Abstract
Sera (n = 188) from 29 patients with first-episode genital herpes simplex virus type 2 (HSV-2) infections were tested by POCkit-HSV-2 and Western blot (WB) to determine the speed of seroconversion. The median time to detection of HSV-2 antibody was 13 days (range, 3 to 102 days) by the POCkit-HSV-2 test versus 13 days (range, 2 to 58 days) for WB.
The seroprevalence of herpes simplex virus type 2 (HSV-2) has increased by more than 30% over the past 2 decades (8). Most HSV-2-seropositive individuals (75 to 90%) are unaware that they are infected (8, 20). Type-specific antibody detection methods can identify genital HSV-2 infections in individuals with mild or unrecognized clinical manifestations (12, 13). A number of tests based on the type-specific glycoprotein G (gG) are in use in the United Kingdom, Australia, and Europe (7, 10, 18, 19). Such tests are much more accurate than currently available tests based on crude antigen mixtures (1) but are not widely available in the United States (3, 14). One product, the POCkit-HSV-2 test, provides a rapid (6-min) point-of-care format for detecting antibody to gG-2 in capillary blood. Serum antibodies also can be detected by the POCkit-HSV-2 test with sensitivities of 96% compared with Western blot (WB) and 92% compared with a gG-2 type-specific enzyme-linked immunosorbent assay (ELISA) (Gull Laboratories, Salt Lake City, Utah). Specificities were 97 and 100% compared with WB and ELISA, respectively (5).
A rigorous and practical measure of sensitivity is the time after infection required for a test to detect seroconversion. We used the POCkit-HSV-2 test and WB to test 188 sera (3 to 11 sera per patient; median, 7 sera) from 29 patients with culture-documented genital HSV-2 infections: 17 patients were HSV seronegative (primary episodes) and 12 were HSV-1 seropositive (nonprimary episodes) at the time they presented with genital herpes. The median time from the onset of symptoms to the first clinic visit was 4.5 days (range, 1 to 7 days). The date of a patient’s first clinic visit was recorded as day 0.
The POCkit-HSV-2 test was performed by allowing the diluted sample, and then the developing reagent, to pass through a membrane containing a dot with affinity-purified gG-2 and a second dot with human immunoglobulin G (IgG). If both dots turned red, the test was scored positive; if only the human IgG dot changed color, the test was negative. If neither dot changed color, the test was invalid. No invalid test results occurred. Thirty sera (16%) did not pass through the membrane on the first attempt; therefore, fresh portions were filtered and tested successfully.
WB results were tallied for two types of antibody profiles. First, early HSV-2 seroconversion gives a limited WB profile that usually lacks the gG-2 band (2, 16). This result is confirmed by preadsorption of the serum against HSV-1 and HSV-2 antigens and repetition of the test (2). Second, we also recorded the earliest time at which a full HSV-2 antibody profile developed. These profiles all contained the gG-2 band, and preadsorption of the serum was not necessary. This result represents the earliest time that a single serum could give an unequivocal profile for HSV-2.
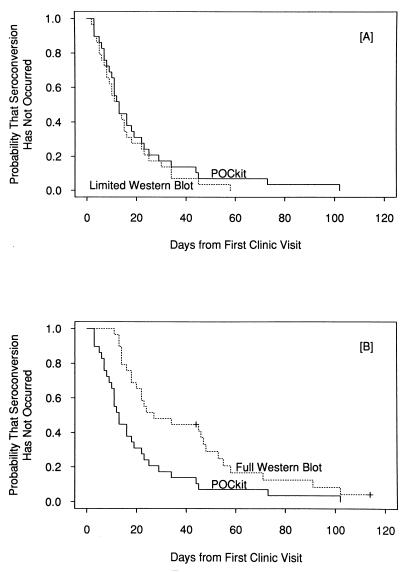
The median number of days required by the POCkit-HSV-2 test to detect HSV-2 antibody in the 29 seroconverting subjects was 13 (range, 3 to 102) (Fig. 1). Antibody responses to primary HSV-2 episodes required 19 days (range, 7 to 102 days), while for those patients with prior HSV-1 antibodies (nonprimary episodes) only 8 days was required (range, 3 to 45 days [P = 0.003; Wilcoxon rank-sum test]). This difference suggests an anamnestic response to type-common epitopes in the gG-2 used in the POCkit-HSV-2 test. Recent epitope mapping studies suggest that the human antibody response is to an immunodominant region of gG that has substantial sequence homology between gG-1 and gG-2 (15). Hashido and colleagues (9) have demonstrated relatively high levels of gG-2 antibodies in nonprimary HSV-2 episodes and lower levels of gG-2 antibodies in response to primary HSV-2 episodes; such titer differences are consistent with a later time to detection of primary gG-2 antibody responses, as seen here. However, their study revealed no increase in the titer of antibody to gG-1 after infection with HSV-2. Thus, it remains unclear why gG-2 antibody responses are more pronounced in HSV-1-seropositive patients.
FIG. 1.
Kaplan-Meier plots showing time to seroconversion determined by the POCkit-HSV-2 test compared with the time to develop a limited (A) or full (B) HSV Western blot profile.
Limited WB profiles developed in a median of 13 days (range, 2 to 58 days) after a patient’s presentation to the clinic (Fig. 1A). Full HSV-2 profiles, including gG-2 bands, were observed at a median of 27 days (range, 11 to >114 days) after presentation (Fig. 1B). A longer time was required for development of complete WB profiles for patients with nonprimary episodes (median, 47.5 days; range, 11 to >114 days) than for those with primary episodes (median, 23 days; range, 13 to 102 days). However, this difference did not reach statistical significance (P = 0.3712; chi-square test). This finding is probably due to the difficulty of detecting HSV-2-specific bands against the background of HSV type-common antibodies in sera from patients with nonprimary episodes.
The time to seroconversion determined by the POCkit-HSV-2 test was comparable to that required to develop a limited WB antibody profile (P = 0.24; Wilcoxon signed-rank test). The POCkit-HSV-2 test was markedly faster than the full WB (P < 0.001 by the paired Prentice-Wilcoxon test [17]) (Fig. 1B). These findings were somewhat surprising since the early WB profiles lacked gG-2 bands and since the production of antibodies to gG-2 is not felt to be among the early responses to HSV-2 infection (16). The WB method in this study uses secondary enzyme-linked reagents directed against human IgG. The POCkit-HSV-2 reagent is directed against human light-chain as well as against human heavy-chain IgG. We have shown that 47 to 52% of sera from first-episode genital HSV-2 patients have IgM to gG-2 but not IgG (6). The POCkit-HSV-2 test may detect IgM to gG-2 in samples with undetectable levels of IgG to gG-2. This conclusion is consistent with the report by Ho et al. of early IgM responses (11) and with our previous findings from a comparison of gG-2 IgG and IgM ELISAs (Gull Laboratories) for detection of seroconversion (4). The gG-2 IgM ELISA was 9 weeks faster (median) in detecting seroconversion in patients with HSV-2 primary episodes and 16 weeks faster (median) in detecting seroconversion in patients with nonprimary episodes than the gG-2 IgG ELISA (4).
In summary, the POCkit-HSV-2 test detects seroconversion within 4 weeks of presentation of 80% of patients with HSV-2 episodes. Further study is required to confirm these findings when the test is used at the point of care with capillary blood. Also, studies to compare this test to other gG-based serology tests are needed to fully evaluate its performance. Currently WB is available to very few patients. A rapid, easily performed test such as POCkit-HSV-2 that is as sensitive as or more sensitive than WB in detecting seroconversion could greatly aid clinicians and laboratories in accurately diagnosing genital HSV-2 infections.
Acknowledgments
This work was supported by National Institutes of Health grant AI-30731 and Diagnology Ltd.
REFERENCES
- 1.Ashley R, Cent A, Maggs V, Nahmias A, Corey L. Inability of enzyme immunoassays to discriminate between infections with herpes simplex virus types 1 or 2. Ann Intern Med. 1991;115:520–526. doi: 10.7326/0003-4819-115-7-520. [DOI] [PubMed] [Google Scholar]
- 2.Ashley R L. Current concepts of laboratory diagnosis of herpes simplex infection. In: Sacks S L, Straus S E, Whitley R J, Griffiths P D, editors. Clinical management of herpes viruses. Washington, D.C: IOS Press; 1995. pp. 137–171. [Google Scholar]
- 3.Ashley R L, Wu L, Pickering J, Tu M, Schnorenberg L. Premarket evaluation of a commercial glycoprotein G-based enzyme immunoassay for herpes simplex virus type-specific antibodies. J Clin Microbiol. 1998;36:294–295. doi: 10.1128/jcm.36.1.294-295.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ashley R L, Pickering J, Pfeiffer N, Wei S, Wu L. Abstracts of the 98th General Meeting of the American Society for Microbiology. Washington, D.C: American Society for Microbiology; 1998. Early diagnosis of first episode genital herpes simplex virus (HSV) infection by enzyme immunoassay (EIA) for type-specific IgM antibodies, abstr. C-392; p. 196. [Google Scholar]
- 5.Ashley R L, Eagleton M. Evaluation of a novel point-of-care test for antibodies to herpes simplex virus type 2. Sex Transm Infect. 1998;74:228–229. [PubMed] [Google Scholar]
- 6.Ashley R L. Type-specific antibodies to HSV-1 and -2: review of methodology. Herpes. 1998;5:33–38. [Google Scholar]
- 7.Enders G, Bartelt U, Risse B, Zauke M. Evaluation of an enzyme immunoassay on the immunoanalyzer Cobas® Core for the specific detection of IgG antibodies to herpes simplex virus type 2. Clin Lab. 1997;43:753–756. [Google Scholar]
- 8.Fleming D T, McQuillan G M, Johnson R E, Nahmias A J, Aral S O, Lee F K, St. Louis M E. Herpes simplex virus type 2 in the United States, 1976 to 1994. N Engl J Med. 1997;337:1105–1111. doi: 10.1056/NEJM199710163371601. [DOI] [PubMed] [Google Scholar]
- 9.Hashido M, Lee F K, Inouye S, Kawana T. Detection of herpes simplex virus type-specific antibodies by an enzyme-linked immunosorbent assay based on glycoprotein G. J Med Virol. 1997;53:319–323. doi: 10.1002/(sici)1096-9071(199712)53:4<319::aid-jmv2>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 10.Ho D W T, Field P R, Sjogren-Jansson E, Jeansson S, Cunningham A L. Indirect ELISA for the detection of HSV-2 specific IgG and IgM antibodies with glycoprotein G (gG-2) J Virol Methods. 1992;36:249–264. doi: 10.1016/0166-0934(92)90056-j. [DOI] [PubMed] [Google Scholar]
- 11.Ho D W T, Field P R, Irving W L, Packham D R, Cunningham A L. Detection of immunoglobulin M antibodies to glycoprotein G-2 by Western blot (immunoblot) for diagnosis of initial herpes simplex virus type 2 genital infections. J Clin Microbiol. 1993;31:3157–3164. doi: 10.1128/jcm.31.12.3157-3164.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koutsky L, Ashley R, Holmes K, Stevens C, Critchlow C, Kiviat N, Lipinski C, Wolner-Hanssen P, Corey L. The frequency of unrecognized type 2 herpes simplex virus infection among women: implications for the control of genital herpes. Sex Transm Dis. 1990;17:90–94. doi: 10.1097/00007435-199004000-00009. [DOI] [PubMed] [Google Scholar]
- 13.Langenberg A, Benedetti J, Jenkins J, Ashley R L, Winter C, Corey L. Development of clinically recognizable genital lesions among women previously identified as having “asymptomatic” herpes simplex virus type 2 infection. Ann Intern Med. 1989;110:882–887. doi: 10.7326/0003-4819-110-11-882. [DOI] [PubMed] [Google Scholar]
- 14.Lee F K, Coleman R M, Pereira L, Bailey P D, Tatsuno M, Nahmias A J. Detection of herpes simplex virus type 2-specific antibody with glycoprotein G. J Clin Microbiol. 1985;22:641–644. doi: 10.1128/jcm.22.4.641-644.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liljeqvist J-A, Trybala E, Svennerholm B, Jeansson S, Sjogren-Jansson E, Bergstrom T. Localization of type-specific epitopes of herpes simplex virus type 2 glycoprotein G recognized by human and mouse antibodies. J Gen Virol. 1998;79:1215–1224. doi: 10.1099/0022-1317-79-5-1215. [DOI] [PubMed] [Google Scholar]
- 16.Lopez C, Arvin A, Ashley R. Immunity to herpesvirus infection in humans. In: Roizman B, Whitley R J, Lopez C, editors. The human herpesvirus. New York, N.Y: Raven Press; 1993. pp. 397–425. [Google Scholar]
- 17.O’Brien P C, Fleming T R. A paired Prentice-Wilcoxon test for censored paired data. Biometrics. 1987;43:169–180. [Google Scholar]
- 18.Slomka M J, Ashley R L, Cowan R M, Cross A, Brown D W G. Monoclonal antibody blocking testes for the detection of HSV-1 and HSV-2 specific humoral responses: comparison with Western blot assay. J Virol Methods. 1995;55:27–35. doi: 10.1016/0166-0934(95)00042-s. [DOI] [PubMed] [Google Scholar]
- 19.Svennerholm B, Olofsson S, Jeansson S, Vahlne A, Lycke E. Herpes simplex virus type-selective enzyme-linked immunosorbent assay with Helix pomatia lectin-purified antigens. J Clin Microbiol. 1984;19:235–239. doi: 10.1128/jcm.19.2.235-239.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wald A, Koutsky L, Ashley R L, Corey L. Genital herpes in a primary care clinic: demographic and sexual correlates of herpes simplex type 2 infections. Sex Transm Dis. 1997;24:149–155. doi: 10.1097/00007435-199703000-00005. [DOI] [PubMed] [Google Scholar]