Pulmonary arterial hypertension (PAH) is characterized by progressive pulmonary vascular remodeling, resulting in an elevated right ventricle (RV) afterload, heart failure, and death. Importantly, RV function is predictive of survival in PAH (1). Currently available treatment options for PAH consist mostly of pulmonary vasodilators; they do not clearly reverse established disease, nor do they directly assist the RV itself.
BMPR2 (bone morphogenetic protein receptor type 2) germline loss-of-function mutations and the resulting decrease in the downstream signaling pathway have been associated with hereditary PAH (2). Interestingly, it has also been reported that patients with idiopathic PAH without a BMPR2 mutation exhibit reduced expression of BMPR2 in pulmonary arteries. Estrogen can also reduce BMPR2 expression, which may contribute to the higher observed prevalence of PAH in females (3). Augmenting BMPR2 signaling is therefore a clear therapeutic approach for both heritable and nonheritable PAH.
In this issue of the Journal, Boehm and colleagues (pp. 272–287) extend a strong body of previous work on the compound FK506 (tacrolimus) in augmenting BMPR2 signaling (4). FK506 was initially identified using a screening approach for drugs that increase BMP signaling mediated by BMPR2, even in the absence of the exogenous ligand (5). This mechanistic, basic translational work led to a phase IIa clinical trial of FK506 in patients with PAH, which demonstrated increases in BMPR2 expression and established the safety and tolerability of the compound (6). In line with these data on using a small molecule, it was found that the administration of BMP9 (a prototypical BMPR2 protein ligand) reverses established experimental pulmonary hypertension (PH) in a humanized mouse model of spontaneous disease after BMPR2 deficiency (Bmpr2+/R899X) (7). The aggregate body of evidence suggests that increasing BMPR2 pathway signaling will ultimately be identified as a successful approach for treating PAH, and indeed, clinical studies are currently ongoing, including studies of compounds such as sotatercept (8), which is sought as a means of increasing BMPR2 signaling via modulation of TGF-β family ligands.
The study by Boehm and colleagues explores the possibility that augmenting BMPR2 signaling may directly improve RV dysfunction in PH. Although deficient BMPR2 signaling has been linked to RV cardiomyocyte dysfunction, its effects on the RV have not been studied in isolation (9). The primary technique used by the authors involved the pulmonary artery banding mouse model, which results in increased RV afterload, with the advantage of avoiding confounding effects on the pulmonary vasculature. In this model, FK506 administration markedly improved RV function, as evidenced by attenuated left ventricle compression, septal flattening, and RV dilatation. Structural mechanisms underlying this functional improvement include reductions in collagen production and proliferation of cardiac fibroblasts, leading to reduced RV fibrosis with concurrent preservation of RV capillary density.
These findings both confirm and extend what is known about BMPR2 in PH. Adding to the known pathogenetic roles of endothelial-cell (7) and smooth-muscle-cell (10) BMP deficiency in experimental PH, their study identifies a fibroblast-mediated, BMP signaling–induced attenuation of RV remodeling. These findings are concordant with prior reports of decreased BMP signaling in pulmonary and liver fibrosis (11, 12). They are also in agreement with the clinical use of tacrolimus for the management of fibrosis in systemic rheumatic diseases. Although its precise mechanism of action in this context remains unknown, it is conceivable that one of the mechanisms by which tacrolimus may dampen fibrosis in rheumatologic conditions is by promoting BMP signaling (13).
The beneficial effects of BMP pathway reconstitution using the pulmonary artery banding model, specifically in cardiac fibroblasts, are of particular clinical relevance, given that RV function closely tracks with the clinical prognosis in PAH. The significance of an FK506-derived reduction in RV fibrosis in mice, however, must be confirmed in humans. Although BMPR2 deficiency was associated with impaired RV function in both humans with PAH (14) and laboratory animals (9), reduced RV function did not correlate with BMP signaling (14) or the degree of RV fibrosis (15). These conflicting observations underscore the importance of verifying the overall impact of BMP modulation by quantifying key clinical endpoints, such as 6-minute-walk distance and cardiac output. The present study by Boehm and colleagues, as well as their previous clinical trial (6), did not show FK506-induced improvements in cardiac output. Such clinical measurements are important in interpreting the role of FK506, given that some degree of RV fibrosis is likely to be beneficial in allowing RV adaptation to an increased afterload, whereas pathologic, excessive fibrosis is likely detrimental because it increases stiffness.
As clinical applications targeting the TGF-β superfamily continue to evolve, non–fibrosis-related, off-target effects of FK506 and other BMP-enhancing interventions must be taken into careful consideration. One potential example is the effect of BMPR2 on cell metabolism (16). BMP signaling has been implicated in dysregulated RV fatty acid metabolism; precisely how FK506 affects the RV and pulmonary vasculature via modulation of triglyceride and other metabolic substrates remains an interesting but unexplored topic. Whether FK506 inhibits collagen synthesis or augments its degradation and whether inflammatory cells such as macrophages contribute to tissue fibrosis also merit further investigation.
The novel discoveries by Boehm and colleagues introduce new, exciting questions that will help define the role of future therapies directed at improving RV fibrosis in PAH. For example, the optimal timing of antifibrotic therapy initiation remains undefined and will likely depend on the degree of existing RV fibrosis. The potential need for dose adjustment in response to a changing RV afterload—which is reduced through vasodilator treatments or increased owing to progression of the underlying pulmonary vascular disease—will require elucidation. The impact of promoting BMP signaling in PAH with concurrent left ventricle fibrosis, as seen in systemic sclerosis, will also require additional studies.
Augmenting BMP signaling may allow simultaneous targeting of both the lung parenchyma and RV fibrosis in patients (Figure 1), particularly in those with pulmonary fibrosis–induced PH. Furthermore, there may be an opportunity to combine compounds such as BMP9 and FK506 to more precisely modulate BMPR2 pathway signaling, or to develop a personalized approach for patients with genetic mutations in BMPR2, with upstream ligands or downstream intracellular signaling mediators. Overall, the discovery of the FK506-mediated reduction in collagen deposition in the overloaded mouse RV represents an important step toward uncovering the therapeutic potential of BMP-signaling augmentation in PAH.
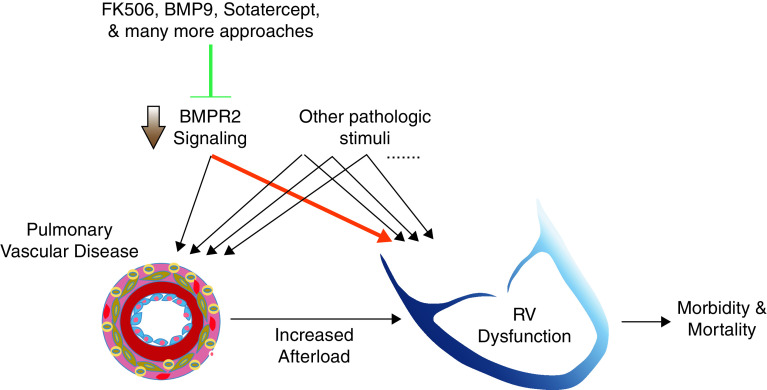
Figure 1.
Increasing BMPR2 (bone morphogenetic protein receptor type 2) signaling may benefit both the lungs and the right ventricle (RV) in pulmonary arterial hypertension. Decreased BMPR2 signaling can contribute to both pulmonary vascular and RV disease, along with other potential pathologic stimuli, and targeting this pathway may therefore have dual beneficial effects in pulmonary arterial hypertension. The highlighted arrow is the focus of the present work by Boehm and colleagues (4).
Footnotes
Supported by National Institutes of Health (NIH) grants F32 HL151076 (M.H.L.), 1P01HL152961 (C.M. and B.B.G.), and 1R01HL135872 (B.B.G.).
Originally Published in Press as DOI: 10.1165/rcmb.2021-0143ED on May 24, 2021
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Sachdev A, Villarraga HR, Frantz RP, McGoon MD, Hsiao JF, Maalouf JF, et al. Right ventricular strain for prediction of survival in patients with pulmonary arterial hypertension. Chest. 2011;139:1299–1309.. doi: 10.1378/chest.10-2015. [DOI] [PubMed] [Google Scholar]
- 2. Girerd B, Weatherald J, Montani D, Humbert M. Heritable pulmonary hypertension: from bench to bedside. Eur Respir Rev. 2017;26:170037. doi: 10.1183/16000617.0037-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Austin ED, Hamid R, Hemnes AR, Loyd JE, Blackwell T, Yu C, et al. BMPR2 expression is suppressed by signaling through the estrogen receptor. Biol Sex Differ. 2012;3:6. doi: 10.1186/2042-6410-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boehm M, Tian X, Ali MK, Mao Y, Ichimura K, Zhao M, et al. Improving right ventricular function by increasing BMP signaling with FK506 Am J Respir Cell Mol Biol 202165272–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Spiekerkoetter E, Tian X, Cai J, Hopper RK, Sudheendra D, Li CG, et al. FK506 activates BMPR2, rescues endothelial dysfunction, and reverses pulmonary hypertension. J Clin Invest. 2013;123:3600–3613. doi: 10.1172/JCI65592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Spiekerkoetter E, Sung YK, Sudheendra D, Scott V, Del Rosario P, Bill M, et al. Randomised placebo-controlled safety and tolerability trial of FK506 (tacrolimus) for pulmonary arterial hypertension. Eur Respir J. 2017;50:1602449. doi: 10.1183/13993003.02449-2016. [DOI] [PubMed] [Google Scholar]
- 7. Long L, Ormiston ML, Yang X, Southwood M, Gräf S, Machado RD, et al. Selective enhancement of endothelial BMPR-II with BMP9 reverses pulmonary arterial hypertension. Nat Med. 2015;21:777–785. doi: 10.1038/nm.3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Humbert M, McLaughlin V, Gibbs JSR, Gomberg-Maitland M, Hoeper MM, Preston IR, et al. Sotatercept for the treatment of pulmonary arterial hypertension. N Engl J Med. 2021;384:1204–1215. doi: 10.1056/NEJMoa2024277. [DOI] [PubMed] [Google Scholar]
- 9. Hautefort A, Mendes-Ferreira P, Sabourin J, Manaud G, Bertero T, Rucker-Martin C, et al. Bmpr2 mutant rats develop pulmonary and cardiac characteristics of pulmonary arterial hypertension. Circulation. 2019;139:932–948. doi: 10.1161/CIRCULATIONAHA.118.033744. [DOI] [PubMed] [Google Scholar]
- 10. Hurst LA, Dunmore BJ, Long L, Crosby A, Al-Lamki R, Deighton J, et al. TNFα drives pulmonary arterial hypertension by suppressing the BMP type-II receptor and altering NOTCH signalling. Nat Commun. 2017;8:14079. doi: 10.1038/ncomms14079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen NY, Collum SD, Luo F, Weng T, Le TT, Hernandez AM, et al. Macrophage bone morphogenic protein receptor 2 depletion in idiopathic pulmonary fibrosis and Group III pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2016;311:L238–L254. doi: 10.1152/ajplung.00142.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Desroches-Castan A, Tillet E, Ricard N, Ouarné M, Mallet C, Belmudes L, et al. Bone morphogenetic protein 9 is a paracrine factor controlling liver sinusoidal endothelial cell fenestration and protecting against hepatic fibrosis. Hepatology. 2019;70:1392–1408. doi: 10.1002/hep.30655. [DOI] [PubMed] [Google Scholar]
- 13. Broen JCA, van Laar JM. Mycophenolate mofetil, azathioprine and tacrolimus: mechanisms in rheumatology. Nat Rev Rheumatol. 2020;16:167–178. doi: 10.1038/s41584-020-0374-8. [DOI] [PubMed] [Google Scholar]
- 14. van der Bruggen CE, Happé CM, Dorfmüller P, Trip P, Spruijt OA, Rol N, et al. Bone morphogenetic protein receptor type 2 mutation in pulmonary arterial hypertension: a view on the right ventricle. Circulation. 2016;133:1747–1760. doi: 10.1161/CIRCULATIONAHA.115.020696. [DOI] [PubMed] [Google Scholar]
- 15. Crnkovic S, Egemnazarov B, Damico R, Marsh LM, Nagy BM, Douschan P, et al. Disconnect between fibrotic response and right ventricular dysfunction. Am J Respir Crit Care Med. 2019;199:1550–1560. doi: 10.1164/rccm.201809-1737OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Graham BB, Robinson JC, Tuder RM. Fatty acid metabolism, bone morphogenetic protein receptor type 2, and the right ventricle. Am J Respir Crit Care Med. 2016;194:655–656. doi: 10.1164/rccm.201603-0592ED. [DOI] [PMC free article] [PubMed] [Google Scholar]