ABSTRACT
Background
Omega-3 fatty acids, including DHA and α-linolenic acid (ALA), are proposed to improve metabolic health by reducing obesity-associated inflammation. Their effects are mediated in part by conversion to oxylipins. ALA is relatively understudied, and direct comparisons to other omega-3 fatty acids are limited.
Objectives
We compared the effects of equal doses of ALA and DHA on plasma oxylipins and markers of metabolic health in women with obesity.
Methods
We carried out a randomized, double-blind, crossover clinical trial where women aged 20–51 with a BMI of 30–51 kg/m2 were supplemented with 4 g/day of ALA or DHA for 4 weeks in the form of ALA-rich flaxseed oil or DHA-rich fish oil. The primary outcome, the plasma oxylipin profile, was assessed at Days 0 and 28 of each phase by HPLC-MS/MS. Plasma fatty acids, inflammatory markers, and the monocyte glucose metabolism were key secondary outcomes. Data were analyzed using a mixed model.
Results
Compared to the baseline visit, there were higher plasma levels of nearly all oxylipins derived from DHA (3.8-fold overall; P < 0.001) and EPA (2.7-fold overall; P < 0.05) after 28 days of fish-oil supplementation, while there were no changes to oxylipins after flaxseed-oil supplementation. Neither supplement altered plasma cytokines; however, adiponectin was increased (1.1-fold; P < 0.05) at the end of the fish-oil phase. Compared to the baseline visit, 28 days of flaxseed-oil supplementation reduced ATP-linked oxygen consumption (0.75-fold; P < 0.05) and increased spare respiratory capacity (1.4-fold; P < 0.05) in monocytes, and countered the shift in oxygen consumption induced by LPS.
Conclusions
Flaxseed oil and fish oil each had unique effects on metabolic parameters in women with obesity. The supplementation regimens were insufficient to reduce inflammatory markers but adequate to elicit increases in omega-3 oxylipins and adiponectin in response to fish oil and to alter monocyte bioenergetics in response to flaxseed oil. This trial was registered at clinicaltrials.gov as NCT03583281.
Keywords: omega-3 fatty acids, docosahexaenoic acid, α-linolenic acid, oxylipins, inflammation, obesity, bioenergetics, metabolism, adiponectin
Introduction
Diets rich in PUFA have many documented benefits, including reduced all-cause mortality (1). The protective effects of omega-3 fatty acids in particular are usually attributed to their lipid-lowering and anti-inflammatory actions (2). Animal studies have demonstrated that supplementation with fish oil or flaxseed oil rich in omega-3 fatty acids reduces obesity-associated inflammatory markers and the progression of chronic diseases, such as type 2 diabetes mellitus and cardiovascular disease (3–6). Results of human trials have been mixed and have so far been more focused on fish oils rich in DHA and EPA. Systemic anti-inflammatory effects, generally a decrease in C-reactive protein (CRP) or in proinflammatory cytokines IL-1β and TNF-α, have been demonstrated in several trials investigating the effects of DHA/EPA (7–10) or α-linolenic (ALA) (11–13) supplementation in subjects with obesity or hypercholesterolemia. It is clear that not all omega-3 PUFAs are equal; for example, disparate effects of DHA-rich compared with EPA-rich oils have been documented (9). So far, direct comparisons between plant-derived ALA and fish oil–derived DHA or EPA in subjects with obesity have been limited.
Many of the biological effects of PUFAs have been attributed to the actions of their oxygenated free fatty acid metabolites, called oxylipins. Oxylipins are produced from precursor PUFAs by groups of intracellular enzymes in the cyclooxygenase, lipoxygenase (LOX), and cytochrome P450 (CYP450) families. Dietary fatty acid intake influences the availability of precursor PUFAs for conversion to oxylipins; however, changes to oxylipin profiles do not necessarily reflect changes in the PUFA composition (14, 15). Many omega-3 oxylipins have been reported to exhibit direct anti-inflammatory or proresolving effects, while both pro- and anti-inflammatory effects have been demonstrated for various omega-6 oxylipins (16).
Multiple chronic diseases are characterized by the presence of mitochondrial dysfunction in immune and other cells (17). The mitochondrial function is closely tied to the production of reactive oxygen species, which influences proinflammatory cytokine production (18–20) and vice versa (21). Innate immune cells isolated from patients with obesity-related chronic diseases, including type 2 diabetes (22) and coronary artery disease (23), exhibit elevated mitochondrial respiration rates compared to those from healthy control populations. Early changes to leukocyte mitochondrial function have not yet been investigated in at-risk (obese) participants who have not developed secondary disease. Fish-oil supplementation has been demonstrated to alter mitochondrial structures and functions in skeletal and cardiac muscle cells (24–26); however, so far no such investigation has been performed in immune cells.
Here, we report the results of a 2-phase, randomized, double-blind, crossover clinical trial (NCT03583281) where females with abdominal obesity were supplemented with 4 g/day of ALA or DHA for 4 weeks in the form of ALA-rich flaxseed oil or DHA-rich fish oil (4:1 DHA/EPA). The primary outcome was the plasma oxylipin profile. Secondary outcomes included clinical makers and anthropometrics, plasma fatty acids, plasma cytokines and adipokines, and monocyte glucose metabolism.
Methods
Trial design
As outlined in Figure 1, this was a randomized, double-blind, crossover trial with two 28-day (±2 days) phases. Each phase was preceded by a minimum 28-day wash-in period and each phase was scheduled to start at 9 ± 2 days from last menses. Participants were randomly assigned to start with either flaxseed oil or DHA-rich fish oil using a simple randomization code generated by a statistician. All participants and all team members except 1 (who had no contact with participants or samples) were blinded to the supplement order. Study visits took place on Days 0, 3, and 28 of each phase. At each visit, participants were asked to report compliance to study protocols and changes in their health status or concomitant medications. Height, weight, and blood pressure were recorded and a fasting blood sample was drawn. A Mobil-O-Graph PWA (I.E.M. GmbH) was used to assess blood vessel elasticity on Days 0 and 28. Fewer than half the participants (37%) were able to provide samples on Day 3 due to scheduling difficulties; therefore, this time point was dropped from the analyses. Participants were instructed to avoid consumption of foods high in omega-3 fatty acids, defined as no more than 0.3 g ALA/serving or 0.1 g EPA + DHA/serving (as per examples on a handout). Three-day food recall and activity questionnaires were to be completed during the third week of each wash-in and supplementation phase. This trial, registered at clinicaltrials.gov as NCT03583281, was approved by the University of Manitoba Research Ethics Board and the St. Boniface Hospital Research Review Committee. Details are provided in Rodway et al. (27) that further describe the study rationale and design, inclusion criteria, and sample size calculations. Some secondary outcomes will be reported separately [fatty acid composition, oxylipin production, and cytokine production by peripheral blood mononuclear cells (PBMC)], while others will not be reported as originally planned due to low sample numbers (vaginal fluid oxylipin profiles and immune composition).
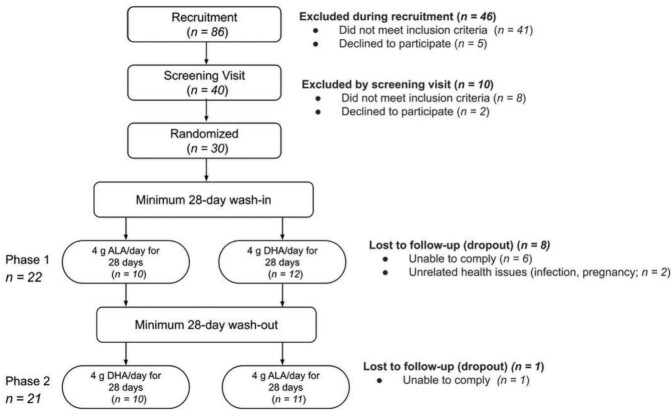
FIGURE 1.
CONSORT chart of study subjects. After contacting 86 individuals and screening 40, 8 individuals did not meet the inclusion criteria. Of 32 eligible individuals, 30 were randomized, while 2 declined to participate. The completion rate varied between phases due to the randomized crossover nature of the study. The analysis of the fish oil arm of the study was based on 22 individuals, and the analysis of flaxseed oil was based on 21 individuals. ALA, α-linolenic acid.
Participants
This trial recruited nonpregnant, nonlactating, premenopausal females between the ages of 20–55 with a BMI > 30 kg/m2 and waist circumference >80 cm (Asian descent) or >88 cm (all other ethnicities). Print, electronic, and social media advertising were used in the Winnipeg, Manitoba, area. Participants made contact by phone or email, and those meeting the general eligibility criteria (age, estimated BMI, nonsmoker, and premenopausal with regular menses) were invited to a screening visit. Written informed consent was given before any medical information was recorded or study-related procedures were conducted. The inclusion/exclusion criteria are described in detail in Rodway et al. (27). In brief, participants were required to be in relatively good health apart from an elevated BMI, as evidenced by a lack of clinically diagnosed diseases and by bloodwork showing liver enzyme, creatinine, and lipid values within specified ranges. Additional exclusion criteria included an unstable body weight, cigarette smoking, excessive consumption of alcoholic beverages, and regular use of nonsteroidal anti-inflammatory drugs or omega-3 PUFA supplements apart from those provided during the study. Participants were required to have regular menses and agreed to maintain stable levels of activity and their usual regimen of vitamin/mineral/dietary supplements, if applicable, during the course of their participation. If infection or illness (including cold, flu, yeast infection, etc.) was reported within 7 days of a study visit, the sample was not used and the participant was asked to repeat the phase or withdraw. Thirty individuals were enrolled by LRR into the study (with oversight from CGT), with 22 and 21 participants completing phases 1 and 2, respectively.
Interventions
Thirty daily doses of ∼4 g of ALA or DHA were provided in opaque, deidentified packaging by the St. Boniface Hospital Pharmacy and distributed to participants at their Day 0 visits. Either 3.70 g (first lot, 16 participants) or 3.58 g (second lot, 5 participants) of ALA per day was achieved by providing 7 flaxseed capsules (7 g total oil; NOW Foods; NPN:80002313). Additionally, 3.86 g of DHA (plus 1.01 g EPA) was achieved by providing 6 DHA-rich fish-oil capsules (6 g total oil; Super DHA gems, Carlson Laboratories; NPN: 80012587), according to a fatty acid analysis of the capsule contents (27). Participants were instructed to consume half their daily capsules with a morning meal and the other half with an afternoon or evening meal. Any missed capsules were to be returned at their Day 28 visit and were counted by the 1 team member not blinded. Self-reported compliance of >85% overall and 100% for the 72 hours preceding the study visit was required. A plasma fatty acid analysis was also used to confirm compliance.
Sample collection
For screening visits, fasting blood samples were collected by venipuncture into BD Vacutainer collection tubes (Becton Dickinson) by a phlebotomist and analyzed by Shared Health Services at St Boniface Hospital. Lithium heparin–containing tubes were used for measurements of a lipid panel, creatinine, and liver enzymes, while K2-EDTA tubes were used to measure glycated hemoglobin.
For study visits, fasting blood samples were collected into 10-mL K2-EDTA Vacutainer collection tubes (Becton Dickinson) and processed within 2 hours. To isolate platelet-poor plasma, blood was centrifuged at 1100 g for 10 minutes at room temperature, then the top three-fourths of the plasma was transferred and centrifuged again at 1500 g for 15 minutes. An antioxidant cocktail (0.2 mg/mL of BHT, 0.2 mg/mL of EDTA, 2 mg/mL of triphenylphosphine, and 2 mg/mL of indomethacin in a solution of 2:1:1 methanol:ethanol:water) was added to plasma aliquots reserved for fatty acid and oxylipin extraction at 3.3% of the sample volume. All plasma aliquots were kept frozen at –80°C until analyzed.
Oxylipin analysis
Following a previously established protocol (28), oxylipins were extracted from plasma samples (thawed on ice) using Strata-X-SPE columns (Phenomenex), analyzed by HPLC-MS/MS (QTRAP 6500; Sciex), and quantified by the stable isotope dilution method (29). Peaks were considered quantifiable if the peak height exceeded 5 times the blank levels in multiple samples. Detailed descriptions of quantification methods, mass transitions, and retention times for the 157-oxylipin panel can be found in previous publications (14, 30, 31).
Fatty acid analysis
Total lipids were extracted from 250 μL of plasma using the Folch method and quantified by gas chromatography as previously described (28, 32–34).
Plasma cytokine analysis
Cytokines and adipokines were quantified by electrochemiluminescence detection according to manufacturer's instructions using a SECTOR Imager (Meso Scale Discovery), with all antibodies, diluents, and standards purchased from Meso Scale Discovery. Precoated multi-spot V-Plex plates were used to quantify IL-1β, TNF-α, IL-10, IL-6, IL-8, IL-17A, and vascular endothelial growth factor (VEGF), while single-spot R-Plex plates coated in house were used to quantify adiponectin and resistin.
Analysis of clinical markers in plasma
Alanine aminotransferase (ALT), aspartate aminotransferase (AST), creatinine, glucose, a lipid panel (total cholesterol, LDL cholesterol, HDL cholesterol, and triglycerides), and CRP were measured for all study visit samples using a Cobas c111 analyzer (Roche).
Monocyte isolation
PBMC were first isolated using SepMate tubes with Lymphoprep Density Gradient Medium (Stem Cell Technologies) according to the manufacturer's instructions. Briefly, blood was mixed 1:1 with PBS:2% FBS [PBS prepared from disks (Calbiochem); FBS (Multicell)], dispensed into SepMate tube inserts, then centrifuged at 1200 g for 10 minutes at room temperature. Plasma was removed to ∼2 cm above the buffy coat, then the remainder was poured into a fresh 50 mL tube, topped up with PBS:2% FBS and centrifuged at 300 g for 8 minutes. The cells were washed once more and spun at 120 g for 10 minutes to deplete platelets. The PBMC were assessed for viability and counted using a hemocytometer and trypan blue dye (Gibco), then resuspended in PBS:2% FBS containing 1 mM of EDTA. The EasySep Human Monocyte Isolation Kit (Stem Cell Technologies) was then used to purify CD14+CD16– monocytes by immunomagnetic negative selection, as per the manufacturer's instructions. Viability (target > 95%) was assessed for every sample using trypan blue, and purity (target > 90%) was assessed periodically by blocking with TruStain FcX (Biolegend), staining with Fluorescein isothiocyanate-conjugated anti-CD14 antibody (Stem Cell Technologies), and examining cells under a fluorescence microscope (Olympus).
Monocyte bioenergetics
Bioenergetics parameters were assessed by modifying previously published protocols (35). Seahorse XF24 culture plates were coated with 45 μg/mL of CellTak (Corning) in 0.1 M of sodium bicarbonate (pH, 7.5) for 20–30 minutes then washed with sterile deionized water. Monocytes were plated at 4×105 cells per well in 200 μL of serum-free Roswell Park Memorial Institute (RPMI) medium. Plates were centrifuged at 300 g for 5 minutes at room temperature; 200 μL of 2× media (20% FBS) was added along with a media control or 10 ng/mL of LPS (from Esherichia coli O111: B4, γ-irradiated; Sigma-Aldrich), with 5 wells per condition as the target. Some samples were limited to 3–4 wells per condition due to low monocyte numbers (because of difficulties in drawing blood or purifying cells). Cells were incubated for 2 hours in a humidified cell-culture incubator (37°C; 5% CO2), after which a prewarmed Seahorse XF Assay medium [XF base medium (Agilent Technologies) with 0.5% FBS (Multicell), 2 mM L-glutamine (Hyclone), 1 mM of sodium pyruvate (Gibco), 10 mM of glucose (Agilent Technologies), and a pH of 7.4] was used to wash the cells twice and replace the culture medium. A mitochondrial stress test protocol was followed where 0.5 M of oligomycin (Sigma-Aldrich), 1 μM of carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone (Sigma-Aldrich), and 10 μM of antimycin A (Sigma-Aldrich) plus 10 μM of rotenone (Sigma-Aldrich) were injected sequentially as the oxygen consumption rate (OCR) and extracellular acidification rate (ECAR) were read on a Seahorse XF24 analyzer (Agilent Technologies).
Statistical analysis
SAS University Edition (SAS Institute Inc.) was used for statistical analyses. Primary outcomes (plasma oxylipins) and secondary outcomes (plasma fatty acids, plasma cytokines and adipokines, anthropometric data, blood lipids and other clinical parameters, nutrient intake data, activity data, and monocyte metabolic flux parameters) were all analyzed using a mixed model (28). Individuals were treated as random effects, while the supplement, day, and phase order were treated as fixed effects and entered as categorical values. The phase order had no effect on the majority of parameters [fixed effect P value (Pr > F) ≥ 0.05], and so was dropped from the model unless otherwise stated, in which case the full data are reported in Supplemental Table 1. Type 3 fixed effects of Day (0 and 28), Supplement (flaxseed oil and fish oil), and Day*Supplement interaction were determined, and differences of least square means with adjusted P values (Tukey-Kramer correction) are reported to compare D28 to D0 for each supplementation and to compare flaxseed oil to fish oil at each day. If necessary, values were log transformed to achieve a normal distribution within each group, as assessed by the distribution of Conditional Studentized Residuals. Values were excluded as outliers if they fell above or below the mean by 6× (oxylipins) or 5× (all other parameters) the SD, unless the paired values for the same participant in the same phase were similarly elevated. Some data are missing due to, for example, an insufficient sample volume, values outside the assay detection range, or an unexpected retention time. Missing values were not imputed. Pearson's correlation was performed to identify associations between parameters. For all analyses, a P value < 0.05 was considered significant.
Results
General results
Recruitment took place between June 2018 and December 2019. A participant flow diagram can be found in Figure 1. Thirty participants were randomized; however, 8 dropped out due to unrelated health issues or an inability to comply with the study protocols or schedule. One additional participant was unable to complete phase 2 due to cessation of operations in response to the coronavirus disease 2019 pandemic in March 2020. No adverse events were reported. Self-declared ethnicities included Caucasian (19 of 22), Asian (2 of 22), and Hispanic (1 of 22). Ten participants received flaxseed oil for phase 1, and all 10 went on to receive DHA-rich fish oil for phase 2. Twelve participants received DHA-rich oil for phase 1, and 11 of these went on to receive flaxseed oil for phase 2. One participant repeated their DHA phase after retroactively reporting a yeast infection that was present at their Day 28 visit. Participant characteristics at the screening visit are described in Table 1. All participants had abdominal obesity according to BMI and waist circumference measurements, as specified in the inclusion criteria. Mean self-reported capsule consumption compliance was >95% for both supplements. This was supported by the plasma fatty acid analysis, which confirmed an overall increase in the supplemented PUFA for each phase (Table 2). One participant reported relatively low compliance (86%) in the flaxseed-oil phase and another completed their flaxseed-oil phase prematurely by 6 days due to availability. A substantial increase in plasma ALA was nonetheless observed in both of these participants (rises of 34% and 124%, respectively). In total, 21 and 22 participants completed the flaxseed-oil and fish-oil phases, respectively, and were analyzed for the primary outcome. Self-reported 3-day food records (Supplemental Table 2) and activity (Supplemental Table 3) indicated similar nutrient intakes and activity levels at Week 3 of each wash-out and supplementation phase.
TABLE 1.
Anthropometrics and plasma analyte concentrations of women with obesity1
| Parameters | |
|---|---|
| Age, years | 38.0 ± 8.6 (21–51) |
| Weight, kg | 99.8 ± 22.1 (70.0–146) |
| BMI, kg/m2 | 36.8 ± 6.10 (30.0–51.4) |
| Waist circumference, cm | 102 ± 12.7 (82.0–134) |
| BP, systolic, mmHg | 128 ± 14.2 (98–155) |
| BP, diastolic, mmHg | 82.5 ± 9.90 (63–99) |
| Creatinine, μmol/L | 68.0 ± 8.90 (48–89) |
| AST, U/L | 17.1 ± 4.73 (11–29) |
| ALT, U/L | 17.1 ± 7.10 (10–37) |
| Cholesterol, mmol/L | 4.60 ± 0.70 (3.1–6.3) |
| HDL cholesterol, mmol/L | 1.50 ± 0.50 (1.0–3.1) |
| LDL cholesterol, mmol/L | 2.53 ± 0.80 (0.8–4.4) |
| TG, mmol/L | 1.30 ± 0.41 (0.5–1.9) |
| CRP, mmol/L | 7.60 ± 6.20 (1–26) |
| HbA1c, % | 5.41 ± 0.30 (4.8–6.3) |
| Compliance,2 flaxseed-oil phase, % | 96.7 ± 4.1 (86.1–100) |
| Compliance,2 fish-oil phase, % | 97.8 ± 2.2 (93.2–100) |
Values are reported as means ± SDs (ranges) from 21 (flaxseed-oil phase) or 22 (fish-oil phase) participants. ALT, alanine aminotransferase; AST, aspartate aminotransferase; BP, blood pressure; CRP, C-reactive protein; HbA1c, glycated hemoglobin; TG, triglyceride.
Calculated from self-reported doses missed.
TABLE 2.
Effects of flaxseed-oil and fish-oil supplementation on plasma FA in women with obesity1
| Supplementation with flaxseed oil | Supplementation with DHA-rich fish oil | Pr > F for type 3 fixed effect | |||||
|---|---|---|---|---|---|---|---|
| D0 | D28 | D0 | D28 | Day | Suppl. | Day*Suppl | |
| SFA, μmol/L | |||||||
| 12:02,3 | 9.77 ± 5.19 | 9.71 ± 4.92 | 13.28 ± 15.9 | 9.53 ± 8.1 | 0.234 | 0.256 | 0.500 |
| 14:02 | 155 ± 97.0 | 139 ± 60.5 | 164 ± 121 | 115 ± 67.14 | 0.004 | 0.069 | 0.106 |
| 16:02 | 3410 ± 1530 | 3390 ± 1720 | 3640 ± 2260 | 3030 ± 15404 | 0.012 | 0.170 | 0.109 |
| 17:02 | 33.5 ± 9.92 | 34.4 ± 14.6 | 36.8 ± 19.9 | 33.6 ± 13.9 | 0.402 | 0.816 | 0.585 |
| 18:02 | 928 ± 346 | 932 ± 397 | 976 ± 519 | 883 ± 412 | 0.174 | 0.308 | 0.392 |
| 20:02 | 35.2 ± 12.8 | 34.7 ± 13.1 | 35.9 ± 17.4 | 36.3 ± 14.7 | 0.918 | 0.689 | 0.346 |
| 22:0 | 83.9 ± 29.2 | 82.7 ± 31.2 | 86.4 ± 39.2 | 83.0 ± 36.2 | 0.361 | 0.516 | 0.667 |
| 24:0 | 61.7 ± 21.5 | 61.8 ± 26.8 | 64.0 ± 29.2 | 59.9 ± 27.4 | 0.303 | 0.835 | 0.291 |
| MUFA, μmol/L | |||||||
| 14:1n-52 | 9.91 ± 6.78 | 9.01 ± 4.62 | 10.6 ± 8.98 | 5.75 ± 3.754,5 | .0004 | 0.016 | 0.030 |
| 16:1n-72,3 | 336 ± 213 | 291 ± 169 | 338 ± 246 | 223 ± 1464 | <0.0001 | 0.100 | 0.234 |
| 16:1n-7t2 | 62.2 ± 30.2 | 58.7 ± 33.0 | 67.8 ± 47.3 | 55.1 ± 32.4 | 0.008 | 0.835 | 0.530 |
| 18:1n-92 | 2780 ± 1150 | 2630 ± 1400 | 3030 ± 1830 | 2300 ± 11004 | 0.003 | 0.742 | 0.463 |
| 18:1n-72,3 | 228 ± 89.8 | 218 ± 111 | 243 ± 141 | 188 ± 77.84 | 0.002 | 0.231 | 0.349 |
| 20:1n-92 | 20.6 ± 13.3 | 20.0 ± 14.1 | 22.5 ± 16.8 | 16.2 ± 9.754,5 | 0.001 | 0.182 | 0.019 |
| 22:1n-92 | 1.08 ± 4.42 | 0.760 ± 5.06 | 2.36 ± 7.84 | 0.380 ± 4.694 | 0.024 | 0.642 | 0.045 |
| 24:1n-92 | 126 ± 38.6 | 120 ± 56.3 | 128 ± 67.1 | 142 ± 54.7 | 0.846 | 0.064 | 0.019 |
| n-6 PUFA, μmol/L | |||||||
| 18:2n-62 | 3800 ± 1180 | 3690 ± 1730 | 3980 ± 1840 | 3380 ± 1400 | 0.018 | 0.939 | 0.946 |
| 18:3n-6 | 49.7 ± 18.8 | 43.2 ± 21.8 | 50.6 ± 23.8 | 26.7 ± 10.64,5 | <0.0001 | 0.009 | 0.002 |
| 20:2n-62 | 24.5 ± 16.4 | 23.2 ± 18.42 | 259 ± 21.5 | 18.0 ± 11.54 | 0.001 | 0.474 | 0.389 |
| 20:3n-6 | 237 ± 158 | 205 ± 144 | 240 ± 164 | 136 ± 80.64,5 | <0.0001 | 0.020 | 0.005 |
| 20:4n-62 | 809 ± 255 | 778 ± 400 | 859 ± 402 | 671 ± 246 | 0.004 | 0.949 | 0.731 |
| 22:2n-62 | 3.40 ± 1.94 | 3.80 ± 3.02 | 4.00 ± 2.83 | 3.02 ± 2.244 | 0.006 | 0.463 | 0.042 |
| 22:4n-62 | 20.9 ± 9.99 | 18.9 ± 9.65 | 23.3 ± 14.6 | 10.4 ± 6.294,5 | <0.0001 | .0003 | <0.0001 |
| 22:5n-62 | 13.7 ± 8.45 | 12.1 ± 8.19 | 16.3 ± 12.9 | 11.7 ± 6.194 | 0.001 | 0.151 | 0.555 |
| n-3 PUFA, μmol/L | |||||||
| 18:3n-3 | 119 ± 64.2 | 179 ± 98.84,5 | 130 ± 75.2 | 113. ± 73.8 | 0.015 | 0.002 | <0.0001 |
| 20:3n-32 | 2.79 ± 1.96 | 4.11 ± 3.734, 5 | 2.77 ± 2.68 | 2.24 ± 1.74 | 0.184 | <0.0001 | 0.003 |
| 20:5n-32 | 91.1 ± 41.0 | 102 ± 50.6 | 81.1 ± 38.4 | 376 ± 1804, 5 | <0.0001 | <0.0001 | <0.0001 |
| 22:5n-32,6 | 49.0 ± 20.2 | 53.9 ± 29.2 | 52.7 ± 26.7 | 44.6 ± 25.3 | 0.142 | 0.733 | 0.419 |
| 22:6n-32,7 | 218 ± 125 | 204 ± 153 | 191 ± 154 | 588 ± 3014,5 | <0.0001 | <0.0001 | <0.0001 |
| Totals, μmol/L | |||||||
| SFA2 | 4720 ± 2020 | 4683 ± 2240 | 5020 ± 2980 | 4250 ± 20804 | 0.020 | 0.195 | 0.148 |
| MUFA2,6 | 3560 ± 1480 | 3350 ± 1750 | 38340 ± 2320 | 2930 ± 13704 | 0.002 | 0.687 | 0.490 |
| PUFA | 5440 ± 1770 | 5320 ± 2560 | 5650 ± 2680 | 5380 ± 2220 | 0.317 | 0.466 | 0.713 |
| n-32 | 480 ± 219 | 543 ± 299 | 457 ± 269 | 1120 ± 5474, 5 | <0.0001 | <0.0001 | <0.0001 |
| n-62,6 | 4960 ± 1560 | 4770 ± 2270 | 5190 ± 2420 | 4260 ± 1690 | 0.007 | 0.844 | 0.798 |
Concentrations are shown as means ± SDs; n = 19–21. Differences of least square means and adjusted P values are in Supplemental Table 5.
Log transformed for statistics.
One outlier removed.
Indicates difference (P < 0.05) from Day 0 of the same phase.
Indicates difference (P < 0.05) from other supplement at the same time point.
Residual distributions may limit validity of the model.
Phase order effect (refer to Supplemental Table 1 for values separated by phase).
Oxylipin profiles
Plasma oxylipin concentrations were measured as the primary outcome of this study and are reported in Table 3, with statistical details and effect sizes in Supplemental Table 4. Note that due to the large number of parameters being examined statistically, there is an increased risk of a type 1 error for a given oxylipin. As such, we emphasize results pertaining to oxylipin groups, defined by the precursor PUFA or enzymatic pathway, rather than individual lipids, whose P values should be interpreted with caution. Flaxseed oil had no effect on any plasma oxylipin (Table 3). Total ALA oxylipins were statistically similar on D0 and D28 of flaxseed-oil supplementation, although the concentrations were higher on D28 of flaxseed oil supplementation compared to D28 of DHA-rich fish oil supplementation (Figure 2A, upper left panel). By contrast, supplementation with DHA-rich fish oil increased the total concentrations of EPA- and DHA-derived oxylipins (Figure 2A, middle and lower left panels) by 2.7-fold and 3.8-fold, respectively. Nearly all individual EPA and DHA oxylipins were significantly increased as well (Table 3). These were the LOX products 8-, 11-, 12-, 15-, and 18-hydroxy-eicosapentaenoic acid (HEPE) and 7-, 10-, 11-, 13-, 14-, 16-, and 17-hydroxy-docosahexaenoic acid (HDoHE), the CYP450 epoxygenase products 19,20-epoxy-docosapentaenoic acid and 16,17- and 19,20-dihydroxy-docosapentaenoic acid, and the CYP450 hydroxylase product 20-HDoHE. Levels of 3 arachidonic acid (ARA) oxylipins, all secondary metabolites of CYP450 epoxygenase, were reduced in response to supplementation with DHA-rich fish oil (Table 3).
TABLE 3.
Effects of flaxseed-oil and fish-oil supplementation on plasma oxylipins in women with obesity1
| Supplementation with flaxseed oil | Supplementation with DHA-rich fish oil | Pr > F for type 3 fixed effect | |||||
|---|---|---|---|---|---|---|---|
| D0 | D28 | D0 | D28 | Day | Suppl. | Day*Suppl | |
| LA oxylipins, nmol/L | |||||||
| 9-HODE2 | 24.6 ± 9.78 | 28.7 ± 19.2 | 23.3 ± 10.5 | 18.9 ± 9.20 | 0.422 | 0.069 | 0.077 |
| 9-oxoODE2 | 4.43 ± 1.75 | 4.38 ± 2.75 | 4.79 ± 2.87 | 3.40 ± 1.78 | 0.070 | 0.479 | 0.415 |
| 13-HODE2 | 11.1 ± 4.19 | 12.8 ± 8.19 | 11.0 ± 5.18 | 8.91 ± 4.26 | 0.438 | 0.119 | 0.101 |
| 13-oxoODE2 | 4.55 ± 2.20 | 5.91 ± 5.51 | 5.09 ± 2.69 | 3.32 ± 1.93 | 0.239 | 0.153 | 0.057 |
| 9,10,13-TriHOME | 1.19 ± 0.564 | 1.11 ± 0.676 | 1.16 ± 0.751 | 0.722 ± 0.366 | 0.039 | 0.155 | 0.160 |
| 9,12,13-TriHOME | 0.763 ± 0.317 | 0.733 ± 0.409 | 0.767 ± 0.476 | 0.519 ± 0.229 | 0.080 | 0.202 | 0.166 |
| 9,10-DiHOME2,3,4 | 0.212 ± 0.063 | 0.200 ± 0.101 | 0.202 ± 0.072 | 0.195 ± 0.136 | 0.111 | 0.506 | 0.948 |
| 12,13-DiHOME2,3 | 0.621 ± 0.203 | 0.549 ± 0.186 | 0.611 ± 0.361 | 0.501 ± 0.176 | 0.040 | 0.440 | 0.739 |
| GLA oxylipins, nmol/L | |||||||
| 13-HOTrE-γ2 | 0.180 ± 0.104 | 0.190 ± 0.132 | 0.195 ± 0.141 | 0.119 ± 0.048 | 0.032 | 0.193 | 0.010 |
| DGLA oxylipins, nmol/L | |||||||
| 15k PGE1 | 0.008 ± 0.002 | 0.011 ± 0.008 | 0.009 ± 0.003 | 0.008 ± 0.003 | 0.207 | 0.232 | 0.026 |
| 15-HETrE | 0.191 ± 0.092 | 0.180 ± 0.086 | 0.167 ± 0.096 | 0.155 ± 0.063 | 0.471 | 0.467 | 0.688 |
| ARA oxylipins, nmol/L | |||||||
| PGK2 | 0.079 ± 0.044 | 0.098 ± 0.072 | 0.085 ± 0.049 | 0.109 ± 0.051 | 0.040 | 0.550 | 0.623 |
| 5-HETE | 1.54 ± 0.681 | 1.76 ± 0.822 | 1.59 ± 0.734 | 1.35 ± 0.516 | 0.968 | 0.465 | 0.024 |
| 5-oxoETE | 0.177 ± 0.102 | 0.140 ± 0.080 | 0.128 ± 0.079 | 0.139 ± 0.073 | 0.389 | 0.252 | 0.100 |
| 8-HETE2 | 0.560 ± 0.193 | 0.557 ± 0.176 | 0.570 ± 0.281 | 0.514 ± 0.165 | 0.795 | 0.790 | 0.606 |
| 9-HETE | 0.344 ± 0.128 | 0.305 ± 0.145 | 0.347 ± 0.166 | 0.267 ± 0.112 | 0.016 | 0.693 | 0.422 |
| 11-HETE2 | 0.230 ± 0.088 | 0.216 ± 0.075 | 0.218 ± 0.089 | 0.191 ± 0.050 | 0.140 | 0.448 | 0.814 |
| 12-HETE | 0.817 ± 0.333 | 0.861 ± 0.398 | 0.788 ± 0.323 | 0.693 ± 0.288 | 0.746 | 0.279 | 0.291 |
| 15-HETE | 0.643 ± 0.228 | 0.767 ± 0.381 | 0.701 ± 0.343 | 0.579 ± 0.263 | 0.683 | 0.449 | 0.020 |
| 15-oxoETE | 0.105 ± 0.045 | 0.125 ± 0.111 | 0.136 ± 0.067 | 0.101 ± 0.075 | 0.171 | 0.926 | 0.039 |
| 5,6-EpETrE | 0.020 ± 0.011 | 0.021 ± 0.010 | 0.023 ± 0.016 | 0.019 ± 0.009 | 0.338 | 0.780 | 0.364 |
| 8,9-DiHETrE | 0.119 ± 0.054 | 0.112 ± 0.049 | 0.110 ± 0.037 | 0.091 ± 0.0285 | 0.006 | 0.232 | 0.158 |
| 11,12-DiHETrE2 | 0.208 ± 0.067 | 0.205 ± 0.077 | 0.209 ± 0.063 | 0.162 ± 0.0455 | 0.002 | 0.204 | 0.005 |
| 14,15-DiHETrE2 | 0.190 ± 0.062 | 0.186 ± 0.071 | 0.182 ± 0.050 | 0.145 ± 0.0335 | 0.002 | 0.131 | 0.012 |
| 16-HETE | 0.162 ± 0.075 | 0.150 ± 0.089 | 0.123 ± 0.070 | 0.091 ± 0.053 | 0.066 | 0.017 | 0.420 |
| 17-HETE2 | 0.068 ± 0.030 | 0.064 ± 0.032 | 0.068 ± 0.040 | 0.064 ± 0.026 | 0.660 | 0.925 | 0.966 |
| 18-HETE2,3 | 0.077 ± 0.045 | 0.065 ± 0.043 | 0.076 ± 0.070 | 0.092 ± 0.092 | 0.810 | 0.478 | 0.185 |
| ALA oxylipins, nmol/L | |||||||
| 9-HOTrE2 | 4.07 ± 2.02 | 5.78 ± 3.955 | 3.93 ± 1.89 | 3.10 ± 1.116 | 0.498 | 0.012 | 0.016 |
| 9-oxoOTrE2 | 0.762 ± 0.337 | 0.839 ± 0.546 | 0.805 ± 0.547 | 0.572 ± 0.202 | 0.407 | 0.232 | 0.365 |
| 13-HOTrE2 | 1.24 ± 0.587 | 1.38 ± 0.63 | 1.30 ± 0.771 | 0.940 ± 0.241 | 0.489 | 0.105 | 0.034 |
| 12,13-EpODE2 | 0.012 ± 0.007 | 0.013 ± 0.007 | 0.012 ± 0.006 | 0.008 ± 0.0056 | 0.285 | 0.095 | 0.011 |
| EPA oxylipins, nmol/L | |||||||
| 8-HEPE2 | 0.322 ± 0.131 | 0.338 ± 0.331 | 0.354 ± 0.216 | 0.697 ± 0.2085,7 | 0.005 | 0.001 | 0.001 |
| 11-HEPE | 0.101 ± 0.042 | 0.104 ± 0.055 | 0.106 ± 0.046 | 0.171 ± 0.0635,6 | 0.010 | 0.030 | 0.002 |
| 12-HEPE2 | 0.180 ± 0.077 | 0.201 ± 0.088 | 0.215 ± 0.291 | 0.453 ± 0.3005,6 | <0.0001 | 0.041 | <0.0001 |
| 15-HEPE2 | 0.122 ± 0.051 | 0.122 ± 0.041 | 0.103 ± 0.049 | 0.248 ± 0.0865,6 | <0.0001 | 0.020 | <0.0001 |
| 18-HEPE | 0.225 ± 0.119 | 0.265 ± 0.120 | 0.202 ± 0.097 | 0.592 ± 0.1835,6 | <0.0001 | <0.0001 | <0.0001 |
| DHA oxylipins, nmol/L | |||||||
| 7-HDoHE | 0.556 ± 0.353 | 0.563 ± 0.462 | 0.395 ± 0.211 | 1.80 ± 0.6975,7 | <0.0001 | <0.0001 | <0.0001 |
| 10-HDoHE | 0.193 ± 0.105 | 0.205 ± 0.144 | 0.142 ± 0.089 | 0.629 ± 0.2085,6 | <0.0001 | <0.0001 | <0.0001 |
| 11-HDoHE | 0.272 ± 0.149 | 0.291 ± 0.196 | 0.196 ± 0.127 | 0.910 ± 0.3015,6 | <0.0001 | <0.0001 | <0.0001 |
| 13-HDoHE2 | 0.397 ± 0.174 | 0.427 ± 0.272 | 0.346 ± 0.185 | 1.12 ± 0.4305,6 | <0.0001 | 0.001 | <0.0001 |
| 14-HDoHE2 | 0.275 ± 0.130 | 0.313 ± 0.196 | 0.201 ± 0.108 | 0.861 ± 0.3465,6 | <0.0001 | 0.005 | <0.0001 |
| 16-HDoHE | 0.227 ± 0.115 | 0.256 ± 0.168 | 0.171 ± 0.095 | 0.652 ± 0.2235,6 | <0.0001 | <0.0001 | <0.0001 |
| 17-HDoHE | 0.795 ± 0.364 | 0.866 ± 0.550 | 0.567 ± 0.300 | 2.224 ± 0.7685,6 | <0.0001 | <0.0001 | <0.0001 |
| RvD1 | 0.041 ± 0.012 | 0.042 ± 0.008 | 0.040 ± 0.015 | 0.048 ± 0.011 | 0.029 | 0.370 | 0.133 |
| 16,17-DiHDoPE | 0.051 ± 0.022 | 0.048 ± 0.021 | 0.043 ± 0.023 | 0.124 ± 0.0305,6 | <0.0001 | <0.0001 | <0.0001 |
| 19,20-EpDoPE2 | 0.044 ± 0.030 | 0.046 ± 0.030 | 0.031 ± 0.021 | 0.132 ± 0.0615,6 | <0.0001 | 0.013 | <0.0001 |
| 19,20-DiHDoPE | 0.743 ± 0.449 | 0.709 ± 0.332 | 0.594 ± 0.336 | 1.649 ± 0.4025,6 | <0.0001 | .0004 | <0.0001 |
| 20- HDoHE2,7 | 0.497 ± 0.273 | 0.588 ± 0.399 | 0.387 ± 0.222 | 1.526 ± 0.7305,6 | <0.0001 | 0.006 | <0.0001 |
| Free FA, nmol/L | |||||||
| ALA | 2350 ± 873 | 2520 ± 761 | 2270 ± 838 | 1981 ± 740 | 0.656 | 0.154 | 0.100 |
| ARA | 183 ± 75.9 | 195 ± 72.2 | 190 ± 81.4 | 177 ± 63.1 | 0.911 | 0.919 | 0.213 |
| DHA | 957 ± 423 | 995 ± 505 | 797 ± 398 | 2320 ± 7025,6 | <0.0001 | 0.0001 | <0.0001 |
| EPA2 | 123 ± 70.8 | 136 ± 59.9 | 109 ± 61.5 | 324 ± 1355,6 | <0.0001 | 0.008 | <0.0001 |
Concentrations are shown as means ± SDs; n = 19–20 for all oxylipins except PGK2 (n = 10–17), 8-HEPE (n = 13–21), 11-HEPE (n = 13–21), and 15-oxoETE (n = 18–19). Two participant samples were excluded from the analysis because more than one-third of their oxylipins qualified as outlier values, suggesting a technical error. Differences of least square means and adjusted P values are in Supplemental Table 4. ALA, α-linolenic acid; ARA, arachidonic acid; DGLA, dihomo-gamma-linolenic acid; DiHDoPE, dihydroxy-docosapentaenoic acid; DiHETrE, dihydroxy-eicosatrienoic acid; DiHOME, dihydroxy-octadecenoic acid; EpDoPE, epoxydocosapentaenoic acid; EpETrE, epoxy-eicosatrienoic acid; EpODE, epoxy-octadecadienoic acid; FA, fatty acid; GLA, gamma-linolenic acid; HDoHE, hydroxy-docosahexaenoic acid; HEPE, hydroxy-eicosapentaenoic acid; HETE, hydroxy-eicosatetraenoic acid; HETrE, hydroxy-eicosatrienoic acid; HODE, hydroxy-octadecadienoic acid; HOTrE, hydroxy-octadecatrienoic acid; LA, linoleic acid; oxoETE, oxo-eicosatetraenoic acid; oxoODE, oxo-octadecadienoic acid; oxoOTrE, oxo-octadecatrienoic acid; PG, prostaglandin; Rv, resolvin; TriHOME, trihydroxy-octadecenoic acid.
Log transformed for statistics.
Residual distributions may limit validity of the model.
One outlier removed.
Indicates difference (P < 0.05) from Day 0 of the same phase.
Indicates difference (P < 0.05) from other supplement at the same time point.
Phase order effect (refer to Supplemental Table 1 for values separated by phase).
FIGURE 2.
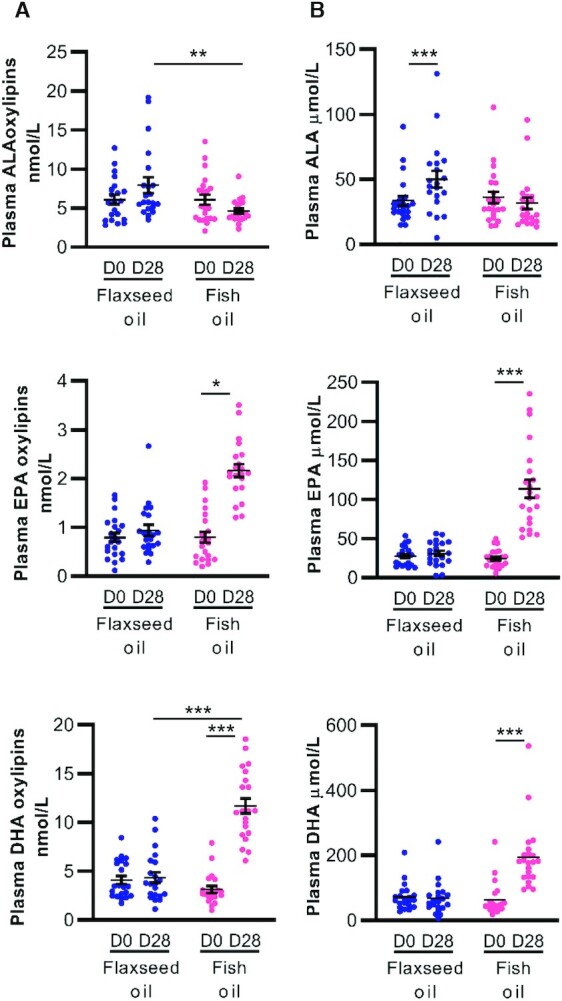
Effects of flaxseed-oil and fish-oil supplementation on plasma ALA, EPA, and DHA oxylipins and their fatty acid precursors in women with obesity. (A) Selected plasma oxylipins (n = 21) and (B) total plasma fatty acids (n = 21–22) are shown for day 0 and day 28 of each phase. Data were analyzed using a mixed model, and pairwise comparisons were assessed by difference of least square means with adjusted P values (Tukey-Kramer correction). *P < 0.05, **P < 0.01, and ***P < 0.001. Complete statistical analyses are provided in Table 3 and Supplemental Table 4 for all oxylipins and in Table 2 and Supplemental Table 5 for all fatty acids. ALA, α-linolenic acid.
Fatty acid profiles
Plasma fatty acid concentrations are reported in Table 2 and Supplemental Table 5. Only 2 plasma fatty acids were higher (by 1.5-fold each) after 28 days of flaxseed-oil supplementation: ALA itself and a low-abundance omega-3 PUFA, 20:3n3 (eicosatrienoic acid, Table 2). Supplementation with DHA-rich fish oil resulted in elevated plasma levels of EPA (4.6-fold) and DHA (3.1-fold; Figure 2B, middle and lower right panels). There were changes in several other plasma fatty acids (reported in Table 2 and Supplemental Table 5) that were not accompanied by changes in their derived oxylipins. For example, supplementation with DHA-rich fish oil resulted in lower levels of several omega-6 PUFAs, including γ-linolenic acid (18:3n6) and dihomo-γ-linolenic acid (20:3n6; Table 2). Oxylipins derived from these PUFAs were unchanged (Table 3). Also, supplementation with DHA-rich fish oil had no measurable effect on plasma ARA (Table 2), despite reductions in 3 ARA oxylipins (Table 3). Overall, both plasma fatty acid and oxylipin profiles were altered substantially by supplementation with DHA-rich fish oil but were relatively resistant to flaxseed-oil supplementation.
Clinical parameters
Anthropometric, vascular, and blood biochemistry measurements are reported in Table 4 and Supplemental Table 6. None of these parameters were altered by flaxseed-oil supplementation. One marker of liver injury, ALT, was slightly (1.18 × fold) but significantly increased after 28 days of supplementation with DHA-rich fish oil, whereas AST was unchanged. HDL cholesterol was increased, while triglyceride levels were decreased, after 28 days of supplementation with DHA-rich fish oil, similar to previous clinical trials that employed longer intervention periods (9). Although supplementation with both ALA and DHA has been previously reported to reduce CRP levels (7, 8, 11, 12), we did not detect a significant effect of either fatty acid supplement on CRP in this study.
TABLE 4.
Effects of flaxseed-oil and fish-oil supplementation on anthropometric and clinical parameters in women with obesity1
| Supplementation with flaxseed oil | Supplementation with DHA-rich fish oil | Pr > F for type 3 fixed effect | |||||
|---|---|---|---|---|---|---|---|
| D0 | D28 | D0 | D28 | Day | Suppl. | Day*Suppl | |
| Anthropometrics | |||||||
| Body weight, kg | 100 ± 21.4 | 100 ± 23.0 | 100 ± 23.3 | 100 ± 24.0 | 0.963 | 0.998 | 0.513 |
| Waist circumference, cm | 102 ± 13.5 | 102 ± 12.9 | 102 ± 13.7 | 103 ± 13.3 | 0.712 | 0.932 | 0.770 |
| BMI, kg/m2 | 36.9 ± 6.12 | 36.7 ± 6.67 | 36.9 ± 6.59 | 37 ± 6.84 | 0.915 | 0.962 | 0.479 |
| Vascular | |||||||
| SBP, mmHg | 127 ± 16.6 | 129 ± 16.7 | 128 ± 14.3 | 127 ± 15.7 | 0.899 | 0.986 | 0.232 |
| DBP, mmHg | 80.5 ± 11.3 | 80.8 ± 10.8 | 80.4 ± 10.1 | 81.4 ± 9.4 | 0.803 | 0.836 | 0.952 |
| AIx, % | 16.3 ± 9.99 | 17.4 ± 11.6 | 20.2 ± 9.46 | 20.5 ± 16.4 | 0.822 | 0.268 | 0.890 |
| PWV, m/s | 6.22 ± 0.806 | 5.97 ± 0.903 | 6.15 ± 1.19 | 5.86 ± 0.995 | 0.467 | 0.947 | 0.340 |
| Blood biochemistry | |||||||
| ALT, U/L2 | 17.9 ± 7.27 | 17.5 ± 6.76 | 18.5 ± 9.37 | 21.8 ± 11.193 | 0.039 | 0.420 | 0.003 |
| AST, U/L | 22.7 ± 5.29 | 24.1 ± 6.51 | 24.3 ± 7 | 26.1 ± 7.32 | 0.075 | 0.306 | 0.816 |
| Creatinine, μmol/L4 | 67.3 ± 8.35 | 68 ± 9.51 | 68.2 ± 7.84 | 68.3 ± 8.06 | 0.604 | 0.810 | 0.759 |
| CRP, mg/L2,5 | 9.28 ± 11.92 | 8.04 ± 7.74 | 8.44 ± 8.69 | 7.97 ± 8.23 | 0.083 | 0.813 | 0.838 |
| Glucose, mmol/L | 5.53 ± 0.653 | 5.62 ± 0.761 | 5.55 ± 0.66 | 5.74 ± 0.761 | 0.035 | 0.731 | 0.489 |
| Cholesterol, mmol/L | 4.74 ± 0.735 | 4.62 ± 0.664 | 4.66 ± 0.84 | 4.81 ± 0.828 | 0.859 | 0.802 | 0.054 |
| HDL cholesterol, mmol/L | 1.42 ± 0.425 | 1.38 ± 0.423 | 1.35 ± 0.401 | 1.50 ± 0.4323 | .0004 | 0.847 | <0.0001 |
| LDL-cholesterol, mmol/L2 | 2.80 ± 0.805 | 2.67 ± 0.654 | 2.72 ± 0.835 | 2.79 ± 0.89 | 0.858 | 0.968 | 0.230 |
| TG, mmol/L | 1.25 ± 0.333 | 1.26 ± 0.381 | 1.41 ± 0.483 | 1.08 ± 0.3393 | 0.001 | 0.910 | .0005 |
Concentrations are shown as means ± SDs; n = 20–22 for all parameters except AIx and PWV (n = 12–16). Differences of least square means and adjusted P values are in Supplemental Table 6. AIx, augmentation index; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CRP, C-reactive protein; DBP, diastolic blood pressure; PWV, pulse wave velocity; SBP, systolic blood pressure.
Log transformed for statistics;
Indicates difference (P < 0.05) from Day 0 of the same phase.
Phase order effect (refer to Supplemental Table 1 for values separated by phase).
Residuals distribution may limit validity of the model.
Cytokines and adipokines
Inflammatory mediator concentrations are reported in Table 5 and Supplemental Table 7. Based on previous studies, we expected to observe a reduction in plasma inflammatory markers with DHA (8, 9) and ALA (13) supplementation. Instead, no changes were detected in any of the measured pro-inflammatory cytokines (IFN-γ, IL-1β, IL-6, IL-8, IL-17A, TNF-α, or VEGF), anti-inflammatory cytokine (IL-10), or resistin. However, we did observe a small but significant increase in plasma adiponectin after 28 days of supplementation with DHA-rich fish oil, consistent with previous reports (36), which is indicative of improved adipocyte function in these participants.
TABLE 5.
Effects of flaxseed-oil and fish-oil supplementation on plasma inflammatory markers in women with obesity1
| Supplementation with flaxseed oil | Supplementation with DHA-rich fish oil | Pr > F for type 3 fixed effect | |||||
|---|---|---|---|---|---|---|---|
| D0 | D28 | D0 | D28 | Day | Suppl. | Day*Suppl | |
| IFN-γ, pg/mL2,3 | 4.34 ± 2.56 | 4.52 ± 2.80 | 3.79 ± 1.71 | 3.51 ± 1.58 | 0.425 | 0.460 | 0.781 |
| IL-10, pg/mL4 | 0.235 ± 0.083 | 0.227 ± 0.081 | 0.231 ± 0.089 | 0.246 ± 0.079 | 0.843 | 0.694 | 0.408 |
| IL-1β, pg/mL2,5 | 0.098 ± 0.056 | 0.099 ± 0.047 | 0.092 ± 0.054 | 0.093 ± 0.051 | 0.656 | 0.481 | 0.887 |
| IL-6, pg/mL6 | 1.31 ± 1.33 | 1.05 ± 1.08 | 1.07 ± 1.09 | 1.03 ± 1.25 | 0.307 | 0.635 | 0.478 |
| IL-8, pg/mL | 2.72 ± 1.17 | 3.26 ± 2.13 | 2.62 ± 1.42 | 2.63 ± 1.37 | 0.278 | 0.393 | 0.250 |
| TNF-α, pg/mL2 | 2.01 ± 0.740 | 1.95 ± 0.620 | 1.81 ± 0.500 | 1.90 ± 0.490 | 0.689 | 0.612 | 0.280 |
| VEGF, pg/mL2 | 37.8 ± 29.6 | 35.1 ± 23.1 | 39.5 ± 21.5 | 31.3 ± 13.7 | 0.185 | 0.810 | 0.197 |
| IL-17A, pg/m2,4 | 0.557 ± 0.278 | 0.491 ± 0.187 | 0.466 ± 0.167 | 0.519 ± 0.146 | 0.956 | 0.834 | 0.026 |
| Resistin, pg/m2 | 2530 ± 747 | 2630 ± 712 | 2710 ± 851 | 2560 ± 672 | 0.786 | 0.919 | 0.263 |
| Adiponectin, μg/mL | 237 ± 77.1 | 242 ± 83.4 | 237 ± 73.2 | 261 ± 87.97 | 0.010 | 0.903 | 0.079 |
Concentrations are shown as Mean ± SD; n = 19–20 for all markers except VEGF (n = 18–19) and IL-17A (n = 16–19). Differences of least square means and adjusted P-values are in Supplemental Table 7. VEGF, vascular endothelial growth factor.
Log transformed for statistics.
Two outliers removed.
One outlier removed.
Phase order effect (refer to Supplemental Table 1 for values separated by phase).
Indicates residuals distribution may limit validity of the model.
Indicates difference (P < 0.05) from Day 0 of the same phase.
Associations between BMI and monocyte bioenergetics
Several studies have reported an altered glucose metabolism in circulating immune cells of patients with chronic disease; however, the direction of change (higher or lower) seems to depend on the cellular population and/or the specific disease condition (22, 23, 37, 38). Here, we measured the live monocyte metabolism ex vivo using a Seahorse XF platform in the presence of glucose. We found that BMI is negatively associated with ECAR and positively associated with OCR and ATP-linked OCR (Figure 3A). Consistent with experiments performed in dendritic cells and macrophages (37, 38), we observed a Warburg effect induced by LPS stimulation of monocytes (Supplemental Figure 1A–C): that is, a slight decrease in OCR accompanied by a large increase in ECAR. Furthermore, the LPS-induced decrease in OCR is amplified with an increasing BMI (Supplemental Figure 1D). Thus, in the least healthy state (highest BMI), the resting OCR is increased but the dampening of OCR by LPS is enhanced.
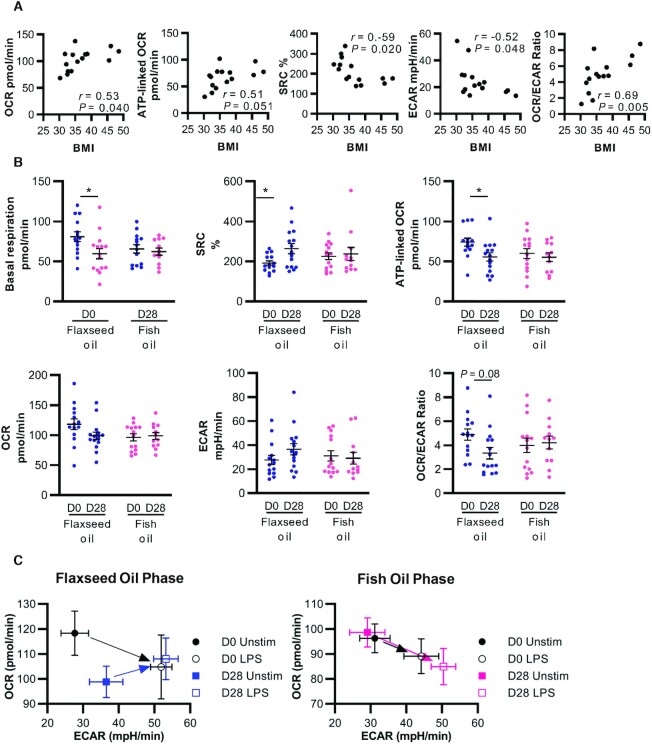
FIGURE 3.
Flaxseed-oil supplementation alters the monocyte glucose metabolism in women with obesity. Bioenergetics parameters of CD14+CD16– monocytes purified from peripheral blood on day 0 and 28 of each phase were assessed using a Seahorse XF mitochondrial stress test in the presence of glucose. (A) Correlations between BMI and parameters at the first study visit (n = 15). Pearson coefficients (r) and P values are indicated. (B) Supplementation effects on selected bioenergetics parameters (n = 12–15). Data were analyzed using a mixed model, and pairwise comparisons were assessed by difference of least square means with adjusted P values (Tukey-Kramer correction). *P < 0.05. Complete statistical analyses of all bioenergetics parameters are in Table 6 and Supplemental Table 8. (C) Visualization of cell energy phenotypes of unstimulated and 2-hour LPS-stimulated cells. OCR and ECAR measurements represent mean values from 10–15 participants. ECAR, extracellular acidification rate; OCR, oxygen consumption rate; SRC, spare respiratory capacity.
Supplementation effects on monocyte bioenergetics
Complete data for monocyte bioenergetics parameters are reported in Table 6 and Supplemental Table 8. Flaxseed-oil supplementation for 28 days reduced basal respiration and ATP-linked OCR in monocytes, and increased spare respiratory capacity (Table 6; Figure 3B). Thus, flaxseed-oil supplementation countered the increase in the monocyte oxidative glucose metabolism observed with a higher BMI. In contrast, supplementation with DHA-rich fish oil had no effect on any bioenergetic parameters.
TABLE 6.
Effects of flaxseed-oil and fish-oil supplementation on monocyte bioenergetics parameters in women with obesity1
| Supplementation with flaxseed oil | Supplementation with DHA-rich fish oil | Pr > F for type 3 fixed effect | |||||
|---|---|---|---|---|---|---|---|
| D0 | D28 | D0 | D28 | Day | Suppl. | Day*Suppl | |
| Unstimulated | |||||||
| Basal resp, pmol/min | 80.8 ± 22.8 | 59.7 ± 24.52 | 65.6 ± 19.4 | 62.3 ± 15.4 | 0.022 | 0.439 | 0.048 |
| Proton leak, pmol/min | 8.89 ± 12.2 | 5.47 ± 8.3 | 5.78 ± 6.2 | 6.98 ± 7.3 | 0.238 | 0.830 | 0.080 |
| Maximum resp, pmol/min | 152 ± 44.4 | 144 ± 31.8 | 147 ± 45.1 | 125 ± 22.8 | 0.129 | 0.244 | 0.471 |
| SRC, pmol/min | 71.0 ± 33.4 | 83.8 ± 30.1 | 80.8 ± 37.5 | 62.4 ± 20.2 | 0.698 | 0.514 | 0.058 |
| Non mito ox, pmol/min | 37.5 ± 21.1 | 39 ± 11.3 | 30.7 ± 6.6 | 36.3 ± 18 | 0.360 | 0.298 | 0.538 |
| ATP-linked OCR, pmol/min | 74.0 ± 18.6 | 55.5 ± 19.92 | 60.0 ± 22.9 | 55.3 ± 18.3 | 0.018 | 0.422 | 0.057 |
| Coupling effic,3 pmol/min | 0.948 ± 0.241 | 0.943 ± 0.124 | 0.889 ± 0.173 | 0.926 ± 0.211 | 0.280 | 0.546 | 0.579 |
| SRC,3,4 % | 1.91 ± 0.42 | 2.63 ± 0.9752 | 2.26 ± 0.649 | 2.37 ± 1.13 | 0.029 | 0.992 | 0.028 |
| OCR,3 pmol/min | 118 ± 33.2 | 98.8 ± 24.3 | 96.3 ± 21.6 | 98.6 ± 20.3 | 0.180 | 0.180 | 0.067 |
| ECAR, mpH/min | 27.7 ± 14.5 | 36.5 ± 18.1 | 31.2 ± 15.9 | 29.1 ± 17.1 | 0.613 | 0.610 | 0.110 |
| OCR/ECAR ratio3 | 4.88 ± 1.75 | 3.33 ± 1.85 | 3.98 ± 2.24 | 4.21 ± 1.85 | 0.293 | 0.785 | 0.021 |
| Change by LPS | |||||||
| ΔBasal resp,5 pmol/min | –26.4 ± 21.2 | –2.08 ± 23.52 | –8.68 ± 17.0 | –19.6 ± 15.5 | 0.213 | 0.867 | .0003 |
| ΔProton leak, pmol/min | 1.96 ± 5.09 | 7.07 ± 5.88 | 5.65 ± 6.12 | 3.31 ± 5.66 | 0.395 | 0.984 | 0.030 |
| ΔMaximum resp, pmol/min | –11.6 ± 23.9 | 20.2 ± 37.22 | 9.44 ± 25.1 | 5.05 ± 16.1 | 0.077 | 0.713 | 0.026 |
| ΔSRC, pmol/min | 14.8 ± 30.9 | 22.3 ± 25.4 | 19.2 ± 30.1 | 24.7 ± 22.8 | 0.409 | 0.662 | 0.899 |
| ΔNon mito ox, pmol/min | 11.1 ± 19.6 | 11.4 ± 16.0 | 4.92 ± 12.8 | 10.5 ± 21.1 | 0.472 | 0.530 | 0.488 |
| ΔATP-linked OCR, pmol/min | –27.9 ± 18.9 | –9.72 ± 21.32 | –14.6 ± 19.6 | –23.0 ± 16.5 | 0.459 | 0.796 | 0.001 |
| ΔCoupling efficiency,3 pmol/min | –0.043 ± 0.163 | –0.125 ± 0.150 | –0.089 ± 0.166 | –0.176 ± 0.229 | 0.082 | 0.347 | 0.942 |
| ΔSRC,3 % | 0.875 ± 1.17 | 0.399 ± 0.725 | 0.701 ± 0.699 | 0.832 ± 1.167 | 0.557 | 0.581 | 0.221 |
| ΔOCR,5 pmol/min | –15.3 ± 17.3 | 9.30 ± 23.12 | –3.76 ± 21.1 | –9.15 ± 21 | 0.071 | 0.525 | 0.003 |
| ΔECAR, mpH/min | 23.3 ± 13.0 | 17.5 ± 11.1 | 13.6 ± 15.2 | 21.1 ± 13.9 | 0.692 | 0.518 | 0.048 |
| ΔOCR/ECAR ratio | –2.86 ± 1.96 | –1.37 ± 1.80 | –1.44 ± 1.87 | –2.32 ± 1.49 | 0.894 | 0.895 | 0.003 |
Concentrations are shown as means ± SDs; n = 12–15 for unstimulated and n = 10–14 for change by LPS. Differences of least square means and adjusted P values are in Supplemental Table 8. ECAR, extracellular acidification rate; mito, mitochondrial; OCR, oxygen consumption rate; ox, oxidation; resp, respiration; SRC, spare respiratory capacity.
Indicates difference (P < 0.05) from Day 0 of the same phase.
Log transformed for statistics.
One outlier removed.
Phase order effect (refer to Supplemental Table 1 for values separated by phase).
Furthermore, flaxseed-oil supplementation countered the changes in oxidative phosphorylation parameters induced by LPS. Specifically, after supplementation, the LPS-induced reductions in basal respiration, ATP-linked OCR, and OCR were lessened (Table 6). This can also be visualized in Figure 3C, where the LPS-induced change in ECAR compared with OCR is traced with an arrow. Two hours of LPS stimulation increased ECAR and decreased OCR in cells harvested on Day 0 of both regimens and on D28 of DHA supplementation. In contrast, in cells harvested on D28 of flaxseed oil supplementation, LPS stimulation increased both ECAR and OCR. Thus, flaxseed oil opposed the enhanced Warburg effect of LPS observed with a higher BMI.
Discussion
Omega-3 PUFAs are a promising group of dietary bioactive compounds proposed to block the progression of chronic disease, in part by modulating obesity-associated inflammation (2). However, before they can be utilized effectively, much remains to be learned in humans with respect to the differences between plant and marine PUFAs and the types of inflammatory markers and processes that are affected. Here, we present the results of a randomized, double-blind, crossover clinical trial that compared the effects of supplementation with ALA-rich oil compared with DHA-rich oil (4:1 DHA/EPA) for 4 weeks in women with obesity. We report that supplementation with DHA-rich fish oil increased the plasma levels of nearly all detectable oxylipins derived from DHA and EPA, including CYP450 products with anticancer activities (39) and LOX products that are precursors to proresolving mediators (40). Plasma oxylipins were unaffected by flaxseed-oil supplementation. There were also no measurable changes to inflammatory cytokines (IFN-γ, IL-1β, IL-6, IL-8, IL-17A, TNF-α, and VEGF), an anti-inflammatory cytokine (IL-10), or resistin with either supplement; however, plasma levels of adiponectin and HDL cholesterol were increased and triglycerides were decreased after 28 days of supplementation with DHA-rich fish oil. We also investigated effects on the monocyte glucose metabolism and found that ALA but not DHA reduced OCR and increased spare respiratory capacity. These appear to be favorable changes since higher a BMI was associated with an increase in oxygen consumption and a decrease in spare respiratory capacity. Finally, flaxseed-oil supplementation also countered the Warburg effect of LPS stimulation. Overall, monocytes in ALA-supplemented participants with obesity shift to a more quiescent phenotype with respect to their glucose metabolism. Thus, we report that fish-oil and flaxseed-oil supplementation have distinct effects in this population: fish oil enhances proresolving lipid mediators and adipocyte function, while flaxseed oil seems to promote immune-metabolic quiescence at the cellular level.
The effects of ALA- and DHA-rich oils on plasma oxylipin profiles described here largely agree with the results of earlier work. We previously reported that the same DHA-rich oil increased most DHA- and EPA-derived oxylipins in healthy-weight men and women, while flaxseed oil had little effect on circulating oxylipins (28). At least 3 independent clinical trials similarly report increased DHA- and EPA-derived oxylipins in plasma after fish-oil supplementation (41–43). Flaxseed-oil supplementation studies designed with higher doses and/or longer durations have reported increases in ALA oxylipins (42, 44), unlike what we observed here using ∼4 g/day for 4 weeks. The degree of precursor PUFA enrichment is likely an important factor contributing to oxylipin profile changes. Here, supplementation with DHA-rich fish oil increased total plasma EPA and DHA levels by more than 200% each, while flaxseed-oil supplementation increased the total plasma ALA by only 50%. We previously reported that better vascular health was associated with higher plasma levels of 12-LOX products, including 12-HEPE and 14-HDoHE (45). Both of these products increased after supplementation with DHA-rich fish oil. Other omega-3 oxylipins have known anti-inflammatory and proresolving effects (46, 47). Thus, omega-3 oxylipins may mediate some of the physiological benefits of short-term supplementation with DHA-rich fish oil and long-term supplementation with flaxseed oil.
In rats, supplementation with pure omega-3 PUFAs reduces circulating omega-6 PUFA oxylipins, including proinflammatory eicosanoids derived from ARA, with DHA having a stronger effect compared to ALA (15). However, human studies examining the impacts of supplementation with ALA, DHA, or a combination of EPA and DHA on ARA-derived oxylipins have reported either a minimal effect (28, 44) or reductions in a few discrete products only (41–43, 48). Similarly, here we report minimal effects of supplementation on omega-6 oxylipins, despite the observation that DHA-rich (but not ALA-rich) oil reduced total plasma levels of several omega-6 PUFAs. Only 3 omega-6 oxylipins were reduced by supplementation with DHA-rich fish here, all secondary CYP450 epoxygenase metabolites of ARA.
Despite potent anti-inflammatory effects reported in animal feeding studies (3–6), the outcomes of supplementation with ALA-rich and DHA-rich oil in humans with obesity have been mixed. These trials commonly use circulating CRP, IL-6, and/or TNF-α as the markers of interest. Many (8, 10) but not all (49, 50) studies providing fish oil rich in both EPA and DHA to subjects with obesity for similar time frames have reported systemic anti-inflammatory effects. A study comparing DHA-rich to EPA-rich oil demonstrated some unique anti-inflammatory effects of each after 10 weeks (9). Some trials of supplementation with ALA-rich oil reported no effect (7, 50, 51), while others that used higher doses [∼17.5 g/day (12, 13)], longer durations [24 weeks (52)], or participants with more severe obesity [BMI ≥ 40 kg/m2 (11)] reported reduced inflammation. Here, we used a moderate dose (∼4 g/day), short time frame (4 weeks), and participants with BMIs of 30–44 kg/m2 without secondary disease. Note that consensus healthy reference ranges are not available for these inflammatory mediators. According to values reported in other studies, levels of IFNγ, IL-6, and TNFα in our participants are not higher than those expected in healthy female controls, but IL-10 levels are lower than expected (53–55). Other factors, such as the background diet and co-administered functional ingredients, likely play an important role: for example, other fatty acids present in the oil or fiber and protein components when milled flaxseed is used as the source of ALA. Gene-diet interactions could also be at play (56). It is important to note that the current study reports only on circulating cytokine levels; other aspects of leukocyte functions and tissue-level cytokine expression may be more sensitive to omega-3 PUFA interventions.
According to current disease models, abdominal obesity is harmful because adipocytes become hypertrophic and begin secreting chemokines and other factors that attract immune cells, which then perpetuate a local, and eventually systemic, inflammatory state (57, 58). A well-established marker of adipocyte dysfunction is reduced levels of adiponectin (59). Here, we report that plasma adiponectin levels are slightly but significantly elevated after supplementation with DHA-rich fish oil, in agreement with previously published studies (7, 36). Since adiponectin is primarily produced by adipocytes, this is evidence of an improved adipocyte function. Although the change in adiponectin occurred in the absence of reduced adiposity, defined by either body weight or BMI, it is possible that beneficial effects related to metabolic and/or cardiovascular functions might still occur. Indeed, high plasma adiponectin is thought to reduce triglycerides and increase HDL cholesterol (60), both of which were observed here after supplementation with DHA-rich fish oil. Adiponectin can also bind to and inactivate oxidized LDL cholesterol, reducing its atherogenic potential without altering its levels (61). Our group previously reported no change in triglyceride levels when the same DHA-rich oil was given to healthy weight men and women for 4 weeks (28). That study population was younger and healthier, according to their blood biochemistry; thus, elevated triglycerides at baseline might be required to detect a significant reduction with supplementation.
The most novel finding of this study is that monocyte OCR and ATP-linked OCR are reduced and spare respiratory capacity is increased after 4 weeks of flaxseed-oil supplementation. Evidence from our study population suggests that these are beneficial effects, since a higher BMI is associated with a higher OCR and ATP-linked OCR but lower spare respiratory capacity. A limitation to this result is the lack of healthy-weight participants and a small sample size. Nevertheless, our results are in agreement with other findings. Hartman et al. (22) reported increased basal respiration in PBMC from patients with type 2 diabetes compared to cells from control patients. Shirai et al. (23) demonstrated a similar OCR elevation in monocytes of coronary artery disease patients, although this was accompanied by a greater increase in ECAR and was measured in LPS/IFNγ–stimulated monocytes without an unstimulated reference. Here, we found that in parallel to a reduction in the resting OCR, flaxseed-oil supplementation also led to a dampened Warburg shift in response to 2 hours of LPS stimulation. In the presence of adequate glucose, the Warburg shift promotes a proinflammatory phenotype in innate immune cells (62, 63). Thus, we predict that the combined effect of a decreasing oxidative glucose metabolism at rest and diminishing the LPS-induced Warburg shift will reduce mitochondrial ROS production in monocytes and suppress a proinflammatory phenotype. Although the mechanism is as yet unclear, this may represent a new means by which ALA impedes the progression of chronic disease, and is of interest for future investigations. Note that although we achieved cellular purity of >90% (tested sporadically), we cannot rule out that population shifts in the contaminating minority component may have contributed to the observed changes.
The role of glucose availability and the effects of obesity, ALA, and other PUFAs on oxidative phosphorylation of lipid substrates remain to be investigated. For example, it is possible that the decrease in OCR observed with flaxseed-oil supplementation may be accompanied by an increase in fatty acid oxidation to support cellular functions. Furthermore, the relationship between the immune cell metabolism and whole-body physiology is controversial and seemingly context dependent. Clarity may be achieved by characterizing the effects of ALA on bioenergetics in other cells and tissues (64, 65), on the response to acute cellular stress (66), and in the context of autoimmune disease (67).
In summary, we report that ALA- and DHA-rich oils each have unique effects on plasma oxylipins and on markers of metabolic health in women with obesity. Four-week supplementation with 4 g/day of DHA (plus 1 g/day of EPA) was sufficient to increase plasma levels of oxylipins derived from EPA and DHA, to improve clinical blood lipid profiles, and to increase plasma adiponectin, but was insufficient to alter systemic inflammatory markers. Four-week supplementation with 4 g/day of ALA was insufficient to alter oxylipin, blood lipid, or inflammatory profiles in our study population; however, the monocyte bioenergetic profiles were markedly changed. These disparate effects provide further evidence that omega-3 PUFAs are not interchangeable and may provide metabolic benefits by entirely different, and possibly complementary, mechanisms. This raises the possibility that the full benefits of plant and marine omega-3 fatty acids may be optimally realized when present together in a given diet.
Supplementary Material
Acknowledgments
We are grateful for the clinical trial volunteers who made this research possible and thank St. Boniface Hospital Research for facilities support. We thank Dr. Paul Fernyhough and Dr. Darrell Smith for the use of the Seahorse XF24 analyzer and for their guidance and technical assistance. We also acknowledge Li Ren and Dennis Joseph for technical support and Dr. Jaime Clark for assistance with diet records.
The authorsʼ responsibilities were as follows – SDP: designed and conducted research, analyzed data, performed statistical analyses, and wrote the paper; LRR: conducted research, analyzed data, and constructed 1 table and 1 figure; KKS: conducted research and analyzed data; TW, NS: conducted research; HMA, PZ: designed research, provided essential reagents/materials, and edited the paper; CGT: designed research, performed statistical analyses, provided essential reagents/material, had primary responsibility for the final content, responsible for ethics approval, and edited the paper; and all authors: read and approved the final manuscript.
Notes
This study was supported by the Canadian Institutes of Health Research and the Canadian Agricultural Partnership - AG Action Manitoba.
Author disclosures: The authors report no conflicts of interest.
Supplemental Tables 1–8 and Supplemental Figure 1 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn/.
Abbreviations used: ALA, α-linolenic acid; ALT, alanine aminotransferase; ARA, arachidonic acid; AST, aspartate aminotransferase; CRP, C-reactive protein; CYP450, cytochrome P450; ECAR, extracellular acidification rate; HDoHE, hydroxy-docosahexaenoic acid; HEPE, hydroxy-eicosapentaenoic acid; LOX, lipoxygenase; OCR, oxygen consumption rate; PBMC, peripheral blood mononuclear cells; VEGF, vascular endothelial growth factor.
Contributor Information
Samantha D Pauls, Department of Food and Human Nutritional Sciences, University of Manitoba, Manitoba, Canada; Canadian Centre for Agri-Food Research in Health and Medicine, Winnipeg, Canada.
Lisa R Rodway, Canadian Centre for Agri-Food Research in Health and Medicine, Winnipeg, Canada; Department of Physiology and Pathophysiology, University of Manitoba, Canada.
Karanbir K Sidhu, Department of Food and Human Nutritional Sciences, University of Manitoba, Manitoba, Canada; Canadian Centre for Agri-Food Research in Health and Medicine, Winnipeg, Canada.
Tanja Winter, Department of Food and Human Nutritional Sciences, University of Manitoba, Manitoba, Canada; Canadian Centre for Agri-Food Research in Health and Medicine, Winnipeg, Canada.
Nikhil Sidhu, Department of Food and Human Nutritional Sciences, University of Manitoba, Manitoba, Canada; Canadian Centre for Agri-Food Research in Health and Medicine, Winnipeg, Canada.
Harold M Aukema, Department of Food and Human Nutritional Sciences, University of Manitoba, Manitoba, Canada; Canadian Centre for Agri-Food Research in Health and Medicine, Winnipeg, Canada.
Peter Zahradka, Department of Food and Human Nutritional Sciences, University of Manitoba, Manitoba, Canada; Canadian Centre for Agri-Food Research in Health and Medicine, Winnipeg, Canada; Department of Physiology and Pathophysiology, University of Manitoba, Canada.
Carla G Taylor, Department of Food and Human Nutritional Sciences, University of Manitoba, Manitoba, Canada; Canadian Centre for Agri-Food Research in Health and Medicine, Winnipeg, Canada; Department of Physiology and Pathophysiology, University of Manitoba, Canada.
References
- 1. Wang DD, Li Y, Chiuve SE, Stampfer MJ, Manson JE, Rimm EB, Willett WC, Hu FB. Association of specific dietary fats with total and cause-specific mortality. JAMA Intern Med. 2016;176(8):1134–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Natto ZS, Yaghmoor W, Alshaeri HK, Van Dyke TE. Omega-3 fatty acids effects on inflammatory biomarkers and lipid profiles among diabetic and cardiovascular disease patients: A systematic review and meta-analysis. Sci Rep. 2019;9(1):18867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Aguilera AA, Díaz GH, Barcelata ML, Guerrero OA, Ros RM. Effects of fish oil on hypertension, plasma lipids, and tumor necrosis factor-alpha in rats with sucrose-induced metabolic syndrome. J Nutr Biochem. 2004;15(6):350–7. [DOI] [PubMed] [Google Scholar]
- 4. Baranowski M, Enns J, Blewett H, Yakandawala U, Zahradka P, Taylor CG. Dietary flaxseed oil reduces adipocyte size, adipose monocyte chemoattractant protein-1 levels and T-cell infiltration in obese, insulin-resistant rats. Cytokine. 2012;59(2):382–91. [DOI] [PubMed] [Google Scholar]
- 5. Storlien LH, Kraegen EW, Chisholm DJ, Ford GL, Bruce DG, Pascoe WS. Fish oil prevents insulin resistance induced by high-fat feeding in rats. Science. 1987;237(4817):885–8. [DOI] [PubMed] [Google Scholar]
- 6. Poudyal H, Panchal SK, Ward LC, Brown L. Effects of ALA, EPA and DHA in high-carbohydrate, high-fat diet-induced metabolic syndrome in rats. J Nutr Biochem. 2013;24(6):1041–52. [DOI] [PubMed] [Google Scholar]
- 7. Baril-Gravel L, Labonte ME, Couture P, Vohl MC, Charest A, Guay V, Jenkins DA, Connelly PW, West S, Kris-Etherton PMet al. Docosahexaenoic acid-enriched canola oil increases adiponectin concentrations: A randomized crossover controlled intervention trial. Nutr Metab Cardiovasc Dis. 2015;25(1):52–59. [DOI] [PubMed] [Google Scholar]
- 8. Browning LM, Krebs JD, Moore CS, Mishra GD, O'Connell MA, Jebb SA. The impact of long chain n-3 polyunsaturated fatty acid supplementation on inflammation, insulin sensitivity and CVD risk in a group of overweight women with an inflammatory phenotype. Diabetes Obes Metab. 2007;9(1):70–80. [DOI] [PubMed] [Google Scholar]
- 9. Allaire J, Couture P, Leclerc M, Charest A, Marin J, Lépine MC, Talbot D, Tchernof A, Lamarche B. A randomized, crossover, head-to-head comparison of eicosapentaenoic acid and docosahexaenoic acid supplementation to reduce inflammation markers in men and women: The Comparing EPA to DHA (ComparED) Study. Am J Clin Nutr. 2016;104(2):280–7. [DOI] [PubMed] [Google Scholar]
- 10. Itariu BK, Zeyda M, Hochbrugger EE, Neuhofer A, Prager G, Schindler K, Bohdjalian A, Mascher D, Vangala S, Schranz Met al. Long-chain n-3 PUFAs reduce adipose tissue and systemic inflammation in severely obese nondiabetic patients: A randomized controlled trial. Am J Clin Nutr. 2012;96(5):1137–49. [DOI] [PubMed] [Google Scholar]
- 11. Faintuch J, Horie LM, Barbeiro HV, Barbeiro DF, Soriano FG, Ishida RK, Cecconello I. Systemic inflammation in morbidly obese subjects: Response to oral supplementation with alpha-linolenic acid. Obes Surg. 2007;17(3):341–7. [DOI] [PubMed] [Google Scholar]
- 12. Zhao G, Etherton TD, Martin KR, West SG, Gillies PJ, Kris-Etherton PM. Dietary alpha-linolenic acid reduces inflammatory and lipid cardiovascular risk factors in hypercholesterolemic men and women. J Nutr. 2004;134(11):2991–7. [DOI] [PubMed] [Google Scholar]
- 13. Zhao G, Etherton TD, Martin KR, Gillies PJ, West SG, Kris-Etherton PM. Dietary alpha-linolenic acid inhibits proinflammatory cytokine production by peripheral blood mononuclear cells in hypercholesterolemic subjects. Am J Clin Nutr. 2007;85(2):385–91. [DOI] [PubMed] [Google Scholar]
- 14. Leng S, Winter T, Aukema HM. Dietary LA and sex effects on oxylipin profiles in rat kidney, liver, and serum differ from their effects on PUFAs. J Lipid Res. 2017;58(8):1702–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Leng S, Winter T, Aukema HM. Dietary ALA, EPA and DHA have distinct effects on oxylipin profiles in female and male rat kidney, liver and serum. J Nutr Biochem. 2018;57:228–37. [DOI] [PubMed] [Google Scholar]
- 16. Gabbs M, Leng S, Devassy JG, Monirujjaman M, Aukema HM. Advances in our understanding of oxylipins derived from dietary PUFAs. Adv Nutr. 2015;6(5):513–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pieczenik SR, Neustadt J. Mitochondrial dysfunction and molecular pathways of disease. Exp Mol Pathol. 2007;83(1):84–92. [DOI] [PubMed] [Google Scholar]
- 18. Forrester SJ, Kikuchi DS, Hernandes MS, Xu Q, Griendling KK. Reactive oxygen species in metabolic and inflammatory signaling. Circ Res. 2018;122(6):877–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bauernfeind F, Bartok E, Rieger A, Franchi L, Núñez G, Hornung V. Cutting edge: Reactive oxygen species inhibitors block priming, but not activation, of the NLRP3 inflammasome. J Immunol. 2011;187(2):613–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bulua AC, Simon A, Maddipati R, Pelletier M, Park H, Kim KY, Sack MN, Kastner DL, Siegel RM. Mitochondrial reactive oxygen species promote production of proinflammatory cytokines and are elevated in TNFR1-associated periodic syndrome (TRAPS). J Exp Med. 2011;208(3):519–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Suematsu N, Tsutsui H, Wen J, Kang D, Ikeuchi M, Ide T, Hayashidani S, Shiomi T, Kubota T, Hamasaki Net al. Oxidative stress mediates tumor necrosis factor-alpha-induced mitochondrial DNA damage and dysfunction in cardiac myocytes. Circulation. 2003;107(10):1418–23. [DOI] [PubMed] [Google Scholar]
- 22. Hartman ML, Shirihai OS, Holbrook M, Xu G, Kocherla M, Shah A, Fetterman JL, Kluge MA, Frame AA, Hamburg NMet al. Relation of mitochondrial oxygen consumption in peripheral blood mononuclear cells to vascular function in type 2 diabetes mellitus. Vasc Med. 2014;19(1):67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shirai T, Nazarewicz RR, Wallis BB, Yanes RE, Watanabe R, Hilhorst M, Tian L, Harrison DG, Giacomini JC, Assimes TLet al. The glycolytic enzyme PKM2 bridges metabolic and inflammatory dysfunction in coronary artery disease. J Exp Med. 2016;213(3):337–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cavaliere G, Trinchese G, Bergamo P, De Filippo C, Mattace Raso G, Gifuni G, Putti R, Moni BH, Canani RB, Meli Ret al. Polyunsaturated fatty acids attenuate diet induced obesity and insulin resistance, modulating mitochondrial respiratory uncoupling in rat skeletal muscle. PLoS One. 2016;11(2):e0149033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Khairallah RJ, Sparagna GC, Khanna N, O'Shea KM, Hecker PA, Kristian T, Fiskum G, Des Rosiers C, Polster BM, Stanley WC. Dietary supplementation with docosahexaenoic acid, but not eicosapentaenoic acid, dramatically alters cardiac mitochondrial phospholipid fatty acid composition and prevents permeability transition. Biochim Biophys Acta. 2010;1797(8):1555–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pepe S, McLennan PL. Cardiac membrane fatty acid composition modulates myocardial oxygen consumption and postischemic recovery of contractile function. Circulation. 2002;105(19):2303–8. [DOI] [PubMed] [Google Scholar]
- 27. Rodway L, SD P, Aukema H, Zahradka P, Taylor C. Rationale and design of a randomized controlled trial examining the effects of marine and plant-sourced omega-3 fatty acid supplements on oxylipin profiles and inflammation in females with obesity (OXBIO trial). Prostaglandins Leukot Essent Fatty Acids. 2021;170:102284. [DOI] [PubMed] [Google Scholar]
- 28. Gabbs M, Zahradka P, Taylor CG, Aukema HM. Time course and sex effects of α-linolenic acid-rich and DHA-rich supplements on human plasma oxylipins: A randomized double-blind crossover trial. J Nutr. 2021;151(3):513–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Deems R, Buczynski MW, Bowers-Gentry R, Harkewicz R, Dennis EA. Detection and quantitation of eicosanoids via high performance liquid chromatography-electrospray ionization-mass spectrometry. Methods Enzymol. 2007;432:59–82. [DOI] [PubMed] [Google Scholar]
- 30. Aukema HM, Winter T, Ravandi A, Dalvi S, Miller DW, Hatch GM. Generation of bioactive oxylipins from exogenously added arachidonic, eicosapentaenoic and docosahexaenoic acid in primary human brain microvessel endothelial cells. Lipids. 2016;51(5):591–9. [DOI] [PubMed] [Google Scholar]
- 31. Monirujjaman M, Devassy JG, Yamaguchi T, Sidhu N, Kugita M, Gabbs M, Nagao S, Zhou J, Ravandi A, Aukema HM. Distinct oxylipin alterations in diverse models of cystic kidney diseases. Biochim Biophys Acta Mol Cell Biol Lipids. 2017;1862(12):1562–74. [DOI] [PubMed] [Google Scholar]
- 32. Aukema HM, Yamaguchi T, Takahashi H, Celi B, Holub BJ. Abnormal lipid and fatty acid compositions of kidneys from mice with polycystic kidney disease. Lipids. 1992;27(6):429–35. [DOI] [PubMed] [Google Scholar]
- 33. Aukema HM, Holub BJ. Effect of dietary supplementation with a fish oil concentrate on the alkenylacyl class of ethanolamine phospholipid in human platelets. J Lipid Res. 1989;30(1):59–64. [PubMed] [Google Scholar]
- 34. Fair DE, Ogborn MR, Weiler HA, Bankovic-Calic N, Nitschmann EP, Fitzpatrick-Wong SC, Aukema HM. Dietary soy protein attenuates renal disease progression after 1 and 3 weeks in Han:sPRD-cy weanling rats. J Nutr. 2004;134(6):1504–7. [DOI] [PubMed] [Google Scholar]
- 35. Kramer PA, Chacko BK, Ravi S, Johnson MS, Mitchell T, Darley-Usmar VM. Bioenergetics and the oxidative burst: protocols for the isolation and evaluation of human leukocytes and platelets. J Vis Exp. 2014;85:51301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gammelmark A, Madsen T, Varming K, Lundbye-Christensen S, Schmidt EB. Low-dose fish oil supplementation increases serum adiponectin without affecting inflammatory markers in overweight subjects. Nutr Res. 2012;32(1):15–23. [DOI] [PubMed] [Google Scholar]
- 37. Krawczyk CM, Holowka T, Sun J, Blagih J, Amiel E, DeBerardinis RJ, Cross JR, Jung E, Thompson CB, Jones RGet al. Toll-like receptor-induced changes in glycolytic metabolism regulate dendritic cell activation. Blood. 2010;115(23):4742–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tannahill GM, Curtis AM, Adamik J, Palsson-McDermott EM, McGettrick AF, Goel G, Frezza C, Bernard NJ, Kelly B, Foley NHet al. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature. 2013;496(7444):238–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhang G, Panigrahy D, Mahakian LM, Yang J, Liu JY, Stephen Lee KS, Wettersten HI, Ulu A, Hu X, Tam Set al. Epoxy metabolites of docosahexaenoic acid (DHA) inhibit angiogenesis, tumor growth, and metastasis. Proc Natl Acad Sci. 2013;110(16):6530–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Weylandt KH, Chiu CY, Gomolka B, Waechter SF, Wiedenmann B. Omega-3 fatty acids and their lipid mediators: Towards an understanding of resolvin and protectin formation. Prostaglandins Other Lipid Mediat. 2012;97(3–4):73–82. [DOI] [PubMed] [Google Scholar]
- 41. Fischer R, Konkel A, Mehling H, Blossey K, Gapelyuk A, Wessel N, von Schacky C, Dechend R, Muller DN, Rothe Met al. Dietary omega-3 fatty acids modulate the eicosanoid profile in man primarily via the CYP-epoxygenase pathway. J Lipid Res. 2014;55(6):1150–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Meuronen T, Lankinen MA, Fauland A, Shimizu BI, de Mello VD, Laaksonen DE, Wheelock CE, Erkkilä AT, Schwab US. Intake of camelina sativa oil and fatty fish alter the plasma lipid mediator profile in subjects with impaired glucose metabolism–A randomized controlled trial. Prostaglandins Leukot Essent Fatty Acids. 2020;159:102143. [DOI] [PubMed] [Google Scholar]
- 43. Schuchardt JP, Ostermann AI, Stork L, Fritzsch S, Kohrs H, Greupner T, Hahn A, Schebb NH. Effect of DHA supplementation on oxylipin levels in plasma and immune cell stimulated blood. Prostaglandins Leukot Essent Fatty Acids. 2017;121:76–87. [DOI] [PubMed] [Google Scholar]
- 44. Greupner T, Kutzner L, Nolte F, Strangmann A, Kohrs H, Hahn A, Schebb NH, Schuchardt JP. Effects of a 12-week high-α-linolenic acid intervention on EPA and DHA concentrations in red blood cells and plasma oxylipin pattern in subjects with a low EPA and DHA status. Food Funct. 2018;9(3):1587–600. [DOI] [PubMed] [Google Scholar]
- 45. Pauls SD, Du Y, Clair L, Winter T, Aukema HM, Taylor CG, Zahradka P. Impact of age, menopause, and obesity on oxylipins linked to vascular health. Arterioscler Thromb Vasc Biol. 2021;41(2):883–97. [DOI] [PubMed] [Google Scholar]
- 46. Ulu A, Harris TR, Morisseau C, Miyabe C, Inoue H, Schuster G, Dong H, Iosif AM, Liu JY, Weiss RHet al. Anti-inflammatory effects of ω-3 polyunsaturated fatty acids and soluble epoxide hydrolase inhibitors in angiotensin-II-dependent hypertension. J Cardiovasc Pharmacol. 2013;62(3):285–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ishihara T, Yoshida M, Arita M. Omega-3 fatty acid-derived mediators that control inflammation and tissue homeostasis. Int Immunol. 2019;31(9):559–67. [DOI] [PubMed] [Google Scholar]
- 48. Caligiuri SP, Aukema HM, Ravandi A, Pierce GN. Elevated levels of pro-inflammatory oxylipins in older subjects are normalized by flaxseed consumption. Exp Gerontol. 2014;59:51–57. [DOI] [PubMed] [Google Scholar]
- 49. Chan DC, Watts GF, Barrett PH, Beilin LJ, Mori TA. Effect of atorvastatin and fish oil on plasma high-sensitivity C-reactive protein concentrations in individuals with visceral obesity. Clin Chem. 2002;48(6):877–83. [PubMed] [Google Scholar]
- 50. Dewell A, Marvasti FF, Harris WS, Tsao P, Gardner CD. Low- and high-dose plant and marine (n-3) fatty acids do not affect plasma inflammatory markers in adults with metabolic syndrome. J Nutr. 2011;141(12):2166–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bloedon LT, Balikai S, Chittams J, Cunnane SC, Berlin JA, Rader DJ, Szapary PO. Flaxseed and cardiovascular risk factors: Results from a double blind, randomized, controlled clinical trial. J Am Coll Nutr. 2008;27(1):65–74. [DOI] [PubMed] [Google Scholar]
- 52. Egert S, Baxheinrich A, Lee-Barkey YH, Tschoepe D, Wahrburg U, Stratmann B. Effects of an energy-restricted diet rich in plant-derived α-linolenic acid on systemic inflammation and endothelial function in overweight-to-obese patients with metabolic syndrome traits. Br J Nutr. 2014;112(8):1315–22. [DOI] [PubMed] [Google Scholar]
- 53. Fletcher MA, Zeng XR, Barnes Z, Levis S, Klimas NG. Plasma cytokines in women with chronic fatigue syndrome. J Transl Med. 2009;7(1):96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sadeghi M, Daniel V, Naujokat C, Weimer R, Opelz G. Strikingly higher interleukin (IL)-1alpha, IL-1beta and soluble interleukin-1 receptor antagonist (sIL-1RA) but similar IL-2, sIL-2R, IL-3, IL-4, IL-6, sIL-6R, IL-10, tumour necrosis factor (TNF)-alpha, transforming growth factor (TGF)-beta and interferon IFN-gamma urine levels in healthy females compared to healthy males: protection against urinary tract injury?. Clin Exp Immunol. 2005;142:312–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Stowe RP, Peek MK, Cutchin MP, Goodwin JS. Plasma cytokine levels in a population-based study: Relation to age and ethnicity. J Geront A Biol Sci Med Sci. 2010;65A(4):429–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Chilton FH, Dutta R, Reynolds LM, Sergeant S, Mathias RA, Seeds MC. Precision nutrition and omega-3 polyunsaturated fatty acids: A case for personalized supplementation approaches for the prevention and management of human diseases. Nutrients. 2017;9(11):1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Choe SS, Huh JY, Hwang IJ, Kim JI, Kim JB. Adipose tissue remodeling: Its role in energy metabolism and metabolic disorders. Front Endocrinol. 2016;7:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Klöting N, Blüher M. Adipocyte dysfunction, inflammation and metabolic syndrome. Rev Endocr Metab Dis. 2014;15(4):277–87. [DOI] [PubMed] [Google Scholar]
- 59. Turer AT, Scherer PE. Adiponectin: Mechanistic insights and clinical implications. Diabetologia. 2012;55(9):2319–26. [DOI] [PubMed] [Google Scholar]
- 60. Yanai H, Yoshida H. Beneficial effects of adiponectin on glucose and lipid metabolism and atherosclerotic progression: Mechanisms and perspectives. Int J Mol Sci. 2019;20(5):1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kakino A, Fujita Y, Ke LY, Chan HC, Tsai MH, Dai CY, Chen CH, Sawamura T. Adiponectin forms a complex with atherogenic LDL and inhibits its downstream effects. J Lipid Res. 2021;62:100001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Raulien N, Friedrich K, Strobel S, Rubner S, Baumann S, von Bergen M, Körner A, Krueger M, Rossol M, Wagner U. Fatty acid oxidation compensates for lipopolysaccharide-induced Warburg effect in glucose-deprived monocytes. Front Immunol. 2017;8:609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kelly B, O'Neill LA. Metabolic reprogramming in macrophages and dendritic cells in innate immunity. Cell Res. 2015;25(7):771–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Avila C, Huang RJ, Stevens MV, Aponte AM, Tripodi D, Kim KY, Sack MN. Platelet mitochondrial dysfunction is evident in type 2 diabetes in association with modifications of mitochondrial anti-oxidant stress proteins. Exp Clin Endocrinol Diabetes. 2012;120:248–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Yu EPK, Reinhold J, Yu H, Starks L, Uryga AK, Foote K, Finigan A, Figg N, Pung YF, Logan Aet al. Mitochondrial respiration is reduced in atherosclerosis, promoting necrotic core formation and reducing relative fibrous cap thickness. Arterioscler Thromb Vasc Biol. 2017;37(12):2322–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kramer PA, Chacko BK, George DJ, Zhi D, Wei CC, Dell'Italia LJ, Melby SJ, George JF, Darley-Usmar VM. Decreased Bioenergetic Health Index in monocytes isolated from the pericardial fluid and blood of post-operative cardiac surgery patients. Biosci Rep. 2015;35(4):e00237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Lee HT, Lin CS, Pan SC, Wu TH, Lee CS, Chang DM, Tsai CY, Wei YH. Alterations of oxygen consumption and extracellular acidification rates by glutamine in PBMCs of SLE patients. Mitochondrion. 2019;44:65–74. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.