Abstract
We have characterized the Drosophila bancal gene, which encodes a Drosophila homologue of the vertebrate hnRNP K protein. The bancal gene is essential for the correct size of adult appendages. Reduction of appendage size in bancal mutant flies appears to be due mainly to a reduction in the number of cell divisions in the imaginal discs. Transgenes expressing Drosophila or human hnRNP K are able to rescue weak bancal phenotype, showing the functional similarity of these proteins in vivo. High levels of either human or Drosophila hnRNP K protein in imaginal discs induces programmed cell death. Expression of the antiapoptotic P35 protein suppresses this phenotype in the eye, suggesting that apoptosis is the major cellular defect caused by overexpression of K protein. Finally, the human K protein acts as a negative regulator of bancal gene expression. We propose that negative autoregulation limits the level of Bancal protein produced in vivo.
Heterogeneous nuclear RNAs (hnRNAs), from which mRNAs are derived by RNA processing, are associated with specific nuclear proteins and form hnRNP complexes in eukaryotes (reviewed in reference 16). Monoclonal antibodies to several different hnRNP proteins immunoprecipitate a set of about 20 similar proteins called hnRNP A to U (58). Extensive biochemical studies have suggested a role for vertebrate hnRNP proteins in splicing and nucleocytoplasmic transport of mRNA (11, 47, 59, 60, 62, 71).
More than 10 homologues of vertebrate hnRNP proteins have been characterized in Drosophila melanogaster, the most abundant being hrp36, hrp40, and hrp48 (45). All hnRNPs described in Drosophila share sequence homology with human hnRNP A and B, containing two N-terminal RNP consensus (RNP-CS) RNA-binding domains and a glycine-rich carboxy-terminal domain (43). Drosophila hnRNP proteins have been shown to be associated with the majority of nascent transcripts (44, 45). In vivo overexpression studies have established a role for Drosophila hnRNP proteins in RNA processing. For instance, overexpression of the hrp36 or hrp38 protein affects the splice site selection of the dopa decarboxylase pre-mRNA (69, 88). Hammond et al. (24) have determined that the hrp48 gene is required in vivo for splicing of the third intron in the P transposable element. These authors have shown that hypomorphic mutations in the hrp48 gene cause larval lethality and developmental defects including reduced numbers of ommatidia in the eye and bristle abnormalities. The squid gene (encoding hrp40) is required for dorsoventral axis formation during oogenesis (33, 65). Several additional Drosophila RNA-binding proteins with RNP-CS motifs are required for diverse processes such as sex determination, vision, behavior, and spermatogenesis (2, 4, 5, 23, 32).
Human hnRNP K is the major poly(rC)-binding protein (46), and several features set it apart from other hnRNP proteins. Unlike most other hnRNP proteins, nucleic acid binding by hnRNP K is not mediated by an RNP-CS RNA-binding domain (reviewed in reference 16); instead, it uses three repeat motifs termed KH (K homology) domains (72, 74). Direct competition studies reveal that hnRNP K preferentially binds single-stranded DNA over single-stranded RNA in vitro (81), and several groups have identified hnRNP K as a sequence-specific DNA-binding protein, suggesting that it might be involved in transcription (21, 29, 56, 81).
Here we report the expression pattern and biochemical properties of the D. melanogaster homologue of vertebrate hnRNP K protein. Null and weak alleles of this gene called bancal (bl) result in adults with shortened appendages. This phenotype appears to result from a reduction in the number of cell divisions in the imaginal discs. Elevated hnRNP K activity induces cell death in imaginal discs, and expression of the antiapoptotic P35 protein suppresses this phenotype in the eye. We also present the first in vivo evidence for a role of the human hnRNP K protein in gene expression control. These observations indicate that a negative feedback regulation mechanism of the bl gene controls the amount of Bancal (Bl) protein produced.
MATERIALS AND METHODS
Fly strains and genetics.
Oregon R was used as a wild-type standard. The enhancer trap line P(lac, w+)176 isolated in our laboratory is located cytologically at position 57A on chromosome 2R and expresses β-galactosidase in the anterior region of the embryo at the blastoderm stage. The P(lac, w+) element is described in reference 6. Precise and imprecise excisions of this P element were isolated by the jump start technique (64). For the identification of bl mutant third-instar larvae, blV6 and blV4 were introduced into a yellow− genetic background and balanced with a chromosome carrying an yellow+ insertion. Mutants are identifiable by their yellow− phenotype.
Cloning of the bl gene.
Cloning of the bl gene was achieved by plasmid rescue (61) using DNA isolated from the P(lac, w+)176 insertion. Phages carrying overlapping genomic DNA fragments were isolated by hybridization screening of an EMBL3 genomic library (Clontech) and chromosomal walking. Genomic DNA fragments hybridizing to poly(A)+ RNA on Northern blots were used to isolate clones from a Zimm embryonic cDNA library. For Northern blot experiments, poly(A)+ mRNAs were isolated from various developmental stages by using a poly(A) tract isolation kit (Promega). Sequencing of the cDNAs and the genomic fragments was done by Genome Express (Grenoble, France), using synthetic primers. To analyze the extent of the deletions generated after mobilization of the P element, Southern blot analysis was performed with the genomic DNA extracted from the different mutant and parental strains. The genomic DNA was digested by EcoRI, run on an agarose gel, transferred to a nitrocellulose membrane, and probed with either the P(lac, w+) DNA or different fragments of the genomic DNA surrounding the insertion site.
Analysis of Bl protein.
Anti-Bl antibodies were made in rats by injecting a histidine-tagged Bl fusion protein produced in bacteria; the bl sequence used encodes for the C-terminal 433 amino acids of the Bl protein. Proteins from various developmental stages were extracted by homogenizing the tissue in the sample buffer (5 parts S buffer:1 part D buffer; S-buffer is 0.2 M Tris [pH 6.8], 5 mM EDTA, 0.01% BBP, and 22% sucrose; D buffer is 18% sodium dodecyl sulfate [SDS] and 0.5 M dithiothreitol) in the presence of protease inhibitors (aprotinin, leupeptin, pepstatin A, antipain, and phenylmethylsulfonyl fluoride; 1 mg/ml, final concentration). The proteins were then separated on SDS-polyacrylamide (10%) gels, transferred to nitrocellulose, and probed as described previously (25). In situ immunodetection of the Bl protein was performed with the anti-Bl antiserum at 1/500 dilution.
RNA binding assays.
Binding of in vitro-produced proteins to ribohomopolymers was carried out essentially as described previously (77), with minor modifications. Briefly, ribonucleotide homopolymer (Pharmacia) binding reactions were carried out with 100,000 cpm of trichloroacetic acid-precipitable protein in a total of 0.5 ml of binding buffer (10 mM Tris-HCl [pH 7.4], 2.5 mM MgCl2, 0.5% Triton X-100, 200 mM NaCl) for 10 min on a rocking platform at 4°C. The beads were pelleted with a brief spin in a microcentrifuge and washed five times with binding buffer prior to resuspension in 50 μl of SDS-polyacrylamide gel electrophoresis (PAGE) sample buffer. Bound proteins were eluted from the nucleic acid by boiling, resolved on an SDS-polyacrylamide (12.5%) gel, and visualized by fluorography.
Transgenic lines and overexpression of the K proteins.
A 2.0-kb EcoRI fragment corresponding to the full-length bl cDNA and a 2.3-kb EcoRI fragment encoding the human hnRNP K protein (HK5 cDNA; a gift from G. Dreyfuss [45]) were inserted in the EcoRI site of the pCaSpeR-hs vector (80) and in the EcoRI site of the pUAST vector (7). The full-length bl cDNA was cloned in the EcoRI polylinker site of pGMR (26). hs-bl, hs-HK5, pUAST-bl, pUAST-HK5, and pGMR-bl transgenic flies were obtained by P-element-mediated transformation (66). The Gal4 system was used to overexpress bl and the human K gene (7), using the ptcGAL4 driver (7). Adult wings were dissected in 100% ethanol, rinsed twice in xylene, mounted in DPX, and observed under a Zeiss AxioPhot microscope using Nomarski optics. Samples for scanning electron microscopy (SEM) were prepared as described in reference 34.
BrdU labeling, apoptosis assays, and cell size analysis.
Imaginal discs from third-instar larvae were dissected and placed in Schneider’s medium containing 5-bromo-2′-deoxyuridine (BrdU; 0.2 mg/ml; Sigma) for 30 min at room temperature. Tissues were then fixed and stained as described previously (3). The anti-BrdU antibody was from Dako. Apoptotic cells were detected as described previously (76), using acridine orange (Sigma) at 1 μg/ml. Cell size was determined using an Arm antibody (described below) to stain the periphery of cells.
In situ hybridization and immunostaining on discs.
Discs were dissected from both control and transgenic third-instar larvae in Ringer’s solution, fixed, and immunostained with anti-Bl antibody (see above) (30). The human hnRNP K primary antibody was kindly provided by G. Dreyfuss. The Teashirt (Tsh) antibody is described in reference 22. A rat polyclonal antibody raised against glutathione S-transferase–Modulo (Mod) was used (36). Arm N2 7A1 monoclonal antibody (Developmental Studies Hybridoma Bank) (57) recognizes an epitope in the amino-terminal part of the Arm protein. Secondary antibodies, either fluorescein isothiocyanate or tetramethylrhodamine isothiocyanate labeled, were from Jackson ImmunoResearch Laboratories (West Grove, Pa.). Confocal microscopy was performed on a Zeiss confocal microscope, and data were processed with Adobe Photoshop.
In situ hybridization was performed with digoxigenin-labeled probes (Boehringer). We used a full-length bl cDNA to detect bl RNA and a full-length mod cDNA to detect mod RNA. After phosphatase alkaline detection, the discs were mounted in 70% glycerol–100 mM Tris-HCl (pH 7.5) and observed under a Zeiss AxioPhot microscope using Nomarski optics.
Sequence accession numbers.
The protein identification numbers for the human, Xenopus, and Caenorhabditis elegans hnRNP K clones are 585911, 542643, and 1707028, respectively. The bl GenBank accession number is AF142631.
RESULTS
Mutations in the bl gene.
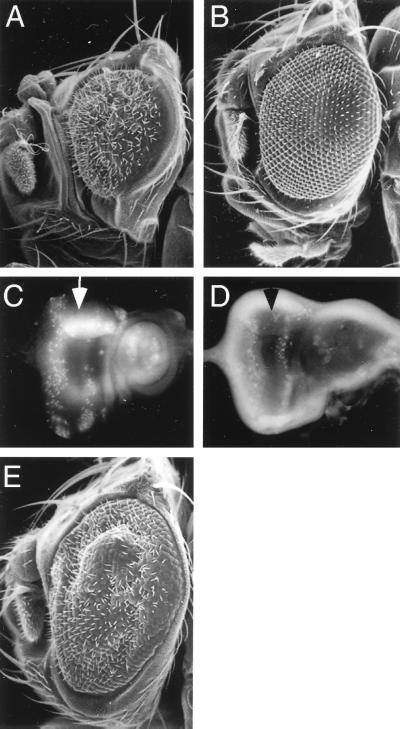
From a viable enhancer-trap line, P(lac, w+)176, that cytologically maps to position 57A (33a), we generated deletion mutations following mobilization of this P element (Materials and Methods). From 370 w− excision events, 9 showed fully penetrant mutant, semiviable, adult phenotypes when homozygous. Homozygotes or transheterozygotes for all mutations affected the size of the adult appendages. Wings, legs, and eyes were smaller than wild type (Fig. 1). The mutations isolated fall into three classes (strong, weak, and nearly wild type) based on the viability of alleles and their effects on the derivatives of imaginal discs (Table 1). Because adult flies with a strong phenotype cannot walk and fall over, we called these mutants bancal, which means “wobbly” in French. Twenty to twenty-five percent of adult flies homozygous for blv5 or blv6 or blv6/blv4 transheterozygotes have smaller than wild-type eyes. Observation of the descendants from the different bl heterozygous flies shows that all homozygotes survive to the second or third instar larval stage; 70 to 80% of mutant individual survive to pupal or adult stages. We have also removed maternal and zygotic bl+ function, by making bl germ line clones (data not shown). Loss of maternal bl product has no effect on the survival characteristics of bl homozygotes (data not shown). The bl mutant phenotype (Fig. 1) and the bl mutant flies used in the rescue experiment described below are blv6/blv4 transheterozygotes. Note that the adult wing is the tissue most affected by the bl mutations (Table 1).
FIG. 1.
The reduction in size of adult appendages observed in the blv6/blv4 mutant is rescued by ubiquitous expression of Bl protein. The blv6/blv4 mutant eyes (B), legs (E), and wing (H) are smaller than wild type (A, D, and G). blv6/blv4, hsp70-bl/hsp70-bl flies show normal adult structures when grown at 25°C (C, F, and I). Eyes were observed by SEM (A to C).
TABLE 1.
Genetic analysis of bl
| Genotype | Viabilitya (%) | Phenotypeb
|
||
|---|---|---|---|---|
| Eye | Leg | Wing | ||
| Phenotype and viability profile of different bl allelic combinations | ||||
| w; blv1/blv2 | 100 | − | + | + |
| w; blv4/blv4 | 10 | + | + | ++ |
| w; blv10/blv10 | 10 | + | + | ++ |
| w; blv4/blv6 | 10 | + | ++ | +++ |
| w; blv5/blv6 | 1–5 | ++ | +++ | ++++ |
| w; blv6/blv6 | 1–5 | ++ | +++ | ++++ |
| Rescue of bl mutations by hs-bl or hs-HK5c P element construct | ||||
| w; blv4/blv6; hs-bl/hs-bl | 62 | − | + | − |
| w; blv4/blv6; hs-HK5/hs-HK5 | 51 | − | + | − |
Score of viability is determined by the percentage of bl homozygotes that give viable adults.
Based on the strength of the defects, the mutations isolated fall into three classes: strong (+++ or ++++), weak (++), and nearly wild type (+). −, the corresponding structure is wild type. See Fig. 1 for the bl phenotype.
The bl gene encodes a Drosophila homologue of the vertebrate hnRNP K protein.
Genomic DNA flanking the 3′ end of the transposon P(lac, w+)176 was isolated by plasmid rescue (61). Genomic phages were isolated, allowing the isolation of 50 kb of genomic DNA surrounding the insertion site (Fig. 2A). Mutant and parental chromosomes were studied by Southern blot analysis (data not shown). Because the distal breakpoint of the deficiencies blv6 and blv5 has not been characterized, we used a transheterozygous allelic combination, blv6/blv4, in all the genetic experiments described in this report. Several embryonic cDNA clones were isolated by using a genomic DNA fragment flanking the P-element insertion site as a probe. One of the longest cDNA clones (CR22) detects mRNAs of about 1.9 and 2.1 kb during the first 10 h of embryogenesis; only the larger transcript is detected during the later stages of development (Fig. 2B). The cDNA CR22 (Fig. 3A) contains an open reading frame (ORF) encoding a 490-amino-acid protein with a predicted molecular weight of 53,900 and a predicted pI of 8.98. Another differentially spliced form of bl transcript that produces an abortive protein has been described (reference 17a and Fig. 3B). Database searches showed that the Bl sequence has strong homology within three maxi-KH domain internal repeats found in the human, Xenopus, and C. elegans hnRNP K proteins (Fig. 3A). The maxi-KH domain, an extended classical KH domain (54), is found in the prokaryotic RNase PNP (63), the Saccharomyces cerevisiae meiosis-specific factor MER1 (55), the Drosophila PSI (70) and Bicaudal-C proteins (40), and the product of the human fragile X gene FMR-1 (73) and the human Src substrate Sam 68 (20).
FIG. 2.
Molecular analysis of bl. (A) Map of the bl genomic region. Restriction sites are represented by vertical bars, EcoRI sites above and XbaI sites below. Breakpoints or deletions found in bl alleles are indicated by shaded boxes. The location and polarity of the insertion were determined by Southern blot and sequence analysis (data not shown). Genomic λ phages are indicated. The triangle indicates the P-element insertion site. (B) Northern blot analysis revealing bl transcripts, using CR22 cDNA as a probe. mRNAs of 1.9 and 2.1 kb are detected during the first 10 h of embryogenesis (0 to 5 h, lane 1; 5 to 10 h, lane 2); only the larger transcript is detected in the later stages of embryogenesis (lane 3), in third-instar larvae (lane 4), and in adult females (lane 5). Each lane contains 5 μg of poly(A)+ mRNA.
FIG. 3.
The Bl protein is structurally and functionally homologous to human K. (A) Amino acid sequence alignment between Bl and human, Xenopus, and C. elegans K proteins. Identicals amino acids are indicated by black boxes. The Bl protein has a putative nuclear localization signal (NLS) in the N-terminal region and three maxi-KH domains (KH1, KH2, and KH3; in yellow boxes). The KH3 domain is located in the C-terminal extremity of the protein and is separated from the KH2 motif by a hinge region composed of 33% glycine residues for Bl and 19% glycine and 16% proline residues for the human hnRNP K protein. The KNS domain (50) present in the human K protein is indicated. A putative M9 motif, responsible for the shuttling activity of human hnRNP A1 and B2 proteins (49), is present in the Bl protein (amino acids 272 to 319; in blue). (B) Another differentially spliced form of bl transcript produces an abortive protein. Shown is sequence alignment between the bl cDNA CR22 and the EST (expressed sequence tag) clone A1389318. The predicted ORFs of CR22 and the EST clone are indicated. In italics are the amino acids that differs between the two ORFs. The KH1 domain is underlined. (C and D) Binding of the Bl and human K proteins to ribonucleotide homopolymers. The Bl protein and the human K protein were produced by in vitro transcription-translation of pBS-CR22 and pBS-HK5, respectively. An amount equivalent to 20% of the material used for each binding reaction is shown in the lanes marked Translation. The translated proteins were incubated with the indicated ribonucleotide homopolymers in the presence of 200 mM NaCl, and bound proteins were analyzed by SDS-PAGE as described previously (73). Positions of the molecular mass markers are indicated on the left. (E) Amino acid sequence comparison between the M9 motifs identified in different organisms. Conserved residues are underlined. The derived consensus is indicated below the sequence alignment. An amino acid critical for the shuttling activity of the M9 motif is boxed (49).
Sequence homology strongly suggests that the bl cDNA CR22 encodes the Drosophila homologue of the vertebrate hnRNP K protein. First, the homology between the human and Drosophila amino acid sequences is not confined to the three maxi-KH domains; sequence similarity extends outside the second maxi-KH domain (Fig. 3A; see Discussion). Second, the positions of the KH motifs are conserved in the different species. Third, extensive sequence analysis revealed that the Bl maxi-KH domains are more homologous to the human hnRNP K maxi-KH domains than to those found in other vertebrate KH domain-containing proteins (data not shown). To investigate whether Bl is functionally as well as structurally similar to human hnRNP K, we transcribed and translated the Bl cDNA in the presence of [35S]methionine and assayed the affinity of the protein for different RNA homopolymers immobilized on agarose beads. This assay has been useful in assessing RNA binding for many other RNA-binding proteins (34, 73, 77). In the presence of 200 mM NaCl, Bl shows strong binding to poly(rC), very little binding to poly(rG) and poly(rU), and no binding to poly(rA) (Fig. 3C). Human hnRNP K protein has the same RNA-binding profile as Bl (Fig. 3D). Taken as whole, these data show that Bl protein is a Drosophila homologue of the vertebrate hnRNP K protein.
Both Drosophila and human K proteins can rescue the bl mutations when expressed ubiquitously.
To confirm that the identified transcription unit corresponds to the bl gene, we performed rescue experiments in which transgenic flies carrying the CR22 cDNA under control of the heat shock-inducible hsp70 promoter were combined with blv4/blv6 transheterozygotes. In the absence of the transgene, only 10% of the expected number of blv4/blv6 transheterozygotes survived to adulthood at 25°C (Table 1). Due to weak and leaky activity of the hsp70 promoter at 25°C, 62% of blv4/blv6 transheterozygotes carrying the transgene gave fully viable adults. In addition, the blv4/blv6 mutant phenotype is rescued (Fig. 1). Similar results were obtained with a hsp70-driven human hnRNP K transgene (Table 1). This rescue experiment is highly sensitive to growth temperature. At 29°C almost 100% of the blv4/blv6 transheterozygotes carrying the transgenes die during the second or third instar larval stage, most likely because of higher leakage of the hsp70 promoter at this temperature. This observation suggests that the amount of K protein synthesis is critical for the normal development of the flies (see Discussion). In summary, the CR22 cDNA isolated corresponds to the bl gene genetically characterized by the bl mutations. Moreover, these results show that the human K protein and Drosophila Bl protein are functionally homologous.
Analysis of Bl expression.
Rats were immunized to obtain polyclonal antiserum specific to the Drosophila Bl protein. Western blot analysis shows that this antiserum recognizes a single protein with a molecular mass of 54 kDa at all stages examined (Fig. 4A). To assess the effects of different bl mutations on protein production, Western blot analysis was performed with imaginal tissue homozygous or heterozygous for different bl alleles (Fig. 4A and B). Bl is undetectable in blv6 (or blv5 not shown) homozygotes, confirming that these are null alleles (Fig. 4A, lane 5). Bl is still detected, but at a lower than wild-type level, in blv4/blv6 larvae (Fig. 4A, lane 4) or in the weak bl alleles blv10 and blv4 (data not shown).
FIG. 4.
Analysis of Bl expression. (A and B) The Bl antiserum is specific for the bl gene product, and blv6/blv4 mutant imaginal discs have a reduced amount of the Bl protein. (A) Equal amounts of protein extracted from wild-type ovaries (1), wild-type embryos (2), and wild-type (3) or blv6/blv4 (4) or blv6/blv6 (5) mutant imaginal discs were resolved by SDS-PAGE and transferred to a nitrocellulose membrane. The bl antiserum detects a single 54-kDa protein among proteins prepared from all tissues except proteins prepared from blv6/blv6 (lane 5) mutant imaginal discs. Bl is still detected in blv6/blv4 imaginal discs (lane 4) but at a lower than wild-type level (lane 3). (B) Ponceau S staining of the nitrocellulose membrane immunoblotted by Bl antiserum in panel A. (C to F) Immunodetection of the Bl protein during embryogenesis. (C) Preferential cytoplasmic localization of Bl, syncytial blastoderm, cycle 12. (D) Nuclear and cytoplasmic localization, cellular blastoderm, cycle 13. Cytoplasmic staining is indicated by a thin arrow. Note that the Bl protein is present in the cytoplasm but not in the nucleus of the pole cells at this stage (thick arrow). (E) Nuclear localization, cellular blastoderm, cycle 14. (F) Embryo at stage 9 during germ band elongation. Bl is weakly detected in cells undergoing mitosis (indicated by asterisks) (19); the long arrow indicates the nuclear staining of amnioserosa cells in panel F. In all views, anterior is left and dorsal is uppermost. (C and D) Confocal sections of the posterior region of the embryo; (E and F) optical views.
The pattern of Bl protein distribution during the early stages of embryogenesis is illustrated in Fig. 4C to F. Bl shows a dynamic redistribution from the cytoplasm to the nuclei at the time of the initiation of massive zygotic transcription in early embryos. Fixed embryos of the nuclear cycles 10-12 (18) are characterized by strong cytoplasmic staining (Fig. 4C). In contrast, embryos of cycle 14 showed exclusively nuclear Bl signal (Fig. 4E), whereas cycle 13 embryos were clearly in transition, demonstrating examples of both patterns (Fig. 4D). From cycle 14 through the rest of embryogenesis and in larvae imaginal discs, the protein is found in all interphase nuclei without any preferential tissue distribution (not shown). Bl is weakly detected in cells undergoing mitosis (Fig. 4F), likely because of the absence of transcription during cell division and dilution of Bl product all over the cell. In summary, the dynamic redistribution of Bl during early development and/or mitosis coincides with the transcription activity of the cell, as described previously (8). These observations are also true for other components of nuclear RNP complexes like the Sm proteins of splicing snRNPs and the Drosophila hnRNP A and B groups (15, 68).
Mutations of the Drosophila hnRNP K gene slow down cell division.
The reduction in size of imaginal structures caused by bl mutations could result from an excess of programmed cell death, fewer cell divisions, and/or a reduction in size of imaginal cells. We have stained mutant imaginal discs with acridine orange to visualize apoptotic cells: no difference in cell death was observed between wild-type and bl mutant wing imaginal discs at the third instar stage (Fig. 5A and B). Careful inspection of cell size does not reveal any obvious differences between wild-type and bl wing mutant imaginal cells (Fig. 5C and D). However, following incorporation of BrdU, we observed that bl mutant imaginal discs show a significant reduction of cells stained with BrdU compared to the wild-type situation (Fig. 5E to H). Incorporation of BrdU occurs during the S phase of the cell cycle when DNA is replicated. Consequently, BrdU staining is an indirect marker of cellular proliferation in a given tissue. In the late third-instar larva, the distribution of BrdU-labeled cells is nonuniform (82). Some regions of the discs contain nonlabeled cells like the zone of nonproliferating cells (the ZNC band) and the M stripe, while other domains of the disc contain strongly labeled nuclei such as the notum region. We found that all bl mutant imaginal discs examined at the third-instar larvae displayed less BrdU-labeled nuclei than wild-type imaginal discs. Figure 5F illustrates a bl mutant third-instar larvae wing imaginal disc with a particularly strong reduction of BrdU staining in the notum region (Fig. 5H). Note that the regions of the bl mutant disc showing less intense BrdU staining vary from one disc to another (data not shown). In summary, our observations suggest that bl mutant discs proliferate less actively than wild-type discs.
FIG. 5.
Cell proliferation is reduced in blv6/blv4 mutant imaginal discs. Apoptotic cells were stained with acridine orange in wild-type (A) and blv6/blv4 mutant (B) wing imaginal discs. Arrows in panels A and B indicate acridine orange-positive cells. Inspection of cell size does not reveal any obvious differences between wild-type (C) and blv6/blv4 mutant (D) imaginal discs. Panels D and C correspond to a magnification of the wing blade. (E and F) Imaginal discs from late third instar were labeled with BrdU and stained with anti-BrdU antibodies. The number of fluorescent nuclei reflects the number and distribution of cells in S phase. A significant reduction in the number of BrdU-positive cells is seen in blv6/blv4 third-instar wing discs (F) compared to wild-type discs (E). Arrows indicate the ZNC, and the notum region is boxed. (G and H) Magnifications of the boxed regions in panels E and F, respectively.
Overexpression of either the Drosophila or human hnRNP K protein induces cell death.
To gain more insight into the function of the K proteins, we studied the consequences of overexpression of either the Drosophila Bl protein or its human counterpart hnRNP K in Drosophila imaginal discs. We constructed flies carrying UAS-bl and UAS-HK5 (human hnRNP K) transgenes in order to target the expression of Bl to specific cells (7). Ubiquitous and high-level expression of K proteins is lethal to flies (see above). Under ptcGAL4 regulation, we targeted UAS-bl or UAS-HK5 expression in a band of cells just anterior to the anteroposterior boundary in most imaginal discs, focusing at the third instar larval stage (Fig. 6A). These mutant flies display loss of some adult epidermal structures, corresponding to the domain of imaginal discs which overexpresses the K proteins. For instance, the intervein tissue, located between veins 3 and 4 of the wings, is reduced in size and the anterior cross-vein is missing (Fig. 6B to D). The absence of epidermal structures in flies overexpressing K could result from excessive cell death during the larval and/or pupal stages. Therefore, we investigated the pattern of cell death in these mutant discs. Normally there is little detectable cell death in the imaginal discs during the larval life. Extensive cell death, however, correlates with the site of overproduction of K proteins (Fig. 6E to G). Similarly, the expression of reaper (rpr), which encodes an effector of programmed cell death in Drosophila (27, 85), is expressed ectopically in cells overexpressing the K proteins (Fig. 6H to J).
FIG. 6.
High levels of hnRNP K proteins induce apoptosis. (A) Immunodetection of the Bl protein in ptcGAL4/UAS-bl wing imaginal disc. The experiment was performed using the anti-Bl antiserum at 1/2,000 dilution to detect only Bl-overexpressing cells. Bl is immunodetected in cells located in a band just anterior (ant) to the anteroposterior boundary. A similar distribution of the human K protein is observed in ptcGAL4/UAS-HK5 wing disc immunostained with mouse monoclonal anti-human K antibody (Fig. 8). post, posterior. (B to D) Wings of wild-type flies (B) or wings of flies overexpressing the Drosophila (C) or the vertebrate (D) K protein under ptcGAL4 regulation lack adult structures. Note the absence of the anterior cross-vein (arrow) and the reduction in size of the intervein tissue between veins 3 and 4 (black dot). These structures originate from the domain that overexpresses the K proteins in imaginal discs (A). (E to G) Apoptotic cells were stained with acridine orange in a wild-type wing imaginal disc (E) and a ptcGAL4/UAS-bl (F) or ptcGAL4/UAS-HK5 (G) wing disc. (H and I) Digoxigenin-labeled rpr cDNA was hybridized to fixed wild-type wing imaginal disc (H) and a ptcGAL4/UAS-bl (I) or a ptcGAL4/UAS-HK5 (J) wing disc. The rpr probe hybridizes to regions that probably overexpress the K proteins.
Expression of the antiapoptotic P35 protein suppresses the phenotype of Bl overexpression in the eye.
It has been shown that ectopic expression of the baculovirus P35 protein, which acts as an inhibitor of ICE proteases (9), inhibits both normal and induced apoptosis in the Drosophila eye imaginal discs (26). To further confirm that high levels of hnRNP K protein induce cell death, we targeted the expression of Bl in the Drosophila eye by using a pGMR-bl construct and then investigated the consequence of P35 coexpression. Expression of the pGMR transgenes is directed by the developmentally regulated Glass transcription factor in cells in, and posterior to, the morphogenetic furrow of the eye imaginal disc (53). Four independent transgenic P(GMR-bl; w+) lines were generated, and no phenotypic difference was observed between the different lines. By SEM, the eyes of flies carrying GMR-bl transgenes appeared normal (data not shown). A second chromosome carrying two copies of P(GMR-bl; w+) was constructed after recombination and designated GMR-bl2. Eyes of heterozygotes for this chromosome are reduced in size and highly disorganized (Fig. 7A). Inspection of GMR-bl2/+ eye by SEM revealed that interommatidial bristles are mislocated. As expected, eye imaginal discs from GMR-bl2/+ flies display a significant increase in the amount of cell death posterior to the furrow (Fig. 7C), while wild-type eye discs show very few apoptotic cells (Fig. 7D). Note that extensive acridine orange staining is observed only in the very posterior region of the eye disc despite the fact that the level of ectopic Bl expression was constant from the furrow to the posterior end of the disc at this stage (data not shown). It seems that there is a time delay between the onset of Bl expression and the appearance of cell death, and apparently the only defect resulting from high levels of Bl expression in postmitotic cells is cell death. To test if P35 can suppress the GMR-bl2 phenotype, GMR-bl2/+ flies were crossed with GMR-P35 flies. Flies heterozygous for both GMR-bl2 and GMR-P35 have eyes very similar in size and organization to a wild-type eye (Fig. 7E). We conclude that cell death is the major cellular defect caused by high levels of Bl expression.
FIG. 7.
P35 is a suppressor of the phenotype of Bl overexpression in the eye. (A and B) Scanning electron micrographs of GMR-bl2/+ (A) and GMR-P35/+ (B) eyes. (C) High levels of Bl protein in postmitotic cells of the eye lead to increased apoptosis visualized by acridine orange staining (C). (D) Wild-type eye imaginal disc stained with acridine orange. Very little cell death occurs posterior to the morphogenetic furrow (arrows in panels C and D) in a wild-type eye disc. (E) Scanning electron micrograph of a GMR-bl2/+; GMR-P35/+ eye.
The human K protein negatively regulates expression of the bl gene in vivo.
Because high levels of K protein cause cell death, we thought that the amount of K protein synthesized must be tightly regulated. One possibility is that K protein acts as its own negative regulator in a dose-sensitive manner (a dose-sensitive negative feedback loop). Since the Drosophila and vertebrate K proteins are functionally conserved, high levels of human K protein would be expected to negatively regulate the production of the Bl protein. To test this hypothesis, ptcGAL4/UAS-HK5 discs were double labeled with specific antibodies to immunodetect the Drosophila and human K proteins. Drosophila K protein was detected in the nuclei of all cells of wild-type wing discs of third-instar larvae (data not shown). Confocal analysis revealed that no Bl protein is detected in cells expressing high levels of the human K protein (Fig. 8A and B). Notably, Bl was weakly detected in cells expressing low levels of human K protein (Fig. 8B). However, overexpression of the K proteins did not affect the nucleoplasmic accumulation of the Tsh protein (Fig. 8C) (1) and the nucleolar expression of the Mod protein (Fig. 8D) (36), showing that cells overexpressing Bl are not generally deficient in protein expression. To determine the level at which this regulation occurs, we investigated the consequences of high levels of human K protein production on the level of the Drosophila bl mRNA detected by in situ hybridization. In situ hybridization to ptcGAL4/UAS-HK5 wing imaginal discs, using bl as a probe, reveals that Drosophila hnRNP K transcripts are not detected in the ptcGAL4/UAS-HK5 domain (Fig. 8E). Since cell death is observed following high levels of K protein production, it is feasible that no transcription was occurring in the ptcGAL4/UAS-HK5-expressing domain. However, this is unlikely since mod mRNA is present at wild-type levels (Fig. 8F) and tsh transcript levels are unaffected in the ptcGAL4 domain (data not shown). These results demonstrate that the human K protein functions in vivo in Drosophila as a direct or indirect negative regulator of bl gene expression and that the regulatory effect depends on the amount of hnRNP K expressed.
FIG. 8.
The human K protein negatively regulates expression of the bl gene. Wing imaginal discs of ptcGAL4/UAS-HK5 third-instar larvae were double stained with anti-human K protein (red) and anti-Bl (green) (A and B), anti-human K protein (red) and anti-Tsh (green) (C), or anti-human K protein (red) and anti-Mod (green) (D). (A and B) Bl is detected in the nuclei of all cells except those expressing the human K protein. Note that like Bl, the human K protein is detected essentially in the nucleus. The arrow in panel B indicates cells expressing both the vertebrate and Drosophila K proteins. Overexpression of the human K protein has no detectable effect on the immunodetection of the Tsh (expressed only in the proximal region of the disc) (C) or Mod (D) protein. Digoxigenin-labeled bl cDNA (E) and mod cDNA (F) were hybridized to fixed ptcGAL4/UAS-HK5 wing imaginal discs. bl transcript is not detected in a band of cells along the anterior-posterior boundary of Gal4-expressing cells (E). Ubiquitous expression of mod transcript is not affected in ptcGAL4/UAS-HK5 wing imaginal discs (F).
DISCUSSION
Bl is the Drosophila homologue of the human hnRNP K protein.
We have cloned the gene, bl, that encodes the Drosophila homologue of the vertebrate hnRNP K protein. Using an hsp70 promoter-driven Drosophila or human hnRNP K transgene, we have been able to rescue a weak bl phenotype. Although we were able to only partially rescue the bl mutant phenotype, the functional equivalence of human hnRNP K and Drosophila bl in the rescue assays strongly argues in favor of a conserved functional homology between Bl and the human hnRNP K protein.
The human and Drosophila K proteins have highest sequence similarity within their KH domains (KH1, 34% identity; KH2, 34% identity; KH3, 44% identity), and Bl preferentially binds poly(rC) in vitro. Dejgaard and Leffers (12) reported that KH3 is responsible for the in vitro poly(rC)-binding activity of human K, while the two other KH motifs act as nonspecific RNA-binding domains. Our observations reinforce the idea that the KH3 domain is critical for RNA-binding specificity of K protein because of its strong conservation throughout evolution. The conservation of a block of amino acids immediately adjacent to the second maxi-KH domain also suggests that this region is functionally relevant for the hnRNP K proteins. This region partially overlaps the binding domain of human hnRNP K to the transcriptional repressor Zik1 (14) (Fig. 3), and we found that Bl interacts in vitro with the human Zik1 protein (data not shown) probably through this conserved domain.
Human hnRNP K protein contains a specific domain, the KNS (hnRNP K nuclear shuttling) domain (50), that promotes bidirectional transport through the nuclear pore complex. This KNS domain lacks the Bl protein. An M9 motif, responsible for the shuttling activity of human A1 and A2/B1 hnRNP between the nucleus and the cytoplasm (49), is present upstream of the third maxi-KH domain in Bl (Fig. 3A and E). The presence of such a motif in Bl supports the idea that the Drosophila hnRNP K protein is a member of the hnRNP family. Note that the M9 motif is conserved in other Drosophila hnRNP proteins (75).
The level of hnRNP K protein is critical for cell survival.
We found that mutations in the bl gene cause developmental defects of imaginal disc derivatives (Fig. 1). Hypomorphic bl mutants, which express low levels of Bl protein (Fig. 4A, lane 4), give rise to adults with shortened appendages, probably due to less active cell proliferation compared to wild-type levels (Fig. 5F). Imaginal discs contain cells which normally progress through the four phases of the cell cycle (G1, S, G2, and M) (reviewed in reference 17). Because cells maintain a constant size as they proliferate, cell growth appears to be a limiting factor in the division of the imaginal cells (17, 39). In bl mutant discs, the imaginal cells are able to grow to a normal size (Fig. 5D). However, the reduction of the number of cells in S phase suggests that proliferation is less active than in wild-type flies. In conclusion, we propose that bl imaginal cells grow more slowly than wild-type cells.
Several lines of evidence suggest that the human hnRNP K protein has functions which are linked to the cell cycle. First, hnRNP K protein has been found to be upregulated in human simian virus 40-transformed keratinocytes compared to normal proliferating keratinocytes (13). Second, hnRNP K is associated in vivo with several members of the Src protein tyrosine kinase family (79, 83, 84). Third, the human hnRNP K can bind and activate the human c-myc promoter (78). However, during our work, we found that overexpressing K proteins does not affect the transcription of the d-myc gene in vivo (data not shown). Finally, because the bl gene encodes an hnRNP protein, it is more likely that pre-mRNA biogenesis is affected in bl mutant flies. It is therefore possible that the bl mutant cells grow slowly because of defective pre-mRNA processing. Interestingly, mutations in housekeeping genes such as ribosomal genes cripple protein synthesis and have a dominant effect of slowing growth (reviewed in reference 37).
Negative regulation of bl gene expression.
The expression level of hnRNP proteins is critical for their normal function (10, 31, 69, 86, 87), and here we have shown that overexpression of Drosophila and human K proteins in imaginal discs promotes cell death, as demonstrated by the increased number of acridine orange-positive cells (Fig. 6E to F) and the induction of rpr gene expression (Fig. 6H to J). Thus, it is very likely that the expression level of hnRNP K proteins is tightly regulated. We found that high levels of the human hnRNP K protein negatively regulate the expression of the bl gene, as demonstrated by the absence of Bl protein (Fig. 8B) and bl mRNA (Fig. 8E) in cells overexpressing the human K protein. Notably, a lower level of Bl protein is detected in cells weakly expressing the human K protein (arrow in Fig. 8B), suggesting that the vertebrate hnRNP K protein is able to repress, probably in a dose-sensitive manner, the expression of the bl gene. Two observations argue in favor of regulation at the transcriptional rather than posttranscriptional level. First, no bl transcripts are detected in cells overexpressing the human K protein (Fig. 8E); second, when expressed under the control of a heterologous promotor (the UAS-TATA promoter), the human and Drosophila K proteins are always highly expressed without any modulation in protein synthesis. In fact, many groups have reported that the human K protein can bind DNA to both activate and repress RNA polymerase II promoters (21, 29, 38, 48, 51, 52, 54, 81). However, we cannot exclude the possibility that overexpression of the human K protein affects either the stability, splicing, or nuclear export of the bl mRNA, since any of these mechanisms of regulation may affect transcript abundance.
Based on functional conservation between the vertebrate and Drosophila K proteins, we imagine that the Bl protein acts to negatively regulate its own expression, allowing us to propose the existence of negative feedback regulation for bl gene expression. Such a regulatory mechanism has been proposed for many genes in Drosophila (28, 41, 42, 67). For example, two mRNAs encoding transformer-2 (tra-2) are produced in the male germ line that differ from each other only by the retention of intron 3 (nonfunctional mRNA) or removal by splicing of intron 3 (functional mRNA) (42). It has been proposed that the Tra-2 protein limits its own synthesis by repressing the splicing of intron 3 (42). Additionally, the cyclin E protein is required directly or indirectly to shut off its own expression in endoreplicating tissues of the Drosophila embryo (67). Because overexpression of Bl is lethal, we speculate that such a regulatory mechanism has evolved for tight control of the level of Bl expression.
ACKNOWLEDGMENTS
We thank Gideon Dreyfuss and Matthew Michael for the human hnRNP K cDNA and the human K protein antibody. We gratefully acknowledge Sara Nakielny, Haruhiko Siomi, and Wes Friesen for critical comments on the manuscript.
This work was funded by the Centre National de la Recherche Scientifique and the Association de la Recherche contre le Cancer (ARC). B.C. received grants from the Ministère de la Recherche et de la Technologie and the ARC.
REFERENCES
- 1.Alexandre E, Graba Y, Fasano L, Gallet A, Perrin L, De Zulueta P, Pradel J, Kerridge S, Jacq B. The Drosophila Teashirt homeotic protein is a DNA-binding protein and modulo, a HOM C regulated modifier of variegation, is a likely candidate for being a direct target gene. Mech Dev. 1996;59:191–204. doi: 10.1016/0925-4773(96)00594-1. [DOI] [PubMed] [Google Scholar]
- 2.Amrein H, Gorman M, Nothiger R. The sex-determining gene tra-2 of Drosophila encodes a putative RNA binding protein. Cell. 1988;55:1025–1035. doi: 10.1016/0092-8674(88)90247-4. [DOI] [PubMed] [Google Scholar]
- 3.Baker N E, Rubin G M. Ellipse mutations in the Drosophila homologue of the EGF receptor affect pattern formation, cell division, and cell death in eye imaginal discs. Dev Biol. 1992;150:381–396. doi: 10.1016/0012-1606(92)90250-k. [DOI] [PubMed] [Google Scholar]
- 4.Bell L R, Maine E M, Schedl P, Cline T W. Sex-lethal, a Drosophila sex determination switch gene, exhibits sex-specific RNA splicing and sequence similarity to RNA binding proteins. Cell. 1988;55:1037–1046. doi: 10.1016/0092-8674(88)90248-6. [DOI] [PubMed] [Google Scholar]
- 5.Bellen H J, Kooyer S, D’Evelyn D, Pearlman J. The Drosophila couch potato protein is expressed in nuclei of peripheral neuronal precursors and shows homology to RNA-binding proteins. Genes Dev. 1992;6:2125–2136. doi: 10.1101/gad.6.11.2125. [DOI] [PubMed] [Google Scholar]
- 6.Bier E, Vaessin H, Shepherd S, Lee K, McCall K, Barbel S, Ackerman L, Carretto R, Uemura T, Grell E, et al. Searching for pattern and mutation in the Drosophila genome with a P-lacZ vector. Genes Dev. 1989;3:1273–1287. doi: 10.1101/gad.3.9.1273. [DOI] [PubMed] [Google Scholar]
- 7.Brand A H, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- 8.Buchenau P, Saumweber H, Arndt-Jovin D J. The dynamic nuclear redistribution of an hnRNP K-homologous protein during Drosophila embryo development and heat shock. Flexibility of transcription sites in vivo. J Cell Biol. 1997;137:291–303. doi: 10.1083/jcb.137.2.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bump N J, Hackett M, Hugunin M, Seshagiri S, Brady K, Chen P, Ferenz C, Franklin S, Ghayur T, Li P, et al. Inhibition of ICE family proteases by baculovirus antiapoptotic protein p35. Science. 1995;269:1885–1888. doi: 10.1126/science.7569933. [DOI] [PubMed] [Google Scholar]
- 10.Caceres J F, Stamm S, Helfman D M, Krainer A R. Regulation of alternative splicing in vivo by overexpression of antagonistic splicing factors. Science. 1994;265:1706–1709. doi: 10.1126/science.8085156. [DOI] [PubMed] [Google Scholar]
- 11.Choi Y D, Grabowski P J, Sharp P A, Dreyfuss G. Heterogeneous nuclear ribonucleoproteins: role in RNA splicing. Science. 1986;231:1534–1539. doi: 10.1126/science.3952495. [DOI] [PubMed] [Google Scholar]
- 12.Dejgaard K, Leffers H. Characterisation of the nucleic-acid-binding activity of KH domains. Different properties of different domains. Eur J Biochem. 1996;241:425–431. doi: 10.1111/j.1432-1033.1996.00425.x. [DOI] [PubMed] [Google Scholar]
- 13.Dejgaard K, Leffers H, Rasmussen H H, Madsen P, Kruse T A, Gesser B, Nielsen H, Celis J E. Identification, molecular cloning, expression and chromosome mapping of a family of transformation upregulated hnRNP-K proteins derived by alternative splicing. J Mol Biol. 1994;236:33–48. doi: 10.1006/jmbi.1994.1116. [DOI] [PubMed] [Google Scholar]
- 14.Denisenko O N, O’Neill B, Ostrowski J, Van Seuningen I, Bomsztyk K. Zik1, a transcriptional repressor that interacts with the heterogeneous nuclear ribonucleoprotein particle K protein. J Biol Chem. 1996;271:27701–27706. doi: 10.1074/jbc.271.44.27701. [DOI] [PubMed] [Google Scholar]
- 15.Dequin R, Saumweber H, Sedat J W. Proteins shifting from the cytoplasm into the nuclei during early embryogenesis of Drosophila melanogaster. Dev Biol. 1984;104:37–48. doi: 10.1016/0012-1606(84)90034-4. [DOI] [PubMed] [Google Scholar]
- 16.Dreyfuss G, Matunis M J, Pinol-Roma S, Burd C G. hnRNP proteins and the biogenesis of mRNA. Annu Rev Biochem. 1993;62:289–321. doi: 10.1146/annurev.bi.62.070193.001445. [DOI] [PubMed] [Google Scholar]
- 17.Edgar B A, Lehner C F. Developmental control of cell cycle regulators: a fly’s perspective. Science. 1996;274:1646–1652. doi: 10.1126/science.274.5293.1646. [DOI] [PubMed] [Google Scholar]
- 17a.Fly base. 1993–1997, copyright date. [Online.] The Drosophila genetic database. Genetics Society of America. http://flybase.bio.indiana.edu. [7 May 1999, last date accessed.]
- 18.Foe V E, Alberts B M. Studies of nuclear and cytoplasmic behaviour during the five mitotic cycles that precede gastrulation in Drosophila embryogenesis. J Cell Sci. 1983;61:31–70. doi: 10.1242/jcs.61.1.31. [DOI] [PubMed] [Google Scholar]
- 19.Foe V E. Mitotic domains reveal early commitment of cells in Drosophila embryos. Development. 1989;107:1–22. [PubMed] [Google Scholar]
- 20.Fumagalli S, Totty N F, Hsuan J J, Courtneidge S A. A target for Src in mitosis. Nature. 1994;368:871–874. doi: 10.1038/368871a0. [DOI] [PubMed] [Google Scholar]
- 21.Gaillard C, Cabannes E, Strauss F. Identity of the RNA-binding protein K of hnRNP particles with protein H16, a sequence-specific single strand DNA-binding protein. Nucleic Acids Res. 1994;22:4183–4186. doi: 10.1093/nar/22.20.4183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gallet A, Erkner A, Charroux B, Fasano L, Kerridge S. Trunk-specific modulation of Wingless signalling in Drosophila by Teashirt binding to Armadillo. Curr Biol. 1998;8:893–902. doi: 10.1016/s0960-9822(07)00369-7. [DOI] [PubMed] [Google Scholar]
- 23.Goralski T J, Edstrom J E, Baker B S. The sex determination locus transformer-2 of Drosophila encodes a polypeptide with similarity to RNA binding proteins. Cell. 1989;56:1011–1018. doi: 10.1016/0092-8674(89)90634-x. [DOI] [PubMed] [Google Scholar]
- 24.Hammond L E, Rudner D Z, Kanaar R, Rio D C. Mutations in the hrp48 gene, which encodes a Drosophila heterogeneous nuclear ribonucleoprotein particle protein, cause lethality and developmental defects and affect P-element third-intron splicing in vivo. Mol Cell Biol. 1997;17:7260–7267. doi: 10.1128/mcb.17.12.7260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harlow E, Lane D. Antibodies: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1988. [Google Scholar]
- 26.Hay B A, Wolff T, Rubin G M. Expression of baculovirus P35 prevents cell death in Drosophila. Development. 1994;120:2121–2129. doi: 10.1242/dev.120.8.2121. [DOI] [PubMed] [Google Scholar]
- 27.Hay B A, Wassarman D A, Rubin G M. Drosophila homologs of baculovirus inhibitor of apoptosis proteins function to block cell death. Cell. 1995;83:1253–1262. doi: 10.1016/0092-8674(95)90150-7. [DOI] [PubMed] [Google Scholar]
- 28.Hepker J, Wang Q T, Motzny C K, Holmgren R, Orenic T V. Drosophila Cubitus Interruptus forms a negative feedback loop with Patched and regulates expression of Hedgehog target genes. Development. 1997;124:549–558. doi: 10.1242/dev.124.2.549. [DOI] [PubMed] [Google Scholar]
- 29.Ito K, Sato K, Endo H. Cloning and characterization of a single-stranded DNA binding protein that specifically recognizes deoxycytidine stretch. Nucleic Acids Res. 1994;22:53–58. doi: 10.1093/nar/22.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnston L A, Schubiger G. Ectopic expression of wingless in imaginal discs interferes with decapentaplegic expression and alters cell determination. Development. 1996;122:3519–3529. doi: 10.1242/dev.122.11.3519. [DOI] [PubMed] [Google Scholar]
- 31.Kamma H, Portman D S, Dreyfuss G. Cell type-specific expression of hnRNP proteins. Exp Cell Res. 1995;221:187–196. doi: 10.1006/excr.1995.1366. [DOI] [PubMed] [Google Scholar]
- 32.Karsch-Mizrachi I, Haynes S R. The Rb97D gene encodes a potential RNA-binding protein required for spermatogenesis in Drosophila. Nucleic Acids Res. 1993;21:2229–2235. doi: 10.1093/nar/21.9.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kelley R L. Initial organization of the Drosophila dorsoventral axis depends on an RNA-binding protein encoded by the squid gene. Genes Dev. 1993;7:948–960. doi: 10.1101/gad.7.6.948. [DOI] [PubMed] [Google Scholar]
- 33a.Kerridge, S. Unpublished data.
- 34.Kiledjian M, Dreyfuss G. Primary structure and binding activity of the hnRNP U protein: binding RNA through RGG box. EMBO J. 1992;11:2655–2664. doi: 10.1002/j.1460-2075.1992.tb05331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kimmel B E, Heberlein U, Rubin G M. The homeo domain protein Rough is expressed in a subset of cells in the developing Drosophila eye where it can specify photoreceptor cell subtype. Genes Dev. 1990;4:712–727. doi: 10.1101/gad.4.5.712. [DOI] [PubMed] [Google Scholar]
- 36.Krejci E, Garzino V, Mary C, Bennani N, Pradel J. Modulo, a new maternally expressed Drosophila gene encodes a DNA-binding protein with distinct acidic and basic regions. Nucleic Acids Res. 1989;17:8101–8115. doi: 10.1093/nar/17.20.8101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lambertsson A. The minute genes in Drosophila and their molecular functions. Adv Genet. 1998;38:69–134. doi: 10.1016/s0065-2660(08)60142-x. [DOI] [PubMed] [Google Scholar]
- 38.Lee M H, Mori S, Raychaudhuri P. Trans-activation by the hnRNP K protein involves an increase in RNA synthesis from the reporter genes. J Biol Chem. 1996;271:3420–3427. doi: 10.1074/jbc.271.7.3420. [DOI] [PubMed] [Google Scholar]
- 39.Madhavan M, Schneiderman H A. Histological analysis of the dynamics of growth of imaginal discs and histoblast nests during the larval development of Drosophila melanogaster. Roux’s Arch Dev Biol. 1977;183:269. doi: 10.1007/BF00848459. [DOI] [PubMed] [Google Scholar]
- 40.Mahone M, Saffman E E, Lasko P F. Localized Bicaudal-C RNA encodes a protein containing a KH domain, the RNA binding motif of FMR1. EMBO J. 1995;14:2043–2055. doi: 10.1002/j.1460-2075.1995.tb07196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marrus S B, Zeng H, Rosbash M. Effect of constant light and circadian entrainment of perS flies: evidence for light-mediated delay of the negative feedback loop in Drosophila. EMBO J. 1996;15:6877–6886. [PMC free article] [PubMed] [Google Scholar]
- 42.Mattox W, McGuffin M E, Baker B S. A negative feedback mechanism revealed by functional analysis of the alternative isoforms of the Drosophila splicing regulator Transformer-2. Genetics. 1996;143:303–314. doi: 10.1093/genetics/143.1.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matunis E L, Matunis M J, Dreyfuss G. Characterization of the major hnRNP proteins from Drosophila melanogaster. J Cell Biol. 1992;116:257–269. doi: 10.1083/jcb.116.2.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Matunis E L, Matunis M J, Dreyfuss G. Association of individual hnRNP proteins and snRNPs with nascent transcripts. J Cell Biol. 1993;121:219–228. doi: 10.1083/jcb.121.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matunis M J, Matunis E L, Dreyfuss G. Isolation of hnRNP complexes from Drosophila melanogaster. J Cell Biol. 1992;116:245–255. doi: 10.1083/jcb.116.2.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Matunis M J, Michael W M, Dreyfuss G. Characterization and primary structure of the poly(C)-binding heterogeneous nuclear ribonucleoprotein complex K protein. Mol Cell Biol. 1992;12:164–171. doi: 10.1128/mcb.12.1.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mayeda A, Munroe S H, Caceres J F, Krainer A R. Function of conserved domains of hnRNP A1 and other hnRNP A/B proteins. EMBO J. 1994;13:5483–5495. doi: 10.1002/j.1460-2075.1994.tb06883.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miau L H, Chang C J, Shen B J, Tsai W H, Lee S C. Identification of heterogeneous nuclear ribonucleoprotein K (hnRNP K) as a repressor of C/EBPbeta-mediated gene activation. J Biol Chem. 1998;273:10784–10791. doi: 10.1074/jbc.273.17.10784. [DOI] [PubMed] [Google Scholar]
- 49.Michael W M, Choi M, Dreyfuss G. A nuclear export signal in hnRNP A1: a signal-mediated, temperature-dependent nuclear protein export pathway. Cell. 1995;83:415–422. doi: 10.1016/0092-8674(95)90119-1. [DOI] [PubMed] [Google Scholar]
- 50.Michael W M, Eder P S, Dreyfuss G. The K nuclear shuttling domain: a novel signal for nuclear import and nuclear export in the hnRNP K protein. EMBO J. 1997;16:3587–3598. doi: 10.1093/emboj/16.12.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Michelotti E F, Michelotti G A, Aronsohn A I, Levens D. Heterogeneous nuclear ribonucleoprotein K is a transcription factor. Mol Cell Biol. 1996;16:2350–2360. doi: 10.1128/mcb.16.5.2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Michelotti E F, Tomonaga T, Krutzsch H, Levens D. Cellular nucleic acid binding protein regulates the CT element of the human c-myc protooncogene. J Biol Chem. 1995;270:9494–9499. doi: 10.1074/jbc.270.16.9494. [DOI] [PubMed] [Google Scholar]
- 53.Moses K, Ellis M C, Rubin G M. The glass gene encodes a zinc-finger protein required by Drosophila photoreceptor cells. Nature. 1989;340:531–536. doi: 10.1038/340531a0. [DOI] [PubMed] [Google Scholar]
- 54.Musco G, Stier G, Joseph C, Castiglione Morelli M A, Nilges M, Gibson T J, Pastore A. Three-dimensional structure and stability of the KH domain: molecular insights into the fragile X syndrome. Cell. 1996;85:237–245. doi: 10.1016/s0092-8674(00)81100-9. [DOI] [PubMed] [Google Scholar]
- 55.Nandabalan K, Price L, Roeder G S. Mutations in U1 snRNA bypass the requirement for a cell type-specific RNA splicing factor. Cell. 1993;73:407–415. doi: 10.1016/0092-8674(93)90239-m. [DOI] [PubMed] [Google Scholar]
- 56.Ostrowski J, Van Seuningen I, Seger R, Rauch C T, Sleath P R, McMullen B A, Bomsztyk K. Purification, cloning, and expression of a murine phosphoprotein that binds the kappa B motif in vitro identifies it as the homolog of the human heterogeneous nuclear ribonucleoprotein K protein. Description of a novel DNA-dependent phosphorylation process. J Biol Chem. 1994;269:17626–17634. [PubMed] [Google Scholar]
- 57.Peifer M, Wieschaus E. The segment polarity gene armadillo encodes a functionally modular protein that is the Drosophila homolog of human plakoglobin. Cell. 1990;63:1167–1176. doi: 10.1016/0092-8674(90)90413-9. [DOI] [PubMed] [Google Scholar]
- 58.Pinol-Roma S, Choi Y D, Matunis M J, Dreyfuss G. Immunopurification of heterogeneous nuclear ribonucleoprotein particles reveals an assortment of RNA-binding proteins. Genes Dev. 1988;2:215–227. doi: 10.1101/gad.2.2.215. [DOI] [PubMed] [Google Scholar]
- 59.Pinol-Roma S, Dreyfuss G. Transcription-dependent and transcription-independent nuclear transport of hnRNP proteins. Science. 1991;253:312–314. doi: 10.1126/science.1857966. [DOI] [PubMed] [Google Scholar]
- 60.Pinol-Roma S, Dreyfuss G. Shuttling of pre-mRNA binding proteins between nucleus and cytoplasm. Nature. 1992;355:730–732. doi: 10.1038/355730a0. [DOI] [PubMed] [Google Scholar]
- 61.Pirrotta V. Vectors for P-mediated transformation in Drosophila. Bio/Technology. 1988;10:437–456. doi: 10.1016/b978-0-409-90042-2.50028-3. [DOI] [PubMed] [Google Scholar]
- 62.Pollard V W, Michael W M, Nakielny S, Siomi M C, Wang F, Dreyfuss G. A novel receptor-mediated nuclear protein import pathway. Cell. 1996;86:985–994. doi: 10.1016/s0092-8674(00)80173-7. [DOI] [PubMed] [Google Scholar]
- 63.Regnier P, Grunberg-Manago M, Portier C. Nucleotide sequence of the pnp gene of Escherichia coli encoding polynucleotide phosphorylase. Homology of the primary structure of the protein with the RNA-binding domain of ribosomal protein S1. J Biol Chem. 1987;262:63–68. [PubMed] [Google Scholar]
- 64.Robertson H M, Preston C R, Phillis R W, Johnson-Schlitz D M, Benz W K, Engels W R. A stable genomic source of P element transposase in Drosophila melanogaster. Genetics. 1988;118:461–470. doi: 10.1093/genetics/118.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Roth S, Schupbach T. The relationship between ovarian and embryonic dorsoventral patterning in Drosophila. Development. 1994;120:2245–2257. doi: 10.1242/dev.120.8.2245. [DOI] [PubMed] [Google Scholar]
- 66.Rubin G M, Spradling A C. Genetic transformation of Drosophila with transposable element vectors. Science. 1982;218:348–353. doi: 10.1126/science.6289436. [DOI] [PubMed] [Google Scholar]
- 67.Sauer K, Knoblich J A, Richardson H, Lehner C F. Distinct modes of cyclin E/cdc2c kinase regulation and S-phase control in mitotic and endoreduplication cycles of Drosophila embryogenesis. Genes Dev. 1995;9:1327–1339. doi: 10.1101/gad.9.11.1327. [DOI] [PubMed] [Google Scholar]
- 68.Segalat L, Lepesant J A. Spatial distribution of the Sm antigen in Drosophila early embryos. Biol Cell. 1992;75:181–185. doi: 10.1016/0248-4900(92)90139-r. [DOI] [PubMed] [Google Scholar]
- 69.Shen J, Zu K, Cass C L, Beyer A L, Hirsh J. Exon skipping by overexpression of a Drosophila heterogeneous nuclear ribonucleoprotein in vivo. Proc Natl Acad Sci USA. 1995;92:1822–1825. doi: 10.1073/pnas.92.6.1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Siebel C W, Kanaar R, Rio D C. Regulation of tissue-specific P-element pre-mRNA splicing requires the RNA-binding protein PSI. Genes Dev. 1994;8:1713–1725. doi: 10.1101/gad.8.14.1713. [DOI] [PubMed] [Google Scholar]
- 71.Singh R, Valcarcel J, Green M R. Distinct binding specificities and functions of higher eukaryotic polypyrimidine tract-binding proteins. Science. 1995;268:1173–1176. doi: 10.1126/science.7761834. [DOI] [PubMed] [Google Scholar]
- 72.Siomi H, Matunis M J, Michael W M, Dreyfuss G. The pre-mRNA binding K protein contains a novel evolutionarily conserved motif. Nucleic Acids Res. 1993;21:1193–1198. doi: 10.1093/nar/21.5.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Siomi H, Siomi M C, Nussbaum R L, Dreyfuss G. The protein product of the fragile X gene, FMR1, has characteristics of an RNA-binding protein. Cell. 1993;74:291–298. doi: 10.1016/0092-8674(93)90420-u. [DOI] [PubMed] [Google Scholar]
- 74.Siomi H, Choi M, Siomi M C, Nussbaum R L, Dreyfuss G. Essential role for KH domains in RNA binding: impaired RNA binding by a mutation in the KH domain of FMR1 that causes fragile X syndrome. Cell. 1994;77:33–39. doi: 10.1016/0092-8674(94)90232-1. [DOI] [PubMed] [Google Scholar]
- 75.Siomi M C, Fromont M, Rain J C, Wan L, Wang F, Legrain P, Dreyfuss G. Functional conservation of the transportin nuclear import pathway in divergent organisms. Mol Cell Biol. 1998;18:4141–4148. doi: 10.1128/mcb.18.7.4141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Spreij T E. Cell death during the development of imaginal discs of Calliphora erythrocephala. Neth J Zool. 1971;21:221–264. [Google Scholar]
- 77.Swanson M S, Dreyfuss G. Classification and purification of proteins of heterogeneous nuclear ribonucleoprotein particles by RNA-binding specificities. Mol Cell Biol. 1988;8:2237–2241. doi: 10.1128/mcb.8.5.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Takimoto M, Tomonaga T, Matunis M, Avigan M, Krutzsch H, Dreyfuss G, Levens D. Specific binding of heterogeneous ribonucleoprotein particle protein K to the human c-myc promoter, in vitro. J Biol Chem. 1993;268:18249–18258. [PubMed] [Google Scholar]
- 79.Taylor S J, Shalloway D. An RNA-binding protein associated with Src through its SH2 and SH3 domains in mitosis. Nature. 1994;368:867–871. doi: 10.1038/368867a0. [DOI] [PubMed] [Google Scholar]
- 80.Thummel C S, Boulet A M, Lipshitz H D. Vectors for Drosophila P-element-mediated transformation and tissue culture transfection. Gene. 1988;74:445–456. doi: 10.1016/0378-1119(88)90177-1. [DOI] [PubMed] [Google Scholar]
- 81.Tomonaga T, Levens D. Heterogeneous nuclear ribonucleoprotein K is a DNA-binding transactivator. J Biol Chem. 1995;270:4875–4881. doi: 10.1074/jbc.270.9.4875. [DOI] [PubMed] [Google Scholar]
- 82.Usui K, Kimura K I. Sensory mother cells are selected from among mitotically quiescent cluster of cells in the wing disc of Drosophila. Development. 1992;116:601–610. [Google Scholar]
- 83.Van Seuningen I, Ostrowski J, Bustelo X R, Sleath P R, Bomsztyk K. The K protein domain that recruits the interleukin 1-responsive K protein kinase lies adjacent to a cluster of c-Src and Vav SH3-binding sites. Implications that K protein acts as a docking platform. J Biol Chem. 1995;270:26976–26985. doi: 10.1074/jbc.270.45.26976. [DOI] [PubMed] [Google Scholar]
- 84.Weng Z, Thomas S M, Rickles R J, Taylor J A, Brauer A W, Seidel-Dugan C, Michael W M, Dreyfuss G, Brugge J S. Identification of Src, Fyn, and Lyn SH3-binding proteins: implications for a function of SH3 domains. Mol Cell Biol. 1994;14:4509–4521. doi: 10.1128/mcb.14.7.4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.White K, Grether M E, Abrams J M, Young L, Farrell K, Steller H. Genetic control of programmed cell death in Drosophila. Science. 1994;264:677–683. doi: 10.1126/science.8171319. [DOI] [PubMed] [Google Scholar]
- 86.Yang X, Bani M R, Lu S J, Rowan S, Ben-David Y, Chabot B. The A1 and A1B proteins of heterogeneous nuclear ribonucleoparticles modulate 5′ splice site selection in vivo. Proc Natl Acad Sci USA. 1994;91:6924–6928. doi: 10.1073/pnas.91.15.6924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zahler A M, Neugebauer K M, Lane W S, Roth M B. Distinct functions of SR proteins in alternative pre-mRNA splicing. Science. 1993;260:219–222. doi: 10.1126/science.8385799. [DOI] [PubMed] [Google Scholar]
- 88.Zu K, Sikes M L, Haynes S R, Beyer A L. Altered levels of the Drosophila HRB87F/hrp36 hnRNP protein have limited effects on alternative splicing in vivo. Mol Biol Cell. 1996;7:1059–1073. doi: 10.1091/mbc.7.7.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]