ABSTRACT
Background
Palmitic acid (PA; 16:0) is added to infant formula in the form of palm oil/palm olein (PO/POL) and stereospecific numbered–2 palmitate (SN2). Several studies have examined the effects of PO/POL and or SN2 in formulas on health outcomes, mainly growth, digestion, and absorption of nutrients. However, the roles of PA, PO/POL, and SN2 on neurodevelopment remains unknown.
Objectives
The objective of this scoping review was to map out studies in infants fed formula with PO/POL or SN2 to identify current knowledge on the role of PA in infant nutrition, specifically neurodevelopment.
Methods
Data sources, including Medline, Embase, CAB Abstracts, and the Cochrane Database, were searched. Eligible articles were randomized controlled trials (RCTs) and observational studies examining outcomes in term singleton infants fed formula containing PO/POL or SN2. Studies examining preterm infants or infants with infections, mixed-feeding interventions, or outcomes not concerned with PO/POL or SN2 were excluded. Screening and data extraction were performed by 2 independent reviewers, and results were charted into 10 outcome categories.
Results
We identified 28 RCTs and 2 observational studies. Only 1 RCT examined a neurodevelopmental outcome, reporting infants fed SN2 formula had higher fine motor skill scores compared to those fed a vegetable oil formula with a lower amount of SN2; however, only after adjustment for maternal education and at an earlier, but not a later time point. Anthropometric measures do not appear to be influenced by PO/POL or SN2 within formulas. Alternatively, it was reported that infants fed PO/POL within formulas had a decreased absorption of calcium, total fat, and PA compared to those fed vegetable oil formulas. However, studies were heterogenous, making it difficult to isolate the effects of PO/POL or SN2 in formulas.
Conclusions
Our review reiterates the need for future studies to address the effects of PO/POL and SN2 on neurodevelopment in infants. This study is registered at Open Science Framework as osf.io/697he.
Keywords: formula-fed, human milk alternative, artificial milk, synthetic triacylglycerides, specific-structured-triacylglycerides, palm-oil, palm-olein, neurodevelopment
Introduction
Palmitic acid (PA; 16:0) is a SFA consumed in the diet or synthesized endogenously via de novo lipogenesis; therefore, it is considered nonessential (1). Within peripheral tissue phospholipids (PLs), PA is typically esterified at the stereospecific numbered (sn)-1 position and is tightly regulated in balance with PUFAs esterified at the sn-2 position (2, 3). This balance has been shown to persist in brain PLs, in balance with both PUFAs and MUFAs (2), where PA represents nearly half of the total brain saturates (4, 5). Preclinical studies have presented contradictory results pertaining to PA incorporation in the brain, firstly demonstrating the delivery of labelled PA (14C, the radioactive isotope of carbon) to adult rats via 24-hour feeding (6), intravenous injection (7), and in situ brain perfusion (8, 9), results in the rapid incorporation of PA into brain tissue lipids unchanged (6–8). However, labelled PA was not detected in the brain after artificially reared rat pups were fed a rat milk substitute containing perdeuterated PA (10). The latter study concluded that the blood-brain barrier selectively restricts the entry of SFAs, including PA, to the brain, and the brain relies only on endogenous PA synthesis (10, 11). Recently, our laboratory demonstrated that brain PA is maintained equally through both endogenous synthesis and uptake and incorporation from dietary sources in the adult mouse brain (12). However, the contribution of either source of PA during neurodevelopment has yet to be explored. Interestingly, PA is also the most abundant SFA in human breast milk, providing roughly 10% of a breast-fed infant's energy intake (1). Although human, cow, mare, goat, and sheep milk all contain ∼20%–30% PA (13), human milk (HM) is unique in that PA is delivered to the infant in a unique configuration, in which ∼70% of PA is esterified at the sn-2 position of the triacylglyceride (TAG), as opposed to 40%–45% and 10%–20% of PA esterified at the sn-2 position in bovine and vegetable fat, respectively (14–16). Infant formulas are currently supplemented with PA in the form of palm oil (PO; 44% PA) or palm olein (POL; 40% PA) to mimic the levels of PA found in maternal milk; however, levels of PA appear to be variable across different formulas and are not always matched to HM based on the vegetable fat blend used (17, 18). Although this option is less costly, due to environmental concerns as well as the sn-1,3 configuration and its possible physiological effects, there is a movement to discontinue the use of PO/POL in infant formula. Furthermore, advances in enzymatic interesterification (EIE) allowed for the development of synthetic structured TAGs with PA esterified predominantly at the sn-2 position, which have been added as functional ingredients to formulas to enhance the absorption of PA, allowing for both the level and arrangement of PA in HM to be modeled in formula. However, neither the addition or removal of PO/POL, nor the addition of synthetic structured TAGs to formula, have considered the role of PA and its forms in the developing brain.
PA plays a structural and functional role in the body, which has been extensively reviewed elsewhere (1, 3). During development specifically, lipids are important for axonal myelination and maintaining membrane structures during the rapid brain growth spurt (19). Importantly, PA has been shown to flux into both gray and white matter (7) and into major phospholipid fractions, including choline and ethanolamine glycerophospholipids, as well as phosphatidylinositol and phosphatidylserine in the rat brain (2, 6, 9). However, greater attention in research has been given to PUFAs, including DHA (22:6n-3) and arachidonic acid (ARA; 20:4n-6), for their roles in neurogenesis, as well as synapse maturation during gestation and more generally for optimal brain development (20, 21). Although it is well documented that levels of PA in the infant diet can influence tissue levels of PA, such as adipose (22–24), evidence for the brain is less documented, particularly in humans. It appears PA levels in brain cortex phospholipids are stable in response to breastfeeding compared with mixed-formula feeding, as well as different types of formula feeding (25); however, the amounts of PA infants received in each feeding were not reported in this study. Furthermore, preclinical studies yield conflicting results regarding the composition of PA in the brain in response to changes in dietary PA levels (26, 27) and arrangement (sn-2 compared with sn-1,3) (28). Altogether, considering the abundance of PA in the brain and breast milk, in addition to the general importance of lipids during development and the mechanism to maintain PA levels de novo, it is plausible that PA may influence neurodevelopment. Therefore, it is prudent to investigate whether dietary PA levels—and furthermore, PA arrangement (sn-2 compared with sn-1,3)—in infant formula will influence neurodevelopmental outcomes in the current review.
In February of 2019, the European Society for Paediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) Committee on Nutrition published a position paper on PO and sn-2 palmitate in infant formula (29). The ESPGHAN suggested there is insufficient evidence regarding the role of PO and whether it should be avoided as a source of fat in infant formula, and that the inclusion of sn-2 palmitate may only have short-term beneficial effects on stool consistency and cannot be considered essential (29). In contrast, a more recent review concluded that the use of PO/POL in formula results in lower fat, DHA, PA and calcium absorption in the infant compared to the use of formulas without PO/POL (30). We decided to replicate and extend both efforts with a more extensive search of the literature and a focus on neurodevelopmental outcomes. Thus, we conducted a scoping review with the objective of further understanding the role of formulas containing PO/POL or sn-2 palmitate on neurodevelopment in term singleton infants, as well as to address any other gaps in the literature surrounding this topic.
Methods
This review followed the methodology for scoping reviews as described in Chapter 11 of the 2015 Joanna Briggs Institute Reviewer's Manual (31), consistent with the Preferred Reporting Items for Systematic Review and Meta-Analysis Extension for Scoping Reviews (32). A protocol was developed a priori based on items applicable to scoping reviews from the Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols 2015 statement (33). Given the exploratory nature of the review, parameters—including data items, outcomes, and prioritization—and data synthesis were not established until final articles were identified. For this reason, in addition to being ineligible for PROSPERO registration, the protocol was registered post hoc on the Open Science Framework (https://osf.io/697he). Articles were extracted from Medline, Embase, CAB Abstracts, and the Cochrane Database; a full search strategy for Medline is presented in Supplemental Table 1. Eligible articles were randomized controlled trials (RCTs) and observational studies examining outcomes in term singleton infants fed formula containing PO/POL or sn-2 palmitate and published in English, Italian, or French, with no limits on the year of publication to capture as much data as possible. Studies with preterm infants, mixed-feeding interventions, infected infants or mothers, and primary outcomes not evaluating the effects of PO/POL and/or sn-2 palmitate in infant formula were excluded. Data including country, sponsorship, study design, clinical trial identifier, groups, population characteristics, objective, sample size, duration of intervention, time points, formulas, outcomes, and results were extracted and charted by 2 independent reviewers (MES, GC). Results from the articles were further charted into 10 outcome categories based on overlap in outcomes reported among the studies, and those relating to the review objective. Infant formulas were coded into 4 main groups: formula containing PO, POL, vegetable oil (VO), and sn-2 palmitate (SN2). Percent differences were calculated for significant differences reported between groups in the results.
Results
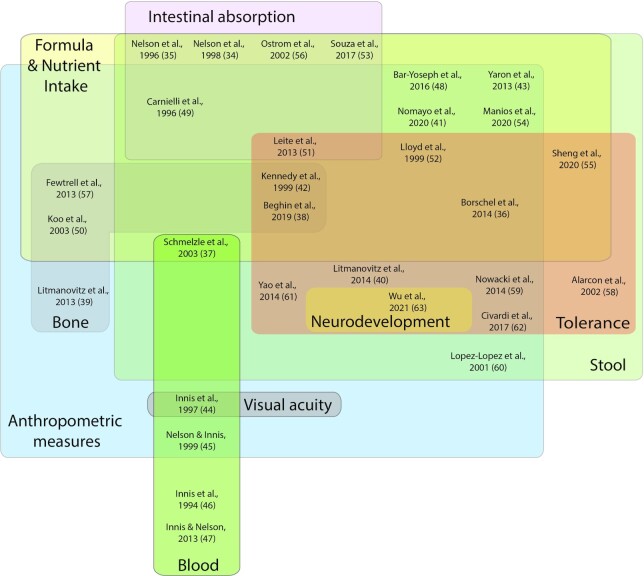
A total of 13,375 articles were screened, of which 28 RCTs and 2 observational studies met the inclusion criteria (Figure 1; Table 1). Infants included in all the studies were between birth and 192 days and were born at 37–42 weeks of gestation. Two studies included an arm of preterm infants, which were included in this review to avoid losing valuable data on term infants (34, 35). The RCT sample sizes and durations varied, ranging from 10–379 infants and from 7 days to 6 months, respectively. One observational study had 6999 infants and lasted 14 days, while the other had 409 infants and was conducted for 7 days. Outcomes were charted into 10 outcome categories, presented in Tables 2–11; visual summaries of the variability of the results and inconsistency of results reported are presented in Figures 2 and 3, respectively.
FIGURE 1.
Schematic describing the search flow of the scoping review. The search flow was adapted from the Preferred Reporting Items for Systematic Review and Meta-Analysis 2009 Statement. A total of 13,375 abstracts and 290 full-text articles were screened by 2 independent reviewers (MES, GC), while conflicts raised were resolved by a third independent reviewer (RJSL). After both stages of screening, 30 articles were included in this scoping review that met the inclusion criteria. Of the 30 articles, 3 were found through the gray literature, shown in additional records identified through other sources.
TABLE 1.
Description of included studies evaluating outcomes in infants fed formulas containing palm oil or palm olein and SN2 palmitate
| Reference, citation | Country, sponsorship | Study design,1 clinical trial identifier (if applicable) | Objective | Formula fat blend, formula code2 | Included human milk group reported to serve as a reference (Y/N) | Characteristics of eligible infants included in the study (birth demographics; age) | N, total recruited; total completed, if stated | Duration of intervention |
|---|---|---|---|---|---|---|---|---|
| Alarcon et al., 2002 (58) | 17 countries, Abbott | Multicenter, prospective, phase IV, open-label, international, observational | To test the hypothesis that the GI tolerance of a new infant formula (Similac Advance) equals or exceeds the tolerance of other milk-based formulas (Enfalac, S-26) and compare the tolerance of this new formula to human milk | Enfalac: 45% palm olein, 20% coconut oil, 20% soy oil, 15% sunflower oil (POL); S-26: Varies by country: Taiwan: oleo, coconut oil, soy oil, safflower oil; Mexico: soy oil, coconut oil (VO-1); New infant formula (Similac Advance): 42% high oleic safflower oil, 30% coconut oil, 28% soy oil (VO-2); Other infant formula: includes Enfalac and S-26; (POL/VO-1); HM + POL; HM + VO-1; HM + VO-2; HM + POL/VO-1; HM | N | 38–42 weeks gestation; ≥2500 g at birth; 28-98 days of age at enrollment | N = 7673; 6999 | 2 weeks |
| Bar-Yoseph et al., 2016 (48) | China (4 different cities), Enzymotec Ltd | Multicenter, controlled, randomized, double-blind (NCT01373541) | To assess the effects of sn-2 palmitate formulas on lipid excretion | Sn-2 palmitate formula: 43% of PA at sn-2 position; InFat, Advanced Lipids [SN23 (43%) + GO]; Control: 13% of PA at sn-2 position; vegetable oil blend [VO3 (13%) + GO] | Y | ≥37 weeks gestation; weight appropriate for gestational age; <14 days of age at inclusion | N = 171; 159 | 24 weeks |
| Beghin et al., 2019 (38) | France (private maternities), Nestlé | Multicenter, controlled, randomized, double-blind (NCT02332967) | To evaluate whether weight gain of infants fed formula enriched with sn-2 palmitate during the first 4 months of life was noninferior to that of infants receiving a control formula, and then to evaluate whether stools were softer in these enriched formulas vs. control. Growth parameters, body composition, bone mineral content, digestive tolerance, and safety were also evaluated | Control formula: 16% of PA at sn-2 position; fat blend not stated [SN2 (16%)]; Experimental formula 1: 43% of PA at sn-2 position; InFat, Advanced Lipids [SN2 (43%)]; Experimental formula 2: 51% of PA at sn-2; InFat, Advanced Lipids [SN2 (51%)] | N/A | 37–42 weeks gestation; 2500–4500 g at birth; 1–7 days of age at enrollment | N = 488; 379 | 6 months |
| Borschel et al., 2014 (36) | USA, Abbott | Controlled, randomized, blinded (NCT01380886) | To examine the growth of healthy terms infants fed a new partially hydrolyzed whey-based formula or a commercially available partially hydrolyzed whey-based formula during the first 4 months of life | Experimental formula: 41% high oleic safflower oil, 27% coconut oil, 29% soy oil, 1.5% mono- and diglycerides (VO3 + GO/HP); Control formula: 46% palm olein, 26% soy oil, 20% coconut oil, 6% high oleic safflower oil or high oleic sunflower oil (POL3 + GO/HP) | N/A | 37–42 weeks gestation; ≥2500 g at birth; 0–8 days of age at enrollment | N = 209; 177 | 119 days |
| Carnielli et al., 1996 (49) | The Netherlands, Nutricia Research | Controlled, randomized, blinded | To investigate the effect of the structural position of palmitic acid on the intestinal absorption of fat and calcium in infants | Regular formula: 13% of PA at sn-2 position; fat blend not stated [SN2 (13%)]; Formula intermediate: 39% of PA at sn-2 position; Betapol, Loders Croklaan [SN2 (39%)]; Formula beta: 66% of PA at sn-2 position; Betapol, Loders Croklaan [SN2 (66%)] | N/A | average of 39.8 ± 1.1 to 39.9 ± 1.1 weeks gestation; average of 3500 ± 400g to 3600 ± 500g at birth; average of 30.1 ± 2.2 to 34.2 ± 2.0 days of age at start of trial | N = 27 | 5 weeks |
| Civardi et al., 2017 (62) | Italy, Heinz Italia S.p.A | Controlled, randomized, double-blind (NCT01197365) | To evaluate the safety and growth ability of an infant formula enriched by functional ingredients (galacto-oligosaccharides, sn-2-palmitate, acidified milk) vs. a standard formula milk | Enriched formula: 39% of PA at sn-2 position; soya oil, structured vegetable fat [SN2 (39%) + GO]; Standard formula: vegetable oils including soy oil (VO) | N/A | 37–42 weeks gestation; birth weight between 10th and 90th percentile according to WHO growth standard; average of 11.1 ± 5.8 to 11.1 ± 6.4 days of age at start of trial | N = 117; 110 | 135 ± 5 days |
| Fewtrell et al., 2013 (57) | United Kingdom, Nutricia Research | Controlled, randomized, double-blind | To investigate whether the type of infant feeding (breast feeding and standard fat blend vs. high sn-2 fat blend) influences later bone mass (10 years) | Control formula: 12% of PA at sn-2 position; fat blend not stated [SN2 (12%)]; High sn-2 formula: 50% of PA at sn-2 position; Betapol, Loders Croklaan [SN2 (50%)]; HM | Y | ≥37 weeks gestation; birth weight > 5th percentile; average of 6.1 ± 1.9 to 70 ± 5 days of age at enrollment | N = 323; 91 | 12 weeks |
| Innis et al., 1997 (44) | Canada & United States, Mead Johnson | Multicenter, controlled, randomized | To determine the effect of infant formula containing differing amounts of LA and LNA with no ARA or DHA (1 containing palm olein, high oleic sunflower oil, coconut and soy oil vs. coconut and soy oil alone) on the blood lipid fatty acids, preferential looking acuity, and growth of healthy term gestation infants with reference to normal breast-fed infants | Formula 1: palm olein, high oleic sunflower oil, coconut oil, soy oil (POL); Formula 2: coconut oil, soy oil (VO); HM | Y | 37–41 weeks gestation; 2500–4500 g at birth; <14 days of age at enrollment | N = 238; 191 | 90 days |
| Innis et al., 1994 (46) | Canada, Mead Johnson | Controlled | To determine whether sn-2 monoacylglycerol containing palmitate is absorbed from human milk by comparing the composition of total and sn-2 position fatty acids in plasma lipids between breast-fed infants and infants fed formula containing similar amounts of palmitate to milk but predominantly esterified to the formula fat sn-1,3 positions | Formula: 4.8% of PA at sn-2 position; fat blend not stated [SN2 (4.8%)]; HM (54.2%) | N | >37 weeks gestation; appropriate weight for gestational age; enrolled by 14 days of age | N = 35 | 3 months |
| Innis & Nelson, 2013 (47) | Canada, CIHR | Controlled, randomized | To gain insight into whether unique aspects of milk TAG palmitate are likely to impact fatty acid metabolism in young infants | Structured-TAG formula: 29.1% of PA at sn-2 position; fat blend not stated [SN2 (29.1%)]; PO-formula: 5% of PA at sn-2 position; POL-based [POL (5%)]; HM | N | 37–42 weeks gestation; 2500–4500 g at birth; <72 hours old at enrollment | N = 39 | 120 days |
| Kennedy et al., 1999 (42) | United Kingdom, Nutricia Research | Controlled, randomized, double-blind | To test the hypothesis that increasing the proportion of sn-2 palmitate in formula for term infants would result in greater skeletal mineral deposition and reduced stool hardness | High sn-2 formula: 50% of PA at sn-2 position; Betapol, Loders Croklaan [SN2 (50%) +CS]; Control formula:12% of PA at sn-2 position; blend of 4 VOs [VO (12%) + CS]; HM | Y | ≥37 weeks; average of 3480 ± 390 g to 3570 ± 420 g at birth; within first 8 days of age at enrollment | N = 323 | 12 weeks |
| Koo et al., 2003 (50) | Canada & United States, Abbott | Controlled, randomized, double-blind | To determine whether POL as used in commercially available infant formulas would result in decreased bone mineralization compared to formula without POL | Milk-based formula with POL: 45% palm olein, 20% coconut oil, 20% soy oil, 15% high oleic sunflower oil (POL); Milk-based formula without POL: 40% high oleic safflower oil, 30% coconut oil, 30% soy oil (VO) | N/A | 37–42 weeks gestation; average of 3329 ± 42 to 3372 ± 42 g at birth; <2 weeks of age at enrollment | N = 128; 102 | 6 months |
| Leite et al., 2013 (51) | Brazil, Abbott | Crossover, controlled, randomized, double-blind (NCT00941564) | To assess the comparative calcium and fat metabolic balance and GI tolerance (including stool consistency) in healthy term infants fed 2 commercially available milk-based powder formulas in Brazil | Nestle NAN PRO 1: 44% palm olein, 21.7% palm kernel oil, 18.5% canola oil (POL3); Similac Advance: 41.4% high oleic sunflower oil, 29.6% coconut oil, 27.6% soy oil (VO3) | N/A | Average of 39.5 ± 1.1 to 39.8 ± 1.2 weeks gestation; 3321 ± 330 to 3333 ± 490 g at birth; 84–156 ± 3 days of age at enrollment | N = 33; 14-day tolerance phase: 32; 4-day metabolic phase: 17 | 14-day tolerance phase, 4-day metabolic phase |
| Litmanovitz et al., 2013 (39) | Israel | Longitudinal, controlled, randomized, double-blind (NCT00874068) | To assess the short-term effect of consuming high sn-2 palmitate formula with regular infant formula, comprising low beta-palmitate on bone strength of term newborns and on anthropometrics | High beta-palmitate formula: 43% of PA at sn-2 position; palm kernel oil, rapeseed oil, sunflower oil, structured palm oil; InFat, Advanced Lipids [SN23 (43%)]; Low beta-palmitate formula: 14% of PA at sn-2 position; palm kernel oil, rapeseed oil, sunflower oil, palm oil [PO3 (14%)]; HM | Y | >37 weeks gestation; appropriate for gestational age; <14 days of age at enrollment | N = 83; 66 | 12 weeks |
| Litmanovitz et al., 2014 (40) | Israel | Placebo-controlled, randomized, double-blind (NCT00874068) | To compare the effects of high sn-2 palmitate and low sn-2 palmitate formulas on infant crying patterns and stool characteristics | High beta-palmitate formula: 44% of PA at sn-2 position; InFat, Advanced Lipids [SN23 (44%)]; Regular formula (low beta-palmitate): 14% of PA at sn-2 position [PO3 (14%)]; HM | Y | Average of 39.1 ± 0.2 to 39.7 ± 0.2 weeks gestation; weight appropriate for gestational age; enrolled in first 2 weeks of life | N = 83; 63 | 12 weeks |
| Lloyd et al., 1999 (52) PI | United States, Abbott | Controlled, randomized | To compare the tolerance of 2 commercially available powder infant formulas that differ in composition | Formula A: 42% high oleic safflower oil, 30% coconut oil, 28% soy oil (VO); Formula B: 45% palm olein, 20% soy oil, 20% coconut oil, 15% high oleic sunflower oil (POL) | N/A | Average of 39.4 ± 0.2 to 39.9 ± 0.2 weeks gestation; 4–188 days of age at start of trial | N = 82; 70 | 3-day breastfeeding phase, 30 ± 5 to 32 ± 6-day weaning phase, 14-day formula feeding phase |
| Lloyd et al., 1999 (52) PII | United States, Abbott | Controlled, randomized | To compare the tolerance of 2 commercially available powder infant formulas that differ in composition | Formula A: 42% high oleic safflower oil, 30% coconut oil, 28% soy oil (VO); Formula B: 45% palm olein, 20% soy oil, 20% coconut oil, 15% high oleic sunflower oil (POL) | N/A | Average of 39.3 ± 0.2 to 39.6 ± 0.2 weeks gestation; 12–17 days of age at start of trail | N = 87; 65 | 7-day standard formula feeding phase, 2-week exclusive formula feeding phase |
| Lopez-Lopez et al., 2001 (60) | Spain, Fundacio Bosch i Gimpera & Laboratories Ordesa & CeRTA | Controlled, randomized, blinded | To investigate the fecal losses of fatty acids, calcium, and magnesium in full-term infants fed with human milk and with 2 formulas with different proportions of palmitic acid at the sn-2 position | Formula β: 44.5% of PA at sn-2 position; 80% Betapol, 10% egg phospholipids, 10% milk fat; Betapol, Loders Croklaan [SN23 (44.5%)]; Formula α: 19% of PA at sn-2 position; 60% vegetable oils, 10% egg phospholipids, 30% milk fat [VO3 (19%)]; HM | N | Average of 39.33 ± 0.74 to 39.58 ± 0.81 weeks gestation; average of 3200 ± 410 to 3360 ± 520 g at birth; enrolled at birth | N = 36 | 2 months |
| Manios et al., 2020 (54) PI | Netherlands. FrieslandCampina Nederland B.V. | Crossover, placebo-controlled, randomized, double-blind (NTR6872) | To evaluate the excretion of PA and PA soaps in stools of healthy term infants | 100% vegetable fat formula: 10.1% of PA at sn-2 position [VO3 (10.1%) + GO)]; 50% bovine milk-based formula: 39% of PA at sn-2 position; 50% bovine milk fat, 50% vegetable fat [SN23 (39%) + GO] | N/A | ≥37 weeks gestation, weight appropriate for gestational age; 9th to 14th week of age at screening | N = 17; 16 | 2 weeks |
| Manios et al., 2020 (54) PII | Netherlands. FrieslandCampina Nederland B.V. | Crossover, placebo-controlled, randomized, double-blind (NTR6872) | To evaluate the excretion of PA and PA soaps in stools of healthy term infants | 100% vegetable fat formula: 10.1% of PA at sn-2 position [VO3 (10.1%) + GO]; 20% bovine milk-based formula: 19.7% of PA at sn-2; 20% bovine milk fat, 80% vegetable fat [SN23 (19.7%) + GO] | N/A | ≥37 weeks gestation, weight appropriate for gestational age; 9th to 14th week of age at screening | N = 18; 17 | 2 weeks |
| Nelson et al., 1996 (35) | United States, Ross Laboratories | Crossover, controlled, randomized | To determine fat absorption by normal infants fed formula containing a blend of vegetable oils | Formula PO/S: 53% palm olein, 47% soy oil (POL); Formula S/C (Similac with iron): 60% soy oil, 40% coconut oil (VO) | N/A | 8 infants: ≥37 weeks gestation, 3 infants: <37 weeks gestation; 8 infants: >2500g at birth; 3 infants: 1760-2410g at birth; 8 infants: 27–137 days of age at the time of the balance studies, 3 infants: 140–161 days of age at the time of the balance studies | N = 11 (3 preterm) | ≥7-day feeding phase, 3- to 4-day metabolic balance phase |
| Nelson et al., 1998 (34) | United States, Abbott | Crossover, controlled, randomized | To test the hypothesis that the inclusion of palm olein (45% of the fat) in the fat blend of a milk-based infant formula decreases the absorption of fat and calcium | Formula PO (Enfamil): 45% palm olein, 20% soy oil, 20% coconut oil, 15% high oleic sunflower oil (POL); Formula HOS (Similac with iron): 42% high oleic safflower oil, 30% coconut oil, 28% soy oil (VO) | N/A | 39–40 weeks gestation (1 infant 37 weeks, and 1 infant 34 weeks); 3545–4850 g at birth (1 infant 2665 g and 1 infant 2975 g); 22–192 days of age at the start of the first study | N = 10 (1 preterm) | 3-day feeding phase, 3-night, 4-day metabolic balance phase |
| Nelson & Innis, 1999 (45) | Canada, Ross Laboratories | Controlled, randomized | To determine whether the position of palmitate in human milk and infant formula TAG influences the position of fatty acids in postprandial plasma chylomicron TAG | Synthesized triacylglycerol formula: 30% of PA at sn-2 position; Betapol, Loders Croklaan [SN2 (30%)]; Standard formula: 5% of PA at sn-2 position, 48% palm olein, 26% soybean oil, 14% high oleic acid sunflower oil, 12% coconut oil [POL (5%)]; HM (57%) | N | 37–42 weeks gestation; 2500–4500 g at birth; <72 hours old at enrollment | N = 87; 58 | 120 days |
| Nomayo et al., 2020 (41) | Germany, Humana GmbH/DMK Baby | Controlled, randomized, double-blind (NCT01603719) | To investigate whether the addition of prebiotic galacto-oligosaccharides in combination with the higher amounts of beta-palmitate from cow's milk fat in infant formula positively affects gut microbiota and the incidence of infections in formula-fed infants | Experimental formula: 20–25% of PA at sn-2 position; cows milk fat, vegetable oil, fish oil; bovine milk fat [SN2 (20%–25%) + GO]; Control formula: <10% PA at sn-2 position; VO (<10%); HM | Y | ≥37 weeks gestation; birth weight 10th to 90th percentile; within first 10 days of life at enrollment | N = 128; 75 | 12 weeks |
| Nowacki et al., 2014 (59) | Taiwan (4 hospitals), Nestlé | Multicenter, controlled, randomized, double-blind (NCT02031003) | To evaluate the effects of feeding infant formulas containing either high sn-2 palmitate or high sn-2 palmitate with the addition of 3 g/L of oligo-fructose compared to a control formula | Sn-2 formula: 40% of PA at sn-2 position; 60% control vegetable oil, 40% high sn-2 palmitate vegetable oil; structured vegetable fat [SN23 (40%)]; Sn-2 + OF formula: 40% of PA at sn-2 position; 60% control vegetable oil, 40% high sn-2 vegetable oil; structured vegetable oil [SN23 (40%) + FO]; Control formula: 12.6% PA at sn-2 position; 100% vegetable fat blend [VO3 (12.6%)]; HM | Y | 37–42 weeks gestation; 25–45 days of age at enrollment | N = 220; 195 | 4 weeks |
| Ostrom et al., 2002 (56) PI | United States, Abbott | Crossover, controlled, randomized, blinded | To compare fat and calcium absorption in healthy infants fed commercially available formula with primarily added calcium salts, casein hydrolysate, and soy protein, with or without PO | Nutramigen Hypoallergenic Protein Hydrolysate Formula with Iron: 45% palm olein, 20% soy oil, 20% coconut oil, 15% high oleic sunflower oil (POL + HP/CS); Alimentum Protein Hydrolysate Formula with Iron: 50% medium chain triacylglyceride oil, 40% safflower oil, 10% soy oil (VO + CS) | N/A | ≥37 weeks gestation; ≥2500 g at birth; average of 75–84 days of age at beginning of first crossover period | N = 16; 10 | 7-day feeding phase, 3-day balance phase |
| Ostrom et al., 2002 (56) PII | United States, Abbott | Crossover, controlled, randomized, blinded | To compare fat and calcium absorption in healthy infants fed commercially available formula with primarily added calcium salts, casein hydrolysate, and soy protein, with or without PO | ProSobee Soy Protein Formula with Iron: 45% palm olein, 20% soy oil, 20% coconut oil, 15% high oleic sunflower oil (POL + HP/CS); Isomil Soy Protein Formula with Iron: 42% high oleic safflower oil, 30% coconut oil, 28% soy oil (VO + CS) | N/A | ≥37 weeks gestation; ≥2500 g at birth; average of 89 days of age at beginning of first crossover period | N = 19; 13 | 7-day feeding phase, 3-day balance phase |
| Schmelzle et al., 2003 (37) | Germany, Numico | Controlled, randomized, double-blind | To evaluate the nutritional efficacy and bifidogenic characteristics of a new infant formula containing partially hydrolyzed whey protein, modified vegetable oil with a high sn-2 palmitate content, prebiotic oligosaccharides, and starch | New infant formula: 41% of PA at sn-2 position; source of sn-2 not stated [SN2 (41%) + GO/FO/HP]; Standard formula: palm oil based (PO) | N/A | 37–42 weeks gestation; weight between 10th and 9th percentiles; <2 weeks old at randomization | N = 154; 102 | 12 weeks |
| Sheng et al., 2020 (55) | China, FrieslandCampina | Cross-sectional, observational | To identify differences in stool characteristics and gut comfort parameters between 4 commercially available infant formulas in China | Milk-fat based formula: 19.9% of PA at sn-2 position; bovine milk fat and structured vegetable oils [SN2 (19.9%) + PF]; Structured vegetable fat blend formula 1: 43.5% of PA at sn-2 position; structured vegetable fat [PO (43.5%) +PF]; Palm oil-free vegetable fat blend-based formula: 10.2% of PA at sn-2 position; palm-oil free fat blend [VO (10.2%) + PF]; Structured vegetable fat blend-based formula: 40.1% of PA at sn-2 position; structured vegetable fat [PO (40.1%) + PF] | N/A | 37–42 weeks gestation; 2500–4000 g at birth; 0–4 months of age at enrollment | N = 433; 409 | 7 days |
| de Souza et al., 2017 (53) | Brazil, Abbott | Crossover, controlled, randomized, double-blind (NCT00941564) | To assess the comparative individual balance of fatty acids and associate their eventual interference with calcium absorption by normal term infants fed formulas containing distinct blends of fats | Nestle NAN PRO 1: 44% palm oil, 21.7% palm kernel oil, 18.5% canola oi (PO3); Similac Advance: 41.4% high oleic sunflower oil, 29.6% coconut oil, 27.6% soy oil (VO3) | N/A | Average of 39.5 ± 1.1 to 39.8 ± 1.2 weeks gestation; average of 3321 ± 330g to 3333 ± 490g at birth; 68-159 ± 3 days of age at enrollment | N = 33; tolerance phase: 32; metabolic balance phase: 17 | 14-day tolerance phase, 4-day metabolic phase |
| Wu et al., 2021 (63) | China, formulas provided by Inner Mongolia Yili Industrial Group Co., Ltd | Controlled, cluster randomized (ChiCTR1800014479) | To assess the hypothesized effect of increasing the amount of PA esterified in the sn-2 position in infant formula on neurodevelopment in early life, and to explore the association of this effect with an alteration in the gut bifidobacteria in infants receiving a control formula vs. an elevated level of sn-2 palmitate | Sn-2 palmitate formula: 46.3% of PA at sn-2 position; Betapol, Loders Croklaan [SN23 (46.3%) + GO]; Control formula: 10.3% of PA at sn-2 position; standard vegetable oil blend [VO3 (10.3%) + GO]; HM | Y | ≥37 weeks gestation; 2500–4500 g at birth; 7–14 days of age at enrollment | N = 199; 174 | 24 weeks |
| Yao et al., 2014 (61) | The Philippines, Wyeth Nutrition (Nestlé) | Controlled, randomized, double-blind (NCT01861600) | To evaluate the effects of a term infant formula containing high sn-2 palmitate or an identical formula containing supplemented oligo-fructose at 2 concentrations (3 or 5 g/L) on stool composition, stool characteristics, and fecal bifidobacteria | High sn-2 palmitate formula: 35.9% of PA at sn-2 position; Betapol, Loders Croklaan [SN23 (35.9%)]; High sn-2 palmitate formula supplemented with 3 g/L of oligofructose: 36.6% of PA at sn-2 position; 60% vegetable fat blend, 40% Betapol; Betapol, Loders Croklaan [SN23 (36.6%) + FO]; High sn-2 palmitate formula supplemented with 5 g/L of oligofructose: 36.9% of PA at sn-2 position; 60% vegetable fat blend, 40% Betapol; Betapol, Loders Croklaan [SN23 (36.9%) + FO]; Control: 100% vegetable oil blend [VO3 (11.7%)]; HM | Y | ≥37 weeks gestation; weight-for-age ≥fifth percentile according to Filipino reference standards; 7–14 days of age at enrollment | N = 375; 369 | 8 weeks |
| Yaron et al., 2013 (43) | Israel, Enzymotec Ltd | 2-center, controlled, randomized, double-blind (NCT01116115) | To assess whether high sn-2 palmitate content in infant formulas affects the intestinal flora | High β-palmitate formula: 44% of PA at the sn-2 position; palm kernel oil, rapeseed oil, sunflower oil, structured palm oil; InFat, Advanced Lipids [SN23 (44%)]; Low β-palmitate formula: 14% of PA at sn-2 position; palm kernel oil, rapeseed oil, sunflower oil, unmodified palm oil [PO3 (14%)]; HM | Y | ≥37 weeks gestation; appropriate for gestational age; <7 days of age at inclusion | N = 36; 30 | 6 weeks |
% Refers to the percentage of palmitic acid esterified at the sn-2 position in formula; 20:4n-6 indicates AA; 18:2n-6 indicates LA; and 18:3n-3 indicates LNA. Abbreviations: AA, arachidonic acid; ARA, arachidonic acid; CeRTA, Centre de Referència en Tecnologia dels Aliments; CF, control formula; CIHR, Canadian Institutes of Health Research; CS, calcium salts; FO, fructo-oligosaccharides; GI, gastrointestinal; GO, galacto-oligosaccharides; HM, human milk; HP, hydrolyzed protein; LA, linoleic acid; LBP, low beta-palmitate formula; LNA, linolenic acid; MF, milk-based formula; N/A, not available; NF, new infant formula; OF, other infant formula; PA, palmitic acid; PF, prebiotic fiber; PI, part one; PII, part two; PO, palm oil; POL, palm olein; sn, stereospecific numbered; SN2, stereospecific numbered–2 palmitate; TAG, triacylglyceride; VO, vegetable oil; VO-1, S-26; VO-2, Similac Advance; VF, vegetable fat.
Reported study design is based on formula groups only.
Formula code in the present review: POL, PO, VO, SN2.
Indicates the addition of long-chain PUFAs, 22:6n-3 (DHA) and 20:4n-6 (ARA) if reported by authors.
TABLE 2.
Results for anthropometric measures in included studies evaluating infants fed formulas containing palm oil or palm olein and sn-2 palmitate
| Reference | Time point(s) | Groups | Outcome |
|---|---|---|---|
| Bar-Yoseph et al., 2016 (48) | 6, 12, 24 weeks | SN21 (43%) + GOVO1 (13%) + GOHM | Body Weight: No differences between groupsLength: No differences between groupsHead Circumference: No differences between groupsBody Composition: Not reported |
| Beghin et al., 2019 (38) | 4 months | SN2 (16%)SN2 (43%)SN2 (51%) | Body Weight: No differences between groupsLength: SN2 (43%) > SN2 (16%), SN2 (51%) > SN2 (16%), no differences between SN2 (43%) and SN2 (51%)Head Circumference: No differences between groupsBody Composition: Mean mass, fat mass, ratio of fat to total body fat: No differences between groups |
| Borschel et al., 2014 (36) | T1: 14–28 daysT2: 28-56, 56-84, 84-119 days | VO1 + GO/HPPOL1 + GO/HP | Body Weight: T1: POL1 + GO/HP > VO1 + GO/HP (weight gain from 14–28 days of age); T2: No differences between groupsLength: T1; T2: No differences between groupsHead Circumference: T1; T2: No differences between groupsBody Composition: T1; T2: Not reported |
| Carnielli et al., 1996 (49) | 1 month | SN2 (13%) SN2 (39%) SN2 (66%) | Body Weight: No differences between groupsLength: No differences between groupsHead Circumference: No differences between groupsBody Composition: T1; T2: Fat mass (% of body weight): No differences between groups |
| Civardi et al., 2017 (62) | 60 ± 5 days, 135 ± 5 days | SN2 (39%) + GO VO | Body Weight: No differences between groupsLength: No differences between groupsHead Circumference: No differences between groupsBody Composition: Not reported |
| Fewtrell et al., 2013 (57) | T1: 12 weeks T2: 10 years | SN2 (12%) SN2 (50%)HM | Body Weight: T1; T2: No differences between groupsLength: T1; T2: No differences between groupsHead Circumference: T1: No differences between groups; T2: Not reportedBody Composition: Not reported |
| Innis et al., 1997 (44) | 90 days | POL VOHM | Body Weight: No differences between groupsLength: No differences between groupsHead Circumference: No differences between groupsBody Composition: Not reported |
| Kennedy et al., 1999 (42) | 3, 6, 12 weeks | SN2 (50%) + CS VO (12%) + CS HM | Body Weight: No differences between groupsLength: No differences between groupsHead Circumference: No differences between groupsBody Composition: Subscapular skinfold thickness: SN2 (50%) + CS > HM, VO (12%) + CS > HM, no differences between SN2 (50%) + CS and VO (12%) + CS; Midupper arm circumference: no differences between groups; Triceps skinfold thickness: no differences between groups |
| Koo et al., 2003 (50) | 1, 3, 6 months | POLVO | Body Weight: No differences between groupsLength: No differences between groupsHead Circumference: No differences between groupsBody Composition: Not reported |
| Leite et al., 2013 (51) | 14-day tolerance phase, 4-day metabolic phase | POL1 VO1 | Body Weight: No differences between groupsLength: No differences between groupsHead Circumference: No differences between groupsBody Composition: Not reported |
| Litmanovitz et al., 2013 (39) | T1: 6 weeks T2: 12 weeks | SN21 (43%)PO1 (14%) HM | Body Weight: T1; T2: No differences between groupsLength: T1: HM > PO1 (14%), no differences between SN21 (43%) and HM or SN21 (43%) and PO1 (14%); T2: no differences between groupsHead Circumference: T1: HM > SN21 (43%), HM > PO1 (14%), no differences between SN21 (43%) and PO1 (14%); T2: No differences between groupsBody Composition: Not reported |
| Litmanovitz et al., 2014 (40) | T1: 6 weeks T2: 12 weeks | SN21 (44%) PO1 (14%) HM | Body Weight: T1: HM > PO1 (14%), HM > SN21 (44%), no differences between SN21 (44%) and PO1 (14%); T2: No differences between groupsLength: T1: HM > SN21 (44%), HM > PO1 (14%), no differences between SN21 (44%) and PO1 (14%); T2: No differences between groupsHead Circumference: T1: HM > SN21 (44%), HM > PO1 (14%), no differences between SN21 (44%) and PO1 (14%); T2: No differences between groupsBody Composition: Not reported |
| Lloyd et al., 1999 (52) PI | 0–2 weeks | POL VO | Body Weight: No differences between groupsLength: Not reportedHead Circumference: Not reportedBody Composition: Not reported |
| Lloyd et al., 1999 (52) PII | Day 8, day 22 | POLVO | Body Weight: No differences between groupsLength: Not reportedHead Circumference: Not reportedBody Composition: Not reported |
| Lopez-Lopez et al., 2001 (60) | 1, 2 months | SN21 (44.5%) VO1 (19%)HM | Body Weight: No differences between groupsLength: No differences between groupsHead Circumference: No differences between groupsBody Composition: Not reported |
| Manios et al., 2020 (54) PI | 2 weeks | VO1 (10.1%) + GOSN21 (39%) + GO | Body Weight: No differences between groupsLength: No differences between groupsHead Circumference: Not reportedBody Composition: Not reported |
| Manios et al., 2020 (54) PII | 2 weeks | VO1 (10.1%) + GO SN21 (19.7%) + GO | Body Weight: No differences between groupsLength: No differences between groupsHead Circumference: Not reportedBody Composition: Not reported |
| Nelson & Innis, 1999 (45) | 7, 30, 60, 90, 120 days | SN2 (30%)POL (5%) HM (57%) | Body Weight: No differences between groupsLength: No differences between groupsHead Circumference: No differences between groupsBody Composition: Not reported |
| Nomayo et al., 2020 (41) | 0-12 weeks | SN2 (20%–25%) + GO VO (<10%) HM | Body Weight: No differences between groupsLength: VO (<10%) > HM, no differences between VO (<10%) and SN2 (20%–25%) + GO or SN2 (20%–25%) + GO and HMHead Circumference: No differences between groupsBody Composition: Not reported |
| Nowacki et al., 2014 (59) | 14, 28 days | SN21 (40%) SN21 (40%) + GOVO1 HM | Body Weight: No differences between groupsLength: No differences between groupsHead Circumference: No differences between groupsBody Composition: Not reported |
| Schmelzle et al., 2003 (37) | T1: 0–6 weeksT2: 0–12 weeks | SN2 (41%) + GO/FO/HPPO | Body Weight: T1: SN2 (41%) + GO/FO/HP > PO (females only); T2: No differences between groupsLength: T1; T2: No differences between groupsHead Circumference: T1: No differences between groups; T2: SN2 (41%) + GO/FO/HP > PO (females only)Body Composition: Gains in total skinfold thickness: T1: No differences between groups; T2: SN2 (41%) + GO/FO/HP > PO (males only) |
| Wu et al., 2021 (63) | 16, 24 weeks | SN21 (46.3%) + GO VO1 (10.3%) + GO | Body Weight: No differences between groupsLength: No differences between groupsHead Circumference: No differences between groupsBody Composition: Not reported |
| Yao et al., 2014 (61) | 4, 8 weeks | SN21 (35.9%)SN21 (36.6%) + FO SN21 (36.9%) + FO VO1 (11.7%) HM | Body Weight: No differences between groupsLength: No differences between groupsHead Circumference: No differences between groupsBody Composition: Not reported |
| Yaron et al., 2013 (43) | 6 weeks | SN21 (44%) PO1 (14%) HM | Body Weight: No differences between groupsLength: No differences between groupsHead Circumference: HM > SN21 (44%), no differences between SN21 (44%) and PO1 (14%)Body Composition: Not reported |
% Refers to the percentage of palmitic acid esterified at the sn-2 position in formula. Abbreviations: ARA, arachidonic acid; CS, calcium salts; FO, fructo-oligosaccharides; GO, galacto-oligosaccharides; HM, human milk; HP, hydrolyzed protein; PO, palm oil; POL, palm olein; sn, stereospecific numbered; SN2, stereospecific numbered–2 palmitate; T1, time point 1; T2, time point 2; VO, vegetable oil.
Indicates the addition of long-chain PUFAs including 22:6n-3 (DHA) and 20:4n-6 (ARA).
TABLE 11.
Results for neurodevelopment in included studies evaluating infants fed formulas containing palm oil or palm olein and sn-2 palmitate
| Ages and Stages Questionnaire, Third Edition (ASQ-3) | |||
|---|---|---|---|
| Reference | Time point(s) | Groups | Outcome |
| Wu et al., 2021 (63) | T1: 16 weeks T2: 24 weeks | SN21 (46.3%) + GOVO1 (10.3%) + GOHM | T1: 5 Developmental Domain Median Unadjusted Scores (communication, gross motor, fine motor, problem-solving, personal, and social): No differences between groups T2: 5 Developmental Domain Median Unadjusted Scores (communication, gross motor, fine motor, problem-solving, personal, and social): No differences between groups |
% Indicates the percentage of palmitic acid esterified at the sn-2 position in formula. Abbreviations: ARA, arachidonic acid; GO, galacto-oligosaccharides; HM, human milk; sn, stereospecific numbered; SN2, stereospecific numbered–2 palmitate; T1, time point 1; T2, time point 2; VO, vegetable oil.
Indicates the addition of long-chain PUFAs, including 22:6n-3 (DHA) and 20:4n-6 (ARA).
FIGURE 2.
Visual summary of the included studies reporting a significant difference in the outcomes between groups, across the 10 outcome categories. The bubble size reflects the number of studies reporting on the outcome of interest, while the value within the bubble confirms the total number of studies reporting on that outcome. Anthropometric outcomes appear to be the least influenced by any differences in formula composition, as this outcome had the most studies reporting on it, alongside low percentage differences between formula groups. GI, gastrointestinal.
FIGURE 3.
Visual summary of the variability of outcomes reported among the 30 articles evaluating outcomes in infants fed palm oil or palm olein and SN2 palmitate. Colors overlapping within the figure indicate consistency between the studies in outcomes reported. Anthropometric outcomes are reported on the most, while neurodevelopment and visual acuity are reported on the least. The outcome of excretion was combined with stool in generating this figure. SN2, stereospecific numbered–2 palmitate.
Anthropometric measures
Many studies did not report differences on anthropometric measures between any of the groups (Table 2). However, 1 study reported infants fed POL formula had a 10% higher body weight gain compared to VO formula–fed infants from 14 to 28 days of age (36). Another study reported a 15% increase in body weight in female but not male infants fed SN2 formula compared to PO formula between 0–6 weeks of age (37). Of note, 1 study reported infants fed SN2 formula with a higher percentage of PA esterified at sn-2 (43%–51%) were slightly (1%) but significantly longer in length than those fed SN2 formula with a lower percentage of PA esterified at sn-2 (16%) at 4 months of age (38). However, HM-fed infants were reported to be 3% longer in length than those fed PO formula and SN2 formula (39, 40), but not those fed VO formula (41). Two studies reported a difference in body composition; firstly, reporting infants fed SN2 and VO formula had a 5%–10% increase in subscapular skinfold thickness compared to HM-fed infants after 12 weeks of diet (42); and secondly, reporting a 27% higher total skinfold thickness in male infants consuming SN2 formula compared to male infants consuming PO formula from 0–12 weeks of age (37). Finally, 3 studies reported that infants fed SN2 formula had a 2%–3% smaller head circumference when compared to those in a HM-fed group (39, 40, 43), while 1 study reported female and not male infants fed SN2 formula had a 13% larger head circumference than infants fed PO formula (37).
Blood measures
Plasma and serum biochemistry
Two studies reported that infants fed POL formula (44, 45) and SN2 formula (45) had 19%–31% lower total plasma cholesterol levels compared to infants fed HM. While plasma TAG was 19% higher in infants fed POL formula compared to infants fed a VO formula (44), 2 studies reported 25%–46% lower apoB in infants fed POL formula compared to those fed HM (44, 45). ApoB was 30% higher in infants fed SN2 formula compared to those fed POL formula, and apoB levels were not different between infants fed HM and those fed SN2 formula (45). Of note, it was not clarified in either of the studies whether apoB-48 or apoB-100 was being measured (44, 45). Additionally, no differences were detected between infants fed SN2 formula and those fed PO formula in serum total albumin, urea, or protein; however, infants fed SN2 formula had higher serum concentrations of several amino acids, including threonine, isoleucine, and lysine, while PO formula–fed infants reported higher levels of tyrosine and prealbumin compared to infants fed the SN2 formula (37).
Fatty acids
The percentage of PA at the sn-2 position of the plasma TAG was reported to be 58%–109% higher in infants fed HM or SN2 formula with a higher percentage of PA esterified at sn-2 (29.1%–57%) than in those fed SN2 formula with a lower percentage of PA esterified at sn-2 (4.8%–5.0%) (45–47). Interestingly, it was consistently reported that levels of DHA esterified at the sn-2 position of the plasma TAG were 100%–178% higher in HM-fed infants compared to both POL and SN2 formula–fed infants (45–47). This is consistent with HM-fed infants having increased levels of plasma phospholpid DHA compared to infants fed POL or SN2 formula (44–47). Additionally, 2 studies reported infants fed VO and SN2 formulas had 19%–64% higher levels of plasma phospholipid DHA (44) and plasma unesterified DHA (47) compared to infants fed POL formula. Results for plasma levels of other fatty acids, including 16:0, 16:1, 18:0, and 18:1n-9, were more variable and can be found in Table 3.
TABLE 3.
Results for blood measures in included studies evaluating infants fed formulas containing palm oil or palm olein and sn-2 palmitate
| Reference | Time point(s) | Groups | Outcome |
|---|---|---|---|
| Plasma biochemistry | |||
| Innis et al., 1997 (44) | T1: 14 days T2: 90 days | POLVOHM | Total Cholesterol: T1; T2: HM > POL, HM > VO, no differences between POL and VOApoB: T1: HM > POL, HM > VO, no differences between POL and VO; T2: HM > POL, HM > VO, no differences between POL and VOApoA-I: T1: No differences between groups; T2: HM > VO, no differences between HM and POLTAG: T1: No differences between groups; T2: POL > VO, no differences between HM and VO or HM and POL |
| Nelson & Innis, 1999 (45) | T1: 30T2: 120 days | SN2 (30%)POL (5%)HM (57%) | Total Cholesterol: T1; T2: HM (57%) > POL (5%), HM (57%) > SN2 (30%), no differences between POL (5%) and SN2 (30%)ApoB: T1: HM (57%) > POL (5%), no differences between HM (57%) and SN2 (30%) or SN2 (30%) and POL (5%); T2: HM (57%) > POL (5%), SN2 (30%) > POL (5%), no differences between HM (57%) and SN2 (30%)ApoA-I: T1; T2: HM (57%) > SN2 (30%), POL (5%) > SN2 (30%), no differences between HM (57%) and POL (5%)TAG: T1; T2: No differences between groups |
| Serum biochemistry | |||
| Schmelzle et al., 2003 (37) | 6 weeks | SN2 (41%) + GO/FO/HPPO | Total albumin: No differences between groupsAverage prealbumin: PO > SN2 (41%) + GO/FO/HPTotal urea: No differences between groupsTotal protein: No differences between groupsTyrosine concentration: PO > SN2 (41%) + GO/FO/HPThreonine concentration: SN2 (41%) + GO/FO/HP > POIsoleucine concentration: SN2 (41%) + GO/FO/HP > POLysine concentration: SN2 (41%) + GO/FO/HP > PONote: SN2 (41%) + GO/FO/HP threonine levels were above normal for HM-fed infants |
| Plasma unesterified fatty acids | |||
| Innis & Nelson, 2013 (47) | 120 days | SN2 (29.1%)POL (5%)HM | 16:0: No differences between groups16:1: Not reported18:0: HM > POL (5%), no differences between HM and SN2 (29.1%)18:1n-9: POL (5%) > SN2 (29.1%), no differences between HM and POL (5%) or HM and SN2 (29.1%)22:6n-3: HM > SN2 (29.1%), HM > POL (5%), SN2 (29.1%) > POL (5%) |
| Cholesterol ester fatty acids | |||
| Innis et al., 1994 (46) | 3 months | SN2 (4.8%)HM (54.2%) | Plasma:16:0: HM (54.2%) > SN2 (4.8%)16:1: HM (54.2%) > SN2 (4.8%)18:0: No differences between groups18:1n-9: No differences between groups22:6n-3: HM (54.2%) > SN2 (4.8%) |
| Nelson & Innis, 1999 (45) | 120 days | SN2 (30%) POL (5%)HM (57%) | Chylomicron:16:0: No differences between groups16:1: Not reported18:0: No differences between groups18:1n-9: No differences between groups22:6n-3: HM (57%) > POL (5%), HM (57%) > SN2 (30%), no differences between SN2 (30%) and POL (5%) |
| Phospholipid fatty acids | |||
| Innis et al., 1994 (46) | 3 months | SN2 (4.8%)HM (54.2%) | Plasma:16:0: SN2 (4.8%) > HM (54.2%)16:1: No differences between groups18:0: HM (54.2%) > SN2 (4.8%)18:1n-9: No differences between groups22:6n-3: HM (54.2%) > SN2 (4.8%) |
| Innis et al., 1997 (44) | 90 days | POL VOHM | Plasma:16:0: POL > HM, POL > VO, no differences between VO and HM16:1: Not reported18:0: HM > POL, VO > HM, VO > POL18:1n-9: POL > HM, POL > VO, HM > VO22:6n-3: HM > POL, HM > VO, VO > PO |
| Innis & Nelson, 2013 (47) | 120 days | SN2 (29.1%)POL (5%)HM | LDL-PL:16:0: No differences between groups16:1: Not reported18:0: No differences between groups18:1n-9: No differences between groups22:6n-3: HM > SN2 (29.1%), HM > POL (5%), no differences between SN2 (29.1%) and POL (5%) |
| Nelson & Innis, 1999 (45) | 120 days | SN2 (30%)POL (5%)HM (57%) | Chylomicron:16:0: No differences between groups16:1: Not reported18:0: HM (57%) > SN2 (30%), HM (57%) > POL (5%), no differences between SN2 (30%) and POL (5%)18:1n-9: SN2 (30%) > HM (57%), POL (5%) > HM (57%), no differences between SN2 (30%) and POL (5%)22:6n-3: HM (57%) > SN2 (30%), HM (57%) > POL (5%), no differences between SN2 (30%) and POL (5%) |
| Phosphatidylcholine fatty acids | |||
| Innis et al., 1997 (44) | 90 days | POLVOHM | Erythrocyte:16:0: POL > VO, POL > HM, no differences between HM and VO16:1: Not reported18:0: VO > POL, VO > HM, HM > POL18:1n-9: POL > VO, HM > VO, no differences between POL and HM22:6n-3: HM > POL, HM > VO, no differences between POL and VO |
| Innis & Nelson, 2013 (47) | 120 days | SN2 (29.1%)POL (5%)HM | Plasma unesterified:16:0: No differences between groups16:1: Not reported18:0: HM > SN2 (29.1%), HM > POL (5%), no differences between SN2 (29.1%) and POL (5%)18:1n-9: No differences between groups22:6n-3: HM > SN2 (29.1%), HM > POL (5%), no differences between SN2 (29.1%) and POL (5%) |
| Erythrocyte phosphoethanolamine fatty acids | |||
| Innis et al., 1997 (44) | 90 days | POL VOHM | 16:0: VO > HM, no differences between POL and VO or POL and HM16:1: Not reported18:0: VO > POL, VO > HM, no differences between POL and HM18:1n-9: POL > HM, POL > VO, HM > VO22:6n-3: HM > POL, HM > VO, no differences between POL and VO |
| Plasma total triglyceride fatty acids | |||
| Innis et al., 1994 (46) | 3 months | SN2 (4.8%)HM (54.2%) | 16:0: No differences between groups16:1: HM (54.2%) > SN2 (4.8%)18:0: HM (54.2%) > SN2 (4.8%)18:1n-9: No differences between groups22:6n-3: No differences between groups |
| Innis & Nelson, 2013 (47) | 120 days | SN2 (29.1%)POL (5%)HM | 16:0: No differences between groups16:1: Not reported18:0: HM > SN2 (29.1%), HM > POL (5%), no differences between SN2 (29.1%) and POL (5%)18:1n-9: No differences between groups22:6n-3: HM > SN2 (29.1%), HM > POL (5%), no differences between SN2 (29.1%) and POL (5%) |
| Nelson & Innis, 1999 (45) | 120 days | SN2 (30%)POL (5%)HM (57%) | 16:0: No differences between groups16:1: Not reported18:0: HM (57%) > POL (5%), no differences between SN2 (30%) and POL (5%) or HM (57%) and SN2 (30%)18:1n-9: No differences between groups22:6n-3: HM (57%) > SN2 (30%), HM (57%) > POL (5%), no differences between SN2 (30%) and POL (5%) |
| Percentage of fatty acid at the sn-2 position in TAG | |||
| Innis et al., 1994 (46) | 3 months | SN2 (4.8%)HM (54.2%) | 16:0: HM (54.2%) > SN2 (4.8%)16:1: HM (54.2%) > SN2 (4.8%)18:0: No differences between groups18:1n-9: SN2 (4.8%) > HM (54.2%)22:6n-3: HM (54.2%) > SN2 (4.8%) |
| Innis & Nelson, 2013 (47) | 120 days | SN2 (29.1%) POL (5%)HM | 16:0: HM > SN2 (29.1%), HM > POL (5%), SN2 (29.1%) > POL (5%)16:1: Not reported18:0: No differences between groups18:1n-9: SN2 (29.1%) > HM, POL (5%) > HM, POL (5%) > SN2 (29.1%)22:6n-3: HM > SN2 (29.1%), HM > POL (5%), no differences between SN2 (29.1%) and POL (5%) |
| Nelson & Innis, 1999 (45) | 120 days | SN2 (30%)POL (5%)HM (57%) | 16:0: HM (57%) > SN2 (30%), HM (57%) > POL (5%), SN2 (30%) > POL (5%)16:1: Not reported18:0: HM (57%) > SN2 (30%), HM (57%) > POL (5%), POL > SN2 (30%)18:1n-9: POL (5%) > HM (57%), POL (5%) > SN2 (30%), no differences between HM (57%) and SN2 (30%)22:6n-3: HM (57%) > SN2 (30%), HM (57%) > POL (5%), SN2 (30%) > POL (5%) |
% Indicates the percentage of palmitic acid esterified at the sn-2 position in formula; 16:0 indicates palmitic acid; 16:1 indicates palmitoleic acid; 18:0 indicates stearic acid; 18:1n-9 indicates oleic acid; and 22:6n-3 indicates DHA. Abbreviations: FO, fructo-oligosaccharides; GO, galacto-oligosaccharides; HM, human milk; HP, hydrolyzed protein; PL, phospholipid; PO, palm oil; POL, palm olein; sn, stereospecific numbered; SN2, stereospecific numbered–2 palmitate; TAG, triacylglyceride; T1, time point 1; T2, time point 2; VO, vegetable oil.
Intake of nutrients and formula
The intakes of nutrients and infant formula are presented in Table 4. Twelve studies reported no differences in formula intakes between infants across feeding groups (34, 38, 41–43, 48–55), while 4 studies reported no differences in nutrient intakes, including total fat, PA, and calcium intakes (35, 49, 56, 57). In contrast, 2 studies reported infants fed VO formula compared to PO and POL formula had a 40%–44% higher intake of calcium (51, 53), while others reported a 90%–115% higher intake of PA in infants fed PO and POL formula compared to VO formula (34, 35, 53).
TABLE 4.
Results for the intake of nutrients and formula in included studies evaluating infants fed formulas containing palm oil or palm olein and sn-2 palmitate
| Nutrient and Formula Intake | |||
|---|---|---|---|
| Reference | Time point(s) | Groups | Outcome |
| Bar-Yoseph et al., 2016 (48) | 6, 12, 24 weeks | SN21 (43%) + GOVO1 (13%) + GOHM | Total fat: Not reported16:0: Not reportedCa+2: Not reportedFormula: No differences between groups22:6n-3: Not reported |
| Beghin et al., 2019 (38) | 2 weeks, 1, 2, 3, 4 months | SN2 (16%)SN2 (43%)SN2 (51%) | Total fat: Not reported16:0: Not reportedCa+2: Not reportedFormula: No differences between groups22:6n-3: Not reported |
| Borschel et al., 2014 (36) | T1: 1-14, 15-28, 1-28 days, T2: 56, 84, 119 days | VO1 + GO/HPPOL1 + GO/HP | Total fat: Not reported16:0: Not reportedCa+2: Not reportedFormula: T1: POL1 + GO/HP > VO1 + GO/HP; T2: No differences between groups22:6n-3: Not reported |
| Carnielli et al., 1996 (49) | ∼4 weeks | SN2 (13%)SN2 (39%)SN2 (66%) | Total fat: No differences between groups16:0: Not reportedCa+2: No differences between groupsFormula: No differences between groups22:6n-3: Not reported |
| Fewtrell et al., 2013 (57) | 10 years | SN2 (12%)SN2 (50%)HM | Total fat: Not reported16:0: Not reportedCa+2: No differences between groupsFormula: Not reported22:6n-3: Not reported |
| Kennedy et al., 1999 (42) | T1: 3,12 weeks T2: 6 weeks | SN2 (50%) + CSVO (12%) + CSHM | Total fat: Not reported16:0: Not reportedCa+2: Not reportedFormula: T1: VO (12%) + CS > SN2 (50%) + CS; T2: No differences between groups22:6n-3: Not reported |
| Koo et al., 2003 (50) | 3, 4, 8, 12, 16, 21, 26 weeks | POLVO | Total fat: Not reported16:0: Not reportedCa+2: Not reportedFormula: No differences between groups22:6n-3: Not reported |
| Leite et al., 2013 (51) | T1: metabolic crossover phase, T2: 14-day tolerance phase, 4-day metabolic phase | POL1 VO1 | Total fat: No differences between groups16:0: Not reportedCa+2: T1: VO1 > POL1Formula: T2: No differences between groups22:6n-3: Not reported |
| Lloyd et al., 1999 (52) PI | 0–2 weeks | POL VO | Total fat: Not reported16:0: Not reportedCa+2: Not reportedFormula: No differences between groups22:6n-3: Not reported |
| Lloyd et al., 1999 (52) PII | 0–2 weeks | POLVO | Total fat: Not reported16:0: Not reportedCa+2: Not reportedFormula: No differences between groups22:6n-3: Not reported |
| Manios et al., 2020 (54) PI | 2 weeks | VO1 (10.1%) + GO SN21 (39%) + GO | Total fat: Not reported16:0: Not reportedCa+2: Not reportedFormula: No differences between groups22:6n-3: Not reported |
| Manios et al., 2020 (54) PII | 2 weeks | VO1 (10.1%) + GOSN21 (19.7%) + GO | Total fat: Not reported16:0: Not reportedCa+2: Not reportedFormula: No differences between groups22:6n-3: Not reported |
| Nelson et al., 1996 (35) | 3- to 4-day metabolic balance phase | POLVO | Total fat: No differences between groups16:0: POL > VOCa+2: No differences between groupsFormula: Not reported22:6n-3: Not reported |
| Nelson et al., 1998 (34) | 3-night, 4-day metabolic balance phase | POLVO | Total fat: No differences between groups16:0: POL > VOCa+2: No differences between groupsFormula: No differences between groups22:6n-3: Not reported |
| Nomayo et al., 2020 (41) | 6, 12 weeks | SN2 (20%–25%) + GOVO (<10%) HM | Total fat: Not reported16:0: Not reportedCa+2: Not reportedFormula: No differences between groups22:6n-3: Not reported |
| Ostrom et al., 2002 (56) PI | 3-day balance phase | POL + HP/CSVO + CS | Total fat: No differences between groups16:0: Not reportedCa+2: No differences between groupsFormula: Not reported22:6n-3: Not reported |
| Ostrom et al., 2002 (56) PII | 3-day balance phase | POL + HP/CSVO + CS | Total fat: No differences between groups16:0: Not reportedCa+2: No differences between groupsFormula: Not reported22:6n-3: Not reported |
| de Souza et al., 2017 (53) | 4-day metabolic phase | PO1VO1 | Total fat: No differences between groups16:0: PO1 > VO1Ca+2: VO1 > PO1Formula: No differences between groups22:6n-3: PO1 > VO1 |
| Schmelzle et al., 2003 (37) | 6, 10 weeks | SN2 (41%) + GO/FO/HPPO | Total fat: Not reported16:0: Not reportedCa+2: Not reportedFormula: PO > SN2 (41%) + GO/FO/HP22:6n-3: Not reported |
| Sheng et al., 2020 (55) | 1–7 days | SN2 (19.9%) + PFPO (43.5%) + PFPO (40.1%) + PFVO (10.2%) + PF | Total fat: Not reported16:0: Not reportedCa+2: Not reportedFormula: No differences between groups22:6n-3: Not reported |
| Yaron et al., 2013 (43) | 6 weeks | SN21 (44%)PO1 (14%)HM | Total fat: Not reported16:0: Not reportedCa+2: Not reportedFormula: No differences between groups22:6n-3: Not reported |
% Indicates the percentage of palmitic acid esterified at the sn-2 position in formula; 16:0 indicates palmitic acid; and 22:6n-3 indicates DHA. Abbreviations: ARA, arachidonic acid; Ca+2, calcium; CS, calcium salts; FO, fructo-oligosaccharides; GO, galacto-oligosaccharides; HM, human milk; HP, hydrolyzed protein; PF, prebiotic fibers; PO, palm oil; POL, palm olein; sn, stereospecific numbered; SN2, stereospecific numbered–2 palmitate; T1, time point 1; T2, time point 2; VO, vegetable oil.
Indicates the addition of long-chain PUFAs including 22:6n-3 (DHA) and 20:4n-6 (ARA).
Stool analysis
Consistency and volume
Heterogeneous results related to stool consistency and stool volume were reported (Table 5). Indeed, while 4 studies reported that POL formula–fed infants had firmer stools than VO formula–fed infants (36, 51, 52, 58), 1 of these 4 studies reported no differences at a later time point (36). Two studies reported infants fed formulas with a lower percentage of PA esterified at sn-2 (13%–16%) had firmer stools than those fed SN2 formulas with a higher percentage of PA esterified at sn-2 (39%–66%) (38, 49), as well as 33%–73% higher stool volume (49). Finally, infants fed formulas containing SN2 (40, 42), PO/POL (40, 58), and VO (58, 42) had firmer stools than infants fed HM, respectively. HM-fed infants also had 86%–106% higher stool volume compared to SN2 and VO formula–fed infants (42).
TABLE 5.
Results for stool analysis in included studies evaluating infants fed Formulas Containing Palm Oil or Palm Olein and sn-2 palmitate
| Reference | Time point(s) | Groups | Outcome |
|---|---|---|---|
| Consistency (degree of firmness) | |||
| Alarcon et al., 2002 (58) | Day 0–14 | POLVO-1VO-2POL/VO-1 HM + POLHM + VO-1HM + VO-2HM + POL/VO-1HM | POL/VO-1 > HM VO-2 > HMPOL/VO-1 > VO-2 POL > VO-2 VO-1 > VO-2 |
| Beghin et al., 2019 (38) | T1: 2 weeks, 1, 2 months T2: 3, 4 months | SN2 (16%)SN2 (43%)SN2 (51%) | T1: SN2 (16%) > SN2 (51%), SN2 (16%) > SN2 (43%), No differences between SN2 (43%) and SN2 (51%) T2: No differences between groups |
| Borschel et al., 2014 (36) | T1: 1-14, 15-28, 1-28, 56, 84 days, T2: 119 days | VO1 + GO/HPPOL1 + GO/HP | T1: POL1 + GO/HP > VO1 + GO/HPT2: No differences between groups |
| Carnielli et al., 1996 (49) | ∼4 weeks | SN2 (13%)SN2 (39%)SN2 (66%) | SN2 (13%) > SN2 (39%)SN2 (13%) > SN2 (66%)SN2 (39%) > SN2 (66%) |
| Civardi et al., 2017 (62) | 60 ± 5 days, 135 ± 5 days | SN2 (39%) + GOVO | No differences between groups |
| Kennedy et al., 1999 (42) | 6, 12 weeks | SN2 (50%) + CS VO (12%) + CSHM | VO (12%) + CS > SN2 (50%) + CS VO (12%) + CS > HM SN2 (50%) + CS > HM |
| Leite et al., 2013 (51) | T1: 14-day tolerance phase T2: 4-day metabolic phase | POL1VO1 | T1: No differences between groups T2: POL1 > VO1 |
| Litmanovitz et al., 2014 (40) | 6, 12 weeks | SN21 (44%)PO1 (14%)HM | SN21 (44%) > HMPO1 (14%) > HM |
| Lloyd et al., 1999 (52) PI | 0–2 weeks | POLVO | POL > VO |
| Lloyd et al., 1999 (52) PII | 0–2 weeks | POLVO | POL > VO |
| Manios et al., 2020 (54) PI | 2 weeks | VO1 (10.1%) + GOSN21 (39%) + GO | VO1 (10.1%) + GO > SN21 (39%) + GO |
| Manios et al., 2020 (54) PII | 2 weeks | VO1 (10.1%) + GOSN21 (19.7%) + GO | No differences between groups |
| Nowacki et al., 2014 (59) | 14, 28 days | SN21 (40%)SN21 (40%) + FO VO1HM | VO1 > HM, SN21 (40%) > HM SN21 (40%) + FO > HM SN21 (40%) > SN21 (40%) + FO VO1 > SN21 (40%) + FO, No differences between SN21 (40%) and VO1 |
| Schmelzle et al., 2003 (37) | 6, 10 weeks | SN2 (41%) + GO/FO/HPPO | PO > SN2 (41%) + GO/FO/HP |
| Sheng et al., 2020 (55) | 1–7 days | SN2 (19.9%) + PFPO (43.5%) + PFPO (40.1%) + PF VO (10.2%) + PF | PO (43.5%) + PF > SN2 (19.9%) + PF, PO (40.1%) + PF > SN2 (19.9%) + PF, VO (10.2%) + PF > SN2 (19.9%) + PF, No differences between PO (43.5%) + PF, PO (40.1%) + PF, and VO (10.2%) + PF |
| Yao et al., 2014 (61) | 8 weeks | SN21 (35.9%)SN21 (36.6%) + FO (3 g/L) SN21 (36.9%) + FO (5 g/L) VO1 (11.7%)HM | VO1 (11.7%) > SN21 (35.9%) VO1 (11.7%) > SN21 (36.6%) + FO (3 g/L)VO1 (11.7%) > SN21 (36.9%) + FO (5g/L) HM > SN21 (35.9%) HM > SN21 (36.6%) + FO (3 g/L) No differences between HM and SN21 (36.9%) + FO (5 g/L) HM > VO1 (11.7%) |
| Volume | |||
| Carnielli et al., 1996 (49) | ∼4 weeks | SN2 (13%)SN2 (39%)SN2 (66%) | SN2 (13%) > SN2 (66%),SN2 (13%) > SN2 (39%) No differences between SN2 (39%) and SN2 (66%) |
| Kennedy et al., 1999 (42) | 6, 12 weeks | SN2 (50%) + CSVO (12%) + CSHM | HM > SN2 (50%) + CS HM > VO (12%) + CS No differences between SN2 (50%) + CS and VO (12%) + CS |
| Sheng et al., 2020 (55) | 1–7 days | SN2 (19.9%) + PFPO (43.5%) + PFPO (40.1%) + PFVO (10.2%) + PF | No differences between groups |
| Frequency | |||
| Alarcon et al., 2002 (58) | Day 0–14 | POLVO-1VO-2POL/VO-1HM + POLHM + VO-1HM + VO-2HM + POL/VO-1HM | HM > POL/VO-1HM > VO-2VO-2 > POL/VO-1 VO-2 > POLVO-2 > VO-1 |
| Borschel et al., 2014 (36) | 1-14, 15-28, 1-28, 56, 84, 119 days | VO1 + GO/HPPOL1 + GO/HP | No differences between groups |
| Kennedy et al., 1999 (42) | 6, 12 weeks | SN2 (50%) + CSVO (12%) + CSHM | No differences between groups |
| Leite et al., 2013 (51) | T1: 14-day tolerance phase T2: 4-day metabolic phase | POL1VO1 | T1: No differences between groups T2: POL1 > VO1 |
| Litmanovitz et al., 2014 (40) | 6, 12 weeks | SN21 (44%)PO1 (14%)HM | HM > SN21 (44%) HM > PO1 (14%) No differences between SN21 (44%) and PO1 (14%) |
| Lloyd et al., 1999 (52) PI | 0–2 weeks | POLVO | VO > POL |
| Lloyd et al., 1999 (52) PII | 0–2 weeks | POLVO | No differences between groups |
| Nowacki et al., 2014 (59) | 14, 28 days | SN21 (40%)SN21 (40%) + FOVO1HM | HM > SN21 (40%) HM > SN21 (40%) + FOHM > VO1 No differences between SN21 (40%)SN21 (40%) + FO and VO1 |
| Schmelzle et al., 2003 (37) | 6, 10 weeks | SN2 (41%) + GO/FO/HPPO | No differences between groups |
| Sheng et al., 2020 (55) | 1–7 days | SN2 (19.9%)PO (43.5%) + PFPO (40.1%) + PFVO (10.2%) + PF | No differences between groups |
% Of palmitic acid esterified at sn-2 position in formula. Abbreviations: ARA, arachidonic acid; CS, calcium salts; FO, fructo-oligosaccharides; GO, galacto-oligosaccharides; HM, human milk; HP, hydrolyzed protein; PF, prebiotic fibers; PO, palm oil; POL, palm olein; sn, stereospecific numbered; SN2, stereospecific numbered–2 palmitate; T1, time point 1; T2, time point 2; VO, vegetable oil; VO-1, S-26; VO-2, Similac Advance.
Indicates the addition of long-chain PUFAs, including 22:6n-3 (DHA) and 20:4n-6 (ARA).
Frequency
Five studies compared infants fed POL formula to those fed VO formula; of these, 2 studies reported infants fed VO formula had a 22%–43% higher frequency of stool than infants fed POL formula (52, 58), while 3 studies reported no differences between the groups (36, 52, 55). Infants fed SN2 formula did not show differences in stool frequency compared to infants fed PO or VO formulas (37, 59) or infants fed SN2 or PO formulas containing a different percentage of PA esterified at sn-2 (14%–44%) (40). Finally, of the 4 studies that included a HM-fed infant group, 3 studies reported infants fed HM had 35%–100% more stool frequency than infants fed a PO or POL formula, VO formula, or SN2 formula (40, 58, 59).
Excretion
The outcomes of excretion included stool composition, stool fatty acid soaps, and bacterial composition of stool (Table 6).
TABLE 6.
Results for excretion in included studies evaluating infants fed formulas containing palm oil or palm olein and sn-2 palmitate
| Reference | Time point(s) | Groups | Outcome |
|---|---|---|---|
| Stool composition: Non-soap | |||
| Bar-Yoseph et al., 2016 (48) | 6 weeks | SN21 (43%) + GOVO1 (13%) + GOHM | Fecal Ca+2: Not reportedTotal fat: Not reportedTotal Fatty Acids: VO1 (13%) + GO > SN21 (43%) + GO, VO1 (13%) + GO > HM, SN21 (43%) + GO > HM16:0: VO1 (13%) + GO > SN21 (43%) + GO, VO1 (13%) > HM, SN21 (43%) + GO > HM22:6n-3: Not reported |
| Carnielli et al., 1996 (49) | ∼4 weeks | SN2 (13%)SN2 (39%)SN2 (66%) | Fecal Ca+2: SN2 (39%) > SN2 (66%), SN2 (13%) > SN2 (66%), no differences between SN2 (19.9%) and SN2 (39%)Total fat: SN2 (39%) > SN2 (66%), SN2 (13%) > SN2 (66%), no differences between SN2 (13%) and SN2 (39%)Total Fatty Acids: Not reported16:0: Not reported22:6n-3: Not reported |
| Kennedy et al., 1999 (42) | 6 weeks | SN2 (50%) + CSVO (12%) + CSHM | Fecal Ca+2: Not reportedTotal fat: Not reportedTotal Fatty Acids: No differences between groups16:0: No differences between groups22:6n-3: Not reported |
| Leite et al., 2013 (51) | 4-day metabolic phase | POL1VO1 | Fecal Ca+2: No differences between groupsTotal fat: POL1> VO1Total Fatty Acids: Not reported16:0: Not reported22:6n-3: Not reported |
| Lopez-Lopez et al., 2001 (60) | T1: 1 month T2: 2 months | SN21 (44.5%)VO1 (19%)HM | Fecal Ca+2: T1; T2 : VO1 (19%) > HM, SN21 (44.5%) > HM, no differences between VO1 (19%) and SN21 (44.5%)Total fat: Not reportedTotal Fatty Acids: T1: VO1 (19%) > HM, SN21 (44.5%) > HM, no differences between VO1 (19%) and SN21 (44.5%); T2: VO1 (19%) > HM, SN21 (44.5%) > HM, VO1 (19%) > SN21 (44.5%)16:0: T1: VO1 (19%) > HM, SN21 (44.5%) > HM, no differences between VO1 (19%) and SN21 (44.5%); T2: VO1 (19%) > HM, SN21 (44.5%) > HM, VO1 (19%) > SN21 (44.5%)22:6n-3: No differences between groups |
| Manios et al., 2020 (54) PI | 2 weeks | VO1 (10.1%) + GOSN21 (39%) + GO | Fecal Ca+2: VO1 (10.1%) + GO > SN21 (39%) + GOTotal fat: Not reportedTotal Fatty Acids: No differences between groups16:0: No differences between groups22:6n-3: Not reported |
| Manios et al., 2020 (54) PII | 2 weeks | VO1 (10.1%) + GOSN21 (19.7%) + GO | Fecal Ca+2: VO1 (10.1%) + GO > SN21 (19.7%) + GOTotal fat: Not reportedTotal Fatty Acids: No differences between groups16:0: No differences between groups22:6n-3: Not reported |
| Nelson et al., 1996 (35) | 3- to 4-day metabolic balance phase | POLVO | Fecal Ca+2: POL > VOTotal fat: POL > VOTotal Fatty Acids: Not reported16:0: POL > VO22:6n-3: Not reported |
| Nelson et al., 1998 (34) | 3-night, 4-day metabolic balance phase | POLVO | Fecal Ca+2: POL > VOTotal fat: POL > VOTotal Fatty Acids: Not reported16:0: POL > VO22:6n-3: Not reported |
| Nowacki et al., 2014 (59) | 28 days | SN21 (40%)SN21 (40%) + FOVO1HM | Fecal Ca+2: VO1 > SN21 (40%) + FO, SN21 (40%) > SN21 (40%) + FO, VO1 > HM, SN21 (40%) > HM, SN21 (40%) + FO > HM, no differences between VO1 and SN21 (40%)Total fat: Not reportedTotal Fatty Acids: Not reported16:0: Not reported22:6n-3: Not reported |
| Ostrom et al., 2002 (56) PI | 3-day balance phase | POL + HP/CSVO + CS | Fecal Ca+2: POL + HP/CS > VO + CSTotal fat: POL + HP/CS > VO + CSTotal Fatty Acids: Not reported16:0: Not reported22:6n-3: Not reported |
| Ostrom et al., 2002 (56) PII | 3-day balance phase | POL + HP/CSVO + CS | Fecal Ca+2: POL + HP/CS > VO + CSTotal fat: No differences between groupsTotal Fatty Acids: Not reported16:0: Not reported22:6n-3: Not reported |
| Sheng et al., 2020 (55) | 5, 6, 7 days | SN2 (19.9%) + PFPO (43.5%) + PFPO (40.1%) + PFVO (10.2%) + PF | Fecal Ca+2: No differences between groupsTotal fat: Not reportedTotal fatty acids: No differences between groups16:0: Not reported22:6n-3: Not reported |
| de Souza et al., 2017 (53) | 4-day metabolic phase | PO1VO1 | Fecal Ca+2: No differences between groupsTotal fat: PO1 > VO1Total fatty acids: PO1 > VO116:0: PO1 > VO1 22:6n-3: PO1 > VO1 |
| Stool content: fatty acid soaps | |||
| Bar-Yoseph et al., 2016 (48) | 6 weeks | SN21 (43%) + GOVO1 (13%) + GOHM | Fatty Acids: VO1 (13%) + GO > HM, SN21 (43%) + GO > HM, VO1 (13%) + GO > SN21 (43%) + GO16:0: VO1 (13%) + GO > SN21 (43%) + GO, SN21 (43%) + GO > HM, VO1 (13%) + GO > HM |
| Kennedy et al., 1999 (42) | 6 weeks | SN2 (50%) + CSVO (12%) + CSHM | Fatty Acids: VO (12%) + CS > SN2 (50%) + CS, SN2 (50%) + CS > HM, VO (12%) + CS > HM16:0: VO (12%) + CS > SN2 (50%) + CS, SN2 (50%) + CS > HM, VO (12%) + CS > HM |
| Manios et al., 2020 (54) PI | 2 weeks | VO1 (10.1%) + GOSN21 (39%) + GO | Fatty Acids: VO1 (10.1%) + GO > SN21 (39%) + GO16:0: VO1 (10.1%) + GO > SN21 (39%) + GO |
| Manios et al., 2020 (54) PII | 2 weeks | VO1 (10.1%) + GOSN21 (19.7%) + GO | Fatty Acids: VO1 (10.1%) + GO > SN21 (19.7%) + GO16:0: VO1 (10.1%) + GO > SN21 (19.7%) + GO |
| Nowacki et al., 2014 (59) | 28 days | SN21 (40%)SN21 (40%) + FOVO1HM | Fatty Acids: SN21 (40%) > SN21 (40%) + FO, VO1 > SN21 (40%) + FO, VO1 > HM, SN21 (40%) + FO > HM, SN21 (40%) > HM, no differences between SN21 (40%) and VO116:0: VO1 > SN21 (40%) + FO, VO1 > SN21 (40%), SN21 (40%) > SN21 (40%) + FO, SN21 (40%) + FO > HM, SN21 (40%) > HM, VO1 > HM |
| Sheng et al., 2020 (55) | 5, 6, 7 days | SN2 (19.9%) + PFPO (43.5%) + PFPO (40.1%) + PFVO (10.2%) + PF | Fatty Acids: VO (10.2%) + PF > SN2 (19.9%) + PF, no differences between SN2 (19.9%) + PF and PO (43.5%) + PF, PO (40.1%) + PF16:0: Not reported |
| Yao et al., 2014 (61) | 8 weeks | SN21 (35.9%)SN21 (36.6%) + FO (3 g/L)SN21 (36.9%) + FO (5 g/L)VO1 (11.7%)HM | Fatty Acids: VO1 (11.7%) > SN21 (35.9%), VO1 (11.7%) > SN21 (36.6%) + FO (3 g/L), VO1 (11.7%) > SN21 (36.9%) + FO (5 g/L), HM > SN21 (35.9%), HM > SN21 (36.9%) + FO (5 g/L), no differences between HM and VO1 (11.7%), SN21 (36.6%) + FO (3 g/L)16:0: VO1 (11.7%) > SN21 (35.9%), VO1 (11.7%) > SN21 (36.6%) + FO (3 g/L), VO1 (11.7%) > SN21 (36.9%) + FO (5 g/L), VO1 (11.7%) > HM, no differences between HM and SN21 (35.9%), SN21 (36.6%) + FO (3 g/L), SN21 (36.9%) + FO (5 g/L) |
| Bacterial composition | |||
| Civardi et al., 2017 (62) | 0 vs. 135 ± 5 day increase | SN2 (39%) + GOVO | Clostridium: No differences between groupsBifidobacteria: SN2 (39%) + GO > VOLactobacillus: Not reported |
| Nomayo et al., 2020 (41) | 12 weeks | SN2 (20%–25%) + GOVO (<10%)HM | Clostridium: Not reportedBifidobacteria: SN2 (20%–25%) + GO > VO (<10%), HM > VO (<10%), no differences between SN2 (20%–25%) + GO and HMLactobacillus: Not reported |
| Schmelzle et al., 2003 (37) | 0- to 6-week increase | SN2 (41%) + GO/FO/HPPO | Clostridium: Not reportedBifidobacteria: SN2 (41%) + GO/FO/HP > POLactobacillus: Not reported |
| Wu et al., 2021 (63) | T1: 16 weeksT2: 24 weeks | SN21 (46.3%) + GOVO1 (10.3%) + GOHM | Clostridium: Not reportedBifidobacteria: T1; T2: SN21 (46.3%) + GO > VO1 (10.3%) + GO, HM > VO1 (10.3%) + GO, no differences between SN21 (46.3%) + GO and HMLactobacillus: Not reported |
| Yao et al., 2014 (61) | 8 weeks, 0- to 8-week increase | SN21 (35.9%)SN21 (36.6%) + FOSN21 (36.9%) + FOVO1 (11.7%)HM | Clostridium: Not reportedBifidobacteria: SN21 (35.9%) > VO1 (11.7%), SN21 (36.6%) + FO (3 g/L) > VO1 (11.7%), SN21 (36.9%) + FO (5 g/L) > VO1 (11.7%), HM > VO1 (11.7%), no differences between SN21 (35.9%), SN21 (36.6%) + FO (3 g/L), SN21 (36.9%) + FO (5 g/L) and HMLactobacillus: Not reported |
| Yaron et al., 2013 (43) | 6 weeks | SN21 (44%)PO1 (14%)HM | Clostridium: Not reportedBifidobacteria: SN21 (44%) > PO1 (14%), HM > PO1 (14%), SN21 (44%) > HMLactobacillus: SN21 (44%) > PO1 (14%), HM > PO1 (14%), no differences between SN21 (44%) and HM |
| Urinary calcium excretion | |||
| Carnielli et al., 1996 (49) | ∼4 weeks | SN2 (13%)SN2 (39%)SN2 (66%) | No differences between groups |
| Leite et al., 2013 (51) | metabolic crossover phase | POL1VO1 | No differences between groups |
| Nelson et al., 1996 (35) | 3- to 4-day metabolic balance phase | POLVO | No differences between groups |
| de Souza et al., 2017 (53) | 4-day metabolic phase | PO1VO1 | No differences between groups |
% Indicates the percentage of palmitic acid esterified at the sn-2 position in formula; 16:0 indicates palmitic acid; and 22:6n-3 indicates DHA. Abbreviations: ARA, arachidonic acid; Ca+2, calcium; CS, calcium salts; FO, fructo-oligosaccharides; GO, galacto-oligosaccharides; HM, human milk; HP, hydrolyzed protein; PF, prebiotic fibers; PO, palm oil; POL, palm olein; sn, stereospecific numbered; SN2, stereospecific numbered–2 palmitate; T1, time point 1; T2, time point 2; VO, vegetable oil.
Indicates the addition of long-chain PUFAs including 22:6n-3 (DHA) and 20:4n-6 (ARA).
Nutrient excretion
Three studies reported infants fed POL formulas had 22%–59% greater total calcium excretion (34, 35, 56) than infants fed VO formulas. It was also reported that infants fed PO or POL formula had 44%–144% greater total fat excretion (34, 35, 51, 53) than infants fed VO formula. Additionally, 2 studies reported that stool from infants fed VO or SN2 formulas had 39%–119% higher total fatty acid stool levels, as well as 74%–150% higher PA stool levels, than HM-fed infants; however, VO formula–fed infants had 30%–46% greater fecal PA than infants fed SN2 formula (48, 60). Lastly, only 2 studies reported on fecal DHA excretion; the first reported that infants fed PO formula had 151% greater DHA excretion than infants fed VO formula (53), while the other reported no differences between SN2 and VO formula–fed infants (60).
Soap content
HM-fed infants had 34%–125% less total soap fatty acids (42, 48, 59) and 40%–151% less soap PA content (42, 48, 59, 61) than infants fed formulas containing SN2 and VO. Differences were reported among formula groups showing that stool from infants fed SN2 formula had 8%–66% less total soap PA than infants fed VO formulas, as well as 5%–60% less total fatty acid soap (48, 54, 61).
Bacterial composition
The most reported bacterial component of infant stool was bifidobacteria (37, 41, 43, 61–63). Infants fed SN2 formula had bifidobacteria levels similar to those fed HM (41, 61, 63), with higher bifidobacteria levels than stools from infants fed VO formula (41, 61, 62) and PO formula (43).
Intestinal absorption and retention of nutrients
Absorption and retention
Studies consistently reported that intestinal absorption, calculated by subtracting nutrient intakes from fecal and sometimes urinary excretion, were 33%–51% greater for calcium (34, 35, 51, 53, 56), 1%–9% greater for total fat (34, 35, 51, 53, 56), and 8%–26% greater for PA (34, 35) in VO formula–fed infants than PO or POL formula–fed infants (Table 7) (34, 35, 51, 53, 56). It was also consistently reported that infants fed VO formula had 43%–75% higher calcium retention than infants fed PO or POL formula (35, 51, 53).
TABLE 7.
Results for intestinal absorption and retention of nutrients in included studies evaluating infants fed formulas containing palm oil or palm olein and sn-2 palmitate
| Reference | Time point(s) | Groups | Outcome |
|---|---|---|---|
| Intestinal absorption | |||
| Carnielli et al., 1996 (49) | ∼4 weeks | SN2 (13%)SN2 (39%)SN2 (66%) | Ca+2: SN2 (66%) > SN2 (39%), SN2 (66%) > SN2 (13%), no differences between SN2 (39%) and SN2 (13%)Total fat: SN2 (66%) > SN2 (39%), SN2 (66%) > SN2 (13%), no differences between SN2 (39%) and SN2 (13%)16:0: SN2 (66%) > SN2 (39%) > SN2 (13%) |
| Leite et al., 2013 (51) | metabolic crossover phase | POL1VO1 | Ca+2: VO1 > POL1Total fat: VO1 > POL116:0: Not reported |
| Nelson et al., 1996 (35) | 3- to 4-day metabolic balance phase | POLVO | Ca+2: VO > POLTotal fat: VO > POL16:0: VO > POL |
| Nelson et al., 1998 (34) | 3-night, 4-day metabolic balance phase | POLVO | Ca+2: VO > POLTotal fat: VO > POL16:0: VO > POL |
| Ostrom et al., 2002 (56) PI | 3-day balance phase | POL + HP/CSVO + CS | Ca+2: VO + CS > POL + HP/CSTotal fat: VO + CS > POL + HP/CS16:0: Not reported |
| Ostrom et al., 2002 (56) PII | 3-day balance phase | POL + HP/CSVO + CS | Ca+2: VO + CS > POL + HP/CSTotal fat: No differences between groups16:0: Not reported |
| de Souza et al., 2017 (53) | 4-day metabolic phase | PO1VO1 | Ca+2: VO1 > PO1Total fat: VO1 > PO116:0: No differences between groups |
| Retention | |||
| Carnielli et al., 1996 (49) | ∼4 weeks | SN2 (13%)SN2 (39%)SN2 (66%) | Ca+2: No differences between groups |
| Leite et al., 2013 (51) | 4-day metabolic phase | POL1 VO1 | Ca+2: VO1 > POL1 |
| Nelson et al., 1996 (35) | 3- to 4-day metabolic balance phase | POLVO | Ca+2: VO > POL |
| de Souza et al., 2017 (53) | 4-day metabolic phase | PO1VO1 | Ca+2: VO1 > POL1 |
% Of palmitic acid esterified at sn-2 position in formula; and 16:0 indicates palmitic acid. Abbreviations: ARA, arachidonic acid; Ca+2, calcium; CS, added calcium salts; HP, hydrolyzed protein; PO, palm oil; POL, palm olein; sn, stereospecific numbered; SN2, stereospecific numbered–2 palmitate; VO, vegetable oil.
Indicates the addition of long-chain PUFAs including 22:6n-3 (DHA) and 20:4n-6 (ARA).
Bone measures
Urinary calcium, which reflects the amount of calcium released during bone resorption, was reported to be indifferent among infants fed VO formula compared to those fed PO or POL formula (35, 51, 53), as well as infants fed SN2 formulas with different levels of PA esterified at the sn-2 position (13%–66%) (Table 6) (49). Results for other bone measures, including bone speed of sound (SOS) and bone mineral content (BMC)/bone mineral density (BMD), are presented in Table 8. Bone SOS was reported to be 3% higher in infants fed SN2 formula containing a higher percentage of PA esterified at sn-2 (43%) compared to those fed formula with a lower percentage of PA esterified at sn-2 (14%), and was more similar to the bone SOS of HM-fed infants (39). A similar result was reported for BMC/BMD, showing that infants fed SN2 formula with a higher percentage of PA esterified at sn-2 (43%–51%) had 5%–8% higher bone BMC/BMD measures compared to infants fed SN2 or VO formula with a lower percentage of PA esterified at sn-2 (12%–16%), and were more similar to the BMC/BMD measures of HM-fed infants (38, 42). Despite these differences, 1 study reported no differences in bone BMC/BMD between SN2 formulas containing different amounts of PA esterified at sn-2 (12%–50%) and HM-fed infants (57), at both 12 weeks and a much later follow-up at 10 years of age. Finally, infants fed VO formula were reported to have 5%–10% higher BMC/BMD measures than those fed POL formula at 3 and 6 months of age (50).
TABLE 8.
Results for bone measures in included studies evaluating infants fed formulas containing palm oil or palm olein and sn-2 palmitate
| Reference | Time point(s) | Groups | Outcome |
|---|---|---|---|
| Bone speed of sound | |||
| Litmanovitz et al., 2013 (39) | T1: 6 weeksT2: 12 weeks | SN21 (43%)PO1 (14%)HM | T1: No differences between groups T2: SN21 (43%) > PO1 (14%), no differences between SN21 (43%) and HM |
| Bone mineral content and density | |||
| Beghin et al., 2019 (38) | 4 months | SN2 (16%)SN2 (43%)SN2 (51%) | BMC: SN2 (51%) > SN2 (16%), SN2 (43%) > SN2 (16%), no differences between SN2 (51%) and SN2 (43%)BMD: Not reported |
| Fewtrell et al., 2013 (57) | 12 weeks, 10 years | SN2 (12%)SN2 (50%)HM | BMC: No differences between groupsBMD: No differences between groups |
| Kennedy et al., 1999 (42) | 12 weeks | SN2 (50%) + CSVO (12%) + CSHM | BMC: SN2 (50%) + CS > VO (12%) + CS, HM > VO (12%) + CS, no differences between SN2 (50%) + CS and HMBMD: SN2 (50%) + CS > VO (12%) + CS, HM > VO (12%) + CS, no differences between SN2 (50%) + CS and HM |
| Koo et al., 2003 (50) | 3, 6 months | POLVO | BMC: VO > POL BMD: VO > POL |
% Indicates the percentage of palmitic acid esterified at the sn-2 position in formula. Abbreviations: ARA, arachidonic acid; BMC, bone mineral content; BMD, bone mineral density; CS, calcium salts; HM, human milk; PO, palm oil; POL, palm olein; sn, stereospecific numbered; SN2, stereospecific numbered–2 palmitate; T1, time point 1; T2, time point 2; VO, vegetable oil.
Indicates the addition of long-chain PUFAs, including 22:6n-3 (DHA) and 20:4n-6 (ARA).
Tolerance of formulas
Tolerance of formulas in the studies was summarized into categories of behavior, indicators of gastrointestinal (GI) tolerance, spit-up/vomiting, and flatulence (Table 9). While 1 study reported no differences in crying/colic between infants fed SN2 formulas containing different percentages of PA esterified at sn-2 (16%–51%) (38), others reported conflicting results, showing that SN2 formulas containing a higher percentage of PA esterified at sn-2 (44%–50%) had both less (40) and more (42) crying/colic than infants fed formula with a lower percentage of PA esterified at sn-2 (12%–14%). Additionally, restlessness and irritability were reported to be higher in infants fed SN2 formula with a lower percentage of PA esterified at sn-2 (16%) compared to those fed SN2 formula with a higher percentage of PA esterified at sn-2 (51%) (38). Infants fed PO or POL formula had more crying/colic (40, 58), more spit-up/regurgitation (58), and poorer indicators of GI tolerance (58) than those fed VO, SN2 formula and HM (40, 58).
TABLE 9.
Results for tolerance of formulas in included studies evaluating infants fed formulas containing palm oil or palm olein and sn-2 palmitate
| Reference | Time point(s) | Groups | Outcome |
|---|---|---|---|
| Behavior | |||
| Alarcon et al., 2002 (58) | Day 14 | POLVO-1VO-2POL/VO-1HM + POLHM + VO-1HM + VO-2HM + POL/VO-1HM | Crying/Colic: POL/VO-1 > VO-2, HM + POL/VO-1 > HM + VO-2Restlessness: Not reportedIrritability: Not reported |
| Beghin et al., 2019 (38) | 2 weeks to 4 months | SN2 (16%)SN2 (43%)SN2 (51%) | Crying/Colic: No differences between groupsRestlessness: SN2 (16%) > SN2 (51%)Irritability: SN2 (16%) > SN2 (51%) |
| Kennedy et al., 1999 (42) | T1: 3 weeks T2: 6, 12 weeks | SN2 (50%) + CSVO (12%) + CSHM | Crying/Colic: T1: SN2 (50%) + CS > VO (12%) + CS; T2: No differences between groupsRestlessness: Not reportedIrritability: Not reported |
| Leite et al., 2013 (51) | 14-day tolerance phase, 4-day metabolic phase | POL1VO1 | Crying/Colic: Not reportedRestlessness: Not reportedIrritability: No differences between groups |
| Litmanovitz et al., 2014 (40) | 6, 12 weeks | SN21 (44%)PO1 (14%)HM | Crying/Colic: T1: PO1 (14%) > SN21 (44%), PO1 (14%) > HM, no differences between SN21 (44%) and HM; T2: PO1 (14%) > SN21 (44%), PO1 (14%) > HM, no differences between SN21 (44%) and HMRestlessness: Not reportedIrritability: Not reported |
| Sheng et al., 2020 (55) | 1–7 days | SN2 (19.9%) + PFPO (43.5%) + PFPO (40.1%) + PFVO (10.2%) + PF | Crying/Colic: No differences between groups in the morning, afternoon, and evening. However, PO (43.5%) + PF > SN2 (19.9%) + PF, PO (40.1%) + PF > SN2 (19.9%) + PF, VO (10.2%) + PF > SN2 (19.9%) + PF at nightRestlessness: Not reportedIrritability: Not reported |
| Indicators of GI intolerance | |||
| Alarcon et al., 2002 (58) | Day 14 | POLVO-1VO-2POL/VO-1HM + POLHM + VO-1HM + VO-2HM + POL/VO-1HM | POL/VO-1 > VO-2HM + POL/VO-1 > HM + VO-2No differences between VO-2 and POL VO-2 < VO-1 |
| Nowacki et al., 2014 (59) | Day 14, 28 | SN21 (40%)SN21 (40%) + FOVO1HM | No differences between groups |
| Sheng et al., 2020 (55) | 1–7 days | SN2 (19.9%) + PFPO (43.5%) + PFPO (40.1%) +PFVO (10.2%) + PF | PO (43.5%) + PF > SN2 (19.9%) + PF, PO (40.1%) + PF > SN2 (19.9%) + PF, VO (10.2%) + PF > SN2 (19.9%) + PF No differences between PO (43.5%) + PF, PO (40.1%) + PF and VO (10.2%) + PF |
| Yao et al., 2014 (61) | 0, 4, 8 weeks | SN21 (35.9%)SN21 (36.6%) + FOSN21 (36.9%) + FOVO1 (11.7%)HM | No differences between groups |
| Wu et al., 2021 (63) | T1: 16 weeks, T2: 24 weeks | SN21 (46.3%) + GOVO1 (10.3%) + GOHM | T1; T2: No differences between groups |
| Spit-up or vomiting | |||
| Alarcon et al., 2002 (58) | Day 14 | POLVO-1VO-2POL/VO-1HM + POLHM + VO-1HM + VO-2HM + POL/VO-1HM | Spit-up: POL/VO-1 > VO-2, HM + POL/VO-1 > HM + VO-2Regurgitation: HM > VO-2, POL/VO-1 > VO-2, HM + POL/VO-1 > HM + VO-2, HM + VO-1 > HM + VO-2 |
| Beghin et al., 2019 (38) | 2 weeks to 4 months | SN2 (16%)SN2 (43%)SN2 (51%) | No differences between groups |
| Borschel et al., 2014 (36) | 0-28, 56, 84, 119 days | VO1 + GO/HPPOL1 + GO/HP | No differences between groups |
| Leite et al., 2013 (51) | 14-day tolerance phase, 4-day metabolic phase | POL1VO1 | No differences between groups |
| Lloyd et al., 1999 (52) PI | 0–2 weeks | POLVO | No differences between groups |
| Lloyd et al., 1999 (52) PII | 0–2 weeks | POLVO | No differences between groups |
| Schmelzle et al., 2003 (37) | 0-6 weeks | SN2 (41%) + GO/FO/HPPO | Vomiting: Reported among reasons for dropout |
| Sheng et al., 2020 (55) | 1–7 days | SN2 (19.9%) + PFPO (43.5%) + PFPO (40.1%) + PFVO (10.2%) + PF | Spit-up: No differences between groupsRegurgitation: No differences between groups |
| Flatulence | |||
| Beghin et al., 2019 (38) | 2 weeks to 4 months | SN2 (16%)SN2 (43%)SN2 (51%) | No differences between groups |
| Civardi et al., 2017 (62) | 60 ± 5 days, 135 ± 5 days | SN2 (39%) + GOVO | No differences between groups |
| Leite et al., 2013 (51) | 14-day tolerance phase, 4-day metabolic phase | POL1VO1 | No differences between groups |
| Schmelzle et al., 2003 (37) | 0-6 weeks | SN2 (41%) + GO/FO/HPPO | Reported among reasons for dropout |
| Sheng et al., 2020 (55) | 1–7 days | SN2 (19.9%) + PFPO (43.5%) + PFPO (40.1%) + PFVO (10.2%) + PF | PO (43.5%) + PF > SN2 (19.9%) + PF, PO (40.1%) + PF > SN2 (19.9%) + PF, VO (10.2%) + PF > SN2 (19.9%) + PF No differences between PO (43.5%) + PF, PO (40.1%) + PF and VO (10.2%) + PF |
% Of palmitic acid esterified at sn-2 position in formula. Abbreviations: ARA, arachidonic; CS, calcium salts; FO, fructo-oligosaccharides; GI, gastrointestinal; GO, galacto-oligosaccharides; HM, human milk; HP, hydrolyzed protein; PF, prebiotic fibers; PO, palm oil; POL, palm olein; sn, stereospecific numbered; SN2, stereospecific numbered–2 palmitate; T1, time point 1; T2, time point 2; VO, vegetable oil; VO-1, S-26; VO-2, Similac Advance.
Indicates the addition of long-chain PUFAs, including 22:6n-3 (DHA) and 20:4n-6 (ARA).
Visual acuity
One study reported no differences in visual acuity between infants fed POL formula compared to those fed VO formula and HM (Table 10) (44).
TABLE 10.
Results for visual acuity in included studies evaluating infants fed formulas containing palm oil or palm olein and sn-2 palmitate
| Reference | Time point | Groups | Outcome |
|---|---|---|---|
| Innis et al., 1997 (44) | 90 days | POL | No differences between groups |
| VO | |||
| HM |
Abbreviations: HM, human milk; POL, palm olein; sn, stereospecific numbered; VO, vegetable oil.
Neurodevelopmental outcomes
One study reported no differences between infants fed SN2 formula compared to those fed VO formula in unadjusted median scores from the Ages and Stages Questionnaire, Third Edition (ASQ-3) across 5 developmental domains (Table 11) (63).
Discussion
The objective of this scoping review was to map the literature and identify current knowledge on the role of PA in infant nutrition, specifically neurodevelopment. Most results, including anthropometric measures, blood measures, intakes of nutrients and formula, stool analyses, excretion, intestinal absorption and retention of nutrients, bone measures, and tolerance of formulas, did not report any negative impacts of sn-2 palmitate in formulas. However, it was consistently reported that infants fed PO or POL formula had significantly less calcium, total fat, and PA absorption, as well as firmer stools, and this may be a negative effect of the distribution of PA within the PO- or POL-TAG. However, we recognize that only a small number of studies reported on this outcome and that there is considerable heterogeneity among the studies influencing this result, which will be discussed further in detail. Nevertheless, anthropometric measures were reported on most frequently, showing consistent evidence that formulas containing PO or POL and SN2 do not influence body weight, length, or head circumference. Importantly, the current literature does not lead to a clear answer on the role of PA or its forms on brain development, as only 1 study reported on a measure of neurodevelopment (63).
Neurodevelopment and palmitate
Brain development is a complex process that starts during gestation, with organogenesis and neurogenesis, and continues during the postnatal phase (64). Neurodevelopment consists of structural changes and neuronal changes, as well as genetic determinants (65). Structural development is usually assessed using an MRI; however, this technique can be challenging to use in the pediatric population and is unpractical because of its cost, especially compared to measuring head circumference, which can predict the brain size (66). Assessing brain volume and head circumference could provide insight to the detection of large deficits (i.e., the development of neurological disorders). Indeed, while a smaller head circumference can be associated with an intellectual disability (67), a larger encephalon could be associated with autism spectrum disorder (68). Head circumference at birth could be influenced by several internal and external factors, including nutrition. For instance, DHA supplementation during the last half of gestation resulted in larger infant sizes, including larger head circumference (69). However, despite the predictive value of head circumference for detecting large deficits beyond an appropriate range, the use of head circumference as a neurodevelopment assessment tool remains unclear (70).
Twenty articles in this review reported on head circumference, with only 4 identifying a difference between groups (37, 39, 40, 43). Yaron and colleagues (43) described a larger head circumference in infants fed HM compared to infants fed SN2 formula containing 44% of PA esterified at the sn-2 position at 6 weeks of age, but no differences were detected in infants fed PO formula with a lower (14%) level of PA esterified at the sn-2 position. Importantly, infants did not show any differences at birth. However, this study included only 12 infants in the HM group and 11 in the SN2 group. Schmelzle and colleagues (37) also reported a larger head circumference in female infants that were fed SN2 formula compared to infants that received a formula containing PO after 12 weeks of diet. However, no differences were found in male infants. Furthermore, 2 studies with a larger sample size reported no differences in head circumference between infants fed PO formula and SN2 formula, with HM-fed infants having larger head circumferences than those in both formula groups (39, 40). Overall, the results of the 20 articles do not indicate that sn-2 palmitate or PO within formula impacts head circumference. Nonetheless, it is challenging to compare studies reporting on head circumference because of the heterogeneity in formula compositions, study durations, and numbers of infants included. Interestingly, 1 of the 20 articles reporting on head circumference also reported on ASQ-3 scores across 5 developmental domains in infants fed SN2 formula compared to those fed VO formula; this was the lone measure of neurodevelopment identified in this review (63). Despite insignificant unadjusted median ASQ-3 scores across the 5 developmental domains between formula groups, median ASQ-3 scores adjusted for maternal education revealed infants fed SN2 formula had higher median scores for fine motor skills compared to infants fed VO formula after 16 weeks of the diet (63). Further analyses revealed the risk of scoring closer to the threshold for fine motor skills was significantly lower in infants fed SN2 formula than those fed VO formula, both when adjusted and unadjusted for maternal education at 16 weeks (63). However, after 24 weeks of formula consumption, scores were not significantly different between groups (63). Importantly, both formulas were matched for added galacto-oligosaccharides, yet only the SN2 formula group showed a higher relative abundance of fecal bifidobacteria (63). This difference in bifidobacteria was associated with decreased odds of 1 domain scoring closer to the threshold among formula-fed infant groups (63), which may give insight to PA surviving digestion and interacting with added prebiotics to yield a favorable effect on the gut microbiome and further neurodevelopment via the gut-brain axis. However, the mechanisms underlying the interaction between higher sn-2 palmitate content in formulas and prebiotics need to be elucidated. Furthermore, differences in median ASQ-3 scores across the 5 developmental domains between formula groups were only observed after adjustment for maternal education. Importantly, differences in adjusted scores suggest that any positive effect of SN2 formula on neurodevelopment observed at 16 weeks might be temporary and is lost by 24 weeks of diet. Therefore, evidence of any benefit is limited. This time-dependent difference in adjusted scores (63), alongside other results in the current review, emphasizes the need for examining long-term effects of dietary interventions during infancy that are inclusive of energy balance (71), as corresponding outcomes assessed in RCTs included in the current review do not include follow-ups at later time points into childhood.
Sn-2 palmitate
Advances in our understanding of PA and its absorption, alongside lipidomic analyses such as mass spectrometry, revealed that the optimal absorption of PA from breast milk was due to the presence of sn-2 palmitate content compared to VO-based formulas (sn-1,3). These discoveries set the stage for the first commercial sn-2 palmitate, launched in 1996 (72, 73). This specific-structured-TAG (SST), commercialized as Betapol, was first developed by IOI Loders Croklaan and was made possible through EIE (72). This catalytic reaction introduces an enzyme (sn-1,3-specific lipase) to a VO blend, allowing the rearrangement of PA at the sn-2 position of the glycerol backbone (72). Other ingredient suppliers followed with patented processes of EIE, including creating a mixture of VOs to supply sn-2 palmitate at levels of 40%–45% to the infant (INFAT, Zhejiang Beijia, Lipomilk). More recently, there has been a movement to not only model but match the sn-2 palmitate content of breast milk within formula by achieving higher levels of sn-2 palmitate (i.e., higher than 40%; Betapol Plus). However, synthetic SSTs are relatively expensive. Accordingly, 3 of the most recent articles included in this review tested a more affordable, natural source of sn-2 palmitate from cows’ milk fat (41, 54, 55). This may be a more practical and affordable option, also given the previous recommendations by the ESPGHAN on beta-palmitate (29). Despite this, the bulk of research on sn-2 palmitate covered in this review used formulas with synthetic SSTs, such as Betapol (42, 45, 49, 57, 60, 61, 63), INFAT (38–40, 43, 48), other structured VOs (59, 62), and other sources not stated (37, 46, 47).
Limitations
The present study has several limitations. Firstly, the included studies were heterogenous in terms of the durations of the interventions used, sample sizes, and outcome measures, as well as how results were reported. Studies also varied in terms of using HM-fed infants as a reference group (39–44, 48, 57, 59, 61, 63), using an experimental group (45–47, 58, 60), or having no reference group (34–38, 49–56, 62), making it difficult to summarize results. Secondly, formulas used in the studies differed in fat blends, sources of sn-2 palmitate content, long-chain PUFA contents (namely, DHA and ARA), prebiotics, hydrolyzed proteins, and calcium salts, not to mention overall energy density, making it difficult to compare the effects of PA between studies. For example, 7 studies included galacto-oligosaccharides (36, 37, 41, 48, 54, 62, 63), 3 studies included fructo-oligosaccharides (37, 59, 61), and 1 included unknown prebiotic fibers (55) in combination with sn-2 palmitate or POL/VO. This leads to difficulty in interpreting results, because prebiotic oligosaccharides have a role in stimulating both the growth and activity of the bacteria in the digestive system, and formulas supplemented with prebiotics have been found to result in better infant stool consistency, stool frequency, and a higher amount of bifidobacteria compared to controls (i.e., nonsupplemented formulas) (41). Therefore, results for these studies should be interpreted with caution, as the effects of SN2 and PO/POL cannot be isolated. Furthermore, the levels of reported PA (percentage by weight) in each formula differed, ranging from 7.37%–27.2% in the included studies, with all formulas containing <10% PA being VO based (34, 35, 50, 51, 53, 55). Additionally, whether the ratio of SFAs to MUFAs to PUFAs influenced outcomes or not could not be determined across the included studies. This was due to the lack of reporting on formula compositions altogether and the inconsistency in fatty acids reported across studies. Lastly, although our review is limited to healthy, term infants and studies on preterm infants were excluded, we included 2 studies that presented data on both term and preterm infants, to avoid losing valuable data on term infants (34, 35). However, the impact of including these infants is likely small, as no noteworthy differences were shown between term and preterm infants within the results (34, 35).
Conclusion
Despite considerable heterogeneity between studies, anthropometric measures, such as head circumference, body weight, and body length, do not appear to be influenced by PO/POL or SN2 within formulas. As shown in the recent review by Padial-Jaudenes et al. (30), it was consistently reported that infants fed PO or POL formulas had decreased intestinal absorptions of calcium, total fat, and PA compared to infants fed VO formulas. However, the small number of studies (n = 5) reporting on this outcome and the heterogeneity between the studies makes drawing concrete conclusions difficult. Nonetheless, our review, along with the ESPGHAN position paper, revealed that there is insufficient evidence regarding the effects of PO/POL and SN2 as sources of fat in formula. Importantly, a gap was identified in the literature surrounding neurodevelopment in infants fed formulas containing PO/POL and SN2. This is consistent with the inconclusive literature surrounding whether the level or arrangement of PA in formula may influence the endogenous synthesis of PA or the brain membrane PA composition. Therefore, preclinical studies untangling whether brain PA is influenced by these factors are warranted, in addition to future studies in infants assessing neurodevelopment as a primary outcome, which is an area that remains largely unexplored. Ideally, longer interventions using formulas containing no other functional ingredients in addition to SN2 and PO/POL would be needed isolate the effects of PA. Larger sample sizes and durations appropriate for assessing neurodevelopment should also be considered. Alternatively, mechanisms underlying the interactions of both SN2 and PO/POL with functional ingredients should be further investigated in relation to neurodevelopment.
Supplementary Material
Acknowledgments
The authors’ responsibilities were as follows—MES and GC: designed the protocol and search strategy, conducted the search, screened articles, extracted the data, and charted the data; MES: wrote the manuscript; GC: wrote sections of the discussion and results within the manuscript; RJSL: resolved conflicts raised between MES and GC at all stages of screening; RPB: conceptualized the scoping review and supervised data collection; and all authors: read and approved the final manuscript.
Notes
All phases of this scoping review were supported by a grant from the Natural Sciences and Engineering Research Council of Canada (482597). MES was supported by the Loblaws Food as Medicine Graduate Award, and the Ontario Graduate Scholarship. GC is supported by the Canadian Institutes of Health Research. RJSL is supported by the Partnership for Clean Competition. RPB holds the Canada Research Chair in Brain Lipid Metabolism.
Author disclosures: RPB has received industrial grants, including those matched by the Canadian government, and/or travel support related to work on brain fatty acid uptake from Arctic Nutrition, Bunge Ltd, DSM, Fonterra, Mead Johnson, and Nestec Inc.; is on the executive committee of the International Society for the Study of Fatty Acids and Lipids and held a meeting on behalf of Fatty Acids and Cell Signaling, both of which rely on corporate sponsorship; has given expert testimony in relation to supplements and the brain; and holds the Canada Research Chair in Brain Lipid Metabolism.
The funder/sponsor did not participate in the work.
Supplemental Table 1 is available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn/.
Abbreviations used: ARA, arachidonic acid; ASQ-3, Ages and Stages Questionnaire, Third Edition; BMC, bone mineral content; BMD, bone mineral density; EIE, enzymatic interesterification; ESPGHAN, European Society for Pediatric Gastroenterology, Hepatology, and Nutrition; GI, gastrointestinal; HM, human milk; PA, palmitic acid; PL, phospholipid; PO, palm oil; POL, palm olein; PRISMA-P, preferred reporting items for systematic review and meta-analysis protocols; PRISMA-Scr, preferred reporting items for systematic review and meta-analysis for scoping reviews; RCT, randomized controlled trial; sn, stereospecific numbered; SN2, stereospecific numbered–2 palmitate; SOS, speed of sound; SST, specific-structured triacylglyceride; TAG, triacylglyceride; VO, vegetable oil.
Contributor Information
Mackenzie E Smith, Department of Nutritional Sciences, University of Toronto, Toronto, Ontario, Canada.
Giulia Cisbani, Department of Nutritional Sciences, University of Toronto, Toronto, Ontario, Canada.
R J Scott Lacombe, Department of Pediatrics, Dell Medical School, The University of Texas at Austin, Austin, TX, USA.
Richard P Bazinet, Department of Nutritional Sciences, University of Toronto, Toronto, Ontario, Canada.
References
- 1.Innis SM. Palmitic acid in early human development. Crit Rev Food Sci Nutr. 2016;56:1952–9. [DOI] [PubMed] [Google Scholar]
- 2.Choi J, Yin T, Shinozaki K, Lampe JW, Stevens JF, Becker LB, Kim J. Comprehensive analysis of phospholipids in the brain, heart, kidney, and liver: brain phospholipids are least enriched with polyunsaturated fatty acids. Mol Cell Biochem. 2018;442:187–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carta G, Murru E, Banni S, Manca C. Palmitic acid: physiological role, metabolism and nutritional implications. Front Physiol. 2017;8:902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McNamara RK, Carlson SE. Role of omega-3 fatty acids in brain development and function: potential implications for the pathogenesis and prevention of psychopathology. Prostaglandins Leukot Essent Fatty Acids. 2006;75:329–49. [DOI] [PubMed] [Google Scholar]
- 5.Sastry PS. Lipids of nervous tissue: composition and metabolism. Prog Lipid Res. 1985;24:69–176. [DOI] [PubMed] [Google Scholar]
- 6.Dhopeshwarkar GA, Mead JF. Fatty acid uptake by the brain: II. Incorporation of [1-14C] palmitic acid into the adult rat brain. Biochim Biophys Acta. 1969;187:461–7. [PubMed] [Google Scholar]
- 7.Kimes AS, Sweeney D, London ED, Rapoport SI. Palmitate incorporation into different brain regions in the awake rat. Brain Res. 1983;274:291–301. [DOI] [PubMed] [Google Scholar]
- 8.Smith QR, Nagura H. Fatty acid uptake and incorporation in brain: studies with the perfusion model. J Mol Neurosci. 2001;16:167–72.; discussion 215–21. [DOI] [PubMed] [Google Scholar]
- 9.Chen CT, Domenichiello AF, Trépanier M-O, Liu Z, Masoodi M, Bazinet RP. The low levels of eicosapentaenoic acid in rat brain phospholipids are maintained via multiple redundant mechanisms. J Lipid Res. 2013;54:2410–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edmond J, Higa TA, Korsak RA, Bergner EA, Lee WN. Fatty acid transport and utilization for the developing brain. J Neurochem. 1998;70:1227–34. [DOI] [PubMed] [Google Scholar]
- 11.Edmond J. Essential polyunsaturated fatty acids and the barrier to the brain: the components of a model for transport. J Mol Neurosci. 2001;16:181–93.; discussion 215–21. [DOI] [PubMed] [Google Scholar]
- 12.Lacombe RJS, Giuliano V, Chouinard-Watkins R, Bazinet RP. Natural abundance carbon isotopic analysis indicates the equal contribution of local synthesis and plasma uptake to palmitate levels in the mouse brain. Lipids. 2018;53:481–90. [DOI] [PubMed] [Google Scholar]
- 13.Pietrzak-Fiećko R, Kamelska-Sadowska AM. The comparison of nutritional value of human milk with other mammals’ milk. Nutrients. 2020;12(5): 1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Innis SM. Dietary triacylglycerol structure and its role in infant nutrition. Adv Nutr. 2011;2:275–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bracco U. Effect of triglyceride structure on fat absorption. Am J Clin Nutr. 1994;60:1002S–9S. [DOI] [PubMed] [Google Scholar]
- 16.Sun C, Wei W, Su H, Zou X, Wang X. Evaluation of sn-2 fatty acid composition in commercial infant formulas on the Chinese market: a comparative study based on fat source and stage. Food Chem. 2018;242:29–36. [DOI] [PubMed] [Google Scholar]
- 17.López-López A, López-Sabater M, Campoy-Folgoso C, Rivero-Urgell M, Castellote-Bargalló A. Fatty acid and sn-2 fatty acid composition in human milk from Granada (Spain) and in infant formulas. Eur J Clin Nutr. 2002;56:1242–54. [DOI] [PubMed] [Google Scholar]
- 18.Mendonça MA, Araújo WMC, Borgo LA, de R Alencar E. Lipid profile of different infant formulas for infants. PLoS One. 2017;12(16):e0177812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hussain G, Schmitt F, Loeffler J-P, Gonzalez De Aguilar JL. Fatting the brain: a brief of recent research. Front Cell Neurosci. 2013;7:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coti Bertrand P, O'Kusky JR, Innis SM. Maternal dietary (n-3) fatty acid deficiency alters neurogenesis in the embryonic rat brain. J Nutr. 2006;136:1570–5. [DOI] [PubMed] [Google Scholar]
- 21.Uauy R, Dangour AD. Nutrition in brain development and aging: role of essential fatty acids. Nutr Rev. 2006;64:S24–33.; discussion S72–91. [DOI] [PubMed] [Google Scholar]
- 22.Widdowson EM, Dauncey MJ, Gairdner DM, Jonxis JH, Pelikan-Filipkova M. Body fat of British and Dutch infants. Br Med J. 1975;1:653–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Farquharson J, Cockburn F, Patrick WA, Jamieson EC, Logan RW. Effect of diet on infant subcutaneous tissue triglyceride fatty acids. Arch Dis Child. 1993;69:589–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sweeney MJ, Etteldorf JN, Throop LJ, Timma DL, Wrenn EL. Diet and fatty acid distribution in subcutaneous fat and in the cholesterol-triglyceride fraction of serum of young infants. J Clin Invest. 1963;42:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Farquharson J, Cockburn F, Patrick WA, Jamieson EC, Logan RW. Infant cerebral cortex phospholipid fatty-acid composition and diet. Lancet. 1992;340:810–3. [DOI] [PubMed] [Google Scholar]
- 26.Geiser F. Influence of polyunsaturated and saturated dietary lipids on adipose tissue, brain and mitochondrial membrane fatty acid composition of a mammalian hibernator. Biochim Biophys Acta. 1990;1046:159–66. [DOI] [PubMed] [Google Scholar]
- 27.Gimenez da Silva-Santi L, Masetto Antunes M, Mori MA, Biesdorf de Almeida-Souza C, Vergílio Visentainer J, Carbonera F, Rabello Crisma A, Nunes Masi L, Massao Hirabara S, Curi Ret al. Brain fatty acid composition and inflammation in mice fed with high-carbohydrate diet or high-fat diet. Nutrients. 2018;10:1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carta G, Murru E, Lisai S, Sirigu A, Piras A, Collu M, Batetta B, Gambelli L, Banni S. Dietary triacylglycerols with palmitic acid in the sn-2 Position modulate levels of N-acylethanolamides in rat tissues. PLoS One. 2015;10:e0120424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bronsky J, Campoy C, Embleton N, Fewtrell M, Mis NF, Gerasimidis K, Hojsak I, Hulst J, Indrio F, Lapillonne Aet al. Palm oil and beta-palmitate in infant formula: a position paper by the European Society for Paediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) Committee on Nutrition. J Pediatr Gastroenterol Nutr. 2019;68:742–60. [DOI] [PubMed] [Google Scholar]
- 30.Padial-Jaudenes M, Castanys-Munoz E, Ramirez M, Lasekan J. Physiological impact of palm olein or palm oil in infant formulas: a review of clinical evidence. Nutrients. 2020;12(12):3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peters MDJ, Godfrey CM, Khalil H, McInerney P, Parker D, Soares CB. Guidance for conducting systematic scoping reviews. Int J Evid Based Healthc. 2015;13:141–6. [DOI] [PubMed] [Google Scholar]
- 32.Tricco AC, Lillie E, Zarin W, O'Brien KK, Colquhoun H, Levac D, Moher D, Peters MDJ, Horsley T, Weeks Let al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169:467–73. [DOI] [PubMed] [Google Scholar]
- 33.Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA, Group PRISMA-P. Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nelson SE, Frantz JA, Ziegler EE. Absorption of fat and calcium by infants fed a milk-based formula containing palm olein. J Am Coll Nutr. 1998;17:327–32. [DOI] [PubMed] [Google Scholar]
- 35.Nelson SE, Rogers RR, Frantz JA, Ziegler EE. Palm olein in infant formula: absorption of fat and minerals by normal infants. Am J Clin Nutr. 1996;64:291–6. [DOI] [PubMed] [Google Scholar]
- 36.Borschel MW, Choe YS, Kajzer JA. Growth of healthy term infants fed partially hydrolyzed whey-based infant formula: a randomized, blinded, controlled trial. Clin Pediatr. 2014;53:1375–82. [DOI] [PubMed] [Google Scholar]
- 37.Schmelzle H, Wirth S, Skopnik H, Radke M, Knol J, Bockler H-M, Bronstrup A, Wells J, Fusch C. Randomized double-blind study of the nutritional efficacy and bifidogenicity of a new infant formula containing partially hydrolyzed protein, a high beta-palmitic acid level, and nondigestible oligosaccharides. J Pediatr Gastroenterol Nutr. 2003;36:343–51. [DOI] [PubMed] [Google Scholar]
- 38.Beghin L, Marchandise X, Lien E, Bricout M, Bernet J-P, Lienhardt J-F, Jeannerot F, Menet V, Requillart J-C, Marx Jet al. Growth, stool consistency and bone mineral content in healthy term infants fed sn-2-palmitate-enriched starter infant formula: a randomized, double-blind, multicentre clinical trial. Clin Nutr. 2019;38:1023–30. [DOI] [PubMed] [Google Scholar]
- 39.Litmanovitz I, Davidson K, Eliakim A, Regev RH, Dolfin T, Arnon S, Bar-Yoseph F, Goren A, Lifshitz Y, Nemet D. High beta-palmitate formula and bone strength in term infants: a randomized, double-blind, controlled trial. Calcif Tissue Int. 2013;92:35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Litmanovitz I, Bar-Yoseph F, Lifshitz Y, Davidson K, Eliakim A, Regev RH, Nemet D. Reduced crying in term infants fed high beta-palmitate formula: a double-blind randomized clinical trial. BMC Pediatr. 2014;14:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nomayo A, Schwiertz A, Rossi R, Timme K, Foster J, Zelenka R, Tvrdik J, Jochum F. Infant formula with cow's milk fat and prebiotics affects intestinal flora, but not the incidence of infections during infancy in a double-blind randomized controlled trial. Mol Cell Pediatr. 2020;7:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kennedy K, Fewtrell MS, Morley R, Abbott R, Quinlan PT, Wells JC, Bindels JG, Lucas A. Double-blind, randomized trial of a synthetic triacylglycerol in formula-fed term infants: effects on stool biochemistry, stool characteristics, and bone mineralization. Am J Clin Nutr. 1999;70:920–7. [DOI] [PubMed] [Google Scholar]
- 43.Yaron S, Shachar D, Abramas L, Riskin A, Bader D, Litmanovitz I, Bar-Yoseph F, Cohen T, Levi L, Lifshitz Yet al. Effect of high beta-palmitate content in infant formula on the intestinal microbiota of term infants. J Pediatr Gastroenterol Nutr. 2013;56:376–81. [DOI] [PubMed] [Google Scholar]
- 44.Innis SM, Akrabawi SS, Diersen-Schade DA, Dobson MV, Guy DG. Visual acuity and blood lipids in term infants fed human milk or formulae. Lipids. 1997;32:63–72. [DOI] [PubMed] [Google Scholar]
- 45.Nelson CM, Innis SM. Plasma lipoprotein fatty acids are altered by the positional distribution of fatty acids in infant formula triacylglycerols and human milk. Am J Clin Nutr. 1999;70:62–9. [DOI] [PubMed] [Google Scholar]
- 46.Innis SM, Dyer R, Nelson CM. Evidence that palmitic acid is absorbed as sn-2 monoacylglycerol from human milk by breast-fed infants. Lipids. 1994;29:541–5. [DOI] [PubMed] [Google Scholar]
- 47.Innis SM, Nelson CM. Dietary triacyglycerols rich in sn-2 palmitate alter post-prandial lipoprotein and unesterified fatty acids in term infants. Prostaglandins Leukot Essent Fatty Acids. 2013;89:145–51. [DOI] [PubMed] [Google Scholar]
- 48.Bar-Yoseph F, Lifshitz Y, Cohen T, Malard P, Xu C. SN2-palmitate reduces fatty acid excretion in Chinese formula-fed infants. J Pediatr Gastroenterol Nutr. 2016;62:341–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Carnielli VP, Luijendijk IH, Van Goudoever JB, Sulkers EJ, Boerlage AA, Degenhart HJ, Sauer PJ. Structural position and amount of palmitic acid in infant formulas: effects on fat, fatty acid, and mineral balance. J Pediatr Gastroenterol Nutr. 1996;23:553–60. [DOI] [PubMed] [Google Scholar]
- 50.Koo WWK, Hammami M, Margeson DP, Nwaesei C, Montalto MB, Lasekan JB. Reduced bone mineralization in infants fed palm olein-containing formula: a randomized, double-blinded, prospective trial. Pediatrics. 2003;111:1017–23. [DOI] [PubMed] [Google Scholar]
- 51.Leite Maria Efigênia de Queiroz, Lasekan J, Baggs G, Ribeiro T, Menezes-Filho J, Pontes M, Druzian J, Barreto DL, de Souza CO, Mattos Aet al. Calcium and fat metabolic balance, and gastrointestinal tolerance in term infants fed milk-based formulas with and without palm olein and palm kernel oils: a randomized blinded crossover study. BMC Pediatr. 2013;13:215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lloyd B, Halter RJ, Kuchan MJ, Baggs GE, Ryan AS, Masor ML. Formula tolerance in postbreastfed and exclusively formula-fed infants. Pediatrics. 1999;103:E7. [DOI] [PubMed] [Google Scholar]
- 53.de Souza CO, Leite MEQ, Lasekan J, Baggs G, Pinho LS, Druzian JI, Ribeiro TCM, Mattos AP, Menezes-Filho JA, Costa-Ribeiro H. Milk protein-based formulas containing different oils affect fatty acids balance in term infants: a randomized blinded crossover clinical trial. Lipids Health Dis. 2017;16:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Manios Y, Karaglani E, Thijs-Verhoeven I, Vlachopapadopoulou E, Papazoglou A, Maragoudaki E, Manikas Z, Kampani T-M, Christaki I, Vonk MMet al. Effect of milk fat-based infant formulae on stool fatty acid soaps and calcium excretion in healthy term infants: two double-blind randomised cross-over trials. BMC Nutr. 2020;6:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sheng XY, Buthmanaban V, Vonk MM, Feitsma AL, Parikh P. Reduced crying and favourable stool characteristics in Chinese infants fed milk fat-based formula. Asia Pac J Clin Nutr. 2020;29::144–51. [DOI] [PubMed] [Google Scholar]
- 56.Ostrom KM, Borschel MW, Westcott JE, Richardson KS, Krebs NF. Lower calcium absorption in infants fed casein hydrolysate- and soy protein-based infant formulas containing palm olein versus formulas without palm olein. J Am Coll Nutr. 2002;21:564–9. [DOI] [PubMed] [Google Scholar]
- 57.Fewtrell MS, Kennedy K, Murgatroyd PR, Williams JE, Chomtho S, Lucas A. Breast-feeding and formula feeding in healthy term infants and bone health at age 10 years. Br J Nutr. 2013;110:1061–7. [DOI] [PubMed] [Google Scholar]
- 58.Alarcon PA, Tressler RL, Mulvaney A, Lam W, Comer GM. Gastrointestinal tolerance of a new infant milk formula in healthy babies: an international study conducted in 17 countries. Nutrition. 2002;18:484–9. [DOI] [PubMed] [Google Scholar]
- 59.Nowacki J, Lee H-C, Lien R, Cheng S-W, Li S-T, Yao M, Northington R, Jan I, Mutungi G. Stool fatty acid soaps, stool consistency and gastrointestinal tolerance in term infants fed infant formulas containing high sn-2 palmitate with or without oligofructose: a double-blind, randomized clinical trial. Nutr J. 2014;13:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lopez-Lopez A, Castellote-Bargallo AI, Campoy-Folgoso C, Rivero-Urgel M, Tormo-Carnice R, Infante-Pina D, Lopez-Sabater MC. The influence of dietary palmitic acid triacylglyceride position on the fatty acid, calcium and magnesium contents of at term newborn faeces. Early Hum Dev. 2001;65(Suppl):S83–94. [DOI] [PubMed] [Google Scholar]
- 61.Yao M, Lien EL, Capeding MRZ, Fitzgerald M, Ramanujam K, Yuhas R, Northington R, Lebumfacil J, Wang L, DeRusso PA. Effects of term infant formulas containing high sn-2 palmitate with and without oligofructose on stool composition, stool characteristics, and bifidogenicity. J Pediatr Gastroenterol Nutr. 2014;59:440–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Civardi E, Garofoli F, Longo S, Mongini ME, Grenci B, Mazzucchelli I, Angelini M, Castellazzi A, Fasano F, Grinzato Aet al. Safety, growth, and support to healthy gut microbiota by an infant formula enriched with functional compounds. Clin Nutr. 2017;36:238–45. [DOI] [PubMed] [Google Scholar]
- 63.Wu W, Zhao A, Liu B, Ye W-H, Su H-W, Li J, Zhang Y-M. Neurodevelopmental outcomes and gut bifidobacteria in term infants fed an infant formula containing high sn-2 palmitate: a cluster randomized clinical trial. Nutrients. 2021;13(2):693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hansen D, Lou HC. Brain development, head circumference and medication. Acta Paediatr. 2000;89:505–7. [DOI] [PubMed] [Google Scholar]
- 65.Richards JE, Xie W. Brains for all the ages: structural neurodevelopment in infants and children from a life-span perspective. Adv Child Dev Behav. 2015;48:1–52. [DOI] [PubMed] [Google Scholar]
- 66.Belfort MB, Ramel SE. NICU diet, physical growth and nutrient accretion, and preterm infant brain development. NeoReviews. 2019;20:e385–96. [DOI] [PubMed] [Google Scholar]
- 67.Dolk H. The predictive value of microcephaly during the first year of life for mental retardation at seven years. Dev Med Child Neurol. 1991;33:974–83. [DOI] [PubMed] [Google Scholar]
- 68.Hazlett HC, Gu H, Munsell BC, Kim SH, Styner M, Wolff JJ, Elison JT, Swanson MR, Zhu H, Botteron KNet al. Early brain development in infants at high risk for autism spectrum disorder. Nature. 2017;542:348–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Carlson SE, Colombo J, Gajewski BJ, Gustafson KM, Mundy D, Yeast J, Georgieff MK, Markley LA, Kerling EH, Shaddy DJ. DHA supplementation and pregnancy outcomes. Am J Clin Nutr. 2013;97:808–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nicolaou L, Ahmed T, Bhutta ZA, Bessong P, Kosek M, Lima AAM, Shrestha S, Chandyo R, Mduma ER, Murray-Kolb Let al. Factors associated with head circumference and indices of cognitive development in early childhood. BMJ Glob Health. 2020;5:e003427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bloomfield FH, Agostoni C. The potential impact of feeding formula-fed infants according to published recommendations. Pediatr Res. 2020;88:526–8. [DOI] [PubMed] [Google Scholar]
- 72.Happe RP, Gambelli L. 12–Infant formula. In: Talbot Geditor. Specialty oils and fats in food and nutrition. Cambridge (UK); Waltham (MA); Kidlington (UK): Woodhead Publishing; 2015. 285–315.. Available from: http://www.sciencedirect.com/science/article/pii/B9781782423768000120 [Google Scholar]
- 73.Wei W, Jin Q, Wang X. Human milk fat substitutes: past achievements and current trends. Prog Lipid Res. 2019;74:69–86. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.