Abstract
Purpose
The aim of this study was to assess the relationship of coronary artery disease (CAD) with levels of homocysteine and sortilin in Egyptian patients.
Background
CAD is a primary contributor to cardiac disease and a prominent cause of death globally.
Patients and Methods
We enrolled 45 patients with CAD evaluated by coronary CT angiography and 42 control subjects without CAD. Plasma-homocysteine and -sortilin levels were measured with a commercial ELISA kit.
Results
Elevated levels of homocysteine and sortilin were observed in the CAD patients compared to controls (13.75±1.40 vs 7.73±2.06 μmol/L, P=0 and 160.91±32.17 vs 143.02±32.30 ng/dL, P=0.02, respectively). Significantly higher total cholesterol, low density–lipoprotein cholesterol and triglycerides (P<0.05) and lower high density–lipoprotein cholesterol (P<0.05) were seen among patients with CAD than the control group. Sortilin levels were positively associated with homocysteine levels (r=0.32, P=0.006), total cholesterol (r=0.61, P=0), low density–lipoprotein cholesterol (r=0.37, P=0.001), triglycerides (r=0.91, P=0), troponin I (r=0.82, P=0), Gensini score (r=0.93, P=0) and high-sensitivity CRP (r=0.87, P=0) in all subjects. Homocysteine has a significantly negative association with high density–lipoprotein cholesterol (r=−0.42, P=0).
Conclusion
Elevated homocysteine and sortilin levels are crucial risk factors of CAD in Egyptian patients.
Keywords: sortilin, homocysteine, coronary artery disease
Introduction
Coronary heart diseases (CHDs), such as angina, stroke, and myocardial infarction, are serious atherosclerotic conditions leading to death. Atherosclerosis is an inflammatory condition of vessels characterized by atheroma (or plaque) buildup through thickening of intimae with accumulation of cholesterol and macrophage foam–cell induction on injured blood-vessel walls.1
Dyslipidemia, hypertension, diabetes mellitus, age, sex, smoking, and obesity are traditional risk factors of developing coronary artery disease (CAD).2 Identification of new biomarkers for the prediction of CAD is helpful in identifying high-risk CAD patients.3 Homocysteine (Hcy) is a sulfur amino acid whose metabolism stands at the intersection of two pathways: remethylation to methionine, which requires folate and vitamin B12, and transsulfuration to cystathionine, which requires pyridoxal 5ʹ-phosphate.4
Metabolic pathway disturbance results in accumulation of intracellular Hcy, which is transferred into the plasma before reaching cytotoxic concentrations.
Hyperhomocysteinemia is caused by disease states, nutritional deficiencies, genetic mutations, and medications, due to alteration of the pathway of its metabolism.5 Increased rates of cardiovascular events are correlated with high levels of Hcy, which has been suggested to be causatively linked to atherosclerosis.6
Large research programs have focused on the identification of new risk factors to prevent CAD, with special attention paid to Hcy, as hyperhomocysteinemia contributes to atherosclerosis through several mechanisms, such as endothelial dysfunction, increased permeability of lipid and inflammatory cells, lipoprotein oxidation, vascular inflammation, and smooth-muscle proliferation.7
Many inflammatory markers, eg, CRP, are well known to play a role in the pathogenesis of atherosclerosis, and have been associated with the risk of cardiovascular disease (CVD).8 Evidence suggests that CRP might have biological impacts on endothelial function, coagulation, fibrinolysis, oxidation of LDL, and plaque stability.9 Increased risk of additional coronary events in patients with CAD has been reported to be associated with high-sensitivity CRP (hs-CRP) levels,10 and hs-CRP is a strong predictor of cardiovascular events.11
Detection of cardiac troponins has grown from being just a diagnostic tool for acute coronary syndrome to a biomarker for risk stratification in individuals without known CVD.12,13
Elevated high-sensitivity troponin I is substantially linked to an increase in worldwide CVD incidence in the general population independently of traditional risk factors.14
High-sensitivity troponin testing may be useful in detecting CHD and triggering more aggressive primary prevention. This indicates a paradigm change from troponin’s use as a diagnostic tool in the acute setting to its use as a main preventive strategy.12
Sortilin has recently received much interest, owing to its suspected roles in lipid-disorder diseases.15 Sotilin, a novel regulator of lipid metabolism, is coded by the cardiovascular-risk gene SORT1 and is likely to facilitate the development of atherosclerosis by affecting lipid metabolism in the liver and macrophages. In atherosclerotic lesions, sortilin has been reported to be overexpressed, but is rarely detectable in normal young vessels, which further indicates that it contributes to and supports the progression of atherosclerosis.15–18
The proatherosclerotic mechanism of sortilin is due to its ability to affect liver- and macrophage-lipid metabolism, leading to increased plasma levels of low-density lipoprotein cholesterol (LDL-C) and macrophage-lipid accumulation. On the other hand, sortilin accelerates the biosynthesis and release of very low–density lipoprotein (VLDL) from the liver, leading to elevated plasma LDL-C levels and atherosclerosis development.16,19
Sortilin inhibits hepatic uptake of plasma LDL-C, resulting in elevated plasma LDL-C levels and atherosclerosis development.20 Sortilin overexpression in macrophages can promote the uptake of LDL-C and intracellular lipid accumulation, which results in the formation of foam cells and atherosclerotic lesion development.21 In this study, we studied whether circulating Hcy and sortilin can be used as biomarkers for screening of CAD in Egyptian population. These biomarkers may be good candidates as therapeutic targets, leading to obviation of complications and decreasedmortality rates, and could help in developing novel therapeutic strategies to stop atherosclerosis progression caused by hyperlipidemia.
Patients and Methods
Study Population
A total of 45 CAD patients (men and women aged 35–65 years) were selected from the Cardiology Department outpatient clinics at Damanhour National Medical Institute, Egypt between June 2020 to September 2020. CAD was diagnosed by coronary angiography revealing >70% stenosis in at least one vessel, and all CAD patients were managed with low-dose aspirin (≤100 mg/day). A total of 42 non-CAD subjects (men and women aged 35–65 years) were also recruited as controls from outpatient clinics of the same institute during routine checkups or scheduled pharmacy refill appointments. The study was approved by the institutional ethics committee in accordance with the Declaration of Helsinki and its amendments, and all participants gave informed consent before initiation of the study. Patients with fever, bacterial/viral infection, history of renal failure, chronic hepatic diseases, arthritis, malignancies, autoimmune disease, or other severe medical illnesses were excluded. Patients who had taken any medication containing vitamins or folic acid in the 3 months before enrollment were also excluded.
Blood Sampling
Blood samples were obtained from each participant after fasting not less than 8 hours. For CAD patients, biochemical analysis was performed within 3 months of coronary CT angiography. Venous blood (10 mL) was withdrawn in an EDTA tube, then centrifuged at 3,000 rpm for 15 min at 4°C. The plasma was immediately frozen at −20°C until analysis. The samples were evaluated in blocks to decrease interassay variability.
Anthropometric Assessment
Weight and height of the enrolled patients were assessed while patients were wearing casual clothes and barefoot. BMI was also calculated.
Coronary Angiography and Blood-Pressure Measurement
The blood pressure of the patients was measured following a 15-minute resting period in the sitting position. All patients underwent diagnostic coronary angiography through radial or femoral access. The Gensini score was utilized to determine CAD severity by an expert cardiologist who was blind to the biochemical results,22 and was calculated by multiplying the luminal narrowing of main coronary artery grading by the site and importance of the lesion factor. Luminal narrowing scores were 32 for total occlusion, 16 for 91%–99% stenosis, 8 for 76%–90% stenosis, 4 for 51%–75% stenosis, 2 for 26%–50% stenosis, and 1 for ≤25% stenosis. The location factor was 1.5 for a mid lesion, 1 for distal LAD (left anterior descending), mid-distal LCX (left circus flex), or RCA (right coronary artery), 5 for left main, and 2.5 for proximal lesions of LAD or LCX. Then, the sum of coronary artery scores was used to calculate the total Gensini score.23
Biochemical Analyses
Plasma glucose was determined using the hexokinase method. Fasting lipid profile was determined using commercial kits. Total cholesterol (TC) and triglyceride (TG) levels were estimated using enzymatic colorimetry. High-density lipoprotein cholesterol (HDL-C) was determined using the precipitation method. LDL-C was calculated by the Friedewald formula: LDL-C = [TC − HDL-C − (TGs/5)]. hs-CRP was assessed using latex-enhanced turbidometric immunoassays (Denka Seiken, Tokyo, Japan). Circulating plasma sortilin and Hcy were measured with an ELISA kit (Cosmo Bio, Carlsbad, CA, USA). For Hcy and sortilin, intra- and interassay coefficients of variation were <10% and <12%, respectively. Sensitivity was calculated to be 0.2–15 μmol/L for Hcy and 30–2,000 pg/mL for sortilin. For each patient, serum levels were measured twice and the results were averaged.
Statistical Analysis
The required sample size was calculated using G*Power 3.1.9.7 (Institut für Experimentelle Psychologie, Heinrich Heine Universität, Dfüsseldorf, Germany). It was estimated that a sample of 74 patients would have a power of 95% to detect a medium–large effect size of 0.85 in outcome measures.
Statistical analyses were done using SPSS 25.0. Data were tested for normality using the Kolmogorov–Smirnov test.24 Fisher’s exact or #χ2 tests (two-sided) were used to compare qualitative data, which are described as numbers and percentages.
Continuous variables are expressed as means ± SD and categorical variables as numbers and percentages. Comparisons between the groups were made with t-tests for parametric statistics. Correlations between variables were calculated using Pearson’s correlations. Area under the receiver-operating characteristic (ROC) curve (AUC) was used to evaluate the measured variables predictive power for CAD. P<0.05 was considered statistically significant.
Results
Participant Characteristics
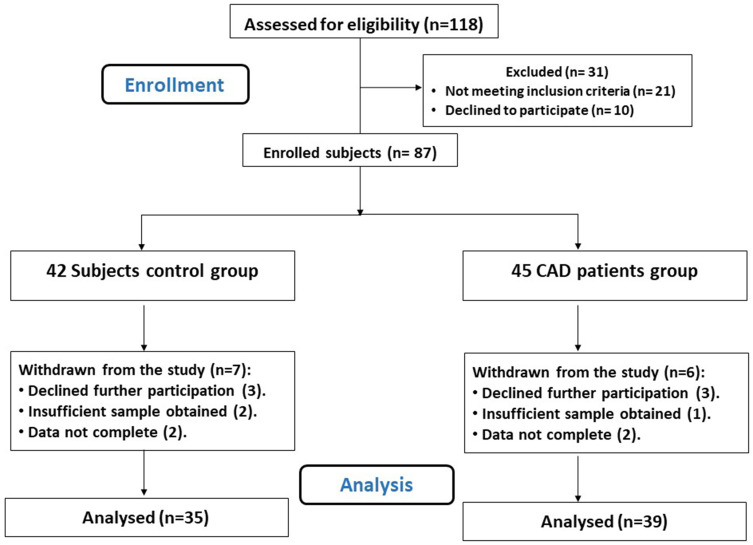
Figure 1 shows that 74 participants, 39 CAD patients, and 35 controls completed the study and were included in the final assessment. Participants were aged 35–65 years (45.28±6.13 and 43.94±4.61 years for the CAD and control groups, respectively), and 66.22% of the study population were men. Demographic and biological characteristics of the study participants are summarized in Table 1.
Figure 1.
Flow diagram of the study.
Table 1.
Demographic and Biochemical Characteristics of Subjects
| Control Group (n=35) | CAD Group (n=39) | P | |
|---|---|---|---|
| Age (years) | 43.94±4.61 | 45.28±6.13 | 0.30 |
| Sex, male/female | 23/12 | 26/13 | 1.00a |
| BMI (kg/m2) | 32.39±1.52 | 32.86±1.79 | 0.22 |
| SBP (mmHg) | 127.66±10.44 | 130.23±7.44 | 0.22 |
| DBP (mmHg) | 82.11±4.27 | 81.74±3.66 | 0.70 |
| Smokers, n (%) | 12 (34.3) | 18 (46.2) | 0.35a |
| Statin use, n (%) | 17 (48.6) | 23 (59.0) | 0.48a |
| Aspirin use, n (%) | 10 (28.6) | 18 (46.2) | 0.15a |
| Hypertension, n (%) | 10 (28.6) | 12 (30.8) | 1.00a |
| Diabetic, n (%) | 10 (28.6) | 14 (30.8) | 0.62a |
| TC (mg/dL) | 164.06±22.65 | 190.32±18.51 | 0* |
| LDL-C (mg/dL) | 102.52±21.35 | 123.28±15.43 | 0* |
| HDL-C (mg/dL) | 34.11±1.79 | 32.57±1.61 | 0* |
| TGs (mg/dL) | 135.74±21.00 | 156.77±24.85 | 0* |
| Hs-CRP (mg/L) | 6.07±0.89 | 7.12±1.20 | 0* |
| Troponin I (ng/mL) | 1.34±0.22 | 1.49±0.22 | 0.005* |
| Gensini score | 1.24±0.26 | 1.50±0.25 | 0* |
| Homocysteine (μmol/L) | 7.73±2.06 | 13.75±1.40 | 0* |
| Sortilin (ng/dL) | 143.02±32.30 | 160.91±32.17 | 0.02* |
Notes: Data expressed as means ± SD or n (%). a#χ2 or Fisher’s exact test (two-sided); *P<0.05 (independent t-test).
Abbreviations: BMI, body-mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; TC, total cholesterol; LDL-C, low density–lipoprotein cholesterol; HDL-C, high density–lipoprotein cholesterol; TGs, triglycerides; Hs-CRP, high-sensitivity CRP.
Both groups were comparable in terms of age, sex, BMI, systolic, and diastolic blood pressure. The prevalence of traditional risk factors was similar between the groups, ie, the CAD group had high percentages of hypertension, diabetes, and frequency of smoking, with no significant differences compared to control group (P>0.05).
Circulating Homocysteine and Sortilin Concentrations
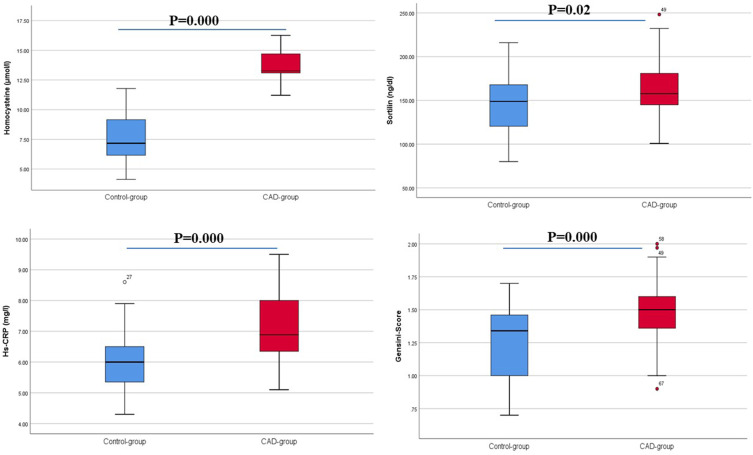
The present study demonstrated that the plasma concentrations of Hcy were significantly higher in the CAD patients than controls (13.75±1.40 vs 7.73±2.06 μmol/L, P=0). Significantly greater sortilin concentrations were observed in CAD patients than controls (160.91±32.17 vs 143.02±32.30 ng/dL, P=0.02), as shown in Table 1 and Figure 2.
Figure 2.
Differences in measured variables between the groups.
Troponin I, hs-CRP, and Mean Angiographic Gensini Scores
Patients with CAD had significantly higher levels of serum troponin I (P=0.005), hs-CRP (P=0), and mean angiographic Gensini scores (P=0) than controls, as shown in Table 1 and Figure 2.
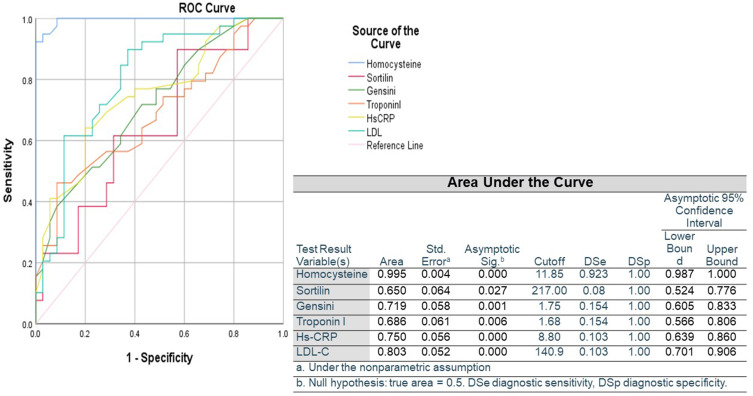
Figure 3 shows the capability of biomarkers in predicting the presence of CAD. Cutoff values of ROC curves were used to calculate diagnostic sensitivity, diagnostic specificity, and AUC of the measured biomarkers. We observed that Hcy was the most sensitive predictor (AUC 0.995, P=0), followed by LDL-C (AUC 0.803, P=0), hs-CRP (AUC 0.750, P=0), Gensini score (AUC 0.719, P=0.001), and troponin I (AUC 0.686, P=0.006), whereas sortilin was the least sensitive (AUC 0.650, P=0.027).
Figure 3.
Area under ROC curve of the ability of measured variables to predict presence of coronary artery disease.
Associations Between Circulating Sortilin and Homocysteine
We observed positive correlations between plasma-sortilin levels and plasma TC (r=0.61, P=0), LDL-C (r=0.37, P=0.001), TGs (r=0.91, P=0), troponin I (r=0.82, P=0), hs-CRP (r=0.87, P=0), Gensini score (r=0.93, P=0), and plasma Hcy levels (r=0.32, P=0.006) in all participants. In contrast, a significant negative association was observed between Hcy and HDL-C (r=−0.42, P=0) and an insignificant negative association between sortilin and HDL-C (r=−0.21, P=0.08), as demonstrated in Table 2.
Table 2.
Correlations among Measured Parameters in Both Groups
| LDL | HDL | TG | Troponin I | Hs-CRP | Gensini | Homocysteine | Sortilin | ||
|---|---|---|---|---|---|---|---|---|---|
| TC | r | 0.91** | −0.34** | 0.63** | 0.61** | 0.58** | 0.62** | 0.65** | 0.61** |
| P | 0 | 0.003 | 0 | 0 | 0 | 0 | 0.02 | 0 | |
| LDL-C | r | −0.30** | 0.40** | 0.41** | 0.35** | 0.42** | 0.51** | 0.37** | |
| P | 0.009 | 0 | 0 | 0.002 | 0 | 0 | 0.001 | ||
| HDL-C | r | −0.31** | −0.26* | −0.39** | −0.22 | −0.42** | −0.21 | ||
| P | 0.007 | 0.02 | 0.001 | 0.06 | 0 | 0.08 | |||
| TGs | r | 0.85** | 0.83** | 0.91** | 0.72** | 0.91** | |||
| P | 0 | 0 | 0 | 0 | 0 | ||||
| Troponin I | r | 0.74** | 0.85** | 0.58** | 0.82** | ||||
| P | 0 | 0 | 0 | 0 | |||||
| Hs-CRP | r | 0.87** | 0.73** | 0.87** | |||||
| P | 0 | 0 | 0 | ||||||
| Gensini | r | 0.71** | 0.93** | ||||||
| P | 0 | 0 | |||||||
| Homocysteine | r | 0.32** | |||||||
| P | 0.006 | ||||||||
Notes: **P<0.01 (two-tailed); *P<0.05 (two-tailed).
Abbreviations: TC, total cholesterol; LDL-C, low density–lipoprotein cholesterol; HDL-C, high density–lipoprotein cholesterol; TGs, triglycerides; Hs-CRP, high-sensitivity CRP.
Table 3 shows the logistic regression model used to determine whether Hcy and sortilin levels were associated with CAD. Our findings showed that Hcy (P=0.025) and sortilin (P=0.025) are independent risk factors of CAD.
Table 3.
Logistic Regression Analysis of Sortilin and Homocysteine as Independent Risk Factors of Coronary Artery Disease
| OR | 95% CI | P | ||
|---|---|---|---|---|
| Lower | Upper | |||
| Homocysteine (μmol/L) | 27.503 | 1.521 | 497.233 | 0.025 |
| Sortilin (ng/dL) | 1.018 | 1.002 | 1.034 | 0.025 |
According to the logistic regression analysis in Table 4, smoking, statin use, aspirin use, diabetes, and hypertension had no significant effect in either group.
Table 4.
Logistic Regression Analysis of Effects of Statin and Aspirin Use and Associated Diseases in Coronary Artery Disease Patients
| OR | 95% CI | P | ||
|---|---|---|---|---|
| Lower | Upper | |||
| Smoker | 0.644 | 0.197 | 2.106 | 0.467 |
| Statins | 0.592 | 0.218 | 1.605 | 0.303 |
| Aspirin | 0.512 | 0.178 | 1.467 | 0.213 |
| Hypertension | 1.138 | 0.335 | 3.874 | 0.836 |
| Diabetes | 0.663 | 0.237 | 1.860 | 0.435 |
Discussion
In many countries, CAD is a widespread cause of death, and the detection of associated risk factors could assist in its prevention.25 Major attempts have been made in the last few years to improve antithrombotic therapy and revascularization strategies and decrease cardiac mortality, particularly in patients with acute coronary syndrome.26 However, in some high-risk patients, suboptimal results are still found.27–29 As such, the discovery of new biomarkers aimed at preventing CAD has been given considerable attention. Our study showed that plasma-Hcy concentration was significantly elevated in CAD patients compared to controls.
Since 1969, when McCully demonstrated that there was a link between high plasma concentration of Hcy and arteriosclerosis, great focus have been centered on Hcy.30 Consistently, Clarke et al showed in his research that hyperhomocysteinemia was an independent vascular and CHD risk factor.31 Similar results were found by Klerk et al in a large meta-analysis supporting the hyperhomocysteinemia and CVD association.32
Consistently with our results, Lee et al observed that in subjects with high Hcy levels, the possibility of CAD risk was considerably increased.33 On the contrary, a large meta-analysis conducted to examine the connection of moderately increased levels of Hcy with CHD, which included 48,175 CHD patients and 67,961 controls, showed an insignificant impact on the risk of CHD.34 Cross‐sectional studies have consistently shown that increased Hcy is correlated with CVD.35 The plaques developed in coronary artery endothelial cells are more vulnerable to detachment by hyperhomocysteinemia.36 However, other studies have not confirmed such correlations.37,38
The role of Hcy in patients with atherosclerosis and associated vascular consequences has been extensively studied. The role of hyperhomocysteinemia and its correlation with CAD has been subjected to extensive research.2,39,40 Moderately increased Hcy (>13–15 μmol/L) has a multifactorial relationship with various genetic and environmental interactions and consequently cardiogenic effects.41
Our results confirm that elevated hs-CRP is correlated with an elevated risk of CAD. We also demonstrated that CAD is similarly related to Hcy and has a tendency toward an association with high TG and low HDL-C. Consistently with this, Seo et al found a significant association between hs-CRP and risk of CAD.11 This in agreement with previous studies illustrating that serum hs-CRP is an independent risk factor of CVD, regardless of LDL-C level.11,42,43 Moreover, hs-CRP may offer considerable additive value in predicting cardiovascular risks.
The inflammatory response is thought to play a major role in atherosclerosis formation and progression. Acute reactant hs-CRP appears to trigger endothelial dysfunction and promote inflammation in the arterial wall, resulting in atherogenesis and an elevated risk of cardiovascular events.11
Our results showed that patients with CAD had significantly higher levels of serum troponin I, in agreement with Jia et al, who found that cardiac troponin I was positively and strongly associated with incident CAD.14
Consistently, Lima et al found that in stable CAD patients, higher resting levels and elevation of high-sensitivity troponin I with exercise were predictors of adverse cardiovascular outcomes beyond traditional cardiovascular risk factors and presence of inducible ischemia.44
Similarly, Zhu et al found that cardiac troponin I was an independent predictor of fatal and non-fatal CVD events and could be used to identify individuals at risk in a general population.45
Our results showed a significant negative relationship between Hcy and HDL-C and a positive association between Hcy and angiographic Gensini scores. Also, ROC-curve analysis demonstrated that Hcy was the most sensitive biomarker for CAD, followed by LDL-C, hs-CRP, Gensini score, and troponin-I, whereas sortilin was the least sensitive. We believe that these results emphasize the importance of recognizing individuals with elevated Hcy that could be at risk of atherothrombotic disease. So, focusing on preventive measures, such as intake of folate, vitamin B6, and B12, which are known to decrease Hcy levels,2 in order to define the role of Hcy and Hcy-lowering therapy in reducing risk in high-risk CAD patients are thus required.
Our results showed that patients with CAD tended to have significantly higher sortilin concentrations than control subjects. In accordance with our results, Oh et al46 demonstrated that sortilin is a protein associated with CAD. A Japanese study, in contrast to our data,17 revealed that subjects with CAD had lower sortilin levels than non-CAD subjects. Other research has shown that sortilin plays a major role in cholesterol metabolism.16,47,48 Previous investigational data have proved that sortilin is implicated in atherosclerotic plaque formation by enhancing the inflammatory pathway in a mouse model.49
Reduced plasma HDL- and LDL-cholesterol associated with reduction of atherosclerotic lesions has been observed in whole-body sortilin-knockout mice16 and vascular calcification.50 Correspondingly, decreased atherosclerotic lesions due to decreased LDL uptake by macrophages have been displayed in macrophage sortilin–deficient mice.21 In contrast, serum LDL-cholesterol levels are diminished following exaggerated expression of sortilin in the liver.51 Our findings, in accordance with Ogawa et al,17 who demonstrated that sortilin levels were significantly associated with LDL-C, TG, hs-CRP, and Hcy levels, which are considered cardiovascular risk factors. HDL possesses potent antiinflammatory characteristics that may have a protective effect against atherogenesis.52 Clinical and epidemiological studies have demonstrated that HDL concentration in atherosclerotic CVD is often negatively associated with plasma-CRP levels.53 The present study established that hs-CRP had a significant association with CAD severity evaluated using angiographic Gensini scores. This in consistent with Masood et al, who carried out a cross-sectional study to investigate the relationship between hs-CRP and coronary atherosclerosis severity on 80 patients subjected to coronary angiography.54
Moukarbel et al discovered that raised CRP were associated with coronary artery–lesion complexity.55 In terms of plaque ulceration and inflammation, attempts have also been made to relate the susceptibility of coronary plaques to serum hs-CRP levels. Espligureal et al observed considerably higher hs-CRP levels in patients suffering from acute coronary syndrome than patients with chronic stable angina.56 There has been confirmation that CRP is a strong predictor of CVD risk among patients undergoing elective revascularization treatment, patients with acute coronary syndromes, and even apparently healthy individuals.57 Peppes et al58 showed that serum levels of myocardial enzymes and inflammatory biomarkers not only increased at the time of an acute coronary event but also — according to angiographic results (Gensini score) — were quantitatively associated with the degree of myocardial damage and the severity of CAD. A negative correlation between HDL levels and the severity of CAD (Gensini score) was identified in this study and a reverse correlation between serum HDL and CRP was observed. The present study revealed a negative correlation between HDL level and Gensini score, the index of CAD severity in CAD patients and controls.
Wadham et al found that via oxidation of HDL, main phospholipid is neutralizing the proinflammatory ability of CRP in endothelial cells, showing an equilibrium between anti-inflammatory and proinflammatory actions inside the vascular wall. Increased CRP concentration is often correlated with factors that reduce the amount and/or quality of HDL, such as age, diabetes, and obesity.59,60 This possibly will finally disrupt the anti-inflammatory and proinflammatory equilibrium to promote the progression of inflammatory CVD. Tarchalski et al61 who conducted a prospective study, showed that coronary atherosclerosis severity was positively associated with TC, LDL-C, and TGs and inversely associated with HDL-C in angina pectoris patients with no previous myocardial infarction.
Our data demonstrated that in Egyptian subjects, Hcy and sortilin levels may provide additive value in predicting cardiovascular risk, and interventions that reduce these levels may be effective in reducing the incidence of cardiovascular events.
Limitations
We had a relatively small sample size. In order to examine the potential clinical relevance of our results to management of CAD, a prospective study is needed.
Conclusion
Hcy and circulating sortilin are novel and major elevated biomarkers in patients with CAD, and may be used as tools for diagnosis and potential therapeutic targets in CAD patients. However, additional human studies are needed to confirm our results.
Funding Statement
The authors did not get any funding or grant from any organizations.
Disclosure
All authors declare no conflict of interest.
References
- 1.Badimon L, Storey RF, Vilahur G. Update on lipids, inflammation and atherothrombosis. Thromb Haemost. 2011;105(S 06):S34–S42. doi: 10.1160/THS10-11-0717 [DOI] [PubMed] [Google Scholar]
- 2.Baños-González MA, Anglés-Cano E, Cardoso-Saldaña G, et al. Lipoprotein (a) and homocysteine potentiate the risk of coronary artery disease in male subjects. Circ J. 2012:1204161700. doi: 10.1253/circj.CJ-12-0039 [DOI] [PubMed] [Google Scholar]
- 3.von Scholten BJ, Reinhard H, Hansen TW, et al. Additive prognostic value of plasma N-terminal pro-brain natriuretic peptide and coronary artery calcification for cardiovascular events and mortality in asymptomatic patients with type 2 diabetes. Cardiovas Diabetol. 2015;14(1):59. doi: 10.1186/s12933-015-0225-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kumar A, Palfrey HA, Pathak R, Kadowitz PJ, Gettys TW, Murthy SN. The metabolism and significance of homocysteine in nutrition and health. Nutr Metab. 2017;14(1):1–12. doi: 10.1186/s12986-017-0233-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gauthier GM, Keevil JG, McBride PE. The association of homocysteine and coronary artery disease. Clin Cardiol. 2003;26(12):563–568. doi: 10.1002/clc.4960261204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wald DS, Law M, Morris JK. Homocysteine and cardiovascular disease: evidence on causality from a meta-analysis. BMJ. 2002;325(7374):1202. doi: 10.1136/bmj.325.7374.1202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schaffer A, Verdoia M, Cassetti E, et al. Relationship between homocysteine and coronary artery disease. Results from a large prospective cohort study. Thromb Res. 2014;134(2):288–293. doi: 10.1016/j.thromres.2014.05.025 [DOI] [PubMed] [Google Scholar]
- 8.Vivona N, Bivona G, Noto D, et al. C-reactive protein but not soluble CD40 ligand and homocysteine is associated to common atherosclerotic risk factors in a cohort of coronary artery disease patients. Clin Biochem. 2009;42(16–17):1713–1718. doi: 10.1016/j.clinbiochem.2009.08.014 [DOI] [PubMed] [Google Scholar]
- 9.Boekholdt SM, Kastelein J. C-reactive protein and cardiovascular risk: more fuel to the fire. Lancet. 2009;375(9709):95–96. doi: 10.1016/S0140-6736(09)62098-5 [DOI] [PubMed] [Google Scholar]
- 10.Kumakura H, Fujita K, Kanai H, et al. High-sensitivity C-reactive protein, lipoprotein (a) and homocysteine are risk factors for coronary artery disease in Japanese patients with peripheral arterial disease. J Atheroscler Thromb. 2014:25478. doi: 10.5551/jat.25478 [DOI] [PubMed] [Google Scholar]
- 11.Seo SM, Baek SH, Jeon HK, et al. Correlations between the level of high-sensitivity C-reactive protein and cardiovascular risk factors in Korean adults with cardiovascular disease or diabetes mellitus: the CALLISTO study. J Atheroscler Thromb. 2013;20(7):616–622. doi: 10.5551/jat.16089 [DOI] [PubMed] [Google Scholar]
- 12.Iribarren C, Chandra M, Rana JS, et al. High-sensitivity cardiac troponin I and incident coronary heart disease among asymptomatic older adults. Heart. 2016;102(15):1177–1182. doi: 10.1136/heartjnl-2015-309136 [DOI] [PubMed] [Google Scholar]
- 13.Omland T, Pfeffer MA, Solomon SD, et al. Prognostic value of cardiac troponin I measured with a highly sensitive assay in patients with stable coronary artery disease. J Am Coll Cardiol. 2013;61(12):1240–1249. doi: 10.1016/j.jacc.2012.12.026 [DOI] [PubMed] [Google Scholar]
- 14.Jia X, Sun W, Hoogeveen RC, et al. High-sensitivity troponin I and incident coronary events, stroke, heart failure hospitalization, and mortality in the ARIC study. Circulation. 2019;139(23):2642–2653. doi: 10.1161/CIRCULATIONAHA.118.038772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dube JB, Johansen CT, Hegele RA. Sortilin: an unusual suspect in cholesterol metabolism: from GWAS identification to in vivo biochemical analyses, sortilin has been identified as a novel mediator of human lipoprotein metabolism. Bioessays. 2011;33(6):430–437. doi: 10.1002/bies.201100003 [DOI] [PubMed] [Google Scholar]
- 16.Kjolby M, Andersen OM, Breiderhoff T, et al. Sort1, encoded by the cardiovascular risk locus 1p13. 3, is a regulator of hepatic lipoprotein export. Cell Metab. 2010;12(3):213–223. doi: 10.1016/j.cmet.2010.08.006 [DOI] [PubMed] [Google Scholar]
- 17.Ogawa K, Ueno T, Iwasaki T, et al. Soluble sortilin is released by activated platelets and its circulating levels are associated with cardiovascular risk factors. Atherosclerosis. 2016;249:110–115. doi: 10.1016/j.atherosclerosis.2016.03.041 [DOI] [PubMed] [Google Scholar]
- 18.Strong A, Rader DJ. Sortilin as a regulator of lipoprotein metabolism. Curr Atheroscler Rep. 2012;14(3):211–218. doi: 10.1007/s11883-012-0248-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kjolby M, Nielsen MS, Petersen CM. Sortilin, encoded by the cardiovascular risk gene SORT1, and its suggested functions in cardiovascular disease. Curr Atheroscler Rep. 2015;17(4):18. doi: 10.1007/s11883-015-0496-7 [DOI] [PubMed] [Google Scholar]
- 20.Gao A, Cayabyab FS, Chen X, et al. Implications of sortilin in lipid metabolism and lipid disorder diseases. DNA Cell Biol. 2017;36(12):1050–1061. doi: 10.1089/dna.2017.3853 [DOI] [PubMed] [Google Scholar]
- 21.Patel KM, Strong A, Tohyama J, et al. Macrophage sortilin promotes LDL uptake, foam cell formation, and atherosclerosis. Circ Res. 2015;116(5):789–796. doi: 10.1161/CIRCRESAHA.116.305811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gensini GG. A more meaningful scoring system for determining the severity of coronary heart disease. Am J Cardiol. 1983;51(3):606. doi: 10.1016/S0002-9149(83)80105-2 [DOI] [PubMed] [Google Scholar]
- 23.Elasfar A, Hekal S, ElGendy E, Abdulaal M, Elsokkary H, Ashmawy M. Assessment of left and right atrial geometrical changes in patients with stable coronary artery disease: left and right atrial strain and strain rate imaging study. Egypt Heart J. 2018;70(2):101–106. doi: 10.1016/j.ehj.2018.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lilliefors HW. On the Kolmogorov-Smirnov test for normality with mean and variance unknown. J Am Stat Assoc. 1967;62(318):399–402. doi: 10.1080/01621459.1967.10482916 [DOI] [Google Scholar]
- 25.Sanchis-Gomar F, Perez-Quilis C, Leischik R, Lucia A. Epidemiology of coronary heart disease and acute coronary syndrome. Ann Transl Med. 2016;4(13):13. doi: 10.21037/atm.2016.06.33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Luca G, Verdoia M, Suryapranata H. Benefits from intracoronary as compared to intravenous abciximab administration for STEMI patients undergoing primary angioplasty: a meta-analysis of 8 randomized trials. Atherosclerosis. 2012;222(2):426–433. doi: 10.1016/j.atherosclerosis.2012.02.041 [DOI] [PubMed] [Google Scholar]
- 27.De Luca G, Ernst N, Van’t Hof AW, et al. Predictors and clinical implications of early reinfarction after primary angioplasty for ST-segment elevation myocardial infarction. Am Heart J. 2006;151(6):1256–1259. doi: 10.1016/j.ahj.2005.06.047 [DOI] [PubMed] [Google Scholar]
- 28.De Luca G, Gibson CM, Bellandi F, et al. Diabetes mellitus is associated with distal embolization, impaired myocardial perfusion, and higher mortality in patients with ST-segment elevation myocardial infarction treated with primary angioplasty and glycoprotein IIb-IIIa inhibitors. Atherosclerosis. 2009;207(1):181–185. doi: 10.1016/j.atherosclerosis.2009.03.042 [DOI] [PubMed] [Google Scholar]
- 29.De Luca G, Van’t Hof AW, Ottervanger JP, et al. Ageing, impaired myocardial perfusion, and mortality in patients with ST-segment elevation myocardial infarction treated by primary angioplasty. Eur Heart J. 2005;26(7):662–666. doi: 10.1093/eurheartj/ehi110 [DOI] [PubMed] [Google Scholar]
- 30.McCully KS. Vascular pathology of homocysteinemia: implications for the pathogenesis of arteriosclerosis. Am J Pathol. 1969;56(1):111. [PMC free article] [PubMed] [Google Scholar]
- 31.Clarke R, Daly L, Robinson K, et al. Hyperhomocysteinemia: an independent risk factor for vascular disease. New Engl J Med. 1991;324(17):1149–1155. doi: 10.1056/NEJM199104253241701 [DOI] [PubMed] [Google Scholar]
- 32.Klerk M, Verhoef P, Clarke R, Blom HJ, Kok FJ, Schouten EG. MTHFR 677C→ T polymorphism and risk of coronary heart disease: a meta-analysis. JAMA. 2002;288(16):2023–2031. doi: 10.1001/jama.288.16.2023 [DOI] [PubMed] [Google Scholar]
- 33.Lee B-J, Lin P-T, Liaw Y-P, Chang S-J, Cheng C-H, Huang Y-C. Homocysteine and risk of coronary artery disease: folate is the important determinant of plasma homocysteine concentration. Nutrition. 2003;19(7–8):577–583. doi: 10.1016/S0899-9007(02)01098-5 [DOI] [PubMed] [Google Scholar]
- 34.Clarke R, Bennett DA, Parish S, et al. Homocysteine and coronary heart disease: meta-analysis of MTHFR case-control studies, avoiding publication bias. PLoS Med. 2012;9(2):e1001177. doi: 10.1371/journal.pmed.1001177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Splaver A, Lamas GA, Hennekens CH. Homocysteine and cardiovascular disease: biological mechanisms, observational epidemiology, and the need for randomized trials. Am Heart J. 2004;148(1):34–40. doi: 10.1016/j.ahj.2004.02.004 [DOI] [PubMed] [Google Scholar]
- 36.Han K, Lu Q, Zhu W, Wang T, Du Y, Bai L. Correlations of degree of coronary artery stenosis with blood lipid, CRP, Hcy, GGT, SCD36 and fibrinogen levels in elderly patients with coronary heart disease. Eur Rev Med Pharmacol Sci. 2019;23(21):9582–9589. [DOI] [PubMed] [Google Scholar]
- 37.Bozkurt A, Toyaksi H, Acartürk E, Tuli A, Çayli M. The effects of hyperhomocysteinemia on the presence, extent, and severity of coronary artery disease. Jpn Heart J. 2003;44(3):357–368. doi: 10.1536/jhj.44.357 [DOI] [PubMed] [Google Scholar]
- 38.Brilakis ES, McConnell JP, Ballman KV, Klee GG, Berger PB. Lack of association between plasma homocysteine and angiographic coronary artery disease in the era of fortification of cereal grain flour with folic acid. Atherosclerosis. 2002;165(2):375–381. doi: 10.1016/S0021-9150(02)00303-9 [DOI] [PubMed] [Google Scholar]
- 39.Akyürek Ö, Akbal E, Güneş F. Increase in the risk of ST elevation myocardial infarction is associated with homocysteine level. Arch Med Res. 2014;45(6):501–506. doi: 10.1016/j.arcmed.2014.08.003 [DOI] [PubMed] [Google Scholar]
- 40.Cioni G, Marcucci R, Gori AM, et al. Increased homocysteine and lipoprotein (a) levels highlight systemic atherosclerotic burden in patients with a history of acute coronary syndromes. J Vasc Surg. 2016;64(1):163–170. doi: 10.1016/j.jvs.2016.01.056 [DOI] [PubMed] [Google Scholar]
- 41.Masud R, Baqai HZ. The communal relation of MTHFR, MTR, ACE gene polymorphisms and hyperhomocysteinemia as conceivable risk of coronary artery disease. App Physiol Nutr Metab. 2017;42(10):1009–1014. doi: 10.1139/apnm-2017-0030 [DOI] [PubMed] [Google Scholar]
- 42.Koenig W, Sund M, Fröhlich M, et al. C-reactive protein, a sensitive marker of inflammation, predicts future risk of coronary heart disease in initially healthy middle-aged men: results from the MONICA (Monitoring Trends and Determinants in Cardiovascular Disease) Augsburg cohort study, 1984 to 1992. Circulation. 1999;99(2):237–242. [DOI] [PubMed] [Google Scholar]
- 43.Xu M, Bi Y, Chen Y, et al. Increased C-reactive protein associates with elevated carotid intima-media thickness in Chinese adults with normal low density lipoprotein cholesterol levels. J Atheroscler Thromb. 2013;20(6):575–584. doi: 10.5551/jat.15172 [DOI] [PubMed] [Google Scholar]
- 44.Lima BB, Hammadah M, Kim JH, et al. Relation of high-sensitivity cardiac troponin i elevation with exercise to major adverse cardiovascular events in patients with coronary artery disease. Am J Cardiol. 2020;136:1–8. doi: 10.1016/j.amjcard.2020.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhu K, Knuiman M, Divitini M, et al. High-sensitivity cardiac troponin I and risk of cardiovascular disease in an Australian population-based cohort. Heart. 2018;104(11):895–903. doi: 10.1136/heartjnl-2017-312093 [DOI] [PubMed] [Google Scholar]
- 46.Oh TJ, Ahn CH, Kim B-R, et al. Circulating sortilin level as a potential biomarker for coronary atherosclerosis and diabetes mellitus. Cardiovas Diabetol. 2017;16(1):92. doi: 10.1186/s12933-017-0568-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gustafsen C, Kjolby M, Nyegaard M, et al. The hypercholesterolemia-risk gene SORT1 facilitates PCSK9 secretion. Cell Metab. 2014;19(2):310–318. doi: 10.1016/j.cmet.2013.12.006 [DOI] [PubMed] [Google Scholar]
- 48.Linsel-Nitschke P, Samani NJ, Schunkert H. Sorting out cholesterol and coronary artery disease. New Engl J Med. 2010;363(25):2462. doi: 10.1056/NEJMcibr1010765 [DOI] [PubMed] [Google Scholar]
- 49.Mortensen MB, Kjolby M, Gunnersen S, et al. Targeting sortilin in immune cells reduces proinflammatory cytokines and atherosclerosis. J Clin Invest. 2014;124(12):5317–5322. doi: 10.1172/JCI76002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goettsch C, Hutcheson JD, Aikawa M, et al. Sortilin mediates vascular calcification via its recruitment into extracellular vesicles. J Clin Invest. 2016;126(4):1323–1336. doi: 10.1172/JCI80851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Musunuru K, Strong A, Frank-Kamenetsky M, et al. From noncoding variant to phenotype via SORT1 at the 1p13 cholesterol locus. Nature. 2010;466(7307):714–719. doi: 10.1038/nature09266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cockerill GW, Rye K-A, Gamble JR, Vadas MA, Barter PJ. High-density lipoproteins inhibit cytokine-induced expression of endothelial cell adhesion molecules. Arterioscler Thromb Vasc Biol. 1995;15(11):1987–1994. doi: 10.1161/01.ATV.15.11.1987 [DOI] [PubMed] [Google Scholar]
- 53.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. New Engl J Med. 2000;342(12):836–843. doi: 10.1056/NEJM200003233421202 [DOI] [PubMed] [Google Scholar]
- 54.Masood A, Jafar SS, Akram Z. Serum high sensitivity C-reactive protein levels and the severity of coronary atherosclerosis assessed by angiographic gensini score. Atherosclerosis. 2011;3:4. [PubMed] [Google Scholar]
- 55.Moukarbel GV, Arnaout MSA, Alam SE. C‐reactive protein is a marker for a complex culprit lesion anatomy in unstable angina. Clin Cardiol. 2001;24(7):506–510. doi: 10.1002/clc.4960240718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Arroyo-Espliguero R, Avanzas P, Cosín-Sales J, Aldama G, Pizzi C, Kaski JC. C-reactive protein elevation and disease activity in patients with coronary artery disease. Eur Heart J. 2004;25(5):401–408. doi: 10.1016/j.ehj.2003.12.017 [DOI] [PubMed] [Google Scholar]
- 57.Brunetti ND, Troccoli R, Correale M, Luigi Pellegrino P, Di BM. C-reactive protein in patients with acute coronary syndrome: correlation with diagnosis, myocardial damage, ejection fraction and angiographic findings. Int J Cardiol. 2006;109(2):248–256. doi: 10.1016/j.ijcard.2005.06.021 [DOI] [PubMed] [Google Scholar]
- 58.Peppes V, Rammos G, Manios E, Koroboki E, Rokas S, Zakopoulos N. Correlation between myocardial enzyme serum levels and markers of inflammation with severity of coronary artery disease and Gensini score: a hospital-based, prospective study in Greek patients. Clin Interv Aging. 2008;3(4):699. doi: 10.2147/CIA.S3551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chambers JC, Eda S, Bassett P, et al. C-reactive protein, insulin resistance, central obesity, and coronary heart disease risk in Indian Asians from the United Kingdom compared with European whites. Circulation. 2001;104(2):145–150. doi: 10.1161/01.CIR.104.2.145 [DOI] [PubMed] [Google Scholar]
- 60.Wadham C, Albanese N, Roberts J, et al. High-density lipoproteins neutralize C-reactive protein proinflammatory activity. Circulation. 2004;109(17):2116–2122. doi: 10.1161/01.CIR.0000127419.45975.26 [DOI] [PubMed] [Google Scholar]
- 61.Tarchalski J, Guzik P, Wysocki H. Correlation between the extent of coronary atherosclerosis and lipid profile. Mol Cell Biochem. 2003;246(1–2):25–30. doi: 10.1023/A:1023443609730 [DOI] [PubMed] [Google Scholar]