Abstract
Hyperkalemia is a common electrolyte abnormality identified in the emergency department (ED) and potentially fatal. However, there is no consensus over the potassium threshold that warrants intervention or its treatment algorithm. Commonly used medications are at best temporizing measures, and the roles of binders are unclear in the emergent setting. As the prevalence of comorbid conditions altering potassium homeostasis rises, hyperkalemia becomes more common, and hence there is a need to standardize management. A panel was assembled to synthesize the available evidence and identify gaps in knowledge in hyperkalemia treatment in the ED. The panel was composed of 7 medical practitioners, including 5 physicians, a nurse, and a clinical pharmacist with collective expertise in the areas of emergency medicine, nephrology, and hospital medicine. This panel was sponsored by the American College of Emergency Physicians with a goal to create a consensus document for managing acute hyperkalemia. The panel evaluated the evidence on calcium for myocyte stabilization and potassium shifting and excretion. This article summarizes information on available therapies for hyperkalemia and proposes a hyperkalemia treatment algorithm for the ED practitioner based on the currently available literature and highlights diagnostic pitfalls and evidence gaps.
Keywords: acute management, algorithm, consensus recommendation, hyperkalemia
1. INTRODUCTION
Hyperkalemia (HK) is a potentially life‐threatening disorder occurring in 1% to 10% of hospitalized and up to 2% to 3% of emergency department (ED) patients.1 It occurs more commonly in patients with diabetes mellitus (DM), renal failure, chronic kidney disease (CKD), and heart failure (HF)2, 3 due to renal impairment or medications that alter potassium excretion.3, 4 Recent estimates show that HK contributes to more than 800,000 annual ED visits in the United States 1, 5 and its prevalence is growing because of the aging population and the growth in associated comorbidities.6
Although there is no global definition of HK because of differences in laboratory assays, the European Resuscitation Council defines mild HK as 5.5–5.9 mEq/L, moderate as 6.0–6.5 mEq/L, and severe as >6.5.7 The Kidney Disease: Improving Global Outcomes (KDIGO) group adopted a similar scale but integrated electrocardiogram (ECG) changes to categorize its severity, such that a new ECG abnormality increases the severity level, for example, mild HK and ECG changes result in moderate severity.8
Although HK has been correlated with increased risk of mortality in multiple patient populations,9, 10 the lack of prospective outcome data, management guidelines, and consensus have posed a challenge regarding its acute management in the ED.
The goal of this manuscript is to gather all the evidence‐based treatments from works published by various specialties (ie, nephrology, cardiology, critical care medicine, and emergency medicine) and have a multidisciplinary group review them to fit the needs of the EM physician. This article reviews efficacy and safety data for the commonly used agents and proposes a management algorithm tailored for the acute care physician by building on previous guidelines on HK management.
1.1. Clinical presentation and diagnosis
Patients with HK may be completely asymptomatic or may present with musculoskeletal, cardiac, or gastrointestinal (GI) disturbances. Generalized fatigue, muscle cramps, and paresthesia are common and may progress to a flaccid paralysis. Nausea, vomiting, and diarrhea can also occur. A more serious effect of HK is noted on ECGs that may lead to arrhythmias and ultimately cardiopulmonary arrest.11, 12 Because HK manifests in non‐specific symptoms, it is prudent to consider the entire clinical picture when diagnosing or treating patients.
1.2. Potassium homeostasis
The total body potassium is approximately 3000 mEq, 98% of which is stored intracellularly.13 In normal physiologic conditions, the intracellular potassium concentration is approximately 140 mEq/L whereas the extracellular concentration ranges between 3.5 and 5 mEq/L. This balance of plasma potassium is maintained by active Na‐K‐ATPase pumps and passive potassium efflux, thus setting the resting membrane potential needed for normal nerve, cardiac, and muscle function.
In healthy individuals, potassium is absorbed in the GI tract and excreted mainly by the kidneys via the principal cells in the distal tubule. Excess dietary potassium is stored in muscles and liver and is mediated by insulin and beta‐2‐adrenergic receptors. Factors that influence renal excretion include plasma potassium, aldosterone level, and sodium delivery to the distal tubules, and potassium storage (cellular uptake) is controlled by insulin, catecholamines, and mineralocorticoid. Because of the rapid uptake and excretion, plasma potassium varies less than 10% over the course of a day.14
HK occurs when excretion or storage mechanisms are disrupted or due to excessive release of potassium from cellular damage (eg, rhabdomyolysis). Table 1 lists some of the medications associated with HK.
TABLE 1.
| Amiloride | |
| Antifungals ‐ Azoles | |
| Angiotensin‐receptor blockers and angiotensin‐converting enzyme inhibitors | |
| Beta‐blockers (Carvedilol, Labetalol, Propranolol) | |
| Birth control (Yazmin 28) | |
| Cyclosporine | |
| Digoxin | |
| Heparin | |
| Herbal supplements–milkweed, lily of the valley, Siberian ginseng, Hawthorn berries | |
| Lithium | |
| Non‐steroidal anti‐inflammatories (NSAIDs) | |
| Penicillin G | |
| Pentamidine | |
| Potassium supplements | |
| Somatostatin | |
| Spironolactone | |
| Succinylcholine | |
| Tacrolimus | |
| Triamterene | |
| Trimethoprim |
1.3. ECG manifestations
HK decreases the transmembrane potassium gradient leading to increased potassium conductance and shortening the duration of the action potential. This results in ST‐segment depression, peaked T waves, and Q‐T interval shortening. As potassium levels rise, prolongation of the PR and QRS duration occurs that leads to a sinewave complex and ultimately to ventricular fibrillation or asystole.15, 16, 17 Hence it is recommended to obtain an ECG early in the evaluation of patients with HK.7, 18 Some of the common ECG changes are listed in Table 2 and categorized as repolarization and depolarization abnormalities, with the latter signifying impending arrhythmia. It is important to note, that although some have proposed that ECG changes are sequential and predictable based on serum K, in fact, the changes depend on multiple factors19, 20 (eg, concurrent electrolyte abnormalities, acid‐base status, prior cardiac injury, etc.) and may be more erratic. Therefore, relying on or expecting progressive ECG changes may be misleading and potentially dangerous.
TABLE 2.
ECG manifestations of hyperkalemia
| Common Hyperkalemia‐Related ECG Changes | |
|---|---|
| Depolarization Changes | Repolarization Changes |
|
|
|
|
|
|
|
|
|
|
Lastly, although the presence of ECG changes supports the diagnosis of HK, their absence does not negate it. Because an ECG is not a test for HK but only its cardiac manifestation, it should never be used to rule out HK.21
1.4. Diagnostic pitfalls
Not all measures of HK represent the actual potassium concentration. Because aggressive treatment of pseudo‐HK is unnecessary and potentially lethal, clinicians must distinguish true HK from pseudo‐HK and spurious HK.
Pseudo‐HK is defined as a serum potassium > 0.4 mEq/L above the plasma or whole blood potassium.22, 23 The primary mechanism of pseudo‐HK is potassium released from blood cells during in vitro coagulation. It occurs in thrombocytosis, polycythemia vera, extreme leukocytosis (as in leukemia), and hereditary spherocytosis.22, 23, 24 In the setting of large numbers of fragile cells, alternative analytic strategies should be considered in consultation with hematologists and nephrologists to identify the accurate potassium value.
On the other hand, spurious HK diagnoses may result from mistakes of sampling, transport, and storage of blood.25 Commonly the result of hemolysis, repeated measurements usually result in the accurate value and thus the error is easily identified.24 In contrast to spurious HK, pseudo‐HK depends on blood cell composition and is not related to collection method errors. This distinction is important because in pseudo‐HK a repeat measurement will report a consistently erroneous potassium value.
1.5. Treatment
The current treatment of HK in the ED varies considerably between practitioners because the available pharmacologic agents have limited data supporting their efficacy.18, 26, 27 Furthermore, there are no patient outcome data with any of the medications discussed in this section and hence the optimal time for intervention and the extent of benefit are not fully understood. In general, acute management recommendations involve a threefold approach (Figure 1): (1) stabilization of the cardiac membranes, (2) redistribution of potassium, and (3) elimination of potassium.
FIGURE 1.
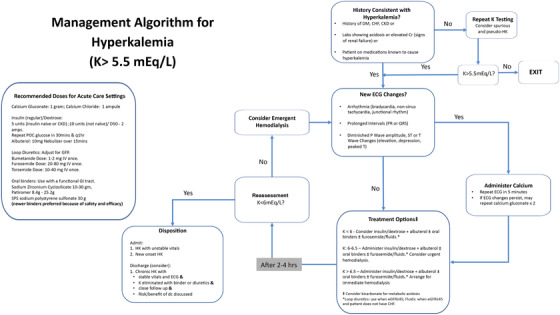
Acute management algorithm for hyperkalemia. Adapted from Rafique Z, et al. Current treatment and unmet needs of hyperkalemia in the emergency department. Eur Heart J Suppl. 2019;21(Suppl A):A12‐A19. Abbreviations: CHF, congestive heart failure; CKD, chronic kidney disease; Cr, creatinine; dc, discharge; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; GI, gastrointestinal; HK, hyperkalemia; IV, intravenous; K, potassium; POC, point of care; SPS, sodium polystyrene sulfonate
Multiple potassium lowering agents are available, and they can be used individually or in combination based on severity of HK or institutional protocol.
1.6. Stabilization
1.6.1. Calcium
The effect of potassium on myocytes is counterbalanced by the concurrent calcium concentration. It improves or totally reverses HK‐related ECG changes (Table 2), arrhythmias, or cardiac arrest.7, 28, 29 The initial dose may be repeated if there is no effect within 5–10 minutes. Some adverse effects of intravenous calcium are peripheral vasodilation, hypotension, bradycardia, and arrhythmias.30 A more serious adverse effect of intravenous calcium is tissue necrosis if extravasation occurs. This can be avoided if calcium gluconate instead of calcium chloride is used. Historically, calcium administration was contraindicated in the setting of digoxin toxicity, but this is not supported by current literature.31, 32
1.7. Redistribution
1.7.1. Insulin/Dextrose
Insulin lowers serum potassium by activating sodium‐potassium ATPase (Na‐K ATPase) and by moving sodium out of the cell in exchange for potassium into the cell. Potassium can start to decrease within 15 minutes of administration and its effect may last several hours.28, 33, 34
The main risk of insulin‐glucose therapy is hypoglycemia, with the incidence of hypoglycemia ranging from 11% to 75%.35, 36 Patients with kidney failure may experience hypoglycemia up to 6 hours after treatment and glucose monitoring is recommended for several hours after insulin administration.37 Dextrose (glucose) is administered with insulin to prevent hypoglycemia and subsequent doses may be warranted if hypoglycemia persists.
1.7.2. Beta 2 agonists
These promote the translocation of potassium into the cell by activation of the Na‐K ATPase pump, and are equally effective given intravenously or via a nebulizer.38 Nebulized albuterol works within 30 minutes with a peak effect at 60 minutes, decreases serum potassium by approximately 1 mEq/L and its effects last for at least 2 hours.34, 35, 39 Small clinical trials of intravenous versus nebulized beta‐2 agonists for treatment of HK show similar efficacy. However, because trials with intravenous salbutamol are even smaller than their nebulized counterparts, dosing and safety profile of the intravenous formulation are not established, and thus the nebulized form is preferred.
Albuterol may be ineffective in some patients, and the mechanism of resistance is not understood. Common side effects are tremor, palpitations, headache, and mild hyperglycemia, which may alleviate insulin‐induced hypoglycemia when used simultaneously.39, 40
1.7.3. Sodium bicarbonate
Sodium bicarbonate promotes uptake of potassium into skeletal muscle by increasing intracellular sodium via sodium‐bicarbonate cotransport and sodium‐hydrogen exchange and in turn increases Na‐K ATPase activity.41 However, recent studies do not support its use in reducing potassium in the absence of metabolic acidosis.28, 42, 43 Intravenous bicarbonate has been studied against other comparators in 2o studies. Ngugi et al.44 showed that bicarbonate reduced serum potassium by 0.47 (±0.31) mEq/L at 30 minutes but was less effective than insulin or albuterol. However, in the placebo‐controlled trial by Allon et al.45, bicarbonate treatment did not lower potassium compared to placebo in the first 60 minutes. Multiple other trials have studied bicarbonate infusion; however, it was combined with other potassium lowering agents and thus its effect has been difficult to elucidate. Currently, there is insufficient evidence to support the use of intravenous sodium bicarbonate for the acute treatment of HK, and it should be used with caution because it can cause sodium and fluid overload.28
1.8. Elimination
1.8.1. Loop diuretics
Loop diuretics (furosemide, bumetanide, and torsemide) inhibit the luminal Na‐K‐2Cl cotransporter by binding to the chloride‐binding site of the thick ascending limb of the loop of Henle and macula densa. They work to lower serum potassium by increasing distal sodium delivery and flow rate to promote potassium secretion into the lumen and subsequent removal. This effect is enhanced with administration of saline solutions. Although loop diuretics are effective at promoting potassium excretion, evidence for use of loop diuretics in the acute management of HK is lacking. These agents may be considered for use as adjuncts in a hyperkalemic emergency.
Adverse effects include ototoxicity, hypotension (hypovolemia), electrolyte imbalances (hypokalemia, hyponatremia), hyperuricema, and hypersensitivity reactions (furosemide, bumetanide, and torsemide are sulfonamide‐containing agents).46
1.8.2. Hemodialysis
Hemodialysis is a modality where blood and a balanced salt solution (dialysate) are perfused to opposite sides of the semipermeable membrane. This process is highly effective at lowering serum as well as total body potassium. As potassium drops with dialysis there is movement of potassium from the intracellular to the extracellular space with the most efficient removal occurring during the first couple of hours of the procedure. Hemodialysis is an option for acute management of HK, but usually reserved for individuals with end‐stage renal disease who have established vascular access.47 In life‐threatening HK, hemodialysis is the best option to reduce potassium effectively, and thus vascular access needs to be established emergently in patients who do not already have one.
Acute complications of hemodialysis include hypotension, cramping, chest pain, back pain, arrhythmias, headache, nausea, and vomiting, many of which are associated with fluctuations in volume and electrolyte concentrations.48
1.8.3. Oral binders
Though routinely used in the management of HK in the emergency setting, oral potassium binders have yet to be approved for the emergency treatment of life‐threatening HK. The newer binders appear to be safe and current investigations are underway to determine their effectiveness on lowering potassium in the acute setting and the subsequent impact on patient outcomes. Currently available binders are sodium polystyrene sulfonate, patiromer, and sodium zirconium cyclosilicate.
Sodium Polystyrene Sulfonate (SPS): SPS is a sulfonated polymer that exchanges sodium for potassium in the GI tract and may be administered orally or per rectum. Doses of 15 g orally administered up to 4 times daily or 30 grams rectally (up to 60 g daily) are Food and Drug Administration (FDA)‐approved doses. Scherr et al.49 reported the first interventional trial using SPS. Thirty‐two patients with HK were enrolled in a single‐arm study and the mean potassium was lowered by 1.0 mEq/L in 23 patients in the first 24 hours. However, there was no placebo arm to measure its efficacy. The best evidence for SPS is reported by Lepage et al.50 in a double‐blind randomized‐control trial. CKD patients with mild HK were randomized to SPS (n = 16) or control (n = 17) groups and given 30 g of SPS or placebo respectively, once daily for 7 days. SPS was found to lower potassium by 1.0 mEq/L in 7 days. Two other randomized studies evaluated SPS against calcium polystyrene sulfonate; however, once again there was no placebo‐control arm to establish efficacy.28, 49, 50, 51, 52 Considering the paucity of evidence, SPS is not recommended in the acute setting.53
Adverse reactions include gastrointestinal symptoms and electrolyte imbalance (eg, hypokalemia). Like other oral binders, SPS can bind to some oral medications and decrease their absorption. Caution is advised around the timing of concurrent administration of other oral medications.54 Uncommon, but serious gastrointestinal complications such as colonic ulcer, ischemia, and intestinal necrosis have been documented with the use of SPS,55 with an incidence between 16 and 23 per 1000 person‐years.56, 57 These risks should be considered when including SPS in the treatment of acute HKHK.
Patiromer: Patiromer is a cation exchange resin that exchanges calcium for potassium and has been shown to reduce recurrent hyperkalemia for patients on renin‐angiotensin‐aldosterone system inhibitor therapy.58 The drug has a reported onset of action of approximately 2 to 7 hours.59, 60 Though a small pilot study showed a trend to reduced potassium within 2 hours, its effectiveness for the acute setting has yet to be established. A larger study is underway to establish its role in the acute management of HK (ClinicalTrials.gov, NCT04443608).
The initial recommended dose of patiromer is 8.4 g/day with titrations made in increments of 8.4 grams at 1‐week intervals for outpatient treatment of HK (maximum dose 25.2 g/day). Patiromer is generally well tolerated, both acutely and chronically.58 Adverse effects include gastrointestinal symptoms and hypomagnesemia.61 Lastly, patiromer may interact with certain positively charged drugs (eg, ciprofloxacin, levothyroxine, and metformin) and reduce their bioavailability, and therefore, is recommended to be administered at least 3 hours apart from other oral medications.61
Sodium Zirconium Cyclosilicate (SZC): SZC is an inorganic zirconium silicate compound that selectively exchanges potassium for sodium and hydrogen in the intestine. The efficacy of SZC in the reduction of potassium has been demonstrated in > 1700 patients.62, 63 A 10 mg dose of SZC reduced potassium by 0.11 mEq/L within 1 hour and by 0.73 mEq/L by 48 hours compared to placebo. Reduction in serum potassium was dose dependent, and patients with higher baseline potassium experienced greater reduction.64, 65 A prospective ED study comparing SZC to placebo identified no safety concerns but was unable to demonstrate effectiveness.66
The starting dose of SZC is 10 grams 3 times daily for up to 48 hours with doses adjusted by 5 grams daily at 1‐week intervals based on serum potassium levels (maximum dose is 15 grams per day). Adverse symptoms include edema, GIl symptoms, and hypokalemia. SZC also interacts with dabigatran (decreasing bioavailability) and atorvastatin and furosemide (increasing bioavailability). Recommendations are to separate administration from other oral medications by at least 2 hours.67
2. MANAGEMENT ALGORITHM AND DISPOSITION (FIGURE 1)
Patients with K > 5.5 mEq/L and presenting to the ED with acute illness are eligible to enter the algorithm.
Step 1: Because pseudo‐HK and spurious HK lead to falsely elevated potassium levels, and many EDs use point‐of‐care blood testing, which cannot detect hemolysis, it is important to verify the potassium level before starting treatment. Hence, the first step in this algorithm is to assess pretest probability of HK by reviewing medical history (DM, congestive HF, CKD), current medications (Table 1) and laboratory abnormalities; note that an isolated K elevation may signify a spurious result. If the history does not fit with laboratory findings, consider retesting from a fresh blood draw.
Step 2: The next step is to evaluate cardiac involvement. Table 2 shows the common ECG changes seen with HK. If new changes are found, administer calcium gluconate intravenous 1 g as per the algorithm (Figure 1), and repeat as needed. Calcium gluconate is preferred because calcium chloride carries the risk of tissue necrosis in case of extravasation. However, in the hemodynamically unstable patient, calcium chloride may be preferred because it carries 3 times the amount of elemental calcium than that of the gluconate formulation.
Step 3: Treatment options are based on K levels and categorized into redistribution and elimination. Hemodialysis is the most effective way to eliminate K; however, it may not always be indicated or readily available. While waiting on a definitive treatment plan for HK management or arranging for hemodialysis, medical management is an appropriate option. For redistribution of potassium, intravenous insulin/dextrose, and nebulized albuterol are a mainstay in HK management. Based on a recent Cochrane review, bicarbonate is not indicated in the acute treatment of HK anymore but can still be used in cases of acidosis.28 As for elimination of potassium, oral binders may be helpful in sequestering potassium in the GI tract and have an acceptable safety profile. However, their efficacy in the acute setting (ED or in‐patient) is limited and these binders should be used only in patients with a functional GI tract (ie, patients with bowel obstruction or postoperative ileus are not good candidates54, 61, 67). Newer binders are preferred over SPS because of their safety profile. Lastly, diuretics have been commonly used to treat HK but there are no studies to show their efficacy or onset of action in the acute setting. Hence, they are not recommended to be used as the sole agent.
Step 4: Reassessment of HK is indicated every 2 to 4 hours because most temporizing agents manifest their maximal effect within 2 hours and are likely wearing off by 4 hours. At this point if K > 6 mEq/L, consider redosing with medications (Step 2) and arranging hemodialysis. If K < 6 mEq/L, consider proceeding to the disposition step.
Disposition: Patients should be frequently assessed for abnormal vital signs, rebound HK, and side effects of medications administered. Hemodynamic instability, persistent new ECG abnormality, and new onset HK should be admitted for further evaluation. Admission should also be considered if HK is recalcitrant to acute treatment or in whom potassium was not eliminated (ie, excreted or dialyzed) but only redistributed. Yet for others, discharge should be considered when all of the following apply: (1) the patient has chronic HK, and a cause for the current exacerbation has been identified and rectified; (2) the patient has stable vital signs and feels well to go home; (3) the patient will have close follow‐up, ideally within 24 to 48 hours; and (4) the risks and benefits of discharge have been discussed with the patient.
2.1. Future research
Our knowledge of HK is fragmented and there are more gaps than knowledge at this time. First, we need to agree on standardized definitions for severity categories to allow for treatment and data gathering standardization. Second, we need to further understand the role of ECG in HK treatment based on patient outcomes. Third, current therapeutic agents (except for binders) have limited data on efficacy and safety and are not approved by the FDA for HK management. Hence, it is essential to study the commonly used agents for their efficacy, onset of action, dosing interval, adverse effects, and most important, patient outcomes. Lastly, there is a need to standardize care so that we can evaluate the efficacy and safety of treatment algorithms.
3. CONCLUSION
HK is a common electrolyte abnormality, and its clinical manifestation may be non‐specific. Medications used in the acute setting are limited to shifting potassium and lack robust safety and efficacy data. Moreover, the lack of guidelines has led to much variability in HK treatment.
This consensus document proposes a HK management and patient disposition protocol for the acute setting, incorporating the best available evidence. A standardized protocol is a significant leap forward on the path to understanding the efficacy and safety of commonly used agents, and this document presents one such protocol. Further clinical studies are needed to address knowledge gaps and to provide much needed patient outcomes data for the treatment of HK in the acute setting.
AUTHOR CONTRIBUTIONS
ZR and JN: design, drafting of manuscript, critical revision, and submission. FP, TA, JJVV, JH, MRW: drafting of manuscript and critical revision.
CONFLICT OF INTEREST
This manuscript is a consensus document of an expert panel on hyperkalemia management, sponsored by the American College of Emergency Physicians.
ZR is a principal investigator for Relypsa Inc and a consultant to Relypsa Inc and AstraZeneca.
FP has received consulting fees from AstraZeneca and Relypsa and grants to his institution from AstraZeneca and Relypsa.
TA has nothing to disclose.
JB reports personal fees from American College of Emergency Physicians, during the conduct of the study; grants from Beckman Coulter, grants from Comprehensive Research Associates, outside the submitted work;
JD reports: I have presented continuing education sessions within the last year on the topic of hyperkalemia supported by educational grants from AstraZeneca. These programs were coordinated through WebMD.
MW reports personal fees from Vifor, personal fees from AstraZeneca, personal fees from Boehringer‐Ingelheim, personal fees from Bayer, personal fees from Janssen, during the conduct of the study;
JN reports a grant for his institution from Relypsa and consulting fees and honoraria from AstraZeneca.
Rafique Z, Peacock F, Armstead T, et al. Hyperkalemia management in the emergency department: An expert panel consensus. JACEP Open. 2021;2:e12572. 10.1002/emp2.12572
Supervising Editor: Valerie De Maio, MD, MSc
Funding and support: By JACEP Open policy, all authors are required to disclose any and all commercial, financial, and other relationships in any way related to the subject of this article as per ICMJE conflict of interest guidelines (see www.icmje.org). The authors have stated that no such relationships exist.
REFERENCES
- 1.Singer AJ, Thode HC Jr, Peacock WF. A retrospective study of emergency department potassium disturbances: severity, treatment, and outcomes. Clin Exp Emerg Med. 2017.Published online 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Collins AJ, Pitt B, Reaven N, et al. Association of serum potassium with all‐cause mortality in patients with and without heart failure, chronic kidney disease, and/or diabetes. Am J Nephrol. 2017.Published online 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bandak G, Sang Y, Gasparini A, et al. Hyperkalemia after initiating renin‐angiotensin system blockade: the Stockholm Creatinine Measurements (SCREAM) project. J Am Heart Assoc. 2017.Published online 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang AR, Sang Y, Leddy J, et al. Antihypertensive medications and the prevalence of hyperkalemia in a large health system. Hypertension. 2016.Published online 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aggarwal S, Topaloglu H, Kumar S. Trends in emergency room visits due to hyperkalemia in the United States. Value Health. 2015. Published online 2015. [Google Scholar]
- 6.Bowe B, Xie Y, Li T, et al. Changes in the US burden of chronic kidney disease from 2002 to 2016: an analysis of the global burden of disease study. JAMA network open. 2018. Published online 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Truhlář A, Deakin CD, Soar J, et al. European resuscitation council guidelines for resuscitation 2015. Resuscitation. 2015;95:148‐201. Section 4. Cardiac arrest in special circumstances. [DOI] [PubMed] [Google Scholar]
- 8.Lindner G, Burdmann EA, Clase CM, et al. Acute hyperkalemia in the emergency department. Eur J Emerg Med. 2020. Published online 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khanagavi J, Gupta T, Aronow WS, et al. Hyperkalemia among hospitalized patients and association between duration of hyperkalemia and outcomes. Arch Med Sci. 2014. Published online 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.An JN, Lee JP, Jeon HJ, et al. Severe hyperkalemia requiring hospitalization: predictors of mortality. Critic Care. 2012;16(6):R225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alfonzo AVM, Isles C, Geddes C, Deighan C. Potassium disorders‐clinical spectrum and emergency management. Resuscitation. Published online 2006. [DOI] [PubMed] [Google Scholar]
- 12.Medford‐Davis L, Rafique Z. Derangements of potassium. Emerg Med Clin North Am. 2014;32(2). [DOI] [PubMed] [Google Scholar]
- 13.Boddy K, King PC, Hume R, Weyers E. The relation of total body potassium to height, weight, and age in normal adults. J clin Path. 1972;25:512‐517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Physiology MC. Physiology of the Circadian Timing System: Predictive versus Reactive Homeostasis. [DOI] [PubMed] [Google Scholar]
- 15.Parham WA, Mehdirad AA, Biermann KM, Fredman CS. Hyperkalemia revisited. Tex Heart Inst J. 2006. Published online 2006. [PMC free article] [PubMed] [Google Scholar]
- 16.Dittrich KL, Walls RM. Hyperkalemia: eCG manifestations and clinical considerations. J Emerg Med. 1986. Published online 1986. [DOI] [PubMed] [Google Scholar]
- 17.Ettinger PO, Regan TJ, Oldewurtel HA. Hyperkalemia, cardiac conduction, and the electrocardiogram: a review. Am Heart J. 1974. Published online 1974. [DOI] [PubMed] [Google Scholar]
- 18.Alfonzo A, Macrury MM. Renal Association Clinical Practice Guidelines‐Treatment of Acute Hyperkalaemia in Adults. 2020. [Google Scholar]
- 19.Ahmed R, Yano K, Mitsuoka T, Xkeda S, Ichimaru M, Hashiba K. Changes in T Wave Morphology During Hypercalcemia and Its Relation to the Severity of Hypercalcemia. 1987. [DOI] [PubMed] [Google Scholar]
- 20.Surnwicz B. Fundamentals of clinical cardiology relationship between electrocardiogram and electrolytes. 1967. [DOI] [PubMed] [Google Scholar]
- 21.Rafique Z, Aceves J, Espina I, Peacock F, Sheikh‐Hamad D, Kuo D. Can physicians detect hyperkalemia based on the electrocardiogram?. Am J Emerg Med. 2020;38(1). [DOI] [PubMed] [Google Scholar]
- 22.Lábadi Á, Nagy Á, Szomor Á, Miseta A, Kovács GL. Factitious hyperkalemia in hematologic disorders. Scand J Clin Lab Invest. 2017. Published online 2017. [DOI] [PubMed] [Google Scholar]
- 23.Sevastos N, Theodossiades G, Archimandritis AJ. Pseudohyperkalemia in serum: a new insight into an old phenomenon. Clin Med Res. 2008. Published online 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stankovic AK, Smith S. Elevated serum potassium values: the role of preanalytic variables. Am J Clin Pathol. 2004. Published online 2004. [DOI] [PubMed] [Google Scholar]
- 25.Davis KR, Crook MA. Seasonal factitious increase in serum potassium: still a problem and should be recognised. Clin Biochem. Published online 2014. [DOI] [PubMed] [Google Scholar]
- 26.Weisberg LS. Management of severe hyperkalemia. Crit Care Med. 2008. Published online 2008. [DOI] [PubMed] [Google Scholar]
- 27.Acker CG, Johnson JP, Palevsky PM, Greenberg A. Hyperkalemia in hospitalized patients: causes, adequacy of treatment, and results of an attempt to improve physician compliance with published therapy guidelines. Arch Intern Med. 1998. Published online 1998. [DOI] [PubMed] [Google Scholar]
- 28.Mahoney BA, Smith WAD, Lo D, Tsoi K, Tonelli M, Clase C. Emergency interventions for hyperkalaemia [Cochrane Database of Systematic Reviews]. Cochrane Database Syst Rev. 2016. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoffman BF, Suckling EE. Effect of several cations on transmembrane potentials of cardiac muscle. Am J Physiol. 1956. Published online 1956. [DOI] [PubMed] [Google Scholar]
- 30.Calcium Gluconate Package Insert . 2008;(October):45793‐45793.
- 31.Van Deusen SK, Birkhahn RH, Gaeta TJ. Treatment of hyperkalemia in a patient with unrecognized digitalis toxicity. J Toxicol Clin Toxicol. 2003. Published online 2003. [DOI] [PubMed] [Google Scholar]
- 32.Levine M, Nikkanen H, Pallin DJ. The effects of intravenous calcium in patients with digoxin toxicity. J Emerg Med. 2011. Published online 2011. [DOI] [PubMed] [Google Scholar]
- 33.Ahee P, Crowe AV. The management of hyperkalaemia in the emergency department. J Accid Emerg Med. 2000. Published online 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lens XM, Montoliu J, Cases A, Campistol JM, Revert L. Treatment of hyperkalaemia in renal failure: salbutamol v. Insulin. Nephrol Dial Transplant. 1989. Published online 1989. [DOI] [PubMed] [Google Scholar]
- 35.Allon M, Copkney C. Albuterol and insulin for treatment of hyperkalemia in hemodialysis patients. Kidney Int. 1990. Published online 1990. [DOI] [PubMed] [Google Scholar]
- 36.Rafique Z, Chouihed T, Mebazaa A. Frank Peacock W. Current treatment and unmet needs of hyperkalaemia in the emergency department. Eur Heart J Suppl. 2019;21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pierce DA, Russell G, Pirkle JL. Incidence of Hypoglycemia in patients with low eGFR treated with insulin and dextrose for hyperkalemia. Ann Pharmacother. 2015. Published online 2015. [DOI] [PubMed] [Google Scholar]
- 38.R M, V P, Brocklebank J. Treatment of hyperkalaemia using intravenous and nebulised salbutamol. Arch Dis Child. 1994. Published online 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Allon M, Dunlay R, Copkney C. Nebulized albuterol for acute hyperkalemia in patients on hemodialysis. Ann Intern Med. 1989. Published online 1989. [DOI] [PubMed] [Google Scholar]
- 40.Mandelberg A, Krupnik Z, Houri S, et al. Salbutamol metered‐dose inhaler with spacer for hyperkalemia: how fast? How safe?. Chest. 1999. Published online 1999. [DOI] [PubMed] [Google Scholar]
- 41.Palmer BF. Regulation of potassium homeostasis. Clin J Am Soc Nephrol. 2014. Published online 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gutierrez R, Schlessinger F, Oster JR, Rietberg B, Perez GO. Effect of hypertonic versus isotonic sodium bicarbonate on plasma potassium concentration in patients with end‐stage renal disease. Miner Electrolyte Metab. 1991. Published online 1991. [PubMed] [Google Scholar]
- 43.Greenberg A. Hyperkalemia: treatment options. Semin Nephrol. 1988. Published online 1998. [PubMed] [Google Scholar]
- 44.Ngugi NN, McLigeyo SO, Kayima JK. Treatment of hyperkalaemia by altering the transcellular gradient in patients with renal failure: effect of various therapeutic approaches. East Afr Med J. 1997. Published online 1997. [PubMed] [Google Scholar]
- 45.Allon M, Shanklin N. Effect of bicarbonate administration on plasma potassium in dialysis patients: interactions with insulin and albuterol. Am J Kidney Dis. 1996. Published online 1996. [DOI] [PubMed] [Google Scholar]
- 46.Loop Diuretics: Dosing and Major Side Effects ‐ UpToDate. Accessed January 28, 2021. https://www.uptodate.com/contents/loop‐diuretics‐dosing‐and‐major‐side‐effects
- 47.Palmer BF, Clegg DJ. Hyperkalemia across the continuum of kidney function. Clin J Am Soc Nephrol. 2018;13(1):155‐157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sherman RA, Daugirdas JT, Ing TS. Handbook of Dialysis: Fifth Edition. 2014. [Google Scholar]
- 49.Scherr L, Ogden DA, Mead AW, Spritz N, Rubin AL. Management of hyperkalemia with a cation‐exchange resin. N Engl J Med. 1961. Published online 1961. [DOI] [PubMed] [Google Scholar]
- 50.Lepage L, Dufour AC, Doiron J, et al. Randomized clinical trial of sodium polystyrene sulfonate for the treatment of mild hyperkalemia in CKD. Clin J Am Soc Nephrol. 2015. Published online 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nasir K, Ahmad A. Treatment of hyperkalemia in patients with chronic kidney disease: a comparison of calcium polystyrene sulphonate and sodium polystyrene sulphonate. J Ayub Med Coll Abbottabad. 2014. Published online. [PubMed] [Google Scholar]
- 52.Nakayama Y, Ueda •Kaoru Sho‐Ichi, Yamagishi •, et al. Compared effects of calcium and sodium polystyrene sulfonate on mineral and bone metabolism and volume overload in pre‐dialysis patients with hyperkalemia. Clin Exp Nephrol;22. [DOI] [PubMed] [Google Scholar]
- 53.Mahoney BA, Smith WA, Lo D, Tsoi K, Tonelli M, Clase C. Emergency interventions for hyperkalaemia. Cochrane Database Syst Rev. 2005. Published online 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kayexalate ® SODIUM POLYSTYRENE SULFONATE Package Insert.
- 55.Harel Z, Harel S, Shah PS, Wald R, Perl J, Bell CM. Gastrointestinal adverse events with sodium polystyrene sulfonate (Kayexalate) use: a systematic review. Am J Med. 2013. Published online 2013. [DOI] [PubMed] [Google Scholar]
- 56.Laureati P, Xu Y, Trevisan M, et al. Initiation of sodium polystyrene sulphonate and the risk of gastrointestinal adverse events in advanced chronic kidney disease: a nationwide study. Nephrol Dial Transplant 2020;35(9):1518‐1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Noel JA, Bota SE, Petrcich W, et al. Risk of hospitalization for serious adverse gastrointestinal events associated with sodium polystyrene sulfonate use in patients of advanced age supplemental content. JAMA Intern Med. 2019;179(8):1025‐1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Montaperto AG, Gandhi MA, Gashlin LZ, Symoniak MR. Patiromer: a clinical review. Curr Med Res Opin. 2016.Published online 2016. [DOI] [PubMed] [Google Scholar]
- 59.Rafique Z, Liu M, Staggers KA, Minard CG, Peacock WF. Patiromer for treatment of hyperkalemia in the emergency department: a pilot study. Acad Emerg Med. 2020;27(1). [DOI] [PubMed] [Google Scholar]
- 60.Bushinsky DA, Williams GH, Pitt B, et al. Patiromer induces rapid and sustained potassium lowering in patients with chronic kidney dkalemia. Kidney Int. 2015. Published online 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Veltassa Package Insert. Published online 2018.
- 62.Ash SR, Singh B, Lavin PT, Stavros F, Rasmussen HS. A phase 2 study on the treatment of hyperkalemia in patients with chronic kidney disease suggests that the selective potassium trap, ZS‐9, is safe and efficient. Kidney Int. 2015.Published online 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Amin AN, Menoyo J, Singh B, Kim CS. Efficacy and safety of sodium zirconium cyclosilicate in patients with baseline serum potassium level ≥ 5.5 mmol/L: pooled analysis from two phase 3 trials. BMC Nephrol. 2019;20(1):440. Published online 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Packham DK, Rasmussen HS, Lavin PT, et al. Sodium Zirconium Cyclosilicate in Hyperkalemia. N Engl J Med. Published online 2015. [DOI] [PubMed] [Google Scholar]
- 65.Kosiborod M, Rasmussen HS, Lavin P, et al. Effect of sodium zirconium cyclosilicate on potassium lowering for 28 days among outpatients with hyperkalemia: the HARMONIZE randomized clinical trial. J Am Med Assoc. Published online 2014. [DOI] [PubMed] [Google Scholar]
- 66.Peacock WF, Rafique Z, Vishnevskiy K, et al. Emergency potassium normalization treatment including sodium zirconium cyclosilicate: a phase II, randomized, double‐blind, placebo‐controlled study (ENERGIZE). Acad Emerg Med. 2020;27(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sodium Zirconium Cyclosilicate Package Insert. Accessed January 27, 2021. www.fda.gov/medwatch [Google Scholar]
- 68.Acker CG, Johnson JP, Palevsky PM, Greenberg A. Hyperkalemia in Hospitalized Patients Causes, Adequacy of Treatment, and Results of an Attempt to Improve Physician Compliance With Published Therapy Guidelines. https://jamanetwork.com/ [DOI] [PubMed] [Google Scholar]
- 69.Shingarev R, Allon M. A Physiologic‐Based Approach to the Treatment of Acute Hyperkalemia. Published online 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hollander‐Rodriguez JC, Calvert JF. Hyperkalemia. Am Fam Physician. 2006;73(2):283‐290. [PubMed] [Google Scholar]


