Abstract
Background
Acute otitis media (AOM) is the most common bacterial infection among young children in the United States. There are limitations and concerns over its treatment with antibiotics and surgery and so effective preventative measures are attractive. A potential preventative measure is xylitol, a natural sugar substitute that reduces the risk of dental decay. Xylitol can reduce the adherence of Streptococcus pneumoniae (S pneumoniae) and Haemophilus influenzae (H influenzae) to nasopharyngeal cells in vitro. This is an update of a review first published in 2011.
Objectives
To assess the efficacy and safety of xylitol to prevent AOM in children aged up to 12 years.
Search methods
We searched CENTRAL (to Issue 12, 2015), MEDLINE (1950 to January 2016), Embase (1974 to January 2016), CINAHL (1981 to January 2016), LILACS (1982 to January 2016), Web of Science (2011 to January 2016) and International Pharmaceutical Abstracts (2000 to January 2016).
Selection criteria
Randomised controlled trials (RCTs) or quasi‐RCTs of children aged 12 years or younger where xylitol supplementation was compared with placebo or no treatment to prevent AOM.
Data collection and analysis
Two review authors independently selected trials from search results, assessed and rated study quality and extracted relevant data for inclusion in the review. We contacted trial authors to request missing data. We noted data on any adverse events of xylitol. We extracted data on relevant outcomes and estimated the effect size by calculating risk ratio (RR), risk difference (RD) and associated 95% confidence intervals (CI).
Main results
We identified five clinical trials that involved 3405 children for inclusion. For this 2016 update, we identified one new trial for inclusion. This trial was systematically reviewed but due to several sources of heterogeneity, was not included in the meta‐analysis. The remaining four trials were of adequate methodological quality. In three RCTs that involved a total of 1826 healthy Finnish children attending daycare, there is moderate quality evidence that xylitol (in any form) can reduce the risk of AOM from 30% to around 22% compared with the control group (RR 0.75, 95% CI 0.65 to 0.88). Among the reasons for dropouts, there were no significant differences in abdominal discomfort and rash between the xylitol and the control groups. Xylitol was not effective in reducing AOM among healthy children during a respiratory infection (RR 1.13, 95% CI 0.83 to 1.53; moderate quality evidence) or among otitis‐prone healthy children (RR 0.90, 95% CI 0.67 to 1.21; low‐quality evidence).
Authors' conclusions
There is moderate quality evidence showing that the prophylactic administration of xylitol among healthy children attending daycare centres can reduce the occurrence of AOM. There is inconclusive evidence with regard to the efficacy of xylitol in preventing AOM among children with respiratory infection, or among otitis‐prone children. The meta‐analysis was limited because data came from a small number of studies, and most were from the same research group.
Plain language summary
Xylitol sugar supplement for preventing middle ear infection in children up to 12 years of age
Review question We reviewed the evidence about the effectiveness and safety of xylitol to prevent acute middle ear infection (acute otitis media; AOM) in children up to 12 years old.
Background AOM is the most common bacterial infection among young children in the United States. Although serious complications are rare, this common childhood ailment imposes a huge impact on the healthcare system. In the United States, it accounted for almost 20 million office visits. Antibiotic treatment of AOM is costly and raises concerns about the development of antibiotic‐resistant strains of bacteria. Surgery is invasive and costly, and because of these factors, effective measures for preventing AOM are sought. An alternative treatment is xylitol or birch sugar. Xylitol has been used for decades as a natural non‐sugar sweetener principally in chewing gums, confectionery, toothpaste and medicines, and can reduce the risk of tooth decay.
Search date
We searched the literature up to January 2016. This is an update of a review that was last published in 2011.
Study characteristics We identified five clinical trials that involved 3405 children, mostly from the same research group. Four trials were conducted in Finland and enrolled healthy children (three trials) or children with an acute respiratory infection (one trial). The fifth trial was conducted in the USA and enrolled otitis‐prone children who were recruited from attendance at general medical practices.
Study funding sources
All five trials received governmental funding; and the Finnish study investigators have a US patent for the use of xylitol to treat respiratory infections.
Key results
Xylitol, administered in chewing gum, lozenges or syrup, can reduce the occurrence of AOM among healthy children with no acute upper respiratory infection from 30% to 22%. There is no difference in side effects (namely, abdominal discomfort and rash). Based on these results we would expect that out of 1000 children up to 12 years of age, 299 would experience an AOM compared with between 194 and 263 children who would experience an AOM if they are provided with xylitol chewing gum. The preventive effect among healthy children with respiratory infection or among otitis‐prone children is inconclusive.
Quality of the evidence The quality of evidence was moderate for healthy children and children with respiratory infections but low for otitis‐prone children.
Summary of findings
Background
Description of the condition
AOM is the most common infection for which antibacterial agents are prescribed for children in the United States (AAP 2013). According to the evidence‐based guidelines of the American Academy of Pediatrics and American Academy of Family Physicians, AOM is defined as the presence of middle ear effusion (thick or sticky fluid behind the eardrum in the middle ear) and a rapid onset of signs or symptoms of middle‐ear inflammation, such as ear pain, otorrhoea (discharge from the ear) or fever (AAP 2013).
By the end of their first year of life, approximately 62% of children have experienced at least one episode of AOM, and by the age of three years, almost 83% of children have experienced at least one episode (Friedman 2006; Jansen 2009). The peak incidence occurs between six and 12 months of age (ACIP 2000). Although serious complications are rare, this common childhood ailment imposes a huge impact on healthcare systems. In the United States, AOM accounts for almost 20 million medical office visits annually (CDC 2015). Childhood AOM is a significant healthcare utilisation concern and accounts for approximately USD 2.88 billion in additional healthcare cost annually (Ahmed 2014)
The key step in the pathogenesis of AOM is colonisation of the upper airway with pathogenic bacteria which move from the nasopharynx to the middle ear by way of the eustachian tube (Murphy 2006). The aetiologic agents include bacteria, viruses and a combination of both (Guven 2006). S pneumoniae and H influenzae are the most common causes of bacterial otitis media (Murphy 2006). Studies using diagnostic tympanocentesis in children with AOM identified S pneumoniae in 28% to 55% of all middle ear aspirates (ACIP 2000).
Despite a large number of published RCTs, there is no consensus on therapy for AOM (Venekamp 2015). The rate of antibiotic use for AOM varies from 31% in the Netherlands (Akkerman 2005) to 95% in the USA and Canada (Froom 2001). AOM is the leading reason for antibiotic prescriptions in the United States (Venekamp 2015). This results in the widespread emergence of multidrug resistant pathogens (ACIP 2000; Friedman 2006). Treatment strategies for AOM include surgery, antibiotic prophylaxis and vaccination. Surgical procedures have limited and short‐term efficacy on the occurrence of persistent or recurrent otitis media (McDonald 2008; Paradise 1999; Wallace 2014). The effectiveness of antibiotic therapy for AOM appears to be limited. When potential adverse events such as vomiting, diarrhoea or rash were considered, one in every 14 children treated with antibiotics experienced an adverse event that would not have occurred if antibiotics were withheld (Venekamp 2015). Moreover, in high‐income countries most cases of AOM remit spontaneously with no complications (Venekamp 2015). Furthermore, pneumococcal vaccines make only a small difference (2% to 7% relative reduction) to numbers of AOM cases (Norhayati 2015).
Description of the intervention
Xylitol, or birch sugar, is chemically a pentitol or 5‐carbon polyol sugar alcohol, naturally found in plums, strawberries, raspberries and rowan berries. It does not cause dental caries because, although a sugar alcohol, it cannot be fermented by cariogenic bacteria in the oral cavity. It has the same relative sweetness as sucrose, and is accordingly an ideal non‐sugar sweetener approved in many countries, and principally used in chewing gums, confectionery, toothpaste and medicines (Maguire 2003; Uhari 2000). Xylitol consumption in the UK is about 1000 tonnes per year (Maguire 2003).
How the intervention might work
Xylitol inhibits the growth and acid production of certain strains of Streptococcus mutans (S mutans) (Uhari 2000). In an in vitro study, it was reported that 1% and 5% xylitol markedly reduced the growth of alpha‐haemolytic streptococci including S pneumoniae and Streptococcus mitis (S mitis) during their logarithmic phase of growth (Kontiokari 1995). Clues to the mechanism are suggested by the finding that 5% xylitol reduced adherence of both S pneumoniae and H influenzae to nasopharyngeal cells (Kontiokari 1998). Xylitol is potentially clinically useful in preventing pneumococcal diseases, including AOM.
Why it is important to do this review
Antibiotic treatment of AOM is costly and raises concerns about the development of antibiotic‐resistant strains of bacteria. Surgery is invasive and costly, and because of these factors, alternative, effective measures to prevent AOM are sought. An alternative treatment is xylitol. This review evaluated the efficacy of xylitol to prevent AOM in children.
Objectives
To assess the efficacy and safety of xylitol to prevent AOM in children aged up to 12 years.
Methods
Criteria for considering studies for this review
Types of studies
We included RCTs and one quasi‐RCT of any type of xylitol intervention versus placebo or no intervention for the prevention of AOM in children aged up to 12 years. We did not include cluster trials due to potential differences in the ancillary treatments among centres. We included studies reporting on any primary or secondary outcomes.
Types of participants
Children up to 12 years of age without AOM.
Types of interventions
We included studies of xylitol in any form (e.g. syrup, chewing gum, lozenges or nasal spray) used to prevent AOM. We considered studies of any dose or duration of administration. Eligible control interventions included placebo or no treatment.
Types of outcome measures
Primary outcomes
Number of children (healthy children, children with a respiratory infection, and otitis‐prone children) with at least one AOM episode during the follow‐up period.
Secondary outcomes
Safety and adverse events.
Antibiotic administration.
Hospitalisation (and its length) secondary to AOM or its complications in studies where at least one hospitalisation was reported.
Mortality secondary to complications of AOM in studies where at least one death was reported.
Number of days missed at school or daycare centre.
Cost (direct such as emergency room or general practitioners visits; hospitalisations; medication and indirect costs such as time taken from work for parents or care givers; the opportunity cost of leisure time; informal nursing care; and out‐of‐pocket expenses for transportation).
Search methods for identification of studies
Electronic searches
For this 2016 update we searched the Cochrane Central Register of Controlled Trials (CENTRAL, 2015, Issue 12 December) (accessed 27 January 2016), which includes the Cochrane Acute Respiratory Infections Specialised Register, MEDLINE (June 2011 to January 2016), Embase (June 2011 to January 2016), CINAHL (June 2011 to January 2016), LILACS (2011 to January 2016), International Pharmaceutical Abstracts (June 2011 to Jan 2016), and Web of Science (2011 to January 2016). We did not apply language or publication restrictions. We used the terms in Appendix 1 to search MEDLINE (OVID) and CENTRAL. We did not combine the search with a strategy for identifying randomised trials in MEDLINE as there were too few results. We adapted this search strategy to search Embase (Appendix 2), CINAHL (Appendix 3), LILACS (Appendix 4) and Web of Science (Appendix 5). Previously we searched: CENTRAL 2011, Issue 3; MEDLINE (1950 to August week 1, 2011); Embase.com (1974 to August 2011); CINAHL (1981 to August 2011); Health and Psychosocial Instruments (1985 to August 2011); Healthstar (OVID) (1966 to August 2011); and the International Pharmaceutical Abstracts (2000 to August 2011).
Searching other resources
We searched the WHO ICTRP and ClinicalTrials.gov trials registries for completed and ongoing trials using the term 'xylitol' (latest search 27 January 2016). We attempted to search for unpublished studies (or grey literature) such as technical reports, dissertations, or studies published in languages other than English, which may not have been indexed to major databases, by contacting authors of identified trials. We also conducted Internet searches using Google Scholar™. We searched the first 100 hits that included xylitol and otitis media in the title. We searched dissertations and theses databases (from inception up to 2016) through ProQuest. We also searched in the Open Grey (System for Information on Grey Literature in Europe) database (from inception up to 2016) and the reference lists of identified articles. We also searched abstracts from the annual meetings of the American Association for Respiratory Care, European Respiratory Care Association, the Australian Asthma and Respiratory Educators Association, Society for Pediatric Research, American Pediatric Society and Pediatric Academic Societies published in Pediatric Research (2002 to 2016). There were no language or publication restrictions. We contacted trial authors if we needed to clarify or obtain relevant data on outcomes not reported in the study. We identified manufacturers and distributors of xylitol products based on information extracted mainly from included studies as well as an Internet search and contacted these agencies to locate unpublished trials. We contacted five xylitol distributors (Academy of Dental Resources, Xylarex, Oral Biotech, Xlear Inc. and Oxyfresh Worldwide, Inc.) and three researchers (October 2009) and requested information on any unpublished trials.
Data collection and analysis
Selection of studies
Two review authors (AA, PS) independently screened titles and abstracts for inclusion of all the potential studies we identified as a result of the search. We retrieved the full text study reports/publication and the same review authors independently screened full text and identified studies for inclusion, and recorded reasons for exclusions. We resolved disagreements through discussion and consensus, or by consulting a third review author (HPL). We identified and excluded duplicates and collated multiple reports of the same study so that each study, rather than each report, is the unit of interest in the review. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram (Figure 1) (Moher 2009) and Characteristics of excluded studies.
1.
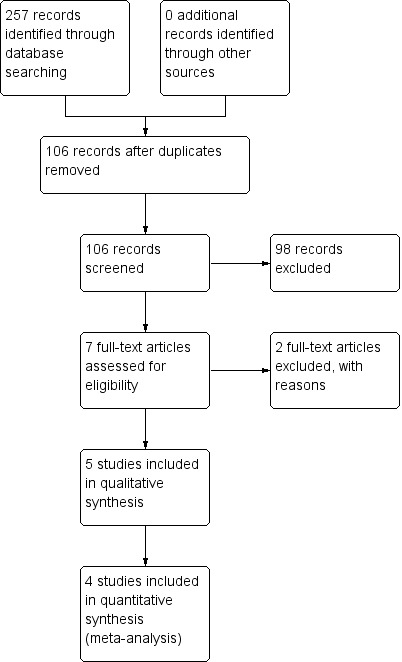
Study flow diagram.
Data extraction and management
We extracted data using a standardised data collection form. Disagreements were resolved by discussion with a third author (HPL). We extracted the following information:
participant characteristics (age, sex, race, representative of, history of recurrent AOM, attendance at daycare or school);
study setting;
type of intervention and control and number studied in each group;
adverse effects;
method for diagnosing AOM;
occurrence of AOM and its complications during follow‐up period;
percentages of children with at least one AOM episode during the follow‐up period;
use of antibiotics to treat AOM or its complications;
time lost from daycare/school (for children) or from work (for parents or care givers) due to AOM;
costs of managing AOM;
randomisation method;
masking method (in randomisation, intervention, outcome assessment); and
whether analysis was by intention‐to‐treat (ITT).
Assessment of risk of bias in included studies
We assessed methodological quality using the information in the original publications. Two review authors (AA, PS) independently evaluated study quality using the Cochrane 'Risk of bias' assessment tool (Higgins 2011). We evaluated six domains (sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting and other issues) based on what was reported in the study and assigned judgements relating to the risk of bias for each domain. Possible judgments for these domains could be high, low or unclear risk of bias.
Measures of treatment effect
We analysed the primary outcome of interest (number of children with at least one AOM episode) using RevMan 5.3 for statistical analysis (RevMan 2014). We used RR and RD and planned to use mean difference (MD) if appropriate. We reported 95% CI for estimates of treatment effects. We used data from all intervention arms in the analyses for studies with more than two arms, where the same xylitol forms were compared in two or more experimental groups (for example, xylitol in chewing gum, syrup or lozenges were compared to a common control group). We analysed the secondary outcomes of interest (adverse events, hospitalisation, mortality, percentage of children needing antibiotic therapy) when the data were available, using RR or RD. We planned to summarise length of hospitalisations, number of days missed at school and cost differences as MD or with 95% CI if data were available.
Unit of analysis issues
We included data from a participant once only, even if the participant was recruited more than once.
Dealing with missing data
We contacted trial authors to request missing data or clarify methods whenever possible, but we received few responses.
Assessment of heterogeneity
We considered a conservative P < 0.1 as significant. We calculated I² statistic values to quantify heterogeneity (Higgins 2003). In addition, we inspected the graphical display of trials estimated treatment effects (along with their 95% CIs) for assessing heterogeneity.
Assessment of reporting biases
We assessed publication bias by checking trials registries and contacting the authors of identified studies to ask if they had other publications or were aware of any other unpublished studies we had missed.
Data synthesis
We used a fixed‐effect model for meta‐analyses.
GRADE and 'Summary of findings' tables
We created three 'Summary of findings' tables for three populations of healthy children, children with a respiratory infection, and otitis‐prone children, in which the xylitol had been evaluated using the following outcomes: final diagnosis of at least one episode of AOM; adverse events; antibiotic administration; hospitalisation; mortality; number of days missed at school or daycare; and cost. We used the five GRADE (Atkins 2004) considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of evidence as it relates to the studies which contribute data to the meta‐analyses for the prespecified outcomes (GRADEpro 2004). We used methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) using GRADEpro GDT software (GRADEproGDT 2015). We justified all decisions to down‐ or up‐grade the quality of studies using footnotes and we made comments to aid readers' understanding of the review where necessary.
Subgroup analysis and investigation of heterogeneity
We performed four subgroup analyses based on the methodology of the identified studies:
younger children unable to chew gum;
older children who were able to chew;
for those whom xylitol was administered during respiratory infection; and
comparison between different types of xylitol vehicles.
Sensitivity analysis
We also looked at the results in subsets of trials with different methods of xylitol administration (syrup, chewing gum or lozenges). We applied Cochrane’s Q test for between‐study heterogeneity (Deeks 2011). We also performed random‐effects meta‐analysis to compare the pooled results to those of the fixed‐effect meta‐analyses.
Results
Description of studies
Results of the search
This is an update of our previous review (Azarpazhooh 2011). For this 2016 update, we reviewed 33 potential citations. The study flow diagram is presented in Figure 1. We identified one new trial on ClinicalTrials.gov (www.clinicaltrials.gov/) completed and published (NCT01044030 as Vernacchio 2014) and a new trial that was not yet open for participant recruitment (NCT02592382). We saved this record for a future update (Characteristics of ongoing studies). Previously, our preliminary searches identified 84 potential citations. We read the titles and abstracts of these studies. We selected four studies for full text appraisal and these met the eligibility criteria for the first version of this review (Hautalahti 2007; Tapiainen 2002; Uhari 1996; Uhari 1998). This review included five clinical trials that involved 3405 children. Details of the participants enrolled in these studies are described in Characteristics of included studies.
Included studies
Vernacchio 2014 performed a pragmatic practice‐based RCT. Ninety‐seven practices of three paediatric practice–based networks (the Slone Center Office‐based Research Network at Boston University, the Pediatric Physicians’ Organization at Children’s (Boston), and the North Carolina Child Health Research Network at the University of North Carolina) referred 778 children, aged six to 71 months, to the study for eligibility review. These children were all otitis‐prone, that is, they had a history of at least three clinically diagnosed episodes of AOM in the previous 12 months with at least one in the previous six months and may have had middle ear effusions at the time of enrolment. Of these children, 326 were randomised to receive either a placebo syrup (n = 166, daily dose of 2.25 g sorbitol three times a day) or a viscous xylitol syrup (n = 160, daily dose of 5 g three times a day) for three months. All of the daily doses were administered by parents or other routine caregivers, not in a controlled daycare setting as in the previous trials. Compliance was monitored by asking parents, at each interview, how much of the recommended syrup the child had taken since the last interview. In total, about 28% of participants in the xylitol group and 24% of participants in the placebo group discontinued treatment before the end of the study period or the occurrence of the primary outcome. The most common reasons were gastrointestinal side effects (18 in the xylitol group and 11 in the placebo group), the participant refusing to take the solution (eight in each group), and the parent becoming too busy or finding the treatment too difficult to administer (eight xylitol, five placebo). At the end of the study, a blinded investigator reviewed medical records from each participant's primary care physician as well as any other healthcare provider whom the parent identified as having treated the participant during the study period. The clinical diagnoses of AOM was registered as reported in the medical records by a wide range of clinicians and were not otherwise verified. If medical records were not available, the diagnosis of AOM was registered as reported by the parents. By the end of the trial, two participants in the xylitol group and three in the placebo group declined further participation. Four participants in the xylitol group and three in the placebo group were lost to follow‐up without any outcome information. The details of the participants enrolled in this study are described in Characteristics of included studies.
Hautalahti 2007 randomised 663 healthy children enrolled in daycare in the city of Oulu, Finland to four groups to receive either a control product (n = 331, daily dose of 0.5 g in the control chewing gum group or in a syrup three times a day, pulled together as one control group) or xylitol (n = 332, daily dose of 9.6 g in chewing gum or in a syrup three times a day, pulled together as one test group) for three months. The total daily amount of received xylitol in this study was equal to that used in earlier trials but the size of a piece of chewing gum and the amount of syrup needed per dose were bigger and dosing was less frequent (three versus five daily doses). In working days, two daily doses were given at the daycare centre and one dose at home. At weekends and holidays all three doses were given at home. Compliance and the consumption of additional xylitol products were monitored by asking parents to record the doses actually given and any other xylitol products on a daily report sheet and also by counting and measuring the unused returned products at the end of the trial. The occurrence of the first AOM diagnosed during any period of respiratory symptoms during the follow‐up was the main outcome measure based on a finding of middle ear effusion in tympanometry (B, C or positive pressure curve) and confirmed with pneumatic otoscopy. Otorrhoea from a tympanostomy tube was counted as AOM. There was dropout of 96 participants (58 in the xylitol group and 38 in the control group). The baseline characteristics (demographic features, known AOM risk factors, or AOM history, earlier use of xylitol products) were similar in all four groups. By the end of trial, the dropouts range was statistically higher in the xylitol group (17%) versus the control group (11%). The reasons for dropouts and the related numbers per each group are as follows: child refused to take the product (control: 15 versus xylitol: 22); abdominal discomfort (control: seven versus xylitol: nine); chewing gum piece too big (control: one versus xylitol: five); duration of the trial too long (control: one versus xylitol: two); forgot to give the preparation when on holiday (control: three versus xylitol: three); illness (control: two versus xylitol: three); rash (control: three versus xylitol: three); parents tired of the trial (control: two versus xylitol: one); difficult to avoid additional xylitol products (control: zero versus xylitol: one); other (control: one versus xylitol: four) or unknown reasons (control: three versus xylitol: five). The details of the patients enrolled in this study are described in the Characteristics of included studies table.
Tapiainen 2002 randomised 1277 healthy children enrolled in daycare in the city of Oulu, Finland after screening with tympanometry to receive either the control mixture (n = 212), xylitol mixture (n = 212), control chewing gum (n = 280), xylitol chewing gum (n = 286) or xylitol lozenges (n = 287) during an acute respiratory infection. The parents began administering the products to their children at the onset of acute respiratory infection (ARI) symptoms, which was defined as the appearance of one or more of the following symptoms: clear or purulent discharge from the nose, congestive nose, cough, conjunctivitis, sore throat or earache. Compliance was monitored by asking the parents to list the doses actually given on the daily symptom sheets and by counting the unused pieces of chewing gum and lozenges returned at the last appointment and measuring the volume of the unused mixture. The follow‐up lasted until resolution of the symptoms or up to three weeks. AOM was diagnosed based on a finding of middle ear effusion in tympanometry (B, C or positive pressure curve) and confirmed with pneumatic otoscopy. A total of 1253 of the 1277 randomised children were eligible for the analysis of the primary outcome. The children who dropped out (n = 24) were excluded from the statistical analysis but those who prematurely stopped using the products but still visited the clinic (n = 35) were included. The reasons for dropouts and the related numbers per each group are as follows: parents got tired (only two in the xylitol syrup and four in the xylitol chewing gum); child disliked the product (two only in the xylitol lozenge); other antibiotics for ARI (only one in the xylitol syrup and one in the control chewing gum); left the area (only one in the control syrup, four in the xylitol chewing gum and two in the xylitol lozenge); and unknown (syrup: zero in the control versus two in the xylitol group; chewing gum: two in control versus one in xylitol; lozenge: two in xylitol). Moreover, the administration was discontinued due to abdominal discomfort (syrup: one in control versus three in xylitol; chewing gum: one in control versus one in xylitol; lozenge: five in xylitol); child disliked the product (one in xylitol chewing gum and 10 in xylitol lozenge); or unknown (syrup: two in control versus two in xylitol; chewing gum: three in control versus three in xylitol; five in xylitol lozenge). The details of the participants enrolled in this study are described in Characteristics of included studies.
Uhari 1996 randomised 306 children in the city of Oulu, Finland daycare (157 in the xylitol group and 149 children in the sucrose group). Each child was instructed to chew two pieces of gum five times a day after meals or snacks until there was no taste (or until five minutes), making a total dose of 8.4 g xylitol per day for two months. Compliance was assessed by asking the parents to report the actual number of pieces of chewing gum used at the end of each month of the trial and also by reporting on the use of additional xylitol products and possible medications. Nasopharyngeal samples were taken before the sugar challenge, at two weeks and at the end of the study. Pneumococci were identified by (alpha) haemolysis on sheep blood agar plates, colony morphology and optochin sensitivity. Parents completed symptom sheets, time and reasons for being absent from daycare, and the use of additional xylitol products and possible medications. At a physician appointment, clinical diagnosis and the drugs prescribed were registered. AOM was diagnosed based on symptoms and signs of ARI and simultaneous signs of middle ear effusion: a cloudy tympanic membrane or impaired tympanic membrane motility in pneumatic otoscopy. The baseline characteristics were similar in the two randomised groups. By the end of trial, there was a dropout of 30 participants; 10 in the xylitol group and 20 in the control group, representing a dropout rate of 6% and 13%, respectively. The reasons for dropouts and the related numbers per each group are as follows: child got tired of eating chewing gum (sucrose: eight versus xylitol one), dental caries (sucrose three versus xylitol four), moved from area (sucrose five versus xylitol one), parents got tired (sucrose two versus xylitol two), insufficient follow‐up data (sucrose: two versus xylitol zero) and loose stools (sucrose zero versus xylitol two). There were no differences in the duration of cough or other symptoms of infections between the groups as reported by parents. There was also no difference in the pneumococcal carriage rates during the study and between the groups as well as the numbers of upper respiratory tract infections without AOM, acute bronchitis, sinusitis and conjunctivitis. The details of the participants enrolled in this study are described in Characteristics of included studies.
Uhari 1998 randomised 857 healthy children enrolled in the city of Oulu, Finland daycare to one of five treatment groups. Participants received control syrup (n = 165), xylitol syrup (n = 159), control chewing gum (n = 178), xylitol gum (n = 179) or xylitol lozenge (n = 176) for three months. Two pieces of chewing gum or lozenges were chewed for at least five minutes five times a day after meals (three doses given by the personnel at the childcare centres during the day and the rest given by the parents at home). Compliance was monitored by asking the parents to list the doses actually given on the daily symptom sheets and also by counting and measuring the unused returned products at the end of the trial. AOM was diagnosed based on a finding of middle ear effusion in tympanometry (B‐ or C‐curve), verified otoscopically with signs of inflammation in the tympanic membrane and the presence of symptoms of ARI (rhinitis, cough, conjunctivitis, sore throat, earache). The baseline characteristics (demographic features, known AOM risk factors or AOM history) and the mean number of forgotten dosages were similar in all five groups. By the end of trial, the dropouts range from 4.5% to 18.9% and were statistically higher in the xylitol syrups (18.9%) and xylitol lozenges (14.8%) as compared to their control groups of respectively control syrups (10.3%) and control chewing gum (4.5%). The reasons for dropouts and the related numbers per each group are as follows: unwilling to continue taking the product (syrup: nine in control versus 14 in xylitol; chewing gum: seven in control versus eight in xylitol; lozenge: 14 in xylitol), left the area (syrup: zero in control versus two in xylitol; chewing gum: zero in control versus two in xylitol; lozenge: one in xylitol), abdominal discomfort (syrup: five in control versus eight in xylitol; chewing gum: zero in control versus one in xylitol; lozenge: seven in xylitol), and unknown reason (syrup: three in control versus six in xylitol; chewing gum: one in control versus one in xylitol; lozenge: four in xylitol). The details of the patients enrolled in this study are described in the Characteristics of included studies table. It should be noted that these trials were all conducted in similar populations in Finland. The methodology appears to be similar with the small change in the control group after the first study (change from sucrose to low‐dose xylitol in the control group), the population in the 2002 study (from healthy children to children with ARI) and the dosing in the 2007 study (five times per day to three times per day).
Excluded studies
We identified a recent trial that evaluated the effectiveness of xylitol paediatric topical oral syrup to reduce the incidence of dental caries among very young children (Milgrom 2009). In the published paper, it was mentioned that the effect of xylitol in reducing AOM will be published in a subsequent study. However, after contacting the primary investigator, it was understood that due to a lack of reliability of data regarding AOM, the plan to publish AOM results has been suspended.
Risk of bias in included studies
Figure 2 summarises the review authors' judgements about each risk of bias domain for each included study.
2.
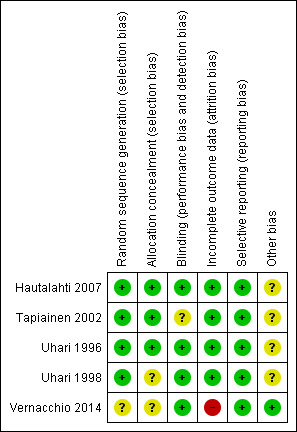
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study
Allocation
With the exception of Vernacchio 2014 for which the sequence generation is unclear, the other four included trials had adequate sequence generation (low risk). For allocation concealment, three trials were considered to have low risk of bias (Hautalahti 2007; Tapiainen 2002; Uhari 1996) and the other two trials an unclear risk (Uhari 1998; Vernacchio 2014).
Blinding
Three out of five included studies had adequate blinding (Hautalahti 2007; Uhari 1996; Vernacchio 2014). The blinding procedure was not mentioned in one study (Uhari 1998) but it was judged to be 'probably done' as per the authors' earlier publication. Tapiainen 2002 was blinded as far as the mixture and chewing gum groups were concerned but open between the xylitol lozenge and control chewing gum groups.
Incomplete outcome data
In Vernacchio 2014, 62/160 allocated to xylitol (38.8%) declined participation, were lost to follow‐up or discontinued intervention versus 58/166 allocated to placebo (34.9%). Intention‐to‐treat (ITT) analysis was performed. The other four trials clarified the number of and the reasons for dropouts, but did not perform an ITT analysis.
Selective reporting
No selective reporting was identified in the five included trials.
Other potential sources of bias
In four Finnish trials the test material was donated by industry (Hautalahti 2007; Tapiainen 2002; Uhari 1996; Uhari 1998). All five trials had governmental funding. No conflict of interest was declared in any of the included trials; however, it should be noted that the study authors in Finnish trials have a US patent for the use of xylitol in treating respiratory infections.
Effects of interventions
See: Table 1; Table 2; Table 3
Summary of findings for the main comparison. Xylitol versus control for prevention of AOM in healthy children.
| Xylitol versus control for prevention of AOM in healthy children | ||||||
| Patient or population: preventing acute otitis media in children up to 12 years of age Setting: daycare in Finland Intervention: xylitol in any form (syrup, gum or lozenge) Comparison: control in any form (gum, syrup) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk with control in any form (gum, syrup) | Corresponding risk with xylitol in any form (syrup, gum or lozenge) | |||||
| Final diagnosis of at least one episode of AOM | Study population | RR 0.75 (0.65 to 0.88) | 1826 (3 RCTs) | ⊕⊕⊕⊝ Moderate¹ | RR 0.74 (0.54, 1.01) with random‐effects meta‐analysis | |
| 299 per 1000 | 224 per 1000 (194 to 263) | |||||
| Moderate | ||||||
| 296 per 1000 | 222 per 1000 (192 to 261) | |||||
| Gastrointestinal‐related adverse events | Study population | RR 1.43 (0.74 to 2.75) | 1826 (3 RCTs) | ⊕⊕⊕⊝ Moderate² | RR 1.41 (0.60, 3.33) with random‐effects meta‐analysis | |
| 17 per 1000 | 24 per 1000 (13 to 47) | |||||
| Moderate | ||||||
| 15 per 1000 | 21 per 1000 (11 to 40) | |||||
| Antibiotic administration | Study population | RR 0.64 (0.42 to 0.97) | 306 (1 RCT) | ⊕⊕⊕⊝ Moderate³ | ||
| 289 per 1000 | 185 per 1000 (121 to 280) | |||||
| Moderate | ||||||
| 289 per 1000 | 185 per 1000 (121 to 280) | |||||
| Hospitalisation ‐ not reported | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported in included studies |
| Mortality ‐ not reported | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported in included studies |
| Number of days missed at school or daycare ‐ not measured | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported in included studies |
| Cost ‐ not measured | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported in included studies |
|
CI: Confidence interval; RR: Risk ratio *The basis for the assumed risk for ‘Study population’ was the average risk in the control groups (i.e. total number of participants with events divided by total number of participants included in the meta‐analysis). The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
¹ There is inconsistency in th findings of the first two studies as compared to the third study of the same group ² 95% CIs are wide and imprecise. Moreover, there are few events and the CI includes appreciable benefit and harm³ The evidence is based on 1 small trial of 306 patients
Summary of findings 2. Xylitol versus control for prevention of AOM in healthy children during a respiratory infection.
| Xylitol versus control for prevention of AOM in healthy children during a respiratory infection | ||||||
| Patient or population: preventing acute otitis media in children up to 12 years of age Setting: daycare in Finland Intervention: xylitol in any form (syrup, gum or lozenge) Comparison: control in any form (gum, syrup) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with control in any form (gum, syrup) | Risk with xylitol in any form (syrup, gum or lozenge) | |||||
| Final diagnosis of at least one episode of AOM | Study population | RR 1.13 (0.83 to 1.53) | 1253 (1 RCT) | ⊕⊕⊕⊝ Moderate¹ | ||
| 115 per 1000 | 130 per 1000 (95 to 176) | |||||
| Moderate | ||||||
| 115 per 1000 | 130 per 1000 (95 to 176) | |||||
| Gastrointestinal‐related adverse events | Study population | RR 2.82 (0.61 to 13.00) | 1277 (1 RCT) | ⊕⊕⊕⊝ Moderate¹ | ||
| 4 per 1000 | 11 per 1000 (2 to 53) | |||||
| Moderate | ||||||
| 4 per 1000 | 12 per 1000 (3 to 53) | |||||
| Antibiotic administration ‐ not measured | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported in included studies |
| Hospitalisation ‐ not reported | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported in included studies |
| Mortality ‐ not reported | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported in included studies |
| Number of days missed at school or daycare ‐ not measured | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported in included studies |
| Cost ‐ not measured | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported in included studies |
|
CI: Confidence interval; RR: Risk ratio *The basis for the assumed risk for ‘Study population’ was the average risk in the control groups (i.e. total number of participants with events divided by total number of participants included in the meta‐analysis). The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
¹ 95% CIs are wide and imprecise. The evidence is based on one trial. Moreover, there are few events and the CI includes appreciable benefit and harm
Summary of findings 3. Xylitol versus control for prevention of AOM in otitis‐prone children.
| Xylitol versus control for prevention of AOM in otitis‐prone children | ||||||
| Patient or population: preventing acute otitis media in children up to 12 years of age Setting: primary care in community Intervention: xylitol in any form (syrup, gum or lozenge) Comparison: control in any form (gum, syrup) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with control in any form (gum, syrup) | Risk with xylitol in any form (syrup, gum or lozenge) | |||||
| Final diagnosis of at least one episode of AOM | Study population | RR 0.90 (0.67 to 1.21) | 326 (1 RCT) | ⊕⊕⊝⊝ Low¹ ² | ||
| 367 per 1000 | 331 per 1000 (246 to 445) | |||||
| Moderate | ||||||
| 368 per 1000 | 331 per 1000 (246 to 445) | |||||
| Gastrointestinal‐related adverse events | Study population | RR 1.04 (0.92 to 1.16) | 326 (1 RCT) | ⊕⊕⊕⊝ Moderate ¹ ³ | ||
| 765 per 1000 | 796 per 1000 (704 to 887) | |||||
| Moderate | ||||||
| 765 per 1000 | 796 per 1000 (704 to 888) | |||||
| Antibiotic administration | Study population | RR 0.90 (0.69 to 1.16) | 326 (1 RCT) | ⊕⊕⊕⊝ Moderate ¹ ³ | ||
| 440 per 1000 | 396 per 1000 (303 to 510) | |||||
| Moderate | ||||||
| 440 per 1000 | 396 per 1000 (303 to 510) | |||||
| Hospitalisation ‐ not reported | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported in included studies |
| Mortality ‐ not reported | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported in included studies |
| Number of days missed at school or daycare ‐ not measured | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported in included studies |
| Cost ‐ not measured | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported in included studies |
|
CI: Confidence interval; RR: Risk ratio *The basis for the assumed risk for ‘Study population’ was the average risk in the control groups (i.e. total number of participants with events divided by total number of participants included in the meta‐analysis). The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
¹ High risk for attrition bias ² 95% CIs are wide and imprecise and includes appreciable benefit and harm ³ 95% CIs are wide and imprecise
See Table 1; Table 2; Table 3 for the main primary outcome 'Number of children (healthy children, children with a respiratory infection, and otitis‐prone children, respectively) with at least one AOM episode during the follow‐up period'. Overall, the quality of available evidence for this outcome was moderate for healthy children (due to inconsistency of the results) moderate for children with respiratory infections (due to imprecise estimates), and low for otitis‐prone children (due to imprecise estimates and high attrition).
Primary outcome
Number of children with at least one AOM episode during the follow‐up period
The included trials reported on the primary outcome of number of children with at least one AOM episode during the follow‐up period when xylitol was administered to healthy children (Hautalahti 2007; Uhari 1996; Uhari 1998), to healthy children with a respiratory infection (Tapiainen 2002), and to otitis‐prone healthy children (Vernacchio 2014). In particular, for the latter, the population was not homogenous, the total dose was increased by 50% (15 g daily in the form of viscous xylitol solution) and the outcome was measured as what had been documented in the patients' medical charts. Due to the heterogeneity among these three groups, the results are presented separately for each outcome.
Xylitol in any form versus control in any form
Figure 3 summarises available data on our primary outcome. We combined the result of three trials among 1826 healthy children to compare the effect of xylitol in any form (syrup, chewing gum or lozenges) versus control of any kind (syrup or gum) (Hautalahti 2007; Uhari 1996; Uhari 1998). There was a statistically significant 25% reduction in the risk of occurrence of AOM among healthy children at daycare centres in the xylitol group compared to the control group (RR 0.75, 95% CI) 0.65 to 0.88 and RD ‐0.07, 95% CI ‐0.12 to ‐0.03; quality of evidence: moderate ‐ downgraded for inconsistency). However, the effect of xylitol in reducing AOM among healthy children during a respiratory infection (Tapiainen 2002: 99 out of 765 children in the xylitol group versus 56 out of 488 children in the control group, RR 1.13, 95% CI 0.83 to 1.53; quality of evidence: moderate ‐ downgraded for imprecision), or among otitis‐prone healthy children (Vernacchio 2014: 53 out of 160 children in the xylitol group versus 61 out of 166 children in the control group, RR 0.90, 95% CI 0.67 to 1.21 and RD ‐0.07, 95% CI ‐0.14 to 0.07; quality of evidence: low ‐ downgraded for imprecision and high attrition) was inconclusive.
3.
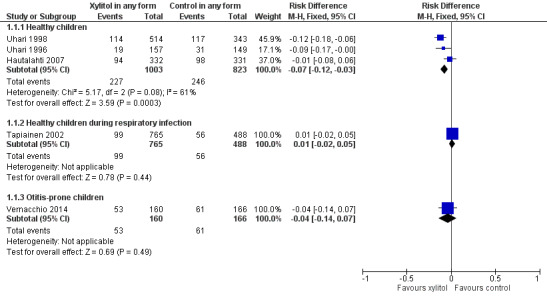
Forest plot of comparison: 1 Xylitol in any form (syrup, gum or lozenge) versus control in any form (gum, syrup), outcome: 1.1 Final diagnosis of at least one episode of AOM.
Secondary outcomes
1. Safety and adverse events
There were no significant differences in the number of children with gastrointestinal‐related adverse events during the follow‐up period between xylitol and control group among 1826 healthy children (Hautalahti 2007; Uhari 1996; Uhari 1998 combined: RR 1.43, 95% CI 0.74 to 2.75), among 1277 healthy children with a respiratory infection (Tapiainen 2002: RR 2.82, 95% CI 0.61 to 13.00), and among 326 otitis‐prone healthy children (Vernacchio 2014: RR 1.04, 95% CI 0.92 to 1.16). Similarly, there was no significant differences in the occurrence of rash during the follow‐up period between xylitol and control group (only reported in Hautalahti 2007: RR 1.00, 95% CI 0.20 to 4.90). The quality of evidence on this outcome was moderate, downgraded for imprecision.
2. Antibiotic administration
Among healthy children: Uhari 1996 and Uhari 1998 reported on antibiotic administration. However, the reported data could not be pooled.
In Uhari 1996, the total number of antimicrobial drugs prescribed in the sucrose group was 60 compared with 34 in the xylitol group. There was a statistically significant 36% reduction in the risk of experiencing at least one exposure to antimicrobial drugs among children in the xylitol group than the children in the sucrose group (RR 0.64, 95% CI 0.42 to 0.97 and RD ‐0.10, 95% CI ‐0.20, ‐0.01; quality of evidence: moderate).
In Uhari 1998, for children unable to chew, during the three‐month follow‐up period, the incidence rate of antimicrobial prescriptions per person years at risk (PYR) was significantly lower in the xylitol syrup group versus the control syrup group (respectively 3.20 versus 4.33, P = 0.012). Similarly, the number of days on antimicrobials/PYR was significantly lower in the xylitol syrup group versus the control syrup group (respectively 25.0 versus 31.7, P < 0.0001). For children able to chew, during the three‐month follow‐up period, the incidence rate of antimicrobial prescriptions/PYR was significantly lower in the xylitol chewing gum group versus the control chewing gum group (respectively 1.66 versus 2.26, P = 0.046), but there was no difference between the xylitol lozenges group and control chewing gum (respectively 1.86 versus 2.26, P = 0.211). The number of days on antimicrobials/PYR was significantly lower (P < 0.0001) in both the xylitol chewing gum group (11.8) and xylitol lozenges (13.8) versus control chewing gum (17.8).
Antibiotic administration was not measured among healthy children with a respiratory infection.
Among otitis‐prone children, Vernacchio 2014 reported no significant differences in antibiotic administration between the xylitol and control groups (RR 0.90, 95% CI 0.69 to 1.16). The quality of evidence was moderate and was downgraded for imprecision and high attrition. Total antibiotic use was 6.8 days per 90 days in the xylitol group versus 6.4 in the placebo group (difference 0.4, 95% CI –1.8 to 2.7, P = 0.7). The incidence of AOM‐related antibiotic use was 5.2 days per participant per 90 days in the xylitol group versus 5.1 in the placebo group (absolute difference 0.1 days, 95% CI for difference –1.9 to 2.2 days. P = 0.9).
3. Hospitalisation (and its length) secondary to AOM or its complications
No case of hospitalisation was reported in the included studies.
4. Mortality secondary to complications of AOM in studies where at least one death was reported
No case of mortality was reported in the included studies.
5. Number of days missed at school or daycare centre
Identified studies did not report on this outcome.
6. Cost
Information on cost was not reported in the included studies.
Subgroup analysis
Based on the methodology of the identified studies, we defined the subgroup analyses of a) younger children unable to chew gum; b) older children who were able to chew and c) comparison between different types of xylitol vehicles. The subgroup analyses were tested among the population of healthy children, healthy children during respiratory infection, as well as otitis‐prone healthy children. It should be noted that although Hautalahti 2007 included the subgroups of chewing gum and syrup (for both xylitol group and control group), the data were presented collectively for control and xylitol group with no breakdown of the AOM in the subgroups. We contacted the trial authors several times but no further information was obtained and therefore the results of the subgroups of Hautalahti 2007 were not included in the subgroup analysis.
Younger children unable to chew gum
Figure 4 presents data from three trials that compared the effect of xylitol syrup versus control syrup in younger children unable to chew gum (Tapiainen 2002; Uhari 1998; Vernacchio 2014). Due to the heterogeneity in these studies, the results were not pooled. Among healthy children, Uhari 1998 showed that xylitol syrup resulted in a statistically significant 30% decrease in the occurrence of AOM among healthy younger children at daycare centres who were unable to chew gum (46 out of 159 children in the xylitol group versus 68 out of 165 children in the control group, RR 0.70, 95% CI 0.52 to 0.95; RD ‐0.12, 95% CI ‐0.23 to ‐0.02). However, the effect of xylitol syrup in reducing AOM among healthy children during a respiratory infection (Tapiainen 2002: 34 out of 207 children in xylitol group versus 32 out of 211 children in control group, RR 1.08, 95% CI 0.70 to 1.69), or among otitis‐prone healthy children (Vernacchio 2014: 53 out of 160 children in the xylitol group versus 61 out of 166 children in the control group, RR 0.90, 95% CI 0.77 to 1.21; RD ‐0.04, 95% CI ‐0.14 to 0.07) was inconclusive.
4.
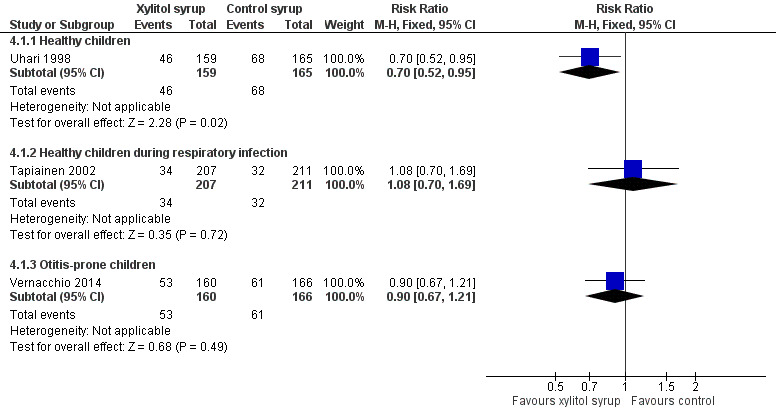
Forest plot of comparison: 3 Xylitol syrup versus control for younger children unable to chew, outcome: 3.1 Final diagnosis of at least one episode of AOM.
Older children who were able to chew
The results of Uhari 1996 and Uhari 1998 were combined to compare the effect of xylitol chewing gum or lozenges versus control chewing gum among the subgroup of older healthy children able to chew gum. Based on the pooled result of these two studies, compared to control chewing gum, xylitol resulted in a statistically significant 34% decrease in the occurrence of AOM if it was administered in chewing gum or lozenges (Figure 5 RR 0.66, 95% CI 0.50 to 0.87; RD ‐0.09, 95% CI ‐0.14 to ‐0.03), and in a statistically significant 41% decrease in the occurrence of AOM if it was administered in chewing gum only (Figure 6 RR 0.59, 95% CI 0.42 to 0.81; RD ‐0.10, 95% CI ‐0.16 to ‐0.04). The effect of xylitol lozenge in reducing AOM was inconclusive (only reported in Uhari 1998, RR 0.80, 95% CI 0.56 to 1.16; RD ‐0.05, 95% CI ‐0.14 to 0.04). Among the healthy children during a respiratory infection (only reported in Tapiainen 2002), there was no difference in the outcome whether xylitol was administered in chewing gum or lozenges (Figure 5 RR 1.34, 95% CI 0.86 to 2.10), in chewing gum only (Figure 6 RR 1.29, 95% CI 0.78 to 2.14), or in lozenges only (RR 1.40, 95% CI 0.85 to 2.29). Vernacchio 2014 did not report this subgroup.
5.
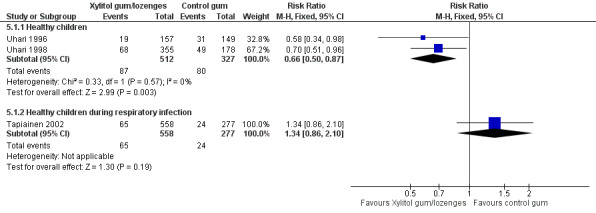
Forest plot of comparison: 4 Xylitol chewing gum/lozenges versus control chewing gum for older children able to chew, outcome: 4.1 Final diagnosis of at least one episode of AOM.
6.
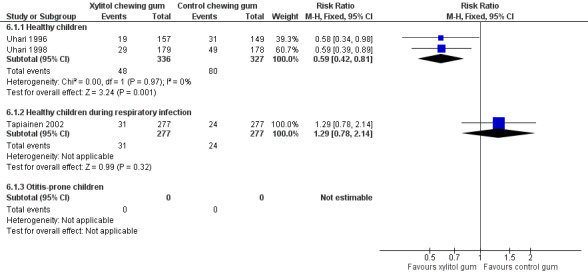
Forest plot of comparison: 5 Xylitol chewing gum versus control chewing gum for older children able to chew, outcome: 5.1 Final diagnosis of at least one episode of AOM.
Comparison of different types of xylitol vehicles
We also compared the outcome of the final diagnosis of at least one episode of AOM between the active ingredients groups to assess whether the mode of delivery for the ingredient makes any difference. We performed this analysis on healthy children (Uhari 1998), and also on children with a respiratory infection (Tapiainen 2002). It should be noted that this comparison is limited to one study in each population group: Uhari 1996 did not test different modes of delivery for xylitol (chewing gum only); and although Hautalahti 2007 used two modes of delivery for xylitol (chewing gum and syrup), the data were collectively presented as one test group. The analyses show that xylitol chewing gum is superior to xylitol syrup in preventing AOM among healthy children (RR 0.59, 95% CI 0.39 to 0.89), but not for children with a respiratory infection (RR 0.68, 95% CI 0.43 to 1.07). There was no difference between xylitol lozenges and xylitol syrup in preventing AOM among healthy children (RR 0.77, 95% CI 0.53 to 1.11), or among children with a respiratory infection (RR 0.74, 95% CI 0.47 to 1.14). Similarly, no difference was noted between xylitol chewing gum and xylitol lozenges in preventing AOM among healthy children (RR 0.73, 95% CI 0.47 to 1.13), or among children with a respiratory infection (RR 0.92, 95% CI 0.59 to 1.46). Vernacchio 2014 did not report this subgroup.
Discussion
Summary of main results
This review has attempted to find evidence for a preventive effect of xylitol in reducing the occurrence of acute otitis media (AOM). Safety and adverse events were not statistically significantly different. This finding is similar to Vernacchio 2007a, where oral xylitol solution at dosages of 5 g three times per day and 7.5 g once daily were found to be well‐tolerated by young children. However, the relative rarity of events and the few patients from whom data are available prevents us from arriving at firm conclusions on safety. Xylitol in connection with dental decay has been used for decades, since it has been shown to reduce mutans streptococci counts in plaque and saliva (Haresaku 2007) and reduce lactic acid production in dental plaque through interference with metabolic pathways (Twetman 2003). In a recent systematic review (Ly 2008) and meta‐analysis (Deshpande 2008), it is claimed that xylitol could benefit people at high risk of caries and should be considered for routine use in clinical dentistry. However, the use of xylitol in dental decay suggests that it may also be useful for preventing AOM, which is the focus of this review.
Overall completeness and applicability of evidence
Considering the concerns over the treatment of AOM using antibiotics and surgery, we wanted to explore the literature for alternative preventive strategies. In the earlier version of this review (Azarpazhooh 2011), following an exhaustive search strategy, we found three clinical trials that investigated the efficacy of xylitol administered prophylactically to children in daycare centres (Hautalahti 2007; Uhari 1996; Uhari 1998). All these trials were double‐blinded, randomised and placebo‐controlled, with appropriate randomisation and masking procedures. While the trials stemmed from the same group of trial authors, the design of the trials was improved following the first one (Uhari 1996), which utilised sucrose in the control group. This study was later criticised due to the possibility of risking dental caries in the participants. Therefore, in the following studies, xylitol was used as a sweetener in the control groups as well, to avoid the increased risk for caries. While the chewing gums were similar in taste, the xylitol mixture tasted sweeter than the control syrup (Hautalahti 2007; Uhari 1998). These studies did not discuss why the low dose of xylitol (0.5 g/day) in the control group can be considered as equivalent to placebo. In this update, we identified a pragmatic practice‐based RCT (Vernacchio 2014), which determined if viscous xylitol solution versus sorbitol solution could reduce the occurrence of clinically diagnosed AOM among otitis‐prone children six months through to five years of age. We elected not to pool the result of this trial with those we identified in 2011 (Azarpazhooh 2011), because as compared to trials included in our 2011 review, the population was not homogenous, the total dose was increased by 50% (15 g daily) and the outcome was measured as what had been documented in the patients' medical charts.
Based on the studies we reviewed, xylitol seems to be a promising alternative to conventional therapies to prevent AOM among healthy children. Even in one study (having controlled for parental education and smoking, breast feeding, use of pacifier, sibling prone to otitis, previous history of AOM, previous use of xylitol and nasopharyngeal carriage of pneumococci) children receiving xylitol had fewer AOM episodes (P = 0.045) (Uhari 1996). The overall results from combining the data from the preventive clinical trials among healthy children (comparing xylitol to a control group of sucrose or minimum non‐therapeutic dose of xylitol plus sorbitol) are encouraging. The magnitude of the reduction in the occurrence of AOM was an overall 25% (1‐ RR 0.75) when the results of all types of administration are considered (i.e. syrup, lozenge or chewing gum) and was 30% (1‐ RR 0.70) if it is administered in a syrup for younger children unable to chew. Among those older children who were able to chew, xylitol in chewing gum form was effective in the prevention of AOM by 41% (1‐ RR 0.59); in contrast, xylitol lozenges were not preventive. Also, it was found that chewing gum is a better vehicle for xylitol administration with an extra 41% preventive effect when compared to xylitol syrup (1‐ RR of 0.59). That said, in this update, the result of the identified pragmatic practice‐based RCT (Vernacchio 2014) showed that 15 g daily dose of a viscous xylitol solution was ineffective in reducing clinically diagnosed AOM among otitis‐prone children. The observed effect in this trial could be the result of lack of adherence to treatment since almost 28% in the xylitol group and 24% in the placebo group discontinued treatment before the end of the study period or the occurrence of the primary outcome. Furthermore, xylitol in reducing AOM among healthy children during a respiratory infection (Tapiainen 2002) was ineffective. The trial authors speculated their findings to the increased bacterial adherence to pharyngeal cells during viral infection, and the increased incidence of otitis media pathogens in the nasopharynx at such times.
Through the literature search and as part of the peer refereeing process, we came across some important papers that can shed more light on the applicability of the results of this review and the potential limitations and obstacles to the applicability of these findings, in particular those of chewing gum. The first obstacle is the knowledge of medical practitioners about medical uses for xylitol. A recent national survey of paediatricians in the USA regarding their knowledge and opinions about xylitol use as a prophylaxis for AOM found that only about half of the participants knew about medical uses for xylitol; of those, most were aware of its use in chewing gum to prevent AOM but had not used it with patients (Danhauer 2010b). Perhaps the most encouraging result of this survey, and a follow‐up focus group survey by the same research group on 10 US paediatricians known to have more experience with xylitol for AOM (Stockwell 2010), was that the majority of the US paediatricians would use xylitol if evidence supported it and wanted information about it via reprints or electronically.
It should also be noted that xylitol chewing gums are commercially available in Finland (the country where four out of five included studies took place) and their use is recommended by the dental organisations. However, in the USA, some school districts prohibit gum chewing altogether, while others leave it up to the discretion of individual teachers. For example, a recent survey of all the Kindergarten through 3rd Grade teachers in Santa Barbara, CA School Districts (Danhauer 2011a), regarding their knowledge about AOM and their willingness to participate in AOM prevention programmes, found that all of the schools and almost all of the teachers did not permit chewing gum on campus or in their classrooms. Other obstacles identified in this pilot survey were supervision, liability, school regulation, compliance issues, potential distractions to classes and risk of choking. These obstacles point to the importance of educational and informational outreach programmes to educate practitioners, parents and school teachers about the medical use of xylitol, in particular when added to chewing gum for both the reduction of dental caries and the occurrence of AOM.
Given the evidence that parental reporting of children's recent AOM history correlates well with medical records (Vernacchio 2007b), in our previous review, we proposed that considering the potential obstacles to AOM prevention programme in daycare and school settings, in‐home use of xylitol needs to be investigated. Fortunately, one such study became available for this update (Vernacchio 2014); however, this study did not find a significant effect for a viscous xylitol solution at a dose of 5 g three times per day in reducing clinically diagnosed AOM among otitis‐prone children. There is some evidence to show that mothers' chewing xylitol gum was a prophylaxis against bacteria and caries in their children. The use of such mother‐child transmission model as a possible vehicle for preventing AOM would be worth further investigations (Danhauer 2011b).
Although the results of this present meta‐analysis show that chewing gum is a better vehicle for xylitol administration, the present obstacles could preclude the use of xylitol chewing gum in school settings for small children who are unable to chew gum. This happens to be the group most susceptible to AOM and therefore, for such children, vehicles other than chewing gum need to be pursued (Danhauer 2011a). For example, we found unpublished evidence internal to Xlear Inc. Orem (Utah, USA) that a xylitol nasal wash three times daily during a three‐month period lowered the recurrence of AOM and the frequency of antibiotic therapy and upper airways infections (Kalanin 2005). Moreover, in our 2016 updated search, we identified a randomised, double‐blinded clinical trial registered with Clinicaltrial.gov that is not yet open for participant recruitment (NCT02592382). This trial, based in Rambam Health Care Campus, Haifa, Israel, is designed to enrol 50 children aged from one to five years who have had three episodes of recurrent otitis media in the last six months prior to entering the study. They will randomly receive isotonic saline nasal spray or 5% xylitol spray (three times daily for two months) and the occurrence of AOM will be measured for five months. We will monitor the progress of this trial for future updates of this review.
Xylitol syrup can also be administered with pacifiers that have reservoirs used to supply medicine to the infant at bed time. That said, a recent web‐based survey of 136 parents of young children in preschool and kindergarten settings (Danhauer 2015), found that most parents were unaware of xylitol's use for AOM and would be unwilling to use xylitol products or comply with a required regimen to prevent AOM in their children. That said, those who knew about xylitol and had children with a history of AOM would be more likely to do so, most would prefer chewing gum for older children and pacifiers for younger children (Danhauer 2015). There remain to be a need for more educational public campaign on the potential benefits of xylitol and the importance of parental and school compliance for successful AOM prevention regimens. Finally, in our protocol, we decided not to include cluster‐design RCTs due to potential differences in ancillary treatments between centres. Although we did not find any cluster‐RCTs in our search, we will look for them in the future updates to this review.
Quality of the evidence
The quality of evidence for the number of children with at least one AOM episode during the follow‐up period was moderate for healthy children due to the inconsistency of the results among trials, moderate for children with respiratory infections due to imprecise estimates, and low for otitis‐prone children due to imprecise estimates and high attrition. Hence, further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Potential biases in the review process
In our previous review, we decided to use fixed‐effect analysis as there was sufficient homogeneity among the three Finnish studies. These studies were all from the same group of researchers in Finland; the studied population was homogenous (healthy children attending daycare centres in Oulu, Finland); the total dose of xylitol was similar (8 to 10 g/day); the control groups include 0 to 0.5 g of xylitol per day plus other ineffective sweetener in preventing AOM; the outcome measurement was similar. Althoug we had calculated I² statistic values which ranged from 0% to 69% in different analyses, the number of trials included in meta‐analyses remain low and test for statistical heterogeneity tend to be underpowered.
Agreements and disagreements with other studies or reviews
Since publication of the protocol of this review in 2008, two systematic reviews have been published (Danhauer 2010a; Danhauer 2011b). Our results are in agreement with these systematic reviews that also concluded that xylitol can be a prophylaxis for AOM, but warrants further study, especially of vehicles other than chewing gum for young children.
Authors' conclusions
Implications for practice.
On the basis of the included trials, we conclude that there is moderate‐quality evidence that the supervised prophylactic administration of xylitol among healthy children attending daycare centres can reduce the occurrence of AOM from 30% to around 22%. If the children are unable to chew (for example, in the first two years of life), the effect size of AOM prevention with xylitol syrup was 30%. If the children were able to chew, the effect size of AOM prevention with xylitol chewing gum was 41%. There is not enough evidence to prove the efficacy of xylitol lozenges for the older children who were able to chew. In comparable situations, chewing gum is a better vehicle for xylitol administration, with an extra 41% preventive effect when compared to xylitol syrup. There is insufficient evidence of low to moderate quality for the efficacy of xylitol administration to prevent AOM only during a respiratory infection, or to otitis‐prone children.
For those young children for whom antibiotics are a major risk, xylitol is a potential alternative for preventing AOM. The benefit appears to be a moderate one but more studies need to be conducted. There were insufficient studies in a variety of groups of children to develop evidence‐based treatment guidelines. It should be noted that the potential obstacles (school classrooms, supervision, liability, school regulation, compliance issues, potential distractions to classes and risk of choking) could preclude the use of xylitol chewing gum as a school‐based prevention programme. For small children who are unable to chew gum, other vehicles (such as nasal wash, pacifier for children or chewing gum for mothers) need to be investigated further.
Implications for research.
In applying these results, it should be appreciated that the thorough literature searches revealed only five randomised clinical trials, four of which are from the same research group. The quality of evidence was moderate for healthy children and for children with respiratory infections and low for otitis‐prone children. Hence, further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Hence, there is still a need for the highest level of evidence, i.e. well‐designed, double‐blind RCTs (preferably a multicentre trial) with adequate sample size, limited or no loss to follow‐up and carefully standardised methods of measurement and analysis, to evaluate the possible use of xylitol for preventing AOM in children up to 12 years of age, in particular targeting 'at risk' groups. Future research may need to focus on a safe and identically tasting placebo rather than low‐dose xylitol. It is still not known what range of xylitol would be most useful for the prevention of AOM. Studies need to be conducted to determine the optimum xylitol exposure for a child on a g/kg body weight/day basis, the best vehicle for toddlers, and whether the first year of life is an appropriate age group to test. Other important outcomes, such as hospitalisation (and length of stay in hospital) secondary to AOM or its complications, percentage of children needing antibiotic therapy, number of days missed at school or daycare centre, and direct and indirect costs, should also be considered. Once the efficacy of the product is established, the next requirement is for high quality studies to evaluate the relative cost‐effectiveness of the prophylactic interventions. In any case, informational outreach programmes are necessary to educate practitioners about medical use of xylitol, in particular chewing gum.
What's new
| Date | Event | Description |
|---|---|---|
| 28 January 2016 | New citation required but conclusions have not changed | Searches updated. One trial registry was identified that is not yet open to recruit patients (NCT02592382); we saved this in the Ongoing studies section for a future update. We included one new trial which has several sources of heterogeneity (Vernacchio 2014). Conclusions were modified accordingly to highlight that xylitol was not effective in reducing acute otitis media among otitis‐prone healthy children. |
| 28 January 2016 | New search has been performed | Hardy Limeback is not a co‐author on this 2016 review update. |
History
Protocol first published: Issue 2, 2008 Review first published: Issue 11, 2011
| Date | Event | Description |
|---|---|---|
| 1 December 2013 | New search has been performed | This review updates the existing review of "Xylitol for preventing acute otitis media in children up to 12 years of age" published in the Cochrane Database of Systematic Reviews,November 2011 (Azarpazhooh 2011). An updated search September 02, 2013 identified no new eligible study. Conclusions have not changed. |
| 2 September 2013 | New search has been performed | Searches updated |
Acknowledgements
The review authors wish to acknowledge Dr Hardy Limeback who was a co‐author on the previous version of this review. We also thank the following people for commenting on the draft protocol: Ann Fonfa, Louis Vernacchio, Jerome Klein, Terry Neeman and Paul Glasziou; and the following for commenting on the draft review: Karl Gallegos, Lorraine Johnson, Jerome Klein, Jeff Danhauer, Carole Johnson, Elaine Beller and Susan Smith. The review authors would also like to thank Elizabeth Dooley, the Managing Editor of Cochrane Acute Respiratory Infections for her patience and editorial support and Justin Clark, the Information Specialist of the Cochrane Acute Respiratory Infections Group and Maria Budda, the librarian at the Faculty of Dentistry, University of Toronto for their assistance with the search strategy. We also thank and acknowledge Maria Ximena Rojas Reyes, Pontificia Universidad Javeriana, Bogotá, Colombia, for her assistance with the 'Summary of findings' tables.
Appendices
Appendix 1. OVID MEDLINE/CENTRAL search strategy
1 exp Otitis Media/ 2 otitis media.tw. 3 (middle ear adj3 (infect* or inflam*)).tw. 4 (aom or ome).tw. 5 or/1‐4 6 Xylitol/ 7 xylitol.tw. 8 Chewing Gum/ 9 chewing gum.tw. 10 birch sugar*.tw. 11 or/6‐10 12 11 and 5
Appendix 2. Embase (Elsevier) search strategy
#10 #5 AND #9 #9 #6 OR #7 OR #8 #8 xylitol:ab,ti OR 'chewing gum':ab,ti OR 'birch sugar':ab,ti #7 'chewing gum'/de #6 'xylitol'/de #5 #1 OR #2 OR #3 OR #4 #4 aom:ab,ti OR ome:ab,ti #3 ('middle ear' NEAR/3 (infect* OR inflam*)):ab,ti #2 'otitis media':ab,ti #1 'otitis media'/exp
Appendix 3. CINAHL (Ebsco) search strategy
S14 S6 and S12 S13 S6 and S12 S12 S7 or S8 or S9 or S10 or S11 S11 TI birch sugar or AB birch sugar S10 TI chewing gum or AB chewing gum S9 (MH "Chewing Gum") S8 TI xylitol or AB xylitol S7 (MH "Xylitol") S6 S1 or S2 or S3 or S4 or S5 S5 TI ( aom or ome ) or AB ( aom or ome ) S4 TI middle ear N3 inflam* or AB middle ear N3 inflam* S3 TI middle ear N3 infect* or AB middle ear N3 infect* S2 TI otitis media or AB otitis media S1 (MH "Otitis Media+")
Appendix 4. LILACS (BIREME) search strategy
(mh:"Otitis Media" OR mh:c09.218.705.663* OR "otitis media" OR "Otite Média" OR "middle ear infection" OR "middle ear infections" OR "middle ear inflammation") AND (mh:xylitol OR xylitol OR xilitol OR "chewing gum" OR "birch sugar") AND db:("LILACS")
Appendix 5. Web of Science (Thomson Reuters) search strategy
Topic=("otitis media" OR ("middle ear" NEAR/3 (infect* or inflam*))) AND Topic=(xylitol OR "chewing gum" OR "birch sugar")
Databases=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH Timespan=All years
Appendix 6. International Pharmaceutical Abstracts search strategy
1. 'otitis media'.tw. 2. 'aom'.tw. 3. 'ome'.tw. 4. (middle ear adj3 infect*).tw. 5. (middle ear adj3 inflamm*).mp. [mp=title, subject heading word, registry word, abstract, trade name/generic name] 6.1 or 2 or 3 or 4 or 5 7. 'xylitol'.tw. 8. 6 and 7
Data and analyses
Comparison 1. Xylitol in any form (syrup, gum or lozenge) versus control in any form (gum, syrup).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Final diagnosis of at least one episode of AOM | 5 | Risk Difference (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Healthy children | 3 | 1826 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.07 [‐0.12, ‐0.03] |
| 1.2 Healthy children during respiratory infection | 1 | 1253 | Risk Difference (M‐H, Fixed, 95% CI) | 0.01 [‐0.02, 0.05] |
| 1.3 Otitis‐prone children | 1 | 326 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.04 [‐0.14, 0.07] |
1.1. Analysis.
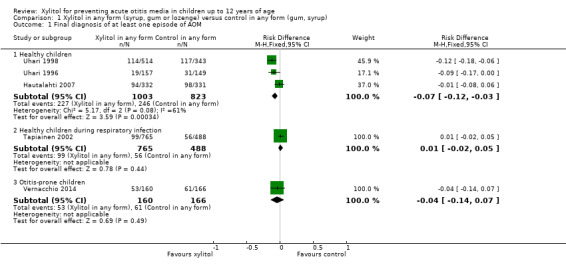
Comparison 1 Xylitol in any form (syrup, gum or lozenge) versus control in any form (gum, syrup), Outcome 1 Final diagnosis of at least one episode of AOM.
Comparison 2. Adverse effect: xylitol versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Gastrointestinal‐related adverse events | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Healthy children | 3 | 1826 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.74, 2.75] |
| 1.2 Healthy children during respiratory infection | 1 | 1277 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.82 [0.61, 13.00] |
| 1.3 Otitis‐prone children | 1 | 326 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.92, 1.16] |
| 2 Rash | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Healthy children | 1 | 663 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.20, 4.90] |
2.1. Analysis.
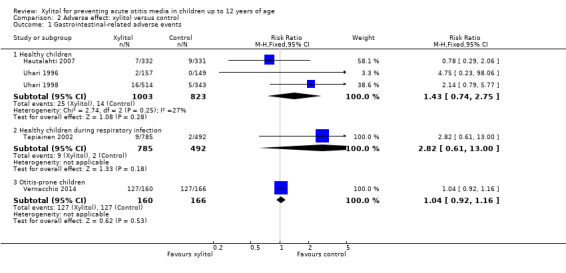
Comparison 2 Adverse effect: xylitol versus control, Outcome 1 Gastrointestinal‐related adverse events.
2.2. Analysis.
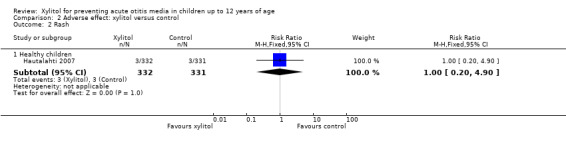
Comparison 2 Adverse effect: xylitol versus control, Outcome 2 Rash.
Comparison 3. Antibiotic administration.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 At least one exposure to antimicrobial drugs | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Healthy children | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Otitis‐prone children | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
3.1. Analysis.
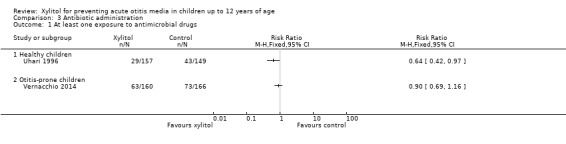
Comparison 3 Antibiotic administration, Outcome 1 At least one exposure to antimicrobial drugs.
Comparison 4. Xylitol syrup versus control for younger children unable to chew.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Final diagnosis of at least one episode of AOM | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Healthy children | 1 | 324 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.52, 0.95] |
| 1.2 Healthy children during respiratory infection | 1 | 418 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.70, 1.69] |
| 1.3 Otitis‐prone children | 1 | 326 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.67, 1.21] |
4.1. Analysis.
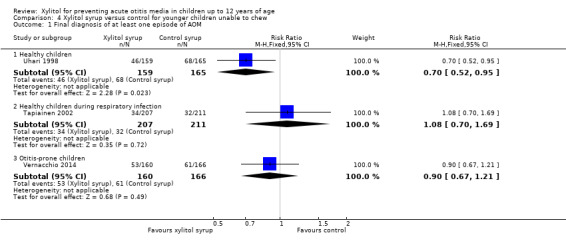
Comparison 4 Xylitol syrup versus control for younger children unable to chew, Outcome 1 Final diagnosis of at least one episode of AOM.
Comparison 5. Xylitol chewing gum/lozenges versus control chewing gum for older children able to chew.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Final diagnosis of at least one episode of AOM | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Healthy children | 2 | 839 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.50, 0.87] |
| 1.2 Healthy children during respiratory infection | 1 | 835 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.34 [0.86, 2.10] |
5.1. Analysis.
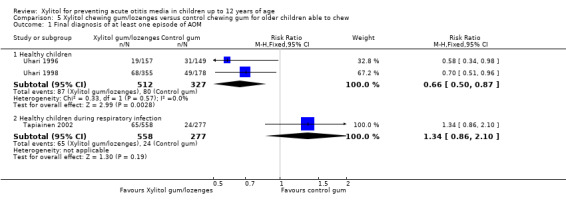
Comparison 5 Xylitol chewing gum/lozenges versus control chewing gum for older children able to chew, Outcome 1 Final diagnosis of at least one episode of AOM.
Comparison 6. Xylitol chewing gum versus control chewing gum for older children able to chew.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Final diagnosis of at least one episode of AOM | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Healthy children | 2 | 663 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.42, 0.81] |
| 1.2 Healthy children during respiratory infection | 1 | 554 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.78, 2.14] |
| 1.3 Otitis‐prone children | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
6.1. Analysis.
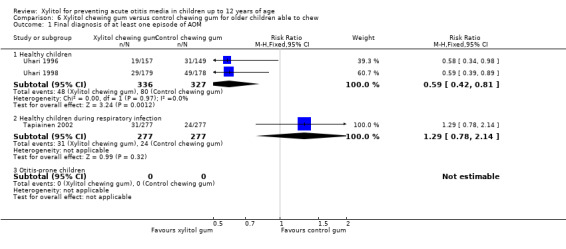
Comparison 6 Xylitol chewing gum versus control chewing gum for older children able to chew, Outcome 1 Final diagnosis of at least one episode of AOM.
Comparison 7. Xylitol lozenges versus control chewing gum for older children able to chew.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Final diagnosis of at least one episode of AOM | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Healthy children | 1 | 354 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.56, 1.16] |
| 1.2 Healthy children during respiratory infection | 1 | 558 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.40 [0.85, 2.29] |
7.1. Analysis.
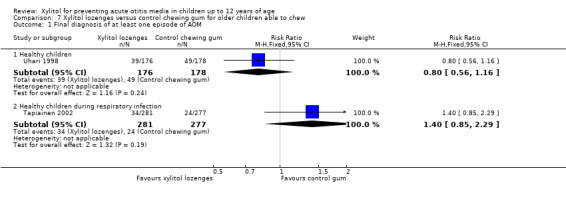
Comparison 7 Xylitol lozenges versus control chewing gum for older children able to chew, Outcome 1 Final diagnosis of at least one episode of AOM.
Comparison 8. Comparison between active ingredients groups: xylitol chewing gum versus xylitol syrup.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Final diagnosis of at least one episode of AOM | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Healthy children | 1 | 338 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.39, 0.89] |
| 1.2 Healthy children during respiratory infection | 1 | 484 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.43, 1.07] |
| 1.3 Otitis‐prone children | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
8.1. Analysis.
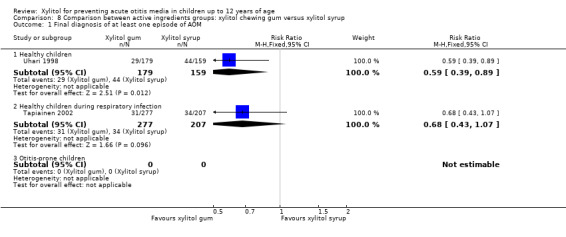
Comparison 8 Comparison between active ingredients groups: xylitol chewing gum versus xylitol syrup, Outcome 1 Final diagnosis of at least one episode of AOM.
Comparison 9. Comparison between active ingredients groups: xylitol lozenges versus xylitol syrup.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Final diagnosis of at least one episode of AOM | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Healthy children | 1 | 335 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.53, 1.11] |
| 1.2 Healthy children during respiratory infection | 1 | 488 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.47, 1.14] |
| 1.3 Otitis‐prone children | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
9.1. Analysis.
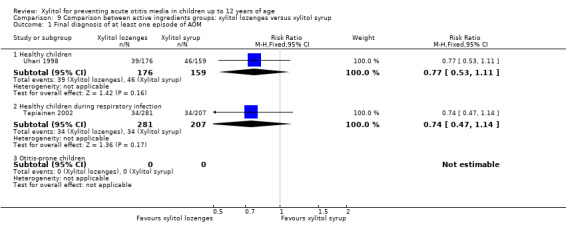
Comparison 9 Comparison between active ingredients groups: xylitol lozenges versus xylitol syrup, Outcome 1 Final diagnosis of at least one episode of AOM.
Comparison 10. Comparison between active ingredients groups: xylitol chewing gum versus xylitol lozenges.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Final diagnosis of at least one episode of AOM | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Healthy children | 1 | 355 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.47, 1.13] |
| 1.2 Healthy children during respiratory infection | 1 | 558 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.59, 1.46] |
| 1.3 Otitis‐prone children | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
10.1. Analysis.
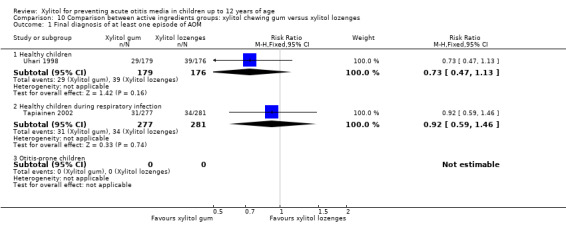
Comparison 10 Comparison between active ingredients groups: xylitol chewing gum versus xylitol lozenges, Outcome 1 Final diagnosis of at least one episode of AOM.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Hautalahti 2007.
| Methods | Double‐blind, placebo‐controlled randomised study | |
| Participants | 663 healthy daycare children with normal ear status and without current ARI (normal tympanograms, A‐curve) recruited from 21 childcare centres in the city of Oulu, Finland between August 2001 and January 2002. Age: 7 months to 7 years Exclusion criteria: current antimicrobial prophylaxis, craniofacial malformations and structural middle ear abnormalities |
|
| Interventions | Test group, unable to chew gum n = 60: 8 mL of a syrup containing 400 g/L xylitol 3 times a day (daily doses of 9.6 g of xylitol)
Test group, able to chew gum n = 272: xylitol chewing gum 3 times a day (daily dose 9.6 g of xylitol)
Placebo group, unable to chew gum n = 57: control syrup containing 20 g/L xylitol diluted in water without any other sweeteners 3 times a day (daily doses of 0.5 g xylitol) Placebo group, able to chew gum n = 274: control chewing gum 3 times a day (daily dose 0.5 g of xylitol) also contained sucrose as a sweetener |
|
| Outcomes | AOM based on a finding of middle ear effusion by pneumatic otoscopy and symptoms of acute respiratory infection | |
| Notes | The daily amount of xylitol was the same as other trials but it was administered only 3 times a day | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation was performed using tables of random numbers and a block approach with a block size of four.” |
| Allocation concealment (selection bias) | Low risk | A randomisation list was given to the product providers and the products were packed using this random allocation sequence |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "All study physicians, authors, and participating families were blinded to the group assignment until data entry and data checking were complete.” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | By the end of trial, the dropout range was statistically higher in test group (17%) versus the control group (11%). The reasons for dropouts were: child refused to take the product; abdominal discomfort; chewing gum piece too big; duration of the trial too long; forgot to give the preparation when on holiday; illness; rash; parents tired of the trial; difficult to avoid additional xylitol products; other or unknown reasons |
| Selective reporting (reporting bias) | Low risk | None identified |
| Other bias | Unclear risk | The material was donated by industry. Governmental source of funding. The study authors declared no conflict of interest but based on their previous paper, they have a US patent for the use of xylitol in treating respiratory infection |
Tapiainen 2002.
| Methods | Double‐blind, placebo‐controlled randomised study | |
| Participants | 1277 healthy daycare children the city of Oulu, Finland after screening with tympanometry during an ARI | |
| Interventions | Children unable to chew gum: Placebo group: n = 212: 5 mL of a syrup containing 20 g/L xylitol diluted in water without any other sweeteners (daily doses of 0.5 g of xylitol) Test group: n = 212: 5 mL of a syrup containing 400 g/L xylitol 5 times a day (daily doses of 10 g of xylitol) Syrups administered after meals with a syringe during a period of 5 minutes to maintain a high concentration of xylitol in the oral cavity for as long as practically possible Children able to chew gum: Placebo group: n = 280, control chewing gum with daily dose 0.5 g of xylitol Test group: n = 286: xylitol chewing gum with daily dose 8.4 g of xylitol Test group: n = 287: xylitol lozenges with daily dose 10 g of xylitol Two pieces of chewing gum or lozenges were chewed for at least 5 minutes 5 times a day after meals. 3 of the doses were given by the personnel at the childcare centres during the day and the rest were given by the parents at home. |
|
| Outcomes | AOM based on a finding of middle ear effusion in tympanometry (B, C or positive pressure curve) and confirmed with pneumatic otoscopy; A total of 1253 of the 1277 randomised children were eligible for the analysis of the primary outcome | |
| Notes | The daily doses of control and xylitol products were equal to those used in earlier trials The parents began administering the products to their children at the onset of symptoms of ARI |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed in blocks of 4 in the mixture groups and in blocks of 3 in chewing gum and lozenge groups, using a random number table to make the proportion of participants in each study group approximately the same at each child care centre." |
| Allocation concealment (selection bias) | Low risk | Each child was given a unique participation number at the time of the initial screening |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The study was blinded as far as the mixture and chewing gum groups were concerned but open as between the xylitol lozenge and control chewing gum groups |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The children who dropped out (n = 24) were excluded from the statistical analysis, but those who prematurely stopped using the products but still visited the clinic (n = 35) were included. The children who dropped out contributed days at risk to the cumulative occurrence analysis for as long as they continued to participate |
| Selective reporting (reporting bias) | Low risk | None identified |
| Other bias | Unclear risk | The material was donated by industry. Governmental source of funding. The study authors declared they have a US patent for the use of xylitol in treating respiratory infection |
Uhari 1996.
| Methods | Double‐blind, placebo‐controlled randomised study | |
| Participants | 306 children from 11 ordinary daycare nurseries for healthy, normal children from the city of Oulu, Finland were enrolled in March 1995 after parental consent. 30 dropouts, which left 276 children Xylitol group (n = 157), mean (SD) age = 5.0 (1.4); mean (SD) duration of daycare (months) = 26.5 (16.9) Sucrose group (n = 149), mean (SD) age = 4.9 (1.5) years; mean (SD) duration of daycare (months) = 25.2 (19.0) Exclusion criteria: children with dental caries |
|
| Interventions | Xylitol chewing gum n = 157: 2 pieces five times (one box) a day after meals or snacks were chewed until there was no taste left or for at least 5 minutes, making a total dose of 8.4 g xylitol a day Sucrose (control) chewing gum n = 149 |
|
| Outcomes | AOM based on symptoms and signs of ARI and simultaneous signs of middle ear effusion: a cloudy tympanic membrane or impaired tympanic membrane motility in pneumatic otoscopy Antimicrobial treatment received during the intervention and nasopharyngeal carriage of S pneumoniae |
|
| Notes | Use of sucrose as the control group has been criticised as possibly increasing the dental caries experience. However, of the children who underwent a dental examination, there was no difference in the rate of dental decay (23/114 in sucrose group versus 21/111 in the xylitol group) The study population age ranges from 2 to 5 years, which is older than the usual peak age for otitis media (6 to 18 months,Journal of Pediatrics 1988; 113:581‐7) Xylitol was administered 5 times a day which may be difficult in a practical situation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A randomisation list was made by using random number tables which were also used to number the cartridges. Block randomisation with a block size of 4 in each daycare was used to ensure equal numbers of children in the 2 groups Quote: "A randomisation list was made by using random number tables.” To ensure equal numbers of children in the 2 groups in each of the nurseries we used block randomisation with a block size of 4 |
| Allocation concealment (selection bias) | Low risk | The list was sealed in an envelope. The observers were blinded to the randomisation scheme Quote: "The cartridges were numbered accordingly, and the list was sealed in an envelope" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "The observers in the trial were unaware of the randomisation scheme" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was a dropout of 30 participants, 10 in xylitol group and 20 in control group representing a dropout rate of 6% and 13%, respectively. The reasons for dropouts were: child got tired of eating chewing gum, dental caries, moved from area, parents got tired, insufficient follow‐up data, loose stools |
| Selective reporting (reporting bias) | Low risk | None identified |
| Other bias | Unclear risk | The material was donated by industry, no conflict of interest was reported. Governmental source of funding |
Uhari 1998.
| Methods | Double‐blind, placebo‐controlled randomised study | |
| Participants | 857 children from 34 typical daycare centres for healthy children from the city of Oulu, Finland were recruited after parental consent between September and December 1996 Exclusion criteria: antimicrobial prophylactics, congenital craniofacial malformation or a structural middle ear abnormality | |
| Interventions | Test group, unable to chew gum n = 159: 5 mL of a syrup containing 400 g/L xylitol 5 times a day (daily doses of 10 g of xylitol) Test group, able to chew gum n = 179: 2 pieces of xylitol chewing gum 5 times a day (3 x in daycare and 2 x in home) after a meal for at least 5 minutes or as long as it tasted sweet (daily dose 8.4 g of xylitol), or 3 xylitol/maltitol lozenges (n = 176) lozenges 5 times a day after a meal for at least 5 minutes or as long as it tasted sweet (daily dose 10 g of xylitol) Control group, unable to chew gum n = 165: control syrup containing 20 g/L xylitol diluted in water without any other sweeteners with the same protocol as xylitol syrup group (daily doses of 0.5 g of xylitol) Control group, able to chew gum n = 178: chewing gum sweetened with sucrose and xylitol (daily dose 0.5 g of xylitol) with the same protocol as xylitol gum group |
|
| Outcomes | AOM based on a finding of middle ear effusion in tympanometry (B‐ or C‐curve), verified otoscopically with signs of inflammation in the tympanic membrane, and the presence of symptoms of acute respiratory infection (rhinitis, cough, conjunctivitis, sore throat, earache) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation was performed using tables of random numbers and using a block randomisation with a block size of six.” |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned but probably done as per their earlier publication |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "The study was double‐blind in the syrup and chewing gum groups and open between the chewing gum and lozenge groups. The xylitol syrup was sweeter than the control syrup, but the taste of the chewing gums was quite similar regardless of the sweeteners used” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | By the end of trial, the dropouts range from 4.5% to 18.9% and were statistically higher in the xylitol syrups (18.9%) and xylitol lozenges (14.8%) as compared to their control groups of, respectively, control syrups (10.3%) and control chewing gum (4.5%). The reasons for dropouts were: unwilling to continue taking the product, left the area, abdominal discomfort, unknown reason |
| Selective reporting (reporting bias) | Low risk | None identified |
| Other bias | Unclear risk | The material was donated by industry, no conflict of interest was reported. Governmental source of funding |
Vernacchio 2014.
| Methods | Double‐blind, pragmatic practice‐based RCT | |
| Participants | 326 otitis‐prone children ages 6 to 71 months with history of at least 3 clinically diagnosed episodes of acute otitis media (with/without middle ear effusions) in the previous 12 months with at least 1 in the previous 6 months, in good general health (see criteria for ‘good health’ on page 290) and English or Spanish speaking All children were identified and referred by participating physicians from 3 paediatric practice‐based networks (the Slone Center Office‐based Research Network at Boston University, the Pediatric Physicians’ Organization at Children’s (Boston), and the North Carolina Child Health Research Network at the University of North Carolina) |
|
| Interventions | Active treatment: Xylitol oral solution (Xylarex; Arbor Pharmaceuticals, Atlanta, GA) consisting of a 66.7% aqueous xylitol solution with the addition of carboxymethylcellulose and potato starch as mucosal adherence agents and natural flavouring. The dose for the xylitol syrup was 7.5 mL 3 times daily for 12 weeks (i.e. 5 g xylitol per dose) Placebo medication: 30% sorbitol oral solution with the addition of carboxymethylcellulose and potato starch as mucosal adherence agents and natural flavouring at a dose of 2.25 g sorbitol 3 times per day |
|
| Outcomes | Primary outcome: comparison of time to first clinically diagnosed AOM episode in the two study groups, using proportional hazards model. Secondary outcomes: 1. Proportion of participants in each group with no AOM episodes and no antibiotic use during the study period 2. Reduction in the 3‐month cumulative incidence of AOM episodes, as measured by the risk difference and the hazard rate for AOM (in Poisson regression) 3. Reduction in antibiotic use per 90 days, as measured by the risk difference and the hazard rate (in Poisson regression) Frequency of occurrence of adverse events in either group, as measured by the risk difference |
|
| Notes | Analyses were based on the intention‐to‐treat principle. For the comparison of incidence of AOM episodes and antibiotic use per 90 days between the two groups, “rates were calculated individually (events divided by days of enrolment), assuming a zero rate for the 12 participants with no follow‐up.” Higher amount of xylitol per dose and per day over previous studies and the addition of mucosal adherence agents to the xylitol solution as compared to other studies |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No direct quote was found. There was an acknowledgement to Qiaoli (Lily) Chen who generated randomisation assignments |
| Allocation concealment (selection bias) | Unclear risk | No information is provided |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blinded study syrups. Sorbitol is similar in appearance and taste to xylitol. “After randomisation, study materials, including the blinded study syrup, appropriate oral syringes, and a study calendar, were shipped to the subject’s home.” The principal investigator reviewed medical records in a blinded fashion. Quote: “At the end of the study, medical records from each subject’s primary care physician and from any other health care provider whom the parent identified as having treated the subject during the study period were obtained and reviewed by the principal investigator (LV) in a blinded fashion.” |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 62/160 allocated to xylitol (38.8%) declined participation, were lost to follow‐up or discontinued intervention versus 58/166 allocated to placebo (34.9%) |
| Selective reporting (reporting bias) | Low risk | As compared to protocol NCT01044030, 2 secondary outcomes were not reported, although they do not represent key outcomes: 1) To determine the effect of viscous‐adherent xylitol on nasopharyngeal and oropharyngeal colonisation with S pneumoniae and non‐typable H influenzae and 2) To compare the antimicrobial resistance patterns of isolates of S pneumoniae and non‐typable H influenzae cultured from the oropharynx and/or nasopharynx of subjects treated with viscous‐adherent xylitol compared to placebo |
| Other bias | Low risk |
Potential bias for outcome assessment: The study did not have any protocol of assessment for healthcare providers. Quote: “For the primary outcome of clinical diagnoses of AOM, the medical record was considered the gold standard. For those cases in which the medical record was not available, the parent’s report of AOM diagnoses was used” The researchers assumed that inaccurate diagnoses would be equal in both groups. Quote: “AOM diagnoses were made by a wide range of clinicians and were not otherwise verified.” “… assuming that AOM diagnoses were equally inaccurate in both study groups, the overall effect would be to bias the study toward a null result.” The trial was performed under FDA New Drug application number 107246 and was registered at www.clinicaltrials.gov (NCT01044030) No financial incentives paid to parents/subjects or to enrolling practices, except a nominal reimbursement for staff time involved in referring potentially eligible parents. |
AOM: acute otitis media ARI: acute respiratory infection n: number SD: standard deviation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Milgrom 2009 | No data on AOM. In the published paper, it was mentioned that the effect of xylitol in reducing AOM will be published in a subsequent study. However, after contacting the primary investigator, it was understood that due to lack of reliability of data regarding AOM, the plan to publish AOM results has been suspended |
AOM: acute otitis media
Characteristics of studies awaiting assessment [ordered by study ID]
Kalanin 2005.
| Methods | Multicentre, randomised, double‐blind, placebo‐controlled study |
| Participants | 82 male and 86 female outpatients from 1 to 15 years of age with recurrent AOM. Mean age was 6.14 years. The population is from Prague and Mid‐Bohemia region, Czech Republic |
| Interventions | Intervention: 3 daily doses of nasal wash, each consisting of 2 squirts per nostril, at regular assigned times daily (after waking up; after lunch; before going to bed). Patients received one of the following treatments as predetermined by their randomisation number: a solution of nasal xylitol (11% pure xylitol; XLEAR®) or distilled water |
| Outcomes | AOM recurrence; frequency of antibiotic therapy; frequency of upper airways infections; adverse events |
| Notes | Xlear Inc. has been contacted for further information about this unpublished report |
AOM: acute otitis media
Characteristics of ongoing studies [ordered by study ID]
NCT02592382.
| Trial name or title | Nasal xylitol in the prevention of otitis media |
| Methods | Double‐blind, RCT |
| Participants | 50 children at the age of 1 to 5 years who had 3 episodes of recurrent otitis media in the last 6 months prior to entering the study. Those with immune deficiency, craniofacial malformations, chronic otitis media, or those who received prophylactic antibiotic treatment prior to entering the study (3 months) will be excluded |
| Interventions | Control: isotonic saline nasal spray; test: 5% xylitol spray (3 times daily for 2 months) |
| Outcomes | Primary outcome measures: prevalence of otitis media and the number of events of acute otitis media during the study period of 5 months Secondary outcome measures: side effects of treatment during a time frame of 2 months |
| Starting date | January 2016 |
| Contact information | Arie Gordin, M, a_gordin@rambam.health.gov.il and Shani Fischershani_fi@clalit.co.il Sponsors and Collaborators: Rambam Health Care Campus, HaEmek Medical Center, Israel, Carmel Medical Center |
| Notes |
Differences between protocol and review
Based on the methodology of the identified studies, we defined the three subgroup analyses of younger children unable to chew gum, older children able to chew gum and xylitol administered during a respiratory infection. These subgroup analyses were not defined in the protocol.
In this 2016 update, we defined a new sensitivity analysis and performed random‐effects meta‐analysis to compare the pooled results to those of the fixed‐effect meta‐analyses. Furthermore, in this 2016 update, we changed the order and wording of the Secondary outcomes from:
Secondary outcomes
Any adverse events of xylitol (for example, gastrointestinal discomfort).
Hospitalisation secondary to AOM or its complications in studies where at least one hospitalisation was reported.
Mortality secondary to complications of AOM in studies where at least one death was reported.
Length of hospital stay.
Percentage of children needing antibiotic therapy.
Number of days missed at school or day care centre.
Cost (direct such as emergency room or general practitioners visits cost; hospitalisations cost; medication cost and indirect such as the time taken from work for parents or care givers; the opportunity cost of leisure time; informal nursing care; and out‐of‐pocket expenses for transportation).
To:
Secondary outcomes
Safety and adverse events.
Antibiotic administration.
Hospitalisation (and its length) secondary to AOM or its complications in studies where at least one hospitalisation was reported.
Mortality secondary to complications of AOM in studies where at least one death was reported.
Number of days missed at school or daycare centre.
Cost (direct such as emergency room or general practitioners visits cost; hospitalisations cost; medication cost and indirect such as the time taken from work for parents or care givers; the opportunity cost of leisure time; informal nursing care; and out‐of‐pocket expenses for transportation)
Contributions of authors
Dr Amir Azarpazhooh (AA) conceptualised and designed the research, conducted and screened the results of search, assessed the quality of and abstracted data from included studies, performed the statistical analysis, interpreted data and drafted the review. Dr Prakeshkumar S Shah (PS) critiqued and edited the review, assessed the quality of included studies, supervised the statistical analysis and data interpretation. Dr Herenia P Lawrence (HPL) critiqued and edited the protocol and the review.
Sources of support
Internal sources
Discipline of Dental Public Health, Faculty of Dentistry, University of Toronto, Canada.
Department of Paediatrics, Mount Sinai Hospital, Canada.
Department of Paediatrics, University of Toronto, Canada.
External sources
No sources of support supplied
Declarations of interest
Amir Azarpazhooh: is an investigator on the Canadian Institue of Health Resarch's funded clinical trial related to this topic. The trial has not been initiated. Herenia P Lawrence: none known Prakeshkumar S Shah: none known
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Hautalahti 2007 {published data only}
- Hautalahti O, Renko M, Tapiainen T, Kontiokari T, Pokka T, Uhari M. Failure of xylitol given three times a day for preventing acute otitis media. Pediatric Infectious Disease Journal 2007;26(5):423‐7. [DOI] [PubMed] [Google Scholar]
Tapiainen 2002 {published data only}
- Tapiainen T, Luotonen L, Kontiokari T, Renko M, Uhari M. Xylitol administered only during respiratory infections failed to prevent acute otitis media. Pediatrics 2002;109(2):E19. [DOI] [PubMed] [Google Scholar]
Uhari 1996 {published data only}
- Uhari M, Kontiokari T, Koskela M, Niemela M. Xylitol chewing gum in prevention of acute otitis media: double blind randomised trial. BMJ 1996;313(7066):1180‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Uhari 1998 {published data only}
- Uhari M, Kontiokari T, Niemela M. A novel use of xylitol sugar in preventing acute otitis media. Pediatrics 1998;102(4 pt 1):879‐84. [DOI] [PubMed] [Google Scholar]
Vernacchio 2014 {published data only}
- Vernacchio L, Corwin MJ, Vezina RM, Pelton SI, Feldman HA, Coyne‐Beasley T, et al. Xylitol syrup for the prevention of acute otitis media. Pediatrics 2014;133(2):289‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Milgrom 2009 {published data only}
- Milgrom P, Ly KA, Tut OK, Mancl L, Roberts MC, Briand K, et al. Xylitol pediatric topical oral syrup to prevent dental caries: a double‐blind randomized clinical trial of efficacy. Archives of Pediatrics and Adolescent Medicine 2009;163(7):601‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
Kalanin 2005 {published data only}
- Kalanin J, BIOCEN Laboratories Ltd. Preventing recurrent otitis by addressing nasal hygiene with a spray of nasal xylitol: evaluation of effectiveness of regular Xlear® (or its placebo) nasal wash three times daily in prevention of acute otitis media (AOM) in a group of children suffering from recurrent AOM episodes. Xlear Inc., Orem, Utah 84057, US 10 October 2005.
References to ongoing studies
NCT02592382 {published data only}
- NCT02592382. Nasal xylitol in the prevention of otitis media. https://clinicaltrials.gov/ct2/show/NCT02592382?term=xylitol&rank=9 (accessed 27 January 2016).
Additional references
AAP 2013
- Lieberthal AS, Carroll AE, Chonmaitree T, Ganiats TG, Hoberman A, Jackson MA, et al. The diagnosis and management of acute otitis media. Pediatrics 2013 Mar;131(3):e964‐99. [DOI] [PubMed] [Google Scholar]
ACIP 2000
- Advisory Committee on Immunization Practices. Preventing pneumococcal disease among infants and young children. Recommendations of the Advisory Committee on Immunization Practices (ACIP). Morbidity and Mortality Weekly Report 2000;49(RR‐9):1‐35. [PubMed] [Google Scholar]
Ahmed 2014
- Ahmed S, Shapiro NL, Bhattacharyya N. Incremental health care utilization and costs for acute otitis media in children. Laryngoscope 2014;124(1):301‐5. [DOI] [PubMed] [Google Scholar]
Akkerman 2005
- Akkerman AE, Kuyvenhoven MM, Wouden JC, Verheij TJ. Analysis of under‐ and overprescribing of antibiotics in acute otitis media in general practice. Journal of Antimicrobial Chemotherapy 2005;56(3):569‐74. [DOI] [PubMed] [Google Scholar]
Atkins 2004
- Atkins D, Best D, Briss PA, Eccles M, Falck‐Ytter Y, Flottorp S, et al. GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
CDC 2015
- Centers for Disease Control and Prevention. Chapter 17: Pneumococcal disease. In: Hamborsky J, Kroger A, Wolfe S editor(s). Epidemiology and Prevention of Vaccine‐Preventable Diseases. 13th Edition. Washington DC: Public Health Foundation, 2015. [Google Scholar]
Danhauer 2010a
- Danhauer JL, Johnson CE, Corbin NE, Bruccheri KG. Xylitol as a prophylaxis for acute otitis media: systematic review. International Journal of Audiology 2010;49(10):754‐61. [DOI] [PubMed] [Google Scholar]
Danhauer 2010b
- Danhauer JL, Johnson CE, Rotan SN, Snelson TA, Stockwell JS. National survey of pediatricians' opinions about and practices for acute otitis media and xylitol use. Journal of the American Academy of Audiology 2010;21(5):329‐46. [DOI] [PubMed] [Google Scholar]
Danhauer 2011a
- Danhauer JL, Johnson CE, Caudle AT. Survey of K‐3rd‐grade teachers' knowledge of ear infections and willingness to participate in prevention programs. Language, Speech, and Hearing Services in Schools 2011;42(2):207‐22. [DOI] [PubMed] [Google Scholar]
Danhauer 2011b
- Danhauer JL, Kelly A, Johnson CE. Is mother‐child transmission a possible vehicle for xylitol prophylaxis in acute otitis media?. International Journal of Audiology 2011;50(10):661‐72. [DOI] [PubMed] [Google Scholar]
Danhauer 2015
- Danhauer JL, Johnson CE, Baker JA, Ryu JA, Smith RA, Umeda CJ. Will parents participate in and comply with programs and regimens using xylitol for preventing acute otitis media in their children?. Language, Speech, and Hearing Services in Schools 2015;46(2):127‐40. [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011:28. [Google Scholar]
Deshpande 2008
- Deshpande A, Jadad AR. The impact of polyol‐containing chewing gums on dental caries: a systematic review of original randomized controlled trials and observational studies. Journal of the American Dental Association 2008;139(12):1602‐14. [DOI] [PubMed] [Google Scholar]
Friedman 2006
- Friedman NR, McCormick DP, Pittman C, Chonmaitree T, Teichgraeber DC, Uchida T, et al. Development of a practical tool for assessing the severity of acute otitis media. Pediatric Infectious Disease Journal 2006;25(2):101‐7. [DOI] [PubMed] [Google Scholar]
Froom 2001
- Froom J, Culpepper L, Green LA, Melker RA, Grob P, Heeren T. A cross‐national study of acute otitis media: risk factors, severity, and treatment at initial visit. Report from the international primary care network (IPCN) and the ambulatory sentinel practice network (ASPN). Journal of the American Board of Family Practice 2001;14(6):406‐17. [PubMed] [Google Scholar]
GRADEpro 2004
- GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEproGDT 2015 [Computer program]
- McMaster University. GRADEpro GDT: GRADEpro Guideline Development Tool (developed by Evidence Prime, Inc.). Available from gradepro.org. Version 16 February 2015. Hamilton: McMaster University, 2015.
Guven 2006
- Guven M, Bulut Y, Sezer T, Aladag I, Eyibilen A, Etikan I. Bacterial etiology of acute otitis media and clinical efficacy of amoxicillin‐clavulanate versus azithromycin. International Journal of Pediatric Otorhinolaryngology 2006;70(7):915‐23. [DOI] [PubMed] [Google Scholar]
Haresaku 2007
- Haresaku S, Hanioka T, Tsutsui A, Yamamoto M, Chou T, Gunjishima Y. Long‐term effect of xylitol gum use on mutans streptococci in adults. Caries Research 2007;41(3):198‐203. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration. www.cochrane‐handbook.org 2011.
Jansen 2009
- Jansen AGSC, Hak E, Veenhoven RH, Damoiseaux RAMJ, Schilder AGM, Sanders EAM. Pneumococcal conjugate vaccines for preventing otitis media. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD001480.pub2] [DOI] [PubMed] [Google Scholar]
Kontiokari 1995
- Kontiokari T, Uhari M, Koskela M. Effect of xylitol on growth of nasopharyngeal bacteria in vitro. Antimicrobial Agents and Chemotherapy 1995;39(8):1820‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kontiokari 1998
- Kontiokari T, Koivunen P, Niemelä M, Pokka T, Uhari M. Symptoms of acute otitis media. Pediatric Infectious Disease Journal 1998;17(8):676‐9. [DOI] [PubMed] [Google Scholar]
Ly 2008
- Ly KA, Milgrom P, Rothen M. The potential of dental‐protective chewing gum in oral health interventions. Journal of the American Dental Association 2008;139(5):553‐63. [DOI] [PubMed] [Google Scholar]
Maguire 2003
- Maguire A, Rugg‐Gunn AJ. Xylitol and caries prevention‐‐is it a magic bullet?. British Dental Journal 2003;194(8):429‐36. [DOI] [PubMed] [Google Scholar]
McDonald 2008
- McDonald S, Langton Hewer CD, Nunez DA. Grommets (ventilation tubes) for recurrent acute otitis media in children. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD004741] [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred reporting items for systematic reviews and meta‐analyses: The PRISMA Statement. BMJ 2009;339:2535. [PMC free article] [PubMed] [Google Scholar]
Murphy 2006
- Murphy TF. Otitis media, bacterial colonization, and the smoking parent. Clinical Infectious Diseases 2006;42(7):904‐6. [DOI] [PubMed] [Google Scholar]
Norhayati 2015
- Norhayati MN, Ho JJ, Azman MY. Influenza vaccines for preventing acute otitis media in infants and children. Cochrane Database of Systematic Reviews 2015, Issue 3. [DOI: 10.1002/14651858.CD010089] [DOI] [PubMed] [Google Scholar]
Paradise 1999
- Paradise JL, Bluestone CD, Colborn DK, Bernard BS, Smith CG, Rockette HE, et al. Adenoidectomy and adenotonsillectomy for recurrent acute otitis media: parallel randomized clinical trials in children not previously treated with tympanostomy tubes. JAMA 1999;282(10):945‐53. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Stockwell 2010
- Stockwell JS, Johnson CE, Danhauer JL. Additional data from physicians having previous experience with xylitol as a prophylaxis for acute otitis media in children. Journal of the American Academy of Audiology 2010;21(8):558. [DOI] [PubMed] [Google Scholar]
Twetman 2003
- Twetman S, Stecksen‐Blicks C. Effect of xylitol‐containing chewing gums on lactic acid production in dental plaque from caries active pre‐school children. Oral Health & Preventive Dentistry 2003;1(3):195‐9. [PubMed] [Google Scholar]
Uhari 2000
- Uhari M, Tapiainen T, Kontiokari T. Xylitol in preventing acute otitis media. Vaccine 2000;19(Suppl 1):144‐7. [DOI] [PubMed] [Google Scholar]
Venekamp 2015
- Venekamp RP, Sanders SL, Glasziou PP, Mar CB, Rovers MM. Antibiotics for acute otitis media in children. Cochrane Database of Systematic Reviews 2015, Issue 6. [DOI: 10.1002/14651858.CD000219] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vernacchio 2007a
- Vernacchio L, Vezina RM, Mitchell AA. Tolerability of oral xylitol solution in young children: implications for otitis media prophylaxis. International Journal of Pediatric Otorhinolaryngology 2007;71(1):89‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vernacchio 2007b
- Vernacchio L, Vezina RM, Ozonoff A, Mitchell AA. Validity of parental reporting of recent episodes of acute otitis media: a Slone Center Office‐Based Research (SCOR) Network study. Journal of the American Board of Family Medicine 2007;20(2):160‐3. [DOI] [PubMed] [Google Scholar]
Wallace 2014
- Wallace IF, Berkman ND, Lohr KN, Harrison MF, Kimple AJ, Steiner MJ. Surgical treatments for otitis media with effusion: a systematic review. Pediatrics 2014 Feb;133(2):296‐311. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Azarpazhooh 2011
- Azarpazhooh A, Limeback H, Lawrence HP, Shah PS. Xylitol for preventing acute otitis media in children up to 12 years of age. Cochrane Database of Systematic Reviews 2011, Issue 11. [DOI: 10.1002/14651858.CD007095.pub2] [DOI] [PubMed] [Google Scholar]


