Abstract
Background
A novel [64Cu]Cu-NOTA-aCD40 immunoPET tracer was developed to image a CD40+ pancreatic tumor model in C57BL/6 mice and to study the biodistribution profile of the agonist CD40 (aCD40) monoclonal antibody (mAb) alone or combined with other mAbs.
Procedures
Copper-64 ([64Cu]Cu) labeled NOTA-aCD40 and NOTA-IgG (10 μg; 7 MBq) were injected intravenously into C57BL/6 mice with subcutaneous mT4 tumors to assess specificity 48 h post injection (p.i.) through positron emission tomography/computed tomography (PET/CT) imaging and biodistribution studies (n = 5). [64Cu]Cu-NOTA-aCD40 was injected alone or simultaneously in combination with a therapeutic mass of cold aCD40 (100 μg), aPD-1 (200 μg) and aCTLA-4 (200 μg) mAbs. A group of mice with or without tumor received the second round of injections 1 or 3 weeks apart, respectively. PET/CT imaging and biodistribution studies were performed at 48 h p.i. The organ dose for [64Cu]Cu was estimated based on biodistribution studies with 2 μg [64Cu]Cu-NOTA-aCD40 (corresponds to 5 mg patient dose) in non-tumor bearing mice.
Results
[64Cu]Cu-NOTA-aCD40 accumulation was 2.3- and 7.8-fold higher than [64Cu]Cu-NOTA-IgG in tumors and spleen, respectively, indicating the specificity of aCD40 mAb in a mouse pancreatic tumor model. Tumor accumulation of [64Cu]Cu-NOTA-aCD40 was 21.2 ± 7.3 %ID/g at 48 h after injection. Co-injection of [64Cu]Cu-NOTA-aCD40 with cold aCD40 mAb alone or with PD-1 and CTLA-4 mAbs reduced both spleen and tumor uptake, whereas liver uptake was increased. With the second round of injections, the liver was the only organ with substantial uptake. With a 2 μg administered dose of [64Cu]Cu-NOTA-aCD40 in a dosimetry study, the liver to spleen ratio was greater compared to the 10 μg dose (2.8 vs 0.37; respectively). The human equivalent for the highest dose organ (liver) was 198 ± 28.7 μSv/MBq.
Conclusions
A CD40-immunoreactive [64Cu]Cu-NOTA-aCD40 probe was developed. The ratio of spleen to liver accumulation exceeded that of the IgG isotype and was greatest with a single small, injected mass. The safety of human patient imaging with [64Cu]Cu was established based on extrapolation of the organ specificity to human imaging.
Keywords: CD40 monoclonal antibody, Positron emission tomography, Pancreatic cancer
Graphical Abstract
1. Introduction
Recent studies have provided compelling evidence as to the utility of agonist CD40 (aCD40) monoclonal antibodies (mAbs) within multicomponent protocols to treat pancreatic cancer [1–15]. In particular, clinical studies combining aCD40 with checkpoint modulators and chemotherapy have shown highly encouraging data [15–19]. CD40 is expressed on a subset of pancreatic cancer cells and the overwhelming majority of peritumoral lymphocytes [20, 21]. For pancreatic ductal adenocarcinoma (PDAC), the aCD40 mAb promotes stromal degradation, dendritic cell (DC) maturation and alters macrophage phenotype, and therefore is an attractive approach for immunotherapy [22–24]. However, while NCT03214250 (combining gemcitabine and Abraxane with aCD40 and aPD-1 immunotherapy) yielded very promising results in which all patients receiving all components demonstrated regression of metastatic pancreatic cancer, T cell activation was not observed, and patients were not cured. Reliably delivering these treatments in human pancreatic cancer is challenging due to the dense stroma and limited vascular supply. Improving our understanding of the mAb delivery could improve outcomes.
We, therefore, created a positron emission tomography (PET) imaging probe to assess the accumulation of the aCD40 mAb and changes in CD40 expression with treatment. Unusually short serum half-life has been reported for aCD40 mAbs (e.g. Dacetuzumab (SGN-40), Selicrelumab (CP-870,893)) and the half-life is dose-dependent [25, 26]. Reducing dosing intervals to compensate for short serum half-life has led to chronic B cell activation, and T cell depletion in some patients [27]. The rationale for developing aCD40 mAb-based PET imaging includes the need to establish the PK as a function of dose and time (to inform the dosing schedule for CD40 immunotherapy) and determine the distribution of CD40-expressing cells with and without therapy. Measuring the correlation between tumor uptake and response could provide a tool for early non-invasive discrimination between responders and non-responders in early treatment.
Eventual human studies of aCD40 pharmacokinetics would also inform possible immunogenicity by evaluating the clearance rate. Although fully human and humanized mAbs have been developed to lower the risk of immunogenicity, due to residual mouse sequences in the complementarity determining regions (CDRs), patients may still develop anti-drug antibodies (ADA) and neutralizing antibodies after repeated injections [28, 29]. For example, according to United States Prescribing Information (USPI), post-treatment ADA has been detected in 30–48% of patients that received more than one injection of atezolizumab, a humanized aPD-L1 IgG1. Similarly, another study showed that up to 37.8% of patients develop ADA following treatment with nivolumab, a human aPD-1 IgG4 (reviewed in [28]).
Encouraging results from recent clinical trials of other radiolabeled mAbs also support the evaluation of radiolabeled agonist and antagonist mAbs as a potential tool for response prediction in cancer patients. Zirconium-89 ([89Zr]Zr: T1/2 = 78.4 h) labeled versions of almost all currently approved PD-1 and PD-L1 targeting mAbs have been developed for PET imaging ([89Zr]Zr-nivolumab [30], [89Zr]Zr-durvalumab [31], [89Zr]Zr-avelumab [32] (NCT03514719), [89Zr]Zr-atezolizumab [33, 34] [89Zr]Zr-pembrolizumab [35]). [89Zr]Zr-pembrolizumab (37 MBq; 2 mg) PET/CT scans from NSCLC patients (NCT02760225) revealed high spleen and liver uptake [35]. Tumor uptake was higher in responding patients than non-responding patients but did not correlate with PD-1 expression. PET scans at 7 days after 89Zr-nivolumab injection in NSCLC patients showed high accumulation in the spleen and liver with low to moderate uptake in the lungs and bone marrow [30]. [89Zr]Zr-nivolumab with or without cold nivolumab co-injection as a predictive imaging biomarker of response is also in phase II in patients with NSCLC (NCT03065764). In another study, following baseline FDG-PET imaging, 14 patients with incurable squamous cell carcinoma of the head and neck (SCCHN) received [89Zr]Zr - durvalumab (37 MBq), at a dose of 2 mg, 10 mg or 50 mg durvalumab (NCT03829007). A PET scan was acquired at day 5 post injection and demonstrated prolonged blood circulation time with increasing mAb dose [31]. The best tumor visualization was achieved using 10 or 50 mg durvalumab with 10 mg durvalumab having the highest tumor to blood ratio.
The longer half-life of copper-64 [[64Cu]Cu: T1/2 (12.7 h); Eβ+(0.65 MeV, 17.4%); Eβ− (0.57 MeV, 39.0%)] and zirconium-89 [89Zr]Zr: T1/2 (78.4 h); β+ (395 keV); γ ray (909 keV)] compared to fluorine-18 [[18F]F: T1/2 (109.7 min); Eβ+(0.63 MeV, 97%)] renders these isotopes suitable for mAb-based PET imaging. The half-life of [89Zr]Zr matches the long circulation time of some mAb and allows imaging at late time points (e.g. 7 days); however, high energy γ radiations emitted by [89Zr]Zr might be a dose-limiting factor particularly for the liver [36–38]. In this study, we developed [64Cu]Cu-NOTA-aCD40 mAb to evaluate whether PET imaging using this probe would be informative for optimization of dosing schedule and response in a pre-clinical context. aCD40 immunoPET imaging may also help us to evaluate whether specific therapies (e.g., chemotherapy or ablative treatments) alter the mAb pharmacokinetics (PK) and biodistribution. Here, we evaluate the biodistribution of a preclinical aCD40 mAb as a function of diagnostic and therapeutic doses in a murine model of pancreatic cancer. In particular, we add therapeutic doses of PD-1 and CTLA-4 checkpoint inhibitors to reveal any changes in the biodistribution profile of aCD40 mAb for combination protocols of immunotherapy.
2. Materials and methods
Anti-mouse aCD40 (BE0016-2), anti-PD-1 (BE0146), anti-CTLA-4 (BE0164) and rat IgG2a (BE0089) mAbs were purchased from Bio X Cell. Binding assays were done using commercially available products including magnetic beads functionalized with nickel-nitrilotriacetic acid (Ni-NTA, Thermo Scientific, 88831), 1.5 mL lobind tubes (Fisher Scientific, 13-698-794), mouse histidine-tagged CD40 (His-CD40, AcroBiosystems, TN5-M52H8) and PBS containing 0.05% Tween-20 (Sigma-Aldrich, P3563). All radiolabeling experiments were conducted under a controlled radiation authorization approved by Stanford University (Palo Alto, CA).
2.1. NOTA conjugation and characterization
Trace metals were removed from all buffers using Chelex-100 cation-exchange resin (Bio-Rad, 142-2832). aCD40 was buffer-exchanged and concentrated to 10 mg/mL in 0.1 M NaHCO3, pH 9.0, in an Amicon Ultra device (Millipore, UFC503096; MWCO = 30 kDa). Protein concentration was determined spectrophotometrically [E280nm = 1.4 (mg/mL)™1cm™1] and also with the Bradford assay using Pierce Coomassie Plus Assay Reagent (Thermo Scientific, 23238). aCD40 was reacted with a 15-fold molar excess of 2-S-(4-isothiocyanatobenzyl)-1,4,7-triazacyclononane-1,4,7-triaceticacid (p-SCN-Bn-NOTA; Macrocyclics, B605) for 2 h at 22 °C. Unconjugated NOTA was washed out from the aCD40-NOTA conjugation mixture by ultrafiltration with 0.1 M CH3CO2Na buffer, pH 5.5, in an Amicon Ultra device (MWCO = 30 kDa). To determine the substitution level of NOTA per aCD40 molecule, aliquots of aCD40 and aCD40-NOTA (5 μL, 20 μM) were analyzed by electrospray ionization mass spectrometry (ESI-MS) on an Agilent 1260 HPLC and Bruker MicroTOF-Q II using a Waters MassPREP 5 × 2.1 mm diphenyl desalting column, at 50 °C, with a flow rate of 0.3 ml/min. Spectra were collected in full scan MS mode with a mass range of 600–4000 Da and collision RF setting of 1200 Vpp. The mean NOTA/aCD40 ratio was determined by calculating the difference in the mass of aCD40 before and after NOTA conjugation, divided by the mass of a single NOTA substituent.
2.2. [64Cu]CuCl2 labeling and stability in mouse plasma
aCD40-NOTA was incubated with [64Cu]CuCl2 (University of Wisconsin) in 0.1 M CH3CO2Na buffer pH 5.5 for 1 h at 40° C to achieve the specific activity of 0.74 MBq/μg. The radiochemical purity was assessed by instant thin-layer chromatography (ITLC; Biodex Medical Systems) developed in 0.1 M sodium citrate solution, pH 5.0. Purity and homogeneity were assessed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) on a 4–20% Tris-Glycine mini gel (Novex™, XP04200PK2). [64Cu]Cu-NOTA-aCD40 (0.74 MBq/μg) was incubated in mouse plasma (Innovative Research, IGMSC57PLAK2E) at 37 °C in duplicate. Samples were analyzed by ITLC and ultrafiltration through a Millipore centrifugal filter device (MWCO =100 kDa) and measuring the percentage of radioactivity filtered vs retained through the device in a γ-counter up to 72 h.
2.3. Immunoreactivity and characterization of mouse model
The immunoreactivity of [64Cu]Cu-NOTA-aCD40 was assessed by a rapid binding assay in triplicate, as reported in Sharma et al. [39]. Briefly, in nine 1.5 mL lobind tubes, 380 μL PBS-0.05% Tween (PBS-T) were added to 20 μL Ni-NTA magnetic beads. The tubes were vortexed followed by a brief spin and supernatant was removed by placing the tubes on a magnetic rack for 1–2 min. The washed beads were resuspended in 390 μL PBS-T. Mouse His-CD40 (1 μg; 0.1 mg/mL) was added to six tubes for 15 min on a rotating mixer at room temperature to coat the beads with CD40. The beads in the other three tubes remained intact as a control arm. His-CD40 coated beads were washed again with 400 μL PBS-T before incubation with 1 ng of the [64Cu]Cu-NOTA-aCD40 in the absence (total binding) or presence of 2 μg unlabeled aCD40 (non-specific binding) for 30 min on a rotating mixer at room temperature. Naked beads in the control arm were similarly incubated with 1 ng of [64Cu]Cu-NOTA-aCD40. Following incubation, supernatant was collected by isolation of the beads using the magnetic rack. The beads were washed two times with 400 μL PBS-T and the supernatant was collected separately in different tubes. The beads, supernatant and washes were measured for radioactivity on a gamma counter. Total binding of [64Cu]Cu-NOTA-aCD40 was determined from the percentage of total radioactivity bound to His-CD40 coated beads minus the percentage of non-specific binding.
All animal studies were performed in compliance with approval from the Administrative Panel on Laboratory Animal Care (APLAC) at Stanford University. PET/CT imaging, biodistribution and therapy studies were conducted in groups of 4–5 female C57BL/6 mice (The Jackson laboratory, Bar Harbor, ME) bearing subcutaneous (s.c.) bilateral mT4 pancreatic tumors. mT4 tumor organoid cells [40] were a gift from David Tuveson, previously shown to display characteristics of human pancreatic cancer. In culture, 62.7% of cells were aCD40+ including 36.5% of EpCAM+ cells (Supplementary Fig. S1). C57BL/6 mice were inoculated with 4 × 105 mT4 cells in 40 μL of a 1:1 mixture of matrigel and PBS. Studies started with tumors of 3–4 mm.
2.4. PET/CT imaging and biodistribution studies
Groups of 5 C57BL/6 mice with mT4 tumors received [64Cu]Cu-NOTA-aCD40 or [64Cu]Cu-NOTA-IgG (7.4 MBq, 10 μg) via tail vein injection. Mice were anesthetized with 2% isoflurane at room temperature, and whole body radioactivity was measured at 0, 24 and 48 h post injection (p.i.) by placing the mice in a dose calibrator (Capintec CRC 15R). Mice were then placed in the prone position and near the center of the field of view of the PET/CT scanner. Thirty-minute scans were acquired on a Siemens Inveon PET/CT machine at 24 and 48 h p.i. Images were reconstructed using a two-dimensional ordered-subset expectation maximization (OSEM 2D) algorithm. At 48 h p.i. biodistribution studies were performed to compare tumor and normal tissue uptake of [64Cu]Cu-NOTA-aCD40 and [64Cu]Cu-NOTA-IgG. Following imaging, mice were sacrificed by cervical dislocation under anesthesia. Tissues of interest were collected, counted using a γ-counter, weighted and the results were reported as % ID/g.
2.5. Monitoring response to aCD40-based immunotherapy protocol
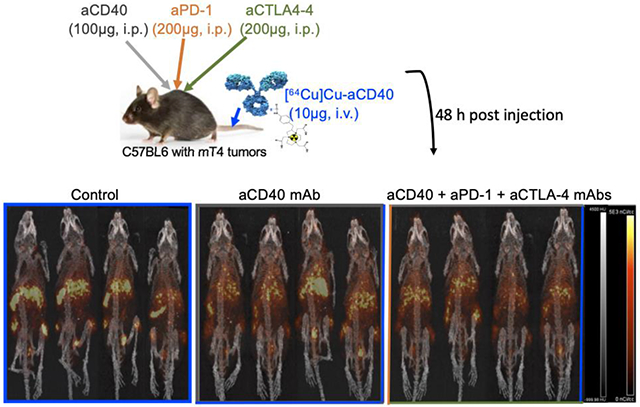
Tumor-bearing mice were injected intraperitoneally (i.p.) with a combination of aPD-1 (200 μg), aCD40 (100 μg) and aCTLA-4 (200 μg) mAbs or CD40 mAbs (100 μg) alone. [64Cu]Cu-NOTA-aCD40 (10 μg) was injected i.v. along with other mAbs and also one week after treatment. Mice were imaged at 48 h p.i. (Scheme 1) and biodistribution was performed at 48 h after the second injection.
Scheme 1.
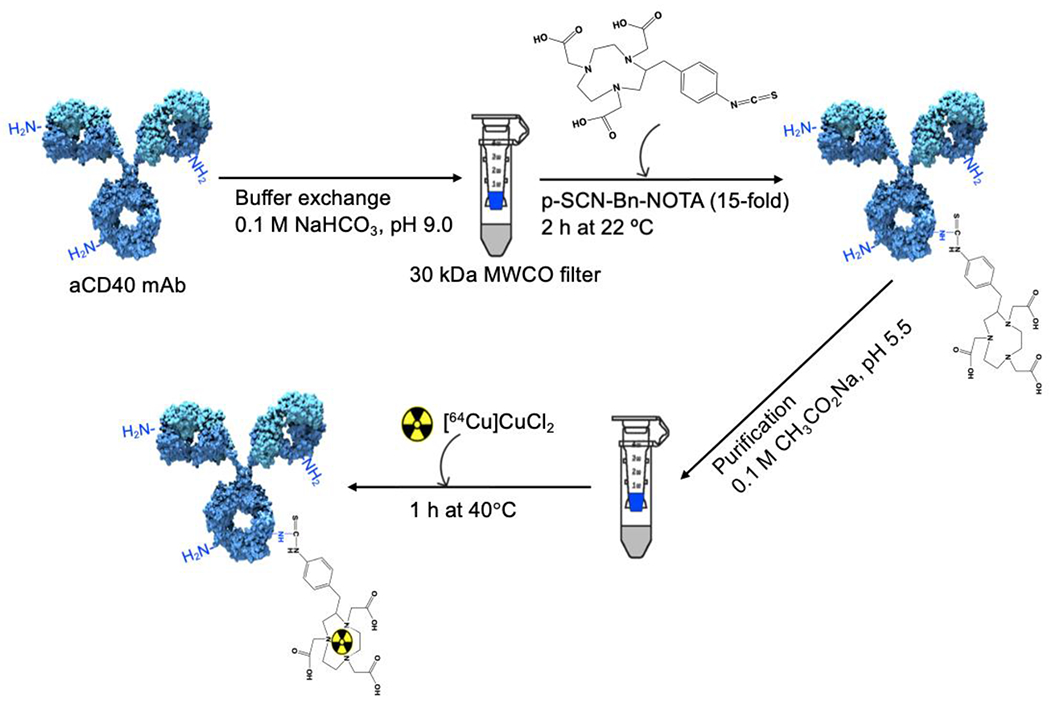
[64Cu]Cu-NOTA-aCD40 synthesis. aCD40 mAb was incubated with 15-fold molar excess p-SCN-Bn-NOTA in 0.1 M NaHCO3, pH 9.0 for 2 h at 22 °C. Unconjugated NOTA molecules were removed using an ultracentrifuge and 0.1 M CH3CO2Na, pH 5.5.64Cu-labeling was performed at 40 °C for 1 h.
2.6. Immunogenicity
To evaluate whether a change in the treatment interval can alter the biodistribution profile of aCD40, non-tumor bearing mice were divided into the control, monotherapy and combination therapy groups. In this study, mice received the second injections after 3 weeks. Mice were imaged and dissected for biodistribution at 48 h after the second injection.
2.7. Radiation dosimetry and pharmacokinetics
The normal tissue accumulation and elimination of [64Cu]Cu-NOTA-aCD40 was studied in groups of 4 non-tumor bearing female C57BL/6 mice following i.v. injection (2 μg; 1–2 MBq). The administered dose of aCD40 mAb corresponds to the proposed human dose (≤5 mg) scaled on a μg/kg basis (FDA guideline). Biodistribution was then performed at selected times (3, 24, 48, and 72 h p.i.). To project the human doses, the radioactivity in the normal organs of human adults was proportionately extrapolated from that in mice using the %kg/g method [(%ID/organ)human = (%ID/organ)mouse × (mouse body weight (kg) / mouse organ weight (g)) × (human organ weight (g)/human body weight (kg))][41]. The time-integrated radioactivity (MBq.h) in normal source organs (Ã0-72h) was derived from biodistribution data using Prism Ver. 4.0 software (GraphPad). The time-integrated radioactivity from 72 h to infinity (Ã72h-∞) was calculated by dividing the radioactivity at the final measured time point by the decay constant for [64Cu]Cu (0.0545 h−1), thus assuming that further elimination occurs only by radioactive decay without additional biological elimination [42, 43]. The combined A(0-∞h) for each organ was used to predict the radiation-absorbed doses in human adults (μSv/MBq) using OLINDA/EXM 1.0 software [44].
Blood samples were collected and the radioactivity concentration in the blood (%ID/mL) was measured by γ-counter and then plotted versus the time. The elimination curves were fitted to a two-compartment pharmacokinetic model and standard pharmacokinetic parameters was estimated.
2.8. Statistical analysis
Data were presented as mean ± standard deviation of the mean (SD) for n = 3–5 replicates. Statistical comparisons were made using an unpaired Student’s t-test or one-way ANOVA. The significance of the value is indicated by asterisks (*P < 0.05, **P < 0.01, and ***P < 0.001).
3. Results
3.1. NOTA conjugation and characterization
The difference in the molecular weight of aCD40 mAb before (145,580.7 g/mol or 145,581 g/mol) and after (146,353.2 g/mol or 146,483 g/mol) NOTA conjugation divided by the molecular weight of NOTA (559.9 g/mol) indicates that each aCD40 mAb molecule is conjugated to on average 1.4–1.6 NOTA as shown by ESI-MS (Fig. 1a). SDS-PAGE confirmed the homogeneity of NOTA-aCD40 conjugates (Fig. 1b).
Fig. 1.
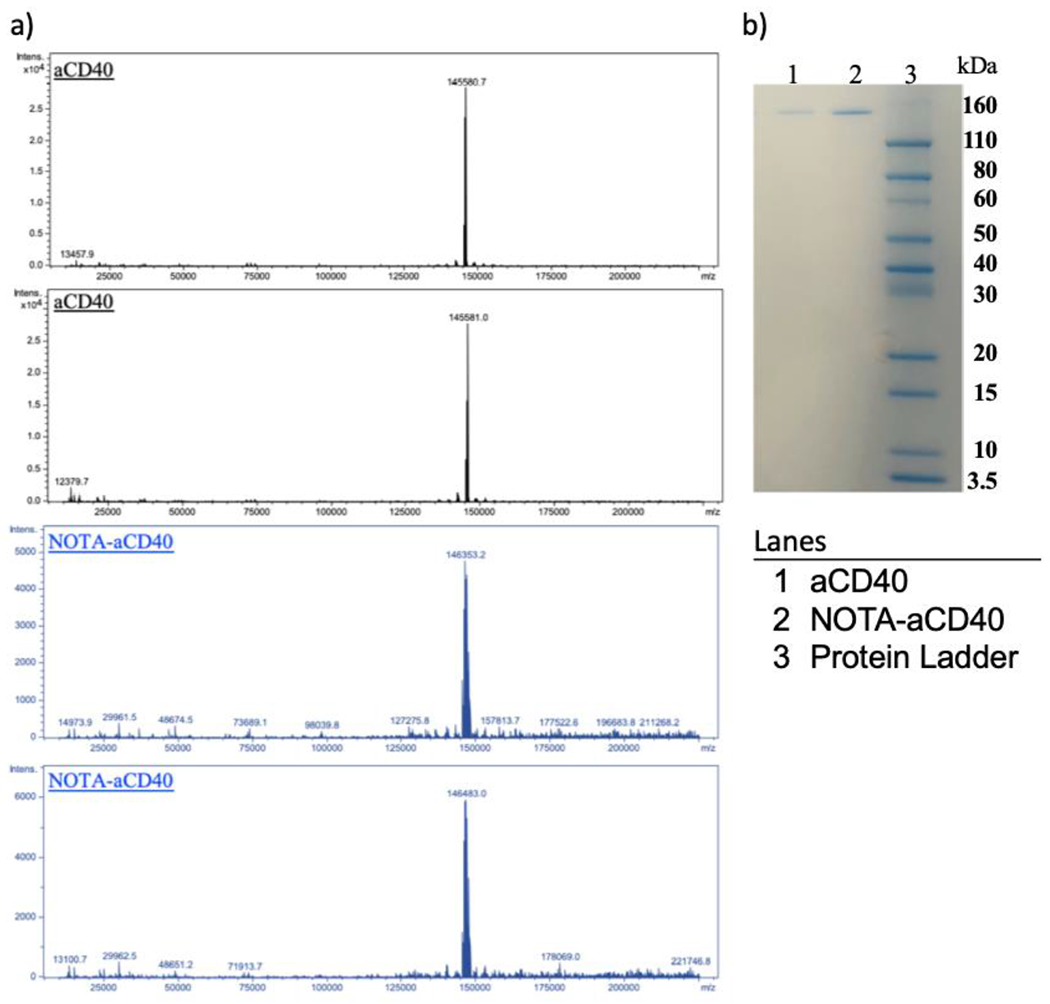
Electrospray ionization–mass spectrometry (ESI-MS) and SDS-PAGE. a) Estimation of NOTA substitution level on aCD40 mAb molecule by mass spectrometry in duplicate. Mass difference between the peak of NOTA-aCD40 in blue (146,353.2 kDa, 146,483 kDa) and aCD40 in black (145,580.7 kDa, 145,581 kDa) accounts for average 1–2 NOTA per CD40 with as absolute mass of NOTA is 559.9. b) SDS-PAGE shows the purity of aCD40 before and after NOTA conjugation. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.2. [64Cu]CuCl2 labeling and stability in mouse plasma
The radiochemical purity of [64Cu]Cu-NOTA-aCD40 at a specific activity of 20 μCi/μg was over 97%. No release of radioactivity was measured by ultrafiltration (MWCO = 100 kDa) or ITLC, indicating the stability of [64Cu]Cu-NOTA-aCD40 in mouse plasma at 37 °C for up to 72 h (Fig. 2).
Fig. 2.
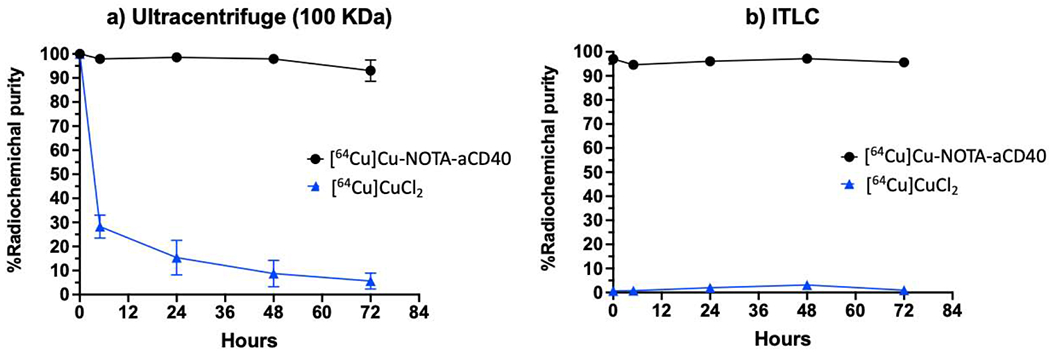
Measurement of [64Cu]Cu-NOTA-aCD40 stability in human plasma at 37 °C using ultracentrifuge with 100 kDa Amicon Ultra device (a) and ITLC (b). No release of radioactivity from [64Cu]Cu-NOTA-aCD40 was observed indicating the stability of the conjugates over a period of 72 h.
3.3. Immunoreactivity
Total binding of [64Cu]Cu-NOTA-aCD40 to His-CD40 coated beads in the unblocking arm was measured and compared to non-specific binding after blocking CD40 antigens with unlabeled aCD40 mAb as well as to naked beads (Fig. 3). Bead-bound activity decreased in the blocking and control arms (45.7 ± 3.6% and 40.55 ± 0.1%, respectively) compared to the main arm (85.2 ± 1.9%). There was no significant difference in the non-specific binding between the blocked and the control arm (P > 0.5).
Fig. 3.
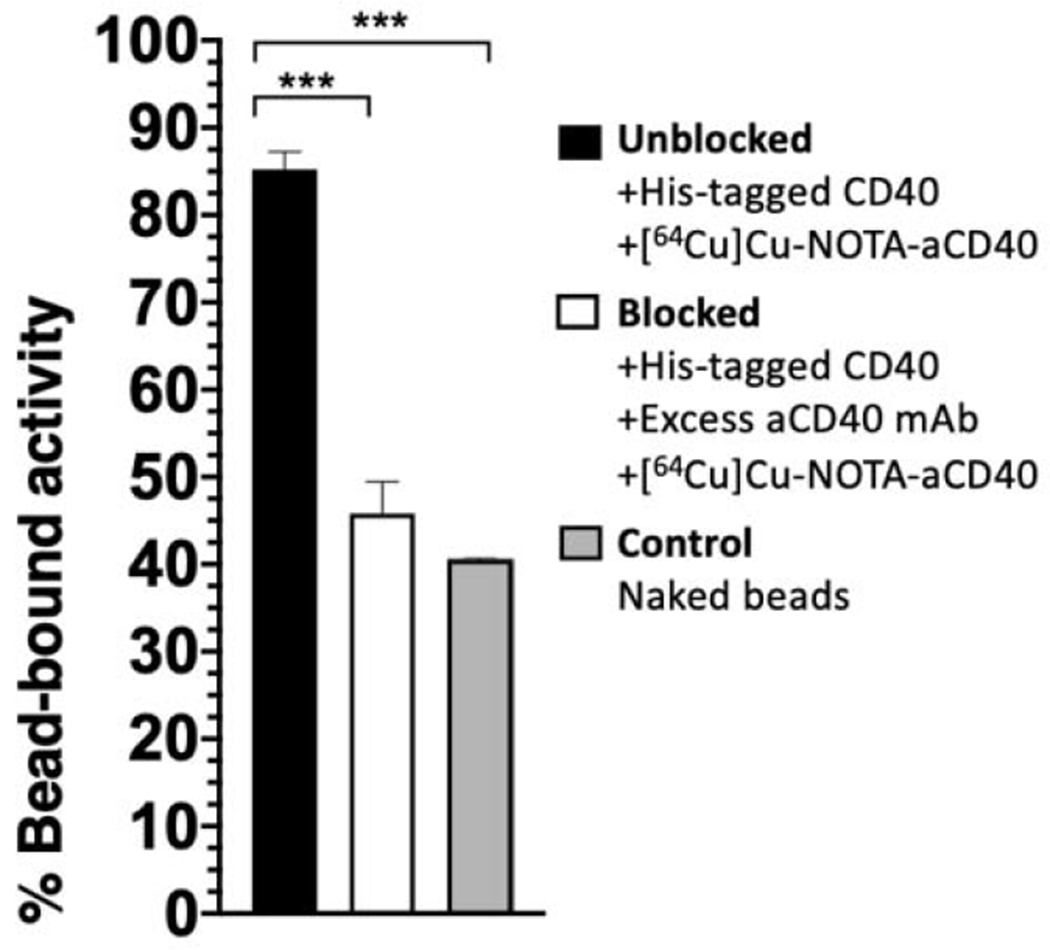
Ni-NTA bead-based [64Cu]Cu-NOTA-aCD40 rapid binding assay. Controls include non-specific binding of [64Cu]Cu-NOTA-aCD40 either to naked beads or to His-CD40 coated beads in the presence of an excess of unlabeled CD40 mAb (blocked arm). Total binding of [64Cu]Cu-NOTA-aCD40 to CD40-coated beads shows a significantly higher binding compared to the controls (*** indicates P < 0.001).
3.4. PET/CT imaging and biodistribution
A biodistribution study was performed to compare [64Cu]Cu-NOTA-aCD40 and [64Cu]Cu-NOTA-IgG at 48 h p.i. in C57BL/6 mice bearing s.c. mT4 xenografts (Fig. 4a). Percent injected dose per gram organ (%ID/g) values are shown in Supplementary Table S1. This study revealed a significantly higher uptake of [64Cu]Cu-NOTA-aCD40 in tumors (21.2 ± 7.3%ID/g vs 9.2 ± 1.9%ID/g; P = 0.007), liver (18.2 ± 4.6%ID/g vs 10.3 ± 1.7%ID/g; P = 0.007), spleen (48.9 ± 15.1%ID/g vs 6.3 ± 0.8%ID/g; P = 0.0002) and bone (4.2 ± 0.9%ID/g vs 2.5 ± 0.2%ID/g; P = 0.002) than that of [64Cu]Cu-NOTA-IgG (Figure 4a). Uptake of [64Cu]Cu-NOTA-IgG was higher in blood (8.8 ± 2%ID/g vs 17.5 ± 0.9%ID/g; P = 0.0002) and heart (3.0 ± 1.1%ID/g vs 6.8 ± 1.3%ID/g; P = 0.0008) compared to [64Cu]Cu-NOTA-aCD40. Whole body retention up to 48 h p.i. was 49.1 ± 7.5%/ID of [64Cu]Cu-NOTA-aCD40 and 31.5 ± 2.8%/ID of [64Cu]Cu-NOTA-IgG (Supplementary Fig. S2 and Table S2). The PET/CT images are correlated with the results of biodistribution studies (Fig. 4b).
Fig. 4.

Biodistribution (a) and PET/CT images (b) in C57BL/6 with bilateral s.c. mT4 tumors at 48 h post injection (p.i.) of [64Cu]Cu-NOTA-aCD40 and [64Cu]Cu-NOTA-IgG. Visualized on the images are tumors (encircled), the liver (L, wide arrowhead) and spleen (S, narrow arrowheads). Asterisks are **** (P < 0.0001), *** (P < 0.001), and ** (P < 0.01).
3.5. Monitoring immune related response
Subcutaneously implanted mT4 pancreatic tumor bearing mice were injected intraperitoneally (i.p.) with a combination of aPD-1 (200 μg), aCD40 (100 μg) and aCTLA-4 (200 μg) mAbs or aCD40 mAbs (100 μg) alone. [64Cu]Cu-NOTA-aCD40 (10 μg) was injected i.v. along with other mAbs and also one week after treatment. Mice were imaged at 48 h after the first and second injection (Scheme 2), and biodistribution was performed following the second scan (Fig. 5a and b) and %ID/g values are shown in Supplementary Table S3. Injection of the therapeutic mass of cold aCD40 mAb alone or in combination with aPD-1 and aCTLA-4 mAbs significantly reduced the tumor (7.6 ± 0.5%ID/g and 9.5 ± 3.1%ID/g) and spleen uptake (12.5 ± 0.2%ID/g, 22.2 ± 8.2%ID/g) of [64Cu]Cu-NOTA-aCD40 compared to the control group [21.2 ± 7.3%ID/g (P < 0.0001) and 48.9 ± 15%ID/g (P < 0.0001)]; respectively (Fig. 4a, Supplementary Fig. S3) 48 h after injection. However, liver uptake was increased from 18.2 ± 4.6%ID/g in the control group to 23.6 ± 0.9%ID/g (P < 0.0001) and 25 ± 4%ID/g (P = 0.008) with single and combination injections. With the second round of injections a week later, the biodistribution profile of [64Cu]Cu-NOTA-aCD40 was not significantly different between the control, monotherapy and combination therapy groups. All groups showed a significantly higher liver (43.8 ± 4%ID/g, 49.4 ± 6.4%ID/g, 42.7 ± 3.1%ID/g) uptake compared to the first injection whereas a significantly reduced spleen (8.9 ± 3.3%ID/g, 4.7 ± 1.1%ID/g, 7.9 ± 1.5%ID/g) and tumor (1.4 ± 0.4%ID/g, 2.6 ± 1.5%ID/g, 1.3 ± 1%ID/g) uptake was observed (P < 0.001 for all). Based on whole body activity (Supplementary Fig. S4 and Table S4), the second round of injections significantly accelerated the clearance of [64Cu]Cu-NOTA-aCD40 in monotherapy and combination therapy (28.4 ± 14.9% and 33.2 ± 12.9%) groups compared to the first injection [50.5 ± 12.9% (P = 0.0019) and 56.7 ± 6.5% (P = 0.015), respectively]. No significant difference in the clearance rate of radiolabeled aCD40 between the first and second injections was observed in the control group [48.8 ± 3.6% vs 31.8 ± 9.2% (P = 0.06), respectively].
Scheme 2.
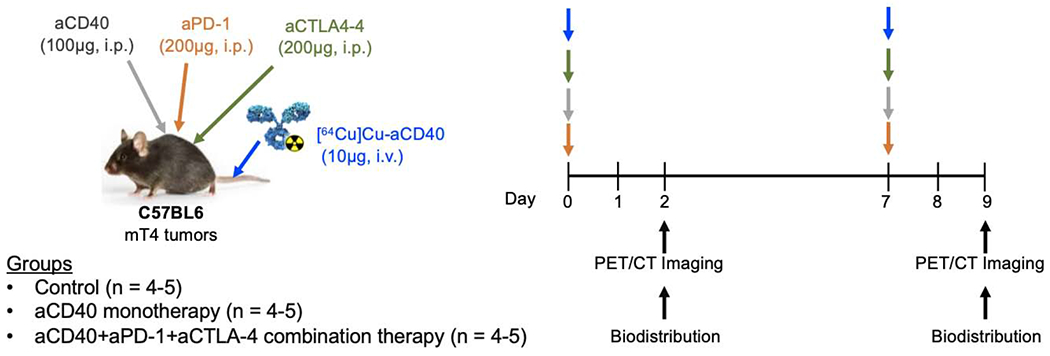
Injection schedule. C57BL/6 mice with bilateral s.c. mT4 tumors first received [64Cu]Cu-NOTA-aCD40 (10 μg) intravenously immediately followed by i.p. injection of a therapeutic dose of aCD40 alone or in combination with aPD-1 and aCTLA-4 mAbs. PET/CT imaging was performed at 48 h p.i. The second round of injections and imaging were repeated a week later.
Fig. 5.
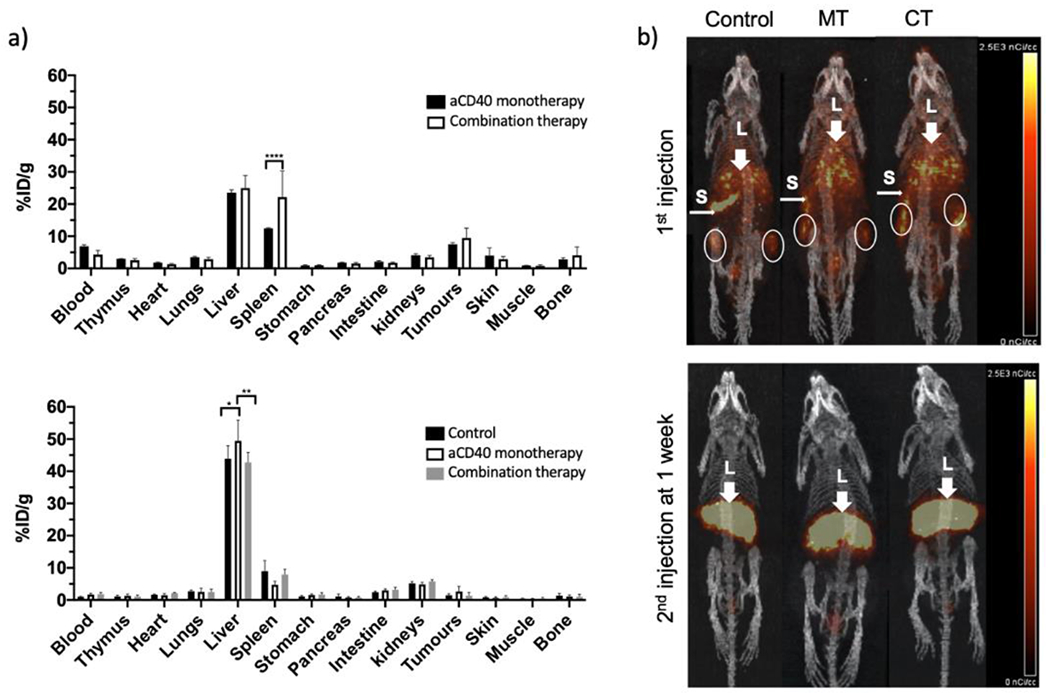
Biodistribution (a) and PET/CT images (b) in C57BL/6 mice with bilateral s.c. mT4 tumors at 48 h after the first (upper row) and second round of injections (bottom row) with [64Cu]Cu-NOTA-aCD40 (control), [64Cu]Cu-NOTA-aCD40 and aCD40 mAb without (monotherapy, MT) or with aPD-1 and aCTLA-4 mAbs (combination therapy, CT). L with thick arrowhead and S with narrow arrowheads represent liver and spleen, respectively. Asterisks are **** (P < 0.0001), **(P < 0.01), and *(P < 0.05).
3.6. Immunogenicity
Non-tumor bearing mice were injected two times with either aCD40 alone or in combination with aPD-1 and aCTLA-4 three weeks apart. Biodistribution and imaging at 48 h after the second injection showed a similar distribution of radioactivity among all the treated and non-treated control mice, and in particular an intense liver uptake (~50%ID/g; Supplementary Table S5). Blood and spleen uptake were less than 0.8%ID/g and 8%ID/g, respectively (Fig. 6a and b).
Fig. 6.

Biodistribution (a) and PET/CT images (b) in non-tumor bearing mice 48 h after the second round of injections with [64Cu]Cu-NOTA-aCD40, aCD40, aPD-1 and aCTLA-4 mAbs. L with thick arrowhead and S with narrow arrowheads represent liver and spleen, respectively.
3.7. Radiation dosimetry and pharmacokinetics
The concentration of radioactivity in normal C57BL/6 mice at selected times post intravenous injection of [64Cu]Cu-NOTA-aCD40 is shown in Fig. 7. %ID/g values are shown in Supplementary Table S6. Significant liver (47.7 ± 7.7%ID/g) and spleen uptake (28.4 ± 5.7%ID/g) was observed as early as 2–3 h p.i. and gradually decreased over time up to 72 h p.i. Similarly, the highest blood concentration was at the earliest time point at 5.7 ± 0.8%ID/g. [64Cu]Cu-NOTA-aCD40 exhibited a biexponential elimination from the blood within the first 2–3 h after intravenous administration to mice (Fig. 8). The distribution half-life (α-phase) and the elimination phase half-life (β-phase) was approximately 0.6 ± 0.05 h and 65.9 ± 64.6 h. The volume of distribution of the central compartment (V1) in mice was 2 ± 0 mL and the volume of distribution at steady state (Vss) was 1232 ± 605 mL. The systemic clearance (CLs) was 0.3 ± 0.1 mL/h (Table 1).
Fig. 7.
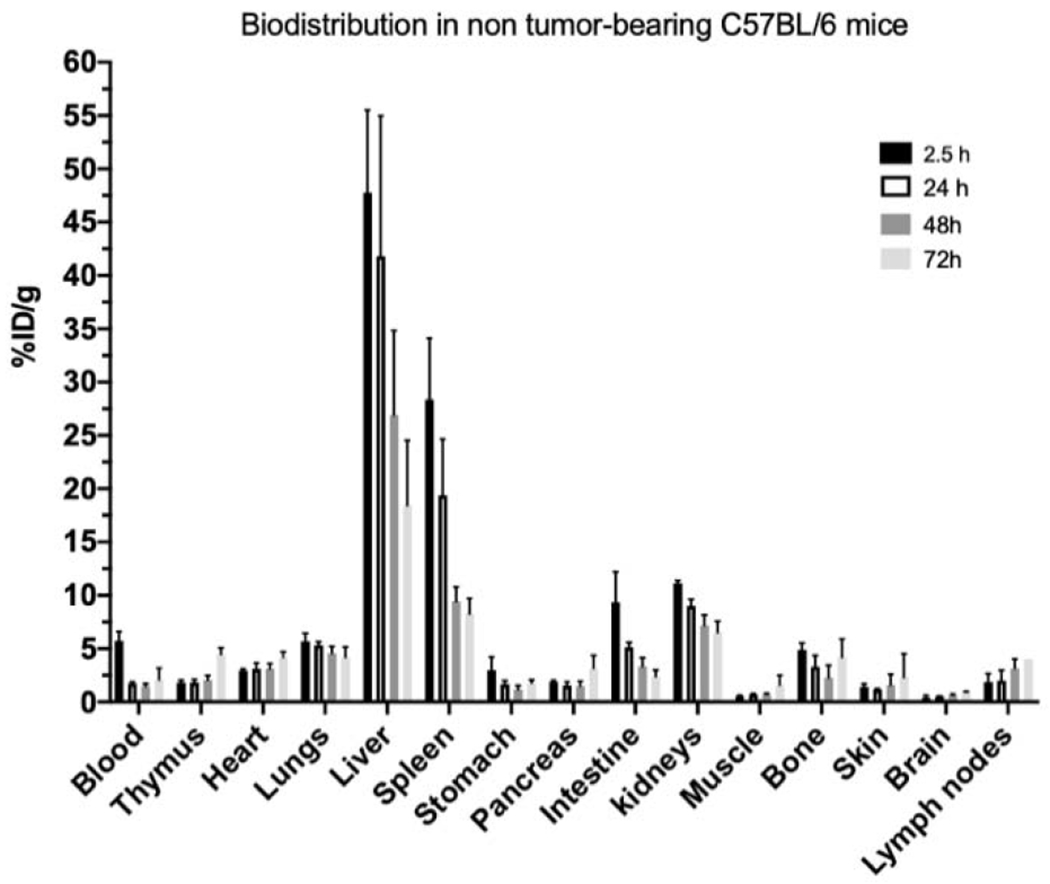
Biodistribution at selected times post injection of [64Cu]Cu-NOTA-aCD40 (2 μg) in non-tumor bearing C57BL/6 mice.
Fig. 8.
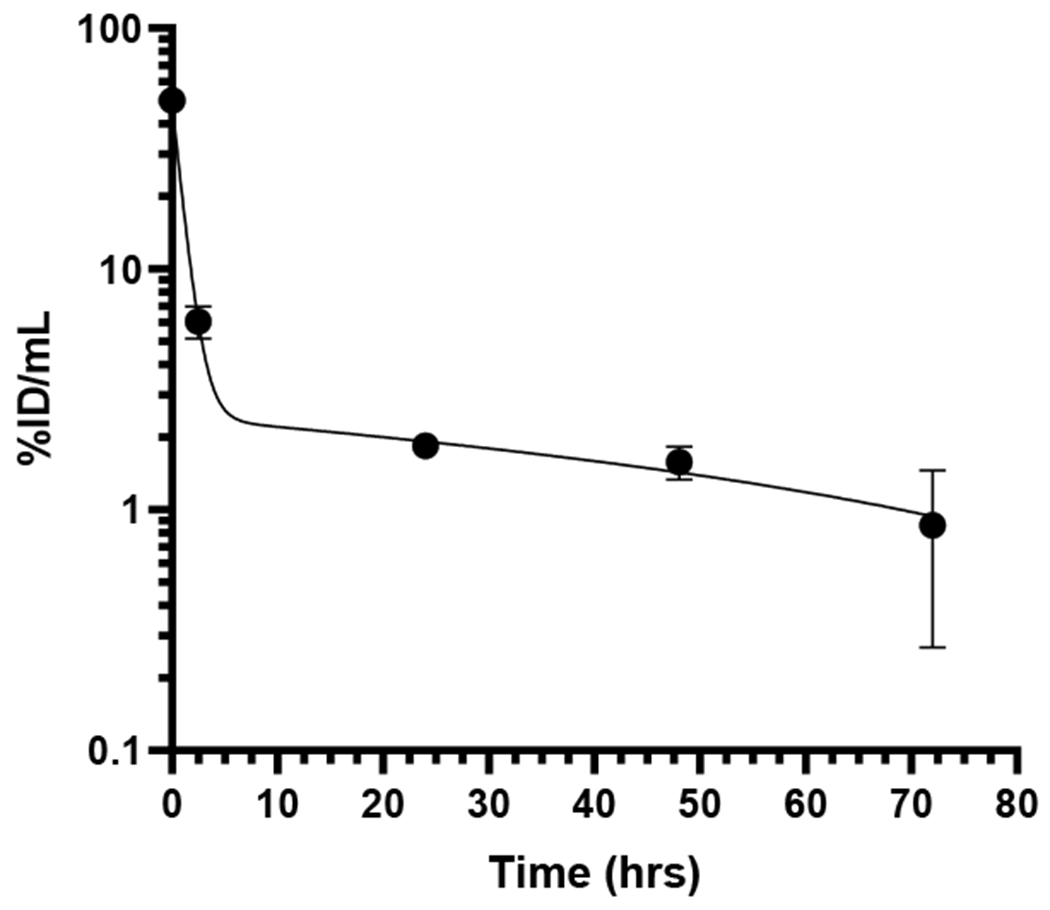
Elimination of [64Cu]Cu-NOTA-aCD40 from the blood vs time post injection.
Table 1.
Pharmacokinetics of [64Cu]Cu-NOTA-aCD40.
| Parameters | |
|---|---|
| t1/2α (h) | 0.6 ± 0.05 |
| t1/2β (h) | 65.9 ± 64.6 |
| V1 (mL) | 2 ± 0 |
| Vss (mL) | 1232 ± 605 |
| CLtotal (mL/h) | 0.3 ± 0.1 |
| AUCtotal (%ID/mL × h) | 347 ± 190 |
Data were fitted using a two-compartment model with i.v. bolus input.
t1/2,α = alpha phase half-life, t1/2,β = beta phase half-life, V1 = volume of distribution (central compartment), Vss = volume of distribution at steady-state, CLtotal = clearance, and AUCtotal = area under the curve.
Estimates of absorbed radiation dose (μSv/MBq) for the administration of [64Cu]Cu-NOTA-aCD40 to humans are shown in Table 2. Liver, spleen and gallbladder received the highest radiation doses, respectively, with the liver being the dose limiting organ. The predicted human doses (μSv/MBq) as effective dose and effective dose equivalent were 34 ± 2.4 and 43.1 ± 5.5 respectively.
Table 2.
Radiation dose estimates for adult male (μSv/MBq) using OLINDA.
| Organs | Mean absorbed dose ± SD (μSv/MBq) |
|---|---|
| Adrenals | 28.1 ± 1.7 |
| Brain | 4.6 ± 0.5 |
| Breasts | 17.9 ± 0.7 |
| Gallbladder | 35.7 ± 2.7 |
| LLI | 48.1 ± 5.2 |
| Small Intestine | 32.2 ± 2.3 |
| Stomach | 25.9 ± 0.5 |
| ULI | 40.9 ± 3.8 |
| Heart | 24.2 ± 1.5 |
| Kidneys | 53.7 ± 3.8 |
| Liver | 198 ± 28.7 |
| Lungs | 28.9 ± 5 |
| Muscle | 19.5 ± 0.8 |
| Ovaries | 22.1 ± 0.9 |
| Pancreas | 24.6 ± 3.4 |
| Red Marrow | 17.5 ± 0.7 |
| Osteogenic cells | 38 ± 1.5 |
| Skin | 16.6 ± 0.6 |
| Spleen | 120.4 ± 56.2 |
| Testes | 18 ± 0.6 |
| Thymus | 16.2 ± 3.6 |
| Thyroid | 18.5 ± 0.7 |
| Urinary Bladder Wall | 20.5 ± 0.8 |
| Uterus | 21.7 ± 0.9 |
| Total Body | 25.3 ± 1.3 |
| Effective Dose Equivalent | 43.1 ± 5.5 |
| Effective dose | 34 ± 2.4 |
Radiation absorbed doses were extrapolated to adult male human patient from the [64Cu]Cu-NOTA-aCD40 injected C57BL/6 mouse biodistribution data using the OLINDA/EXM version 1.0 computer program.
4. Discussion
[64Cu]Cu-NOTA-aCD40 was stable in mouse plasma at 37 °C, with no appreciable release of [64Cu]Cu from CD40 mAb detected up to 72 h, using ultrafiltration and ITLC methods. Similarly, [64Cu]CuCl2 was incubated with mouse plasma as a positive control and more than 95% of radioactivity was filtered from plasma after 72 h. Thus, [64Cu]Cu did not bind to large plasma proteins (e.g. transcuprein (270 kDa)), which is in agreement with other studies [45–48]. A plausible explanation for initial retention of 27% of the [64Cu]CuCl2 in the Amicon filter at 6 h is nonspecific interaction of copper with the filter which is obviated by gradual dilution after each filtration.
A high percentage of non-specific binding of [64Cu]Cu-NOTA-aCD40 (−40%) was shown in the blocked and control arms of binding assay. Replacing Ni-NTA beads and His-CD40 with streptavidin beads and biotinylated CD40 in the future may decrease the non-specific binding as shown by Sharma et al. with 89Zr-deferoxamine (DFO)-Trastuzumab and His-HER2 versus biotinylated HER2 [39]. The choice of Ni-NTA beads was due to availability of His-CD40 antigen. In vivo, [64Cu]Cu-NOTA-aCD40 tumor accumulation in a CD40+ pancreatic tumor was ~21 %ID/g and significantly greater than the IgG isotype, also suggesting specific tumor binding. Injection of the therapeutic mass of cold aCD40 mAb alone or in combination with aPD-1 and aCTLA-4 mAbs significantly reduced the tumor and spleen uptake of [64Cu]Cu-NOTA-aCD40 compared to the control group at 48 h after injection, whereas liver uptake was increased. The highest spleen to liver ratio (2.7-fold), and the highest tumor uptake was observed with the injection of 10 μg of [64Cu]Cu-NOTA-aCD40 without cold mAbs.
The second round of injections one or three weeks apart resulted in high liver uptake with minimal accumulation in other major organs. Identical observations following increasing dosing intervals from one week to three weeks rule out the role of CD40 blocking or down-regulation. This finding could be explained by immunogenicity of the rat IgG CD40 mAb suggesting that repeated doses of rat mAbs may not be effective. These findings indicate that diagnostic (10 μg) and therapeutic (100 μg) masses of aCD40 mAb (alone or combined with other mAbs) do not result in identical biodistribution profiles. Mass-dependency and possible immunogenicity must be taken into consideration when dosing immunotherapy combinations with multiple mAbs.
[64Cu]Cu-NOTA-aCD40 (2 μg; equivalent to a 5 mg patient dose) was eliminated from the blood following i.v. injection in mice, and the elimination was well described by a two compartment (α-phase and β-phase) model. Decreasing the mass dose of [64Cu]Cu-NOTA-aCD40 from 10 μg to 2 μg resulted in a 3-fold higher liver uptake compared to the spleen suggesting that co-injection of cold and radiolabeled mAbs might be required to achieve the optimum spleen or tumor to liver uptake ratio in patients. The low uptake of [64Cu]Cu-NOTA-aCD40 in the blood as early as 2.5 h p.i. revealed the short serum half-life of the aCD40 mAb which agrees with the findings of clinical studies with various CD40 mAbs [26]. A plausible explanation for unusual short serum half-life of this mAb could be due to the large sink of CD40 antigens in the body [26].
The annual dose limit of the radiation doses to the radiosensitive organs (whole body and active blood-forming organs) should not exceed 50 mSv (5 rem)[49]. Based on the projected human doses, the maximum injected activity per annum would be limited by the liver. The safe injected activity per annum would be 252.5 MBq and aCD40 imaging with [64Cu]Cu could then be accomplished within safe limits. [64Cu]Cu and [89Zr]Zr labeled human aCD40 mAb ([64Cu]Cu-DOTA-hCD40 and [89Zr]Zr-DFO-hCD40) are being developed for dosimetry and human studies.
5. Conclusion
In this study, ~20%ID/g of aCD40 accumulated in a CD40 expressing pancreatic tumor and this accumulation was greater than a control mAb. The ratio of spleen to liver accumulation exceeded that of the control mAb and was greatest with a single small, injected mass. The feasibility of human aCD40 imaging was established based on extrapolation of the organ specificity to human imaging.
Supplementary Material
Highlights.
Agonist CD40 antibody accumulates a ~21 %ID/g in murine pancreatic tumors
Human aCD40 imaging feasibility was established
Immunogenicity must be considered with repeated doses of rat IgGs in mice
Acknowledgments
The authors would like to acknowledge the Stanford Center for Innovation in In-Vivo Imaging (SCi3) and, in particular Frezghi Habte for his assistance with pre-clinical imaging. We also thank Theresa McLaughlin for assistance with mass spectrometry (Stanford University Mass Spectrometry). This work was supported by R01CA253316 and R01CA210553.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Richards DM, Sefrin JP, Gieffers C, Hill O, and Merz C. Concepts for agonistic targeting of CD40 in immuno-oncology. Hum Vaccin Immunother 2020;16:377–87 DOI: 10.1080/21645515.2019.1653744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Sarmiento L, Svensson J, Barchetta I, Giwercman A, and Cilio CM. Copy number of the X-linked genes TLR7 and CD40L influences innate and adaptive immune responses. Scand J Immunol 2019;90:e12776 DOI: 10.1111/sji.12776. [DOI] [PubMed] [Google Scholar]
- [3].Stone ML, Graham K, Aldridge D, Jain K, Pan X, Pachter J, et al. A CD40 agonist potentiates the efficacy and immune-stimulatory capacity of chemotherapy in combination with a focal adhesion kinase inhibitor in a mouse model of pancreatic ductal adenocarcinoma. Cancer Res 2019;79 DOI: 10.1158/1538-7445.sabcs18-115.31641034 [DOI] [Google Scholar]
- [4].Vonderheide RH. CD40 agonist antibodies in cancer immunotherapy. Annu Rev Med 2020;71:47–58 DOI: 10.1146/annurev-med-062518-045435. [DOI] [PubMed] [Google Scholar]
- [5].Knorr DA, Dahan R, and Ravetch JV. Toxicity of an Fc-engineered anti-CD40 antibody is abrogated by intratumoral injection and results in durable antitumor immunity. Proc Natl Acad Sci U S A 2018;115:11048–53 DOI: 10.1073/pnas.1810566115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Mayes PA, Hance KW, and Hoos A. The promise and challenges of immune agonist antibody development in cancer. Nat Rev Drug Discov 2018;17:509–27 DOI: 10.1038/nrd.2018.75. [DOI] [PubMed] [Google Scholar]
- [7].Beatty GL, Li Y, and Long KB. Cancer immunotherapy: activating innate and adaptive immunity through CD40 agonists. Expert Rev Anticancer Ther 2017;17:175–86 DOI: 10.1080/14737140.2017.1270208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hoves S, Ooi CH, Wolter C, Sade H, Bissinger S, Schmittnaegel M, et al. Rapid activation of tumor-associated macrophages boosts preexisting tumor immunity. J Exp Med 2018;215:859–76 DOI: 10.1084/jem.20171440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Nimanong S, Ostroumov D, Wingerath J, Knocke S, Woller N, Gurlevik E, et al. CD40 signaling drives potent cellular immune responses in heterologous cancer vaccinations. Cancer Res 2017;77:1918–26 DOI: 10.1158/0008-5472.CAN-16-2089. [DOI] [PubMed] [Google Scholar]
- [10].Perry CJ, Munoz-Rojas AR, Meeth KM, Kellman LN, Amezquita RA, Thakral D, et al. Myeloid-targeted immunotherapies act in synergy to induce inflammation and antitumor immunity. J Exp Med 2018;215:877–93 DOI: 10.1084/jem.20171435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Singh M, Vianden C, Cantwell MJ, Dai Z, Xiao Z, Sharma M, et al. Intratumoral CD40 activation and checkpoint blockade induces T cell-mediated eradication of melanoma in the brain. Nat Commun 2017;8:1447 DOI: 10.1038/s41467-017-01572-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ward-Kavanagh LK, Kokolus KM, Cooper TK, Lukacher AE, and Schell TD. Combined sublethal irradiation and agonist anti-CD40 enhance donor T cell accumulation and control of autochthonous murine pancreatic tumors. Cancer Immunol Immunother 2018;67:639–52 DOI: 10.1007/s00262-018-2115-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Stromnes IM, Burrack AL, Hulbert A, Bonson P, Black C, Brockenbrough JS, et al. Differential effects of depleting versus programming tumor-associated macrophages on engineered T cells in pancreatic ductal Adenocarcinoma. Cancer Immunol Res 2019;7:977–89 DOI: 10.1158/2326-6066.CIR-18-0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Elgueta R, Benson MJ, de Vries VC, Wasiuk A, Guo Y, and Noelle RJ. Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol Rev 2009;229:152–72 DOI: 10.1111/j.1600-065X.2009.00782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ahonen CL, Doxsee CL, McGurran SM, Riter TR, Wade WF, Barth RJ, et al. Combined TLR and CD40 triggering induces potent CD8+ T cell expansion with variable dependence on type I IFN. J Exp Med 2004;199:775–84 DOI: 10.1084/jem.20031591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Beatty GL, Chiorean EG, Fishman MP, Saboury B, Teitelbaum UR, Sun W, et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science 2011;331:1612–6 DOI: 10.1126/science.1198443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Caisova V, Uher O, Nedbalova P, Jochmanova I, Kvardova K, Masakova K, et al. Effective cancer immunotherapy based on combination of TLR agonists with stimulation of phagocytosis. Int Immunopharmacol 2018;59:86–96 DOI: 10.1016/j.intimp.2018.03.038. [DOI] [PubMed] [Google Scholar]
- [18]. https://www.sciencedaily.com/releases/2019/03/190331192532.htm.
- [19]. https://www.cancer.gov/about-cancer/treatment/clinical-trials/intervention/cd40-agonistic-monoclonal-antibody-apx005m.
- [20].He S, Zhao H, Fei M, Wu Y, Wang L, Zhu X, Li D Expression of the co-signaling molecules CD40-CD40L and their growth inhibitory effect on pancreatic cancer in vitro. Oncol Rep 2012;28:262–8. [DOI] [PubMed] [Google Scholar]
- [21].Unek T, Unek IT, Agalar AA, Sagol O, Ellidokuz H, Ertener O, et al. CD40 expression in pancreatic cancer. Hepatogastroenterology 2013;60:2085–93. [PubMed] [Google Scholar]
- [22].Byrne KT and Vonderheide RH. CD40 stimulation obviates innate sensors and drives T cell immunity in cancer. Cell Rep 2016;15:2719–32 DOI: 10.1016/j.celrep.2016.05.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Eriksson E, Milenova I, Wenthe J, Stahle M, Leja-Jarblad J, Ullenhag G, et al. Shaping the tumor stroma and sparking immune activation by CD40 and 4-1BB signaling induced by an armed oncolytic virus. Clin Cancer Res 2017;23:5846–57 DOI: 10.1158/1078-0432.CCR-17-0285. [DOI] [PubMed] [Google Scholar]
- [24].Eriksson E, Moreno R, Milenova I, Liljenfeldt L, Dieterich LC, Christiansson L, et al. Activation of myeloid and endothelial cells by CD40L gene therapy supports T-cell expansion and migration into the tumor microenvironment. Gene Ther 2016;24:92–103 DOI: 10.1038/gt.2016.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Drachman JG, McDonald M, Harrop KL, Munshi NC, Niesvizky R, Berenson JR, et al. A phase I humanized anti-CD40 monoclonal Antibody (SGN-40) in patients with multiple myeloma. Blood 2005;106:2572 DOI: 10.1182/blood.V106.11.2572.2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ruter J, Antonia SJ, Burris HA, Huhn RD, and Vonderheide RH. Immune modulation with weekly dosing of an agonist CD40 antibody in a phase I study of patients with advanced solid tumors. Cancer Biol Ther 2010;10:983–93 DOI: 10.4161/cbt.10.10.13251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Hussein M, Berenson JR, Niesvizky R, Munshi N, Matous J, Sobecks R, et al. A phase I multidose study of dacetuzumab (SGN-40; humanized anti-CD40 monoclonal antibody) in patients with multiple myeloma. Haematologica 2010;95:845–8 DOI: 10.3324/haematol.2009.008003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Davda J, Declerck P, Hu-Lieskovan S, Hickling TP, Jacobs IA, Chou J, et al. Immunogenicity of immunomodulatory, antibody-based, oncology therapeutics. J Immunother Cancer 2019;7, 105 DOI: 10.1186/s40425-019-0586-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Harding FA, Stickler MM, Razo J, and DuBridge RB. The immunogenicity of humanized and fully human antibodies: residual immunogenicity resides in the CDR regions. MAbs 2010;2:256–65 DOI: 10.4161/mabs.2.3.11641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Niemeijer AN, Leung D, Huisman MC, Bahce I, Hoekstra OS, van Dongen G, et al. Whole body PD-1 and PD-L1 positron emission tomography in patients with non-small-cell lung cancer. Nat Commun 2018;9:4664 DOI: 10.1038/s41467-018-07131-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kawai O, Ishii G, Kubota K, Murata Y, Naito Y, Mizuno T, et al. Predominant infiltration of macrophages and CD8(+) T cells in cancer nests is a significant predictor of survival in stage IV nonsmall cell lung cancer. Cancer 2008;113:1387–95 DOI: 10.1002/cncr.23712. [DOI] [PubMed] [Google Scholar]
- [32].Wei L, Shi J, Jagoda E, Wong K, Opina C, Strauss J, et al. Development of 89Zr-Avelumab for clinical studies. J Nucl Med 2019;60:1060. [Google Scholar]
- [33].Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 2014;515:563–7 DOI: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Lamberts LE, Williams SP, Terwisscha van Scheltinga AG, Lub-de Hooge MN, Schroder CP, Gietema JA, et al. Antibody positron emission tomography imaging in anticancer drug development. J Clin Oncol 2015;33:1491–504 DOI: 10.1200/JCO.2014.57.8278. [DOI] [PubMed] [Google Scholar]
- [35].Niemeijer A, Oprea-Lager D, Huisman M, Boellaard R, Hoekstra O, De Wit - Van Der Veen L, et al. P1.04-12 tumor uptake and biodistribution of 89Zr-labeled pembrolizumab in patients with metastatic non-small-cell lung cancer. J Thorac Oncol 2019;14:S443–S DOI: 10.1016/j.jtho.2019.08.915. [DOI] [Google Scholar]
- [36].Borjesson PK, Jauw YW, de Bree R, Roos JC, Castelijns JA, Leemans CR, et al. Radiation dosimetry of 89Zr-labeled chimeric monoclonal antibody U36 as used for immuno-PET in head and neck cancer patients. J Nucl Med 2009;50:1828–36 DOI: 10.2967/jnumed.109.065862. [DOI] [PubMed] [Google Scholar]
- [37].Holland JP, Divilov V, Bander NH, Smith-Jones PM, Larson SM, and Lewis JS. 89Zr-DFO-J591 for immunoPET of prostate-specific membrane antigen expression in vivo. J Nucl Med 2010;51:1293–300 DOI: 10.2967/jnumed.110.076174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Zettlitz KA, Tavare R, Knowles SM, Steward KK, Timmerman JM, and Wu AM. ImmunoPET of malignant and normal B cells with 89Zr- and 124I-labeled obinutuzumab antibody fragments reveals differential CD20 internalization in vivo. Clin Cancer Res 2017;23:7242–52 DOI: 10.1158/1078-0432.CCR-17-0855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Sharma SK, Lyashchenko SK, Park HA, Pillarsetty N, Roux Y, Wu J, et al. A rapid bead-based radioligand binding assay for the determination of target-binding fraction and quality control of radiopharmaceuticals. Nucl Med Biol 2019;71:32–8 DOI: 10.1016/j.nucmedbio.2019.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Boj SF, Hwang CI, Baker LA, Chio II, Engle DD, Corbo V, et al. Organoid models of human and mouse ductal pancreatic cancer. Cell 2015;160:324–38 DOI: 10.1016/j.cell.2014.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Kirschner AS, Ice RD, and Beierwaltes WH. Radiation dosimetry of 131I-19-iodocholesterol. J Nucl Med 1973;14:713–7. [PubMed] [Google Scholar]
- [42].Aghevlian S, Cai Z, Lu Y, Hedley DW, Winnik MA, and Reilly RM. Radioimmunotherapy of PANC-1 human pancreatic cancer xenografts in NRG mice with panitumumab modified with metal-chelating polymers complexed to 177Lu. Mol Pharm 2019;16:768–78 DOI: 10.1021/acs.molpharmaceut.8b01040. [DOI] [PubMed] [Google Scholar]
- [43].Aghevlian S, Cai Z, Hedley D, Winnik MA, and Reilly RM. Radioimmunotherapy of PANC-1 human pancreatic cancer xenografts in NOD/SCID or NRG mice with Panitumumab labeled with Auger electron emitting, 111In or beta-particle emitting, 177Lu. EJNMMI Radiopharm Chem 2020;5:22 DOI: 10.1186/s41181-020-00111-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Stabin MG, Sparks RB, and Crowe E. OLINDA/EXM: the second-generation personal computer software for internal dose assessment in nuclear medicine. J Nucl Med 2005;46:1023–7. [PubMed] [Google Scholar]
- [45].Kim H, Lee SJ, Kim JS, Davies-Venn C, Cho HJ, Won SJ, et al. Pharmacokinetics and microbiodistribution of 64Cu-labeled collagen-binding peptides in chronic myocardial infarction. Nucl Med Commun 2016;37:1306–17 DOI: 10.1097/MNM.0000000000000590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].De Silva RA, Jain S, Lears KA, Chong HS, Kang CS, Sun X, et al. Copper-64 radiolabeling and biological evaluation of bifunctional chelators for radiopharmaceutical development. Nucl Med Biol 2012;39:1099–104 DOI: 10.1016/j.nucmedbio.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Cooper MS, Ma MT, Sunassee K, Shaw KP, Williams JD, Paul RL, et al. Comparison of (64)Cu-complexing bifunctional chelators for radioimmunoconjugation: labeling efficiency, specific activity, and in vitro/in vivo stability. Bioconjug Chem 2012;23:1029–39 DOI: 10.1021/bc300037w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Zhang K, Aruva MR, Shanthly N, Cardi CA, Rattan S, Patel C, et al. PET imaging of VPAC1 expression in experimental and spontaneous prostate cancer. J Nucl Med 2008;49:112–21 DOI: 10.2967/jnumed.107.043703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].CFR - Code of Federal Regulations Title 21. 2020. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


