ABSTRACT
Background
Emerging evidence links underhydration and habitual low water intake to higher cardiometabolic risk, but evidence is limited in community-dwelling older adults.
Objectives
The objective is to examine if higher water intake and better hydration are associated with better cardiometabolic health.
Methods
This cross-sectional analysis using general linear models included 2238 participants from the Framingham Heart Study Second Generation and First Generation Omni cohorts with an estimated glomerular filtration rate >30 mL·min–1·1.73 m–2 and a valid FFQ for assessment of water intake. Of these participants, 2219 had fasting spot urinary creatinine data and 950 had 24-h urine creatinine data to assess hydration. Cardiometabolic risk factors included fasting glucose, triglycerides (TGs), total cholesterol (TC), HDL cholesterol, and calculated LDL cholesterol; glycated hemoglobin (HbA1c); C-reactive protein (CRP); and systolic (SBP) and diastolic (DBP) blood pressure.
Results
The combined cohorts were on average aged 70 y; 55% were women. Mean (95% CI) daily total water intakes were 2098 (2048, 2150) mL for men and 2109 (2063, 2156) mL for women. Total daily water, beverage (including plain water), and plain water intakes demonstrated significant positive trends with HDL cholesterol (P < 0.01). TG concentrations were significantly lower among the highest plain water consumers (P < 0.05). The 24-h urine concentration, as measured by creatinine, was positively associated with LDL cholesterol and TG concentrations ( P < 0.01) and inversely associated with HDL cholesterol concentrations (P < 0.002). Neither water intake nor urine concentration was associated with glucose or HbA1c (P > 0.05).
Conclusions
Our findings of a consistent pattern between circulating lipid concentrations and different water sources and hydration markers support an association between hydration and lipid metabolism in older adults and add to the growing evidence that inadequate water intake and underhydration may lead to higher cardiometabolic risk.
Keywords: water intake, hydration, cardiometabolic risk, HDL cholesterol, triglycerides
Introduction
Water is essential to life and is both the largest constituent of the human body (37–65% of body mass) (1) and the most abundant nutrient in the diet. If consumed in accordance with the Institute of Medicine adequate intakes of 2.7 and 3.7 L of total water each day, respectively, for adult women and men (2), adult women and men would consume ∼985 kg and 1350 kg of water yearly. Of this, ∼70–80% of total water intake is estimated to come from drinking water and other beverages (2).
Although water is an essential nutrient, it has often been overlooked in nutrition and health research (3). Over the past 25 y, epidemiologic evidence has slowly emerged tying lower water intake or biomarkers associated with underhydration (4) with various aspects of cardiometabolic health, such as obesity; lower HDL cholesterol and higher triglyceride (TG), glucose, and insulin concentrations; higher glycated hemoglobin (HbA1c) concentrations; and higher incidence of type 2 diabetes (T2D) and cardiovascular disease (5–18).
Limited data exist on hydration and cardiometabolic health in community-dwelling older adults. Stookey et al. documented a high prevalence of plasma hypertonicity in community-dwelling older adults (19) and found that hyperglycemia progressed faster to T2D in the presence of plasma hypertonicity (20), suggestive of insufficient hydration. In the same cohort of older adults, baseline plasma hypertonicity was also associated with increased risk of incident disability over 4 y and death over 8 y of follow-up (21). Moreover, Saleem et al. (22) documented that copeptin, a surrogate biomarker for arginine vasopressin (AVP), was an independent risk factor for insulin resistance and the metabolic syndrome in two cohorts of adults with mean ages of 59 and 64 y. It is important to note, however, that although hypertonicity or high copeptin can be suggestive of insufficient hydration, both biomarkers may also reflect other factors, such as high solute intake or high stress (as any stressful event can trigger AVP release, not only osmotic stress). Neither of these studies reported water or beverage intake.
There remain many gaps in our understanding of water intake, hydration, and metabolic health of older individuals. To help address these gaps, we undertook the present investigation of the role of water intake and hydration on markers of cardiometabolic health in older adult participants from the Framingham Heart Study (FHS) cohorts to test the hypothesis that higher water intake and better hydration are associated with better cardiometabolic health.
Methods
The participants in this cross-sectional analysis were derived from the FHS Second Generation (Gen2) (23) and First Generation FHS Omni (Omni1) (24) cohorts, with the latter cohort composed of minority residents of Framingham created to enhance the minority representation in the FHS cohorts. The examinations for the Gen2 and Omni1 cohorts occur concurrently and use the same protocols. Each cohort undergoes clinic examinations about every 4 y. For the present study, we use data from participants involved in the ninth Gen2 cohort and the fourth Omni1 cohort examinations (2011–2014). Of the 2731 participants who attended Gen2 examination 9 (n = 2430)/Omni1 examination 4 (n = 301), 2340 had valid FFQ data for assessment of water intake (see details below). We excluded 53 participants with an estimated glomerular filtration rate ≤30 mL·min–1·1.73 m–2 (25) and 49 participants with missing data on cardiometabolic risk factors and covariate information. Our final sample included 2238 participants (n = 2025 Gen2 examination 9/n = 213 Omni1 examination 4; Figure 1). Of these 2238 participants, 2219 also had spot urinary creatinine data and 950 Gen2 participants had 24-h urine creatinine data for assessment of hydration.
FIGURE 1.
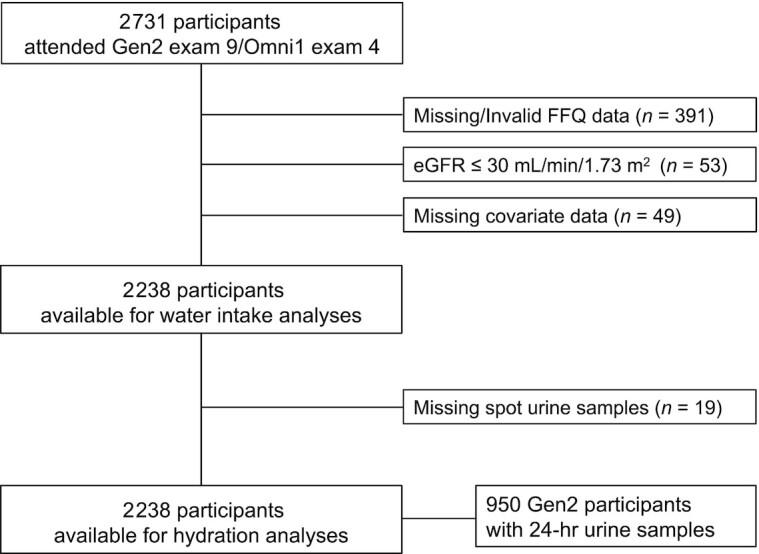
Flowchart of participants from the Framingham Heart Study Second Generation and First Generation Omni cohorts included in analyses. eGFR, estimated glomerular filtration rate; Gen2, FHS Second Generation cohort; Omni1, FHS First Generation Omni cohort.
The original FHS protocols were approved by the Institutional Review Board at Boston University Medical Center, and written informed consent was obtained from all participants. The present study was reviewed and approved by the Tufts University Health Sciences Institutional Review Board.
Dietary assessment and water intake
Dietary intake was assessed in the FHS cohorts using the Harvard semiquantitative FFQ (26, 27). The FFQ questionnaires were mailed to the participants before the examination, and the participants were asked to bring the completed questionnaire with them to their appointment, where they were reviewed for completeness. The FFQ consisted of a list of 126 foods with a standard serving size and a selection of 9 frequency categories ranging from “never or <1 serving/month” to “≥6 servings/day.” Participants were asked to report their frequency of consumption of each food item during the past year. Nutrient content of each food was derived from the Harvard nutrient composition database, which included food composition values from the USDA and is supplemented by additional published sources and communications with laboratories and manufacturers (27). The FFQ has been studied thoroughly for reproducibility and validity for nutrient and food intakes (26–29) and dietary patterns (30) using long-term diet records and biomarkers of nutrient status (31–38).
A modification to the FFQ adding tap and bottled (plain) water consumption was implemented in 2008, allowing for the assessment of total water and beverage intake starting at examinations 9 and 4, respectively, for the Gen2 and Omni1 cohorts. The FFQ used at these examinations included 27 questions on water and other beverage consumption. We assessed water intake from plain water (bottled/tap water), other beverages, and food. Water from beverages (beverage intake) included plain water, 100% fruit and vegetable juices (including prune juice, orange juice calcium fortified, orange juice regular, grapefruit juice, apple juice, other fruit juice, tomato juice), sugar-sweetened beverages (including carbonated beverages with caffeine, carbonated beverages without caffeine, and other sugared beverages—punch, lemonade, sports beverages, and sugared ice tea), nonnutritive sweetened carbonated beverages (including low-calorie beverages with caffeine and low-calorie beverages without caffeine), skim milk, low fat (1–2%) milk, whole milk, soy milk, herbal and decaffeinated tea, caffeinated tea including green tea, coffee with caffeine, decaffeinated coffee, dairy coffee beverages, regular beer, light beer, red wine, white wine, and liquor beverages. Water from foods included water contained in fruits and vegetables, soups, cereals and grains, nonmilk dairy, meats, and fish. We also characterized total water intake from both beverages and food.
Water content of each food and beverage item was calculated from the Harvard FFQ nutrient tables, which are based on USDA and market share databases. Reported water content per 100 g of food and gram weight per serving of each food listed on the FFQ were used to derive water content (g/serving) for each line item on the FFQ. Market share data were used to determine specific foods for some line items (e.g., yogurt) and the distribution of foods for combined items (e.g., “raisins or grapes” and “apples or pears”). For each participant, consumed water for every line item was calculated by multiplying the water content g/serving by the number of servings reported per day. Line item data were then summed into the broader categories of water intake g/d from beverages, plain water, and food. We expressed water intake as mL/d (1 mL water = 1 g) and also in mL/kg/d, using the latter to quantify water intake relative to body size.
Hydration
Biomarkers of urine concentration have been shown to vary with total water intake in community-dwelling adults (39–43). Furthermore, highly concentrated urine is suggestive of underhydration, a state in which total body water is maintained despite insufficient water intake through sustained antidiuretic effort (higher AVP secretion, low urine volume) (4). For this analysis, we assessed hydration using urinary creatinine concentration as a proxy for urine dilution (44). Although urinary creatinine is not commonly used as an indicator of hydration, the ratio of spot urine osmolality to creatinine has found to be consistent in the steady state (i.e., in the absence of a water-loading challenge) (45). Spot urine specimens were collected from all participants at their clinic examination, typically between 07:00 and 09:00 after an overnight fast. In addition, 24-h urine creatinine concentration was assessed in a subset of the Gen2 cohort who provided 24-h urine samples at examination 9 (n = 950).
Cardiometabolic risk factors
Outcomes included the following cardiometabolic risk factors: fasting plasma glucose, TGs, total cholesterol (TC), HDL cholesterol, and calculated LDL cholesterol; HbA1c; serum C-reactive protein (CRP); and systolic blood pressure (SBP) and diastolic blood pressure (DBP). All testing, except for HDL cholesterol, was performed on a Roche Cobas 501 using Roche reagents (Roche Diagnostics). HDL cholesterol was an offline precipitation step with dextran sulfate–Mg2+, followed by measurement of cholesterol as described above in the non–apo-B–containing supernatant. LDL cholesterol was calculated per the Friedewald equation modified by Martin et al. (46) as TC – HDL cholesterol – (TG/adjustable factor). SBP and DBP were measured twice by a physician using a sphygmomanometer and averaged.
Covariates
Potential confounders of the relation between water intake or hydration and the cardiometabolic risk factors were considered as covariates, including age (years); sex; education (<college, ≥college); BMI (kg/m2); regular smoking in the prior year (yes/no); pharmacologic treatment for hypertension, dyslipidemia, or diabetes (all yes/no); and a physical activity index (PAI). PAI was expressed in metabolic equivalents and calculated by averaging the number of hours spent on specific activities (i.e., sleep, sedentary, slight activity, moderate activity, and heavy activity) with each activity weighted by the oxygen consumption required to perform the activity. For the analyses of urinary creatinine, we also included prevalent hypertension and diabetes as covariates. Prevalent hypertension was defined as SBP ≥140 mm Hg or DBP ≥90 mm Hg (based on the average of 2 physician readings) or current use of antihypertensive medication. Presence of diabetes was defined as a plasma fasting glucose concentration ≥7.0 mmol/L or current use of hypoglycemic drug therapy.
Statistical analyses
All analyses were conducted with SAS statistical software (version 9.4; SAS Institute). Outcome variables were examined for normality, and natural log transformations were applied when needed. Mean values and percentages of participant characteristics and water intake were calculated and compared by sex because of the different water requirements for men and women using general linear models with significance defined as a 2-tailed P ≤ 0.05. General linear models were also used to examine associations between quartile categories of water intake and cardiometabolic risk factors to test the hypothesis that higher water intake is associated with better cardiometabolic health. All results are presented as least squares means and 95% CIs, with the exposures presented both as mL/d and mL/(kg·d) of water intake. Outcomes needing log transformation are presented as geometric means and 95% CIs. To assess trends across quartile categories, the median intake of each quartile category was assigned to individuals with intake in that category, and this variable was used as a continuous measure in our models. Models were adjusted for age (years), sex, cohort, BMI, education (<college, ≥college), current smoker (yes/no), physical activity score, and medication use (yes/no) as appropriate. Lipid outcomes were adjusted for lipid-lowering medication use, glucose and HbA1c for diabetic medication use, and blood pressure for antihypertensive use. Identical analyses were done using quartile categories of urinary creatinine (spot and 24-h) and cardiometabolic risk factors with water intake exposures presented as mg/dL (spot) and mg/(L·d) (24 h) and with additional adjustment for prevalent hypertension and diabetes (yes/no).
We did not consider energy in our original models for water from beverage and food because we viewed energy intake as a possible mediator of the impact of water intake on cardiometabolic risk. However, we performed sensitivity analyses adjusting for energy intake to determine if it might influence our findings.
Results
The combined Gen2 and Omni1 cohorts were on average aged 70 y (range: 44–95 y) and 55% of the participants were women. Men were more likely to have attended college or obtained a college or graduate degree (Table 1). Men were also more likely to have higher BMI, waist circumference, DBP, glucose, and energy intake; have a greater prevalence of hypertension and T2D; and use medications, including medications for hypertension, hypercholesterolemia, and diabetes. Women, on the other hand, had higher TC, HDL cholesterol, LDL cholesterol, and CRP than the men.
TABLE 1.
Characteristics for study participants from the Framingham Heart Study Second Generation and First Generation Omni cohorts by sex: mean or % (95% CI)1
| Characteristic | All (n = 2238) | Men (n = 999) | Women (n = 1239) | P value |
|---|---|---|---|---|
| Age, y | 69.9 (69.6, 70.2) | 70.0 (69.5, 70.5) | 69.8 (69.3, 70.2) | 0.53 |
| Education—highest grade completed, % | ||||
| High school or less | 24.8 (23, 26.6) | 20.4 (17.8, 23.1) | 28.3 (25.9, 30.7) | <0.001 |
| Some college/associate's degree | 30.3 (28.3, 32.2) | 26.4 (23.5, 29.3) | 33.3 (30.7, 35.9) | <0.001 |
| College | 22.2 (20.4, 23.9) | 26 (23.3, 28.6) | 19.1 (16.8, 21.5) | <0.001 |
| Postgraduate | 21.6 (19.9, 23.3) | 26.1 (23.5, 28.7) | 18 (15.7, 20.3) | <0.001 |
| Current smoker, % | 5.2 (4.3, 6.1) | 4.4 (3, 5.8) | 5.8 (4.6, 7) | 0.14 |
| BMI,2 kg/m2 | 27.9 (27.7, 28.1) | 28.6 (28.2, 28.9) | 27.4 (27.1, 27.7) | <0.001 |
| Waist circumference, cm2 | 100 (100, 101) | 104 (104, 105) | 97.3 (96.6, 98.0) | <0.001 |
| Systolic blood pressure, mm Hg | 127 (126,127) | 127 (126,128) | 126 (125,127) | 0.18 |
| Diastolic blood pressure, mm Hg | 72 (71.6, 72.4) | 73.2 (72.6, 73.8) | 71.1 (70.5, 71.6) | <0.001 |
| Total cholesterol,2 mg/dL | 180 (178, 182) | 165 (163, 167) | 193 (191, 195) | <0.001 |
| HDL cholesterol,2 mg/dL | 59.3 (58.6, 60.1) | 51.7 (50.8, 52.6) | 66.3 (65.3, 67.3) | <0.001 |
| LDL cholesterol,2,3 mg/dL | 94.2 (93.0, 95.4) | 87.8 (86.1, 89.5) | 99.7 (98.0, 101.4) | <0.001 |
| Triglycerides,2 mg/dL | 103 (101, 105) | 101 (98, 104) | 104 (101, 106) | 0.18 |
| C-reactive protein,2 mg/L | 1.6 (1.5, 1.6) | 1.4 (1.3, 1.5) | 1.7 (1.6, 1.8) | <0.001 |
| Glucose,2 mg/dL | 102 (101, 103) | 106 (104, 107) | 99 (98, 100) | <0.001 |
| Hypertension, % | 71 (69.1, 72.9) | 74.6 (71.8, 77.5) | 68 (65.5, 70.6) | <0.001 |
| Diabetes, % | 13 (11.6, 14.4) | 15.8 (13.7, 17.9) | 10.8 (8.9, 12.6) | <0.001 |
| Medication use, % | ||||
| Antihypertensive | 56.4 (54.3, 58.4) | 61.2 (58.2, 64.3) | 52.4 (49.7, 55.2) | <0.001 |
| Cholesterol lowering | 52.3 (50.2, 54.4) | 59 (55.9, 62) | 46.9 (44.1, 49.7) | <0.001 |
| Antidiabetic | 11 (9.7, 12.3) | 13.8 (11.9, 15.8) | 8.7 (7, 10.5) | <0.001 |
| Energy intake,2 kcal/d | 1829 (1804, 1856) | 1930 (1889, 1971) | 1752 (1719, 1786) | <0.001 |
Maximum number of participants from the Framingham Heart Study Second Generation and Omni First Generation cohorts who had valid FFQs, estimated glomerular filtration rate >30 mL·min–1·1.73 m–2, complete covariate information, and at least 1 spot or 24-h urinary creatinine value.
Geometric means and 95% CIs are presented.
Calculated LDL cholesterol.
Average daily total water intake (the sum of water from foods and beverages) was the same in men and women when expressed in mL (P = 0.76) but was higher in women when characterized as mL/kg body weight (P < 0.001) (Table 2). Daily beverage intake (plain water and other beverages) in mL was slightly higher in men (P = 0.02) but substantially higher in women when expressed as mL/kg body weight (P < 0.001). Plain water intake and water intake from foods were significantly higher in women irrespective of how they were expressed (P < 0.001). Overall, ∼30% of total water came from foods, whereas 70% came from beverages. Within the beverage category, plain water was the main source of water, accounting for ∼35% of water from beverages (∼40% for women and 30% for men). Coffee was the second highest source of water from beverages (data not shown). Detailed distributions of beverage intakes based on selected percentiles are shown in Supplemental Table 1. Spot urinary creatinine concentrations and 24-h urinary creatinine concentrations (obtained only in a subset of participants from the Gen2 cohort) were significantly higher in men (P < 0.001) (Table 2).
TABLE 2.
Daily water intake and urinary creatinine for study participants from the Framingham Heart Study Second Generation and First Generation Omni cohorts by sex: geometric means (95% CI)1
| Characteristic | All (n = 2238) | Men (n = 999) | Women (n = 1239) | P value2 |
|---|---|---|---|---|
| Total water intake | ||||
| mL | 2104 (2070, 2139) | 2098 (2048, 2150) | 2109 (2063, 2156) | 0.76 |
| mL/kg | 27.5 (27.0, 28.0) | 24.4 (23.7, 25.0) | 30.2 (29.5, 30.9) | <0.001 |
| Beverage intake3 | ||||
| mL | 1422 (1392, 1454) | 1462 (1415, 1510) | 1392 (1352, 1433) | 0.02 |
| mL/kg | 18.6 (18.1, 19.0) | 17.0 (16.4, 17.6) | 19.9 (19.3, 20.5) | <0.001 |
| Plain water | ||||
| mL | 496 (478, 513) | 424 (398, 451) | 553 (530, 578) | <0.001 |
| mL/kg | 6.6 (6.4, 6.9) | 5.0 (4.6, 5.3) | 8.0 (7.7, 8.3) | <0.001 |
| Water from food | ||||
| mL | 641 (629, 653) | 609 (592, 626) | 668 (652, 684) | <0.001 |
| mL/kg | 8.4 (8.2, 8.5) | 7.1 (6.9, 7.3) | 9.6 (9.3, 9.8) | <0.001 |
| Urinary creatinine, mg/dL | ||||
| Spot urine | 87.9 (85.5, 90.3) | 109.5 (105.4, 113.8) | 73.6 (71.1, 76.2) | <0.001 |
| 24-h urine | 77.5 (75.2, 79.9) | 98.6 (94.8,102.5) | 63.0 (60.8, 65.4) | <0.001 |
1N for urinary creatinine: total = 2219, men = 990, women = 1239; 24-h creatinine: total = 941, men = 435, women = 506.
2P value comparing geometric means in men and women.
Water from beverages (beverage intake) included plain water, 100% fruit and vegetable juices, sugar-sweetened and nonnutritive-sweetened carbonated beverages, milk (skim/low fat and whole), soy milk, tea, coffee (with and without caffeine), dairy coffee beverages, and beer, wine, and liquor beverages.
Total daily water, beverage, and plain water intakes all demonstrated significant positive trends with HDL cholesterol concentrations across quartile categories of intake characterized as mL (Table 3). TG concentrations were observed to be significantly lower among the highest plain water consumers (P < 0.05), but the inverse associations were only marginally statistically significant (P < 0.10) for total water and beverage intakes. We observed no associations between total water intake, beverage intake, or plain water intake and TC, LDL cholesterol, glucose, HbA1c, SBP, DBP, or CRP. The associations between daily water intakes characterized as mL/kg and the cardiometabolic risk factors were very similar to those for water intake expressed in mL (Supplemental Table 2). We further adjusted for energy intake in a sensitivity analysis, which generally resulted in a modest strengthening of the observed associations between the cardiometabolic risk factors and intakes of total water, beverages and plain water (Supplemental Table 3) .
TABLE 3.
Geometric means (95% CIs) for cardiometabolic risk factors for study participants from the Framingham Heart Study Second Generation and First Generation Omni cohorts by quartile categories of daily water intake (mL)1
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | ||
|---|---|---|---|---|---|
| Characteristic | (n = 558) | (n = 559) | (n = 560) | (n = 561) | P-trend |
| Total cholesterol, mg/dL2 | |||||
| Total water intake3 | 175 (171, 178) | 176 (173, 180) | 177 (174, 181) | 176 (172, 179) | 0.43 |
| Beverage intake4 | 173 (170, 176) | 178 (174, 181) | 176 (173, 180) | 177 (174, 181) | 0.07 |
| Plain water intake | 173 (170, 177) | 177 (173, 180) | 178 (174, 181) | 176 (172, 179) | 0.20 |
| LDL cholesterol,2 mg/dL | |||||
| Total water intake | 91.9 (89.0, 94.9) | 93.1 (90.1, 96.2) | 93.1 (90.1, 96.2) | 91.2 (88.3, 94.2) | 0.65 |
| Beverage intake | 91.1 (88.3, 94.0) | 94.2 (91.2, 97.3) | 92.5 (89.5, 95.6) | 91.9 (88.9, 95.0) | 0.92 |
| Plain water intake | 90.7 (87.9, 93.6) | 93.7 (90.6, 97.0) | 93.6 (90.7, 96.6) | 91.7 (88.7, 94.8) | 0.69 |
| HDL cholesterol,2 mg/dL | |||||
| Total water intake | 56.6 (55.0, 58.2) | 56.8 (55.1, 58.5) | 58.2 (56.5, 60.0) | 58.9 (57.2, 60.7) | 0.004 |
| Beverage intake | 56.0 (54.4, 57.6) | 57.0 (55.4, 58.7) | 58.3 (56.5, 60.0) | 59.8 (58.0, 61.6) | <0.001 |
| Plain water intake | 56.7 (55.1, 58.4) | 55.8 (54.1, 57.6) | 58.7 (57.0, 60.4) | 58.2 (56.5, 59.9) | 0.01 |
| Triglycerides,2 mg/dL | |||||
| Total water intake | 102 (97, 106) | 101 (97, 106) | 99 (94, 104) | 98 (94, 103) | 0.10 |
| Beverage intake | 102 (97, 106) | 102 (97, 107) | 99 (94, 104) | 98 (93, 103) | 0.08 |
| Plain water intake | 102 (97, 106) | 105 (100, 110) | 98 (93, 102) | 99 (94, 104) | 0.05 |
| Fasting glucose,5 mg/dL | |||||
| Total water intake | 103 (101, 104) | 102 (100, 103) | 102 (101, 104) | 104 (102, 105) | 0.24 |
| Beverage intake | 102 (101, 104) | 102 (100, 104) | 103 (101, 104) | 103 (102, 105) | 0.23 |
| Plain water intake | 103 (102, 105) | 101 (100, 103) | 103 (101, 104) | 102 (101, 104) | 0.73 |
| HbA1c,5 % | |||||
| Total water intake | 5.78 (5.74, 5.83) | 5.78 (5.74, 5.82) | 5.81 (5.76, 5.85) | 5.79 (5.75, 5.83) | 0.66 |
| Beverage intake | 5.80 (5.76, 5.84) | 5.78 (5.74, 5.83) | 5.78 (5.74, 5.83) | 5.79 (5.75, 5.84) | 0.83 |
| Plain water intake | 5.79 (5.75, 5.84) | 5.78 (5.74, 5.83) | 5.80 (5.76, 5.84) | 5.77 (5.73, 5.82) | 0.44 |
| C-reactive protein, mg/L | |||||
| Total water intake | 1.57 (1.41, 1.74) | 1.47 (1.32, 1.64) | 1.43 (1.28, 1.60) | 1.44 (1.29, 1.61) | 0.13 |
| Beverage intake | 1.55 (1.39, 1.72) | 1.43 (1.28, 1.60) | 1.48 (1.32, 1.66) | 1.44 (1.29, 1.61) | 0.32 |
| Plain water intake | 1.49 (1.34, 1.66) | 1.52 (1.35, 1.70) | 1.45 (1.31, 1.62) | 1.45 (1.30, 1.62) | 0.47 |
| Systolic blood pressure,6 mm Hg | |||||
| Total water intake | 126 (125, 128) | 128 (126, 130) | 128 (126, 129) | 128 (126, 130) | 0.10 |
| Beverage intake | 126 (125, 128) | 127 (125, 129) | 128 (126, 130) | 128 (126, 130) | 0.06 |
| Plain water intake | 126 (125, 128) | 127 (125, 129) | 128 (126, 130) | 128 (126, 130) | 0.11 |
| Diastolic blood pressure,6,7 mm Hg | |||||
| Total water intake | 72.0 (71.0, 73.0) | 73.0 (71.9, 74.0) | 72.6 (71.6, 73.6) | 72.2 (71.1, 73.2) | 0.98 |
| Beverage intake | 72.1 (71.1, 73.1) | 72.6 (71.6, 73.6) | 73.0 (71.9, 74.0) | 72.1 (71.0, 73.1) | 0.96 |
| Plain water intake | 72.1 (71.1, 73.1) | 72.2 (71.2, 73.3) | 72.8 (71.8, 73.8) | 72.6 (71.6, 73.6) | 0.26 |
Adjusted for age (years), sex, cohort, BMI (kg/m2), education (<college, ≥college), current smoker (yes/no), and physical activity score.
Additional adjustment for cholesterol-lowering medication use (yes/no).
Total water from foods and beverages.
Water from beverages (beverage intake) included plain water, 100% fruit and vegetable juices, sugar-sweetened and nonnutritive-sweetened carbonated beverages, milk (skim/low fat and whole), soy milk, tea, coffee (with and without caffeine), dairy coffee beverages, and beer, wine, and liquor beverages.
Additional adjustment for insulin lowering medication (yes/no).
Additional adjustment for antihypertensive medication use (yes/no).
Arithmetic mean.
We observed significant positive trends between 24-h urine creatinine concentrations and LDL cholesterol (P-trend = 0.004) and TG (P-trend = 0.006) concentrations, as well as a significant inverse trend between the 24-h urine creatinine concentrations and HDL cholesterol concentrations (P-trend = 0.002) (Table 4). The spot urine creatinine concentrations were unrelated to any of the cardiometabolic risks with the exception of TGs, with which they were positively associated (P = 0.01). As with water intake, we observed no associations between urine creatinine measures and glucose, HbA1c, SBP, DBP, or CRP.
TABLE 4.
Geometric means (95% CIs) for cardiometabolic risk factors for study participants from the Framingham Heart Study Second Generation and First Generation Omni cohorts by quartile categories of urinary creatinine concentrations (spot and 24 h)1
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | ||
|---|---|---|---|---|---|
| Characteristic | (n = 546/238)2 | (n = 550/235) | (n = 546/227) | (n = 543/231) | P-trend |
| Total cholesterol,3 mg/dL | |||||
| Spot creatinine, mg/dL | 175 (172, 179) | 177 (173, 180) | 176 (172, 179) | 178 (175, 182) | 0.21 |
| 24-h creatinine, mg/dL | 173 (168, 179) | 174 (168, 179) | 178 (172, 183) | 178 (172, 184) | 0.08 |
| HDL cholesterol, mg/dL | |||||
| Spot creatinine, mg/dL | 57.3 (55.6, 59.1) | 58.5 (56.8, 60.3) | 57.1 (55.5, 58.9) | 57.7 (56.0, 59.5) | 0.98 |
| 24-h creatinine, mg/dL | 60.2 (57.4, 63.1) | 57.4 (55.0, 60.0) | 57.9 (55.3, 60.6) | 54.9 (52.4, 57.5) | 0.002 |
| LDL cholesterol, mg/dL | |||||
| Spot creatinine, mg/dL | 93.4 (90.3, 96.6) | 92.3 (89.3, 95.4) | 93.0 (90.0, 96.1) | 93.5 (90.4, 96.6) | 0.81 |
| 24-h creatinine, mg/dL | 90.4 (85.6, 95.3) | 90.1 (85.7, 94.7) | 93.5 (88.8, 98.4) | 97.6 (92.6,102.9) | 0.004 |
| Triglycerides, mg/dL | |||||
| Spot creatinine, mg/dL | 96.6 (92.0, 101.4) | 99.0 (94.4, 103.8) | 99.2 (94.7, 104.0) | 103.4 (98.6, 108.4) | 0.01 |
| 24-h creatinine, mg/dL | 89.5 (83.0, 96.5) | 98.7 (92.0,105.9) | 99.0 (92.1,106.5) | 102.5 (95.2,110.4) | 0.006 |
| Glucose,4 mg/dL | |||||
| Spot creatinine, mg/dL | 102 (100, 103) | 102 (101, 104) | 102 (101, 104) | 103 (101, 104) | 0.28 |
| 24-h creatinine, mg/dL | 101 (98, 103) | 101 (98, 103) | 101 (99.0, 104) | 102 (100, 105) | 0.29 |
| HbA1c,4 % | |||||
| Spot creatinine, mg/dL | 5.77 (5.73, 5.82) | 5.77 (5.73, 5.81) | 5.79 (5.75, 5.83) | 5.79 (5.74, 5.83) | 0.52 |
| 24-h creatinine, mg/dL | 5.74 (5.68, 5.81) | 5.75 (5.69, 5.81) | 5.76 (5.70, 5.83) | 5.76 (5.70, 5.83) | 0.60 |
| C-reactive protein, mg/L | |||||
| Spot creatinine, mg/dL | 1.42 (1.26, 1.59) | 1.44 (1.28, 1.61) | 1.42 (1.27, 1.59) | 1.55 (1.38, 1.73) | 0.15 |
| 24-h creatinine, mg/dL | 1.38 (1.15, 1.66) | 1.49 (1.25, 1.76) | 1.43 (1.19, 1.70) | 1.38 (1.15, 1.66) | 0.83 |
| Systolic blood pressure,5 mm Hg | |||||
| Spot creatinine, mg/dL | 127 (126, 129) | 128 (126, 130) | 127 (126, 129) | 127 (126, 129) | 0.63 |
| 24-h creatinine, mg/dL | 127 (125, 130) | 126 (123, 128) | 129 (126, 131) | 126 (124, 129) | 0.92 |
| Diastolic blood pressure,5,6 mm Hg | |||||
| Spot creatinine, mg/dL | 72.5 (71.5, 73.4) | 72.4 (71.4, 73.3) | 72.5 (71.6, 73.5) | 72.8 (71.8, 73.8) | 0.48 |
| 24-h creatinine, mg/dL | 71.5 (70.0, 73.1) | 72.3 (70.9, 73.8) | 72.9 (71.5, 74.4) | 72.8 (71.2, 74.3) | 0.18 |
Adjusted for age (years), sex, cohort, BMI (kg/m2), education (<college, ≥college), current smoker (yes/no), physical activity score, hypertension (yes/no) and diabetes (yes/no). HbA1c, glycated hemoglobin.
Number of participants per quartile category for the total sample and 24-h urine subsample.
Additional adjustment for cholesterol-lowering medication use (yes/no).
Additional adjustment for diabetes medication use (yes/no).
Additional adjustment for antihypertensive medication use (yes/no).
Arithmetic mean.
Water from food was unrelated to any of the cardiometabolic risk factors considered except for an inverse relation with CRP concentrations expressed as mL (P-trend < 0.001) and mL/kg (P-trend = 0.004) (Supplemental Table 4). As seen for total water, beverage and plain water intakes, further adjustment for energy intake in a sensitivity analysis generally resulted in a modest strengthening of the observed associations between the cardiometabolic risk factors and water from foods (Supplemental Table 5).
Discussion
There is limited, but accumulating, evidence that water intake and hydration may play a role in cardiometabolic health in younger adults (9–14), but apart from the well-documented adverse effects of dehydration (47–49), we know little of the health effects of habitual low water intake or evidence of underhydration on chronic conditions in older adults. We undertook the present study to extend our understanding of hydration and cardiometabolic health in older adults, using measures of water intake as well as measures of urine concentration to assess hydration. Our most consistent finding was that water intake and hydration were beneficially associated with circulating lipid concentrations. Specifically, total water, beverage intake, and plain water intake all demonstrated beneficial associations with HDL cholesterol concentrations, and similar beneficial associations were seen with TG concentrations. These findings are supported by the observations that better hydration (as assessed by 24-h urine creatinine concentrations) was associated with lower TG and LDL cholesterol and higher HDL cholesterol concentrations. Hydration status based on spot urine collections was also beneficially associated with TG concentrations. The consistent pattern of findings observed with the circulating lipid concentrations across the different water sources and hydration markers supports an association between hydration and lipid metabolism. Although underhydration due to habitual low daily water intake is thought to play a role in cardiometabolic risk through osmotic stimulation of AVP release, which together with corticotrophin-releasing hormone, mediates adrenocorticotropic hormone release, a key player in the stress response (50–53), and through the indirect effect of substitution of water as a calorie-free beverage for other caloric beverages (9), an understanding of the mechanisms relating hydration and lipid metabolism will need to await future work as our findings cannot directly address potential mechanisms.
We also observed that CRP concentrations were significantly lower among individuals with the highest intakes of water from foods. However, given that there was no evidence that water intakes from nonfood sources or hydration markers was related to CRP concentrations, the association with water from foods may be a consequence of the composition of the foods, such as vegetables and fruit, providing higher water intakes. Moreover, the mean CRP concentrations were only modestly elevated, and the differences between means for the highest and lowest quartile categories of daily water intake from food were small.
The associations we observed between water intake and urine concentration with circulating lipids, to the best of our knowledge, have not been previously reported in older adults. Two previous studies have reported associations between higher copeptin (an equimolar surrogate for AVP secretion) and blood lipids in community-dwelling adults (22, 54). The current analysis adds evidence for a direct association between low water intake or high urine concentration and a poorer blood lipid profile in older adults.
We were unable to replicate observations from earlier studies in generally younger individuals that observed cross-sectional or longitudinal associations between copeptin or water intake and measures of glycemic control (9, 12–14, 16, 17, 54–57). This discrepancy may be due to the older age of our population [mean age of 70 y compared with mean ages of ∼58 and 49 in Swedish (11) and Dutch (55) cohorts] and worse metabolic health. Mean plasma fasting glucose in our sample was 5.7 mmol/L compared with 5.1 and 4.6–4.9 mmol/L in the Swedish and Dutch cohorts. We also did not observe any associations between water intake or hydration and systolic or diastolic blood pressure, but more than half of the current sample was taking hypertensive medication, limiting our ability to observe any possible associations. A further complicating factor is that certain blood pressure medications exert an influence on water (and sodium) balance.
The work benefited from a large, well-characterized population-based cohort of older men and women with a large array of cardiometabolic phenotypes. Another strength of this study was the inclusion of both reported water intakes from beverages and food sources and urinary markers of hydration. However, there are also notable limitations. Although commonly used in epidemiologic studies, FFQs are not without their limitations. Although they do not provide truly quantitative intakes and likely underreport energy intake, FFQs provide good estimates of relative intake, giving us the ability to distinguish between high and low consumers of a particular food. Moreover, validation studies of the Harvard FFQ, which we used in the present study, demonstrated that beverage intake assessed by multiple 7-d diet records completed over the course of 1 y generally correlates well with intake assessed by this FFQ completed at the end of the year. For example, correlations for most of the important beverage water sources in women (29) and men (28), respectively, were 0.81 and 0.88 for skim/low-fat milk, 0.84 and 0.78 for orange juice, 0.78 and 0.93 for coffee, 0.93 and 0.77 for tea, 0.84 in both men and women for cola beverages, and 0.94 and 0.83 for beer. The correlation for other juice (0.89) was assessed only in men, as was that for plain water, which was more modest than for other beverages at 0.52. Also, given the possible impact of age on water consumption and hydration biomarkers, the wide age range of our participants may have limited the strength of the observed associations.
Although urinary creatinine is a proxy for urine dilution (44), urine concentration is usually measured more by its specific gravity or osmolality, which has been shown to vary with water intake (58, 59). In the absence of measured urine osmolality or specific gravity in this data set, urine creatinine was the best proxy for urine concentration available. Moreover, as the findings based on urinary creatinine largely corroborate the associations found based on water intake, the use of urinary creatinine does not diminish the validity of our findings. The cross-sectional nature of the study is another limitation, as we cannot assess the temporal relation between water intake and hydration and the metabolic risk factors. Many hydration factors are affected by age, and our sample covered a wide age range, which may result in greater misclassification of hydration status in older participants based on the intake and urine creatinine concentrations, but such misclassification would tend to result in underestimation of the associations with cardiometabolic risk factors. We initially hypothesized that some of the impact of water intake on cardiometabolic risk might be mediated by a reduced energy intake, but adjusting for energy intake modestly strengthened the observed associations, contrary to our expectations. While we were unable to accurately control for potential confounding or modification by total energy intake based on the FFQ, we addressed this by including BMI and a physical activity index as covariates in our models, although the latter is based on self-report and also subject to misclassification. Finally, despite the inclusion of the FHS Omni1 cohort participants in this study, the study participants remain largely Caucasians of European ancestry.
Our findings add to the growing evidence that inadequate water intake and underhydration may lead to higher cardiometabolic risk. Understanding the role of hydration for metabolic health is critical, given the potential public health impact of inadequate hydration, particularly among older individuals. Based on our findings in conjunction with earlier evidence, prospective studies of hydration and changes in cardiometabolic health are warranted to address limitations of the existing evidence and the current gaps in our knowledge of this relation.
Supplementary Material
Acknowledgments
The authors’ responsibilities were as follows—PFJ, JDS, and ETP: designed the research; PFJ and GR: conducted the research; GR: analyzed data and performed statistical analysis; PFJ and ETP: jointly drafted the paper and had primary responsibility for final content; and all authors: contributed to critically reviewing the manuscript and read and approved the final version.
Notes
Supported in part by Danone Research, the USDA Agricultural Research Service (agreement #58-1950-4-003), and the National Heart, Lung, and Blood Institute Framingham Heart Study (contract HHSN2682015000011). The views expressed in this article are of those of the authors and do not necessarily represent the views of the funding organizations.
Author disclosures: ETP is a full-time employee of Danone Research. JDS served as a consultant for Danone Research. All other authors report no conflicts of interest.
Supplemental Tables 1–5 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn/.
Abbreviations used: AVP, arginine vasopressin; CRP, C-reactive protein; DBP, diastolic blood pressure; FHS, Framingham Heart Study; Gen2, FHS Second Generation cohort; HbA1c, glycated hemoglobin; Omni1, FHS First Generation Omni cohort; PAI, physical activity index; SBP, systolic blood pressure; TC, total cholesterol; TG, triglyceride; T2D, type 2 diabetes.
Contributor Information
Paul F Jacques, USDA Human Nutrition Research Center on Aging, Tufts University, Boston, MA, USA.
Gail Rogers, USDA Human Nutrition Research Center on Aging, Tufts University, Boston, MA, USA.
Jodi Dunmeyer Stookey, Hydration Sciences Lab, Arizona State University, Tempe, AZ, USA.
Erica T Perrier, Health, Hydration & Nutrition Science, Danone Research, Palaiseau, France.
References
- 1.Perrier ET, Armstrong LE, Daudon M, Kavouras S, Lafontan M, Lang F, Peronnet F, Stookey JD, Tack I, Klein A. From state to process: defining hydration. Obes Facts. 2014;7(2):6–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Panel on Dietary Reference Intakes for Electrolytes and Water , Food and Nutrition Board, Institute of Medicine of the National Academies. Dietary reference intakes for water, potassium, sodium, chloride, and sulfate. Washington (DC): The National Academies Press; 2005. [Google Scholar]
- 3.Rush EC. Water: neglected, unappreciated and under researched. Eur J Clin Nutr. 2013;67(5):492–5. [DOI] [PubMed] [Google Scholar]
- 4.Kavouras SA. Hydration, dehydration, underhydration, optimal hydration: are we barking up the wrong tree?. Eur J Nutr. 2019;58(2):471–3. [DOI] [PubMed] [Google Scholar]
- 5.Stookey JD, Barclay D, Arieff A, Popkin BM. The altered fluid distribution in obesity may reflect plasma hypertonicity. Eur J Clin Nutr. 2007;61(2):190–9. [DOI] [PubMed] [Google Scholar]
- 6.Padrao P, Sousa AS, Guerra RS, Alvares L, Santos A, Borges N, Afonso C, Amaral TF, Moreira P. A cross-sectional study on the association between 24-h urine osmolality and weight status in older adults. Nutrients. 2017;9(11):1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosinger AY, Lawman HG, Akinbami LJ, Ogden CL. The role of obesity in the relation between total water intake and urine osmolality in US adults, 2009–2012. Am J Clin Nutr. 2016;104(6):1554–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang T, Ravi N, Plegue MA, Sonneville KR, Davis MM. Inadequate hydration, BMI, and obesity among US adults: NHANES 2009–2012. Ann Fam Med. 2016;14(4):320–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carroll HA, Betts JA, Johnson L. An investigation into the relationship between plain water intake and glycated Hb (HbA1c): a sex-stratified, cross-sectional analysis of the UK National Diet and Nutrition Survey (2008–2012). Br J Nutr. 2016;116(10):1770–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Enhorning S, Bankir L, Bouby N, Struck J, Hedblad B, Persson M, Morgenthaler NG, Nilsson PM, Melander O. Copeptin, a marker of vasopressin, in abdominal obesity, diabetes and microalbuminuria: the prospective Malmo Diet and Cancer Study cardiovascular cohort. Int J Obes. 2013;37(4):598–603. [DOI] [PubMed] [Google Scholar]
- 11.Enhorning S, Struck J, Wirfalt E, Hedblad B, Morgenthaler NG, Melander O. Plasma copeptin, a unifying factor behind the metabolic syndrome. J Clin Endocrinol Metab. 2011;96(7):E1065–72. [DOI] [PubMed] [Google Scholar]
- 12.Enhorning S, Wang TJ, Nilsson PM, Almgren P, Hedblad B, Berglund G, Struck J, Morgenthaler NG, Bergmann A, Lindholm Eet al. . Plasma copeptin and the risk of diabetes mellitus. Circulation. 2010;121(19):2102–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roussel R, El Boustany R, Bouby N, Potier L, Fumeron F, Mohammedi K, Balkau B, Tichet J, Bankir L, Marre Met al. . Plasma copeptin, AVP gene variants, and incidence of type 2 diabetes in a cohort from the community. J Clin Endocrinol Metab. 2016;101(6):2432–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roussel R, Fezeu L, Bouby N, Balkau B, Lantieri O, Alhenc-Gelas F, Marre M, Bankir L. Low water intake and risk for new-onset hyperglycemia. Diabetes Care. 2011;34(12):2551–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Milla-Tobarra M, Garcia-Hermoso A, Lahoz-Garcia N, Notario-Pacheco B, Lucas-de la Cruz L, Pozuelo-Carrascosa DP, Garcia-Meseguer MJ, Martinez-Vizcaino V. The association between water intake, body composition and cardiometabolic factors among children—The Cuenca study. Nutr Hosp. 2016;33(Suppl 3):19–26. [DOI] [PubMed] [Google Scholar]
- 16.Johnson EC, Bardis CN, Jansen LT, Adams JD, Kirkland TW, Kavouras SA. Reduced water intake deteriorates glucose regulation in patients with type 2 diabetes. Nutr Res. 2017;43:25–32. [DOI] [PubMed] [Google Scholar]
- 17.Wannamethee SG, Welsh P, Papacosta O, Lennon L, Whincup PH, Sattar N. Copeptin, insulin resistance, and risk of incident diabetes in older men. J Clin Endocrinol Metab. 2015;100(9):3332–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wannamethee SG, Welsh P, Lennon L, Papacosta O, Whincup PH, Sattar N. Copeptin and the risk of incident stroke, CHD and cardiovascular mortality in older men with and without diabetes: the British Regional Heart Study. Diabetologia. 2016;59(9):1904–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stookey JD, Pieper CF, Cohen HJ. Is the prevalence of dehydration among community-dwelling older adults really low? Informing current debate over the fluid recommendation for adults aged 70+years. Public Health Nutr. 2005;8(8):1275–85. [DOI] [PubMed] [Google Scholar]
- 20.Stookey JD, Pieper CF, Cohen HJ. Hypertonic hyperglycemia progresses to diabetes faster than normotonic hyperglycemia. Eur J Epidemiol. 2004;19(10):935–44. [DOI] [PubMed] [Google Scholar]
- 21.Stookey JD, Purser JL, Pieper CF, Cohen HJ. Plasma hypertonicity: another marker of frailty?. J Am Geriatr Soc. 2004;52(8):1313–20. [DOI] [PubMed] [Google Scholar]
- 22.Saleem U, Khaleghi M, Morgenthaler NG, Bergmann A, Struck J, Mosley TH Jr, Kullo IJ. Plasma carboxy-terminal provasopressin (copeptin): a novel marker of insulin resistance and metabolic syndrome. J Clin Endocrinol Metab. 2009;94(7):2558–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feinleib M, Kannel WB, Garrison RJ, McNamara PM, Castelli WP. The Framingham Offspring Study: design and preliminary data. Prev Med. 1975;4(4):518–25. [DOI] [PubMed] [Google Scholar]
- 24.Quan SF, Howard BV, Iber C, Kiley JP, Nieto FJ, O'Connor GT, Rapoport DM, Redline S, Robbins J, Samet JMet al. . The Sleep Heart Health Study: design, rationale, and methods. Sleep. 1997;20:1077–85. [PubMed] [Google Scholar]
- 25.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF III, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene Tet al. . A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, Hennekens CH, Speizer FE. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122(1):51–65. [DOI] [PubMed] [Google Scholar]
- 27.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135(10):1114–26. [DOI] [PubMed] [Google Scholar]
- 28.Feskanich D, Rimm EB, Giovannucci EL, Colditz GA, Stampfer MJ, Litin LB, Willett WC. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. J Am Diet Assoc. 1993;93(7):790–6. [DOI] [PubMed] [Google Scholar]
- 29.Salvini S, Hunter DJ, Sampson L, Stampfer MJ, Colditz GA, Rosner B, Willett WC. Food-based validation of a dietary questionnaire: the effects of week-to-week variation in food consumption. Int J Epidemiol. 1989;18(4):858–67. [DOI] [PubMed] [Google Scholar]
- 30.Hu FB, Rimm E, Smith-Warner SA, Feskanich D, Stampfer MJ, Ascherio A, Sampson L, Willett WC. Reproducibility and validity of dietary patterns assessed with a food-frequency questionnaire. Am J Clin Nutr. 1999;69(2):243–9. [DOI] [PubMed] [Google Scholar]
- 31.Jacques PF, Sulsky SI, Sadowski JA, Phillips JC, Rush D, Willett WC. Comparison of micronutrient intake measured by a dietary questionnaire and biochemical indicators of micronutrient status. Am J Clin Nutr. 1993;57(2):182–9. [DOI] [PubMed] [Google Scholar]
- 32.Ascherio A, Stampfer MJ, Colditz GA, Rimm EB, Litin L, Willett WC. Correlations of vitamin A and E intakes with the plasma concentrations of carotenoids and tocopherols among American men and women. J Nutr. 1992;122(9):1792–801. [DOI] [PubMed] [Google Scholar]
- 33.Wilson KM, Vesper HW, Tocco P, Sampson L, Rosen J, Hellenas KE, Tornqvist M, Willett WC. Validation of a food frequency questionnaire measurement of dietary acrylamide intake using hemoglobin adducts of acrylamide and glycidamide. Cancer Causes Control. 2009;20(3):269–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Holmes MD, Powell IJ, Campos H, Stampfer MJ, Giovannucci EL, Willett WC. Validation of a food frequency questionnaire measurement of selected nutrients using biological markers in African-American men. Eur J Clin Nutr. 2007;61(11):1328–36. [DOI] [PubMed] [Google Scholar]
- 35.Cho E, Zeisel SH, Jacques P, Selhub J, Dougherty L, Colditz GA, Willett WC. Dietary choline and betaine assessed by food-frequency questionnaire in relation to plasma total homocysteine concentration in the Framingham Offspring Study. Am J Clin Nutr. 2006;83(4):905–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Romieu I, Stampfer MJ, Stryker WS, Hernandez M, Kaplan L, Sober A, Rosner B, Willett WC. Food predictors of plasma beta-carotene and alpha-tocopherol: validation of a food frequency questionnaire. Am J Epidemiol. 1990;131(5):864–76. [DOI] [PubMed] [Google Scholar]
- 37.Garland M, Sacks FM, Colditz GA, Rimm EB, Sampson LA, Willett WC, Hunter DJ. The relation between dietary intake and adipose tissue composition of selected fatty acids in US women. Am J Clin Nutr. 1998;67(1):25–30. [DOI] [PubMed] [Google Scholar]
- 38.Hunter DJ, Rimm EB, Sacks FM, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Comparison of measures of fatty acid intake by subcutaneous fat aspirate, food frequency questionnaire, and diet records in a free-living population of US men. Am J Epidemiol. 1992;135(4):418–27. [DOI] [PubMed] [Google Scholar]
- 39.Armstrong LE, Johnson EC, Munoz CX, Swokla B, Le Bellego L, Jimenez L, Casa DJ, Maresh CM. Hydration biomarkers and dietary fluid consumption of women. J Acad Nutr Diet. 2012;112(7):1056–61. [DOI] [PubMed] [Google Scholar]
- 40.Armstrong LE, Pumerantz AC, Fiala KA, Roti MW, Kavouras SA, Casa DJ, Maresh CM. Human hydration indices: acute and longitudinal reference values. Int J Sport Nutr Exerc Metab. 2010;20(2):145–53. [DOI] [PubMed] [Google Scholar]
- 41.Perrier E, Rondeau P, Poupin M, Le Bellego L, Armstrong LE, Lang F, Stookey J, Tack I, Vergne S, Klein A. Relation between urinary hydration biomarkers and total fluid intake in healthy adults. Eur J Clin Nutr. 2013;67(9):939–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Perrier E, Vergne S, Klein A, Poupin M, Rondeau P, Le Bellego L, Armstrong LE, Lang F, Stookey J, Tack I. Hydration biomarkers in free-living adults with different levels of habitual fluid consumption. Br J Nutr. 2013;109(9):1678–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Johnson EC, Munoz CX, Le Bellego L, Klein A, Casa DJ, Maresh CM, Armstrong LE. Markers of the hydration process during fluid volume modification in women with habitual high or low daily fluid intakes. Eur J Appl Physiol. 2015;115(5):1067–74. [DOI] [PubMed] [Google Scholar]
- 44.Barr DB, Wilder LC, Caudill SP, Gonzalez AJ, Needham LL, Pirkle JL. Urinary creatinine concentrations in the U.S. population: implications for urinary biologic monitoring measurements. Environ Health Perspect. 2005;113(2):192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Godevithanage S, Kanankearachchi PP, Dissanayake MP, Jayalath TA, Chandrasiri N, Jinasena RP, Kumarasiri RP, Goonasekera CD. Spot urine osmolality/creatinine ratio in healthy humans. Kidney Blood Pressure Res. 2010;33(4):291–6. [DOI] [PubMed] [Google Scholar]
- 46.Martin SS, Blaha MJ, Elshazly MB, Toth PP, Kwiterovich PO, Blumenthal RS, Jones SR.. Comparison of a novel method vs the Friedewald equation for estimating low-density lipoprotein cholesterol levels from the standard lipid profile. JAMA. 2013;310(19):2061–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.El-Sharkawy AM, Sahota O, Lobo DN. Acute and chronic effects of hydration status on health. Nutr Rev. 2015;73(Suppl 2):97–109. [DOI] [PubMed] [Google Scholar]
- 48.Lemetais G, Melander O, Vecchio M, Bottin JH, Enhorning S, Perrier ET. Effect of increased water intake on plasma copeptin in healthy adults. Eur J Nutr. 2018;57(5):1883–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Popkin BM, D'Anci KE, Rosenberg IH. Water, hydration, and health. Nutr Rev. 2010;68(8):439–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bankir L. Antidiuretic action of vasopressin: quantitative aspects and interaction between V1a and V2 receptor-mediated effects. Cardiovasc Res. 2001;51(3):372–90. [DOI] [PubMed] [Google Scholar]
- 51.Papadimitriou A, Priftis KN. Regulation of the hypothalamic-pituitary-adrenal axis. Neuro Immuno Modulation. 2009;16(5):265–71. [DOI] [PubMed] [Google Scholar]
- 52.Tanoue A, Ito S, Honda K, Oshikawa S, Kitagawa Y, Koshimizu TA, Mori T, Tsujimoto G. The vasopressin V1b receptor critically regulates hypothalamic-pituitary-adrenal axis activity under both stress and resting conditions. J Clin Invest. 2004;113(2):302–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Taveau C, Chollet C, Bichet DG, Velho G, Guillon G, Corbani M, Roussel R, Bankir L, Melander O, Bouby N. Acute and chronic hyperglycemic effects of vasopressin in normal rats: involvement of V1A receptors. Am J Physiol Endocrinol Metab. 2017;312(3):E127–35. [DOI] [PubMed] [Google Scholar]
- 54.El Boustany R, Tasevska I, Meijer E, Kieneker LM, Enhorning S, Lefevre G, Mohammedi K, Marre M, Fumeron F, Balkau Bet al. . Plasma copeptin and chronic kidney disease risk in 3 European cohorts from the general population. JCI Insight. 2018;3(13):e121479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Abbasi A, Corpeleijn E, Meijer E, Postmus D, Gansevoort RT, Gans RO, Struck J, Hillege HL, Stolk RP, Navis Get al. . Sex differences in the association between plasma copeptin and incident type 2 diabetes: the Prevention of Renal and Vascular Endstage Disease (PREVEND) study. Diabetologia. 2012;55(7):1963–70. [DOI] [PubMed] [Google Scholar]
- 56.Meijer E, Bakker SJ, Halbesma N, de Jong PE, Struck J, Gansevoort RT. Copeptin, a surrogate marker of vasopressin, is associated with microalbuminuria in a large population cohort. Kidney Int. 2010;77(1):29–36. [DOI] [PubMed] [Google Scholar]
- 57.Enhorning S, Brunkwall L, Tasevska I, Ericson U, Persson M, Lemetais G, Vanhaecke T, Dolci A, Perrier ETet al. . Water supplementation reduces copeptin and plasma glucose in adults with high copeptin: the H2O metabolism pilot study. J Clin Endocrinol Metab. 2019;104(6):1917–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Johnson EC, Huffman AE, Yoder H, Dolci A, Perrier ET, Larson-Meyer DE, Armstrong LE. Urinary markers of hydration during 3-day water restriction and graded rehydration. Eur J Nutr. 2020;59(5):2171–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Perrier E, Demazieres A, Girard N, Pross N, Osbild D, Metzger D, Guelinckx I, Klein A. Circadian variation and responsiveness of hydration biomarkers to changes in daily water intake. Eur J Appl Physiol. 2013;113(8):2143–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


