Abstract
Background:
Men who have sex with men (MSM) who have bacterial sexually transmitted infections (STIs) are at increased risk for HIV infection. We enhanced and updated past summary risk estimates.
Methods:
We systematically reviewed (PROSPERO #CRD42018084299) peer-reviewed studies assessing increased risk of HIV infection among MSM attributable to: Chlamydia trachomatis (CT), Mycoplasma genitalium (MG), Neisseria gonorrhoeae (NG), Treponema pallidum (TP), and/or Trichomonas vaginalis (TV). We searched three databases through December 2017. We excluded studies with self-reported data or simultaneous STI and HIV assessment. We conducted dual screening and data extraction, meta-analytically pooled risk ratios (RR), and assessed potential risk of bias.
Results:
We included 26 studies yielding k=39 RR for HIV acquisition due to one of TP, NG, or CT. We did not identify eligible data for MG or TV nor for HIV transmission. HIV acquisition risk increased among MSM infected with TP (k=21, RR 2.68, 95% CI 2.00–3.58), NG (k=11, RR 2.38, 95% CI 1.56–3.61), and CT (k=7, RR 1.99, 95% CI 1.59–2.48). Sub-analysis RR for all three pathogens were >= 1.66 and remained statistically significant across geography and methodological characteristics. Pooled RR increased for data with the lowest risk of bias for NG (k=3, RR 5.49, 95% CI 1.11–27.05) and TP (k=4, RR 4.32, 95% CI 2.20–8.51). We observed mostly moderate to high heterogeneity and moderate to high risk of bias.
Conclusion:
MSM infected with TP, NG, or CT have twice or greater risk of HIV acquisition, although uncertainties exist due to data heterogeneity and risk of bias.
Keywords: HIV, MSM, STI, systematic review
SUMMARY
This review highlights the temporal relationship between STI and HIV for MSM. Results indicate that MSM infected with TP, NG, or CT have twice or greater risk of HIV acquisition.
INTRODUCTION
With an estimated 357 million new cases of Chlamydia trachomatis, Neisseria gonorrhea, syphilis, and Trichomonas vaginalis annually, the global burden of sexually transmitted infections (STIs) is rising [1]. This burden is disproportionately high among men who have sex with men (MSM). For instance, MSM in the United States comprised 68.2% of reported syphilis cases and 38.5% of reported gonorrhea cases in 2017; an estimated 13.3–25% of MSM are infected with at least one bacterial STI [2–5].
As early as 1992, studies reported increased risk of HIV transmission and acquisition in the presence of STIs [6–13]. Mechanisms include ulcers that facilitate HIV entry, a localized immune response involving CD4 cell proliferation, and increased HIV shedding [14–15].
Rationale for systematic review
Several systematic reviews have examined the effect of STIs on HIV risk in MSM and heterosexuals. However, there is unexplained variation in the magnitude of effects [8,14,16,17]. This may reflect differing eligibility criteria and including studies that assess HIV and STI concurrently, where the temporality of STI and HIV diagnoses is unknown [18–20]. Advances in diagnosis, prevention (e.g., pre-exposure prophylaxis, PrEP), and treatment can also influence effect size [21–22].
Accurate and up-to-date estimates of STI-related HIV infection risk support mathematical modeling of HIV prevention strategy benefits. The modifiable risk of HIV attributed to STIs bears on implementation of PrEP and other strategies. This paper provides unprecedented attention to the temporal relationship between STI and HIV diagnoses in our analysis of this effect.
MATERIALS & METHODS
This MSM-focused manuscript stems from a parent systematic review on the effect of six STI pathogens (Chlamydia trachomatis, Herpes Simplex Virus type 2 (HSV-2), Mycoplasma genitalium, Neisseria gonorrhoeae, Treponema pallidum, Trichomonas vaginalis) on HIV acquisition and transmission among high-risk populations.
We followed Cochrane Collaboration recommendations [23], registered our protocol in the PROSPERO database (CRD42018084299)[24–25], used the Population, Exposure, Comparator, Outcomes (PECO) schema for study screening and data extraction, followed Grading of Recommendations Assessment, Development and Evaluation Guideline (GRADE) methods to assess risk of bias at the PECO level [26] and used Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines for reporting [27].
Searches and screening
We developed search strategies and searched PubMed in December 2017 and Web of Science and Embase in January 2018. Two authors conducted dual, independent screening of studies; 5% of excluded records were reviewed by other authors for quality assurance. [Appendices A–C].
Study eligibility
We included peer-reviewed studies comparing STI-infected and STI-uninfected MSM on the risk of HIV acquisition (HIV-susceptible partner had STI) or transmission (HIV-infected partner had STI). We included men who have sex with men, men who have sex with men and women, and transgender women, as defined by studies.
We included data where we could establish that STI assessment occurred prior to HIV diagnosis. We excluded studies using self-reported data, where the timing of STI and HIV assessment was two or more years apart, and where diagnosis timing was unclear. We included outcomes with sufficient data to calculate the effect size in the form of risk ratio (RR) and 95% confidence intervals (CI).
Data extraction and standardization
We used pre-structured data extraction tables in Google Sheets that captured: effect size; study participant and partner demographics; ART, PrEP, and condom use; exposure to other interventions; STI diagnoses and treatment, diagnostic technologies, and timing; data related to risk of bias; location; and year(s) of data collection. Two raters entered data into the spreadsheet and used formulas to identify discrepancies, which they resolved via discussion. When essential data were missing or ambiguous, we contacted study authors for clarification. Coauthors reviewed data extraction of 5% of studies (randomly-selected) and those identified as especially nuanced for quality assurance.
Risk of bias assessment
We adapted and used the Making GRADE the Irresistible Choice (MAGIC) approach for assessing potential risk of bias for each effect size across nine bias domains dictated by study design [Appendix E] [23,26,28,29]. We incorporated nuances of timing and accuracy for STI exposure and HIV outcome assessments. For example, studies received higher ratings for HIV RNA tests, shorter intervals between STI and HIV assessments, or analysis of STI exposure as time-sensitive. We rated each domain on the scale: “very low,” “low,” “medium”, and “high” risk of bias.
Data analysis and synthesis
We used Stata v14.230 for statistical analysis, calculated RR and 95% CI for effect estimates, and used the Zhang and Yu [31] method to calculate RR when studies reported odds ratios. We grouped effect sizes according to pathogen and timing of STI diagnosis (baseline vs. incident). We pooled data using a random-effects model when we identified two or more conceptually combinable effect sizes and reported the I2 statistic (as percentage) for heterogeneity [23]. When more than one effect size was reported by one study for a given STI, we prioritized reports of infection at ‘any’ anatomical site or aggregated site-specific estimates and prioritized adjusted over unadjusted estimates. We conducted sensitivity analyses by removing each estimate individually and recalculating the pooled estimate using remaining data. We plotted RRs (x-axis) against their log of the standard error (y-axis) for meta-analyzed pooled estimate with ≥10 effect sizes to explore the small-study effects.
We explored the effect of studies’ geographic setting and certain methodological characteristics on effect estimates. To depict the estimates with the lowest risk of bias, we conducted two meta-analyses (Models 1 and 2) after omitting data from case-control studies, unadjusted effect size estimates, or an interval greater than 12 months between STI and HIV diagnosis. Model 1 additionally excluded data from medical records.
This study occurred through a cooperative agreement with the U.S. Centers for Disease Control and Prevention under the National Center for HIV, Viral Hepatitis, STD, and TB Prevention Epidemiologic and Economic Modeling Agreement.
RESULTS
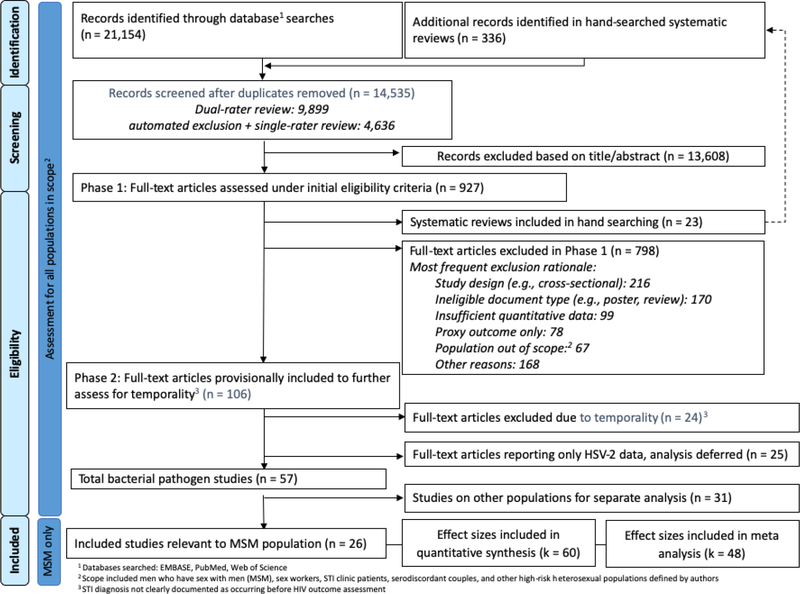
Our searches returned 14,535 unique records; we excluded 13,608 after reviewing titles and/or abstracts (Figure 1).
Figure 1.
Identification and screening of bibliographic records for systematic review of the effect of STI diagnosis on the risk of HIV seroconversion among MSM (search up to January 2018)
Initial full-text review excluded 23 systematic reviews plus 798 articles [Appendix D]. Further review excluded an additional 24 articles for ineligible or unclear temporality between STI and HIV diagnosis. Due to a recent review [32], we excluded 25 more studies reporting on HSV-2 infection but not chlamydia, gonorrhea, or syphilis. Of the 57 eligible studies, we included 26 addressing MSM (Table 1) in this review. From these 26 studies, we calculated 60 effect sizes for risk of HIV acquisition associated with diagnosis of syphilis, gonorrhea, chlamydia, or a combination of bacterial STIs (Table 1). We found no eligible studies for the added risk of HIV transmission due to STI in HIV-infected MSM, nor on the effect of Mycoplasma genitalium or trichomonas on HIV acquisition among MSM.
Table 1.
Characteristics of included studies assessing the effect of STI on the risk of HIV acquisition among MSM (n=26)
| Author, Year | Country | Eligible Age | Data or Recruitment Source | Sample | Study Period | Study Design | STI Pathogen | Assessment | Risk Ratio (calculated, may differ from effect size in study text) | Confounders Adjusted For | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Beymer 201633 | USA | NR | Existing data from Los Angeles LGBT Center (Latino) | N=3111 Age <25: 32% Latino: 100% |
2009–2014 | Retrospective Cohort | CT: Rectal or ureteral | Test type NR | 1.53 (1.06, 2.20) | Not adjusted | |
| NG: Rectal or ureteral | Test type NR | 1.65 (1.17, 2.31) | |||||||||
| TP: Any site | Serology | 3.41 (1.78, 6.56) | |||||||||
| Any STI | Test type NR (CT, NG); serology (TP) | 1.97 (1.44, 2.69) | |||||||||
| Cheung 201634 | Australia | NR | Existing data from the Melbourne Sexual Health Centre | 6391 PY | 2007–2013 | Retrospective Cohort | CT: Rectal or ureteral | NAAT | 2.30 (1.40, 3.78) | Not adjusted | |
| CT: Ureteral | NAAT | 2.30 (1.20, 4.30) | |||||||||
| CT: Rectal | NAAT | 2.40 (1.50, 3.90) | |||||||||
| NG: Any site | Culture | 2.30 (1.40, 3.78) | |||||||||
| NG: Ureteral | Culture | 1.80 (0.70, 4.20) | |||||||||
| NG: Rectal | Culture | 3.10 (1.80, 5.20) | |||||||||
| NG: Pharyngeal | Culture | 1.50 (0.60, 3.00) | |||||||||
| TP: Any site | Serology | 1.90 (0.70, 5.16) | |||||||||
| Any of CT, NG, or TP: Any site | Mixed | 2.60 (1.70, 3.98) | |||||||||
| Desai 201735 | UK | ≥ 15 | Existing data from STI clinic | N=26192 Mean age: 34 |
2012–2013 | Retrospective Cohort | CT: Site NR | Medical records | 2.20 (1.40, 3.46) | Not reported | |
| NG: Site NR | Medical records | 2.10 (1.40, 3.15) | |||||||||
| TP: Any site | Medical records | 4.10 (2.00, 8.40) | |||||||||
| Any STI: Any site | Medical records | 1.40 (1.00, 1.96) | |||||||||
| Any STI: Rectal | Medical records | 2.10 (1.30, 3.30) | |||||||||
| Garofalo 201636 | USA | 16–20 | Launched a new cohort (Crew 450) | N = 415 | 2009–2015 | Prospective Cohort | CT or NG: Ureteral | NAAT | 0.45 (0.06, 3.29) | Not adjusted | |
| Giuliani 201437 | Italy | ≥ 16 | Launched a new cohort from among aclients of the San Gallicano Dermatological Institute in Rome and via participant-driven recruitment | N = 1862 | 1985–2009 | Prospective Cohort | NG at baseline: Rectal or phalangeal | Culture | 1.22 (0.52, 2.86) | Not reported | |
| NG preceding 1 year: Rectal or phalangeal | Culture | 1.66 (1.01, 2.73) | |||||||||
| NG > 1 year preceding: Rectal or phalangeal | Culture | 1.63 (1.06, 2.49) | |||||||||
| TP at enrollment: Any site | Serology | 0.93 (0.57, 1.73) | |||||||||
| TP preceding 1 year: Any site | Serology | 7.71 (5.00, 11.89) | |||||||||
| TP >1 year preceding: Any site | Serology | 1.16 (0.83, 0.62) | |||||||||
| Harrison 199938 | Brazil | 18–50 | Launched a new cohort (nested in HIVNET) | N=750 Median age: 28.2 |
1995–1997 | Prospective Cohort | NG: Site NR | NR | 4.50 (1.10, 18.41) | Interval censoring, factors based on baseline associations with seropositivity (not specified) | |
| Jia 201539 | China | ≥ 18 | Launched a new cohort in Beijing | N=3625 | 2007–2012 | Prospective Cohort | TP: Any site | Serology | 1.47 (1.09, 1.98) | Not adjusted | |
| Jin 201040 | Australia | NR | Launched a new cohort in Sydney | N=1427 Median age: 35 |
2001–2007 | Prospective Cohort | CT: Rectal | NAAT | 2.72 (0.54, 11.56) | Not adjusted | |
| NG: Rectal | NAAT | 7.12 (2.04, 24.85) | Number of episodes of nonconcordant UAI by sexual role and knowledge of partner HIV status | ||||||||
| TP: Any site | Serology | 1.37 (0.33, 5.69) | Not adjusted | ||||||||
| Kelley 201541 | USA | ≥ 18 | Launched a new cohort (InvolveMENt Study) | N=562 Median age: 27.6 |
2010–2014 | Prospective Cohort | CT or NG: Ureteral | NAAT | 0.00 (0.00, 2.16e 16 ) | Rectal STI: propensity score | |
| CT or NG: Rectal | NAAT | 2.70 (1.40, 5.21) | |||||||||
| TP: Any site | Serology | 0.00 (0.00, 2.46e 16 ) | |||||||||
| Koblin 201342 | USA | ≥ 18 | Multicenter EXPLORE Study cohort | N=1553 Mean age: 37 White: 72.5% |
2009–2010 | Prospective Cohort | CT, NG: Ureteral or rectal, or TP: Any site | Test type NR (CT, NG); serology (TP) | 1.70 (0.60, 4.82) | Younger age, URAI with HIV+/ unknown partner | |
| Lam 201743 | Thailand | ≥ 18 | Bangkok MSM Cohort Study (BMCS) | N=1744 Mean age = 26 |
2006–20014 | Prospective Cohort | CRF01_AE HIV Subtype | CT: Rectal | NAAT | 1.48 (1.02, 2.15) | Not reported |
| NG: Rectal | NAAT | 1.07 (0.68, 1.68) | |||||||||
| TP: Any site | Serology | 2.50 (1.44, 4.34) | |||||||||
| Non-CRF01_AE HIV Subtype | CT: Rectal | NAAT | 1.95 (0.92, 4.13) | ||||||||
| NG: Rectal | NAAT | 1.41 (0.59, 3.37) | |||||||||
| TP: Any site | Serology | 0.95 (0.13, 6.94) | |||||||||
| Li 201244 | China | NR | Launched a new cohort in Beijing | N=962 Median age = 27 |
2009–2011 | Prospective Cohort | TP: Any site | Serology | 2.62 (1.53, 4.49) | NR (study published in Chinese, may be reported in text) | |
| Meireles 201545 | Portugal | ≥ 18 | Launched a new cohort from among clients of CheckpointLX, a Lisbon community-based HIV counseling and testing center (Lisbon MSM Cohort) | N=804 Mean age: 30.3 |
2011–2014 | Prospective Cohort | NG: Any site | Medical record | 0.00 (0.00, 0.00) | UAI with a steady partner and UAI with occasional partners during follow-up | |
| TP: Any site | Medical record | 3.89 (0.47, 32.19) | |||||||||
| Mitchell 201746 | UK | ≥ 16 | Existing data from the UK genitourinary medicine clinic activity dataset (GUMCADv2) | N=228,764* | 2011–2014 | Retrospective Cohort | Bacterial STI: Any site | Medical record | 3.98 (3.24, 4.89) | Age, ethnic group, world region of birth and sexual health clinic location | |
| Page-Shafer 199747 | Multiple† | NR | Data drawn from across six prospective cohort studies (Tricontinental Seroconverter Study)‡ | N=345 Mean age=35.3 |
1982–1994 | Case-control | NG: Any site | Medical record | 1.51 (0.90, 2.54) | Receptive anal intercourse, condom use | |
| TP: Any site | Medical record | 1.11 (0.52, 2.34) | |||||||||
| Pathela 201348 | USA | NR | Existing data from New York City public STI clinics | N=552 | 2008–2010 | Retrospective Cohort | CT or NG: Rectal | Medical record | 2.58 (1.33, 5.00) | Not adjusted | |
| TP: Any site | Medical record | 1.27 (0.49, 3.29) | |||||||||
| Sanders 201349 | Kenya | 18–49 | Launched a new cohort in coastal Kenya via walk-in counseling and testing centers | N=449 | 2005–2011 | Prospective Cohort | NG: Ureteral or rectal | Stain | 14.70 (8.30, 26.03) | NG: Classification as MSMW vs. MSM, age, sexual exposure & risk in past week, RAI, & group sex | |
| Solomon 201450 | Multiple† | NR | iPrEx Study PrEP trial | N=2499 18–24 age: 50% |
2007–2009 | Prospective Cohort | TP: Any site | Serology | 2.60 (1.60, 4.22) | Not adjusted | |
| Thienkrua 201651 | Thailand | ≥ 18 | Bangkok MSM Cohort Study (BMCS) | N=1744 | 2006–2012 | Prospective Cohort | TP: Site NR | Test NR | 3.16 (1.73, 5.77) | Not reported | |
| van Griensven 201352 | Thailand | ≥ 18 | Launched a new cohort in Bangkok | N=1744 Mean age = 26 |
2006–2010 | Prospective Cohort | CT: Rectal | NAAT | 2.89 (1.96, 4.26) | Not reported | |
| NG: Rectal | NAAT | 2.15 (1.29, 3.58) | |||||||||
| TP: Any site | Serology | 1.82 (1.05, 3.15) | |||||||||
| Wang 201453 | China | ≥ 18 | Launched a new cohort in Minyang | N=701 | 2009–2012 | Prospective Cohort | TP: Any site | Serology | 2.96 (1.31, 6.70) | Not adjusted | |
| Xu 201054 | China | ≥ 18 | Launched a new cohort in Shenyang | N=231 Median age = 27 |
2006–2007 | Prospective Cohort | TP: Any site | Serology | 10.06 (1.20, 84.56) | Having >5 male sexual partners in the past 12 months | |
| Xu 201355 | China | ≥ 18 | Launched a new cohort in Kunming | N=378 Median age =28 |
2001–2012 | Prospective Cohort | TP: Any site | Serology | 17.70 (3.60, 87.02) | Not reported | |
| Yang 201056 | China | ≥ 18 | Launched a new cohort in Nanjing | N=397 Age < 30: 66.2% |
2008–2009 | Prospective Cohort | TP: Any site | Serology | 2.82 (1.31, 6.07) | Not adjusted | |
| Zetola 200957 | USA | NR | Existing data from San Francisco municipal STI clinic | N=13662 | 2003–2007 | Multiple, case-control used in meta-analysis | CT, NG, or TP: Any site | Test type NR (CT, NG); serology (TP) | 2.44 (1.66, 3.57) | Number of partners within the 3 months before HIV testing, reason for HIV testing | |
| Zhao 201358 | China | ≥ 18 | Launched a new cohort in Nanyang | N=429 | 2010–2011 | Prospective Cohort | TP: Any site | Serology | 3.38 (1.13, 10.11) | Not adjusted | |
Legend: CT = Chlamydia; HR = Hazard ratio; HSV-2 = Herpes Simplex Virus type 2; IRR = Incidence rate ratio; MSM = Men who have sex with men; MSMW = Men who have sex with men and women; NAAT = Nucleic acid amplification; NG = Gonorrhea; NR = Not reported; NSGI = Non-specific genital infection; OR = Odds ratio; PrEP = Pre-exposure prophylaxis; PY = Person-years; RAI = Receptive anal intercourse; RR = Risk ratio; STI = Sexually transmitted infection; TP = Syphilis; UAI = Unprotected anal intercourse; URAI= Unprotected receptive anal intercourse
Mitchell 2017: Sample size of 228 764 reflects persons at risk; entire study evaluated records of 229 937 clinic patients.
Multiple-country study sites: Page-Shafer 1997: Australia, Canada, United States, Netherlands; Solomon 2014: Brazil, Ecuador, Peru, Thailand, United States
Page-Shafer 1997: Case-control analysis nested within data from: Amsterdam Cohort Study, San Francisco Men's Health Study, San Francisco General Hospital Cohort Study, Sydney AIDS Prospective Study, and Vancouver Lymphadenopathy-AIDS Study
Risk effect key: Bold = effect size included in meta analyses of the effect of CT, NG, or TP. Not bold = effect size included in meta-analysis of multiple pathogens, see Appendix G. Italic = effect size excluded from analyses.
Included studies were published from 1997–2017, with data collection as early as 1982 (retrospective testing of stored sera) [47]. The average age of study participants varied from 29–37 years. Half (13) of studies addressed populations in Organization for Economic Cooperation and Development (OECD) countries. The greatest numbers of studies took place in China (7), the United States (6), and Thailand (3) (Table 2). Nineteen studies reported rates of overall condom use, however only two stratified condom data across STI-diagnosed and -undiagnosed populations [33,41] Exposures to other relevant interventions were rarely reported: one study reported on participants using PrEP [50], three on the percentage of participants who were circumcised [42, 49–52], and none on ART use by participants’ partners.
Table 2.
Characteristics of included studies and effect sizes assessing the effect of bacterial STI on the risk of HIV seroconversion among MSM
| Total Studies (n=26) | Total Effect Sizes (k=60*) | |||
| Characteristics of Included Studies | n | % | k | % |
|
| ||||
| Data Collection Start Year | ||||
| 1982–1999 | 3 | 11.5% | 9 | 15.5% |
| 2000–2009 | 18 | 69.2% | 39 | 65.0% |
| 2010–2017 | 5 | 19.2% | 12 | 20.0% |
| Publication Year | ||||
| 1982–1999 | 2 | 7.7% | 3 | 5.0% |
| 2000–2009 | 1 | 3.8% | 1 | 1.7 |
| 2010–2017 | 23 | 88.5% | 56 | 93.3% |
| Geographical Distribution | ||||
| OECD Countries | ||||
| United States | 6 | 23.1% | 12 | 20.0% |
| Australia | 2 | 7.7% | 12 | 20.0% |
| United Kingdom | 2 | 7.7% | 6 | 10.0% |
| Other | 3 | 11.5% | 10 | 16.7% |
| Non-OECD Countries | ||||
| China | 7 | 26.9% | 7 | 11.7% |
| Thailand | 3 | 11.5% | 10 | 16.7% |
| Other | 2 | 7.7% | 2 | 3.3% |
| Mixed Countries | 1 | 3.8% | 1 | 1.7% |
| Study Design | ||||
| Prospective Cohort | 18 | 69.2% | 30 | 50.0% |
| Retrospective Cohort | 6 | 23.1% | 27 | 45.0% |
| Case Control | 2 | 7.7% | 3 | 5.0% |
| Reporting of Intervention Coverage (Multiple Responses Possible) | % (coverage range) | |||
| Condom Use | 19 | 73.1% (15%-81%) | 40 | 66.7% |
| STI Treatment | 10 | 38.5% (90%-100%) | 32 | 53.3% |
| Male Population Circumcised | 3 | 11.5% (10%-9.4%) | 5 | 8.3% |
| HIV-Uninfected Population on PrEP | 1 | 3.8% (50%) | 1 | 16.7% |
| Total Effect Sizes (k=60 * ) | ||||
| Characteristics of Included Effect Sizes | k | % | ||
|
| ||||
| Effect Size Type | ||||
| Hazard ratio | 26 | 43.3% | ||
| Risk ratio | 21 | 35.0% | ||
| Odds ratio | 4 | 0.7% | ||
| Percentage | 6 | 10.0% | ||
| Incidence rate ratio | 3 | 5.0% | ||
| Pathogen | ||||
| Syphilis alone | 22 | 36.7% | ||
| Gonorrhea alone | 17 | 28.3% | ||
| Chlamydia alone | 9 | 15.0% | ||
| Multiple pathogens (any of TP, NG, and/or CT) |
12 | 20.0% | ||
| STI Diagnostic Method | ||||
| Lab-confirmed (stain, culture, or serology) | 33 | 55.0% | ||
| Lab-confirmed (NAAT) | 12 | 20.0% | ||
| Medical exam/Clinical records | 13 | 21.7% | ||
| Anatomical Site | ||||
| Single | 18 | 30.0% | ||
| Multiple/unspecified | 31 | 51.6% | ||
| Multiple-specified | 11 | 18.3% | ||
| Timing of STI Assessment | ||||
| Baseline Only | 23 | 38.3% | ||
| Baseline or Follow-up | 33 | 55.0% | ||
| Follow-Up Only | 4 | 6.7% | ||
| HIV Diagnostic Procedure - Baseline | ||||
| RNA Test | 6 | 10.0% | ||
| Western Blot (WB) or p24 Test | 1 | 1.7% | ||
| 4th-Generation ELISA | 3 | 5.0% | ||
| 3rd-Generation ELISA | 15 | 25.0% | ||
| Any ELISA + WB to Confirm Positives | 3 | 5.0% | ||
| Unspecified or Mixed ELISA | 30 | 50.0% | ||
| Not Reported | 2 | 3.3% | ||
| HIV Diagnostic Procedure -Outcome | ||||
| RNA Test | 4 | 6.7% | ||
| Western Blot (WB) or p24 Test | 1 | 1.7% | ||
| 4th-Generation ELISA | 3 | 5.0% | ||
| 3rd-Generation ELISA | 15 | 25.0% | ||
| Any ELISA + WB to Confirm Positives | 10 | 16.7% | ||
| Unspecified or Mixed ELISA | 25 | 41.7% | ||
| Not Reported | 2 | 3.3% | ||
48 effect sizes were included in meta-analysis
Legend: ELISA=Enzyme-linked immunosorbent assay; HR=Hazard ratio; IRR=Incidence rate ratio; NAAT=Nucleic acid amplification test; OECD=Organisation for Economic Co-operation and Development; OR = Odds ratio; PrEP= Pre-exposure prophylaxis; RNA = Ribonucleic acid; RR=Rate ratio; STI=Sexually transmitted infection; WB = Western Blot
Eighteen effect sizes addressed STI infection assessed in a single anatomical site (rectal=13, ureteral=4, pharyngeal=1). The remaining effect sizes reflected STI assessed at any site or via serology.
Nucleic acid amplification tests were used to assess chlamydia exposures in most effect sizes (6, 66.7%) while most gonorrhea exposures (10, 58.9%) were assessed via culture or gram stain. Across pathogens, 13 (21.7%) effect sizes reflected STIs reported in medical records based on unspecified diagnostic technologies.
HIV assessment practices varied across studies and between baseline and follow-up. At baseline, more than half (30) of effect sizes used ELISA tests of unspecified or multiple generations. Only 11 effect sizes reflected baseline HIV assessment that used an RNA test (6) or other method (5) to identify early HIV infection (e.g., censoring participants who tested HIV-positive at the first interval). At follow-up, almost half of effect sizes (25) used unspecified HIV diagnostics: Four involved RNA testing of all samples at the endpoint and one incorporated Western Blot testing of all samples (Table 2).
Thirty effect sizes came from retrospective cohort and case-control studies using routine clinical data (without regularly-scheduled follow-ups). Twenty-three effect sizes reflected STI diagnosis measured only at study baseline, six reported only on incident STIs, and 31 reported on STI diagnosed at any point prior to HIV infection. Duration of scheduled follow-up intervals varied and was reported for 24 (40.0%) effect sizes; where reported, the median interval was 6 months (range 2–12). Only two effect sizes were drawn from prospective cohort studies with assessment intervals under four months and precluded possible HIV infection at baseline.
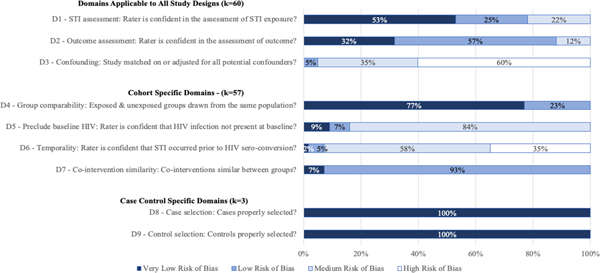
There were two case-control studies reporting three effect sizes and 24 cohort studies (18 prospective, six retrospective) reporting 57 effect sizes. Potential bias varied by risk domain (Figure 2, Appendix F).
Figure 2.
Assessment of risk of bias for effect sizes included in the meta-analysis of the effect of STI diagnosis on the risk of HIV acquisition among MSM.
For cross-design domains (D1–3), risk of bias related to STI assessment (D1) and outcome assessment (D2) was low, with 22 % and 12% of effect sizes, respectively, rated “medium” (none rated “high”). Risk for confounding (D3) was high, with only 5% rated “low” and none “very low.” Twenty-four effect sizes were adjusted, however none accounted for all of the following factors known to alter HIV risk: infection with other STIs, unprotected receptive anal intercourse, condom use, partner type, partner HIV status, and injection drug use. Only three effect sizes adjusted for at least three of these confounders. Risk related to comparability of exposed and unexposed populations (D4) was low, with all effect sizes rated “low” or “very low.” The risk of bias domain with the most undesirable score was inability to rule out undetected HIV infection at baseline (D5, 87% rated “medium”) and risk that undetected HIV infection was present at the time of STI diagnosis (D6, 92% rated “medium” or “high”). All effect sizes were rated “very low” for risk due to co-intervention similarity (D7). All three case-control studies were rated “very low” for risk due to case and control selection (D8–9).
Effects of STI on risk of HIV acquisition
Of the 60 included effect sizes, we omitted 12 that overlapped and could distort pooled estimates. The following reports results for 39 effect sizes addressing exposure to one pathogen. Appendix G reports pooled estimates for nine effect sizes reflecting exposure to mixed bacterial pathogens.
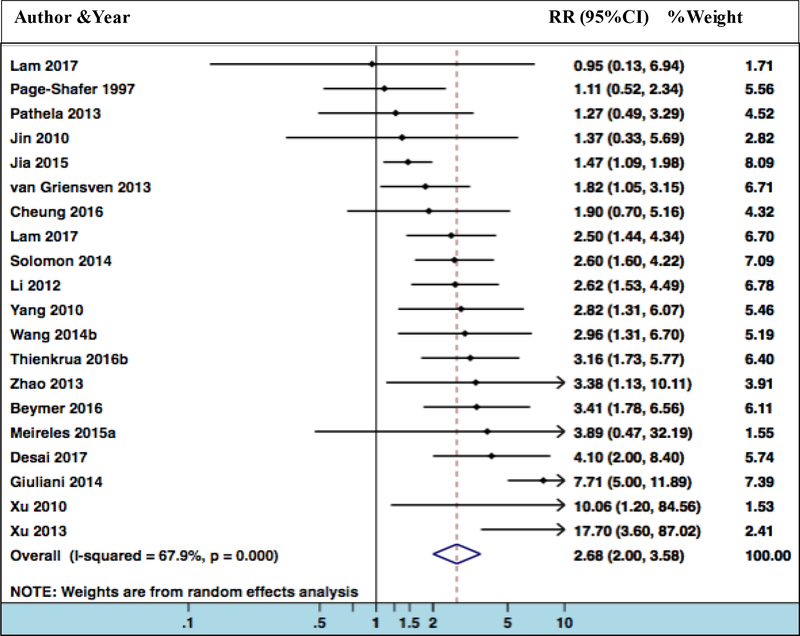
Meta-analysis suggests that syphilis more than doubles HIV acquisition risk (k=21, RR 2.68, 95% CI 2.00–3.58), although with a high degree of heterogeneity (I2=66.3%, p<0.01) (Figure 3).
Figure 3. Forest plot for risk ratios of diagnosis of syphilis and risk of HIV acquisition.
Heterogeneity chi-squared = 59.32 (d.f. = 20)
Estimate of between-study variance Tau-squared = 0.2413
Test of ES=1, z= 6.71 p = 0.000
Studies included in Model 1: Giuliani 2014, Lam 2017, Thienkrua 2016b, Xu 2010
Studies included in Model 2: Desai 2017, Giuliani 2014, Lam 2017, Thienkrua 2016b, Xu 2010
Data from Kelly 2015 was removed since it had no effect on the pooled estimate (i.e., % weight = 0) but it would have distorted the figure.
Stratified meta-analysis (Table 3) suggests that risk was similar in studies conducted in OECD-member countries (k=9, RR 2.61, 95% CI 1.44–4.74) and non-OECD countries (k=11, RR 2.52, 95% CI 1.85–3.44) (one study reported on data pooled across OECD and non-OECD countries and was not included in either of the above analyses). Stratification by risk of bias found the smallest effect estimate with higher risk due to temporality (k=10, RR 1.93, 95% CI 1.36–2.75) and the largest in the multivariate adjustment sub-group with adjusted RR (k=10, RR 3.34, 95% CI 2.11–5.28).
Table 3.
Summary of results on the effect of STI diagnosis on risk of HIV Acquisition among MSM by multivariate adjustment, geography, temporality, high quality data and combined (k=39)
| Syphilis | Gonorrhea | Chlamydia | ||||
|---|---|---|---|---|---|---|
| Geography | OECD* | Non-OECD | OECD | Non-OECD | OECD | Non-OECD |
| Pooled RR (95%) | 2.61 (1.44, 4.74) | 2.52 (1.85, 3.44) | 1.90 (1.51, 2.40) | 2.88 (1.00, 8.28) | 1.90 (1.49, 2.42) | 2.04 (1.27, 3.26) |
| I2, p value | 73.0%, p<0.001 | 50.1%, p=0.029 | 26.3%, p=0.237 | 92.5%, p<0.001 | 0.0%, p=0.459 | 66.4%, p=0.051 |
| K | 9 | 11 | 6 | 5 | 4 | 3 |
| Multivariate Adjustment | Unadjusted RR | Adjusted RR | Unadjusted RR | Adjusted RR | Unadjusted RR | Adjusted RR |
| Pooled RR (95%) | 2.10 (1.63, 2.70) | 3.34 (2.11, 5.28) | 1.66 (1.26, 2.19)† | 3.48 (1.59, 7.59) | 1.99 (1.59, 2.48) | -- |
| I2, p value | 17.2%, p=0.280 | 74.1%, p<0.001 | 37.3%, p=0.172 | 89.3%, p<0.001 | 30.9%, p=0.192 | -- |
| K | 11 | 10 | 5 | 6 | 7 | -- |
| Risk of Bias: Temporality ‡ | Less Risk | More Risk | Less Risk | More Risk | Less Risk | More Risk |
| Pooled RR (95%) | 3.33 (2.44, 4.56) | 1.93 (1.36, 2.75) | 2.58 (1.53, 4.32) | 1.81 (1.26, 2.60) | 1.78 (1.46, 2.16) | 2.89 (1.96, 4.26) |
| I2, p value | 51.0%, p=0.026 | 42.4%, p=0.075 | 87.0%, p<0.001 | 0.0%, p=0.342 | 0.0%, p=0.567 | NA |
| K | 11 | 10 | 9 | 2 | 6 | 1 |
| Risk of Bias: Testing § | Less Risk | More Risk | Less Risk | More Risk | Less Risk | More Risk |
| Pooled RR (95%) | 2.83 (2.03, 3.94) | 2.18 (1.16, 4.11) | 2.34 (1.42, 3.85) | 2.25 (1.46, 3.47) | 1.96 (1.50, 2.55) | 2.20 (1.40, 3.46) |
| I2, p value | 70.2%, p=0.000 | 51.6%, p=0.083 | 87.1%, p<0.001 | 3.7%, p=0.308 | 40.4%, p=0.136 | NA |
| K | 16 | 5 | 9 | 2 | 6 | 1 |
| High Quality Data ¶ | Model 1 | Model 2 | Model 1 | Model 2 | Model 1 | Model 2 |
| Pooled RR (95%) | 4.32 (2.20, 8.51) | 4.25 (2.51, 7.21) | 5.49 (1.11, 27.05) | 4.23 (1.66, 10.77) | -- | -- |
| I2, p value | 75.3%, p=0.007 | 67.3%, p=0.016 | 93.8%, p<0.001 | 90.0%, p<0.001 | -- | -- |
| K | 4 | 5 | 3 | 5 | -- | -- |
| Combined | Combined | Combined | Combined | |||
| Pooled RR (95%) | 2.68 (2.00, 3.58) | 2.38 (1.56, 3.61)** | 1.99 (1.59, 2.48) | |||
| I2, p value | 66.3%, p=0.000 | 84.2%, p= p<0.001 | 30.9%, p=0.192 | |||
| K | 21 | 11 | 7 | |||
| SA RR Range || | 2.39–2.83 | 1.81–2.60 | 1.78–2.13 | |||
OECD countries are members of the Organisation for Economic Co-operation and Development (OECD). Study data is drawn from the following OECD countries: Australia, Italy, Portugal, United Kingdom, United States, and a multi-country study. Study data is drawn from the following non-OECD countries: Brazil, China, Kenya, Thailand.
If Meireles 2015 (RR=0.002, CI= 0.001, 0.003) is included then the pooled estimate for unadjusted RR would be 0.52 (0.07, 4.05).
Risk of bias in temporality is defined as more risk where there was an interval of >12 months between STI exposure and HIV outcome assessments and less risk where the interval <=12 months. Incident STI exposure treated as a fixed variable is classified as higher risk of bias.
Risk of bias in testing is defined as more risk if STI exposure and/or HIV outcome were drawn from medical records and less risk if investigators reported using laboratory test for both STI and HIV.
Model 1: Data excluded if: HIV and STI assessment was based on medical records (vs. if directly confirmed by lab test), if there was no attempt to match or adjust for confounders, a case-control study design was used, and/or assessment intervals were > 12 months.
Model 2: Data excluded if: There was no attempt to match or adjust for confounders and/or assessment intervals were >12 months.
Sensitivity analysis RR range when one study removed from analysis
If Meireles 2015 (RR=0.002, CI= 0.001, 0.003) is included then the combined estimated RR would be 1.359 (0.420, 4.391).
K = Number of effect size estimates included; RR = Risk ratio; SA = Sensitivity analysis
Pooled estimates for the sub-group of higher-quality data that met the definitions of Model 1 (k=4) and Model 2 (k=5) showed that risk may increase more than four times, although with wide confidence intervals and a high degree of heterogeneity (I2> 60%). In sensitivity analysis of the overall model, removal of any one study resulted in an RR of 2.39 to 2.83.
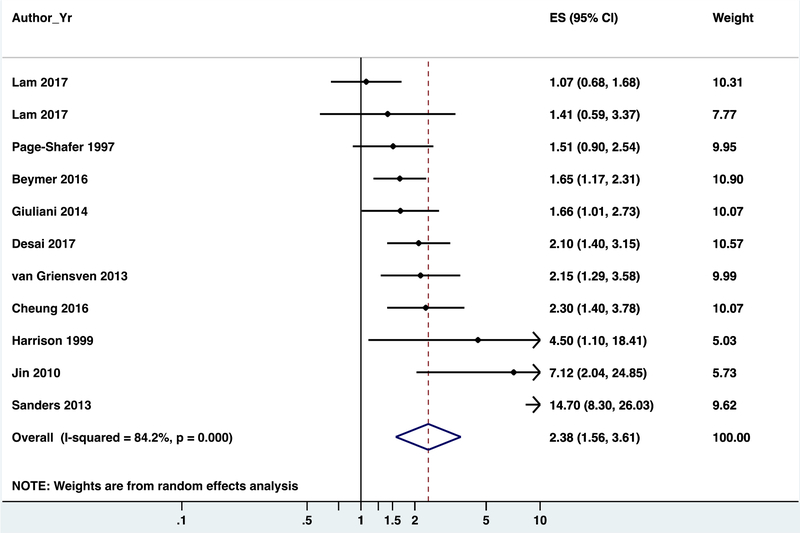
We observed a similar overall pooled estimate for the effect of gonorrhea on HIV risk (k=11, RR 2.38, 95% CI 1.56–3.61) with a higher degree of heterogeneity than syphilis (I2=84.2%, p<0.01) (Figure 4).
Figure 4. Forest plot for risk ratios of diagnosis of gonorrhea and risk of HIV acquisition among MSM.
Heterogeneity chi-squared = 59.30 (d.f. = 9)
Estimate of between-study variance Tau-squared = 0.3743
Test of ES=1: z= 3.69 p = 0.000
If Meireles 2015a (RR=0.002, CI= 0.001, 0.003) is included then the combined estimated RR would be 1.359 (0.420, 4.391).
Studies included in Model 1: Giuliani 2014, Sanders 2013
Studies included in Model 2: Desai 2017, Giuliani 2014, Harrison 1999, Sanders 2013
Estimates differed when stratified by risk of bias in temporality, with a larger estimate for the lower-risk group (k=9, RR 2.58, 95% CI 1.53–4.32) and smaller estimate for higher-risk group (k=2, RR 1.81, 95% CI, 1.26–2.60). Differences in estimates for studies conducted in OECD (k=6, RR 1.90, 95% CI 1.51–2.40) vs. non-OECD (k=5, RR 2.88, 95% CI 1.00–8.28) countries were greater for gonorrhea than other pathogens. Pooling of unadjusted effect sizes resulted in smaller effect size (k=5, RR 1.66, 95% CI 1.26–2.19) than pooling of adjusted effect sizes (k=6, RR, 3.48 95% CI 1.59–7.59). There was a greater increase in pooled RR after restricting to higher-quality data (Model 1 not statistically significant; Model 2: k=5, RR 4.23, 95% CI 1.66–10.77). Removing any one study in sensitivity analysis resulted in RR between 1.73 and 2.60 (Table 3).
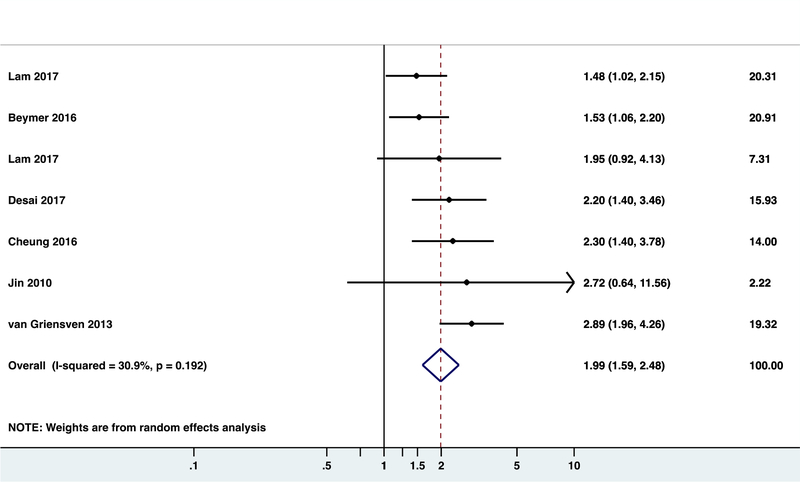
We identified fewer effect sizes for chlamydia. The pooled estimate was smaller (k=7, RR 1.99, 95% CI 1.59–2.48) with less heterogeneity (I2=30.9%, p=0.192) (Figure 5). No multivariate-adjusted data were reported. Of the seven effect sizes, six meeting criteria for lower risk of bias in temporality had a lower RR (1.78, 95% CI 1.46–2.16). Sensitivity analysis produced RR of 1.78 to 2.13 (Table 3).
Figure 5. Forest plot for risk ratios of diagnosis of chlamydia and risk of HIV acquisition among MSM.
Heterogeneity chi-squared = 8.49 (d.f. = 5)
Estimate of between-study variance Tau-squared = 0.0348
Test of ES=1 : z= 5.66 p = 0.00
Studies included in Model 1: N/A
Studies included in Model 2: N/A
We observed asymmetrical distribution of RR by the log of the standard error of RR for the effect sizes related to the crude meta-analysis for gonorrhea. Most RR clustered around the top of the figure around the pooled RR line, with only one small study at the bottom right of the plot, implying that fewer studies with small sample size reported reduction in risk of HIV acquisition due to gonorrhea. Similar plot for the RRs related to syphilis was somewhat symmetrical [Appendix H].
DISCUSSION
We provide comprehensive estimates of the increased risk of HIV acquisition among MSM diagnosed with chlamydia, gonorrhea, and syphilis. Regardless of pathogen, geography, and data stratification model, our review finds risk was substantially higher for MSM infected with each pathogen compared to those without it: approximately two times higher for chlamydia and as much as four times for syphilis and gonorrhea, based on higher-quality data.
Our results are consistent with past reviews. Two investigated syphilis as a risk factor for HIV among MSM in China, estimating RR at 3.33 (95% CI, 1.97–5.62) [18] and 3.22 (95% CI 1.96–8.21 [19]. Others included MSM in pooled estimates but did not report data specific to MSM [8, 14,16]. We did not identify studies reporting HIV transmission data for MSM.
We strengthen evidence that STIs increase the risk of HIV acquisition by addressing uncertainty about the magnitude of this risk by pathogen and ambiguity around the extent to which observed heterogeneity can be explained by methodology. We rigorously assess bias, particularly temporality, which may explain variation in the magnitude of effects for the same STI pathogen across previous reviews [8,14,16,17]. Because our review reflects data published through 2017, we present estimates in the context of advances in STI and HIV diagnosis [28,59].
A challenge to any review is the limitations of observational studies. Primary studies in this and previous reviews reported outcomes comparing participants with and without a specified STI but did not compare STI-infected participants to individuals confirmed as STI-free. This likely pulls effect sizes towards the null, resulting in underestimation of the actual effect.
Further, most primary studies did not systematically measure and/or report data on factors such as exposure to HIV infection, participants’/partners’ sexual risk behaviors, drug use, and ART, PrEP, and circumcision status. Incomplete analysis of confounding factors may underlie the similarity among our estimates across pathogens. Because HIV and STIs share risk factors, it is possible that an unaccounted risk factor was more common in STI-exposed populations than STI-unexposed populations and was the main contributor to observed estimates.
Fewer than half of studies adjusted effect sizes. Of the 16 that did, ten included condom use [38,40–43, 45,47,49,51] or other sexual risk factors [60] in multivariate models. Six other studies reported no significant association between condom use or other sexual risk factors and HIV seroconversion risk in univariate analysis [37,48,50, 54–56]. In our sub-analysis of multivariate-adjusted data on the effects of syphilis on HIV acquisition, six [37,43,45,47,51,55]of ten included effect sizes reflected condom and/or other risk data in their model and/or reported it as nonsignificant; for gonorrhea, four [37,38,47,49] of five effect sizes included in sub-analysis of multivariate-adjusted data accounted for condom use. Thus, our estimates largely account for much of the available sexual risk data although variation in how that behavioral data was reported means uncertainty persists in spite of multivariate adjustment.
We found substantial heterogeneity (I2 > 60%) in most effect sizes, including sub-analyses. To account for heterogeneity, we used random-effects models that resulted in wider 95% CIs. To optimally inform mathematical modeling and policy decisions, uncertainty around point estimates should be incorporated. We did not find multivariate-adjusted data on the effect of chlamydia on HIV acquisition. We included only one study in which some participants used PrEP because it was the only study that stratified HIV outcomes by STI diagnosis and controlled for PrEP exposure, as our protocol required. Given the efficacy of PrEP in reducing HIV acquisition61 and research and modeling that has linked PrEP uptake with an increase in unprotected sex and new STIs,62−66 better understanding of the relationship between PrEP, STI infection, and HIV acquisition is desirable.
We observed funnel plot asymmetry only for the crude meta-analysis of gonorrhea, which can be due to publication bias, heterogeneity of studies, or chance.
Finally, studies specifically designed to examine the effect of STIs on HIV acquisition have ethical and operational limitations: randomizing persons to infection or treatment for an STI is unethical, powering an observational cohort study of high-risk MSM to examine risk of HIV would be cost- and time-prohibitive. Many studies in our analysis were not designed to answer our research question, instead addressing STI diagnosis in secondary analysis or through retrospective data collection. We attempted to account for some methodologic issues in applying these measures of effect, including assessment of bias by temporality and other quality measures.
MSM infected with chlamydia, gonorrhea, and syphilis have twice or greater risk of HIV acquisition, although uncertainty exists due to data heterogeneity and risk of bias. Future studies should report the coverage for PrEP, ART, and condom use by study arms, allowing more nuanced estimates of STI on HIV risk.
Supplementary Material
Acknowledgment
We would like to extend our special gratitude to Wei Chang for her support in screening and extraction of Chinese-language studies, Devon McCabe for project management support, and authors of included studies who provided additional data not reported in their manuscripts.
Funding
The U.S. Centers for Disease Control and Prevention, National Center for HIV, Viral Hepatitis, STD, and TB Prevention Epidemiologic and Economic Modeling Agreement (NEEMA, # 5U38PS004649).
Prospero Number: CRD42018084299
Footnotes
Competing Interest
None known.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- 1.Newman L, Rowley J, Vander Hoorn S, et al. Global Estimates of the Prevalence and Incidence of Four Curable Sexually Transmitted Infections in 2012 Based on Systematic Review and Global Reporting. PLoS One 2015; 10(12): e0143304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.CDC. Sexually Transmitted Disease Surveillance 2017. Atlanta: Centers for Disease Control and Prevention, 2018. [Google Scholar]
- 3.Johnson Jones ML, Chapin-Bardales J, Bizune D, et al. Extragenital Chlamydia and Gonorrhea Among Community Venue-Attending Men Who Have Sex with Men - Five Cities, United States, 2017. MMWR Morbidity and mortality weekly report 2019; 68(14): 321–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dewart CM, Bernstein KT, DeGroote NP, Romaguera R, Turner AN. Prevalence of Rectal Chlamydial and Gonococcal Infections: A Systematic Review. Sexually transmitted diseases 2018; 45(5): 287–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan PA, Crowley C, Rose JS, et al. A Network Analysis of Sexually Transmitted Diseases and Online Hookup Sites Among Men Who Have Sex With Men. Sexually transmitted diseases 2018; 45(7): 462–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wasserheit JN. Epidemiological synergy. Interrelationships between human immunodeficiency virus infection and other sexually transmitted diseases. Sexually transmitted diseases 1992; 19(2): 61–77. [PubMed] [Google Scholar]
- 7.Rottingen JA, Cameron DW, Garnett GP. A systematic review of the epidemiologic interactions between classic sexually transmitted diseases and HIV: how much really is known? Sexually transmitted diseases 2001; 28(10): 579–97. [DOI] [PubMed] [Google Scholar]
- 8.Sexton J, Garnett G, Rottingen JA. Metaanalysis and metaregression in interpreting study variability in the impact of sexually transmitted diseases on susceptibility to HIV infection. Sexually transmitted diseases 2005; 32(6): 351–7. [DOI] [PubMed] [Google Scholar]
- 9.Freeman EE, Weiss HA, Glynn JR, Cross PL, Whitworth JA, Hayes RJ. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. AIDS (London, England) 2006; 20(1): 73–83. [DOI] [PubMed] [Google Scholar]
- 10.Bonell C, Hickson F, Beaumont M, Weatherburn P. Sexually transmitted infections as risk factors for HIV infection among MSMs: systematic review. Sexually transmitted diseases 2008; 35(2): 209. [DOI] [PubMed] [Google Scholar]
- 11.Boily MC, Baggaley RF, Wang L, et al. Heterosexual risk of HIV-1 infection per sexual act: systematic review and meta-analysis of observational studies. The Lancet Infectious diseases 2009; 9(2): 118–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mutua FM, M’Imunya J M, Wiysonge CS. Genital ulcer disease treatment for reducing sexual acquisition of HIV. The Cochrane database of systematic reviews 2012; (8): Cd007933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hilber AM, Francis SC, Chersich M, et al. Intravaginal practices, vaginal infections and HIV acquisition: systematic review and meta-analysis. PLoS One 2010; 5(2): e9119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson LF, Lewis DA. The effect of genital tract infections on HIV-1 shedding in the genital tract: a systematic review and meta-analysis. Sexually transmitted diseases 2008; 35(11): 946–59. [DOI] [PubMed] [Google Scholar]
- 15.Ward H, Ronn M. Contribution of sexually transmitted infections to the sexual transmission of HIV. Current opinion in HIV and AIDS 2010; 5(4): 305–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arora P, Nagelkerke NJ, Jha P. A systematic review and meta-analysis of risk factors for sexual transmission of HIV in India. PLoS One 2012; 7(8): e44094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Houlihan CF, Larke NL, Watson-Jones D, et al. Human papillomavirus infection and increased risk of HIV acquisition. A systematic review and meta-analysis. AIDS (London, England) 2012; 26(17): 2211–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li HM, Peng RR, Li J, et al. HIV incidence among men who have sex with men in China: a meta-analysis of published studies. PLoS One 2011; 6(8): e23431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feng Y, Bu K, Li M, Zhang X, Jin S, Wang L. [Meta-analysis of HIV infection incidence and risk factors among men who have sex with men in China]. Zhonghua liu xing bing xue za zhi = Zhonghua liuxingbingxue zazhi 2015; 36(7): 752–8. [PubMed] [Google Scholar]
- 20.Wald A, Link K. Risk of human immunodeficiency virus infection in herpes simplex virus type 2-seropositive persons: a meta-analysis. The Journal of infectious diseases 2002; 185(1): 45–52. [DOI] [PubMed] [Google Scholar]
- 21.Papp JR, Schachter J, Gaydos CA, Van Der Pol B. Recommendations for the laboratory-based detection of Chlamydia trachomatis and Neisseria gonorrhoeae−−2014. MMWR Recommendations and reports : Morbidity and mortality weekly report Recommendations and reports 2014; 63(Rr-02): 1–19. [PMC free article] [PubMed] [Google Scholar]
- 22.CDC. 2015 Sexually Transmitted Diseases Treatment Guidelines. Available: https://www.cdc.gov/std/tg2015/default.htm [accessed 12 June 2017]. CDC; 2015. [Google Scholar]
- 23.Cochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0. Available from: http://www.cochrane-handbook.org [accessed 18 September 2015]. The Cochrane Collaboration; 2011. [Google Scholar]
- 24.Malekinejad M, al. e.Risk of HIV transmission and acquisition among HIV high-risk populations infected with other sexually transmitted infections. 2018. https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=84299 (accessed November 5, 2018 2018).
- 25.PROSPERO International Prospective Register of Systematic Reviews. https://www.crd.york.ac.uk/prospero/ (accessed November 25, 2018 2018).
- 26.GRADE handbook for grading quality of evidence and strength of recommendations. Available from: http://www.guidelinedevelopment.org/handbook/ [accessed 21 Jan 2018]. GRADE Working Group; 2013. [Google Scholar]
- 27.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ (Clinical research ed) 2009; 339: b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tool to Assess Risk of Bias in Cohort Studies. http://help.magicapp.org/knowledgebase/topics/32139-grade-and-methodology-for-development-of-guideline (accessed November 25, 2018.
- 29.How to rate Risk of bias in Observational studies. http://help.magicapp.org/knowledgebase/articles/294933-how-to-rate-risk-of-bias-in-observational-studies (accessed November 25, 2018.
- 30.StataCorp. Stata Statistical Software: Release 14.2. College Station, TX: StataCorp LP; 2015 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.