Abstract
Fine particulate matter <2.5 μm (PM2.5) air pollution is implicated in global mortality, especially from cardiovascular causes. A large body of evidence suggests a link between PM2.5 and elevation in blood pressure (BP), with the latter implicated as a potential mediator of cardiovascular events. We sought to determine if the outcomes of intensive BP lowering (systolic BP < 120 mm Hg) on cardiovascular events are modified by PM2.5 exposure in in the Systolic Blood Pressure Intervention Trial (SPRINT). We linked annual PM2.5 exposure estimates derived from an integrated model to subjects participating in SPRINT. We evaluated the effect of intensive BP-lowering by PM2.5 exposure on the primary outcome in SPRINT using cox-proportional hazard models. A total of 9286 participants were linked to PM2.5 levels (mean age 68±9 years). Intensive BP-lowering decreased risk of the primary outcome more among patients exposed to higher PM2.5 (Pinteraction=0.047). The estimate for lowering of primary outcome was numerically lower in highest than in the lower quintiles. The benefits of intensive BP-lowering were larger among patients chronically exposed to PM2.5 levels above U.S. National Ambient Air Quality Standards of 12 μg/m3 (HR 0.47, 95% CI: 0.29–0.74) compared to those living in cleaner locations (HR 0.81, 95% CI: 0.68–0.97), Pinteraction=0.037. This exploratory non-prespecified post-hoc analysis of SPRINT suggests that the benefits of intensive BP lowering on the primary outcome was greater in patients exposed to higher PM2.5, suggesting that the magnitude of benefit may depend upon the magnitude of antecedent PM2.5 exposure.
Keywords: Air pollution, particulate matter, hypertension, cardiovascular disease
Summary:
Air pollution has been implicated in cardiovascular risk, partly through effects on blood pressure. We sought to determine if intensive blood pressure lowering (systolic blood pressure < 120 mm Hg) on cardiovascular events is modified by air pollution exposure in the SPRINT trial. SPRINT participants (n=9,286) were linked with annual PM2.5 exposure. We showed that intensive BP-lowering decreased risk of the composite cardiovascular outcome in higher PM2.5 more than cleaner areas (Pinteraction=0.047). Thus, this analysis of SPRINT suggests that the magnitude of cardiovascular benefit of intensive blood pressure lowering may depend upon the magnitude of antecedent PM2.5 exposure
Introduction
A considerable body of evidence implicates particulate matter <2.5 microns (PM2.5) as the principal air pollutant posing the greatest threat to global health.1–6 An estimated 8.9 million avoidable deaths in 2015 were attributable to ambient air pollution alone7, 120% larger than the estimates in the Global Burden of Disease (GBD) study which attributed 4.2 million deaths to ambient air pollution.8 Prior studies have shown that exposure to PM2.5 is associated with acute and chronic elevations in blood pressure (BP)9 as well as incident hypertension10. It has indeed been hypothesized that a portion of the cardiovascular morbidity and mortality from air pollution may in part be mediated by elevations in BP, and that this may help explain the large global footprint of mortality and disability attributable to air pollution.11, 12 Compelling data in the United States and Canada continue to demonstrate that even low levels of PM2.5 pose health risks, with recent studies showing a continuous relationship between mortality and PM2.5 at levels below current annual National Ambient Air Quality Standards (NAAQS) of <12 ug/m.3,13–15 Empirical evidence from time series analyses also continue to suggest a relationship between blood pressure and extreme levels of air pollution, such as that encountered in China, implying a relationship across a broad range of exposures.16, 17
If high BP is truly a mediator of the health effects from PM2.5 exposures (i.e., part of the causal-pathway), strategies that lower PM2.5 should lower BP, as has indeed been shown in a growing number of short-term intervention studies.18–20,21 Conversely given that PM2.5 adversely affects individuals with prior cardiovascular disease and/or those with multiple risk factors, it is also possible that individuals with high levels of blood pressure may be disproportionately affected and that the benefits of BP-lowering may be differentially influenced by the magnitude of concomitant exposure to ambient PM2.5. In this exploratory, non-prespecified post-hoc study, we sought to test the hypothesis that the effect of BP-lowering strategies on cardiovascular outcomes is influenced by on PM2.5 exposure levels, using a contemporary trial of intensive BP lowering, the Systolic Blood Pressure Intervention Trial (SPRINT).
Methods
All data and materials have been made publicly available at the National Heart, Lung and Blood Institute online data repository and can be accessed at https://biolincc.nhlbi.nih.gov.
Study Design and Analytical Plan:
The trial design, inclusion and exclusion criteria, assessments and outcomes for SPRINT are published elsewhere22, 23. Briefly, SPRINT included patients with hypertension, age 50 years or older, systolic BP (SBP) 130–180 mm Hg with one or more of the following CVD risk factors: history of clinical or subclinical CVD other than stroke, estimated glomerular filtration rate (eGFR) of 20–59 mL/min/1.73 m2 using the four-variable MDRD equation, 10-year risk for CVD ≥15% calculated using the Framingham Risk Score, or age ≥75 years. Main exclusion criteria included diabetes, a history of stroke, heart failure, proteinuria ≥1 g/day, or eGFR <20 mL/min/1.73 m2. Eligible participants were randomly assigned to either an SBP target of <120 mm Hg or one of <140 mm Hg. All major classes of antihypertensive medications were included in the SPRINT formulary. Participants were seen monthly for the first 3 months and then every 3 months thereafter. Intensive participants were also seen monthly to titrate medications until SBP goal was reached or the investigator decided to not titrate further.
In this exploratory, non-prespecified, post-hoc analysis of SPRINT we proposed investigating the association between PM2.5 and cardiovascular events and study the heterogeneity of treatment benefit by PM2.5 exposure. The original proposal and analysis plan were approved by the SPRINT steering committee (Data Supplement) and incorporated primary outcomes per PM2.5 increments (in quartiles) and by study randomization. We performed several subsequent exploratory analysis, where we examined the occurrence of the outcomes in SPRINT in relation to PM2.5 both as a continuous variable, in quintiles (vs proposed quartiles, due to narrow range of PM2.5 in the linked dataset) and in relationship to a binary threshold value of the annual United States National Ambient Air Quality Standards (NAAQS) threshold. We were particularly interested in the differential effects of the treatment arms in SPRINT according to the magnitude of concomitant exposure to ambient PM2.5 given the known relationship between PM2.5 and blood pressure. Additional details are provided in the Statistical Analytical Plan (see below).
Exposure characterization:
We linked annual PM2.5 exposure derived from an integrated model (satellite aerosol optical depth with chemical transport) to participants in SPRINT using ZIP code of residence, preserving confidentiality. The U.S. National Ambient Air Quality Standards (NAAQS) has set average annual PM2.5 at 12 ug/m3. We studied the impact of intensive BP lowering by quintiles of PM2.5 exposure during the calendar year of trial enrollment, with respect to the primary outcome (myocardial infarction, other acute coronary syndromes, stroke, heart failure, or cardiovascular death). The primary exposure of interest was ZIP-level average annual PM2.5. Each patient was assigned the average annual PM2.5 using ZIP code of residence of the calendar year at trial entry. Modelled PM2.5 level measures are highly correlated over time (trial year 1 vs year 2, Spearman’s rho=0.90; year 2 vs year 3: Spearman’s rho=0.96), and therefore the entry PM2.5 is a rough approximation of the level of exposure that an individual experienced over the study period.
PM2.5 estimates were obtained from a validated integrated exposure model that estimated PM2.5 by combining aerosol optical depth retrievals from multiple sources (NASA MODIS, MISR, and SeaWIFS instruments) with chemical transport model estimates (GEOS-Chem), and subsequently calibrated against global ground-based observations of PM2.5, using geographically-weighted regression24. The data thus obtained corresponded to grids of 0.01×0.01 degrees (~1×1 km). The data were imported in QGIS and mapped to the 2013 ZIP shapefiles available from (www.census.gov) and mean (area-average) ZIP-level PM2.5 was calculated using zonal statistics.
Study Measurements:
Participant demographics were collected at baseline. Clinical and laboratory data were collected at baseline, and every 3 months thereafter. BPs in SPRINT were measured at each visit by trained staff with an automated device (Omron-HEM-907 XL) using standardized procedures23. Seated BP measurements (X3) were conducted early in the visit after 5 min rest and proper positioning of the participant in a chair with back support and proper cuff size determination, but before stressful exam components such as blood draws and questionnaires. Self-reported CVD outcomes were collected every 3 months using structured interviews in both treatment arms of the study.
Study Outcomes:
The primary outcome in SPRINT was a composite CVD outcome of myocardial infarction (MI), acute coronary syndrome (other than MI), stroke, acute decompensated heart failure, or death from CVD causes. Secondary outcomes included the individual components of the primary outcome, death from any cause, and the composite of the primary outcome or death from any cause. An adjudication committee blinded to treatment assignment adjudicated all outcomes using a prespecified protocol.
Statistical Analysis:
We used Cox proportional hazards regression models stratified according to clinic, to estimate the Hazard Ratios and 95% confidence intervals for first occurrence of the primary outcome and its subcomponents for intensive vs standard treatment arms (referent) by PM2.5 exposure quintile. There were no violations for the proportional hazards assumptions. To assess for effect modification of treatment arm among SPRINT participants by PM2.5, we included the product term (SBP treatment arm × PM2.5 quintile (as an ordinal variable or as a continuous variable)) in the Cox proportional hazards regression in the full sample. This was repeated for secondary outcomes separately without correction for multiple testing. Measures of interaction and p-values for the primary outcome are presented along with hazard ratios for subgroups by quintile of PM2.5 exposure. We additionally assessed interaction between PM2.5 (< vs ≥ 12 ug/m3, categorical variable) and treatment effect, using the NAAQS threshold. We also constructed a Cox proportional hazard model including an interaction term between study treatment arm and PM2.5 quintile (treated as nominal variable) for the incidence of the primary outcome. All analyses were performed using Statistical Package for Social Sciences (version 20) and R project 3.4.2
Results
Population characteristics
Among 9,361 trial participants, 9,286 were linked to PM2.5 levels. Mean average annual exposure among SPRINT participants was 9.5 ± 2.7 μg/m3, with 1328 (14.3%) facing levels at or above annual NAAQS of 12 μg/m3. Supplemental figure S1 shows the histogram of PM2.5 exposure among the study participants. The characteristics of patients by PM2.5 exposures are shown in Table 1. Participants exposed to higher PM2.5 were more likely to be African American, free of pre-existing cardiovascular disease, and not on statin therapy. There were no clinically-important differences in baseline SBP, DBP, or number of antihypertensive agents. When patients were stratified by quintile of PM2.5 and trial assignment, all characteristics (with 3 minor exceptions) were balanced between the arms within each quintile (Supplemental Table S1).
Table 1:
Characteristics of study participants by PM2.5 quintile
| Characteristic | Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 |
|---|---|---|---|---|---|
| Number of patients | 1858 | 1857 | 1857 | 1857 | 1857 |
| PM2.5 (μg/m3) | 5.3 ± 1.9 | 8.7 ± 0.4 | 9.7 ± 0.3 | 10.8 ± 0.3 | 12.8 ± 1.1 |
| Age, years | 68.1 ± 9.6 | 68.2 ± 9.3 | 67 ± 9.6 | 68 ± 9.3 | 68 ± 9.1 |
| Female, n (%) | 601 (32%) | 637 (34%) | 630 (34%) | 719 (39%) | 717 (39%) |
| Black Race, n (%) | 348 (19%) | 434 (23%) | 657 (35%) | 686 (37%) | 797 (43%) |
| Current Smoker, n (%) | 219 (12%) | 225 (12%) | 257 (14%) | 244 (13%) | 285 (15%) |
| History of clinical CVD, n (%) | 389 (21%) | 302 (16%) | 283 (15%) | 300 (16%) | 277 (15%) |
| History of subclinical CVD, n (%) | 118 (6.4%) | 72 (3.9%) | 92 (5.0%) | 89 (4.8%) | 113 (6.1%) |
| Statin, n (%) | 884 (48%) | 827 (45%) | 793 (43%) | 762 (41%) | 767 (42%) |
| Aspirin, n (%) | 933 (50%) | 980 (53%) | 935 (51%) | 940 (51%) | 945 (51%) |
| Assigned to Intensive lowering strategy | 931 (50%) | 946 (51%) | 915 (49%) | 916 (49%) | 927 (50%) |
| Chronic Kidney Disease, n (%) | 504 (27%) | 613 (33%) | 474 (26%) | 543 (29%) | 497 (27%) |
| eGFR, ml/min/1.73 m2 | 73 ± 21 | 69 ± 20 | 73 ± 21 | 71 ± 20 | 72 ± 20 |
| Framingham 10-year risk | 20.4 ± 11.2 | 20.8 ± 11.0 | 20.4 ± 11.2 | 19.6 ± 10.4 | 19.2 ± 10.2 |
| Body Mass Index | 29.4 ± 5.4 | 29.7 ± 5.7 | 30.1 ± 5.9 | 29.9 ± 5.8 | 30.2 ± 6.0 |
| Baseline Blood Pressure (mm Hg) | |||||
| Systolic | 139 ± 15 | 141 ± 16 | 140 ± 15 | 139 ± 16 | 139 ± 16 |
| Diastolic | 77 ± 12 | 79 ± 12 | 79 ± 12 | 78 ± 12 | 79 ± 12 |
| Number of antihypertensives | 1.8 ± 1.0 | 1.8 ± 1.1 | 1.9 ± 1.1 | 1.9 ± 1.0 | 1.9 ± 1.0 |
| Not on antihypertensives | 150 (8.1%) | 198 (11%) | 196 (11%) | 171 (9%) | 158 (9%) |
| Fasting Laboratory (mg/dL) | |||||
| Total cholesterol | 187 ± 41 | 190 ± 42 | 192 ± 41 | 191 ± 41 | 190 ± 41 |
| HDL cholesterol | 52 ± 14 | 53 ± 15 | 53 ± 15 | 54 ± 14 | 54 ± 15 |
| Triglycerides | 132 ± 93 | 128 ± 88 | 126 ± 80 | 123 ± 87 | 120 ± 103 |
| Glucose | 98 ± 12 | 100 ± 14 | 99 ± 13 | 98 ± 14 | 99 ± 14 |
Effects of Intensive BP-lowering by PM2.5 Levels on Cardiovascular Events
We followed participants for a median of 3.3 years (range 2.9 – 3.9) with 559 total primary outcome events over the study period. The number of events for each of the outcomes by quintile of PM2.5 and study assignment are shown in Supplemental Table S2. When PM2.5 was treated as a continuous variable, the higher the PM2.5 the greater the reduction in the primary outcome by more intense BP reduction (Pinteraction=0.047), Figure 1A. Given the lower limit of detection of the PM2.5 model of 3 μg/m3 (likely reflecting participants in Puerto Rico), we performed additional analyses excluding patients living in areas with PM2.5 ≤ 3 μg/m3 (Figure 1B) using PM2.5 as a continuous variable. In this subset, the treatment effect was stronger in participants exposed to higher PM2.5 with respect to primary outcome (PM2.5 × assignment Pinteraction = 0.011).
Figure 1:
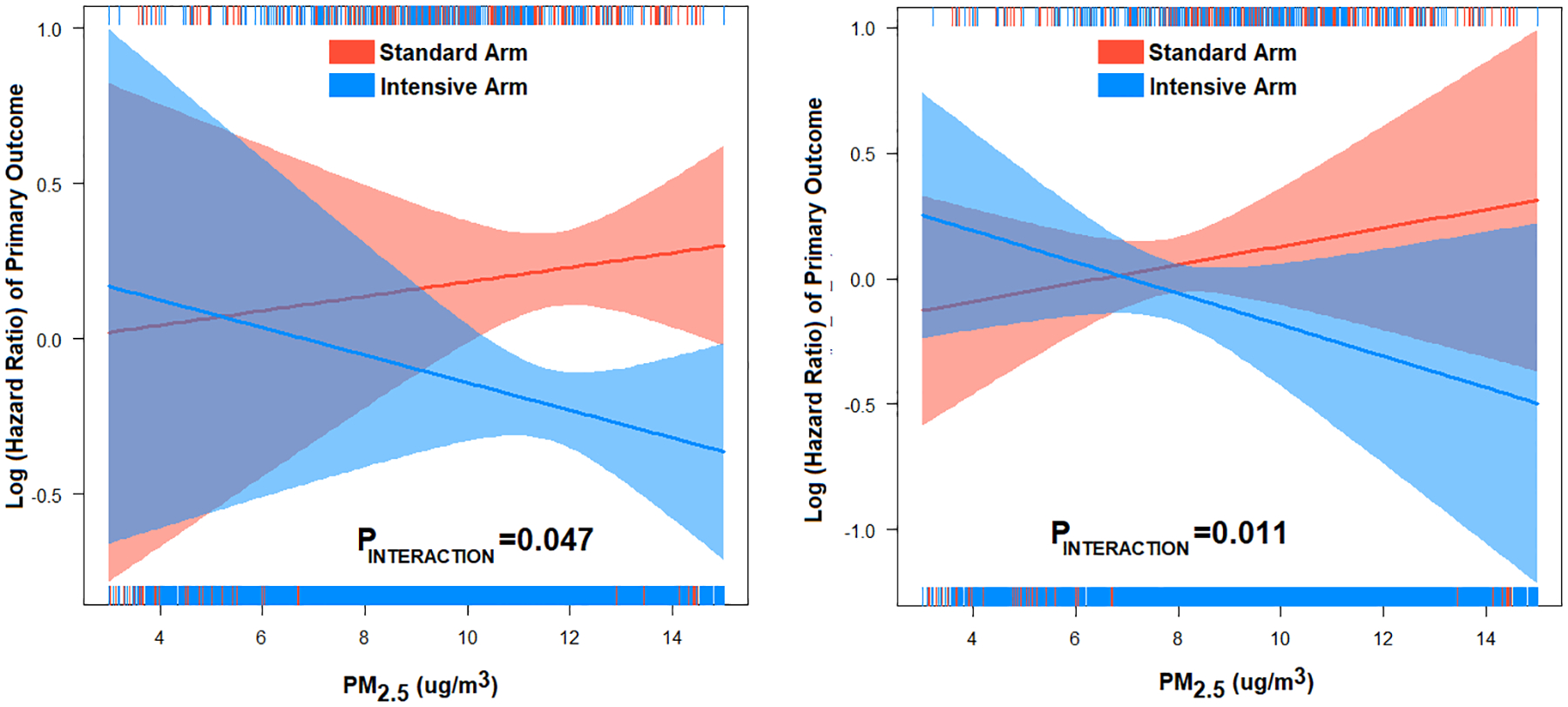
Association between PM2.5 (continuous variable) and hazard ratio (Log) of primary outcome in in the intensive vs standard BP in the (A) entire cohort (n=9,286) and (B) excluding PM2.5≤3 μg/m3 (n=8,718). PINTERACTION is between PM2.5 and study assignment. The association between PM2.5 and primary outcome was not statistically significant in the standard arm or the intensive arm.
The effect of intensive BP control on the primary outcome although smaller, persisted among patients residing in areas with low PM2.5 classified by using a binary threshold of 12 μg/m3 which corresponds to the US NAAQS annual standard (<12 μg/m3: HR 0.81, 95% CI: 0.68–0.97) relative to patients exposed to higher levels (≥12 μg/m3: HR 0.46, 95% CI: 0.29–0.74), (Pinteraction=0.037), Figure 2.
Figure 2:
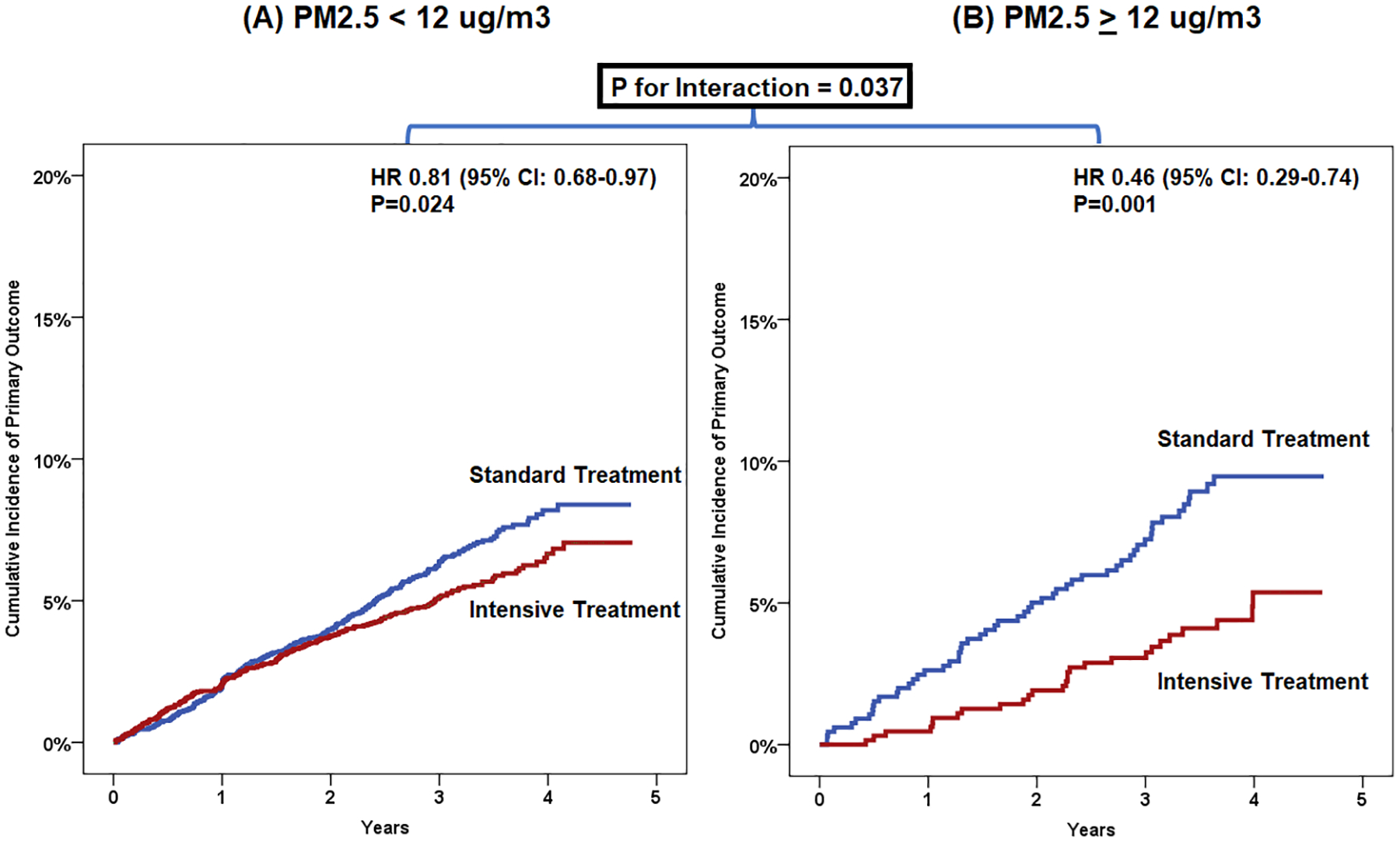
Primary outcome with intensive treatment vs standard treatment stratified by PM2.5 exposure category (above or below the NAAQS cut-off of PM2.5 of 12 ug/m3, nominal variable)
When stratified by PM2.5 quintiles, (Q1: HR 1.00 [0.72–1.41]; Q2: 0.77 [0.51–1.18]; Q3: HR 0.92 [0.62–1.37]; Q4: HR 0.63 [0.44–0.91] and Q5: HR 0.53 [0.36–0.79]) (Figure 3), the interaction with intervention was not significant, (Pinteraction=0.099). When categorized into upper two quintiles (Q4-Q5 grouped) vs lower 3 quintiles (Q1-Q3 grouped), there was an interaction between PM2.5 group and study treatment: Pinteraction=0.013 for Q4-Q5 vs Q1-Q3). Analyses of PM2.5 quintiles and P values for interaction term (PM2.5 × study assignment) as nominal variable and in different models separately are shown in table 2.
Figure 3:
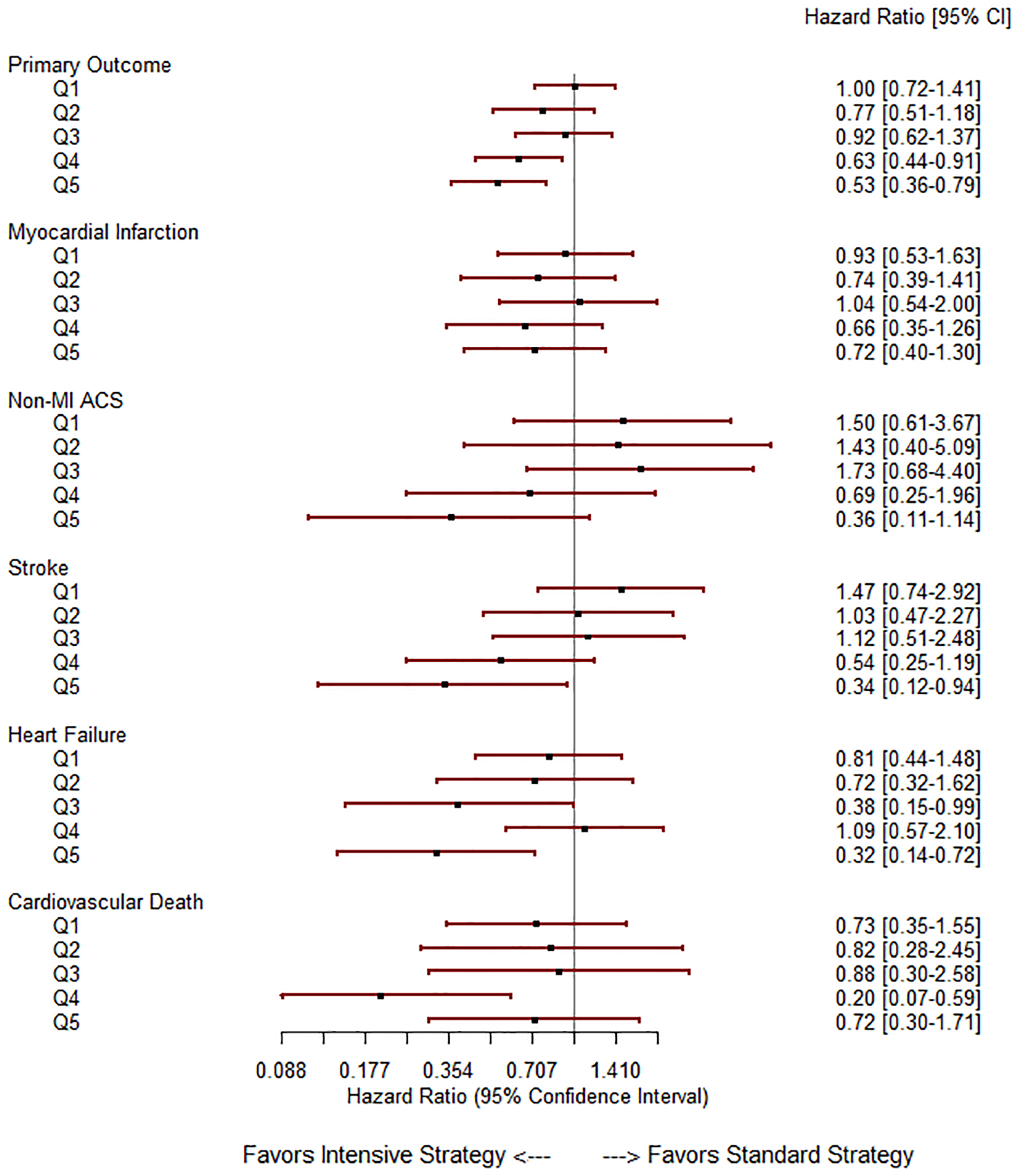
Forest plot depicting the effect of intensive BP lowering by PM2.5 exposure quintile. Statistical tests for varying effect of treatment by PM2.5 level were performed using Cox PH models and corresponding p-values (for interaction term, PM2.5 (ordinal variable) × study assignment) are displayed in the right-hand column. The solid lines represent no difference in the effect and values to the left of the HR 1.0-line favor intensive BP lowering
Table 2:
Analyses of the interaction between PM2.5 (treated as continuous, ordinal, and nominal), and study treatment for the incidence of the primary outcome
| Treatment of PM2.5 Variable | P Value for interaction between PM2.5 and Study Assignment |
|---|---|
| PM2.5 treated as continuous | 0.047 |
| PM2.5 Quintiles treated as continuous | 0.01 |
| PM2.5 Quintiles treated as nominal (all in one model) | |
| Overall | 0.099 |
| Q2 vs Q1 | 0.27 |
| Q3 vs Q1 | 0.76 |
| Q4 vs Q1 | 0.066 |
| Q5 vs Q1 | 0.016 |
| PM2.5 Quintiles treated as nominal (separate analyses) | |
| Q2 vs Q1 | 0.33 |
| Q3 vs Q1 | 0.81 |
| Q4 vs Q1 | 0.068 |
| Q5 vs Q1 | 0.017 |
| Q4–5 vs Q1–3 | 0.013 |
Adjusting for race (Black vs non-Black) did not alter the interaction term between PM2.5 and study assignment with respect to primary outcome (PM2.5 × study assignment, Pinteraction=0.047). The interaction between study assignment and PM2.5 with respect to primary outcome remained statistically significant, when only including Black participants (PM2.5 × study assignment, Pinteraction=0.035).
Secondary outcomes:
When PM2.5 was modelled as a continuous variable, there was a significant interaction between study arm and PM2.5 with respect to stroke (Pinteraction=0.026), but not other secondary outcomes (Supplemental table S3). In the subset excluding PM2.5 ≤ 3 μg/m3, the treatment effect was stronger in participants exposed to higher PM2.5 with respect to non-MI ACS (PM2.5 × assignment Pinteraction = 0.027), and stroke (PM2.5 × assignment Pinteraction = 0.007), but not other secondary outcomes. When PM2.5 was modelled as categorical (above or below NAAQS threshold of 12 μg/m3), there was a similar finding with stroke (PM2.5 <12 μg/m3: HR 0.68 [0.49–0.96] vs PM2.5 ≥12 μg/m3: HR 0.27 [0.10–0.72], Pinteraction=0.046).
When PM2.5 quintiles were treated as ordinal variable, the numerical relationship between higher levels of PM2.5 exposure were also observed individually with stroke [Q1: HR 1.47 (0.74–2.92); Q2: HR 1.03 (0.47–2.27); Q3: HR 1.12 (0.51–2.48); Q4: HR 0.54 (0.25–1.19); and Q5; HR 0.34 (0.12–0.94), Pinteraction=0.01; and non-MI ACS [Q1: HR 1.50 (0.61–3.67); Q2: HR 1.43 (0.40–5.09); Q3: HR 1.73 (0.68–4.40); Q4: HR 0.69 (0.25–1.96) and Q5: HR 0.36 (0.11–1.14), Pinteraction=0.025; PM2.5 quintiles treated as ordinal variable], but not with heart failure, CV death, or myocardial infarction. Hazard ratios of intensive vs standard BP in each quintile are shown in Figure 3.
Discussion
The results from this post-hoc analysis of SPRINT suggests that intensive BP-lowering decreased the primary outcome in patients with the highest PM2.5 exposure. Notably the benefits of intensive BP-lowering were larger among patients exposed to PM2.5 levels above the NAAQS annual standard of 12 μg/m3, compared to those living in cleaner locations, although benefits of intensive BP lowering persisted in patients exposed to levels below NAAQS limits.
The experimental design of SPRINT provided a unique opportunity to study the relationship between annual PM2.5 levels and CVD outcomes. Participants in SPRINT were randomized and treated to two different BP levels, resulting in a 13 mm Hg average SBP separation during the average 3.26 years of follow-up, allowing a platform to examine the effect of antecedent chronic PM2.5 exposure at two separate BP levels, and its effect on the benefits of intensive BP-lowering. These exploratory analyses suggest linear trend in the benefit of intensive BP-lowering influenced by ambient PM2.5, such that SPRINT participants exposed to higher levels of PM2.5 had larger benefits with intensive lowering of BP. Those living in areas with air pollution levels below the NAAQS annual limit of 12 μg/m3 showed lower albeit significant, persistent reduction in the primary endpoint with intensive BP-lowering compared with those living above the NAAQS limit.
The association between increase in air pollutants (in particular PM2.5) and rise in blood pressure is well known, and has been documented extensively including in 4 recent meta-analyses.9, 10, 25, 26 Short term increases in ambient PM2.5 by 10 μg/m3 are consistently reported to be associated with 1 to 3 mm Hg elevations in both systolic and diastolic BP over the ensuing few days9. Longer-term exposures have been associated with chronic elevations in BP and with an increased prevalence or incidence of hypertension, although results are less consistent.27–29 A growing number of short-term intervention studies have also provided corroborative evidence, supporting a causal-relationship, by showing that reductions in PM2.5 levels result in a lowering of BP.30, 31 As such, elevations in BP may mediate some of the adverse cardiovascular health effects of PM2.5.31–33 A large body of evidence also implicates exposure to PM2.5 in cardiovascular events such as cardiovascular mortality, acute myocardial infarction, stroke and heart failure. Time-series and case-crossover studies across the globe have explored the association between short-term changes in air pollution and daily changes in stroke, MI and heart failure. Indeed, increases in PM2.5 have been associated with all 3 outcomes.31 In a systematic review and meta-analysis of 94 studies until 2014, involving 28 countries, a 10 μg/m3 increase in PM2.5 and PM10 concentration was associated with 1% increase in relative risks for admission to the hospital with stroke and stroke mortality34. In an analysis of the ESCAPE cohort from Europe, there was an association between annual PM2.5 and stroke among subjects ≥ 60 years of age (hazard ratio [HR]: 1.40; 95% CI: 1.05 to 1.87 per 5 μg/m3 increase in PM2.5.
The results of the current analysis, if confirmed in other trials, may have important implications for preventive therapies for individuals living in locations with high levels of PM2.5. Our findings suggest that intensive blood pressure lowering may be particularly important in populations facing PM2.5 levels higher than the current NAAQS standard of 12 μg/m3 and that these patients could benefit even more from intensive BP-lowering. The role for preventive therapies in areas associated with high levels of air pollution levels has been an important question for which there is not much evidence in terms of randomized controlled clinical trials and/or clinical outcomes. Position statements by the American Heart Association (AHA) and the European Society of Cardiology have generally advocated primary and secondary preventive measures, but have been constrained by a general paucity of evidence for reduction in hard events with control of risk factors.35, 36 There is some evidence that therapeutic interventions such as statins and dietary components such as ω3 fatty acids may reduce the impact of air pollution on surrogate measures37. In a recent study of more than half a million persons in the National Institutes of Health–American Association for Retired Persons Diet and Health Study, followed for 17 years, the association between fine particulate matter and nitric oxide exposure with cardiovascular events was modified by Mediterranean diet intake, as measured by Mediterranean diet index.38 The association between air pollutants (PM2.5 and NO2) and cardiovascular events was no longer present in patients who had highest consumption of Mediterranean diet.
This study has several limitations that must be acknowledged. As a non-prespecified post-hoc analysis of a randomized trial, our findings should merit abundant caution and require confirmation in future studies. The relatively narrow PM2.5 range and the low statistical power, especially for secondary outcomes may require caution in the interpretation of the results of PM2.5 on secondary outcomes such as stroke and ACS. Exposure misclassification is always a concern, given the fact that this was modelled based on residential zip code, which may not reflect the location where the trial participants may have spent the majority of time. Another limitation is the fact that PM2.5 exposure could be a marker of socioeconomic status or other poorly defined social determinant(s) that co-segregate with exposure. The correlations between PM2.5 and socioeconomic factors and PM2.5 exposure is high, limiting our ability to make firm conclusions with more research needed to define this relationship. Residual confounding from spatially covarying risk factors is always a possibility and it is possible that poorly characterized social determinants could co-segregate with exposures, and contribute to the current observations. However, in multiple previous studies, adjustment of factors such as education, socioeconomic status and other demographic variables failed to eliminate the relationship between PM2.5 and health risks.39, 40 Air pollutants rarely occur in isolation to each other or in the absence of other environmental exposures (e.g., noise, temperature). As a prominent example, near-roadway environments lead to exposures to noise, particulate and gaseous traffic-related air pollution, as well as psychological stressors.11 The independent and potentially additive (or synergistic) cardiovascular risks posed by these multiple exposures, impacting most of the world’s population on a daily basis has yet to be fully understood. Regardless, PM2.5 is acknowledged as the single largest triggering event for myocardial infarction worldwide and the leading environmental cause for preventable morbidity and mortality.41 As such, there is a critical need for treatments that lower the risk posed by air pollution. The importance as well as the putative design and plausibility of clinical trials to mitigate exposures have been previously reviewed.42, 43 Positive results and confirmation of these findings from SPRINT would provide much-needed scientific evidence to protect public health and provide yet another line of evidence supporting a “causal role” for PM2.5 in CVD.
Conclusions
This exploratory non-prespecified post-hoc analysis of SPRINT suggests that the cardiovascular benefit of intensive BP-lowering could be dependent upon the level of PM2.5 exposure. Benefits of BP lowering, however, persisted in patients exposed to PM2.5 lower than NAAQS threshold. Further research is needed to identify strategies to mitigate risk of PM2.5 exposure in high-risk individuals.
Supplementary Material
Perspectives.
Intensive blood pressure lowering is effective in reducing cardiovascular risk in patients exposed to high levels of air pollution. Intensive blood pressure lowering (goal SBP <120 mmHg) can be beneficial in selected patients with who live in neighborhoods with elevated air pollution exposure. Although this is a post-hoc analysis of a large randomized controlled trial, results show a linear trend in the benefit of intensive BP lowering with air pollution exposure. The mechanisms of the interaction between air pollution and benefit of intensive BP lowering need to be further investigated.
Novelty and Significance.
What is new? This study suggests that the cardiovascular benefit of intensive BP-lowering could be dependent upon the level of PM2.5 exposure.
What is relevant? Patients with hypertension exposed to high PM2.5 may derive strong cardiovascular benefit from intensive lowering of blood pressure to goal systolic blood pressure of less than 120 mmHg.
Acknowledgments
The Systolic Blood Pressure Intervention Trial is funded with Federal funds from the National Institutes of Health (NIH), including the National Heart, Lung, and Blood Institute (NHLBI), the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), the National Institute on Aging (NIA), and the National Institute of Neurological Disorders and Stroke (NINDS), under Contract Numbers HHSN268200900040C, HHSN268200900046C, HHSN268200900047C, HHSN268200900048C, HHSN268200900049C, and Inter-Agency Agreement Number A-HL-13-002-001. It was also supported in part with resources and use of facilities through the Department of Veterans Affairs. The SPRINT investigators acknowledge the contribution of study medications (azilsartan and azilsartan combined with chlorthalidone) from Takeda Pharmaceuticals International, Inc. All components of the SPRINT study protocol were designed and implemented by the investigators. The investigative team collected, analyzed, and interpreted the data. All aspects of manuscript writing and revision were carried out by the coauthors. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH, the U.S. Department of Veterans Affairs, or the United States Government. For a full list of contributors to SPRINT, please see the supplementary acknowledgement list:
https://www.sprinttrial.org/public/dspScience.cfm
We also acknowledge the support from the following CTSAs funded by NCATS:
CWRU: UL1TR000439, OSU: UL1RR025755, U Penn: UL1RR024134& UL1TR000003, Boston: UL1RR025771, Stanford: UL1TR000093, Tufts: UL1RR025752, UL1TR000073 & UL1TR001064, University of Illinois: UL1TR000050, University of Pittsburgh: UL1TR000005, UT Southwestern: 9U54TR000017-06, University of Utah: UL1TR000105-05, Vanderbilt University: UL1 TR000445, George Washington University: UL1TR000075, University of CA, Davis: UL1 TR000002, University of Florida: UL1 TR000064, University of Michigan: UL1TR000433, Tulane University: P30GM103337 COBRE Award NIGMS, Wake Forest University: UL1TR001420. This manuscript was prepared using SPRINT Research Materials obtained from the NHLBI.
Sources of Funding
This study is funded by 5R01ES019616-07 and 1R01ES026291 to Sanjay Rajagopalan
Footnotes
Disclosures: none of the authors have conflict of interest pertinent to the contents of this manuscript
References
- 1.Landrigan PJ, Fuller R, Acosta NJR, Adeyi O, Arnold R, Basu NN, Balde AB, Bertollini R, Bose-O’Reilly S, Boufford JI, Breysse PN, Chiles T, Mahidol C, Coll-Seck AM, Cropper ML, Fobil J, Fuster V, Greenstone M, Haines A, Hanrahan D, Hunter D, Khare M, Krupnick A, Lanphear B, Lohani B, Martin K, Mathiasen KV, McTeer MA, Murray CJL, Ndahimananjara JD, Perera F, Potocnik J, Preker AS, Ramesh J, Rockstrom J, Salinas C, Samson LD, Sandilya K, Sly PD, Smith KR, Steiner A, Stewart RB, Suk WA, van Schayck OCP, Yadama GN, Yumkella K and Zhong M. The Lancet Commission on pollution and health. Lancet. 2018;391:462–512. [DOI] [PubMed] [Google Scholar]
- 2.Brook RD, Rajagopalan S, Pope CA, Brook JR, Bhatnagar A, Diez-Roux AV, Holguin F, Hong Y, Luepker RV, Mittleman MA, Peters A, Siscovick D, Smith SC, Whitsel L and Kaufman JD. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the american heart association. Circulation. 2010;121. [DOI] [PubMed] [Google Scholar]
- 3.Newby DE, Mannucci PM, Tell GS, Baccarelli AA, Brook RD, Donaldson K, Forastiere F, Franchini M, Franco OH, Graham I, Hoek G, Hoffmann B, Hoylaerts MF, Künzli N, Mills N, Pekkanen J, Peters A, Piepoli MF, Rajagopalan S and Storey RF. Expert position paper on air pollution and cardiovascular disease. European heart journal. 2015;36:83–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Munzel T, Sorensen M, Gori T, Schmidt FP, Rao X, Brook J, Chen LC, Brook RD and Rajagopalan S. Environmental stressors and cardio-metabolic disease: part I-epidemiologic evidence supporting a role for noise and air pollution and effects of mitigation strategies. Eur Heart J. 2017;38:550–556. [DOI] [PubMed] [Google Scholar]
- 5.Thurston GD, Kipen H, Annesi-Maesano I, Balmes J, Brook RD, Cromar K, De Matteis S, Forastiere F, Forsberg B, Frampton MW, Grigg J, Heederik D, Kelly FJ, Kuenzli N, Laumbach R, Peters A, Rajagopalan ST, Rich D, Ritz B, Samet JM, Sandstrom T, Sigsgaard T, Sunyer J and Brunekreef B. A joint ERS/ATS policy statement: What constitutes an adverse health effect of air pollution? An analytical framework. European Respiratory Journal. 2017;49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lippmann M, Chen L-C, Gordon T, Ito K and Thurston GD. National Particle Component Toxicity (NPACT) Initiative: integrated epidemiologic and toxicologic studies of the health effects of particulate matter components. Research report (Health Effects Institute). 2013:5–13. [PubMed] [Google Scholar]
- 7.Burnett R, Chen H, Szyszkowicz M, Fann N, Hubbell B, Pope CA, Apte JS, Brauer M, Cohen A, Weichenthal S, Coggins J, Di Q, Brunekreef B, Frostad J, Lim SS, Kan H, Walker KD, Thurston GD, Hayes RB, Lim CC, Turner MC, Jerrett M, Krewski D, Gapstur SM, Diver WR, Ostro B, Goldberg D, Crouse DL, Martin RV, Peters P, Pinault L, Tjepkema M, van Donkelaar A, Villeneuve PJ, Miller AB, Yin P, Zhou M, Wang L, Janssen NAH, Marra M, Atkinson RW, Tsang H, Quoc Thach T, Cannon JB, Allen RT, Hart JE, Laden F, Cesaroni G, Forastiere F, Weinmayr G, Jaensch A, Nagel G, Concin H and Spadaro JV. Global estimates of mortality associated with long-term exposure to outdoor fine particulate matter. Proceedings of the National Academy of Sciences. 2018;115:9592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen AJ, Brauer M, Burnett R, Anderson HR, Frostad J, Estep K, Balakrishnan K, Brunekreef B, Dandona L and Dandona R. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: an analysis of data from the Global Burden of Diseases Study 2015. The Lancet. 2017;389:1907–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liang R, Zhang B, Zhao X, Ruan Y, Lian H and Fan Z. Effect of exposure to PM2.5 on blood pressure: a systematic review and meta-analysis. J Hypertens. 2014;32:2130–40; discussion 2141. [DOI] [PubMed] [Google Scholar]
- 10.Cai Y, Zhang B, Ke W, Feng B, Lin H, Xiao J, Zeng W, Li X, Tao J, Yang Z, Ma W and Liu T. Associations of Short-Term and Long-Term Exposure to Ambient Air Pollutants With Hypertension: A Systematic Review and Meta-Analysis. Hypertension. 2016;68:62–70. [DOI] [PubMed] [Google Scholar]
- 11.Munzel T, Sorensen M, Gori T, Schmidt FP, Rao X, Brook FR, Chen LC, Brook RD and Rajagopalan S. Environmental stressors and cardio-metabolic disease: part II-mechanistic insights. Eur Heart J. 2017;38:557–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rajagopalan S, Al-Kindi SG and Brook RD. Air pollution and cardiovascular disease: JACC state-of-the-art review. J Am Coll Cardiol. 2018;72:2054–2070. [DOI] [PubMed] [Google Scholar]
- 13.Di Q, Wang Y, Zanobetti A, Wang Y, Koutrakis P, Choirat C, Dominici F and Schwartz JD. Air Pollution and Mortality in the Medicare Population. N Engl J Med. 2017;376:2513–2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Christidis T, Erickson AC, Pappin AJ, Crouse DL, Pinault LL, Weichenthal SA, Brook JR, van Donkelaar A, Hystad P, Martin RV, Tjepkema M, Burnett RT and Brauer M. Low concentrations of fine particle air pollution and mortality in the Canadian Community Health Survey cohort. Environmental health : a global access science source. 2019;18:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pappin AJ, Christidis T, Pinault LL, Crouse DL, Brook JR, Erickson A, Hystad P, Li C, Martin RV, Meng J, Weichenthal S, van Donkelaar A, Tjepkema M, Brauer M and Burnett RT. Examining the Shape of the Association between Low Levels of Fine Particulate Matter and Mortality across Three Cycles of the Canadian Census Health and Environment Cohort. Environmental health perspectives. 2019;127:107008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao X, Sun Z, Ruan Y, Yan J, Mukherjee B, Yang F, Duan F, Sun L, Liang R, Lian H, Zhang S, Fang Q, Gu D, Brook JR, Sun Q, Brook RD, Rajagopalan S and Fan Z. Personal black carbon exposure influences ambulatory blood pressure: air pollution and cardiometabolic disease (AIRCMD-China) study. Hypertension. 2014;63:871–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brook RD, Sun Z, Brook JR, Zhao X, Ruan Y, Yan J, Mukherjee B, Rao X, Duan F, Sun L, Liang R, Lian H, Zhang S, Fang Q, Gu D, Sun Q, Fan Z and Rajagopalan S. Extreme Air Pollution Conditions Adversely Affect Blood Pressure and Insulin Resistance: The Air Pollution and Cardiometabolic Disease Study. Hypertension. 2016;67:77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Langrish JP, Mills NL, Chan JK, Leseman DL, Aitken RJ, Fokkens PH, Cassee FR, Li J, Donaldson K, Newby DE and Jiang L. Beneficial cardiovascular effects of reducing exposure to particulate air pollution with a simple facemask. Part Fibre Toxicol. 2009;6:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weichenthal S, Mallach G, Kulka R, Black A, Wheeler A, You H, St-Jean M, Kwiatkowski R and Sharp D. A randomized double-blind crossover study of indoor air filtration and acute changes in cardiorespiratory health in a First Nations community. Indoor Air. 2013;23:175–84. [DOI] [PubMed] [Google Scholar]
- 20.Chen R, Zhao A, Chen H, Zhao Z, Cai J, Wang C, Yang C, Li H, Xu X, Ha S, Li T and Kan H. Cardiopulmonary benefits of reducing indoor particles of outdoor origin: a randomized, double-blind crossover trial of air purifiers. J Am Coll Cardiol. 2015;65:2279–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shi J, Lin Z, Chen R, Wang C, Yang C, Cai J, Lin J, Xu X, Ross JA and Zhao Z. Cardiovascular benefits of wearing particulate-filtering respirators: a randomized crossover trial. Environ Health Perspect. 2016;125:175–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ambrosius WT, Sink KM, Foy CG, Berlowitz DR, Cheung AK, Cushman WC, Fine LJ, Goff DC Jr., Johnson KC, Killeen AA, Lewis CE, Oparil S, Reboussin DM, Rocco MV, Snyder JK, Williamson JD, Wright JT Jr., Whelton PK and Group SSR. The design and rationale of a multicenter clinical trial comparing two strategies for control of systolic blood pressure: the Systolic Blood Pressure Intervention Trial (SPRINT). Clin Trials. 2014;11:532–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson KC, Whelton PK, Cushman WC, Cutler JA, Evans GW, Snyder JK, Ambrosius WT, Beddhu S, Cheung AK, Fine LJ, Lewis CE, Rahman M, Reboussin DM, Rocco MV, Oparil S, Wright JT Jr. and Group SR. Blood Pressure Measurement in SPRINT (Systolic Blood Pressure Intervention Trial). Hypertension. 2018;71:848–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Donkelaar A, Martin RV, Li C and Burnett RT. Regional estimates of chemical composition of fine particulate matter using a combined geoscience-statistical method with information from satellites, models, and monitors. Environ Sci Technol. 2019;53:2595–2611. [DOI] [PubMed] [Google Scholar]
- 25.Giorgini P, Di Giosia P, Grassi D, Rubenfire M, Brook RD and Ferri C. Air Pollution Exposure and Blood Pressure: An Updated Review of the Literature. Curr Pharm Des. 2016;22:28–51. [DOI] [PubMed] [Google Scholar]
- 26.Yang BY, Qian Z, Howard SW, Vaughn MG, Fan SJ, Liu KK and Dong GH. Global association between ambient air pollution and blood pressure: A systematic review and meta-analysis. Environ Pollut. 2018;235:576–588. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Z, Laden F, Forman JP and Hart JE. Long-Term Exposure to Particulate Matter and Self-Reported Hypertension: A Prospective Analysis in the Nurses’ Health Study. Environ Health Perspect. 2016;124:1414–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chan SH, Van Hee VC, Bergen S, Szpiro AA, DeRoo LA, London SJ, Marshall JD, Kaufman JD and Sandler DP. Long-Term Air Pollution Exposure and Blood Pressure in the Sister Study. Environ Health Perspect. 2015;123:951–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen H, Burnett RT, Kwong JC, Villeneuve PJ, Goldberg MS, Brook RD, van Donkelaar A, Jerrett M, Martin RV and Kopp A. Spatial association between ambient fine particulate matter and incident hypertension. Circulation. 2014;129:562–569. [DOI] [PubMed] [Google Scholar]
- 30.Langrish JP, Li X, Wang S, Lee MMY, Barnes GD, Miller MR, Cassee FR, Boon Na, Donaldson K, Li J, Li L, Mills NL, Newby DE and Jiang L. Reducing personal exposure to particulate air pollution improves cardiovascular health in patients with coronary heart disease. Environmental health perspectives. 2012;120:367–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rajagopalan S, Al-Kindi SG and Brook RD. Air Pollution and Cardiovascular Disease: JACC State-of-the-Art Review. Journal of the American College of Cardiology. 2018;72:2054–2070. [DOI] [PubMed] [Google Scholar]
- 32.Brook RD and Rajagopalan S. Particulate matter, air pollution, and blood pressure. J Am Soc Hypertens. 2009;3:332–350. [DOI] [PubMed] [Google Scholar]
- 33.Li H, Cai J, Chen R, Zhao Z, Ying Z, Wang L, Chen J, Hao K, Kinney PL, Chen H and Kan H. Particulate Matter Exposure and Stress Hormone Levels: A Randomized, Double-Blind, Crossover Trial of Air Purification. Circulation. 2017;136:618–627. [DOI] [PubMed] [Google Scholar]
- 34.Shah AS, Lee KK, McAllister DA, Hunter A, Nair H, Whiteley W, Langrish JP, Newby DE and Mills NL. Short term exposure to air pollution and stroke: systematic review and meta-analysis. BMJ. 2015;350:h1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brook RD, Rajagopalan S, Pope III CA, Brook JR, Bhatnagar A, Diez-Roux AV, Holguin F, Hong Y, Luepker RV and Mittleman MA. Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation. 2010;121:2331–2378. [DOI] [PubMed] [Google Scholar]
- 36.Newby DE, Mannucci PM, Tell GS, Baccarelli AA, Brook RD, Donaldson K, Forastiere F, Franchini M, Franco OH, Graham I, Hoek G, Hoffmann B, Hoylaerts MF, Kunzli N, Mills N, Pekkanen J, Peters A, Piepoli MF, Rajagopalan S, Storey RF, Esc Working Group on Thrombosis EAfCP, Rehabilitation and Association ESCHF. Expert position paper on air pollution and cardiovascular disease. Eur Heart J. 2015;36:83–93b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dietary Tong H. and pharmacological intervention to mitigate the cardiopulmonary effects of air pollution toxicity. Biochim Biophys Acta. 2016;1860:2891–8. [DOI] [PubMed] [Google Scholar]
- 38.Lim CC, Hayes RB, Ahn J, Shao Y, Silverman DT, Jones RR and Thurston GD. Mediterranean Diet and the Association Between Air Pollution and Cardiovascular Disease Mortality Risk. Circulation. 2019; 139:1766–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Di Q, Wang Y, Zanobetti A, Wang Y, Koutrakis P, Choirat C, Dominici F and Schwartz JD. Air Pollution and Mortality in the Medicare Population. New England Journal of Medicine. 2017;376:2513–2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Di Q, Dai L, Wang Y, Zanobetti A, Choirat C, Schwartz JD and Dominici F. Association of Short-term Exposure to Air Pollution With Mortality in Older Adults. JAMA. 2017;318:2446–2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nawrot TS, Perez L, Künzli N, Munters E, Nemery B and Kunzli N. Public health importance of triggers of myocardial infarction: a comparative risk assessment. Lancet. 2011;377:732–740. [DOI] [PubMed] [Google Scholar]
- 42.Brook RD, Newby DE and Rajagopalan S. Air pollution and cardiometabolic disease: an update and call for clinical trials. Am J Hypertens. 2017;31:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brook RD, Newby DE and Rajagopalan S. The global threat of outdoor ambient air pollution to cardiovascular health: time for intervention. JAMA cardiology. 2017;2:353–354. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


