Abstract
Osteoarthritis (OA) is an age-related degenerative disease of cartilaginous tissues that is accompanied by hyperalgesia. Molecular cause and effect relationships between OA and pain remain to be elucidated. In this study, we have developed an experimental ex vivo organ co-culture system with dorsal root ganglia (DRGs) and knee synovial tissues from OA patients or unaffected human subjects. Our results suggest that tissues may generate symptomatic pain by altering the functional properties of sensory neurons. Specifically, we find that the expression levels of genes associated with neuronal pathways (e.g., SP, NK1, NK2, NPYR1, NPYR2, α2δ1) or inflammation (COX2/PTGS2 and IL6/interferon β2) are clearly elevated in DRG explants cultured in the presence of OA derived synovial tissues. These findings are consistent with a model in which cytokines and pain molecules produced by knee synovium sensitize nociceptive neurons in tissues peripheral to joint cartilage.
INTRODUCTION
Pain is a prominent and disabling symptom of OA, and arthritic pain is associated with inferior functional outcomes and reduced quality of life when compared with a range of other chronic conditions[1]. To develop new therapies to prevent disease progression and relieve pain [2], it is necessary to understand the pathological mechanisms leading to joint degeneration and pain symptoms. Our laboratory and others have mechanistically linked OA to pathological changes in the metabolism of extracellular matrix (ECM) proteins[3–15] that may be controlled by epigenetic [16], epigenomic [17] and systemic [18–20] processes. However, the cause of pain in knee OA remains elusive because the primary site of pathology in OA (i.e., cartilage) does not have neuronal pain receptors that can directly detect tissue injury due to mechanical damage. Nociceptors are located throughout the joint in tissues peripheral to cartilage, including capsule ligaments, menisci, periosteum and subchondral bone[21]. Nociceptive input from the joint is processed in different types of spinal cord neurons, and inflammation could reduce the pain threshold for nociception.
How painful mechanical stimuli from knee OA are converted into electrical signals that propagate along sensory nerves to the central nervous system remains to be fully explored. One attractive theory is that movement of the joint generates shear stresses on the axolemma of the “free” nerve endings, resulting in the opening of neuroimmune pain pathways and/or gated ion channels, such as calcium or sodium channel causing ectopic neuronal firing in injured tissue. Painful stimuli are exaggerated (hyperalgesia) by changes in the dorsal root ganglion (DRG) that cause in-growth of normally non-pain transmitting nerve terminals into the superficial pain transmitting areas of the Dorsal Horn (‘nerve sprouting’). DRG neurons also express mRNAs that cause atrophy of normal C-fiber nociceptors in these same areas. Nerve sprouting causes non-painful sensory input to be interpreted and passed to the brain as a pain producing signal [22]. Defining the origins and blocking neuropathic pathways is a critical strategy for joint pain management in OA. For example, some pain mediators such as substance P(SP)/tachykinin 1 (TAC1) and Neuropeptide Y (NPY)and their cognate receptors, as well as cyclooxygenase 2 (COX2 or PTGS2) and the calcium channel α2δ1 subunit play impartment roles in the transmission of pain in peripheral and central nervous system [3, 23]. In this study, we have investigated whether synovium from OA patients can modulate the expression of genes associated with neuronal pathways (e.g., SP/TAC1, NK1, NK2, NPYR1, NPYR2, α2δ1) or inflammation (COX2/PTGS2 and IL6/interferon β2) in DRG using an explant culture system.
MATERIALS AND METHODS
Human tissue acquisition and preparation of knee joint explants.
Normal (grade 0, 1)[24] cartilage, synovial fluid, and synovial tissues, and knee joint capsules were obtained through the tissue repository systems of either the gift of Hope Tissue Donor Network or the Department of Orthopedic Surgery tissue repository system in which patients with no knee symptoms and no history of previous knee injury were recruited from the Rush University Medical Center with full consent. The tissue donors were strictly screened and are negative for HIV and hepatitis. Exclusion criteria also include individuals with presence or history of malignant diseases, a history of connective tissue disease, or with conditions or diseases of unknown etiology. The tissues were collected within 24 hours of death by a pathologist. All donor tissues are assessed and graded using Collin’s Score system[24]
The Collin’s Score involves careful examination of the surface of the articular cartilage. Abnormalities in the gross appearance of the cartilage including fibrillations, full-thickness defects and osteophytes of joint are scored from 0 to 4 based on the gross morphological scale of Collins. Grade 0 shows no cartilage lesions. Grade 1 shows only minor disruptions of cartilage surface, minor fibrillations, flaking, shallow pits or grooves or small blisters without affecting surface geometry. Grade 0 and 1 are considered as “an almost perfect normal” joint. Grade 2 shows deep fibrillation and fissuring, flaking, and/or blistering, early marginal hyperplasia and possibly small osteophytes. Grade 3 shows extensive fibrillation and fissuring and 30% or less of the articular surface is eroded down to the subchondral bone, and osteophytes. Grade 4 shows lips or shelves at the articular margin, greater than 30% of the articular surface eroded down to the subchondral bone, as well as gross geometric changes and osteophytes.
Homogenously-sized cartilage, synovium and joint capsule explants with a 4 mm diameter were isolated from synovial tissues by applying a biopsy punch as previously described1. The biopsy punch ensures that the layer of synovium tissue remains intact within the tissue structure. The tissues were aseptically weighed before use and each explant has comparable thickness and size to minimize data variability.
DRG co-culture experiments.
The DRGs were harvested from adult Sprague-Dawley rats (250–300 g). Bilateral lumbar DRGs from L1 to L5 (10 DRGs per rat) were carefully removed by aseptical dissection using a stereoscopic microscope. DRGs were co-cultured with knee joint synovium tissue isolated from OA patients with painful joint patients or control tissue from asymptomatic human subjects. DRGs were cultured 5 days with human joint synovial tissue (Fig. 1).
Fig 1.
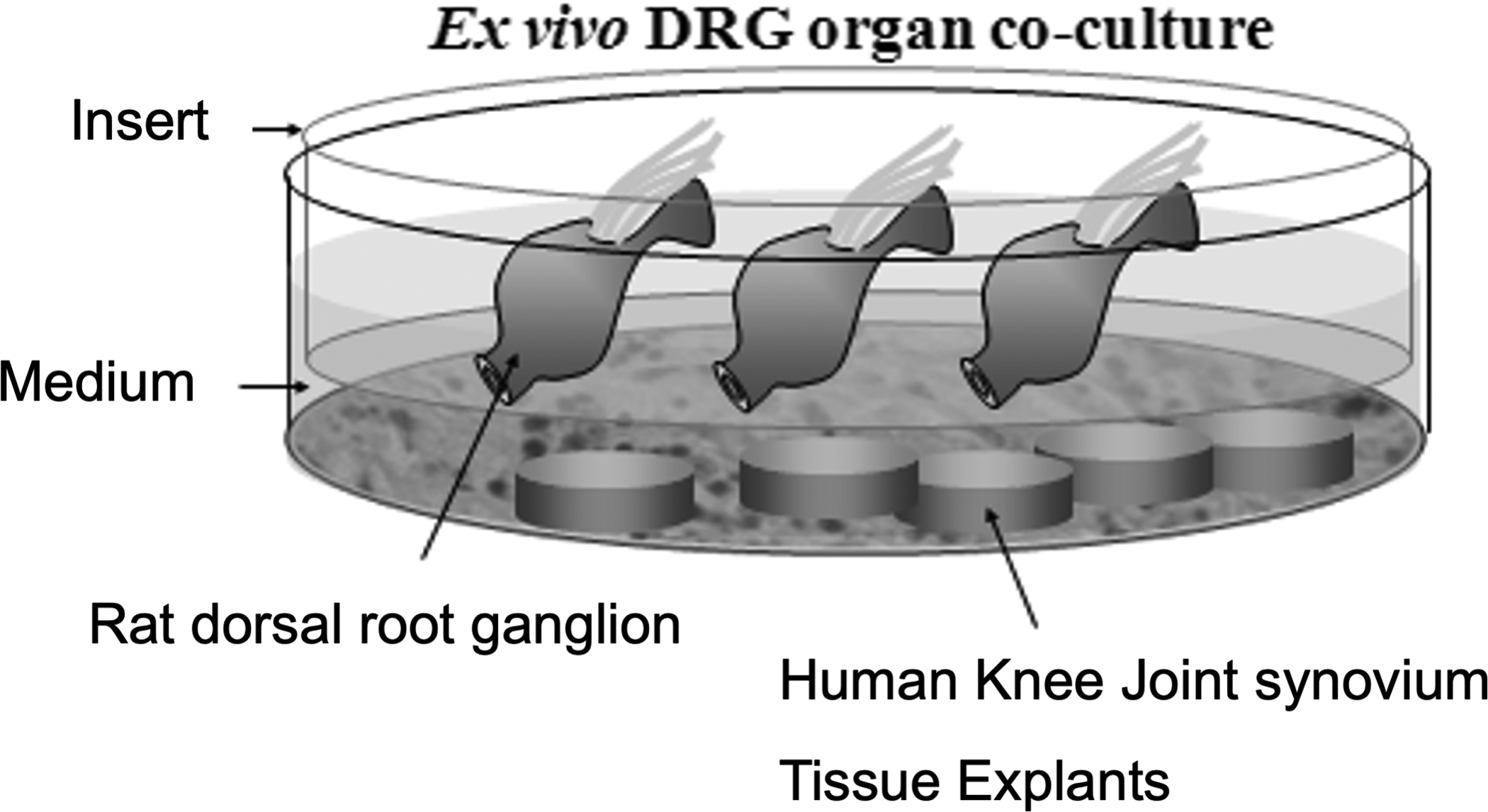
Diagram of the co-culture model for assessing the effects of OA synovium on neuronal modifications in human knee joint tissue.
Total RNA extraction, Reverse Transcription, Real-Time Polymerase Chain Reaction: For total RNA extraction, 500 mg to 1 g of joint capsule explants or DRGs (pulverized under liquid nitrogen) were homogenized in Trizol reagent (3 ml) and the samples were subjected to real-time quantitative reverse transcriptase PCR (RT-qPCR) experiments. Total RNA isolated from either tissue or cells was subjected to reverse transcription using ThermoScript RT kit (Invitrogen). For RT-qPCR, cDNA preparations were amplified using the MyiQ Real-Time PCR Detection System (Bio-Rad). Relative mRNA expression was determined using the ΔΔCT method, as detailed by the manufacturer (Bio-Rad). The standard deviations in samples are based on results from different donors in three separate experiments.
RESULTS
Results from our group and others suggest that pain-related neuropeptides released from synovial fibroblasts, such as substance P (SP/TAC1), induce inflammatory processes the neurokinin/tachykinin receptors (NK1, NK2). These G-protein-coupled receptors are pathophysiologically linked to joint degeneration[25]. Because synovium in OA is heavily enriched with newly formed blood vessels but also nerves [26], we examined whether OA synovial tissues influence sensory neurons in DRGs. Before we initiated our experimental ex vivo co-culture of rat DRGs and human OA synovial tissues, we first ensured that basal gene expression of bilateral DRGs harvested from different lumbar levels (L3-L5) are equal. Harvested bilateral rat DRGs were mechanically homogenized to prepare total RNA for RT-qPCR analyses. There were no significant differences in gene expression among the levels of DRGs (L3-L5) for the housekeeping gene GAPDH or other selected genes (e.g., α2δ1), (Fig. 2).
Fig 2.
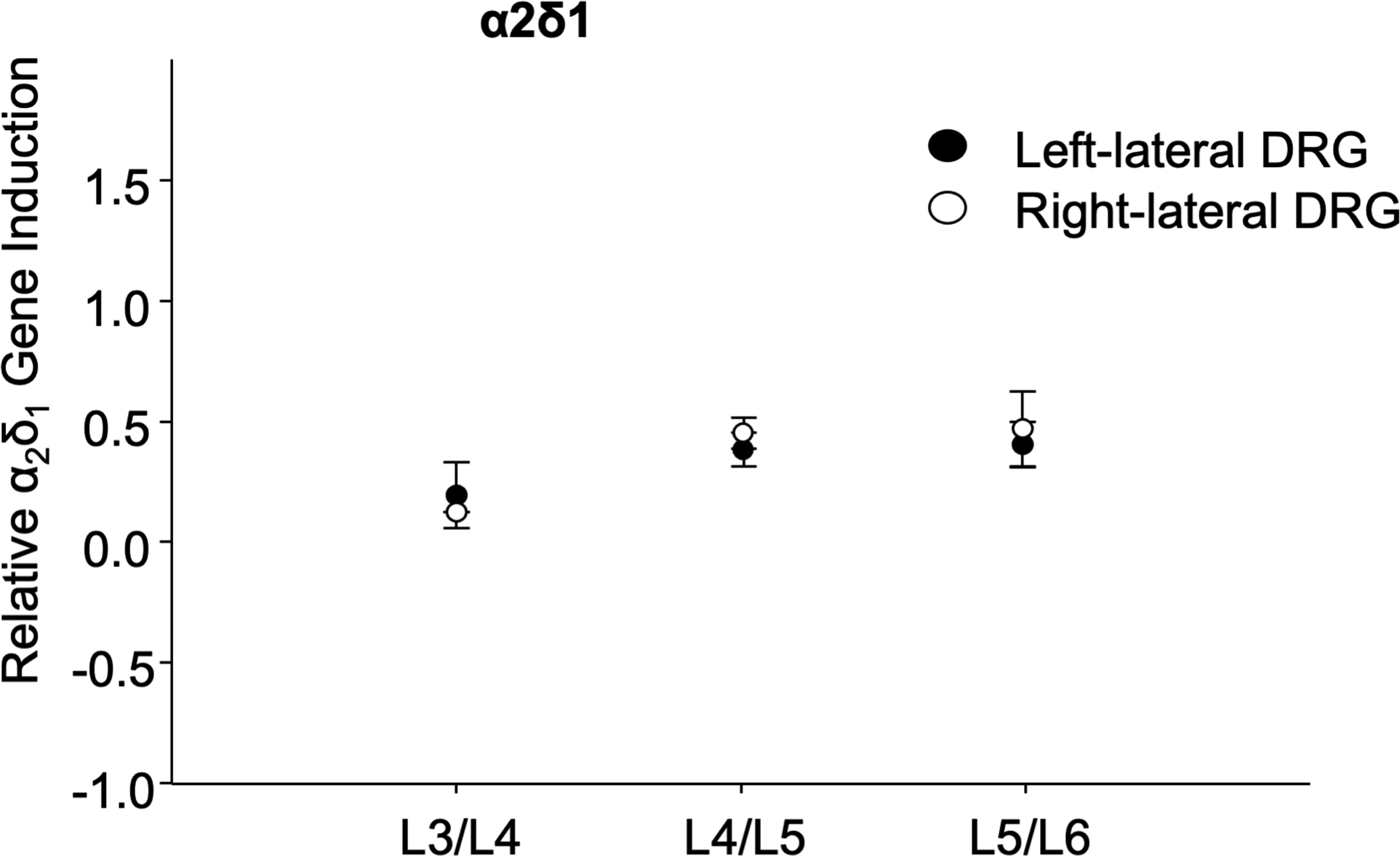
Similar level of basal gene expression in bilateral DRGs harvested from different lumbar levels (L3-L5). Harvested bilateral rat DRGs were mechanically homogenized to prepare total RNA and α2δ1 RT-qPCR.
To determine the length of incubation time of DRGs, we performed a time-course experiments of DRGs by harvesting explants after 1 to 7 days in culture. Each DRG was cultured in the presence or absence IL-1β stimulation at 10 ng/mL and analyzed by qPCR. The results indicate that, gene expression levels are steady from day 1 up to day 7, but that gene expression gradually decreases after day 7 (data not shown). Therefore, we selected an incubation period of five days for the explant co-culture experiments.
We co-cultured the DRGs together with human synovial explant tissues prepared from normal or surgically removed OA synovial tissues from patients who have a history of symptomatic knee joint pain. After 5 days of incubation, the DRGs were subjected to total RNA extraction, and analyzed by RT-qPCR using rat-specific primer sets. We observed markedly increased gene expression levels of substance P and its cognate receptors NK1 and NK2 from the DRGs that were co-cultured with OA synovium. In contrast, DRGs that were co-cultured with normal synovium (isolated from knee joints with Colin’s Grade G0) demonstrated gene expression levels similar to those observed in controls (DRGs co-cultured with media) (Fig. 3A). We also examined expression of neuropeptide receptors that are associated with chronic pain. For example, neuropeptide Y which transduces the nociceptive signals via neuropeptide Y receptors has been shown to be highly expressed in peripheral sensory neurons in chronic pain animal models [27]. Our RT-qPCR analyses clearly demonstrate a striking induction of both receptors (NPY1R, NPY2R) that may lead to enhanced nociceptive signals by neuropeptide Y (Fig. 3B). Transgenic mice that constitutively over-express the α2δ−1 subunit exhibit enhanced electrical currents that alter voltage-gated calcium channel activation in sensory neurons. These changes exaggerate and prolong neuronal responses in the dorsal horn to peripheral mechanical stimulation and pain behaviors[28]. In addition, a previous behavioral study using the α2δ ligand gabapentin, an effective for neuropathic pain, showed alleviation of hyperalgesia in the monosodium iodoacetate (MIA)-induced model for knee joint OA pain[29]. Based on these previous findings, we tested the potential influence of synovial tissues on sensory neurons in DRGs in our ex vivo co-culture system. Indeed, the DRGs co-cultured with OA synovial tissues highly upregulated α2δ1 expression, whereas DRGs that were co-cultured with either synovium from G0 or G1 knee joints showed no significant alterations in gene expression levels compared to media (control) (Fig. 3C).
Fig 3.
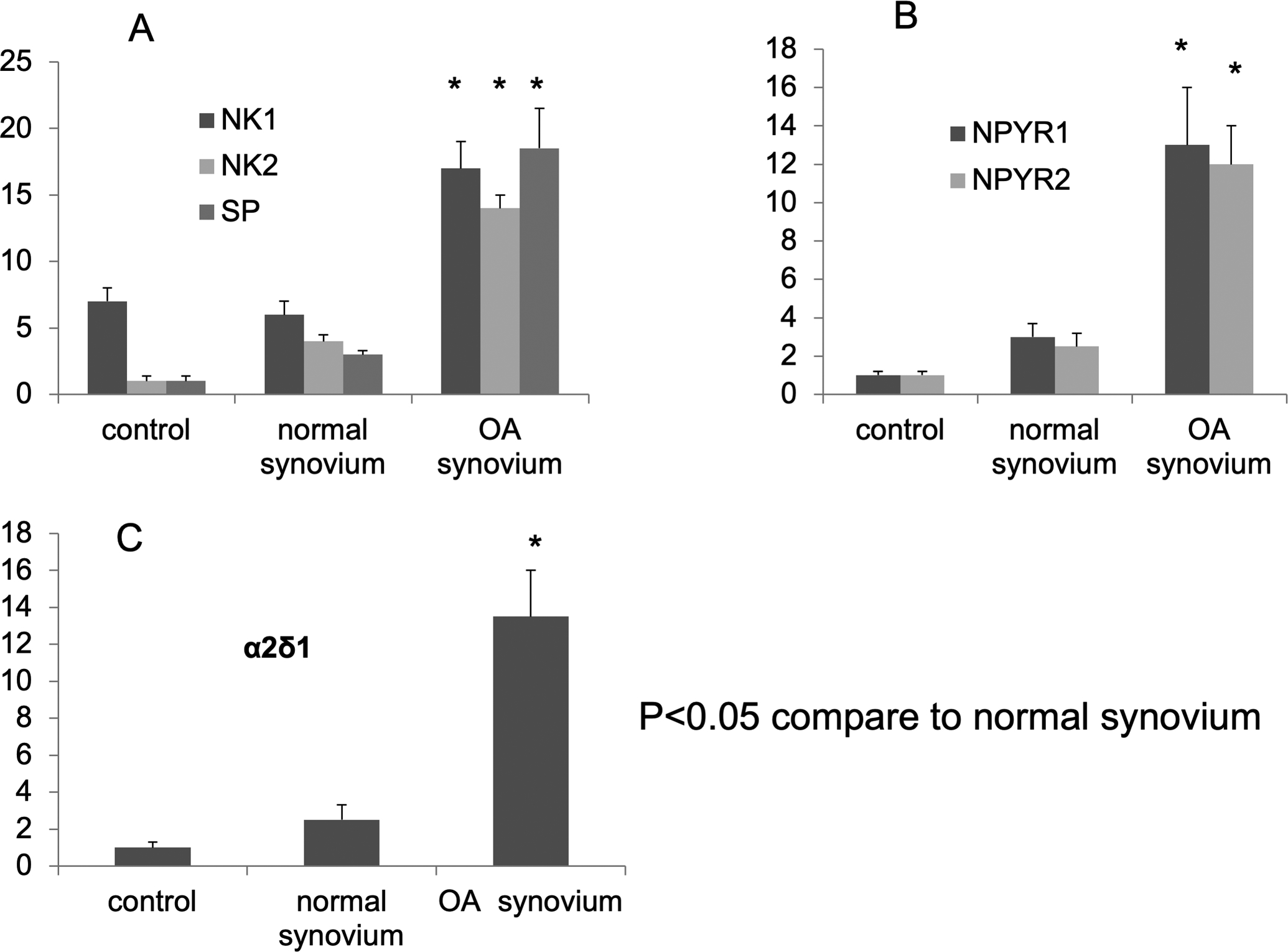
[A] DRGs that were co-cultured with normal synovium and OA synovium. After 5 days of incubation, the DRGs were subjected to total RNA extraction, and analyzed by RT-qPCR. Our data showed the gene expression of Substance P and tachykinin receptor (NK1,NK2) increased in rat DRGs which were co-cultured with human OA synovium. [B] the gene expression of NPY receptor NPY1R and NPY2R increased in DRGs which were co-cultured with OA synovium.
[C] the gene expression of α2δ1 increased in DRGs which were co-cultured with OA synovium
We have previously established the pathophysiologic links between prostaglandin E2 (PGE2), a downstream product of COX2, and OA [7]. Our results indicate that the COX2-PGE2 axis combined with IL-1β synergistically accelerates expression of pain-associated molecules such as inducible nitric oxide synthase (iNOS) and IL-6. Thus, we examined if co-culture of OA synovium tissues changes inflammatory pain modulators such as COX2 or IL-6 in the sensory neurons in DRGs. Our qPCR results demonstrated markedly induced COX2 (Fig. 4A; 7-fold induction, p<0.05) and IL-6 (Fig. 4B; p<0.05) which is less dramatic in terms of the fold-induction. Collectively, our results suggest that synovium tissues in OA potentially influence activation of the sensory neurons in DRGs which may lead to sensitization of knee joint in OA condition. Furthermore, these results may provide initial evidence of nociceptive “cross-talk” between peripheral joint tissues with the peripheral sensory neurons in DRGs for pathological pain pathway in OA.
Fig 4.

[A] DRGs that were co-cultured with normal synovium and OA synovium. After 5 days of incubation, the DRGs were subjected to total RNA extraction, and analyzed by RT-qPCR Our data showed the gene expression of COX2 increased in DRGs which were co-cultured with OA synovium.
[B] IL-6 in inflamed tissue contributes both to the inflammation itself and to pain hypersensitivity. Our RT-qPCR data showed the gene expression of IL-6 increased in DRGs which were co-cultured with OA synovium.
DISCUSSION
In this we assessed whether there are biological changes in neuronal tissues in response to factors secreted by knee joint explants that are co-cultured ex vivo. The key finding of our study is that DRGs respond to factors secreted by synovial tissue from OA patients. The resulting major changes in the expression of neuronal and inflammatory proteins in DRGs is consistent with in vivo studies [26] and our conceptual model that there are mutual cause and effect relationships between OA associated pain and inflammation.
Joint cartilage and synovium injury influences DRG neurons and sensitizes symptomatic pain perception through the dynamic interactions between neuropathic pathways and OA tissues. Our results suggest that alterations in the functional properties of sensory neurons are a key determinant for the severity of symptomatic OA pain. Early intervention in OA associated pain at the level of the central nerve system (CNS) may represent a novel therapeutic paradigm for the treatment of chronic OA pain
ACKNOWLEDGMENTS
This work was supported by grants from NIH R01AR053220 (HJ Im), the Arthritis Foundation (HJ Im), and the National Arthritis Research Foundation (HJ Im).
References
- 1.Sprangers MA, de Regt EB, Andries F, van Agt HM, Bijl RV, de Boer JB, Foets M, Hoeymans N, Jacobs AE, Kempen GI et al. : Which chronic conditions are associated with better or poorer quality of life? J Clin Epidemiol 2000, 53(9):895–907. [DOI] [PubMed] [Google Scholar]
- 2.Griffith CJ, LaPrade RF: Medial plica irritation: diagnosis and treatment. Curr Rev Musculoskelet Med 2008, 1(1):53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Im HJ, Muddasani P, Natarajan V, Schmid TM, Block JA, Davis F, van Wijnen AJ, Loeser RF: Basic fibroblast growth factor stimulates matrix metalloproteinase-13 via the molecular cross-talk between the mitogen-activated protein kinases and protein kinase Cdelta pathways in human adult articular chondrocytes. J Biol Chem 2007, 282(15):11110–11121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Im HJ, Pacione C, Chubinskaya S, Van Wijnen AJ, Sun Y, Loeser RF: Inhibitory effects of insulin-like growth factor-1 and osteogenic protein-1 on fibronectin fragment- and interleukin-1beta-stimulated matrix metalloproteinase-13 expression in human chondrocytes. J Biol Chem 2003, 278(28):25386–25394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Loeser RF, Forsyth CB, Samarel AM, Im HJ: Fibronectin fragment activation of proline-rich tyrosine kinase PYK2 mediates integrin signals regulating collagenase-3 expression by human chondrocytes through a protein kinase C-dependent pathway. J Biol Chem 2003, 278(27):24577–24585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muddasani P, Norman JC, Ellman M, van Wijnen AJ, Im HJ: Basic fibroblast growth factor activates the MAPK and NFkappaB pathways that converge on Elk-1 to control production of matrix metalloproteinase-13 by human adult articular chondrocytes. J Biol Chem 2007, 282(43):31409–31421. [DOI] [PubMed] [Google Scholar]
- 7.Li X, Ellman M, Muddasani P, Wang JH, Cs-Szabo G, van Wijnen AJ, Im HJ: Prostaglandin E2 and its cognate EP receptors control human adult articular cartilage homeostasis and are linked to the pathophysiology of osteoarthritis. Arthritis Rheum 2009, 60(2):513–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Loeser RF, Chubinskaya S, Pacione C, Im HJ: Basic fibroblast growth factor inhibits the anabolic activity of insulin-like growth factor 1 and osteogenic protein 1 in adult human articular chondrocytes. Arthritis Rheum 2005, 52(12):3910–3917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim JS, Ellman MB, An HS, van Wijnen AJ, Borgia JA, Im HJ: Insulin-like growth factor 1 synergizes with bone morphogenetic protein 7-mediated anabolism in bovine intervertebral disc cells. Arthritis Rheum 2010, 62(12):3706–3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang K, Zhang C, Zhang XW, Bao JP, Wu LD: Effect of dehydroepiandrosterone on aggrecanase expression in articular cartilage in a rabbit model of osteoarthritis. Mol Biol Rep 2010. [DOI] [PubMed] [Google Scholar]
- 11.Huang K, Wu LD: Suppression of aggrecanase: a novel protective mechanism of dehydroepiandrosterone in osteoarthritis? Mol Biol Rep 2010, 37(3):1241–1245. [DOI] [PubMed] [Google Scholar]
- 12.Thirunavukkarasu K, Pei Y, Wei T: Characterization of the human ADAMTS-5 (aggrecanase-2) gene promoter. Mol Biol Rep 2007, 34(4):225–231. [DOI] [PubMed] [Google Scholar]
- 13.Bao JP, Chen WP, Wu LD: Lubricin: a novel potential biotherapeutic approaches for the treatment of osteoarthritis. Mol Biol Rep 2010. [DOI] [PubMed] [Google Scholar]
- 14.Xu P, Yao J, Hou W: Relationships between COL2A1 gene polymorphisms and knee osteoarthritis in Han Chinese women. Mol Biol Rep 2010. [DOI] [PubMed] [Google Scholar]
- 15.Huang J, Ballou LR, Hasty KA: Cyclic equibiaxial tensile strain induces both anabolic and catabolic responses in articular chondrocytes. Gene 2007, 404(1–2):101–109. [DOI] [PubMed] [Google Scholar]
- 16.Chen WP, Bao JP, Hu PF, Feng J, Wu LD: Alleviation of osteoarthritis by Trichostatin A, a histone deacetylase inhibitor, in experimental osteoarthritis. Mol Biol Rep 2010, 37(8):3967–3972. [DOI] [PubMed] [Google Scholar]
- 17.Yan D, Davis FJ, Sharrocks AD, Im HJ: Emerging roles of SUMO modification in arthritis. Gene 2010, 466(1–2):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bayram B, Sayin E, Gunes HV, Degirmenci I, Turkoglu Z, Doganer F, Cosan DT: DD genotype of ace gene I/D polymorphism is associated in a turkish study population with osteoarthritis. Mol Biol Rep 2010. [DOI] [PubMed] [Google Scholar]
- 19.Hu PF, Bao JP, Wu LD: The emerging role of adipokines in osteoarthritis: a narrative review. Mol Biol Rep 2010. [DOI] [PubMed] [Google Scholar]
- 20.Bao JP, Chen WP, Feng J, Hu PF, Shi ZL, Wu LD: Leptin plays a catabolic role on articular cartilage. Mol Biol Rep 2010, 37(7):3265–3272. [DOI] [PubMed] [Google Scholar]
- 21.Bauer S, Jendro MC, Wadle A, Kleber S, Stenner F, Dinser R, Reich A, Faccin E, Godde S, Dinges H et al. : Fibroblast activation protein is expressed by rheumatoid myofibroblast-like synoviocytes. Arthritis Res Ther 2006, 8(6):R171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Djouhri L, Koutsikou S, Fang X, McMullan S, Lawson SN: Spontaneous pain, both neuropathic and inflammatory, is related to frequency of spontaneous firing in intact C-fiber nociceptors. J Neurosci 2006, 26(4):1281–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heredia Mdel P, Delgado C, Pereira L, Perrier R, Richard S, Vassort G, Benitah JP, Gomez AM: Neuropeptide Y rapidly enhances [Ca2+]i transients and Ca2+ sparks in adult rat ventricular myocytes through Y1 receptor and PLC activation. J Mol Cell Cardiol 2005, 38(1):205–212. [DOI] [PubMed] [Google Scholar]
- 24.Muehleman C, Berzins A, Koepp H, Eger W, Cole AA, Kuettner KE, Sumner DR: Bone density of the human talus does not increase with the cartilage degeneration score. Anat Rec 2002, 266(2):81–86. [DOI] [PubMed] [Google Scholar]
- 25.Im HJ, Li X, Muddasani P, Kim GH, Davis F, Rangan J, Forsyth CB, Ellman M, Thonar EJ: Basic fibroblast growth factor accelerates matrix degradation via a neuro-endocrine pathway in human adult articular chondrocytes. J Cell Physiol 2008, 215(2):452–463. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.Im HJ, Kim JS, Li X, Kotwal N, Sumner DR, van Wijnen AJ, Davis FJ, Yan D, Levine B, Henry JL et al. : Alteration of sensory neurons and spinal response to an experimental osteoarthritis pain model. Arthritis Rheum 2010, 62(10):2995–3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Upadhya MA, Dandekar MP, Kokare DM, Singru PS, Subhedar NK: Involvement of neuropeptide Y in the acute, chronic and withdrawal responses of morphine in nociception in neuropathic rats: behavioral and neuroanatomical correlates. Neuropeptides 2009, 43(4):303–314. [DOI] [PubMed] [Google Scholar]
- 28.Li CY, Zhang XL, Matthews EA, Li KW, Kurwa A, Boroujerdi A, Gross J, Gold MS, Dickenson AH, Feng G et al. : Calcium channel alpha2delta1 subunit mediates spinal hyperexcitability in pain modulation. Pain 2006, 125(1–2):20–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rahman W, Bauer CS, Bannister K, Vonsy JL, Dolphin AC, Dickenson AH: Descending serotonergic facilitation and the antinociceptive effects of pregabalin in a rat model of osteoarthritic pain. Mol Pain 2009, 5:45. [DOI] [PMC free article] [PubMed] [Google Scholar]


