://health.gov/healthypeople/objectives‐and‐data/browse‐objectives/overweight‐and‐obesity/reduce‐proportion‐children‐and‐adolescents‐obesity‐nws‐04. Accessed June 12, 2021.
- 45.Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018;67:328‐357. [DOI] [PubMed] [Google Scholar]
- 46.Awai HI, Newton KP, Sirlin CB, Behling C, Schwimmer JB. Evidence and recommendations for imaging liver fat in children, based on systematic review. Clin Gastroenterol Hepatol 2014;12:765‐773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McPherson S, Hardy T, Dufour J‐F, Petta S, Romero‐Gomez M, Allison M, et al. Age as a confounding factor for the accurate non‐invasive diagnosis of advanced NAFLD fibrosis. Am J Gastroenterol 2017;112:740‐751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vos MB, Abrams SH, Barlow SE, Caprio S, Daniels SR, Kohli R, et al. NASPGHAN Clinical Practice Guideline for the diagnosis and treatment of nonalcoholic fatty liver disease in children: recommendations from the Expert Committee on NAFLD (ECON) and the North American Society of Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN). J Pediatr Gastroenterol Nutr 2017;64:319‐334. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Table S1‐S6


